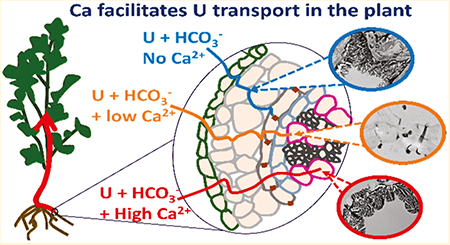
Abstract
The role of calcium (Ca) on the cellular distribution of U(VI) in Brassica juncea roots and root-to-shoot translocation was investigated using hydroponic experiments, microscopy, and spectroscopy. Uranium accumulated mainly in the roots (727–9376 mg kg−1) after 30 days of exposure to 80 μM dissolved U in water containing 1 mM HCO3− at different Ca concentrations (0–6 mM) at pH 7.5. However, the concentration of U in the shoots increased 22 times in experiments with 6 mM Ca compared to 0 mM Ca. In the Ca control experiment, transmission electron microscopy–energy-dispersive spectroscopy analyses detected U–P-bearing precipitates in the cortical apoplast of parenchyma cells. In experiments with 0.3 mM Ca, U–P-bearing precipitates were detected in the cortical apoplast and the bordered pits of xylem cells. In experiments with 6 mM Ca, U–P-bearing precipitates aggregated in the xylem with no apoplastic precipitation. These results indicate that Ca in carbonate water inhibits the transport and precipitation of U in the root cortical apoplast and facilitates the symplastic transport and translocation toward shoots. These findings reveal the considerable role of Ca in the presence of carbonate in facilitating the transport of U in plants and present new insights for future assessment and phytoremediation strategies.
Keywords: uranium, calcium, carbonate, plant roots, cellular distribution, transport pathways, translocation
Graphical Abstract
INTRODUCTION
The consequences of uranium (U) contamination in the environment caused by the demand for weapon manufacturing and nuclear energy include negative ecological effects, the potential risks of U entry into food chains, and the subsequent human health exposure.1–7 Uranium accumulates in plant roots, crosses cell structure barriers, and is transported to the shoots.8–10 However, the mechanisms affecting the transport of U in plant root and subsequent root-to-shoot translocation are still largely unknown.
Aqueous chemical speciation controls the accumulation and transport of U(VI) in plants in oxidizing environments. Uranium is mainly accumulated as U(VI) phosphate on the root surface through precipitation and/or adsorption mechanisms.10,11 Phosphate complexation with U(VI) decreases U accumulation in all plant tissues.10,12 The presence of a relatively high calcium (Ca) concentration in carbonate water can inhibit U accumulation in plants likely as a result of interactions with neutrally charged calcium uranyl carbonate complexes (Ca–U–CO3).13 The complexation of U(VI) with carbonate and citrate enhances U root-to-shoot translocation.14–17 Such an increase in U translocation in planta is generally attributed in the literature to the following electrostatic interactions. First, the negatively charged uranyl carbonate/citrate and the cell wall components (e.g., phosphate) repulse each other electrostatically, avoiding U precipitation and increasing U root-to-shoot translocation. Second, the positively charged uranyl cations (UO22+) and the negatively charged cell wall components of roots attract each other, enhancing U precipitation in the roots and decreasing U translocation.10,14 Surprisingly, U precipitates were recently detected with endogenous phosphorus (P) in the cell walls of the root surface reacted with negatively charged uranyl carbonate (U–CO3), suggesting that U–CO3 complexes can still lead to the precipitation and retention of U in root cell walls.13 The specific conditions and mechanisms (e.g., the presence or absence of a major element, such as Ca, and the formation of ternary Ca–U–CO3 complexes) that can affect the cellular distribution of U in the roots and root-to-shoot translocation at circumneutral pH are still not well-understood. Both Ca and carbonate represent important environmental and physiological constituents that can affect metal accumulation in plants.18–20 Understanding the mechanisms governing U transport in plants and the elements involved in such mechanisms is important given their relevance to phytoremediation and human health concerns.
In this research, we investigated the cellular distribution of U in the roots and root-to-shoot translocation in plants exposed to U and carbonate at different Ca concentrations using hydroponic experiments and microscopy and spectroscopy analyses. Brassica juncea was used as a hyperaccumulator model plant for research investigating U bioaccumulation that showed a significant potential for U accumulation in shoots.9,21,22 The exposure experiments were conducted in hydroponic systems using a constant concentration of HCO3− at pH 7.5 under a range of Ca concentrations. The experimental conditions, including the concentrations of U, Ca, and carbonate and the pH of exposure solutions, were selected (1) to represent environmental locations where U–CO3 and Ca–U–CO3 aqueous complexes can be formed in natural waters and sediments4,13,23 and (2) to investigate the mechanisms of U root-to-shoot translocation under a range of Ca and U concentrations in which translocation of U in plants in carbonate water has been detected in the literature.10,14 Our results contribute to further understand the complex mechanisms of U transport and root-to-shoot translocation by providing insights about the important role of Ca in the rhizosphere where U carbonate complexes (U–CO3 and Ca–U–CO3) can commonly occur at circumneutral pH.
MATERIALS AND METHODS
Seedling Growth and U Treatment.
B. juncea was selected in this work as a research model for phytoremediation studies.9,21,24 Plant seeds were soaked on humid filter paper previously washed with ethanol to allow for seed germination. After 1 week, seedlings were transferred to a non-closed hydroponic system (46 L), which was open to the atmosphere and supplied with NPK liquid fertilizer prepared in purified water. Seedlings were kept at this condition for 2 months under 21–25 °C night and day temperature in a 12/12 h light/dark cycle. Healthy 2 month old seedlings were then selected for exposure experiments and acclimatized to the chemical composition of the exposure solutions for 5 days in 400 mL solution containing simplified Hoagland nutrients but without U at pH 5.8–6.2. The chemical composition of the exposure solutions (0.5 mM MgSO4, 2 mM NH4NO3, 1 mM KCl, 1 mM NaHCO3, and 3 mM CaCl2·2H2O) was depleted of phosphate to avoid any improper U phosphate complexation/precipitation.
Seedlings were then exposed for 30 days to 80 μM U added as UO2 (NO3)2 (1000 mg L−1, Sigma-Aldrich). The concentration of U was selected according to previous studies: (1) showing U root-to-shoot translocation in carbonate water and (2) detecting U accumulation in cells using microscopy and spectroscopy techniques.9,10,14,25,26 The exposure solutions consisted of fresh ultrapure water containing simplified Hoagland nutrients but presenting three different Ca treatments: (1) first treatment corresponds to 0 mM Ca, where ultrapure water was free of any added Ca; (2) the second treatment was prepared by adding 0.3 mM Ca to the solution; (3) and the third treatment was prepared by adding 6 mM Ca to the solution. All of the exposure solutions contained 2 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer to keep the pH stable at 7.5. Three replicate polypropylene (PP) bottles (n = 3) were used for each Ca treatment and for controls (solutions with no added U). Each bottle contained one seedling and 400 mL of exposure solution. All of the PP bottles were cleaned in dilute nitric acid bath (10%) and washed in ultrapure water before using them in the experiments.
Analytical Methods.
The total U content in acid-digested root and shoots was analyzed using inductively coupled plasma optical emission spectrometry (ICP–OES, PerkinElmer Optima 5300DV) and inductively coupled plasma mass spectrometry (ICP–MS, PerkinElmer NexION 300D). Both ICP–OES and ICP–MS were calibrated using a five-point calibration curve. The quality of the results was ensured by conducting periodical measurements of quality control and quality assurance standards. The accuracy and precision of measurements during the course of each analytical run were verified by checking the percent recovery (90–110%) and the standard deviation for the analysis of an initial calibration verification solution and different continuing calibration verification solutions (ICV and CCV). Statistical analyses were conducted using the software XLSTAT13,27,28 to analyze the significance of the effect of Ca treatments (n = 3) on U accumulation in roots and shoots at p < 0.05 (Table S1 of the Supporting Information). Visual MINTEQ29,30 was used to assess U aqueous speciation using inputs based on the chemical composition of the exposure solutions in our study. Root cross sections were analyzed using transmission electron microscopy (TEM) and electron microprobe analysis (EPMA). More details about statistical analyses and plant (roots and shoots) preparation for analytical and spectroscopy analyses are presented in the Supporting Information.
RESULTS AND DISCUSSION
Effect of Ca on U Accumulation and Root-to-Shoot Translocation.
Calcium in carbonate water significantly decreased (p < 0.05) U accumulation in the roots and increased U root-to-shoot translocation (Figure 1 and Table S2 of the Supporting Information). In experiments free of Ca, the mass of U in the roots was 3.35 ± 0.8 mg (which corresponds to 9376 ± 1237 mg kg−1 of dry biomass of roots). However, the mass of U in shoots was only 0.0042 ± 0.002 mg (2.3 ± 0.6 mg kg−1 of dry biomass of shoots), indicating very low root-to-shoot translocation. At a low Ca concentration (0.3 mM), Ca slightly increased U mass in the roots to 4.2 ± 1.3 mg (12692 ± 6492 mg kg−1) as well as in the shoots with up to 0.022 ± 0.005 mg (10 ± 0.2 mg kg−1). However, at a high Ca concentration (6 mM), the mass of U in the shoots increased to 0.133 ± 0.07 mg (51 ± 25 mg kg−1) with a significant increase (p < 0.05) in U translocation and a significant decrease in U accumulation in the roots, where the mass of U was 0.37 ± 0.1 mg (727 ± 90 mg kg−1). The results indicate that Ca and carbonate facilitated the translocation of U (Table S2 of the Supporting Information). In fact, the percentage of U mass in the shoots (with respect to the total U mass accumulated in plants) increased from 0.12 to 26.4% when Ca increased from 0 to 6 mM, whereas the percentage of U mass in the roots decreased from 99.88 to 73.6%, respectively. Translocation depends upon a combination of chemical and biological mechanisms not yet well-understood.10 A different behavior of U accumulation was obtained in our previous study, where no root-to-shoot translocation and no significant accumulation (p < 0.05) in the plant roots were detected when plants were exposed to similar Ca treatment (6 mM) in carbonate water.13 However, in that study, plants were exposed to at least 27.2 less dissolved U concentration than the present study.13 The difference in U uptake and accumulation in plants with respect to the initial U concentration is consistent with possible toxic effects of U on plant tissues.31,32 Seregin et al. suggested that an increase of metal concentration can disrupt the physiological and structural characteristics of translocation barriers in root cells, resulting in an increase in metal translocation.33 Further investigations are necessary to understand how the mechanisms of U toxicity in plants could affect the transport of U into plant roots (e.g., cell wall permeability and transport protein activity) and its translocation to the shoots.
Figure 1.
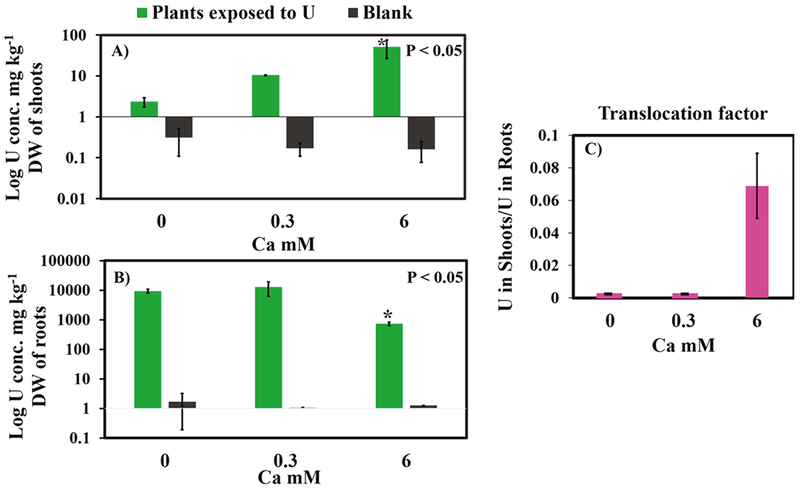
Uranium content (mg kg−1) of (A) shoots and (B) roots presented in logarithmic scale for plants exposed to 80 μM initial Ui concentration at different Ca concentrations. (C) Translocation factor calculated as the ratio of the U content in shoots to that of roots. Multiple pairwise comparisons were conducted using Kruskal–Wallis with the Conover–Iman test to compare the U content between plant samples at the different Ca concentrations. (*) Significant difference (p < 0.05) in the U content between Ca treatments. The standard deviation was determined from triplicate experiments at each Ca treatment (n = 3).
The translocation factor, calculated as the ratio of the U content in the shoots to that of roots,34 was found below 1 under all Ca treatments, reflecting the main accumulation of U in the roots. However, the concentration of U in the shoots increased 22 times when the Ca concentration was increased from 0 to 6 mM. Uranium translocation also increased in previous studies in different plant species (e.g., Arabidopsis thaliana, oilseed rape, sunflower, and wheat) exposed to U (6.5–100 μM) and carbonate in Hoagland nutrient solutions containing Ca (>0.3 mM).10,35 Laurette et al. attributed the translocation of U at these conditions to the formation of the negatively charged U–CO3 complexes, which likely react less with the negatively charged cell wall components compared to the positively charged UO22+.10 Chemical equilibrium modeling based on the experimental conditions used in our study suggests that the enhancement of U root-to-shoot translocation at 6 mM Ca corresponds to an increase of neutrally charged Ca–U–CO3 from 0 to 72% and a decrease of the negatively charged U–CO3 from 99.5 to 15% (Table S3 of the Supporting Information). The presence of positively charged UO22+ was negligible under all treatments, and only 0.1–0.5% of UO2–OH were predicted to be present. The enhancement of U root-to-shoot translocation in the presence of neutrally charged Ca–U–CO3 is in good agreement with previous studies showing that neutral molecules are more readily transported from the roots to the shoots than charged molecules.36
Uranium Cellular Distribution in B. juncea Roots.
The presence and variation of U distribution in root cells caused by different Ca treatments was confirmed by TEM, scanning transmission electron microscopy (STEM), and electron microprobe analyses. Bright-field TEM imaging and electron diffraction analyses indicate that precipitation of U occurred as crystalline needle-like grains, which contained P, under all Ca treatments as indicated by the energy-dispersive spectroscopy (EDS) spectra (panels M–Q of Figures 2). Uranium accumulated at specific sites in the roots with respect to the Ca concentration. In experiments free of Ca, the U–P-bearing precipitates aggregated and attached to the cell walls in the cortex tissue (panels A–D of Figures 2) with no detectable precipitation inside the cells. In experiments with 0.3 mM Ca, U–P-bearing precipitates were detected in the intercellular space of the cortex tissue and the xylem (panels E–H of Figures 2). In contrast, in experiments with 6 mM Ca, intracellular U–P-bearing precipitates were observed in the vacuole of parenchymal tissue (panels I–L of Figures 2) and inside the xylem near the cell wall.
Figure 2.
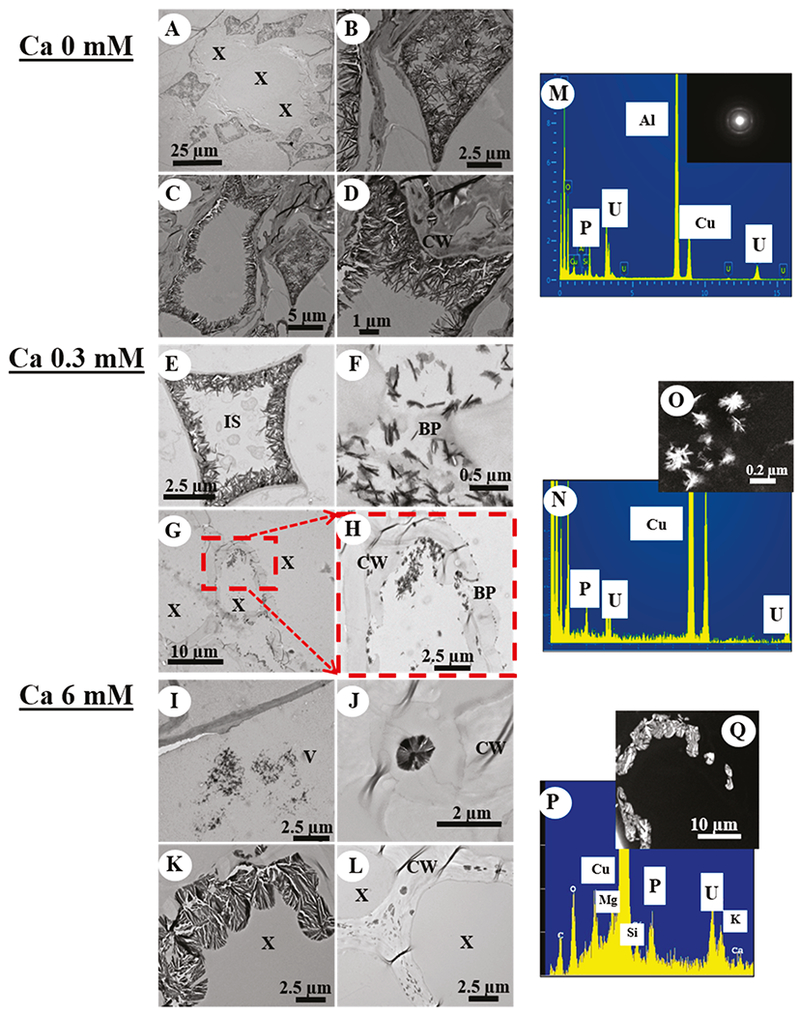
TEM images, EDS spectra, and electron diffraction patterns for B. juncea roots exposed to 80 μM initial Ui concentration at (A–D and M) 0 mM Ca, (E–H and N–O) 0.3 mM Ca, and (I–L and P–Q) 6 mM Ca. Abbreviations: X, xylem; CW, cell wall; IS, intercellular space; BP, bordered pit; and V, vacuole.
Generally, U needs to pass through two pathways to be transported inside the plant from the roots toward the shoots: symplast and apoplast. In the symplastic pathway, U can enter the cells by crossing the cell membrane and via plasmodesmata to be transported from one cell to another toward the vascular tissue. In the apoplastic pathway, U only undergoes a short-distance transport through the cell walls and intercellular space of epidermis and cortex tissues. To enter the vascular tissue from the cortex, U needs to move symplastically through the endodermis, because apoplastic movement is blocked by a barrier in endodermal cell walls, termed the Casparian strip.
In this study, U showed two different transport mechanisms with respect to the Ca concentration in carbonate water that agreed with the measured accumulation of U in roots and shoots. TEM images indicated the apoplast as the primary transport pathway of U in the absence of Ca, resulting in a high precipitation of U with endogenous P in the cortex region. Such precipitation corresponded to a high accumulation of U in the cortical apoplast and no root-to-shoot translocation. In the presence of a low Ca concentration (0.3 mM), U–P precipitation was also detected in the cortex region with no intracellular precipitation, suggesting that the apoplast remained the main transport pathway for U. However, some U–P-bearing precipitates were observed passing through the bordered pits and inside the xylem cells, indicating that Ca enabled U to cross the membrane of endodermal cells, which allowed U to move into xylem and increased the transport of U toward shoots. Uranium noticeably penetrated more into the vascular tissue in experiments with a high Ca concentration (6 mM) with no detectable apoplastic precipitation, suggesting that Ca facilitated the mechanism of symplastic transport of U within the roots toward the shoots. The symplast represents a selective and regulated pathway that involves the transport of solutes from cell to cell by plasmodesmata.37,38 Thus, the mediation of U transport through the symplast by Ca can both enhance root-to-shoot translocation and, at the same time, decrease U accumulation in the roots, in comparison to root cortical apoplastic transport. This explains the significant decrease (p < 0.05) of the U content in plant roots when we detected a significant U accumulation (p < 0.05) in the shoots at 6 mM Ca.
The root-to-shoot translocation of U in carbonate water was attributed in previous studies to an electrostatic repulsion between the negatively charged U–CO3 complexes and the negatively charged cell walls in the cortical apoplast, leading to a symplastic transport.10,14 In our study, U accumulated in the cortical apoplast of plant roots when the negatively charged U–CO3 complexes predominated in water free of Ca at pH 7.5. These observations suggest that the apoplastic transport can still occur in the roots in the presence of the negatively charged U–CO3 complexes. However, the transport of U in root cells changes from apoplastic to symplastic when the Ca concentration increases in carbonate water, resulting in a significant (p < 0.05) U accumulation in the shoots. Such an effect suggests that (1) the predominance of neutrally charged Ca–U–CO3 complexes (72%) at a high Ca concentration facilitates U penetration through the cell membrane, enhancing U transport inside the cells of the roots toward the shoots, (2) a high Ca concentration induces specific physiological mechanisms in plant roots, facilitating U transport in the cells, or (3) a combination of 1 and 2. Because the symplastic transport mechanisms occurred under both the increase of the Ca concentration and the increase of neutrally charged Ca–U–CO3 in water, the results of this study suggest synergetic chemical and physiological effects of Ca. It is likely that Ca facilitates symplastic transport when the neutral aqueous complexes Ca–U–CO3 predominate in water. This is in good agreement with the literature that found that neutrally charged molecules have a higher root-to-shoot translocation than charged molecules as a result of a higher membrane permeability and a high affinity to aquaporins channels.36,39,40 Given that Ca is a potential messenger that participates in the regulation of both membrane permeability and transport proteins,41 further research is needed to understand the specific physiological mechanisms that facilitate U transport through root cells involving Ca.
CONCLUSION
The results obtained in this study reveal that U root-to-shoot translocation in carbonate water is caused by Ca, as opposed to what is known in the literature thus far.11 ,14,35 TEM images suggest that the mechanistic pathway of U in the roots (from the epidermis toward the vascular tissues) of plants in carbonate water is determined by the concentration of Ca. A low Ca concentration (0.3 mM) induces apoplastic transport and precipitation of U with roots endogenous to P, resulting in a high root accumulation and low root-to-shoot translocation. However, a high Ca concentration (6 mM) was found to inhibit the apoplastic transport of U and to facilitate the symplastic transport mechanisms, resulting in a decrease in U root accumulation and an increase in U root-to-shoot translocation. The increase of neutrally charged Ca–U–CO3 concentrations could be a key factor increasing the affinity of U to cross the cell membrane and facilitating its translocation toward the shoots. Unlike the study by El Hayek et al. that showed a significant inhibition of U uptake in plant roots in the presence of Ca–U–CO3 complexes at U ≤ 2.92 μM,13 the results of the present study indicate that, at a higher U concentration, the presence of these complexes can help to increase U entrance into root cells and subsequently U root-to-shoot translocation. These findings are relevant to many environments where uranyl carbonate and calcium uranyl carbonate complexes can be formed in natural waters and sediments.23 Further investigations should assess how the presence of natural organic matter and bacteria in the rhizosphere could alter the Ca-induced mechanisms through the transport of U in the roots. Overall, U root-to-shoot translocation has important implications in human health exposure and phytoremediation approaches. Our findings put new insights into the accumulation and transport of U by highlighting the effect of an important physiological element in plant and opening new pathways for future genetic studies to understand the biological mechanisms behind the Ca effect (e.g., the involvement of Ca-dependent proteins and membrane transport proteins).
Supplementary Material
ACKNOWLEDGMENTS
Funding for this research was provided by the National Science Foundation (CAREER 1652619, NM EPSCoR IIA-1301346, and CREST 1345169) and the National Institute of Environmental Health Sciences SuperfUnd Research Program (Award 1 P42 ES025589). Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. Electron microprobe and scanning transmission electron microscopy were performed in the Electron Microbeam Analysis Facility, Department of Earth and Planetary Sciences and Institute of Meteoritics, University of New Mexico, a facility supported by the State of New Mexico, National Aeronautics and Space Administration (NASA), and the National Science Foundation. TEM images were also generated in the Health Sciences Center (HSC) Electron Microscopy Facility, supported by the University of New Mexico Health Sciences Center.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsearthspace-chem.9b00171
Additional materials and methods, content of U in plant roots and shoots at the three different Ca treatments compared according to the Kruskal–Wallis test, which was used as a non-parametric test alternative to ANOVA (Table S1), measured mass of accumulated U (mg) considering the dry biomass of plant roots and shoots and percentage of U mass in roots or shoots at the three different Ca treatments (Table S2), and percent distribution (%) of U species calculated using Visual MINTEQ for exposure solutions at pH 7.5 (Table S3) (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Jovanovic SV; Pan P; Wong L Bioaccessibility of uranium in soil samples from Port Hope, Ontario, Canada. Environ. Sci. Technol 2012, 46, 9012–9018. [DOI] [PubMed] [Google Scholar]
- (2).Prat O; Vercouter T; Ansoborlo E; Fichet P; Perret P; Kurttio P; Salonen L Uranium speciation in drinking water from drilled wells in Southern Finland and its potential links to health effects. Environ. Sci. Technol 2009, 43 (10), 3941–3946. [DOI] [PubMed] [Google Scholar]
- (3).Winde F; Erasmus E; Geipel G Uranium contaminated drinking water linked to leukaemia-Revisiting a case study from South Africa taking alternative exposure pathways into account. Sci. Total Environ 2017, 574, 400–421. [DOI] [PubMed] [Google Scholar]
- (4).Blake JM; De Vore CL; Avasarala S; Ali A-M; Roldan C; Bowers F; Spilde MN; Artyushkova K; Kirk MF; Peterson E; Rodriguez-Freire L; Cerrato JM Uranium mobility and accumulation along the Rio Paguate, Jackpile Mine in Laguna Pueblo, New Mexico. Environ. Sci. Process. Impacts 2017, 19, 605–621. [DOI] [PubMed] [Google Scholar]
- (5).Blake JM; Avasarala S; Artyushkova K; Ali A-MS; Brearley AJ; Shuey C; Robinson WP; Nez C; Bill S; Lewis J; Hirani C; Pacheco JSL; Cerrato JM Elevated concentrations of U and co-occurring metals in abandoned mine wastes in a northeastern Arizona Native American Community. Environ. Sci. Technol 2015, 49 (14), 8506–8514. [DOI] [PubMed] [Google Scholar]
- (6).Avasarala S; Lichtner PC; Ali A-MS; González-Pinzón R; Blake JM; Cerrato JM Reactive transport of U and V from abandoned uranium mine wastes. Environ. Sci. Technol 2017, 51 (21), 12385–12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Velasco CA; Artyushkova K; Ali AS; Osburn CL; Gonzalez-estrella J; Lezama-pacheco JS; Cabaniss SE; Cerrato JM Organic functional group chemistry in mineralized deposits containing U(IV) and U(VI) from the Jackpile Mine in New Mexico. Environ. Sci. Technol 2019, 53, 5758–5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Ebbs SD; Brady DJ; Kochian LV Role of uranium speciation in the uptake and translocation of uranium by plants. J. Exp. Bot 1998, 49 (324), 1183–1190. [Google Scholar]
- (9).Qi F; Zha Z; Du L; Feng X; Wang D; Zhang D; Fang Z; Ma L; Jin Y; Xia C Impact of mixed low-molecular-weight organic acids on uranium accumulation and distribution in a variant of mustard (Brassica juncea var. tumida). J. Radioanal. Nucl. Chem 2014, 302 (1), 149–159. [Google Scholar]
- (10).Laurette J; Larue C; Mariet C; Brisset F; Khodja H; Bourguignon J; Carriére M Influence of uranium speciation on its accumulation and translocation in three plant species: Oilseed rape, sunflower and wheat. Environ. Exp. Bot 2012, 77, 96–107. [Google Scholar]
- (11).Saenen E; Horemans N; Vanhoudt N; Vandenhove H; Biermans G; Van Hees M; Wannijn J; Vangronsveld J; Cuypers A Effects of pH on uranium uptake and oxidative stress responses induced in Arabidopsis thaliana. Environ. Toxicol. Chem 2013, 32 (9), 2125–2133. [DOI] [PubMed] [Google Scholar]
- (12).Soudek P; Petrova T; Benesova D; Dvorakova M; Vanek T Uranium uptake by hydroponically cultivated crop plants. J. Environ. Radioact 2011, 102 (6), 598–604. [DOI] [PubMed] [Google Scholar]
- (13).El Hayek E; Torres C; Rodriguez-Freire L; Blake JM; De Vore CL; Brearley AJ; Spilde MN; Cabaniss S; Ali A-MS; Cerrato JM Effect of calcium on the bioavailability of dissolved uranium(VI) in plant roots under circumneutral pH. Environ. Sci. Technol 2018, 52, 13089–13098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Laurette J; Larue C; Llorens I; Jaillard D; Jouneau PH; Bourguignon J; Carrière M Speciation of uranium in plants upon root accumulation and root-to-shoot translocation: A XAS and TEM study. Environ. Exp. Bot 2012, 77, 87–95. [Google Scholar]
- (15).Mihalík J; Henner P; Frelon S; Camilleri V; Feévrier L Citrate assisted phytoextraction of uranium by sunflowers: Study of fluxes in soils and plants and resulting intra-planta distribution of Fe and U. Environ. Exp. Bot 2012, 77, 249–258. [Google Scholar]
- (16).Vera Tomeé F; Blanco Rodríguez P; Lozano JC The ability of Helianthus annuus L. and Brassica juncea to uptake and translocate natural uranium and 226Ra under different milieu conditions. Chemosphere 2009, 74 (2), 293–300. [DOI] [PubMed] [Google Scholar]
- (17).Soudek P; Petrová Š; Buzek M; Lhotský O; Vaněk T Uranium uptake in Nicotiana sp. under hydroponic conditions. J. Geochem. Explor 2014, 142, 130–137. [Google Scholar]
- (18).Ji R; Zhou L; Liu J; Wang Y; Yang L; Zheng Q; Zhang C; Zhang B; Ge H; Yang Y; Zhao F; Luan S; Lan W Calcium-dependent protein kinase CPK31 interacts with arsenic transporter AtNIP1;1 and regulates arsenite uptake in Arabidopsis thaliana. PLoS One 2017, 12 (3), e0173681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Van Der Heijden G; Bel J; Craig C-A; Midwood AJ; Mareschal L; Ranger J; Dambrine E; Legout A Measuring plant-available Mg, Ca, and K pools in the soil—An isotopic dilution assay. ACS Earth Sp. Chem 2018, 2 (4), 292–313. [Google Scholar]
- (20).Lauria DC; Ribeiro FCA; Conti CC; Loureiro FA Radium and uranium levels in vegetables grown using different farming management systems. J. Environ. Radioact 2009, 100, 176–183. [DOI] [PubMed] [Google Scholar]
- (21).Straczek A; Duquene L; Wegrzynek D; Chinea-Cano E; Wannijn J; Navez J; Vandenhove H Differences in U root-to-shoot translocation between plant species explained by U distribution in roots. J. Environ. Radioact 2010, 101 (3), 258–266. [DOI] [PubMed] [Google Scholar]
- (22).Huang JW; Blaylock MJ; Kapulnik Y; Ensley BD Phytoremediation of uranium-contaminated soils: Role of organic acids in triggering uranium hyperaccumulation in plants. Environ. Sci. Technol 1998, 32 (13), 2004–2008. [Google Scholar]
- (23).Cumberland SA; Douglas G; Grice K; Moreau JW Uranium mobility in organic matter-rich sediments: A review of geological and geochemical processes. Earth-Sci. Rev 2016, 159, 160–185. [Google Scholar]
- (24).Rader ST; Maier RM; Barton MD; Mazdab FK Uptake and fractionation of Thallium by Brassica juncea in a geogenic Thallium-amended substrate. Environ. Sci. Technol 2019, 53 (5), 2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Carrière M; Gouget B; Gallien JP; Avoscan L; Gobin R; Verbavatz JM; Khodja H Cellular distribution of uranium after acute exposure of renal epithelial cells: SEM, TEM and nuclear microscopy analysis. Nucl. Instrum. Methods Phys. Res., Sect. B 2005, 231 (1−4), 268–273. [Google Scholar]
- (26).Gerber U; Zirnstein I; Krawczyk-Bärsch E; Lünsdorf H; Arnold T; Merroun ML Combined use of flow cytometry and microscopy to study the interactions between the gram-negative betaproteobacterium Acidovorax facilis and uranium(VI). J. Hazard. Mater 2016, 317, 127–134. [DOI] [PubMed] [Google Scholar]
- (27).Le Menn N; Marchand S; de Revel G; Demarville D; Laborde D; Marchal R N,S,O-Heterocycles in aged champagne reserve wines and correlation with free amino acid concentrations. J. Agric. Food Chem 2017, 65 (11), 2345–2356. [DOI] [PubMed] [Google Scholar]
- (28).Renedo M; Amouroux D; Duval B; Carravieri A; Tessier E; Barre J; Bérail S; Pedrero Z; Cherel Y; Bustamante P Seabird tissues as efficient biomonitoring tools for Hg isotopic investigations: Implications of using blood and feathers from chicks and adults. Environ. Sci. Technol 2018, 52 (7), 4227–4234. [DOI] [PubMed] [Google Scholar]
- (29).Gustafsson JP Visual MINTEQ, Version 3.1; https://vminteq.lwr.kth.se/download/.
- (30).Hu S; Lu Y; Peng L; Wang P; Zhu M; Dohnalkova AC; Chen H; Lin Z; Dang Z; Shi Z Coupled kinetics of ferrihydrite transformation and As(V) sequestration under the effect of humic acids: A mechanistic and quantitative study. Environ. Sci. Technol 2018, 52 (20), 11632–11641. [DOI] [PubMed] [Google Scholar]
- (31).Khalid N; Aqeel M; Noman A System biology of metal tolerance in plants: An integrated view of genomics, transcriptomics, metabolomics, and phenomics In Plant Metallomics and Functional Omics; Sablok G, Ed.; Springer: Cham, Switzerland, 2019; pp 107–144, DOI: 10.1007/978-3-030-19103-0_6. [DOI] [Google Scholar]
- (32).Noman A; Aqeel M; Javed MT; Zafar S; Ali Q; Islam W; Irshad MK; Buriro M; Kanwal H; Khalid N; Khan S Histological changes in Hibiscus rosa-sinensis endorse acclimation and phytoremediation of industrial polluted sites. J. Anim. Plant Sci 2017, 27 (5), 1637–1648. [Google Scholar]
- (33).Seregin IV; Shpigun LK; Ivanov VB Distribution and toxic effects of cadmium and lead on maize roots. Russ. J. Plant Physiol 2004, 51 (4), 525–533. [Google Scholar]
- (34).El Hayek E; El Samrani A; Lartiges B; Kazpard V; Benoit M; Munoz M Potential of Opuntia ficus-indica for air pollution biomonitoring: A lead isotopic study. Environ. Sci. Pollut. Res 2015, 22 (22), 17799–17809. [DOI] [PubMed] [Google Scholar]
- (35).Saenen E; Horemans N; Vanhoudt N; Vandenhove H; Biermans G; Van Hees M; Wannijn J; Vangronsveld J; Cuypers A The pH strongly influences the uranium-induced effects on the photosynthetic apparatus of Arabidopsis thaliana plants. Plant Physiol. Biochem 2014, 82, 254–261. [DOI] [PubMed] [Google Scholar]
- (36).Nason SL; Miller EL; Karthikeyan KG; Pedersen JA Plant-induced changes to rhizosphere pH impact leaf accumulation of Lamotrigine but Not Carbamazepine. Environ. Sci. Technol. Lett 2018, 5 (6), 377–381. [Google Scholar]
- (37).Sattelmacher B The apoplast and its significance for plant mineral nutrition. New Phytol. 2001, 149 (22), 167–192. [DOI] [PubMed] [Google Scholar]
- (38).White PJ The pathways of calcium movement to the xylem. J. Exp. Bot 2001, 52 (358), 891–899. [DOI] [PubMed] [Google Scholar]
- (39).Limmer MA; Burken JG Plant translocation of organic compounds: Molecular and physicochemical predictors. Environ. Sci. Technol. Lett 2014, 1 (4), 156–161. [Google Scholar]
- (40).Ma JF; Yamaji N; Mitani N; Xu X; Su Y; Mcgrath SP; Zhao F Transporters of arsenite in rice and their role in arsenic accumulation in rice grain. Proc. Natl. Acad. Sci. U. S. A 2008, 105 (29), 9931–9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Wu Y; Liu X; Wang W; Zhang S; Xu B Calcium regulates the cell-to-cell water flow pathway in maize roots during variable water conditions. Plant Physiol. Biochem 2012, 58, 212–219. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


