Abstract
Common strategies for conversion of lignocellulosic biomass to chemical products center on deconstructing biomass polymers into fermentable sugars. The subsequent fermentation results in the loss of carbon to cells and metabolic by-products. Here, we demonstrate an alternative strategy, a growth-coupled bioconversion, that prevents carbon loss to by-products by feeding cells a non-sugar substrate, by-passing central metabolism, and linking a key metabolic step to generation of acetyl-CoA that is required for biomass and energy generation. Specifically, we converted levulinic acid (LA), an established degradation product of lignocellulosic biomass, to butanone (a.k.a. methyl-ethyl ketone - MEK), a widely used industrial solvent. Our strategy combines a catabolic pathway from Pseudomonas putida that enables conversion of LA to 3-ketovaleryl-CoA, a CoA transferase that generates 3-ketovalerate and acetyl-CoA, and a decarboxylase that generates 2-butanone. By removing the ability of E. coli to consume LA and supplying excess acetate as a carbon source, we built a strain of E. coli that could convert LA to butanone at high yields, but at the cost of significant acetate consumption. Using flux balance analysis as a guide, we built a strain of E. coli that linked acetate assimilation to production of butanone. This strain was capable of complete bioconversion of LA to butanone with a reduced acetate requirement and increased specific productivity. To demonstrate the bioconversion on real world feedstocks, we produced LA from furfuryl alcohol, a compound readily obtained from biomass. These LA feedstocks were found to contain inhibitors that prevented cell growth and bioconversion of LA to butanone. We used a combination of column chromatography and activated carbon to remove the toxic compounds from the feedstock, resulting in LA that could be completely converted to butanone. This work motivates further collaboration between chemical and biological catalysis researchers to explore alternative conversion pathways and the technical hurdles that prevent their rapid deployment.
Keywords: Bioconversion, Levulinic Acid, Sustainability, Green Chemistry, Metabolic Engineering, Escherichia coli, acetate, growth-coupling, flux balance analysis
1. Introduction
Lignocellulosic biomass is a feedstock for renewable production of a wide range of chemical products (Biddy et al., 2016; Langholtz et al., 2016). While most biomass-based processes, particularly those leveraging biological catalysts, utilize glucose and other plant sugars as carbon and energy sources, sugars are not the only feedstock that can be derived from biomass. For instance, levulinic acid (LA), one of a proposed top 12 platform molecules derived from lignocellulosic biomass (Bozell and Petersen, 2010), can be produced from both hemicellulose (through C5-sugars) and cellulose (through C6-sugars) (Alonso et al., 2013; González Maldonado et al., 2012; Han et al., 2014). Conversion of cellulose to LA has been studied extensively (Alonso et al., 2013; Smith, 2017) and high yield of LA from cellulose has been achieved, for example in the Biofine process (Hayes et al., 2005). Conversion of hemicellulose to LA is a four-step process consisting of hydrolysis of hemicellose to xylose, dehydration of xylose to furfural, hydrogenation of furfural to furfuryl alcohol (FurOH) and rehydration of FurOH to form LA. Recent studies have shown that >95% of the hemicellulose in the lignocellulosic biomass can be successfully converted to furfural in an integrated process (Alonso et al., 2017). Furfural can be selectively converted to FurOH with high yield (>95%) (Audemar et al., 2015; Merlo et al., 2009) and FurOH can be rehydrated to LA with ~70% yield (Mellmer et al., 2015). Together, these high-yield processes can be used to produce a single platform chemical from biomass, thereby by-passing the recalcitrant steps of releasing fermentable sugars and simplifying the overall biorefinery concept.
Successful LA production from biomass led to the exploration of routes for chemical upgrading to value added chemicals (Bozell et al., 2000; Isikgor and C. Remzi Becer, 2015; Pileidis and Titirici, 2016), including those with applications as biofuels (Lange et al., 2010; Serrano-Ruiz et al., 2010) and solvents (Chan-Thaw et al., 2013; Demolis et al., 2014; Luterbacher et al., 2014). These catalytic processes motivate an analogous search for biological conversions of LA, which offer high selectivity and moderate process conditions, to value added chemicals via one of two strategies. First, cells can catabolize LA to produce the central metabolites acetyl-CoA and propionyl-CoA, and convert them to a wide range of biochemical products. This process is analogous to feeding cells glucose and engineering cellular metabolism to direct carbon flux to desired compounds. The challenge here is finding pathways where yields are superior to those using alternative carbons feedstocks (e.g. glucose). Alternatively, LA could be biocatalytically converted into molecules with similar structures without going through central metabolism. While a direct bioconversion limits the diversity of molecules that can be produced, it offers the potential for higher yields as none of the substrate carbon will enter central carbon metabolism where it can be respired or shunted to other metabolic end-products.
To date, biological upgrading of LA has been limited to products that natural microbes generate, such as polyhydroxyalkanoates (Berezina and Yada, 2016; Martin and Prather, 2009). This is in large part due to a lack of understanding of the LA catabolic pathways. With the discovery of enzymes capable of LA catabolism in Pseudomonas putida (Rand et al., 2017b) comes the opportunity to explore novel bioconversion pathways. In this study, we build upon our previous work with the LA catabolism pathway in Escherichia coli by further engineering strains to convert LA into the solvent 2-butanone (methyl-ethyl ketone or MEK). Butanone is a widely used industrial chemical, most commonly used as a solvent but also in the production of methyl isopropenyl ketone, polyesters, diacetyl, and methyl-pseudoionone (Hoell et al., 2009). Previous studies have demonstrated the conversion of LA to butanone catalytically, but the approach suffers from energy intensive process conditions and low yields (Gong et al., 2010; Gong and Lin, 2011; Serrano-Ruiz et al., 2010). Biologically, butanone has been produced through the dehydration of 2,3-butanediol with a vitamin B12 dependent diol dehydratase (Ghiaci et al., 2014; Yoneda et al., 2014) and by the direct decarboxylation of LA (a γ-keto acid) into butanone using acetoacetate decarboxylase (adc) from Clostridium acetobutylicum, although the enzyme was susceptible to inhibition by LA in the in vitro system, limiting its overall productivity (Min et al., 2013).
Here, we present a novel route for the bioconversion of LA into butanone, that leverages a growth-coupling strategy to circumvent LA inhibition and drive the reaction to completion. In prior work (Rand et al., 2017b), we engineered E. coli LS5218 to consume LA as the sole carbon and energy source by deleting fadE and introducing a heterologous LA catabolizing pathway, LvaABCDE (obtained from Pseudomonas putida). In this work, we engineered the LA-catabolizing strain to produce butanone by leveraging the native deregulation of acetoacetyl-CoA transferase (AtoDA) and 3-hydroxyacyl-CoA dehydrogenase (FadB) from strain LS5218 and introducing a heterologous acetoacetate decarboxylase (Adc). We then used a genome-scale model of E. coli metabolism to evaluate strain design strategies with flux balance analysis (FBA) to couple the cellular growth and energy generation to butanone production. This method enabled complete bioconversion of LA to butanone in laboratory media. We then extended these conditions to a more integrated bioprocess by producing butanone from LA made from furfuryl alcohol (furfuryl alcohol can be produced in high yield directly from the hemicellulose fraction of lignocellulosic biomass). The integrated process required separation of LA from toxic by-products generated during the hydration of furfuryl alcohol to LA. Finally, we performed the bioconversion in a fed-batch bioreactor at higher cell density and were able to achieve full bioconversion of 20 mM LA in 10 hours. The resulting process serves as an example of an alternative strategy for producing industrial chemicals from renewable resources via a combination of chemical catalysis and metabolic engineering.
2. Results and Discussion
2.1. Establishing Butanone Production
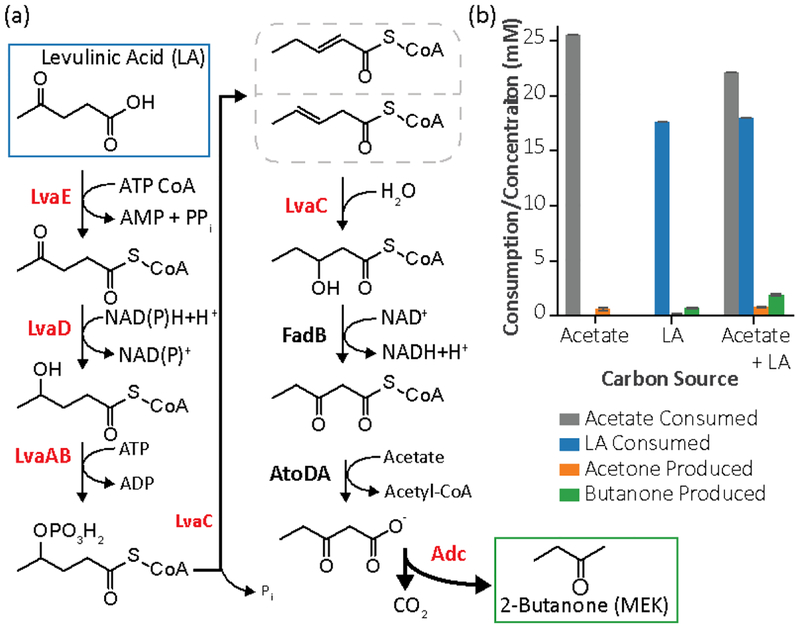
Production of butanone can be performed by decarboxylation of 3-ketovalerate by the acetoacetate decarboxylase (Adc) from the Clostridium acetobutylicum acetone pathway. Adc has been used to heterologously produce acetone in E. coli and other organisms (Bermejo et al., 1998; Hanai et al., 2007). In C. acetobutylicum, acetone is produced through the condensation of two acetyl-CoA molecules to acetoacetyl-CoA (a β-ketoacyl-CoA), which can be liberated to acetoacetate by a CoA transferase and then decarboxylated to acetone by acetoacetate decarboxylase (Cary et al., 1990; Mermelstein et al., 1993). Our strategy for producing butanone (Fig. 1a) integrated a common pathway for producing ketones from β-keto acids with a recently discovered pathway for catabolizing levulinic acid (LA). Here, LA is catabolized to 3-hydroxyvaleryl-CoA (3HV-CoA) through P. putida enzymes encoded by lvaABCDE. Next, a 3-hydroxyacyl-CoA dehydrogenase (encoded by fadB) oxidizes 3HV-CoA to 3-ketovaleryl-CoA (3KV-CoA). Then, an acetoacetyl-CoA transferase (encoded by atoDA) transfers the CoA from 3KV-CoA to acetate to form of 3-ketovalerate and acetyl-CoA. Finally, acetoacetate decarboxylase (encoded by adc from C. acetobutylicum) decarboxylates 3-ketovalerate to yield butanone and CO2.
Fig. 1. Establishing a butanone producing strain of E. coli.
(a) The pathway for butanone production combines the LA catabolism pathway (LvaABCDE) from P. putida and a decarboxylase (Adc) from C. acetobutylicum. Heterologous enzymes are labeled in red. (b) Addition of the butanone pathway to eMEK1 and culturing on minimal media containing acetate alone, LA alone, or both acetate and LA. Error bars represent the standard deviation of biological triplicates.
To test the pathway, lvaABCDE and adc were co-expressed from plasmids pJMR32 and pJMR95, respectively. The plasmid pJMR32, a low copy vector linking expression of lvaABCDE to the Tet promoter, was used in prior studies to establish LA catabolism in E. coli (Rand et al., 2017b). The plasmid pJMR95 is a medium copy plasmid containing the Ptrc promoter for adc expression. Both plasmids were transformed into variants of E. coli LS5218 (Rand et al., 2017a) to leverage the native deregulation of atoDA that results from an atoC(Con) mutation and the deregulation of fadB from the fadR* mutation found in this strain (Spratt et al., 1981). We tested production in the non-optimized strain, eMEK1 (LS5218 ΔfadE), harboring plasmids pJMR32 and pJMR95. We cultured the strain in RK batch media (Korz et al., 1995), supplemented with 23 mM acetate, 18 mM LA, or both carbon sources. After 24 hours, we found that eMEK1 could produce butanone only in the presence of LA, acetone in the presence of acetate, and both ketones in the presence of both acetate and LA (Fig. 1b). In the case where both acetate and LA were supplied, eMEK1 produced about 130 mg/L (1.9 mM) of butanone with a 10% yield of butanone on LA. In each case, we observed complete consumption of each carbon source.
2.2. Genome Scale Modeling and Initial Optimization
While our initial experiments demonstrated the feasibility of the pathway, its performance was far from satisfactory. Therefore, we interrogated a genome scale model of E. coli eMEK1 to identify strategies that would increase the yield of butanone on LA using the Cameo library for Python (Cardoso et al., 2018). The model was created by supplementing the iJO1366 reconstruction of E. coli metabolism (Orth et al., 2011) with the reactions performed by the LA catabolism pathway, acetoacetyl-CoA transferase, and 3HV/acetoacetate decarboxylase (Fig. 2a). With a maximum substrate uptake rate of 10 mmol gDW−1 hr−1, the model predicted a maximum specific growth rate of 0.71 hr−1 when LA served as the sole carbon source. The model predicted a nearly direct trade-off between butanone production and biomass production, with no butanone production at the limit of maximum growth rate (0.71 hr−1) and no growth at the limit of maximum butanone production (9.4 mmol gDW−1 hr−1) (Fig. 2bi). The addition of externally supplied acetate increased the maximum predicted butanone production rate to 10 mmol gDW−1 hr−1 (complete conversion of LA to butanone) by allowing E. coli to grow on acetate and convert all LA to butanone (Fig. 2bii). To prevent LA from being used as a carbon source, we deleted the reaction corresponding to AtoB, which decreased the maximum predicted growth rate to 0.24 hr−1 while maintaining the maximum predicted butanone production rate to 10 mmol gDW−1 hr−1 (Fig. 2biii). It is important to note that, because the iJO1366 model does not include reactions for odd-chain β-oxidation, the in silico deletion of β-oxidation-related reactions was not necessary.
Fig. 2. Design and implementation of a growth-coupling strategy for butanone production.
(a) Growth coupling is achieved in eMEK8 by preventing complete LA catabolism (deleting thiolases - ΔfadA, ΔfadI, ΔatoB) and native acetate incorporation (ΔackA, Δpta, Δacs). (b) Model analysis of pathway designs. Solution space of FBA with (i) E. coli grown on LA as its sole carbon source (ii) E. coli grown on both LA and acetate (iii) deletion of LA catabolism genes preventing E. coli growth on LA (iv) additional deletion of native acetate incorporation. (c) Ability of eMEK4 and eMEK8 to grow on each carbon source. (d) Comparison of carbon utilization in eMEK4 and eMEK8. The amount of each carbon source was varied, where 1:1 represents 20 mM LA and 23 mM acetate. Error bars represent the standard deviation of biological triplicates.
In order to test these predictions, we created a new set of strains. We removed thiolase encoding genes, fadA, fadI and atoB, to prevent complete LA catabolism and increase LA flux towards butanone precursors (3HV-CoA and 3HV). We deleted fadJ simultaneously with fadI as fadB and fadJ provide similar functions. We tested butanone and acetone production in this more optimized strain, eMEK4 (LS5218 ΔfadEAIJ ΔatoB), using pJMR95 (adc from C. acetobutylicum) and pJMR32 (lvaABCDE). This strain did not show an appreciable amount of acetone production under any conditions and produced 395 mg/L (5.5 mM) butanone when both LA and acetate were supplied in roughly equimolar ratios (SI Appendix, Fig. S1). These values correspond to a 59% yield of butanone on LA. Because E. coli eMEK4 can grow on acetate alone, we also performed experiments where either acetate or LA were supplied in excess. In general, butanone production scaled directly with acetate consumption. In the case where acetate was supplied in 2× molar excess, butanone titer increased to 900 mg/L (12.6 mM), reflecting a 74% measurable yield of butanone on LA. In all four cases that we tested, E. coli eMEK4 consumed all provided acetate without significant LA catabolism.
2.3. Coupling LA-butanone conversion to acetate assimilation and cell growth
To further increase the yield of butanone from LA and acetate, we computationally evaluated three strategies for producing 3-ketovalerate (3KV) from 3-ketovaleryl-CoA (3KV-CoA) (Fig. S2) (Lan et al., 2013). The first method uses a thioesterase to hydrolyze 3KV-CoA to 3KV, which results in a strain incapable of growth without another carbon source but is theoretically capable of complete conversion of LA to butanone. The other two strategies couple butanone production to energy generation and/or cell growth. For example, the CoA moiety from 3KV-CoA can be transferred to succinate, thereby generating 3KV, via a succinyl-CoA transferase encoded by pcaIJ in Pseudomonas putida. With the addition of an equimolar feed of succinate and deletion of all reactions forming succinyl-CoA (SUCOAS, AKGDH, 3OXCOAT, PPCSCT) other than the PcaIJ reaction, we were able to show that the maximum growth rate (0.48 hr−1) required production of butanone at a rate of 0.25 mmol gDW−1 hr−1. While promising, this approach required a large number of deletions and maximum growth occurred with a relatively low amount of butanone production. Alternatively, we found that butanone production could be coupled to acetate assimilation via E. coli’s native acetyl-CoA transferase, AtoDA. By knocking out acetate fermentation (reactions ACKr and PTAr) and acetyl-CoA synthesis from acetate (reaction ACS), the only way for E. coli to make acetyl-CoA was through the transfer of CoA from 3HV-CoA to exogenous acetate. In this case, a maximum predicted butanone production rate of 10 mmol gDW−1 hr−1 was achieved (i.e. complete bioconversion) with a maximum predicted growth rate of 0.21 hr−1. This approach predicted the possibility of a strongly growth-coupled bioconversion of LA to butanone. This growth-coupled strain, eMEK8, was constructed by deleting ackA, pta and acs in strain eMEK4.
In a separate evolution experiment using E. coli K12 MG1655, we discovered a variant of the pJMR32 plasmid with a single nucleotide mutation causing a V111F variant on the Rep101 protein that improved catabolism of LA. We then reintroduced the V111F mutation into the Rep101 gene into pJMR32 creating plasmid pJMR32QC and discovered that this mutation significantly increased the growth rate of eMEK8 when compared to eMEK8 containing the plasmid without the V11F mutation (Fig. S3a). Because a mutation in the origin is likely to affect the copy number of the plasmid, we performed qPCR and determined that the mutant plasmid had a copy number threefold higher than the original plasmid (Fig. S3b).
To experimentally test the coupling strategy, we grew cultures of eMEK8 with plasmids pJMR32QC and pJMR95 in minimal media using LA and/or acetate as the carbon source (Fig. 2c, Fig. S4). These data show no growth of eMEK8 on LA or acetate alone but significant growth when both substrates are supplied, indicating that growth of eMEK8 is successfully coupled to LA catabolism. We then examined how different ratios of LA and acetate affect the production of butanone (Fig. 2d, Fig. S5). Importantly, in the case where LA was supplied at half the molar ratio as acetate (1:0.5), the LA was consumed in its entirety while acetate remained in the media. When supplied in equimolar ratios, the acetate and LA consumption are nearly identical (~15 mM) and the measured yield of butanone on LA is 76% (855 mg/L or 11.9 mM). These results show the coupling strategy employed for eMEK8 saves significant amounts of acetate when compared to eMEK4, which consumes all supplied acetate in every case.
2.4. Strain Characterization and Evaluation of Carbon Utilization
To determine the time-dependent behavior of LA and acetate consumption and butanone production, we compared the concentration of each species in cultures of strains eMEK4 and eMEK8 each containing pJMR32QC and pJMR95. Cultures were grown in RK minimal media containing a slight molar excess of acetate. Comparison of these two strains highlights the utility of coupling acetate assimilation with the LA-butanone bioconversion. In strain eMEK4 (Fig. 3a), the cultures reached stationary phase after 16 hours due to acetate depletion, leaving unconverted LA in the media. In contrast, strain eMEK8 (Fig. 3b) consumed LA and acetate in equimolar amounts throughout the culture and reached stationary phase after 20 hours due to LA depletion, further supporting that acetate assimilation flux was coupled to the LA-butanone bioconversion. Two points stand out from this comparison. First, the lower biomass content of the eMEK8 demonstrated an increase in specific productivity from the coupling strategy. Furthermore, evaporation of the product (apparent after 16 hours) suggests that the measured butanone yields are lower than actual butanone yields. This, combined with LA consumption data, suggests the possibility that eMEK8 is converting all the LA to butanone.
Fig. 3. Characterization of eMEK4 and eMEK8 with plasmids pJMR32QC and pJMR95.
(a) Uncoupled strain eMEK4 entered stationary phase after 16 hours (gray) when all the acetate was consumed, at which point no more LA was consumed. (b) Coupled strain eMEK8 entered stationary phase at 20 hours after the LA is consumed completely and no more acetate is consumed. Error bars represent the standard deviation of biological triplicates.
We then performed a carbon balance on eMEK8 in a bioreactor to better understand the strain’s carbon utilization. Due to evaporation of the butanone, we placed an absorber on the off-gas stream of the bioreactor and tracked the substrates and products to account for the atomic carbon during the fermentation (Fig. S6). At the beginning of the fermentation, all carbon was either in the form of LA or acetate (Fig. 4). By tracking the amount of LA and acetate consumed, we determined a total of 21.52 mmol of atomic carbon were consumed. At the end of the fermentation, we accounted for 5.16 mmol of atomic carbon in CO2 evolution and 2.13 mmol of carbon in biomass, which account for 34% of the carbon consumed. We can further account for 2.68 mmol and 7.28 mmol of atomic carbon from butanone in the media and absorber, respectively, at 20 hours (to reduce the effects of butanone evaporation) bringing the carbon balance to 80%. Looking at the measured molar yield butanone on LA over the course of the fermentation (Fig. S7), we measure complete conversion of consumed LA to butanone at 8 hours and observe decreasing the measurement of butanone over the course of the fermentation due to evaporation. These data suggest that eMEK8 is converting all of the LA to butanone, which allows 92% of the total carbon consumed to be accounted for in products.
Fig. 4. Evaluation of carbon utilization of eMEK8.
Total atomic carbon of substrate consumed (left) and biomass, CO2 and butanone (right) measured in a 24-hour fermentation. Butanone data from the 20-hour timepoint. (N = 1)
Finally, we sought to increase the rate at which LA was converted to butanone by performing the bioconversion in a higher density fed-batch culture in a bioreactor (Fig. S8). To test this, we grew strains to higher cell density (OD 8.5) on glucose and then fed LA and acetate to the culture. This higher-density fermentation required 10 hours to consume all available LA. representing a ~60% decrease in process time as compared to the lower density cultures.
2.5. Butanone from FurOH-derived LA
In an integrated bioprocess, LA could be derived from biomass in a catalytic process prior to bioconversion to butanone. For such a process to be feasible, the catalytic process must be performed at high yields, generate minimal side products, and use solvents that are either tolerated in fermentation or easily separated. To this end, we tested several solvents for the conversion of furfuryl alcohol (FurOH) to LA and found that increased LA yields are obtained using a solvent system composed of a polar aprotic solvent and water. (Fig. S9a). When the reaction was performed in a pure aqueous system, we observed poor LA yield on FurOH (<10%). Alternatively, high LA yields were obtained for a solvent system composed of 80 wt % organic solvent (1,4-dioxane, THF, GVL and acetone) and 20 wt % water. Acetone gave the highest LA yield at greater than 80%, which is about 10% higher than previously reported results (Mellmer et al., 2015), and was chosen as the solvent for further study.
To optimize the catalytic process, we tested the effect of FurOH and solvent composition on LA yield. We found that the LA yield was independent of initial FurOH concentration (Fig. S9b). Thus, higher concentrations of FurOH can be used which is beneficial for industrial applications as it is expected to reduce separation and overall capital costs. We also found that LA yield at high FurOH concentrations were similar for various solvent system composition studied (Fig. S9c). This behavior is an important advantage when working at high FurOH concentrations, as the rehydration of FurOH to LA consumes one mole of water for each mole of LA produced, thereby changing the composition of the solvent as the reaction proceeds.
By sight, it was apparent that the crude, FurOH-derived, LA was much less pure than the commercially purchased LA (Fig. S10). Because we suspected that these impurities may have toxic effects on cell growth, we tested several different dilutions of FurOH-derived LA for LA consumption and butanone production (Fig. 5a). At low concentrations (4 mM, 20% of previous experiments), eMEK8 was able to completely consume the LA and convert it to butanone. When we doubled the LA concentration to 8 mM (40% of previous experiments), we noticed inhibited growth of the cells but still observed LA consumption and butanone production. Finally, at 16 mM (80% of previous experiments), we observed no cell growth, LA consumption, or butanone production. This suggested that side products from the catalytic process are indeed toxic to our cells.
Fig. 5. Evaluation of butanone production from FurOH-derived LA.
(a) At 4 mM and 8 mM FurOH-derived LA, the strain eMEK8 grew and produced measurable butanone. The strain was not able to grow on 16 mM FurOH-derived LA and no substrate was consumed. (b) Growth and butanone production were restored when the cells were grown on the purified FurOH-derived LA. Error bars represent the standard deviation of biological triplicates.
First, we suspected that the unknown product causing the orange-brown coloration may be the toxic product. A solvent test using thin layer chromatography (TLC) suggested that water was an acceptable solvent for separation. After performing the separation on a chromatography column packed with silica gel (Fig. 6a), the orange-brown product was removed although a pale-yellow color remained. Attempting to produce butanone from the column-purified LA, however, yielded neither cell growth nor butanone.
Fig. 6. Spectroscopic characterization of LA samples throughout the separation process.
(a) Overview of the separation process. (b) Three-dimensional excitation-emission (EEM) scan of a 1 ppm quinine sulfate standard in 1N H2SO4, with 1 quinine sulfate unit (qsu) representing the emission value of the standard at excitation/emission of 350/450. (c) EEM scan of a 100 mM LA standard in deionized water. (d) EEM scan of unpurified FurOH-derived LA. (e) EEM scan of the column-purified LA. (f) EEM scan of the column-purified LA after treating the column-purified LA with activated carbon. (g) Absorbance scans of the samples throughout the purification process.
Because we had noticed that the column-purified LA fluoresced under UV light, we next suspected that the toxic effects could be caused by humic acids, as has been shown previously in cyanobacteria (Korosh et al., 2018). To further investigate this hypothesis, we performed 3-dimensional excitation-emission (EEM) scans, which show characteristic peaks for humic, fulvic, and aromatic compounds (Fig. 6e). The presence of EEM peaks in the humic acid region of both crude FurOH-derived LA (Fig. 6b) and the column-purified LA (Fig. 6c) further suggested that humic acids may be causing a toxic effect on cell growth. By treating the column-purified LA with 50 mg/mL of activated carbon for 1 hour, the EEM peaks from humic acids (Fig. 6d) and yellow coloration (Fig. 6g) were no longer visible. Using this clean LA, we were able to restore cell growth and butanone production (Fig. 5b).
3. Conclusions
We have engineered Escherichia coli to produce 2-butanone from levulinic acid (LA) by introducing the LA degradation pathway from Pseudomonas putida and an acetoacetate decarboxylase from Clostridium acetobutylicum. Our initial experiments in a minimally-engineered strain showed a small amount of butanone production. To improve butanone production, we added acetate to allow for more efficient CoA transfer and the maximum theoretical yield increased to 1 mol butanone per mol LA. This strategy increased butanone production improved slightly to 130 mg/L. We then removed competing pathways to butanone production by deleting the genes for short-chain fatty acid catabolism and achieved a 74% measurable yield of butanone on LA (900 mg/L) using a more than a 2× excess of acetate.
We then analyzed strategies for producing butanone from LA using a genome-scale metabolic model. From this analysis, we built a strain for which butanone production was coupled to acetate assimilation through the CoA transfer from 3-ketovaleryl-CoA. With the coupled strain (eMEK8), we achieved the same measurable yield (76%) of butanone on LA while reducing the acetate requirement to half.
Next, we examined how the fermentation would perform on a 150 mL bioreactor scale. First, we performed a carbon balance on the eMEK8 and demonstrated the likelihood of complete bioconversion of LA to butanone, with the difference in measured yield attributed to butanone evaporation. We then examined how this unit operation performed at higher cell density by performing a two-stage fermentation: the first stage as cell growth on glucose and the second stage as butanone production on LA. With this method, we were able to reduce the consumption time from 24 hours at low cell density to 10 hours. This suggests the ability to perform a fed-batch process for near-continual butanone production.
Finally, we demonstrated the bioconversion using a FurOH-derived LA, mimicking a biomass-derived LA that could be used in an industrial process. We discovered that the FurOH-derived LA solutions contained toxic contaminants, likely humic acids and an unknown orange-brown compound. We found that the LA could be purified from the toxic side-products using column chromatography and activated carbon. Following the separation, bioconversions with eMEK8 were able to produce butanone from the FurOH-derived LA. This provides an additional example of integrating chemical and biological catalysis to produce sustainable chemicals (Schwartz et al., 2014) and motivates further research in chemical catalysis to produce fermentation-ready substrates.
4. Materials and Methods
4.1. Chemicals, Strains, and Media
All chemicals were obtained from Sigma-Aldrich or Fisher Scientific. Bacterial strains and plasmids used in this study are summarized in Table 1. E. coli strains were grown at 30°C unless otherwise noted. Riesenberg-Korz (RK) minimal media was prepared according to the batch medium recipe by Korz et al. (Korz et al., 1995; Riesenberg et al., 1991) with ferric ammonium citrate substituted for Fe(III) citrate. Kanamycin was used at final concentration of 50 μg/mL and carbenicillin was used at a final concentration of 100 μg/mL.
Table 1:
Strains and plasmids used in this study
| Strains | Relevant genotype/property | Source or Reference |
|---|---|---|
| Escherichia coli | ||
| DH5α | F− Φ80lacZΔM15 Δ(lacZYA-argF) U169 recA1 endA1 hsdR17 (rk−,mk+) phoA supE44 thi-1 gyrA96 relA1 λ− | Invitrogen |
| LS5218 | F+ λ+ fadR601 atoC512(Const) | ECGSC |
| eMEK1 | LS5218 ΔfadE | (Rand et al., 2017b) |
| eMEK4 | LS5218 ΔfadE ΔfadA ΔfadIJ ΔatoB | This work |
| eMEK8 | LS5218 ΔfadE ΔfadA ΔfadIJ ΔatoB ΔackApta Δacs | This work |
| Plasmids | Relevant genotype/property | Source or Reference |
| pMP11 | constitutive S. pyogenes cas9, repA101 (ts), oriR101, AmpR, λ Red genes under PBAD promoter, gRNA for pBR322 ori under PTet promoter | (Mehrer et al., 2018) |
| pgRNA | constitutive gRNA, pBR322 ori, KanR | (Mehrer et al., 2018; Qi et al., 2013) |
| pgRNA-atoC | pgRNA targeted to atoC | This work |
| pgRNA - fadE | pgRNA targeted to fadE | This work |
| pgRNA – fadA | pgRNA targeted to fadA | This work |
| pgRNA - fadIJ | pgRNA targeted to fadIJ | This work |
| pgRNA - atoB | pgRNA targeted to atoB | This work |
| pgRNA - pta | pgRNA targeted to pta | This work |
| pgRNA - acs | pgRNA targeted to acs | This work |
| pBbS2k-mCherry | KanR, SC101 ori, PTet promoter, mCherry | Addgene (Lee et al., 2011) |
| pJMR32 | pBbS2k carrying lva operon in replace of mCherry | (Rand et al., 2017b) |
| pJMR32QC | pJMR32 with V111F mutation in Rep101 | This work |
| pMSB-mCherry | Ptrc promoter, pBR322 ori, AmpR, mCherry | (Politz et al., 2013) |
| pJMR95 | pMSB-mCherry with C. acetobutylicum adc replacing mCherry | This work |
Plasmid construction was completed using Phusion® High Fidelity DNA Polymerase (NEB) for PCR reactions and Gibson assembly (Gibson et al., 2009). Gibson reaction mixtures (2 μL) were transformed into chemically competent E. coli DH5α and cells were plated on LB media with the appropriate antibiotics. Plasmids were verified by sequencing of the cloning junctions. Sequence files for plasmids are appended as supplementary material (Datasets S1 and S2).
4.2. Genome Scale Modeling
Flux balance analysis (FBA) was performed on the iJO1366 model of E. coli metabolism (Orth et al., 2011) using the Cameo Python library (Cardoso et al., 2018). Heterologous reactions for LA metabolism and MEK production were added into the model directly (Table S1). Carbon uptake reactions (EX_LA_e and/or EX_ac_e) were set to 10 mmol gDW−1 hr−1. Reactions atoB, ACS, ACKr, and PTAr were knocked-out as necessary to simulate deletions of genes atoB, acs, ackA, and pta, respectively.
4.3. CRISPR-Cas9 Recombineering
Genome deletions were performed using a CRISPR-Cas9 recombineering system described previously (Mehrer et al., 2018), which was adapted from Li and co-workers (Li et al., 2015).
4.4. Quantitative PCR
The relative copy numbers of pJMR32 and pJMR32QC were quantified using qPCR. Individual colonies of cells containing each plasmid were grown overnight in biological triplicate. Once cultures reached stationary phase, each was diluted in distilled/deionized water to a concentration of ~1,000 cells/μL (using the conversion factor OD 0.4 = 105 cells/μL). Quantitative PCR was performed in 10 μL mixture containing 1× SYBR Green PCR Master Mix (BioRad), 150 nM forward primer, 150 nM reverse primer, and 1 μL of sample. Primers used to determine template concentration were specific to rrsA (for E. coli) and lvaC (for plasmid DNA). Each sample was prepared in technical duplicate. The standard curve for cell concentration was made with a 1:10 dilution series of wild type cells from 10,000 to 1 cell/μL, and the standard curve for plasmid concentration was made with a dilution series of purified plasmid DNA from 100,000 to 1 plasmids/μL. Plasmid concentration was measured using the Qubit 3.0 Fluorometer. The reactions were prepared on an AriaMx skirted 96-well plate (Agilent Technologies) and the plate was sealed with an adhesive cover (Bio-Rad). Reactions were run on an AriaMx Real-Time PCR System (Agilent Technologies) using the following cycling conditions: 3 min at 95°C, followed by 35 cycles of 15s at 95°C, 30s at 55°C, and 1 min at 72°C. Melt curves were generated by increasing the temperature from 55°C to 95°C using increments of 5°C every 5s.
After each run, Cq values, the cycles in which fluorescence was detected, were exported for analysis. Technical duplicates with a standard deviation in Cq value greater than 0.3 were not used in the analysis. Standard curves were constructed by plotting Cq values vs. the log of cell/plasmid concentration. Each standard curve used 4 – 5 data points and had an R2 value of at least 0.99. The plasmid copy number was calculated by determining the cell or plasmid concentration in each sample using the standard curves and then dividing the plasmid concentration by the cell concentration. The copy number values were normalized so that pJMR32 had a relative copy number of 1.
4.5. Butanone Production
Butanone production was tested in strains containing pJMR32(QC) (lvaABCDE) and pJMR95 (adc). Overnight pre-cultures were inoculated with single colonies and grown in RK batch media (Korz et al., 1995; Riesenberg et al., 1991) with 2.5% (w/v) glucose. Butanone production cultures were performed in RK batch media with 20 mM LA and 23 mM acetate (no glucose) and inoculated with 50 μL of overnight culture. The pJMR032(QC) plasmids were induced with 50 μg/mL aTc. The pJMR95 plasmid was not induced, as leaky expression of the adc gene was determined to be sufficient. Bioconversion experiments in shake tubes were performed for 24 hours with 5 mL of media in glass test tubes (20 × 150 mm, Fisher Scientific) at 30°C and 250 rpm agitation in a I26 shaker (New Brunswick Scientific).
4.6. Analytical Chemistry
To quantify acetone and butanone, 1.5 mL culture samples were centrifuged at 18,000 × g for 3 min, the resulting supernatant was filter sterilized, and analyzed on a GC-FID (Shimadzu, model GC-2010) equipped with a Restek Stabilwax-DA column (60 m, 0.53 mm ID) and an AOC-20i autoinjector. The protocol for GC-FID: 40°C, hold for 4 min, increase to 250°C at 5°C/min, hold for 1 min, with H2 constant flow, linear velocity 40 cm/sec. Injection and detector temperature was 250°C. External standards were prepared by diluting 100 mg of each acetone and butanone in 10 mL of water (10 mg/mL) and diluting this solution to concentrations of 1000, 500, 250, 100, 50, 25, and 10 mg/L.
Acetate and LA were quantified on an HPLC (Shimadzu) equipped with an autosampler, quaternary pump, degasser, photodiode array, and refractive index detector. Samples were prepared as described for acetone and butanone quantification. For each sample, 10 μL was injected and separated for 25 min on a Restek Organic Acids column with a guard column (oven temperature of 60°C) with a mobile phase of 5 mM sulfuric acid at a flow rate of 0.6 mL/min. For quantification of all analytes, external calibration curves were constructed to correlate peak area with concentration. Standards were obtained by preparing a 10 mg/mL solution of acetate and LA in water; the concentrated solution was then diluted to concentrations of 1000, 500, 250, 125, and 62.5 mg/L.
4.7. Bioreactor Experiments
Larger scale bioconversion experiments were performed in an Applikon MiniBio 250 bioreactor with 150 mL of RK media containing 20 mM LA with excess acetate (~23 mM). The bioreactor was maintained at 30°C with constant 150 mL/min aeration (i.e. 1 vvm) and 500 rpm agitation with three Rushton impellers. The pH was controlled with aqueous ammonia and phosphoric acid to a set-point of 6.8 and the level was controlled with the addition of sterile water to maintain a constant working volume. For the carbon balance experiment, inoculation and induction were performed simultaneously with 1.5 mL overnight culture, 150 μL of 50 mg/mL aTc (30 × scale compared to shake-tube experiments). Carbon dioxide evolution was tracked using the K-33 ICB 10% CO2 sensor (CO2meter.com) every 30 seconds and the data were integrated by the trapezoid rule to determine the total CO2 evolution. A gas absorber was prepared by adding 500 mL of MilliQ water to a gas washing bottle (Pyrex 31770–500), placing the gas washing bottle in an insulating box, and filling the box with ice. Samples of 2 mL were pulled every 4 hours to quantify LA, acetate, and butanone concentrations from the bioreactor. Biomass was measured by centrifuging 50 mL of culture at 4800 × g for 10 min, washing once with deionized water, centrifuging again, and lyophilizing the cells. Biomass was estimated at approximately 47% carbon by mass (Youngquist et al., 2012).
High density experiments were performed by inoculating 150 mL of RK media with 1.25% (w/v) glucose and allowed to grow for 21 hours. Temperature, pH, and liquid level control were performed as stated previously. Dissolved oxygen (DO) was controlled to 50% of the maximum DO at operating conditions with air. After 21 hours, 1.5 mL of each 20% (w/v) acetate, 2M LA, aTc to 500 ng/mL, and glucose-free feeding solution (Korz et al., 1995) were added. After carbon addition and induction, 2 mL samples were taken hourly for HPLC analysis and OD measurement.
4.8. Preparation of Furfuryl Alcohol-Derived LA
Catalyst preparation:
ZSM-5 (Zeolyst; CBV 2314; SiO2 /Al2O3 = 23) was purchased in the ammonium form. The proton form was obtained by calcination in air. Briefly, NH4-ZSM-5 was heated in a flow of air (60 cm3(STP)/min) from 298 K to 873 K in 6 hours and was kept at 873 K for 4 hours. After calcination, the catalyst was cooled to 298 K by natural convection.
Materials:
Furfuryl alcohol (Sigma Aldrich; 98%) was distilled at 443 K and stored in a refrigerator at 277 K prior to use. Levulinic acid (Aldrich; 98%), acetone (Fisher Scientific; HPLC Grade), 1,4-dioxane (Sigma Aldrich; ≥99%), tetrahydrofuran (Sigma Aldrich; ≥99%), ɣ-valerolactone (Sigma Aldrich; ≥99%), dimethyl sulfoxide (Sigma Aldrich; ≥99%), and acetonitrile (Sigma Aldrich; ≥99%) were used without further purification.
Catalytic Activity Measurements:
In a typical reaction, 7 mL solutions were prepared containing 0.2 M reactant (FurOH) in an acetone/water solvent (80 wt% acetone) and containing appropriate amounts of catalyst with reactant to catalyst ratio (w/w) of 1.7. These solutions were added in thick-wall glass reactors (Alltech; 10 mL) with a magnetic stir bar. Then, the reactors were placed in an oil bath at 393 K, stirring at 900 rpm. After the reaction was completed in 20 minutes, the reactors were removed from the oil bath and cooled in an ice bath at 273 K. The content of the reactor was filtered through a 0.2 μm membrane (VWR International; PTFE) and analyzed for FurOH and LA using GC and HPLC analysis.
Analysis:
Concentrations of levulinic acid were monitored by high-performance liquid chromatograph (Waters Alliance 2695) equipped with a differential refractometer (Waters 410), a photodiode array detector (Waters 996), and an ion-exclusion column (Bio-Rad; Aminex HPX-87H; 7.8 Å~300 mm, 5 μm). A mobile phase of 5 mM sulfuric acid at a flow rate of 0.6 mL/min was used. Concentrations of furfuryl alcohol were quantified using gas chromatograph (Shimadzu GC-2010) equipped with a flame ionization detector and a ZB-50 GC column (Phenomenex; L = 30mm; ID = 0.25 mm; FT = 0.25 μm). The quantification of the concentrations of reactant and product was carried out using external calibrations with known standards. The following equations were used to calculate the conversion of FurOH and the yield of LA:
is the initial concentration of furfuryl alcohol in the feed, CFurOH and CLA are the concentrations of furfuryl alcohol and levulinic acid in the product, respectively.
Organic Solvent Removal:
Solid acid catalyst was filtered and acetone in the mixture was removed at 303 K under vacuum using a rotary evaporator. The product (levulinic acid) in water was recovered as yellow, transparent oil. The yield of levulinic acid after separation was measured using HPLC as described above.
4.9. Purification of Furfuryl Alcohol-Derived LA
The FurOH-derived LA was purified using column chromatography and an activated carbon treatment. To prepare the column, a slurry of 60–200 μm silica gel (ACROS Organics) in deionoized (DI) water was added to a 1½ inch ID × 10 inch length chromatography column (Chemglass Life Sciences) to a 10 cm height of silica gel. Prior to performing the separation, the silica gel packing was washed with at least 2 L of DI water. To perform the separation, 5 mL of 0.8 M crude FurOH-derived LA was added to the column; water was used as the mobile phase. For each run, 24 fractions of 3 mL were collected and analyzed on the HPLC as described in the analytical chemistry section. The fractions with an LA concentration of higher than 100 mM were treated by rocking with 50 mg/mL of activated carbon (Norit SX Ultra, Cabot) for 1 hour at room temperature. The fractions were then centrifuged at 4800 × g for 5 minutes, decanted, and filtered through a 0.22 μm filter for sterilization and removal of any remaining activated carbon.
Supplementary Material
Acknowledgements
This work was funded in part by the US National Science Foundation (CBET-1149678), a grant from the Draper Technology Innovation Fund, and a grant from the William F. Vilas Trust. This material is based upon work supported in part by the Great Lakes Bioenergy Research Center, U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research under Award Numbers DE-SC0018409 and DE-FC02-07ER64494. TC is the recipient of a National Institutes of Health Biotechnology Training Program Fellowship (NIGMS-5 T32 GM08349). The authors are grateful to Matt Long for his assistance in educating us to use the Cameo modeling library. The authors are also grateful to Travis Korosh and Austin Comer for their assistance with humic acid identification using excitation-emission scans as well as Siddarth Krishna and Nathaniel Eagan for assistance in selecting the activated carbon for humic acid removal.
Abbreviations:
- LA
Levulinic Acid
- 3KV
3-ketovalerate
- CoA
Coenzyme A
- FBA
Flux Balance Analysis
- FurOH
Furfuryl alcohol
- MEK
Methyl-ethyl ketone, 2-butanone
Footnotes
Conflict of Interest Statement:
BFP, CRM, JMR, MRI have filed a patent application on the technology described. The other authors have nothing to declare.
References
- Alonso DM, Gallo JMR, Mellmer M. a, Wettstein SG, Dumesic J. a, 2013. Direct conversion of cellulose to levulinic acid and gamma-valerolactone using solid acid catalysts. Catal. Sci. Technol 3, 927–931. 10.1039/C2cy20689g [DOI] [Google Scholar]
- Alonso DM, Hakim SH, Zhou S, Won W, Hosseinaei O, Tao J, Garcia-Negron V, Motagamwala AH, Mellmer MA, Huang K, Houtman CJ, Labbé N, Harper DP, Maravelias CT, Runge T, Dumesic JA, 2017. Increasing the revenue from lignocellulosic biomass: Maximizing feedstock utilization. Sci. Adv 3 10.1126/sciadv.1603301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audemar M, Ciotonea C, De Oliveira Vigier K, Royer S, Ungureanu A, Dragoi B, Dumitriu E, Jérôme F, 2015. Selective hydrogenation of furfural to furfuryl alcohol in the presence of a recyclable cobalt/SBA-15 catalyst. ChemSusChem 8, 1885–1891. 10.1002/cssc.201403398 [DOI] [PubMed] [Google Scholar]
- Berezina N, Yada B, 2016. Improvement of the poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHBV) production by dual feeding with levulinic acid and sodium propionate in Cupriavidus necator. N. Biotechnol 33, 231–236. 10.1016/j.nbt.2015.06.002 [DOI] [PubMed] [Google Scholar]
- Bermejo LL, Welker NE, Papoutsakis ET, 1998. Expression of Clostridium acetobutylicum ATCC 824 genes in Escherichia coli for acetone production and acetate detoxification. Appl. Environ. Microbiol 64, 1079–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biddy MJ, Scarlata C, Kinchin C, 2016. Chemicals from Biomass: A Market Assessment of Bioproducts with Near-Term Potential. 10.2172/1244312 [DOI]
- Bozell JJ, Moens L, Elliott DC, Wang Y, Neuenscwander GG, Fitzpatrick SW, Bilski RJ, Jarnefeld JL, 2000. Production of levulinic acid and use as a platform chemical for derived products. Resour. Conserv. Recycl 28, 227–239. 10.1016/S0921-3449(99)00047-6 [DOI] [Google Scholar]
- Bozell JJ, Petersen GR, 2010. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem 12, 539 10.1039/b922014c [DOI] [Google Scholar]
- Cardoso JGR, Jensen K, Lieven C, Lærke Hansen AS, Galkina S, Beber M, Özdemir E, Herrgård MJ, Redestig H, Sonnenschein N, 2018. Cameo: A Python Library for Computer Aided Metabolic Engineering and Optimization of Cell Factories. ACS Synth. Biol 7, 1163–1166. 10.1021/acssynbio.7b00423 [DOI] [PubMed] [Google Scholar]
- Cary JW, Petersen DJ, Papoutsakis ET, Bennett GN, 1990. Cloning and Expression of Clostridium acetobutylicum ATCC 824 Acetoacetyl-Coenzyme A : Acetate / Butyrate : Coenzyme A- Transferase in Escherichia coli 56, 1576–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan-Thaw CE, Marelli M, Psaro R, Ravasio N, Zaccheria F, 2013. New generation biofuels: γ-valerolactone into valeric esters in one pot. RSC Adv 3, 1302–1306. 10.1039/c2ra23043g [DOI] [Google Scholar]
- Demolis A, Essayem N, Rataboul F, 2014. Synthesis and Applications of Alkyl Levulinates. ACS Sustain. Chem. Eng 2, 1338–1352. [Google Scholar]
- Ghiaci P, Norbeck J, Larsson C, 2014. 2-Butanol and butanone production in Saccharomyces cerevisiae through combination of a B12 dependent dehydratase and a secondary alcohol dehydrogenase using a TEV-based expression system. PLoS One 9, e102774 10.1371/journal.pone.0102774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Young L, Chuang R-Y, Venter JC, Hutchison CA, Smith HO, 2009. Enzymatic assembly of DNA molecules up to several hundred kilobases. Nat. Methods 6, 343–5. 10.1038/nmeth.1318 [DOI] [PubMed] [Google Scholar]
- Gong Y, Lin L, 2011. Oxidative decarboxylation of levulinic acid by silver(I)/persulfate. Molecules 16, 2714–2725. 10.3390/molecules16032714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Lin L, Shi J, Liu S, 2010. Oxidative decarboxylation of levulinic acid by cupric oxides. Molecules 15, 7946–7960. 10.3390/molecules15117946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González Maldonado GM, Assary RS, Dumesic J, Curtiss LA, 2012. Experimental and theoretical studies of the acid-catalyzed conversion of furfuryl alcohol to levulinic acid in aqueous solution. Energy Environ. Sci 5, 6981 10.1039/c2ee03465d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Sen SM, Alonso DM, Dumesic JA, Maravelias CT, 2014. A strategy for the simultaneous catalytic conversion of hemicellulose and cellulose from lignocellulosic biomass to liquid transportation fuels. Green Chem 16, 653–661. 10.1039/c3gc41511b [DOI] [Google Scholar]
- Hanai T, Atsumi S, Liao JC, 2007. Engineered synthetic pathway for isopropanol production in Escherichia coli. Appl. Environ. Microbiol 73, 7814–7818. 10.1128/AEM.01140-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes DJ, Ross J, Hayes MHB, Fitzpatrick S, 2005. The Biofine process: production of levulinic acid, furfural and formic acid from lignocellulosic feedstocks. Biorefineries-Industrial Process. Prod 1 10.1002/9783527619849 [DOI] [Google Scholar]
- Hoell D, Mensing T, Roggenbuck R, Sakuth M, Sperlich E, Urban T, Neier W, Strehlke G, 2009. 2-Butanone, in: Ullmann’s Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, Germany: 10.1002/14356007.a04_475.pub2 [DOI] [Google Scholar]
- Isikgor FH, Remzi Becer C, 2015. Lignocellulosic Biomass: a sustainable platform for production of bio-based chemicals and polymers. Polym. Chem 6, 4497–4559. 10.1039/c3py00085k [DOI] [Google Scholar]
- Korosh TC, Dutcher A, Pfleger BF, McMahon KD, 2018. Inhibition of Cyanobacterial Growth on a Municipal Wastewater Sidestream Is Impacted by Temperature. mSphere 3 10.1128/mSphere.00538-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korz DJ, Rinas U, Hellmuth K, Sanders EA, Deckwer WD, 1995. Simple fed-batch technique for high cell density cultivation of Escherichia coli. J. Biotechnol 39, 59–65. [DOI] [PubMed] [Google Scholar]
- Lan EI, Dekishima Y, Chuang DS, Liao JC, 2013. Metabolic engineering of 2-pentanone synthesis in Escherichia coli. AIChE J 59, 3167–3175. 10.1002/aic.14086 [DOI] [Google Scholar]
- Lange JP, Price R, Ayoub PM, Louis J, Petrus L, Clarke L, Gosselink H, 2010. Valeric biofuels: A platform of cellulosic transportation fuels. Angew. Chemie - Int. Ed 49, 4479–4483. 10.1002/anie.201000655 [DOI] [PubMed] [Google Scholar]
- Langholtz MH, Stokes BJ, Eaton LM, 2016. 2016 Billion-Ton Report: Advancing Domestic Resources for a Thriving Bioeconomy. 10.2172/1271651 [DOI]
- Lee T, Krupa R. a, Zhang F, Hajimorad M, Holtz WJ, Prasad N, Lee S, Keasling JD, 2011. BglBrick vectors and datasheets: A synthetic biology platform for gene expression. J. Biol. Eng 5, 12 10.1186/1754-1611-5-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lin Z, Huang C, Zhang Y, Wang Z, Tang Y, Chen T, Zhao X, 2015. Metabolic engineering of Escherichia coli using CRISPR-Cas9 meditated genome editing. Metab. Eng 31, 13–21. 10.1016/j.ymben.2015.06.006 [DOI] [PubMed] [Google Scholar]
- Luterbacher JS, Martin Alonso D, Dumesic JA, 2014. Targeted chemical upgrading of lignocellulosic biomass to platform molecules. Green Chem 16, 4816–4838. 10.1039/C4GC01160K [DOI] [Google Scholar]
- Martin CH, Prather KLJ, 2009. High-titer production of monomeric hydroxyvalerates from levulinic acid in Pseudomonas putida. J. Biotechnol 139, 61–67. 10.1016/j.jbiotec.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Mehrer CR, Incha MR, Politz MC, Pfleger BF, 2018. Anaerobic production of medium-chain fatty alcohols via a β-reduction pathway. Metab. Eng 48, 63–71. 10.1016/j.ymben.2018.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellmer MA, Gallo JMR, Martin Alonso D, Dumesic JA, 2015. Selective production of levulinic acid from furfuryl alcohol in THF solvent systems over H-ZSM-5. ACS Catal 5, 3354–3359. 10.1021/acscatal.5b00274 [DOI] [Google Scholar]
- Merlo AB, Vetere V, Ruggera JF, Casella ML, 2009. Bimetallic PtSn catalyst for the selective hydrogenation of furfural to furfuryl alcohol in liquid-phase. Catal. Commun 10, 1665–1669. 10.1016/j.catcom.2009.05.005 [DOI] [Google Scholar]
- Mermelstein LD, Papoutsakis ET, Petersen DJ, Bennett GN, 1993. Metabolic engineering of Clostridium acetobutylicum ATCC 824 for increased solvent production by enhancement of acetone formation enzyme activities using a synthetic acetone operon. Biotechnol. Bioeng 10.1002/bit.260420906 [DOI] [PubMed] [Google Scholar]
- Min K, Kim S, Yum T, Kim Y, Sang BI, Um Y, 2013. Conversion of levulinic acid to 2-butanone by acetoacetate decarboxylase from Clostridium acetobutylicum. Appl. Microbiol. Biotechnol 97, 5627–5634. 10.1007/s00253-013-4879-9 [DOI] [PubMed] [Google Scholar]
- Orth JD, Conrad TM, Na J, Lerman JA, Nam H, Feist AM, Palsson BØ, 2011. A comprehensive genome-scale reconstruction of Escherichia coli metabolism--2011. Mol. Syst. Biol 7, 535 10.1038/msb.2011.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pileidis FD, Titirici MM, 2016. Levulinic Acid Biorefineries: New Challenges for Efficient Utilization of Biomass. ChemSusChem 9, 562–582. 10.1002/cssc.201501405 [DOI] [PubMed] [Google Scholar]
- Politz MC, Copeland MF, Pfleger BF, 2013. Artificial repressors for controlling gene expression in bacteria. Chem. Commun. (Camb) 49, 4325–7. 10.1039/c2cc37107c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi LS, Larson MH, Gilbert LA, Doudna J. a, Weissman JS, Arkin AP, Lim W. a, 2013. Repurposing CRISPR as an RNA-guided platform for sequence-specific control of gene expression. Cell 152, 1173–83. 10.1016/j.cell.2013.02.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand JM, Gordon GC, Mehrer CR, Pfleger BF, 2017. Genome sequence and analysis of Escherichia coli production strain LS5218. Metab. Eng. Commun 5, 78–83. 10.1016/j.meteno.2017.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand JM, Pisithkul T, Clark RL, Thiede JM, Mehrer CR, Agnew DE, Campbell CE, Markley AL, Price MN, Ray J, Wetmore KM, Suh Y, Arkin AP, Deutschbauer AM, Amador-Noguez D, Pfleger BF, 2017. A metabolic pathway for catabolizing levulinic acid in bacteria. Nat. Microbiol 2, 1624–1634. 10.1038/s41564-017-0028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesenberg D, Schulz V, Knorre W. a., Pohl H-D, Korz D, Sanders E. a., Roß a., Deckwer W-D, 1991. High cell density cultivation of Escherichia coli at controlled specific growth rate. J. Biotechnol 20, 17–27. 10.1016/0168-1656(91)90032-Q [DOI] [PubMed] [Google Scholar]
- Schwartz TJ, O’Neill BJ, Shanks BH, Dumesic JA, 2014. Bridging the chemical and biological catalysis gap: Challenges and outlooks for producing sustainable chemicals. ACS Catal 4, 2060–2069. 10.1021/cs500364y [DOI] [Google Scholar]
- Serrano-Ruiz JC, West RM, Dumesic JA, 2010. Catalytic Conversion of Renewable Biomass Resources to Fuels and Chemicals. Annu. Rev. Chem. Biomol. Eng 1, 79–100. 10.1146/annurev-chembioeng-073009-100935 [DOI] [PubMed] [Google Scholar]
- Smith RL, 2017. Production of Platform Chemicals from Sustainable Resources. 10.1007/978-981-10-4172-3 [DOI]
- Spratt SK, Ginsburgh CL, Nunn WD, 1981. Isolation and genetic characterization of Escherichia coli mutants defective in propionate metabolism. J. Bacteriol 146, 1166–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda H, Tantillo DJ, Atsumi S, 2014. Biological production of 2-butanone in Escherichia coli. ChemSusChem 7, 92–95. 10.1002/cssc.201300853 [DOI] [PubMed] [Google Scholar]
- Youngquist JT, Lennen RM, Ranatunga DR, Bothfeld WH, M. WD II, Pfleger BF, 2012. Kinetic modeling of free fatty acid production in Escherichia coli based on continuous cultivation of a plasmid free strain. Biotechnol. Bioeng 109, 1518–1527. 10.1002/bit.24420 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.