Abstract
Purpose
We previously demonstrated that the median survival of patients with poor prognosis non–small cell lung cancer (NSCLC) considered unfit for first-line platinum chemotherapy was <4 months. We evaluated whether VeriStrat could be used as a prognostic or predictive biomarker in this population.
Experimental design
We conducted a randomised double-blind trial among patients with untreated advanced NSCLC considered unfit for platinum chemotherapy because of poor performance status (PS) or multiple comorbidities. All patients received active supportive care (ASC) and were treated with either oral erlotinib or placebo daily. Five hundred twenty-seven patients had plasma samples for VeriStrat classification: good (VeriStrat Good [VSG]) or poor (VeriStrat Poor [VSP]). Main end-point was overall survival.
Results
Fifty-five percent patients had VSG, and 83% had Eastern Cooperative Oncology Group (ECOG) 2–3 at baseline. VeriStrat was strongly associated with survival. Among patients managed with ASC only, the adjusted hazard ratio (HR) was 0.54 (p < 0.001) for VSG versus VSP. The association was consistent across patient factors: HR = 0.25 (p = 0.004) and HR = 0.56 (p < 0.001) for ECOG 0–1 and 2–3, respectively, HR = 0.49 (0070 < 0.001) for age≥75 years and HR = 0.59 (p = 0.007) for stage IV. Several ECOG 2–3 patients had long survival: 2-year survival was 8% for VSG patients who had ASC, compared with 0% for VSP. VeriStrat status did not predict benefit from erlotinib treatment because the HRs for erlotinib versus placebo were similar between VSG and VSP patients.
Conclusions
VeriStrat was not a predictive marker for survival when considering first-line erlotinib for patients with NSCLC who had poor PS and were not recommended for platinum doublet therapies. However, VeriStrat was an independent prognostic marker of survival. It represents an objective measurement that could be considered alongside other patient factors to provide a more refined assessment of prognosis for this particular patient group. VSG patients could be selected for treatment trials because of better survival, while VSP patients can continue to be treated conservatively or offered trials of less toxic agents.
Trial registration ISRCTN Number
ISRCTN02370070.
Keywords: Non-small cell lung cancer, Biomarker, Prognostic, Predictive, VeriStrat, Proteonomic, Poor performance ECOG 2&3, Active supportive care
Highlights
-
•
83% advanced NSCLC patients unfit for chemotherapy have poor performance status.
-
•
VeriStrat (proteomic blood test) is an independent prognostic marker for survival.
-
•
Patients classified as VeriStrat Good were less likely to die than those classified as VeriStrat Poor.
-
•
VeriStrat can refine patient prognosis in order to alter treatment management.
1. Introduction
Despite major advances made recently with immunotherapies for advanced non–small cell lung cancer (NSCLC), optimal treatment of patients with poor performance NSCLC (Eastern Cooperative Oncology Group, [ECOG] performance status [PS] ≥2) remains undefined, even though they represent a significant undertreated population with unmet need. In the 2018 UK National Lung Cancer Audit Annual Report, 41% of the 39,199 patients newly diagnosed in the UK were classified as having poor PS (ECOG ≥2) (Reference: https://www.rcplondon.ac.uk/projects/national-lung-cancer-audit). We previously demonstrated that the median survival of poor prognosis NSCLC patients considered unfit for first-line platinum chemotherapy was <4 months, from our multicentre TOPICAL phase III trial [1]. The significant progress using first-line immunotherapies, with or without platinum-based chemotherapy, is mainly confined to patients with good PS (ECOG 0–1).
Oral epidermal growth factor receptor (EGFR) tyrosine-kinase inhibitors (TKIs) including gefitinib, erlotinib and afatinib are established as first-line agents for EGFR-mutant advanced NSCLC and as subsequent lines of therapy for relapsed wild-type patients previously treated with immunotherapy and chemotherapy [2], [3], [4], [5], [6], [7], [8].
VeriStrat® (Biodesix, USA) is a commercially available proteomic test [9] to identify patients likely or unlikely to benefit from TKI treatment. Using pretreatment serum or plasma samples, this test assigns patients to either ‘VeriStrat Good (VSG)’ or ‘VeriStrat Poor (VSP)’ status, as an indicator of prognosis and response to treatment. The test uses matrix-assisted laser desorption/ionisation time of flight (MALDI-TOF) mass spectrometry and compares the intensity of eight mass spectral features with the intensity of those of a reference set [9].
Several studies show that VeriStrat is a prognostic marker such that VSG patients have better outcomes including survival than VSP patients [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]. However, the vast majority of the patients included in these studies had predominantly good PS (ECOG 0–1). Only one study included some patients who had poor status (ECOG 3) [10], and it cannot be assumed that the same prognostic relationship holds for these particular patients.
The TOPICAL trial [1] was based on patients with advanced NSCLC considered unfit for first-line platinum-based chemotherapy, primarily because of having poor PS (83% were ECOG ≥2, including 29% who were ECOG 3) or multiple comorbidities. The primary aim in this article is to examine the prognostic value of VeriStrat in the half of patients who received standard active supportive care (ASC) only, including palliative radiotherapy (i.e. the placebo arm). This could identify patient subgroups with better or worse outcomes, who might then be managed differently by their clinician compared with the traditional selection criteria based mainly on PS as a prognostic selection marker.
2. Materials and methods
TOPICAL represents a unique study population because all patients were chemotherapy naïve (so no potential interaction effects of prior cytotoxic therapy); and most (83%) had poor PS status (ECOG 2–3) reflecting real-world practice.
2.1. Patients and procedures
TOPICAL has been described before [1]. Newly diagnosed patients with stage IIIB/IV NSCLC were considered unfit for first-line platinum-based doublet chemotherapy because of ECOG 2–3 and/or they had multiple comorbidities. Majority of patients had EGFR wild-type tumours [1]. All patients received ASC including palliative radiotherapy for symptomatic sites. They were randomised to 150-mg oral erlotinib or placebo daily, until progressive disease or toxicity. They attended clinic every month in the first year, then every 2 months thereafter. Computed tomography scans were performed at 3 and 6 months and when clinically indicated. Rash was graded using the following criteria: erythema alone, erythema with papules, erythema with papules and pustules and erythema with papules and confluent pustules. Patients were categorised as having first-cycle rash when any of the above symptoms were diagnosed approximately one month after starting erlotinib treatment.
Before starting erlotinib/placebo, patients provided a 10-ml blood sample, collected in an ethylene diamine tetra-acetic acid tube, which was posted immediately to the central laboratory at University College London, where plasma aliquots were stored at −80 °C. Samples were later tested with VeriStrat by the Biodesix laboratory (US), where personnel were blinded to patient/tumour characteristics and outcomes [9]. Of 670 TOPICAL patients, 535 had plasma samples available; of which, 8 had an indeterminate VeriStrat classification.
2.2. Statistical methods
The primary trial end-point was overall survival (OS), measured from the date of randomisation until death from any cause. Progression-free survival (PFS) was measured from randomisation until Response Evaluation Criteria in Solid Tumours (RECIST) progression or death from any cause. Patients who did not die, or did not have a PFS event, were censored at the date they were last known to be alive.
We evaluated two potential clinical uses of the VeriStrat test separately: as a (i) prognostic marker or (ii) predictive biomarker for erlotinib relative to placebo. Kaplan–Meier curves and Cox proportional univariable and multivariable hazards regression were used to examine the association between VeriStrat status and OS and PFS, using hazard ratios (HRs), including adjustment for patient and tumour characteristics. The prognostic value of VeriStrat was determined by examining the HR for VSG versus VSP. The predictive value of VeriStrat was determined by examining the HR for erlotinib versus placebo in each VeriStrat status group and tested using an interaction term between treatment and VeriStrat status. Various exploratory subgroup analyses were carried out, but no allowance was made for multiple testing.
3. Results
Baseline characteristics did not differ between the 527 (out of 670 total) patients contributing to the analyses below and the 143 who were not included (either no blood sample available for VeriStrat testing, or the 8 patients who had an indeterminate VS status). Fifty-five percent (288/527) of patients had VSG status (Table 1). Eighty-three percent (438/527) had ECOG 2–3 (including 143 with ECOG 3). The proportion with VSP increased with worsening PS: 36% (32/89), 45% (133/295) and 52% (74/143) for ECOG 0–1, 2 and 3, respectively (trend p = 0.02). Five hundred eighteen patients had died (most due to lung cancer), and 523 had progressed or died (PFS event). Four hundred forty patients received additional anticancer therapies; of which, at least 195 had palliative radiotherapy.
Table 1.
Baseline characteristics in the 527 patients classified as having a VeriStrat Poor or Good status.
| Characteristics | VeriStrat Poor N = 239 |
VeriStrat Good N = 288 |
P-value for differenceb |
|---|---|---|---|
| Age at entry, median (range) | 76 (51–90) | 78 (51–91) | 0.006 |
| Sex | Number of patients (%) | ||
| Male | 159 (66.5) | 156 (54.2) | 0.004 |
| Female | 80 (33.5) | 132 (45.8) | |
| ECOG performance status | |||
| 0-1 (only 9 ECOG 0) | 32 (13.4) | 57 (19.8) | 0.06 |
| 2 | 133 (55.6) | 162 (56.2) | |
| 3 | 74 (31.0) | 69 (24.0) | |
| Stage | |||
| IIIB | 93 (38.9) | 92 (31.9) | 0.10 |
| IV | 146 (61.1) | 196 (68.1) | |
| Histology | |||
| Adenocarcinoma | 66 (27.6) | 129 (44.8) | <0.001 |
| Squamous | 121 (50.6) | 93 (32.3) | |
| Other | 52 (21.8) | 66 (22.9) | |
| Smoking status | |||
| Current smoker | 89 (37.2) | 100 (34.7) | 0.14 |
| Former smoker | 142 (59.4) | 167 (58.0) | |
| Never smoked | 8 (3.4) | 21 (7.3) | |
| Known EGFR status | n = 144 | n = 166 | 0.006 |
| Mutant positive | 6 (4.2) | 21 (12.6) | |
| Wild-type | 138 (95.8) | 145 (87.4) | |
| Trial treatment | 0.04 | ||
| Erlotinib | 115 (48.1) | 164 (56.9) | |
| Placebo | 124 (51.9) | 124 (43.1) | |
| Rash statusa | n = 197 | n = 267 | 0.12 |
| Placebo | 103 (52.3) | 114 (42.7) | |
| No rash (erlotinib) | 39 (19.8) | 65 (24.3) | |
| Rash (erlotinib) | 55 (27.9) | 88 (33.0) | |
ECOG, Eastern Cooperative Oncology Group; EGFR, epidermal growth factor receptor.
First-cycle rash in the erlotinib group (among patients who started treatment and alive at 28 days).
P-value for the difference between VeriStrat Poor and Good.
3.1. VeriStrat as a prognostic biomarker in patients who had ASC only
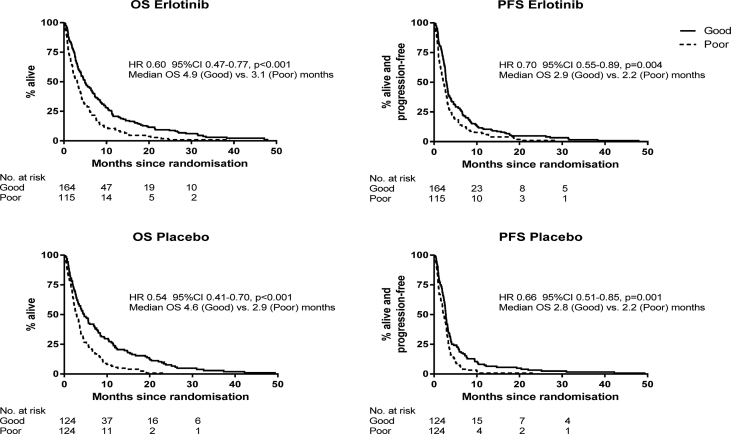
Patients who had ASC only (i.e. placebo) who were classified as VSG had much better OS and PFS than those with VSP classification (Fig. 1). The median OS was 4.6 months for VSG (95% CI 3.3–6.9), compared with 2.9 months VSP (95% CI 2.3–3.5), with HR 0.54; 46% reduction in mortality (p < 0.001). VeriStrat can identify a subgroup of patients who have better outcomes in those who do not receive systemic therapies.
Fig. 1.
VeriStrat as a prognostic marker: Kaplan–Meier curves for VeriStrat Good and Poor according to the treatment group (placebo patients had active supportive care only). The unadjusted hazard ratios (HRs) for VeriStrat Good vs. Poor are shown (adjusted HRs in Table 3). CI, confidence interval; OS, overall survival.
We noted the relatively long survival for several VSG patients (Fig. 1). The 2-year survival rates (ASC only) were 7.6% (95% CI 2.8–12.4) for VSG patients but 0% for VSP patients. At 3 years, the rate was 2.9% for VSG.
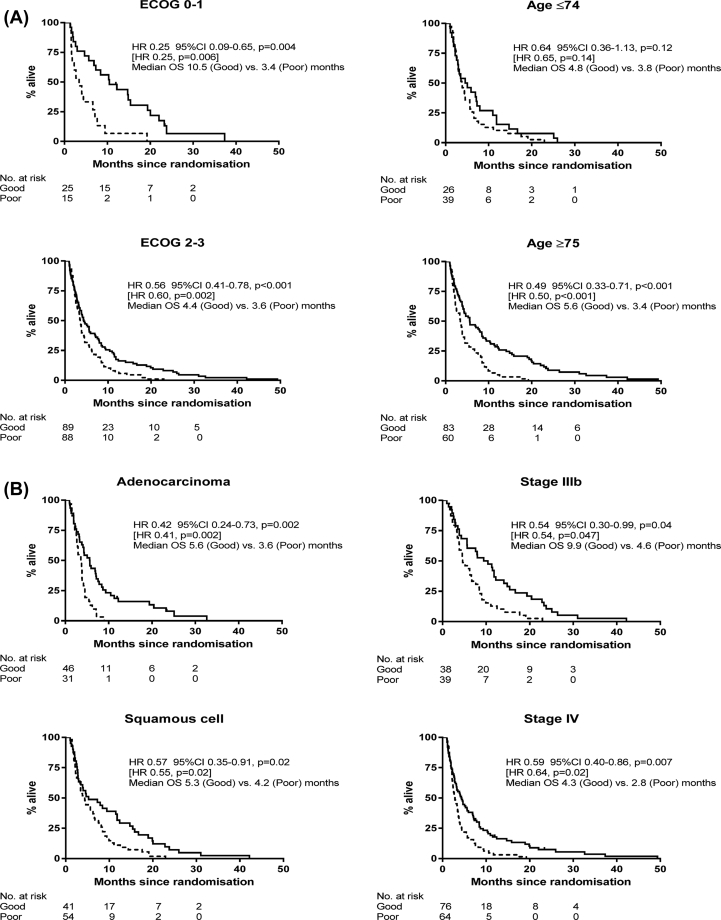
Fig. 2 shows the results separately by baseline ECOG status, age, stage and histology (these subgroup HRs could form a forest plot, but we want to show the whole OS curves to visualise the tails). There were strong associations between VeriStrat status and OS, regardless of ECOG. The HRs were 0.25 for ECOG 0–1 (75% reduction in mortality or striking difference in median OS from 3.4 to 10.5 months) and 0.56 for ECOG 2–3 (44% mortality reduction or difference in median OS from 3.6 to 4.4 months). Some VSG patients with ECOG 2–3 demonstrated very long survival, even among those who were considered to have the worse prognosis at baseline. The 2-year survival rate for ASC only patients with ECOG 2–3 was 8.1% (95% CI 2.3–13.8) for VSG compared with 0% for VSP. In addition, the adjusted HRs (VSG vs. VSP) were 0.45 (95% CI 0.27–0.75, p = 0.002) and 0.63 (95% CI 0.42–0.94, p = 0.02) for females and males, respectively.
Fig. 2.
A. VeriStrat as a prognostic marker among patients who had active supportive care only, according to ECOG and age: Overall survival for VeriStrat Good (solid line) and Poor (dashed line). Hazard ratios (HRs) for VeriStrat Good vs. Poor are shown (adjusted for age, sex, ECOG, stage, histology and smoking, excluding the factor of interest). The HRs in square brackets are when EGFR-positives are excluded. Fig. 2B. VeriStrat as a prognostic marker among patients who had active supportive care only, according to histology and stage: Overall survival for VeriStrat Good (solid line) and Poor (dashed line). Hazard ratios (HRs) for VeriStrat Good vs. Poor are shown (adjusted for age, sex, ECOG, stage, histology and smoking, excluding the factor of interest). The HRs in square brackets are when EGFR-positives are excluded.
Furthermore, the clear separation between VSG and VSP patients was seen in different age groups, with a large effect (HR 0.49, p < 0.001) even among older patients, ≥75 years, and with a very long tail for VSG patients (Fig. 2A), similarly for stage, where the VSG vs. VSP HR for stage IV patients was 0.59 (p = 0.007), and again some VSG patients demonstrated long OS (Fig. 2B). All of these subgroup comparisons were statistically significant.
Table 2 summarises OS among specific factors indicative of poor prognosis, with and without consideration of the VeriStrat test. For example, median OS for patients with ECOG 2–3 is 3.8 months, but patients who are also VSG have a median OS of 4.4 months. The corresponding 1-year OS rate increases from 12 to 17%, respectively. The effect was more pronounced for elderly patients. The median OS for all patients ≥75 years was 4.3 months, compared with 5.6 months if they were VSG; and the 1-year OS rate increases from 19 to 29%, whereas the 2-year rate almost doubles from 5 to 9%. In each of these high-risk groups (ECOG 2–3, elderly, or stage IV), about half of patients are classified as VSG, representing a significant number of patients whose treatment plan could be adapted accordingly, because their outcomes are noticeably better than expected. Similarly, if patients had been classified as VSP the median OS and 1 and 2-year survival rates were all clearly lower than when ignoring VeriStrat status. Results were similar after excluding patients with EGFR-positive tumours.
Table 2.
Comparison of overall survival (OS) without or with VeriStrat status—when considering specific factors associated with poor prognosis and histology, all patients had active supportive care only.
| Survival | All patients (ignoring VeriStrat) | VeriStrat Good | VeriStrat Poor | % with VeriStrat Good |
|---|---|---|---|---|
| Age ≥75 years (n = 143) | ||||
| Median OS, months | 4.3 [4.1] | 5.6 | 3.4 | 58 |
| 1-year rate (95% CI) | 19 (12–25) [17] | 29 (19–38) [26] | 5 (0–10) | |
| 2-year rate (95% CI) | 5 (1–9) [4] | 9 (3–15) [7] | 0 | |
| ECOG 2–3 (n = 177) | ||||
| Median OS, months | 3.8 | 4.4 | 3.6 | 50 |
| 1-year rate | 12 (7–17) | 17 (9–25) | 7 [1–12] | |
| 2-year rate | 4 (1–7) | 8 (2–14) [7] | 0 | |
| Stage IV (n = 140) | ||||
| Median OS, months | 3.4 [3.3] | 4.3 [4.1] | 2.8 | 54 |
| 1-year rate | 11 (6–16) [9] | 18 (9–26) [15] | 3 (0–7) | |
| 2-year rate | 4 (1–7) [2] | 7 (1–14) [4] | 0 | |
| Adenocarcinoma (n = 77) | ||||
| Median OS, months | 4.1 [3.9] | 5.6 | 3.6 | 60 |
| 1-year rate | 11 (4–18) [9] | 19 (7–30) [15] | 0 | |
| 2-year rate | 5 (1–12) [3] | 8 (1–16) [5] | 0 | |
| Squamous cell (n = 95) | ||||
| Median OS, months | 4.6 | 5.3 [6.3] | 4.2 [4.4] | 43 |
| 1-year rate | 22 (14–30) [20] | 32 (17–46) | 11 (3–19) | |
| 2-year rate | 3 (0–7) [3] | 7 (0–15) | 0 | |
ECOG, Eastern Cooperative Oncology Group; CI, confidence interval.
The aforementioned results were the same when patients with EGFR positive tumours were excluded, except where shown in square brackets.
3.2. VeriStrat as a prognostic biomarker in all patients
We analysed all patients together because this provides additional information on the variability of the factors examined to support the assessment of VeriStrat as a prognostic marker. Table 3 shows that VeriStrat and ECOG were independent risk factors with the strongest association with both OS and PFS. Stage and histology were also statistically significantly associated with outcomes. The adjusted OS HR was 0.58 (VSG versus VSP), similar to 0.54 for ASC patients only, and for PFS, it was 0.67, representing a 42% and 33% reduction in risk, respectively (both p < 0.001). These effects were after allowance for the treatment group, ECOG and age. The results were generally similar when only analysing the 283 patients known to have EGFR wild-type tumours (adjusted OS HR 0.53 p < 0.001, and adjusted PFS HR 0.68 p = 0.003) and when differentiating patients who did or did not develop first-cycle rash after receiving erlotinib (Supplemental Table 1). VeriStrat and ECOG were the two strongest prognostic factors for OS/PFS.
Table 3.
Multivariable Cox regression analyses showing the association between each factor and overall or progression-free survival, including the VeriStrat test as a prognostic biomarker.
| Factor | Overall survival hazard ratio (95% CI), p-value | Progression-free survival hazard ratio (95% CI), p-value | ||
|---|---|---|---|---|
| Ignoring first-cycle rash | ||||
| VeriStrat (Good vs. Poor) | 0.58 (0.48–0.70) | <0.001 | 0.67 (0.56–0.81) | <0.001 |
| Treatment (erlotinib vs. placebo) | 0.93 (0.87–1.11) | 0.41 | 0.85 (0.71–1.02) | 0.08 |
| Age | 1.00 (0.99–1.02) | 0.74 | 1.00 (0.99–1.02) | 0.51 |
| Sex (females vs. males) | 0.82 (0.68–0.98) | 0.03 | 0.78 (0.65–0.94) | 0.009 |
| ECOG | ||||
| 0-1 | 1.0 | <0.001 | 1.0 | <0.001 |
| 2 | 1.30 (1.01–1.67) | 1.11 (0.87–1.43) | ||
| 3 | 2.04 (1.53–2.72) | 1.85 (1.39–2.47) | ||
| Stage (IV vs. IIIB) | 1.21 (1.00–1.46) | 0.04 | 1.23 (1.02–1.48) | 0.03 |
| Histology | ||||
| Squamous | 1.0 | 0.02 | 1.0 | 0.05 |
| Adenocarcinoma | 1.26 (1.02–1.55) | 1.16 (0.95–1.43) | ||
| Other | 1.34 (1.07–1.69) | 1.33 (1.06–1.68) | ||
| Smoking status | ||||
| Never/former smoker | 1.0 | 0.25 | 1.0 | 0.30 |
| Current smoker | 1.12 (0.92–1.35) | 1.10 (0.92–1.33) | ||
| EGFR (positive vs wild-type) | 0.53 (0.33–0.83) | 0.006 | 0.65 (0.42–1.01) | 0.06 |
| Allowing for first cycle rash | ||||
| VeriStrat (Good vs. Poor) | 0.61 (0.50–0.74) | <0.001 | 0.71 (0.58–0.87) | <0.001 |
| Treatment | ||||
| Placebo | 1.0 | <0.001 | 1.0 | 0.002 |
| Erlotinib, no rash | 1.29 (1.01–1.65) | 1.07 (0.84–1.37) | ||
| Erlotinib, rash | 0.76 (0.61–0.94) | 0.71 (0.57–0.89) | ||
| Age | 1.00 (0.99–1.02) | 0.67 | 1.01 (0.99–1.02) | 0.48 |
| Sex (females vs. males) | 0.83 (0.68–1.01) | 0.06 | 0.82 (0.67–0.99) | 0.04 |
| ECOG | ||||
| 0-1 | 1.0 | 0.001 | 1.0 | 0.004 |
| 2 | 1.26 (0.97–1.63) | 1.07 (0.83–1.38) | ||
| 3 | 1.75 (1.28–2.38) | 1.55 (1.14–2.11) | ||
| Stage (IV vs. IIIB) | 1.29 (1.06–1.57) | 0.01 | 1.31 (1.07–1.59) | 0.008 |
| Histology | ||||
| Squamous | 1.0 | 0.10 | 1.0 | 0.22 |
| Adenocarcinoma | 1.20 (0.96–1.51) | 1.10 (0.88–1.37) | ||
| Other | 1.28 (1.00–1.64) | 1.25 (0.97–1.60) | ||
| Smoking status | ||||
| Never/former smoker | 1.0 | 0.31 | 1.0 | 0.25 |
| Current smoker | 1.11 (0.90–1.36) | 1.13 (0.92–1.38) | ||
| EGFR (positive vs wild-type) | 0.55 (0.34–0.89) | 0.01 | 0.70 (0.44–1.11) | 0.13 |
ECOG, Eastern Cooperative Oncology Group; CI, confidence interval; EGFR, epidermal growth factor receptor; HR, hazard ratio.
Each HR is adjusted for all the other factors in the table except for EGFR status (because there were only 27 who were EGFR positive). The HRs for EGFR status are from a separate multivariable Cox regression which contains all the factors in the table.
3.3. VeriStrat as a prognostic biomarker in patients given erlotinib
For completeness, we also examined the prognostic role of VeriStrat among the trial patients who received erlotinib, acknowledging that erlotinib is currently only used for patients harbouring EGFR activating mutations. Median survival in our predominantly EGFR wild-type patients treated with erlotinib was 4.9 months (VSG; 95% CI 3.9–6.6) and 3.1 (VSP; 95% CI 2.1–3.8), with HR 0.60 (40% mortality reduction), p < 0.001; Fig. 1. As with patients who had ASC only, the VeriStrat test can clearly distinguish between good and poor prognosis patients receiving erlotinib. VSG patients almost always had better outcomes than VSP patients, which were seen across ECOG, age, stage and histology (Supplemental Figs. 1–4).
3.4. VeriStrat as a predictive biomarker
VeriStrat could not identify patients who might benefit from first-line erlotinib in the TOPICAL patients (Supplemental Table 2). The HRs for erlotinib versus placebo were generally similar between the VSG and VSP groups, even when first-cycle rash was taken into account. Among patients who had erlotinib and developed first-cycle rash, the HR was 0.75 (95% CI 0.56–1.00) if they were classified as VSG and 0.71 (95% CI 0.50–1.02) if they were classified as VSP, that is, the benefit seen with erlotinib was independent of the VeriStrat status. Similarly, the corresponding HRs for erlotinib patients who did not develop first-cycle rash were 1.34 (95% CI 0.96–1.87) and 1.19 (95% CI 0.81–1.76). The test for interaction between erlotinib rash status and VeriStrat was not statistically significant (p = 0.82). The same conclusions were made when only analysing the 283 patients known to have had EGFR wild-type tumours (Supplemental Table 2).
4. Discussion
TOPICAL is the largest randomised trial to evaluate VeriStrat in a group of patients with an expected poor prognosis, considered unfit for first-line platinum-based chemotherapy (median OS less than 4 months). VeriStrat was an independent prognostic biomarker for both OS and PFS in this poor prognosis group who were often unfit, elderly (median = 77 years) and with multiple comorbidities. Patients with VSG status were 42% less likely to die (OS HR = 0.58) or 33% less likely to progress or die (PFS HR = 0.67), than those with VSP status. These are large clinical effects, not often seen with traditional prognostic markers. Our findings could similarly apply to patients considered unfit for first-line treatment with a check-point inhibitor, combination check-point inhibitors or in combination with platinum-doublet therapy.
We focused on patients who had ASC (i.e. the placebo group in TOPICAL). Using the ECOG scale to assess PS in advanced NSCLC is routine practice to select patients clinically fit to receive systemic treatment. Because NSCLC patients with ECOG 2–3 have limited survival, they are usually excluded from many first-line chemotherapy and immunotherapy combination trials. However, a major finding from TOPICAL is that even within this poor performance population (83% had ECOG 2–3), the VeriStrat test can further distinguish patients who have a shorter or longer survival. This has never been reported before.
Table 4 summarises the randomised trials and observational studies of VeriStrat as a prognostic marker, including TOPICAL which is the third largest study. Although there are various treatment regimens, the studies consistently show that VSG patients have better OS and PFS than VSP patients, with all HRs less than one [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24]. However, all studies recruited patients with predominantly good PS, and most had already been previously treated. Only one study included some patients who had ECOG 3 [10], but even these only represented a fraction of the patients (33% of all study patients had ECOG 2–3), and they all had prior chemotherapy. The TOPICAL trial stands out from the rest, with 56% who had ECOG 2 and 27% with ECOG 3, and no patient had prior systemic treatment. The percentage of patients classified as VSG was generally lower in TOPICAL (55%) than the other trials (61–72%), which reflects the modest correlation between VeriStrat status and ECOG score.
Table 4.
Summary of clinical trials and observational studies that have examined the prognostic association between the VeriStrat test in advanced NSCLC (stage IIIb/IV, progressive/recurrent disease) and outcomes, including TOPICAL.
| Hazard ratio for Good vs Poor (95% CI)a |
|||||||
|---|---|---|---|---|---|---|---|
| Reference (first author) | Treatment | Line of therapy | Number patients | % with PS 2 | % with VeriStrat ‘Good’ | Overall survival | Progression-free survival |
| Randomised clinical trials | |||||||
| Carbone 2012 [10] | Erlotinib vs placebo | Second/third | 436 | 33 (PS 2–3) | 61 | 0.67 (0.45–1.01)b | 0.56 (0.40–0.80)b |
| 10.5 vs 4.0 monthsc | 3.7 vs 1.8 monthsc | ||||||
| Peters 2007 [11] | Erlotinib vs docetaxel | Second | 80e | 9 | 72 | 0.49 (0.28–0.86)# | 0.73 (0.44–1.22)# |
| Stinchcombe 2013 [12] | Gemcitabine vs erlotinib vs both | First | 98 | 28 | 64 | 0.53 (0.32–0.90) | 0.51 (0.30–0.86) |
| Gregorc 2014 [13] | Erlotinib vs pemetrexed or docetaxel | Second | 263 | 6 | 70 | 0.53 (0.35–0.80) | 0.57 (0.44–0.75)# |
| Gadgeel 2017 [14] | Erlotinib vs afatinib | Second | 675 | 0.3 | 61 | 0.41 (0.35–0.49) | 0.65 (0.54–0.77) |
| Spigel 2018 [15] | Erlotinib vs placebo (all had pazopanib) | Second/third | 88 | 14 | 72 | 0.42 (0.26–0.69)# | 0.44 (0.26–0.73)# |
| Buttigliero 2019 [16] | Tivantinib vs placebo (all had erlotinib) | Second/third | 996 | 0.2 | 72 | Tiva: 0.33 (0.26–0.42)# Plac: 0.45 (0.35–0.58)# |
0.52 (0.40–0.67) |
| TOPICAL trial | Erlotinib vs placebo | First | 527 | 56% (plus 27% PS 3) | 55 | 0.58 (0.48–0.70) | 0.67 (0.56–0.81) |
| Single-arm clinical trials | |||||||
| Taguchi 2007 [9] | Erlotinib | First | 96 | 26 | 72 | 0.53 (0.30–0.94) | 0.53 (0.33–0.85)# |
| Amann 2010 [17] | Erlotinib | First | 88 | 25 | 73 | 0.44 (0.18–1.08) | 0.51 (0.28–0.90)# |
| Carbone 2010 [18] | Erlotinib + bevacizumab | Second | 34 | 0 | 76 | 0.14 (0.03–0.58)# | 0.04 (0.01–0.24)# |
| Dingemans 2012 [19] | Sorafenib | ≥1 prior line | 55f | 5 | 58 | 0.77 (0.59–1.11)# | 0.71 (0.53–1.0)# |
| Kuiper 2012 [20] | Erlotinib + sorafenib | First | 50 | 0 | 66 | 0.30 (0.12–0.74)# | 0.40 (0.17–0.94)# |
| Akerley 2013 [21] | Erlotinib + bevacizumab | First | 41 | 0 | 76 | 16.5 vs 4.6 monthsd | 4.4 vs 1.4 monthsd |
| Gautschi 2013 [22] | Erlotinib + bevacizumab | First | 114 | 5 | 76 | 0.48 (0.29–0.78)# | 0.77 (0.48–1.22)# |
| Observational studies | |||||||
| Taguchi 2007 [9] | Gefitinib | ≥ second | 67 | 24 | 58 | 0.74 (0.55–0.99) | 0.56 (0.28–0.89)# |
| Lazzari 2012 [23] | Gefitinib | ≥1 prior line | 108 | 18 | 69 | 0.44 (0.26–0.72) | 0.52 (0.30–0.92) |
| Grossi 2016 [24] | Pemetrexed + platinum | First | 76 | 3 | 66 | 0.23 (0.11–0.46) | 0.39 (0.22–0.71) |
ECOG, Eastern Cooperative Oncology Group; CI, confidence interval; EGFR, epidermal growth factor receptor; HR, hazard ratio; OS, overall survival; PS, performance status.
None of the studies except Carbone 2012 and TOPICAL included patients with PS 3.
Adjusted HRs (for various patient/tumour characteristics), except where indicated by # which are unadjusted HRs.
Placebo group only.
Median OS (or PFS) among patients with Good vs Poor VeriStrat, all received erlotinib.
Median OS (or PFS) among patients with Good vs Poor VeriStrat.
Not EGFR positive.
All had KRAS mutant tumours.
In TOPICAL, both ECOG and VeriStrat were independent markers of prognosis and could be considered together to provide a more accurate clinical picture than either on their own. The ability of VeriStrat to distinguish patients who have better or worse survival was consistently seen among traditional adverse risk factors (e.g. elderly patients, or stage IV disease). VeriStrat possibly captures a disease-related additional immune and proinflammatory profile in the blood [25] that confers a survival advantage and is a more sensitive and objective marker than consideration of ECOG status, which is affected by clinician subjectivity and personal interpretation. The value to patients and clinicians is that consideration of ECOG 2 or 3 alone could underestimate their actual prognosis. The relative effects (HRs) and absolute risk differences at 1 and 2 years (Table 2) are in line with those seen with new lung cancer treatments in the last few years.
For patients who would usually receive ASC alone including palliative radiotherapy, VeriStrat could be used as a selection biomarker together with ECOG status. Those classified as VSG, even if they have poor ECOG status or are elderly, could potentially be treated more aggressively with well-tolerated, newer generation systemic agents or selected for experimental treatment trials compared with conventional approach. Given that about half of patients tested would be classified as VSG, this represents a significant number who might be given a different treatment plan and treated more aggressively than if they were not tested. VSP patients could be treated more conservatively because of their very poor outcomes. Alternatively, VSP patients might also be considered for less toxic experimental trials (e.g. immunomonotherapies) to attempt to improve their abysmal outcome as in the IPSOS trial with atezolizumab or other single agent [26].
First-line erlotinib is currently only recommended for treating advanced NSCLC patients with common EGFR activating mutation. Therefore, our analyses relating to VeriStrat as a predictive biomarker for erlotinib was for research interest only, simply because we had a group of poor PS patients who received erlotinib whom we previously demonstrated that patients who developed first-cycle rash was associated with improved OS compared to placebo (HR=0.76) [1]. However, we showed that VeriStrat was not a predictive marker for first-line erlotinib in our predominantly EGFR wild-type particular patient group, even when first-cycle rash was taken into account. Median OS was 4.9 months (erlotinib) and 4.6 months (placebo) among VSG patients, and for VSP patients they were 3.1 and 2.9 months respectively (Fig. 1). This is consistent with several randomised studies of pretreated patients [10], [11], [14], [16], while two other trials of second line therapy found evidence of the (negative) predictive value of VeriStrat, but all of these studies were conducted among primarily good PS patients [13], [14].
A limitation of TOPICAL was that we included patients with ECOG 2 who might now be treated with newer generation single-agent cytotoxic agents, but when TOPICAL was planned in 2002, provision of chemotherapy to such poor prognosis patients was not routine practice because many studies of second-/third-generation chemotherapy agents did not improve survival, and there were significant treatment-related toxicities associated with platinum-based chemotherapy. Another limitation was that EGFR status was not assessed on all trial patients which is now standard practice, an important consideration when examining VeriStrat as a predictive biomarker for an EGFR inhibitor and as a prognostic marker when analysing all patients together. Nevertheless, of the 670 patients randomised for the TOPICAL trial, tumour DNA was available for 390 patients (58%); and the incidence of EGFR mutation was only 7% (with a quarter classified as uncommon EGFR mutations), whereas the rest were EGFR wild-type NSCLC as reported previously [1]. Our results were very similar when only examining patients with known EGFR wild-type tumours (Supplemental Tables 1–2).
In conclusion, TOPICAL shows that VeriStrat was not a predictive marker for first-line erlotinib in our trial. However, we provide new evidence on the added clinical value of VeriStrat in a major EGFR wild-type NSCLC elderly population with poor PS and multiple comorbidities, unfit for first-line combination systemic therapy. Among patients who might receive ASC only, VeriStrat is an objective and reproducible measurement that is strongly associated with OS. It could add value to other clinical parameters including PS to aid patient selection and management by identifying longer term survivors who might be able to tolerate and benefit from appropriate upfront therapies or selection for treatment trials. While patients who have a poorer prognosis could have conservative treatments or be offered interventional trials with agents known to have relatively little toxicity, cost-effective studies on the use of VeriStrat as a selection marker would be useful.
Disclaimer
No funding source had any role in design and conduct of the study; collection, management, analysis and interpretation of the data and preparation, review or approval of the manuscript.
Provenance and peer review
Not commissioned; externally peer reviewed.
Data sharing statement
S.M.L. and A.H. had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author contribution
S.M.L. and A.H. were involved in study concept and design. All authors except A.E. and A.H. were involved in acquisition of data. A.E. and A.H. performed the statistical analysis. S.M.L. and A.H. interpreted the data. S.M.L. and A.H. drafted the manuscript. All authors critically revised the manuscript for important intellectual content. All authors approved the final manuscript submitted and the authorship list.
Ethical approval
The main TOPICAL trial had multicentre ethical approval from the South West Multi-Centre Research Ethics Committee (reference number MREC/04/6/32), and the translational work had approval from the West Midlands—Solihull Research Ethics Committee (reference number 18/WM/0206). All patients gave informed consent to provide blood and tissue samples for biological research.
Conflict of interest statement
M.H. has received honoraria from AstraZeneca and travel, accommodations or other expenses paid by MSD. R.L. has received honoraria from and has provided an uncompensated advisory/consultancy role to Roche, Pfizer and Merck Serono and has received research funding from Merck Serono and travel, accommodations or other expenses paid by Roche. A.H. has received fees for teaching from Roche, Merck Serono and Boehringer Ingelheim and for trial review committee membership from Roche and owns shares in Illumina and ThermoFisher. The remaining authors declare no conflict of interest.
Acknowledgements
University College London was the study sponsor. The main TOPICAL trial was funded by Cancer Research UK (C1438/A4147), with an educational grant from Roche. The VeriStrat analyses were performed by Biodesix, Colorado, USA.
Footnotes
Preliminary results presented at the 16th World Conference on Lung Cancer meeting, held in Denver, Colorado, USA, on Sep 6-9, 2015.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2019.07.025.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Lee S.M., Khan I., Upadhyay S. First-line erlotinib in patients with advanced non-small-cell lung cancer unsuitable for chemotherapy (TOPICAL): a double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2012;13(11):1161–1170. doi: 10.1016/S1470-2045(12)70412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shepherd F.A., Rodrigues Pereira J., Ciuleanu T. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353(2):123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 3.Kawaguchi T., Ando M., Asami K. Randomized phase III trial of erlotinib versus docetaxel as second- or third-line therapy in patients with advanced non-small-cell lung cancer: docetaxel and Erlotinib Lung Cancer Trial (DELTA) J Clin Oncol. 2014;32(18):1902–1908. doi: 10.1200/JCO.2013.52.4694. [DOI] [PubMed] [Google Scholar]
- 4.Garassino M.C., Martelli O., Broggini M. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): a randomised controlled trial. Lancet Oncol. 2013;14(10):981–988. doi: 10.1016/S1470-2045(13)70310-3. [DOI] [PubMed] [Google Scholar]
- 5.Chan B.A., Hughes B.G. Targeted therapy for non-small cell lung cancer: current standards and the promise of the future. Transl Lung Cancer Res. 2015;4(1):36–54. doi: 10.3978/j.issn.2218-6751.2014.05.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soria J.C., Felip E., Cobo M. Afatinib versus erlotinib as second-line treatment of patients with advanced squamous cell carcinoma of the lung (LUX-Lung 8): an open-label randomised controlled phase 3 trial. Lancet Oncol. 2015;16(8):897–907. doi: 10.1016/S1470-2045(15)00006-6. [DOI] [PubMed] [Google Scholar]
- 7.Herbst R.S., Prager D., Hermann R. TRIBUTE: a phase III trial of erlotinib hydrochloride (OSI-774) combined with carboplatin and paclitaxel chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2005;23:5892–5899. doi: 10.1200/JCO.2005.02.840. [DOI] [PubMed] [Google Scholar]
- 8.Giaccone G., Herbst R.S., Manegold C. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 1. J Clin Oncol. 2004;22:777–784. doi: 10.1200/JCO.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Taguchi F., Solomon B., Gregorc V. Mass spectrometry to classify non-small-cell lung cancer patients for clinical outcome after treatment with epidermal growth factor receptor tyrosine kinase inhibitors: a multicohort cross-institutional study. J Natl Cancer Inst. 2007 6;99(11):838–846. doi: 10.1093/jnci/djk195. [DOI] [PubMed] [Google Scholar]
- 10.Carbone D.P., Ding K., Roder H. Prognostic and predictive role of the VeriStrat plasma test in patients with advanced non-small-cell lung cancer treated with erlotinib or placebo in the NCIC Clinical Trials Group BR.21 trial. J Thorac Oncol. 2012;7(11):1653–1660. doi: 10.1097/JTO.0b013e31826c1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters S., Stahel R.A., Dafni U. Randomized phase III trial of erlotinib versus docetaxel in patients with advanced squamous cell non-small cell lung cancer failing first-line platinum-based doublet chemotherapy stratified by VeriStrat good versus VeriStrat poor. The European thoracic oncology platform (ETOP) EMPHASIS-lung trial. J Thorac Oncol. 2017;12(4):752–762. doi: 10.1016/j.jtho.2016.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Stinchcombe T.E., Roder J., Peterman A.H. A retrospective analysis of VeriStrat status on outcome of a randomized phase II trial of first-line therapy with gemcitabine, erlotinib, or the combination in elderly patients (age 70 years or older) with stage IIIB/IV non-small-cell lung cancer. J Thorac Oncol. 2013;8(4):443–451. doi: 10.1097/JTO.0b013e3182835577. [DOI] [PubMed] [Google Scholar]
- 13.Gregorc V., Novello S., Lazzari C. Predictive value of a proteomic signature in patients with non-small-cell lung cancer treated with second-line erlotinib or chemotherapy (PROSE): a biomarker-stratified, randomised phase 3 trial. Lancet Oncol. 2014;15(7):713–721. doi: 10.1016/S1470-2045(14)70162-7. [DOI] [PubMed] [Google Scholar]
- 14.Gadgeel S., Goss G., Soria J.C. Evaluation of the VeriStrat® serum protein test in patients with advanced squamous cell carcinoma of the lung treated with second-line afatinib or erlotinib in the phase III LUX-Lung 8 study. Lung Cancer. 2017;109:101–108. doi: 10.1016/j.lungcan.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Spigel D.R., Burris H.A., 3rd, Greco F.A., Shih K.C., Gian V.G., Lipman A.J. Erlotinib plus either pazopanib or placebo in patients with previously treated advanced non-small cell lung cancer: a randomized, placebo-controlled phase 2 trial with correlated serum proteomic signatures. Cancer. 2018;124(11):2355–2364. doi: 10.1002/cncr.31290. [DOI] [PubMed] [Google Scholar]
- 16.Buttigliero C., Shepherd F.A., Barlesi F., Schwartz B., Orlov S., Favaretto A.G. Retrospective assessment of a serum proteomic test in a phase III study comparing erlotinib plus placebo with erlotinib plus tivantinib (MARQUEE) in previously treated patients with advanced non-small cell lung cancer. The Oncologist. 2019;24(6):e251–e259. doi: 10.1634/theoncologist.2018-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amann J.M., Lee J.W., Roder H. Genetic and proteomic features associated with survival after treatment with erlotinib in first-line therapy of non-small cell lung cancer in Eastern Cooperative Oncology Group 3503. J Thorac Oncol. 2010;5(2):169–178. doi: 10.1097/JTO.0b013e3181c8cbd9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carbone D.P., Salmon J.S., Billheimer D. VeriStrat classifier for survival and time to progression in non-small cell lung cancer (NSCLC) patients treated with erlotinib and bevacizumab. Lung Cancer. 2010;69(3):337–340. doi: 10.1016/j.lungcan.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dingemans A.M., Mellema W.W., Groen H.J. A phase II study of sorafenib in patients with platinum-pretreated, advanced (Stage IIIb or IV) non-small cell lung cancer with a KRAS mutation. Clin Cancer Res. 2013;19(3):743–751. doi: 10.1158/1078-0432.CCR-12-1779. [DOI] [PubMed] [Google Scholar]
- 20.Kuiper J.L., Lind J.S., Groen H.J. VeriStrat(®) has prognostic value in advanced stage NSCLC patients treated with erlotinib and sorafenib. Br J Canc. 2012;107(11):1820. doi: 10.1038/bjc.2012.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akerley W., Boucher K., Rich N. A phase II study of bevacizumab and erlotinib as initial treatment for metastatic non-squamous, non-small cell lung cancer with serum proteomic evaluation. Lung Cancer. 2013;79(3):307–311. doi: 10.1016/j.lungcan.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Gautschi O., Dingemans A.M., Crowe S. VeriStrat® has a prognostic value for patients with advanced non-small cell lung cancer treated with erlotinib and bevacizumab in the first line: pooled analysis of SAKK19/05 and NTR528. Lung Cancer. 2013;79(1):59–64. doi: 10.1016/j.lungcan.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 23.Lazzari C., Spreafico A., Bachi A. Changes in plasma mass-spectral profile in course of treatment of non-small cell lung cancer patients with epidermal growth factor receptor tyrosine kinase inhibitors. J Thorac Oncol. 2012;7(1):40–48. doi: 10.1097/JTO.0b013e3182307f17. [DOI] [PubMed] [Google Scholar]
- 24.Grossi F., Rijavec E., Genova C. Serum proteomic test in advanced non-squamous non-small cell lung cancer treated in first line with standard chemotherapy. Br J Canc. 2017;116(1):36–43. doi: 10.1038/bjc.2016.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fidler M.J., Fhied C.L., Roder J. The serum-based VeriStrat test is associated with proinflammatory reactants and clinical outcome in non-small cell lung cancer patients. BMC Canc. 2018;18:310. doi: 10.1186/s12885-018-4193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.A Study of Atezolizumab Compared With Chemotherapy in Treatment Naïve Participants With Locally Advanced or Recurrent or Metastatic Non-Small Cell Lung Cancer Who Are Deemed Unsuitable For Platinum-Containing Therapy (IPSOS). https://clinicaltrials.gov/ct2/show/NCT03191786.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.