Abstract
Background
Although rare, NTRK gene fusions are known to be oncogenic drivers in pancreatic ductal adenocarcinoma (PDAC). We report the response of a metastatic CTRC-NTRK1 gene fusion-positive PDAC to targeted treatment with the oral tropomyosin receptor kinase (TRK) inhibitor larotrectinib and the eventual development of resistance to treatment.
Patient, methods and results
A 61-year-old woman presented with a 2.5-cm mass in the body of the pancreas and a 1.2-cm liver lesion on routine follow-up for endometrial cancer that was in complete remission. Liver biopsy confirmed a primary PDAC unrelated to the endometrial cancer. The patient was treated with gemcitabine, nab-paclitaxel and ADI-PEG 20 for 12 months until disease progression and toxicity emerged [best overall response (BOR): partial response (PR)]. The patient switched to a modified regimen of folinic acid, fluorouracil, irinotecan and oxaliplatin for 4 months until neuropathy occurred. Oxaliplatin was withheld until disease progression 6 months later (BOR: stable disease). Despite recommencing oxaliplatin, the disease continued to progress. At this time, somatic profiling of the liver lesion revealed a CTRC-NTRK1 gene fusion. Treatment with larotrectinib 100 mg twice daily was commenced with BOR of PR at 2 months. The patient progressed after 6 months and was re-biopsied. Treatment was switched to the investigational next-generation TRK inhibitor selitrectinib (BAY 2731954, LOXO-195) 100 mg twice daily. After 2 months, the disease progressed and dabrafenibtrametinib combination therapy was initiated due to existence of a BRAF-V600E mutation. However, the cancer continued to progress and the patient died 2 months later.
Conclusions
Targeted TRK inhibition with larotrectinib in PDAC harbouring a CTRC-NTRK1 gene fusion is well tolerated and can improve quality of life for the patient. However, acquired resistance to therapy can emerge in some patients. Next-generation TRK inhibitors such as selitrectinib are currently in development to overcome this resistance (NCT02576431; NCT03215511).
Keywords: NTRK gene fusion, TRK fusion cancer, tropomyosin receptor kinase inhibition, pancreatic adenocarcinoma, larotrectinib, selitrectinib
Key Message
NTRK gene fusions are known to be oncogenic drivers in pancreatic ductal adenocarcinoma (PDAC), thus providing a potential target for therapy with tropomyosin receptor kinase (TRK) inhibitors. This case report describes a patient with CTRC-NTRK1 gene fusion-positive PDAC treated with larotrectinib, a selective TRK inhibitor, who subsequently developed resistance due to an acquired BRAF mutation.
Background
The most common type of malignant cancer of the pancreas is pancreatic ductal adenocarcinoma (PDAC). More than one-half of PDAC cases are identified with locally advanced or metastatic disease at presentation, with metastases mainly found in the liver, lungs and peritoneum [1]. The overall survival across all stages of disease is 8%; this decreases to 3% in patients who present with advanced disease [1]. PDAC is characterised by several germline or acquired genetic mutations. In total, 90%–95% of PDAC tumours are found to harbour an oncogenic KRAS mutation; other frequent mutations are TP53 (75%), SMAD4 (22%), CDKNA/B (18%) [2] and BRCA1/2 (4.6–8%) [3]. Although rare, NTRK gene fusions are also known to be oncogenic drivers in <1% of PDAC cases [4], thus providing a potential target for therapy. NTRK gene fusions occur in up to 1% of all solid tumours in both adult and paediatric patients and have been shown to be oncogenic drivers that are actionable with tropomyosin receptor kinase (TRK) inhibitors, such as larotrectinib [5–7].
Patient, methods and results
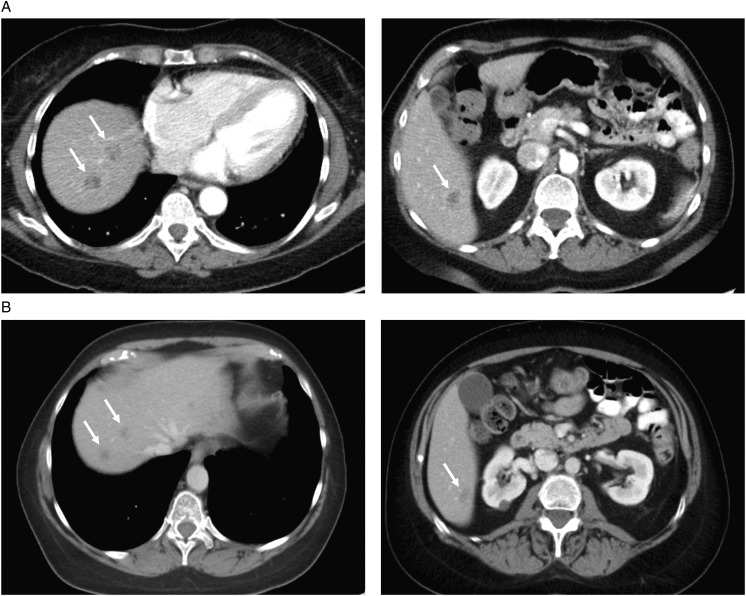
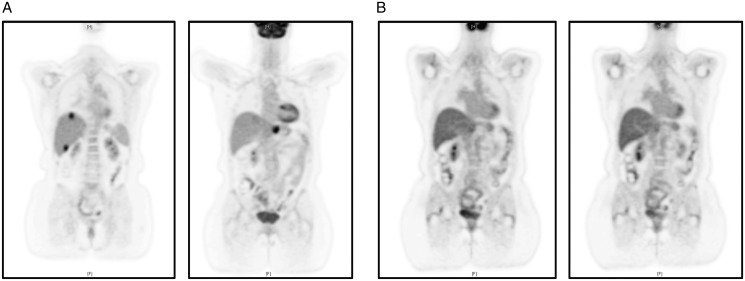
A 61-year-old woman with stage 1A, grade 1 endometrial cancer underwent a total abdominal hysterectomy with sentinel lymph node dissection followed by two rounds of intravaginal radiation and achieved complete remission without subsequent recurrence [8]. Seven months later, routine follow-up computed tomography imaging revealed a 2.5-cm mass in the body of the pancreas and a 1.2-cm liver lesion; positron emission tomography confirmed 2-fluoro-2-deoxy-D-glucose (FDG)-avidity in these areas (Figures 1A and 2A). Liver biopsy demonstrated a poorly differentiated adenocarcinoma that was CK7+/CK20–, consistent with a primary PDAC; this was morphologically different from the prior endometrial cancer. Germline profiling was negative for mutations in BRCA1/2, PALB2, ATM and DNA mismatch repair genes. Of specific note, there was insufficient tissue present for genomic profiling of the PDAC at this time; both the primary and metastatic tumour sites were very small and the yield was anticipated to be low.
Figure 1.
Computed tomography imaging. Computed tomography taken at (A) baseline before initiation of larotrectinib and (B) showing the best overall response of partial response to treatment with larotrectinib.
Figure 2.
[18F]2-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) imaging. (A) FDG-PET imaging taken at baseline before initiation of larotrectinib. 2-fluoro-2-deoxy-D-glucose (FDG)-avidity is visible in the primary pancreatic tumour and liver metastases. (B) FDG-PET imaging showing the best overall response of partial response to treatment with larotrectinib. FDG-avidity in previously hypermetabolic pancreatic and liver lesions is resolved.
The patient began treatment with gemcitabine, nab-paclitaxel and ADI-PEG 20 as part of a phase IB/II clinical trial, continuing this regimen for 12 months until disease progression/emerging toxicity (haemolytic uremic syndrome) occurred. The best overall response (BOR) during this period was partial response (PR). The patient was switched to mFOLFIRINOX (a modified regimen of FOLinic acid, Fluorouracil, IRINotecan, and OXaliplatin) and underwent eight cycles over 4 months; the BOR was stable disease. The patient experienced neuropathy and therefore oxaliplatin was withheld for the next 11 cycles until disease progression 6 months later; the BOR during this period was stable disease. Oxaliplatin was recommenced in the context of disease progression and the patient received two cycles of mFOLFIRINOX over 1 month; however, the disease continued to progress.
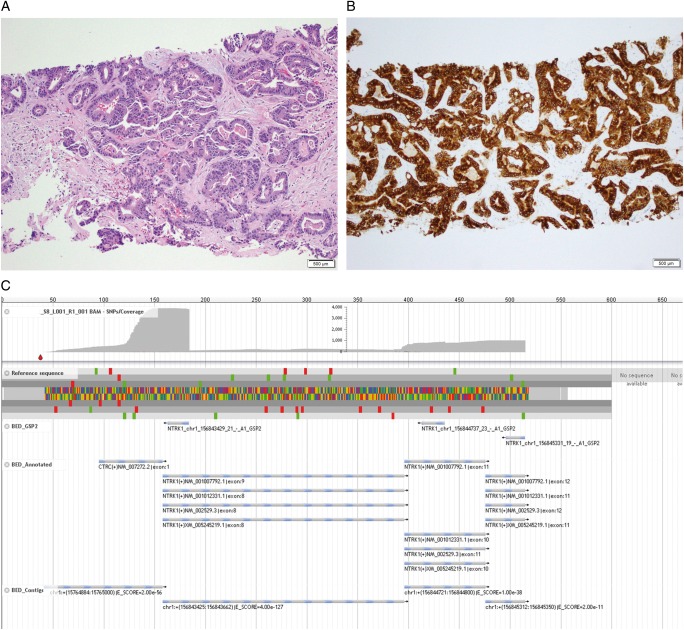
A second liver biopsy was undertaken as there was now sufficient tissue to perform next-generation sequencing for somatic profiling. Liver biopsy analysis confirmed moderately differentiated liver adenocarcinoma which was morphologically and immunophenotypically compatible with pancreatic cancer (Figure 3A). Immunohistochemistry showed tumour cells that were CK7+ and negative for CK20, CDX2, PAX8, WT1, ER and PgR, supporting this diagnosis. TrkA immunohistochemistry further showed tumour cells strongly and diffusely positive for TrkA expression (Figure 3B). Somatic DNA profiling using Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT™; Memorial Sloan Kettering Cancer Center, New York, NY, USA) [9, 10] revealed an in-frame fusion between genes CTRC exon1 and NTRK1 exon8. Additional molecular findings from MSK-IMPACT™ included gain of SDHC, deletion of CDKN2B, CDKN2A and SMAD4, an ARID2 intragenic deletion, microsatellite stable status and a low tumor burden (0.9 mutation per megabase). Archer® FusionPlex® Custom Solid Panel with Anchored Multiplex PCR (AMP™; ArcherDX, Inc., Boulder, CO, USA)) [11] confirmed the presence and transcription of the in-frame CTRC-NTRK1 gene fusion (Figure 3C and Table 1).
Figure 3.
Liver biopsy analysis. (A) Haemotoxylin and eosin (H&E): A core biopsy of the patient’s liver mass demonstrated a moderately differentiated adenocarcinoma, morphologically compatible with pancreatobiliary origin (H&E, 100× original magnification). (B) TrkA immunohistochemistry (IHC): Immunohistochemical staining for TrkA (NTRK1) demonstrated diffuse, strong cytoplasmic expression (TrkA IHC, clone EP1058Y, Abcam, Cambridge, UK, 100× original magnification). (C) Archer® software: Fusion analysis was carried out on the tumoral RNA with the MSK-IMPACT™ panel and demonstrated an in-frame fusion between CTRC (NM_007272) exon1 and NTRK1 (NM_002529) exon8, including the kinase domain of NTRK1 (JBrowse software).
Table 1.
NGS and Archer® results pre- and post-larotrectinib treatment
| NGS pre-larotrectinib | Archer® pre-larotrectinib | NGS at POD on larotrectinib | |
|---|---|---|---|
| Liver mass, right | Liver mass, right | Liver lesion | |
| Clinically validated panel Somatic | |||
| alterations | Negative | Negative | BRAF exon15 alteration |
| (p. V600E [c.1799T>A]) | |||
| Investigational panel | |||
| Somatic alterations | CTRC-NTRK1 gene rearrangementa | CTRC-NTRK1 gene rearrangementa | CTRC-NTRK1 gene rearrangementa |
| (c.88: CTRC_c.850 + 45: NTRK1del) | (c.88: CTRC_c.850 + 46: NTRK1del) | ||
| SDHC gain (1q23.3)b | |||
| CDKN2B deletion (9p21.3) | |||
| CDKN2Ap16INK4A deletion (9p21.3) | |||
| CDKN2Ap14ARF deletion (9p21.3) | |||
| SMAD4 exon9 deletion | SMAD4 exon9 deletion | ||
| (p. R361_C363del [c.1081_1089delCGCTTTTGT]) | (p. R361_C363del [c.1081_1089delCGCTTTTGT]) | ||
| ARID2 rearrangementc | |||
| (c.419-2860_c.638-69del) | |||
| ARAF exon2 alteration | |||
| (p. V21G [c.62T>G]) | |||
| TBX3 exon7 alteration | |||
| (p. G509A [c.1526G>C]) | |||
The CTRC-NTRK1 rearrangement is a deletion which results in the in-frame fusion of CTRC to NTRK1 and includes the kinase domain of NTRK1. One of the breakpoints is within exon2 of CTRC.
The SDHC copy number gain falls slightly below the cut-off criteria for amplification. Confirmatory testing by an alternate method is suggested, if clinically indicated.
The ARID2 rearrangement is an intragenic deletion of exon5. The functional significance is undetermined.
NGS, next-generation sequencing; POD, progression of disease.
Based on the identification of the actionable CTRC-NTRK1 gene fusion, the patient was enrolled in NAVIGATE (NCT02576431), a phase II basket trial of the first-in-class oral selective TRK inhibitor larotrectinib [7], approved for the treatment of cancers harbouring NTRK gene fusions. The patient received larotrectinib at a dose of 100 mg b.i.d.; treatment was well tolerated. The BOR was PR at 2 months, with disease progression at 6 months. Imaging demonstrated resolution of FDG-avidity in previously hypermetabolic pancreatic and liver lesions (Figure 2A and 2B). Repeat liver biopsy was carried out at the time of disease progression. MSK-IMPACT™ revealed a new BRAF-V600E mutation, suggesting acquired resistance to larotrectinib mediated by mutation of the kinase domain of BRAF (Table 1). The patient was subsequently enrolled in a study with the next-generation TRK inhibitor selitrectinib (BAY 2731954, LOXO-195; 100 mg b.i.d.; NCT03215511) for patients with NTRK gene fusions who have progressed on a prior TRK inhibitor. After 2 months, best response of disease progression necessitated a switch in treatment and the patient initiated dabrafenib–trametinib combination therapy; however, the cancer continued to progress, and the patient died 2 months later.
Discussion
This case is of special interest for several reasons. The patient presented with metastatic PDAC after a history of unrelated endometrial cancer; while there was no germline mutation to explain the tumour co-occurrence, ultimately, somatic profiling revealed a CTRC-NTRK1 gene fusion that was therapeutically actionable in the PDAC. The patient received targeted therapy with larotrectinib that was well tolerated and led to a BOR of PR with excellent quality of life. The patient developed resistance to larotrectinib after 6 months which was associated with the emergence of an acquired BRAF mutation as a new oncogenic driver.
Despite initial durable responses to TRK inhibition therapy, it is expected that acquired resistance to therapy will ultimately emerge in a fraction of patients, often mediated by solvent-front mutations that directly interfere with binding by larotrectinib [12] or by the emergence of bypass mutations. On-target kinase domain mutations are the most common mechanism of acquired drug resistance to TRK inhibitors found in patients [13]. However, a mechanistic study of off-target resistance to TRK inhibitors recently reported by Cocco et al. [14] indicated that a subset of patients with gastrointestinal malignancies treated with TRK inhibitors may develop resistance due to MAP kinase pathway-activating genomic alterations. Exploration of molecular mechanisms of resistance used preclinical patient-derived xenograph (PDX) models established from the pre-treatment tumour of a patient with a CTRC-NTRK1 gene fusion-positive PDAC who had an acquired resistance to larotrectinib. In these PDX models, prolonged treatment with larotrectinib resulted in the outgrowth of tumours with a newly acquired BRAF-V600E mutation, thus replicating the development of this bypass mutation in the clinical setting and in this case report. The frequency of resistance mutations in response to TRK inhibitor treatment in patients with TRK fusion cancer and the relative frequency of solvent-front versus bypass resistance mutations remains to be determined [14].
Conclusion
In all, the patient lived for 14 months after an actionable gene fusion was identified, relatively late in the patient’s disease course. This present case illustrates the clinical benefit of larotrectinib in the treatment of patients harbouring NTRK gene fusions, as well as the mechanism behind acquired resistance to TRK inhibitors and the clinical implications of this resistance.
Acknowledgements
Medical writing support, including assisting the authors with the development of the outline and initial draft, incorporation of comments, and preparation of tables and figures, was provided by Cindy Cheung, MBBS; editorial support, including fact-checking, referencing, figure preparation, formatting, proofreading and submission, was provided by Annabel Ola, MSc; both of Scion (London, UK), supported by Bayer Healthcare according to Good Publication Practice guidelines (https://annals.org/aim/fullarticle/2424869/good-publication-practice-communicating-company-sponsored-medical-research-gpp3).
Funding
This work was supported by the Cancer Center Support Grant at the National Institutes of Health (NIH P30-CA008748) and the David M. Rubenstein Center for Pancreatic Cancer Research (no grant numbers apply). This paper was published as part of a supplement financially supported by Bayer AG and Loxo Oncology, Inc., a wholly owned subsidiary of Eli Lilly and Company.
Disclosure
EMO’R has participated in an advisory board related to larotrectinib. Research funding to the author’s institution is provided by Polaris and Celgene. JFH has received honoraria from Cor2ED, WebMD, and Axiom Healthcare strategies as well as research funding from Bayer and Loxo.
References
- 1. Chiaravalli M, Reni M, O'Reilly EM.. Pancreatic ductal adenocarcinoma: state-of-the-art 2017 and new therapeutic strategies. Cancer Treat Rev 2017; 60: 32–43. [DOI] [PubMed] [Google Scholar]
- 2. Krantz BA, O'Reilly EM.. Biomarker-based therapy in pancreatic ductal adenocarcinoma: an emerging reality?. Clin Cancer Res 2018; 24(10): 2241–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singh RR, Goldberg J, Varghese AM. et al. Genomic profiling in pancreatic ductal adenocarcinoma and a pathway towards therapy individualization: a scoping review. Cancer Treat Rev 2019; 75: 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cocco E, Scaltriti M, Drilon A.. NTRK fusion-positive cancers and TRK inhibitor therapy. Nat Rev Clin Oncol 2018; 15(12): 731–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kummar S, Lassen UN.. TRK inhibition: a new tumor-agnostic treatment strategy. Targ Oncol 2018; 13(5): 545–556. [DOI] [PubMed] [Google Scholar]
- 6. Vaishnavi A, Le AT, Doebele RC.. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discov 2015; 5(1): 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Drilon A, Laetsch TW, Kummar S. et al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. N Engl J Med 2018; 378(8): 731–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.American Joint Committee on Cancer. AJCC Cancer Staging Manual, 8th edition. Springer International Publishing, 2017.
- 9. Middha S, Zhang L, Nafa K. et al. Reliable pan-cancer microsatellite instability assessment by using targeted next-generation sequencing data. JCO Precis Oncol 2017; 2017; (1): 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zehir A, Benayed R, Shah RH. et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10, 000 patients. Nat Med 2017; 23(6): 703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Marchiò C, Scaltriti M, Ladanyi M. et al. ESMO recommendations on the standard methods to detect NTRK fusions in daily practice and clinical research. Ann Oncol 2019; doi:10.1093/annonc/mdz204. [DOI] [PubMed] [Google Scholar]
- 12. Drilon A, Nagasubramanian R, Blake JF. et al. A next-generation TRK kinase inhibitor overcomes acquired resistance to prior TRK kinase inhibition in patients with TRK fusion-positive solid tumors. Cancer Discov 2017; 7(9): 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Estrada-Bernal A, Le AT, Tuch B. et al. Abstract C65: tRK kinase domain mutations that induce resistance to a pan-TRK inhibitor. Mol Cancer Ther 2015; 14(12 Suppl 2): C65–C65. [Google Scholar]
- 14. Cocco E, Schram AM, Kulick A. et al. Resistance to TRK inhibition mediated by convergent MAPK pathway activation. Nat Med 2019; 25(9): 1422–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]