Abstract
Background:
The aims of this study were to analyze the metastasis pattern and prognosis of male breast cancer (MBC) and compare it with female breast cancer (FBC), and to determine the independent factors affecting the prognosis of MBC patients.
Methods:
Metastatic MBC diagnosed in the Surveillance, Epidemiology and End results (SEER) database from 2010 to 2015 were selected. Chi-squared test was used to compare clinicopathological characteristics. Survival differences were compared by Kaplan–Meier analysis. Cox proportional hazard model was used to determine the prognostic factors affecting overall survival.
Results:
A total of 2754 MBC patients were identified, of which 196 had distant metastasis. Compared with nonmetastatic MBC, metastatic MBC patients had a higher proportion of <60 years old and grade III–IV, and were more likely to receive chemotherapy and radiotherapy, while the proportion of surgery, central portion of the breast, and Her2–/HR+ was lower. Compared with metastatic FBC, metastatic MBC patients had a higher proportion of ⩾60 years old, central portion of the breast, surgery, simultaneous bone and lung metastasis, while the proportion of Her2+/HR–, triple negative, liver metastasis only, and simultaneous bone and liver metastasis was lower. MBC patients with lung alone, bone alone, and simultaneous lung and bone metastasis had a higher hazard ratio (2.41; 3.06; 2.52; p < 0.0001) compared with nonmetastatic patients.
Conclusions:
Compared with nonmetastatic MBC patients, metastatic MBC patients had unique clinicopathological features, and were also different from metastatic FBC patients. However, there was no difference in prognosis between metastatic MBC and FBC patients. Distant metastasis was an independent risk factor for the prognosis of MBC patients.
Keywords: breast cancer, male, metastasis, prognosis, SEER
Introduction
MBC is a rare disease, which has different clinicopathological and immunohistochemical features from FBC.1–4 According to the latest data from the American Cancer Society, it accounts for about 0.98% and 1.18% of breast cancer morbidity and mortality, respectively.5 The incidence of MBC has increased by 20–25% in the past few decades and continues to rise,6,7 and even reached 15% in some specific populations.8 The prognosis of MBC is worse than that of female patients due to older age and advanced stage at diagnosis.9–11 Distant metastasis is an important factor influencing the prognosis of breast cancer. Nearly 20–30% of breast cancer patients with early age will finally develop metastatic lesions after diagnosis,12,13 and 90% of breast cancer deaths are caused by metastasis leading to resistance to treatment.14 Two previous studies based on the Surveillance, Epidemiology and End results (SEER) database have shown that stage IV accounts for about 7–9% of all MBC patients.15–17 Because distant solid organ metastasis data in the SEER database was collected from 2010, previous studies were unable to study the specific metastasis sites of stage IV MBC patients.
Therefore, we identified MBC data recorded from 2010 to 2015 in the SEER database for this study. We studied metastatic MBC patients horizontally and longitudinally to determine their clinicopathological features and differences from metastatic FBC patients, and, at the same time, to determine independent factors affecting the prognosis of MBC patients.
Methods
Patient selection
For this study, we signed the SEER research data agreement to access SEER information with the username10067-Nov2018. Data were obtained following approved guidelines. The ethics committees considered this research to be on nonhuman subjects because the subjects were patients who had been researched by the United States Department of Health and Human Services and were publicly accessible and deidentified. Thus, this study was exempted by the ethics committee of the Third Affiliated Hospital of Soochow University.
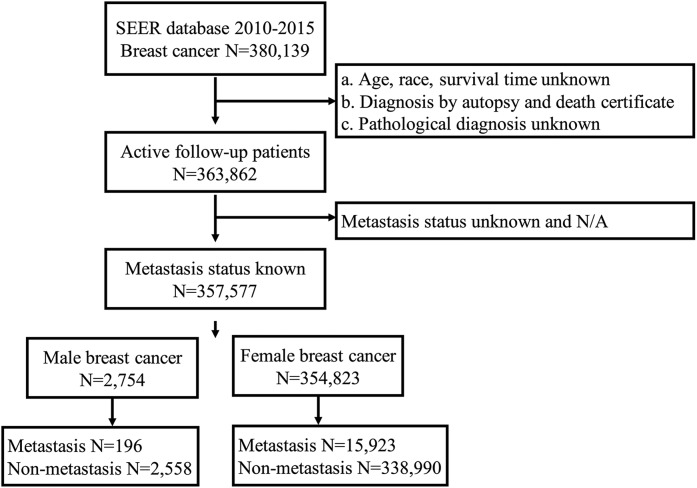
The SEER database is one of the world’s largest open cancer databases, established by the National Cancer Institute of the United States, and accounts for about 28% of the U.S. population. The data we selected came from Incidence-SEER 18 Registries Custom Data (with additional treatment fields), released April 2019, based on the November 2018 submission. MBC patients with definite metastasis from 1 January 2010 to 31 December 2015 were included in this study. The specific screening process is shown in Figure 1. In short, we excluded patients whose age, race, survival time, pathological diagnosis, and metastasis were unknown, and whose pathological results were from autopsy or death certificate.
Figure 1.
Flowchart of patient selection.
Detailed selection of MBC and FBC patients diagnosis at 2010–2015 from SEER database.
FBC, female breast cancer; MBC, male breast cancer; SEER, surveillance, epidemiology and end results database.
Variable classification
Age at diagnosis, race, primary site, laterality, grade, breast subtype, chemotherapy, radiation, surgery, and metastasis were obtained from the database. Age was divided into <60 years old and ⩾60 years old. Metastasis of distant organs is defined in SEER as the state of metastasis of distant organs at the time of the first diagnosis of cancer. Distant metastatic sites included bone, brain, liver, and lung, according to the different metastatic sites, the distant metastasis was divided into 15 groups, which were single organ metastasis (bone, liver, brain, lung), two kinds of organ metastasis (bone and liver, bone and brain, bone and lung, liver and brain, liver and lung, brain and lung), three kinds of organ metastasis (bone, liver and brain; bone, liver and lung; bone, brain and lung; liver, brain and lung), and four organs metastasis (bone, liver, brain and lung). The degree of differentiation of tumors was divided into three groups: grade I (well differentiated) and grade II (moderately differentiated), grade III (poorly differentiated) and grade IV (undifferentiated), and unknown.
Statistical analysis
We use descriptive statistics to summarize demographic and clinical variables. Chi-squared test or Fisher’s exact test was used to compare the clinicopathological characteristics between different cohorts. Kaplan–Meier curves and log-rank test were conducted to analyze the overall survival (OS) of different metastasis organs in MBC and FBC patients. In addition, we use univariate and multivariate Cox proportional hazard models to find other variables that may affect prognosis. Statistical significance was considered at two-sided p value <0.05. All data were obtained using SEER*Stat Software version 8.3.5. All statistical analyses were performed using SPSS Statistics 25 (IBM, New York, NY, USA).
Results
Population characteristics
From the SEER database, we finally identified 2754 MBC patients from 2010 to 2015. Among these MBC patients, 196 cases (7%) had distant metastasis, while 2558 cases (93%) did not. Compared with nonmetastatic MBC patients, MBC patients with distant metastasis had a higher proportion of <60 years old (35% versus 25%), grade III–IV (40% versus 32%), and were more likely to receive chemotherapy (49% versus 36%) and radiotherapy (35% versus 26%), while the proportion of surgery (36% versus 94%), central portion of the breast (27% versus 42%), and Her2–/HR+ (58% versus 79%) was lower. Detailed patient clinical characteristics is summarized in Table 1.
Table 1.
Clinical characteristics of male and female breast cancer.
| Characteristics | MBC without metastasis |
MBC with metastasis |
FBC with metastasis |
p value* | p value** | |||
|---|---|---|---|---|---|---|---|---|
|
n
2558 |
% 93 |
n
196 |
% 7 |
n
15,923 |
% 4 |
|||
| Age | 0.001 | 0.013 | ||||||
| <60 | 631 | 25 | 69 | 35 | 7016 | 44 | ||
| ⩾60 | 1927 | 75 | 127 | 65 | 8907 | 56 | ||
| Race | 0.060 | 0.403 | ||||||
| White | 2078 | 81 | 146 | 74 | 12,033 | 76 | ||
| Black | 360 | 14 | 39 | 20 | 2710 | 17 | ||
| Others | 120 | 5 | 11 | 6 | 1180 | 7 | ||
| Primary Site | <0.0001 | <0.0001 | ||||||
| Upper-outer | 301 | 12 | 15 | 8 | 3663 | 23 | ||
| Lower-outer | 95 | 4 | 2 | 1 | 832 | 5 | ||
| Upper-inner | 101 | 4 | 4 | 2 | 975 | 6 | ||
| Lower-inner | 46 | 2 | 6 | 3 | 546 | 3 | ||
| Central portion | 1082 | 42 | 53 | 27 | 937 | 6 | ||
| Other | 933 | 36 | 116 | 59 | 8970 | 56 | ||
| Laterality | <0.0001 | 0.601 | ||||||
| Left | 1351 | 53 | 103 | 53 | 7798 | 49 | ||
| Right | 1198 | 47 | 84 | 43 | 7380 | 46 | ||
| Other | 9 | 0 | 9 | 5 | 745 | 5 | ||
| Grade | <0.0001 | 0.752 | ||||||
| I-II | 1599 | 63 | 77 | 39 | 6469 | 41 | ||
| III-IV | 829 | 32 | 79 | 40 | 6005 | 38 | ||
| Unknown | 130 | 5 | 40 | 20 | 3449 | 22 | ||
| Breast Subtype | <0.0001 | 0.019 | ||||||
| Her2–/HR+ | 2019 | 79 | 114 | 58 | 8655 | 54 | ||
| Her2+/HR– | 21 | 1 | 5 | 3 | 1205 | 8 | ||
| Her2+/HR+ | 260 | 10 | 30 | 15 | 2333 | 15 | ||
| Triple negative | 36 | 1 | 16 | 8 | 1853 | 12 | ||
| Other | 222 | 9 | 31 | 16 | 1877 | 12 | ||
| Chemotherapy | <0.0001 | 0.326 | ||||||
| No/Unknown | 1641 | 64 | 100 | 51 | 7563 | 47 | ||
| Yes | 917 | 36 | 96 | 49 | 8360 | 53 | ||
| Radiation | 0.012 | 0.406 | ||||||
| No/Unknown | 1883 | 74 | 128 | 65 | 10,842 | 68 | ||
| Yes | 675 | 26 | 68 | 35 | 5081 | 32 | ||
| Surgery | <0.0001 | 0.042 | ||||||
| No | 160 | 6 | 126 | 64 | 11,340 | 71 | ||
| Yes | 2398 | 94 | 70 | 36 | 4489 | 28 | ||
| Unknown | 0 | 0 | 0 | 0 | 94 | 1 | ||
Comparison between MBC without metastasis and MBC with metastasis.
Comparison between MBC with metastasis and FBC with metastasis.
FBC, female breast cancer; MBC, male breast cancer.
In addition, we compared the clinicopathological features of patients with metastatic breast cancer between different genders (Table 1). A total of 354,823 FBC patients were enrolled in the study, of which 15,923 were patients with distant metastasis, accounting for 4% of the total. Compared with metastatic FBC patients, MBC patients with distant metastasis had a higher proportion of ⩾60 years old (65% versus 56%), surgery (36% versus 28%), and central portion of the breast (27% versus 6%), while the proportion of Her2+/HR– (3% versus 8%), triple negative (8% versus 12%), and was lower. There was no difference in race, laterality, grade, chemotherapy, and radiation.
Metastasis pattern
In the cohort of MBC with distant metastasis, the most common single site of metastases was bone with 81 cases, which takes up 41% of patients with distant metastasis, followed by lung metastasis with 26 (13%) cases, only 5 (3%) and 2 (1%) patients were with liver and brain metastasis, respectively. Most patients had distant metastasis of a single organ, accounting for 58%. There were 58 (30%) MBC patients who had distant metastasis of two organs, 43 of whom had bone and lung metastasis; 21 (12%) and 3 (2%) patients were diagnosed with three and four organ metastases, respectively. Detailed results are presented in Table 2.
Table 2.
Comparison of organ metastasis patterns between male and female patients with breast cancer.
| Parameter | Male |
Female |
p value | ||
|---|---|---|---|---|---|
|
N = 196 |
N = 15,923 |
||||
| n | % | n | % | ||
| Bone metastasis only | 81 | 41 | 6948 | 44 | 0.517* |
| Brain metastasis only | 2 | 1 | 250 | 2 | 0.744** |
| Liver metastasis only | 5 | 3 | 1274 | 8 | 0.005* |
| Lung metastasis only | 26 | 13 | 1848 | 12 | 0.471* |
| Bone and brain | 4 | 2 | 298 | 2 | 1** |
| Bone and liver | 9 | 5 | 1483 | 9 | 0.023* |
| Bone and lung | 43 | 22 | 1716 | 11 | <0.0001* |
| Brain and liver | 1 | 1 | 47 | 0 | 1** |
| Brain and lung | 0 | 0 | 133 | 1 | 0.415*** |
| Liver and lung | 1 | 1 | 433 | 3 | 0.094** |
| Bone, brain, and liver | 1 | 1 | 118 | 1 | 1** |
| Bone, brain, and lung | 9 | 5 | 205 | 1 | <0.0001* |
| Bone, liver, and lung | 10 | 5 | 895 | 6 | 0.754* |
| Brain, liver, and lung | 1 | 1 | 55 | 0 | 1** |
| Bone, brain, liver, and lung | 3 | 2 | 220 | 1 | 1** |
Pearson chi-squared test.
Chi-squared test of continuity correction.
Fisher’s exact test.
Additionally, we compared differences in metastasis patterns between males and females (Table 2). The results showed that, in terms of single organ metastasis, the incidence of liver metastasis in MBC patients was significantly lower than that in FBC patients (3% versus 8%; p = 0.005). In terms of multiple organ metastasis, the incidence of both bone and liver metastasis in MBC patients was also lower than that in FBC patients (5% versus 9%; p = 0.023), while the proportion of both bone and lung in MBC patients was higher than that in FBC patients (22% versus 11%; p < 0.0001), as well as in patients with bone, brain, and lung metastases (5% versus 1%; p < 0.0001).
Survival and prognosis of MBC patients with metastasis
In metastatic MBC patients, there were mainly bone metastasis alone, lung metastasis alone, and simultaneous bone and lung metastasis, which accounted for more than three-quarters of the total metastasis population. Therefore, we included these three groups of people in the survival and prognostic analysis to explore the impact of distant metastasis on prognosis.
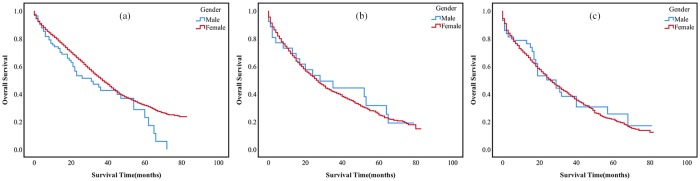
Kaplan–Meier analysis showed that there was no statistical difference in OS between MBC and FBC patients with distant metastasis (Figure 2). However, there was significant difference between metastatic MBC and nonmetastatic MBC patients (Figure 3). Moreover, there was no difference in survival among the three groups mentioned earlier.
Figure 2.
OS rate of MBC and FBC patients at different metastasis sites.
(a) OS of bone alone metastasis between MBC and FBC patients, p = 0.05; (b) OS of lung alone metastasis between MBC and FBC patients, p = 0.772; (c) OS of both bone and lung metastasis between MBC and FBC patients, p = 0.766.
FBC, female breast cancer; MBC, male breast cancer; OS, overall survival.
Figure 3.
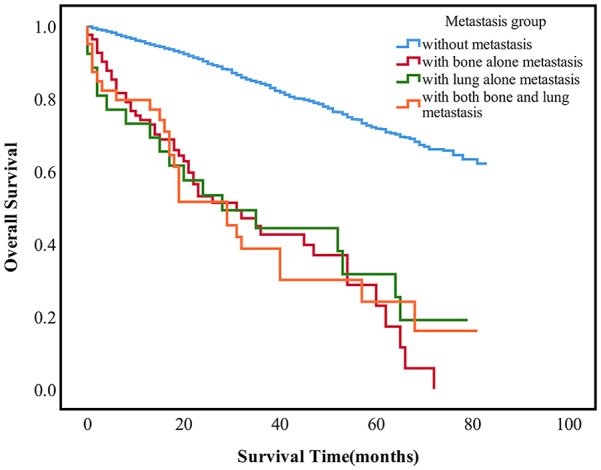
The survival difference among the different metastasis sites in MCB patients, p < 0.0001.
MBC, male breast cancer.
We then performed multivariate analysis on variables that were meaningful in univariate analysis. As shown in Table 3, multivariate analysis showed that age, grade, breast subtype, chemotherapy, surgery, and metastasis were independent factors for OS (p < 0.0001). In details, patients ⩾60 years old had a worse OS than patients <60 years old (HR:1.90, 95%CI:1.53–2.37, p < 0.0001), and a worse prognosis was found in grade III–IV (HR:1.62, 95%CI:1.36–1.93, p < 0.0001), breast subtype of triple negative (HR:3.32, 95%CI:2.10–5.26, p < 0.0001) and patients with distant metastasis (HR:2.40, 95%CI:1.47–3.91, p < 0.0001; HR:3.08, 95%CI:2.22–4.27, p < 0.0001; HR:2.51, 95%CI:1.65–3.80, p < 0.0001). Patients receiving chemotherapy and surgery had a better prognosis (HR:0.64, 95%CI:0.53–0.77, p < 0.0001; HR:0.32, 95%CI:0.25–0.41, p < 0.0001).
Table 3.
Univariate and multivariate survival analysis of male breast cancer patients with lung alone, bone alone and simultaneous lung and bone metastasis.
| Characteristics | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| p value | Hazard ratio | 95%CI | p value | |
| Age | <0.0001 | <0.0001 | ||
| <60 | Reference | |||
| ⩾60 | 1.90 | 1.53–2.37 | <0.0001 | |
| Race | 0.028 | 0.057 | ||
| White | Reference | |||
| Black | 1.24 | 1.00–1.53 | 0.047 | |
| Others | 0.78 | 0.51–1.20 | 0.256 | |
| Primary Site | 0.001 | 0.103 | ||
| Upper-outer | Reference | |||
| Lower-outer | 0.64 | 0.35–1.18 | 0.152 | |
| Upper-inner | 1.24 | 0.74–2.07 | 0.418 | |
| Lower-inner | 0.95 | 0.47–1.94 | 0.893 | |
| Central portion | 1.24 | 0.92–1.67 | 0.153 | |
| Other | 1.30 | 0.97–1.75 | 0.078 | |
| Laterality | 0.170 | NA | ||
| Left | ||||
| Right | ||||
| Other | ||||
| Grade | <0.0001 | <0.0001 | ||
| I–II | Reference | |||
| III–IV | 1.62 | 1.36–1.93 | <0.0001 | |
| Unknown | 1.58 | 1.18–2.13 | 0.002 | |
| Breast Subtype | <0.0001 | <0.0001 | ||
| Her2–/HR+ | Reference | |||
| Her2+/HR– | 0.90 | 0.40–2.07 | 0.807 | |
| Her2+/HR+ | 1.24 | 0.95–1.62 | 0.121 | |
| Triple negative | 3.32 | 2.10–5.26 | <0.0001 | |
| Other | 1.25 | 0.96–1.61 | 0.094 | |
| Chemotherapy | <0.0001 | <0.0001 | ||
| No/Unknown | Reference | |||
| Yes | 0.64 | 0.53–0.77 | <0.0001 | |
| Radiation | 0.518 | NA | ||
| No/Unknown | ||||
| Yes | ||||
| Surgery | <0.0001 | <0.0001 | ||
| No | Reference | |||
| Yes | 0.32 | 0.25–0.41 | <0.0001 | |
| Metastasis | <0.0001 | <0.0001 | ||
| None | Reference | |||
| Lung Only | 2.40 | 1.47–3.91 | <0.0001 | |
| Bone Only | 3.08 | 2.22–4.27 | <0.0001 | |
| Lung and Bone | 2.51 | 1.65–3.80 | <0.0001 | |
Discussion
In this study, we systematically analyzed the distant metastasis of MBC patients through the SEER database. The results showed that MBC patients not only had a higher distant metastasis rate than FBC patients, but also had different metastasis patterns. They had unique clinicopathological features. In addition, a multivariate analysis was conducted to determine independent factors affecting the prognosis of MBC patients.
The distant metastasis rate of MBC was 7% in our study, which was consistent with previous studies,15,16 while the distant metastasis rate in FBC was 4%. The distant metastasis rate of MBC was 1.75 times higher than that of female patients. At present, it is believed that it is mainly lack of awareness of breast cancer in male patients or delays in diagnosis that might be the cause of this phenomenon.7,18,19 A study found that only 29% of 100 Croatian MBC patients were diagnosed within 3 months of symptoms, compared with 58% of 500 Croatian FBC patients at the same time.20 In addition, Hong and colleagues suggested that the prolonged period of symptom duration of MBC was also the reason for the difference in the rate of distant metastasis between MBC and FBC patients,17 and NI and colleagues’ study of 64 cases of male breasts without breast cancer showed columnar cell changes in 39 cases (61%), which was considered to be a transitional stage in the development of some low-grade ductal carcinoma in situ and invasive breast cancer.21 In our opinion, in addition to the above reasons, differences in gene mutation may also cause this phenomenon. There are differences in genomics between MBC and FBC.22,23 It was found that the mutation rate of CHEK2 c.1100delC in MBC was higher than that in FBC, and the mutation rate of CHEK2 c.1100delC was positively correlated with the rate of metastasis.24,25
There were differences in age of diagnosis, primary site, grade, subtypes, and treatment methods (including chemotherapy, radiotherapy, and surgery) between metastatic MBC and nonmetastatic MBC. The difference in gene expression exists not only between different genders, but also at different ages of the same sex. Hallamies and colleagues found that median age of the CHEK2c.1100delC carriers was 56 years, and half of the patients were <50 years old in MBC patients.24 Poorly differentiated tumors seem to be more prone to distant metastasis, which seems to be associated with a higher frequency of local invasion of poorly differentiated tumors.26 Previous studies have found that breast cancer subtypes were independent factors affecting the occurrence of metastasis. Compared with the other three subtypes, patients with luminal A (Her2–/HR+) had the lowest incidence of distant metastasis.27,28 In our study, metastatic MBC patients had a lower proportion of luminal A compared with nonmetastatic patients; in contrast, the proportions of other three subtypes were higher. As expected, patients with metastatic MBC tend to lose the opportunity for surgery, and were more likely to choose radiotherapy and chemotherapy.
The median age of diagnosis of MBC was 5–10 years older than that of FBC patients in many studies.29–31 This may be that the proportion of MBC patients with distant metastasis ⩾60 years old is higher than that of FBC patients. The rate of metastasis in the central portion of the breast in metastatic MBC was significantly higher than that in female patients, but on the contrary in the upper outer of the breast, which may be related to anatomical difference between male and female breasts. As with the findings reported by Li and colleagues,32 there were differences in molecular subtypes in patients with metastatic breast cancer of different genders.
As far as we know, this is the first time that the distant metastasis patterns of MBC and FBC patients have been compared in detail through a large cancer database. Our study found that the metastasis rates of bone, lung, liver, and brain metastasis in metastatic MBC patients were 82%, 47%, 16%, and 11%, respectively. Under the same conditions, the metastasis rates of various organs in women were 75%, 35%, 28%, and 8%, respectively. Bone metastasis rates were higher than in previous studies, with bone metastasis rates of 50% in metastatic MBC patients in a previous German study,33 and 75% in another study based on the SEER database.34 However, the highest incidence of bone metastasis and the lowest incidence of brain metastasis were consistent with previous studies. Although the rate of single liver metastasis was only 3%, it seems that the rate of liver metastasis combined with other metastases was not low, accounting for 13% of the total metastases, and also in brain metastases (1% versus 10%). We believed that once a tumor had distant metastasis of one organ, it may accelerate metastasis in other parts, although single liver or brain metastasis was not common, but when the tumor had metastasis in other parts, it accelerated liver and brain metastases. This requires the attention of clinicians. We also found that, although there was no difference in the rate of single lung and bone metastasis between MBC and FBC patients, the risk of simultaneous bone and lung metastasis in male patients was twice as high as that in female patients. Male patients have a higher smoking rate, at about 1.5 times that of women.35 Smoking is a risk factor for cancer metastasis, including bone and lung metastasis.36,37 Studies have found that the liver microenvironment is an important factor affecting liver metastasis of breast cancer. For example, lysyl oxidase inhibits liver metastasis,38 while osteopontin and vascular endothelial growth factor promote liver metastasis.39,40 This may be one of the reasons for the difference in liver metastasis rate between male and female patients with breast cancer.
Although metastatic MBC patients had unique clinicopathological features and metastatic pattern, we found that there was no difference in OS compared with metastatic FBC patients. Our results were consistent with those of other similar studies, such as stage IV breast cancer patients,34 gastric cancer patients with liver metastasis,41 and colorectal signet ring cell carcinoma patients with distant metastasis.42 Multivariate analysis showed that distant metastasis was an independent risk factor affecting the prognosis of MBC. Metastatic MBC patients had a worse OS rate compared with nonmetastatic MBC patients (p < 0.0001). There was no survival difference between patients with single lung or bone metastasis and patients with both bone and lung metastasis. We also found that the prognosis of HER–/HR+ was similar to that of HER+/HR–, and that triple-negative breast cancer patients had the highest risk of death, which was consistent with previous studies, possibly because HER+ patients benefited from the use of trastuzumab.43,44 In addition, age, chemotherapy, surgery, and histological grade were also important factors affecting the prognosis of MBC. Because there are fewer MBC patients, for a long time, the treatment of MBC refers to FBC.2 Although radiotherapy seem to have no effect on prognosis in our cohort, we do not know what organs have received radiotherapy, and studies have shown that radiotherapy can improve the prognosis of MBC patients,45–47 so this conclusion needs to be further verified.
Our research still has some limitations. Firstly, there are only liver, brain, lung, and bone metastasis in distant parenchymal organ metastasis in SEER database; however, it has been reported that MBC can also metastasize to other sites, such as oral mucosa,48 or choroidal sites.49 Secondly, reasons for the difference in breast cancer metastasis between men and women still need further exploration. Finally, our conclusions may apply only to patients from the United States.
To sum up, through this study, we found that metastatic MBC patients have unique clinicopathological features and metastatic patterns, and that these differed from metastatic FBC patients. However, there was no difference in prognosis between MBC and FBC patients with metastasis. Distant metastasis was an independent risk factor for the prognosis of MBC patients.
Acknowledgments
Authors Jun Xie and Yao-Yu Ying contributed equally to this work and should be considered co-first authors. The authors acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER database.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iD: Jun Xie  https://orcid.org/0000-0003-3909-9231
https://orcid.org/0000-0003-3909-9231
Contributor Information
Jun Xie, Department of Respiration, Third Affiliated Hospital of Soochow University, First People’s Hospital of Changzhou, Changzhou, China.
Yao-Yu Ying, Department of Epidemiology and Biostatistics, Soochow University, Suzhou, Jiangsu, China.
Bin Xu, Department of Tumor Biological Treatment, Third Affiliated Hospital of Soochow University, First People’s Hospital of Changzhou, Changzhou, China.
Yan Li, Department of Respiration, Third Affiliated Hospital of Soochow University, First People’s Hospital of Changzhou, Changzhou, China.
Xian Zhang, Department of Respiration, Third Affiliated Hospital of Soochow University, First People’s Hospital of Changzhou, Changzhou, China.
Chong Li, Department of Respiration, Third Affiliated Hospital of Soochow University, First People’s Hospital of Changzhou, Juqian Road No.185, Changzhou 213000, China.
References
- 1. Gucalp A, Traina TA, Eisner JR, et al. Male breast cancer: a disease distinct from female breast cancer. Breast Cancer Res Treat 2019; 173: 37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Losurdo A, Rota S, Gullo G, et al. Controversies in clinicopathological characteristics and treatment strategies of male breast cancer: a review of the literature. Crit Rev Oncol Hematol 2017; 113: 283–291. [DOI] [PubMed] [Google Scholar]
- 3. Piscuoglio S, Ng CK, Murray MP, et al. The genomic landscape of male breast cancers. Clin Cancer Res 2016; 22: 4045–4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Masci G, Caruso M, Caruso F, et al. Clinicopathological and immunohistochemical characteristics in male breast cancer: a retrospective case series. Oncologist 2015; 20: 586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69: 7–34. [DOI] [PubMed] [Google Scholar]
- 6. Stang A, Thomssen C. Decline in breast cancer incidence in the United States: what about male breast cancer? Breast Cancer Res Treat 2008; 112: 595–596. [DOI] [PubMed] [Google Scholar]
- 7. Giordano SH, Cohen DS, Buzdar AU, et al. Breast carcinoma in men: a population-based study. Cancer 2004; 101: 51–57. [DOI] [PubMed] [Google Scholar]
- 8. Abdelwahab Yousef AJ. Male breast cancer: epidemiology and risk factors. Semin Oncol 2017; 44: 267–272. [DOI] [PubMed] [Google Scholar]
- 9. Scott-Conner CE, Jochimsen PR, Menck HR, et al. An analysis of male and female breast cancer treatment and survival among demographically identical pairs of patients. Surgery 1999; 126: 775–780; discussion 780–771. [PubMed] [Google Scholar]
- 10. Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet 2006; 367: 595–604. [DOI] [PubMed] [Google Scholar]
- 11. Gomez-Raposo C, Zambrana Tevar F, Sereno Moyano M, et al. Male breast cancer. Cancer Treat Rev 2010; 36: 451–457. [DOI] [PubMed] [Google Scholar]
- 12. Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005; 365: 1687–1717. [DOI] [PubMed] [Google Scholar]
- 13. O’Shaughnessy J. Extending survival with chemotherapy in metastatic breast cancer. Oncologist 2005; 10(Suppl. 3): 20–29. [DOI] [PubMed] [Google Scholar]
- 14. Soni A, Ren Z, Hameed O, et al. Breast cancer subtypes predispose the site of distant metastases. Am J Clin Pathol 2015; 143: 471–478. [DOI] [PubMed] [Google Scholar]
- 15. Harlan LC, Zujewski JA, Goodman MT, et al. Breast cancer in men in the United States: a population-based study of diagnosis, treatment, and survival. Cancer 2010; 116: 3558–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leone JP, Zwenger AO, Iturbe J, et al. Prognostic factors in male breast cancer: a population-based study. Breast Cancer Res Treat 2016; 156: 539–548. [DOI] [PubMed] [Google Scholar]
- 17. Hong JH, Ha KS, Jung YH, et al. Clinical features of male breast cancer: experiences from seven institutions over 20 years. Cancer Res Treat 2016; 48: 1389–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zongo N, Ouedraogo S, Korsaga-Some N, et al. Male breast cancer: diagnosis stages, treatment and survival in a country with limited resources (Burkina Faso). World J Surg Oncol 2018; 16: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Henriques Abreu M, Henriques Abreu P, Afonso N, et al. Patterns of recurrence and treatment in male breast cancer: a clue to prognosis? Int J Cancer 2016; 139: 1715–1720. [DOI] [PubMed] [Google Scholar]
- 20. Rudan I, Rudan N, Basic N, et al. Differences between male and female breast cancer. III. Prognostic features. Acta Med Croatica 1997; 51: 135–141. [PubMed] [Google Scholar]
- 21. Ni YB, Mujtaba S, Shao MM, et al. Columnar cell-like changes in the male breast. J Clin Pathol 2014; 67: 45–48. [DOI] [PubMed] [Google Scholar]
- 22. Young IE, Kurian KM, Annink C, et al. A polymorphism in the CYP17 gene is associated with male breast cancer. Br J Cancer 1999; 81: 141–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kornegoor R, Moelans CB, Verschuur-Maes AH, et al. Promoter hypermethylation in male breast cancer: analysis by multiplex ligation-dependent probe amplification. Breast Cancer Res 2012; 14: R101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hallamies S, Pelttari LM, Poikonen-Saksela P, et al. CHEK2 c.1100delC mutation is associated with an increased risk for male breast cancer in Finnish patient population. BMC Cancer 2017; 17: 620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med 2016; 375: 443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Higashino M, Ayani Y, Terada T, et al. Clinical features of poorly differentiated thyroid papillary carcinoma. Auris Nasus Larynx 2019; 46: 437–442. [DOI] [PubMed] [Google Scholar]
- 27. Xiao W, Zheng S, Liu P, et al. Risk factors and survival outcomes in patients with breast cancer and lung metastasis: a population-based study. Cancer Med 2018; 7: 922–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xiao W, Zheng S, Yang A, et al. Breast cancer subtypes and the risk of distant metastasis at initial diagnosis: a population-based study. Cancer Manag Res 2018; 10: 5329–5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miao H, Verkooijen HM, Chia KS, et al. Incidence and outcome of male breast cancer: an international population-based study. J Clin Oncol 2011; 29: 4381–4386. [DOI] [PubMed] [Google Scholar]
- 30. McKinley N, McCain S, Kirk S. Long Term Follow Up of Male Breast Cancer. Ulster Med J 2017; 86: 177–180. [PMC free article] [PubMed] [Google Scholar]
- 31. Gnerlich JL, Deshpande AD, Jeffe DB, et al. Poorer survival outcomes for male breast cancer compared with female breast cancer may be attributable to in-stage migration. Ann Surg Oncol 2011; 18: 1837–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li X, Yang J, Krishnamurti U, et al. Hormone receptor-positive breast cancer has a worse prognosis in male than in female patients. Clin Breast Cancer 2017; 17: 356–366. [DOI] [PubMed] [Google Scholar]
- 33. Foerster R, Schroeder L, Foerster F, et al. Metastatic male breast cancer: a retrospective cohort analysis. Breast Care (Basel) 2014; 9: 267–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu SG, Zhang WW, Liao XL, et al. Men and women show similar survival outcome in stage IV breast cancer. Breast 2017; 34: 115–121. [DOI] [PubMed] [Google Scholar]
- 35. Szklo AS, de Souza MC, Szklo M, et al. Smokers in Brazil: who are they? Tob Control 2016; 25: 564–570. [DOI] [PubMed] [Google Scholar]
- 36. Yao S, Zhang Y, Tang L, et al. Bone remodeling and regulating biomarkers in women at the time of breast cancer diagnosis. Breast Cancer Res Treat 2017; 161: 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Scanlon EF, Suh O, Murthy SM, et al. Influence of smoking on the development of lung metastases from breast cancer. Cancer 1995; 75: 2693–2699. [DOI] [PubMed] [Google Scholar]
- 38. Erler JT, Bennewith KL, Nicolau M, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature 2006; 440: 1222–1226. [DOI] [PubMed] [Google Scholar]
- 39. Mi Z, Bhattacharya SD, Kim VM, et al. Osteopontin promotes CCL5-mesenchymal stromal cell-mediated breast cancer metastasis. Carcinogenesis 2011; 32: 477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ghattass K, El-Sitt S, Zibara K, et al. The quinoxaline di-N-oxide DCQ blocks breast cancer metastasis in vitro and in vivo by targeting the hypoxia inducible factor-1 pathway. Mol Cancer 2014; 13: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Qiu MZ, Shi SM, Chen ZH, et al. Frequency and clinicopathological features of metastasis to liver, lung, bone, and brain from gastric cancer: a SEER-based study. Cancer Med 2018; 7: 3662–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shi T, Huang M, Han D, et al. Chemotherapy is associated with increased survival from colorectal signet ring cell carcinoma with distant metastasis: a surveillance, epidemiology, and end results database analysis. Cancer Med 2019; 8: 1930–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dawood S, Broglio K, Buzdar AU, et al. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol 2010; 28: 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lobbezoo DJ, van Kampen RJ, Voogd AC, et al. Prognosis of metastatic breast cancer subtypes: the hormone receptor/HER2-positive subtype is associated with the most favorable outcome. Breast Cancer Res Treat 2013; 141: 507–514. [DOI] [PubMed] [Google Scholar]
- 45. Madden NA, Macdonald OK, Call JA, et al. Radiotherapy and male breast cancer: a population-based registry analysis. Am J Clin Oncol 2016; 39: 458–462. [DOI] [PubMed] [Google Scholar]
- 46. Abrams MJ, Koffer PP, Wazer DE, et al. Postmastectomy radiation therapy is associated with improved survival in node-positive male breast cancer: a population analysis. Int J Radiat Oncol Biol Phys 2017; 98: 384–391. [DOI] [PubMed] [Google Scholar]
- 47. Jardel P, Vignot S, Cutuli B, et al. Should adjuvant radiation therapy be systematically proposed for male breast cancer? A systematic review. Anticancer Res 2018; 38: 23–31. [DOI] [PubMed] [Google Scholar]
- 48. Lee ZH, Lewing NW, Moak S, et al. Male breast cancer metastasis to the oral mucosa and face. J Plast Reconstr Aesthet Surg 2014; 67: 277–278. [DOI] [PubMed] [Google Scholar]
- 49. Oleksy P, Pogrzebielski A, Karska-Basta I, et al. A case of choroidal metastasis in a male breast cancer. Klin Oczna 2010; 112: 311–313. [PubMed] [Google Scholar]