Abstract
Background
The aim of this study was to investigate the diagnostic value of (F/T)/PSAD for prostate cancer detection in the Chinese population.
Material/Methods
Data were collected retrospectively from patients with prostate cancer or benign prostatic hyperplasia from July 2009 to September 2014. SPSS 19.0 software was used for the receiver operating characteristic curve (ROC), and calculating sensitivity, specificity, and positive predictive values (PPV) and negative predictive values (NPV), respectively. Comparison of the area under ROC (AUC) was performed using the MedCalc v. 10.4.7.0 software.
Results
A total of 660 patients (including 251 patients with prostate cancer and 409 patients with prostatic hyperplasia) were included. Prostate volume (PV), prostate-specific antigen density (PSAD), free-serum PSA (FPSA)/PSAD, and free-to-total PSA (F/T)/PSAD had similar AUC (P>0.05), and had significantly higher AUC (P<0.001) than F/T, total-serum PSA (TPSA), and free-serum PSA (FPSA). Based on the optimal cutoff value, the sensitivity of (F/T)/PSAD and FPSA/PSAD was similar (P>0.05), and significantly higher than the PV and PSAD (P<0.05). The logistic regression model using a combination of age, FPSA, PV, PSAD, FPSA/PSAD, and (F/T)/PSAD showed higher AUC than each one alone (P<0.001).
Conclusionss
(F/T)/PSAD can be used as a predictor for prostate cancer in the Chinese population aged >50 years and has a significantly lower false negative rate than PSAD and PV with a cutoff value of ≤0.731. A new parameter, FPSA/PSAD, has similar diagnostic accuracy comparable to (F/T)/PSAD. The diagnostic value of a combination of age, FPSA, PV, PSAD, FPSA/PSAD, and (F/T)/PSAD needs further investigation.
MeSH Keywords: Diagnosis, Prostate-Specific Antigen, Prostatic Neoplasms
Background
Prostate-specific antigen (PSA) is an essential marker for the screening of prostate cancer with high tissue specificity but has rates for missed diagnosis and misdiagnosis [1–4]. The elevated PSA level is due to a variety of factors [5]. Many benign prostate diseases are also associated with PSA levels ranging from 4 to 10 ng/mL [6–8], resulting in unnecessary prostate biopsies. Some studies have shown a high incidence of prostate cancer when the PSA level is lower than 4.0 ng/mL [9,10].
Moreover, many studies focused their attention on other related parameters, such as free-to-total PSA (F/T) values and prostate-specific antigen density (PSAD), showing higher diagnostic accuracy than that with PSA itself [11–13]. Two parameters, PSAD, and F/T are most commonly used to improve the diagnostic accuracy of PSA. Veneziano et al. [14] combined these 2 parameters and proposed a new parameter (F/T)/PSAD, which was superior to F/T or PSAD alone for the diagnosis of prostate cancer in the Italian population. A previous study in the Chinese population with PSA of 4~10 ng/mL also showed significantly higher specificity with (F/T)/PSAD than F/T and PSAD alone [15]. However, sensitivity and specificity are still not satisfactory. In addition, Lee et al. reported that combination of age, PSA, prostate volume (PV), and digital rectal examination (DRE) status had better prediction ability for prostate cancer than PSA alone [16].
Thus, we further evaluated the diagnostic accuracy of (F/T)/PSAD in 660 Chinese patients with PSA values ranging from 2.5–20 ng/mL and obtained additional evidence for the use of (F/T)/PSAD values in clinical practice for prostate cancer diagnosis. An appropriate new diagnostic parameter FPSA/PSAD was also calculated and analyzed in this study. In addition, the optimal risk prediction model was constructed to increase the diagnostic accuracy of (F/T)/PSAD for prostate cancer diagnosis.
Material and Methods
Baseline data
Approval for this study was from the Ethics Committee of the Chinese People’s Liberation Army General Hospital, and data collected retrospectively from patients with prostate cancer or benign prostatic hyperplasia who underwent systematic prostate puncture in our hospital from July 2009 to September 2014. Prostate biopsy was by following the guidelines of the Urology Branch of Chinese Medical Association (CUA). Biopsied patients had: 1) hard prostate or irregular shape by DRE; 2) presence of low echo nodules in the periphery of the prostate by transrectal ultrasound; 3) serum-total PSA (TPSA) concentration >10 ng/mL without limitation on F/T and PSAD; 4) 4 ng/mL <serum TPSA concentration ≤10 ng/mL with the limitation of F/T <0.16 or PSAD >0.15. Biopsy results diagnosed patients with prostate cancer or benign prostatic hyperplasia.
The patients enrolled in this study met the following criteria. Inclusion criteria were: 1) patient age ≥50 years; 2) first prostate puncture (12+X needle); 3) PSA 2.5–20 ng/mL. Exclusion criteria were: 1) history of prostate cancer, prostate surgery, or taking 5-alpha-reductase inhibitor/drug for treating endocrine dyscrasia in prostate cancer; 2) urinary tract infection or obstruction; 3) diagnosed prostatitis; and 4) undergone prostate massage, DRE, transrectal ultrasound, or cystoscopy within 2 weeks before PSA detection, which might affect serum PSA level.
Prostate puncture
Informed consent was obtained before performing prostate puncture. Oral antibiotics and cleansing enema were used to prevent infection. With the guidance of color ultrasound, total 12 plus 1–3 needle punctures performed on several sites, including midline of prostate glands, the tip, middle, and bottom of both sides of sagittal and the tip, middle, and bottom of both sides of the peripheral zone of the prostate gland. After a prostate puncture, oral antibiotics were continued for 3 days.
Transrectal ultrasound
Examination of the prostate was by the transrectal ultrasound using Acuson Sequoia512 (Siemens Medical Solutions USA). PV was calculated according to the formula: PV=0.52×anterior/posterior diameter (cm)×left/right diameter (cm)×upper/lower diameter (cm).
PSA detection
Venous blood was collected before DRE, transrectal ultrasound, or cystoscopy which might affect the serum PSA level. The TPSA and FPSA levels were determined by the chemiluminescence assay using a fully automated chemiluminescent immunoassay analyzer (Sorin Company, Italy, Liaison-XL). The following parameters were calculated:
| [ 14]. |
In addition, a new parameter was also calculated and analyzed in this study: FPSA/PSAD=(FPSA×PV)/TPSA
Statistical analysis
SPSS 19.0 software was used for statistical analysis (SPSS Inc., Chicago, IL, USA) unless otherwise specified. A P value <0.05 was considered statistically significant. The continuous variables were described by the mean ± standard deviation (SD). Two independent tests, t-test and analysis of variance (ANOVA) were used to compare data between groups when the data met the normal distribution. Otherwise, a nonparametric test was used, and the median (interquartile range) was used to show the data. Comparison of percentages was by chi-square test. The receiver operating characteristic curves (ROC) evaluated the diagnostic value of TPSA, FPSA, PV, F/T, PSAD, FPSA/PSAD, and (F/T)/PSAD in patients to determine prostate cancer or exclude prostatic hyperplasia with a prostate biopsy. Youden index was used to determine the optimal cutoff value. The area under the curve (AUC) was calculated and evaluated using a nonparametric test. The comparison between the AUC of 2 ROC curves was performed by the method of DeLong et al. 1988 [17] using MedCalc v. 10.4.7.0 software (MedCalc Software bvba, Mariakerke, Belgium). Based on the optimal cutoff value, the diagnostic accuracy was assessed using sensitivity, specificity, and positive predictive value (PPV) and negative predictive value (NPV). A stepwise logistic regression analysis was used to find an optimal risk prediction model. The goodness-of-fit of the model (χ2) was evaluated by the Hosmer-Lemeshow test with P>0.05 as a threshold, with higher P-value indicating a better fit.
Results
Characteristics of included patients
A total of 660 patients were in this study, including 251 patients with prostate cancer (38%) and 409 patients with prostatic hyperplasia (62%). Nonparametric test (Mann-Whitney U test) compared the data between the prostate cancer group and prostatic hyperplasia group because of non-normal distribution. Table 1 shows no significant difference in age between patients with prostate cancer and prostatic hyperplasia. A significant difference (P<0.001) was present in TPSA, FPSA, PV, F/T, PSAD, FPSA/PSAD, and (F/T)/PSAD.
Table 1.
Comparison of baseline data between 2 groups.
| Group | Age (year) | tPSA (ng/mL) | fPSA (ng/mL) | PV (cm3) | F/T | PSAD (ng/mL/cm3) | (F/T)/PSAD | fPSA/PSAD |
|---|---|---|---|---|---|---|---|---|
| Prostate cancer (n=251) | 70 (63, 75) | 9.64 (6.11, 13.8) | 0.9 (0.5, 1.53) | 31.12 (23.20, 46.70) | 0.095 (0.064, 0.151) | 0.281 (0.168, 0.421) | 0.327 (0.166, 0.646) | 2.885 (1.738, 5.818) |
| Prostatic hyperplasia (n=409) | 74 (71, 77) | 8.83 (8.14, 9.52) | 2.35 (1.56, 3.04) | 93.51 (63.53, 119.22) | 0.221 (0.130, 0.302) | 0.025 (−0.124, 0.151) | 1.612 (0.821, 2.394) | 11.177 (6.888, 18.607) |
| Z | −1.011 | −5.317 | −4.690 | −13.800 | −10.388 | −14.487 | −14.672 | −14.244 |
| P | 0.312 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Median (interquartile range) was used to show the data and nonparametric test (Mann-Whitney U test) was used to compare data between prostate cancer group and prostatic hyperplasia group because of non-normal distribution. PSA – prostate specific antigen; fPSA – free PSA; tPSA – total PSA; PV – prostate volume; F/T – the ratio of fPSA to tPSA; PSAD – prostate specific antigen density.
Diagnostic value of TPSA, FPSA, PV, F/T, PSAD, FPSA/PSAD, and (F/T)/PSAD
The ROC curves of TPSA, FPSA, PV, F/T, PSAD, and (F/T)/PSAD for diagnosis of prostate cancer are shown in Figure 1. The ROC curves of equal to or lower than 6 parameters can be drawn together by MedCalc v. 10.4.7.0 software, thus, the ROC curve of the new parameter FPSA/PSAD are shown in Figure 2. The AUCs of TPSA, FPSA, PV, F/T, PSAD, FPSA/PSAD, and (F/T)/PSAD were 0.623, 0.609, 0.820, 0.741, 0.836, 0.830, and 0.840, respectively. All these parameters had significant diagnostic value based on the nonparametric test (P<0.001). Table 2 shows a comparison of results between the AUC of ROC curves. The results showed that PV, PSAD, FPSA/PSAD, and (F/T)/PSAD had similar AUC (P>0.05), and they had significantly higher AUC (P<0.001) than F/T, TPSA, and FPSA.
Figure 1.
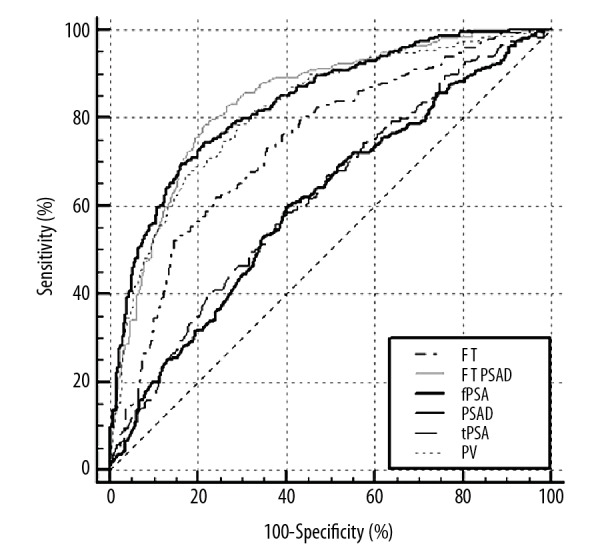
ROC of (F/T)/PSAD, PSAD, F/T, TPSA, FPSA, and PV for prostate cancer diagnosis. ROC – receiver operating characteristic curves; (F/T) – free-to-total PSA; PSAD – prostate-specific antigen density; TPSA – serum-total PSA; FPSA – serum-free PSA; PV – prostate volume.
Figure 2.
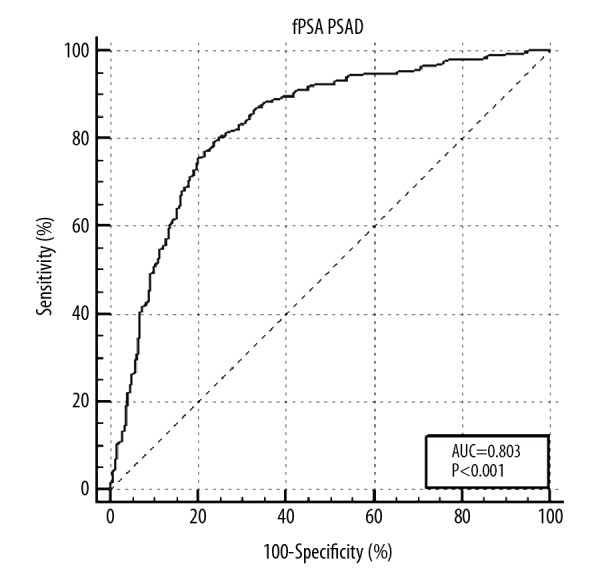
ROC of FPSA/PSAD for prostate cancer diagnosis. ROC – receiver operating characteristic curves; FPSA – serum-free prostate specific antigen; PSAD – prostate-specific antigen density.
Table 2.
Comparison results of ROC curves (P-values).
| Parameter | tPSA | fPSA | PV | F/T | PSAD | (F/T)/PSAD | fPSA/PSAD |
|---|---|---|---|---|---|---|---|
| tPSA | NA | NA | NA | NA | NA | NA | NA |
| fPSA | 0.718 | NA | NA | NA | NA | NA | NA |
| PV | <0.001 | <0.001 | NA | NA | NA | NA | NA |
| F/T | <0.001 | <0.001 | <0.001 | NA | NA | NA | NA |
| PSAD | <0.001 | <0.001 | 0.306 | <0.001 | NA | NA | NA |
| (F/T)/PSAD | <0.001 | <0.001 | 0.199 | <0.001 | 0.684 | NA | NA |
| fPSA/PSAD | <0.001 | <0.001 | 0.376 | <0.001 | 0.720 | 0.274 | NA |
ROC – receiver operating characteristic curves; PSA – prostate specific antigen; fPSA – free PSA; tPSA – total PSA; PV – prostate volume; F/T – the ratio of fPSA to tPSA; PSAD – prostate specific antigen density; PPV – positive predictive value; NPV – negative predictive value; NA – not applicable.
Table 3 shows the optimal cutoff values for PV, PSAD, FPSA/PSAD, and (F/T)/PSAD of ≤39.226 (cm), ≥0.198 (ng/mL/cm3), ≤6.263 cm3, and ≤0.731, respectively for predicting prostate cancer. Based on these cutoff values, sensitivity, specificity, PPV, and NPV were calculated. Among them, the sensitivity of (F/T)/PSAD and FPSA/PSAD was similar between each other (P>0.05), and it was significantly higher than PV and PSAD (P<0.05). There was no significant difference in the specificity, PPV, and NPV among PV, PSAD, FPSA/PSAD, and (F/T)/PSAD (P>0.05).
Table 3.
Comparison of diagnostic accuracy among tPSA, fPSA, F/T, PSAD, and (F/T)/PSAD using the diagnostic parameters.
| Parameter | AUC | Optimal cutoff value | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|
| tPSA | 0.623 | 8.505 (ng/mL)* | 0.582 | 0.606 | 0.475 | 0.703 |
| fPSA | 0.609 | 1.075 (ng/mL)# | 0.547 | 0.739 | 0.547 | 0.739 |
| PV | 0.820 | 39.226 (cm3)# | 0.673 | 0.826 | 0.703 | 0.805 |
| F/T | 0.741 | 0.120# | 0.622 | 0.760 | 0.614 | 0.766 |
| PSAD | 0.836 | 0.198 (ng/mL/cm3)* | 0.697 | 0.836 | 0.723 | 0.818 |
| fPSA/PSAD | 0.830 | 6.263 (cm3)# | 0.773 | 0.785 | 0.688 | 0.850 |
| (F/T)/PSAD | 0.840 | 0.731# | 0.785 | 0.785 | 0.691 | 0.856 |
If the data was greater than or equal to this value, the patients can be diagnosed with prostate cancer when using this parameter for predicting prostate cancer;
If the data was lower than or equal to this value, the patients can be diagnosed with prostate cancer when using this parameter for predicting prostate cancer.
PSA – prostate specific antigen; fPSA – free PSA; tPSA – total PSA; PV – prostate volume; F/T – the ratio of fPSA to tPSA; PSAD – prostate specific antigen density; PPV – positive predictive value; NPV – negative predictive value.
Determination of optimal risk prediction model
All the parameters (including age, TPSA, FPSA, PV, F/T, PSAD, and (F/T)/PSAD) were analyzed using multiple regression analysis. The results showed that FPSA (OR=0.523, 95% CI=0.315–0.871, P=0.013), PV (OR=0.977, 95% CI=0.963–0.992, P=0.002), PSAD (OR=272.056, 95%=20.492–3611.858, P<0.001), and (F/T)/PSAD (OR=0.347, 95% CI=0.196–0.615, P<0.001) were independent predictors of prostate cancer regardless of age (Table 4). Hosmer-Lemeshow test showed good regression model fitting (P=0.890). Thus, the diagnostic value of the model was evaluated. The ROC curve was shown in Figure 3. The AUC for this model was 0.868, which was significantly higher (P<0.001) than that of TPSA, FPSA, PV, F/T, PSAD, FPSA/PSAD, and (F/T)/PSAD (Table 5). In addition, based on the Yuden index of 0.613, the sensitivity, specificity, PPV, and NPV of this model were 0.809, 0.804, 0.717, and 0.873, respectively, which were similar (P>0.05) to FPSA/PSAD and (F/T)/PSAD. However, the NPV of this model was significantly higher (P=0.044) than that of PSAD.
Table 4.
Results of logistic regression analysis.
| Coefficient | SE of coefficient | P-value | OR | 95% CI of OR | |||
|---|---|---|---|---|---|---|---|
| Lower limit | Upper limit | ||||||
| Model | Age | 0.040 | 0.014 | 0.004 | 1.041 | 1.013 | 1.069 |
| fPSA | −0.647 | 0.260 | 0.013 | 0.523 | 0.315 | 0.871 | |
| PV | −0.023 | 0.007 | 0.002 | 0.977 | 0.963 | 0.992 | |
| PASD | 5.606 | 1.319 | 0.000 | 272.056 | 20.492 | 3611.858 | |
| (F/T)/PSAD | −1.059 | 0.292 | 0.000 | 0.347 | 0.196 | 0.615 | |
| fPSAPSAD | 0.120 | 0.063 | 0.055 | 1.128 | 0.997 | 1.275 | |
| Constant | −2.224 | 1.075 | 0.039 | 0.108 | NA | NA | |
Model equation: logP/1−P=−2.224+0.040*Age−0.647*fPSA−0.023*PV+5.606*PSAD−1.059*((F/T)/PSAD)+0.120*(fPSA/PSAD), P=probability of prostatic cancer. PSA – prostate specific antigen; fPSA ,– free PSA; tPSA – total PSA; PV – prostate volume; F/T – the ratio of fPSA to tPSA; PSAD – prostate specific antigen density; SE – standard error; OR – odds radio; CI – confidence interval.
Figure 3.
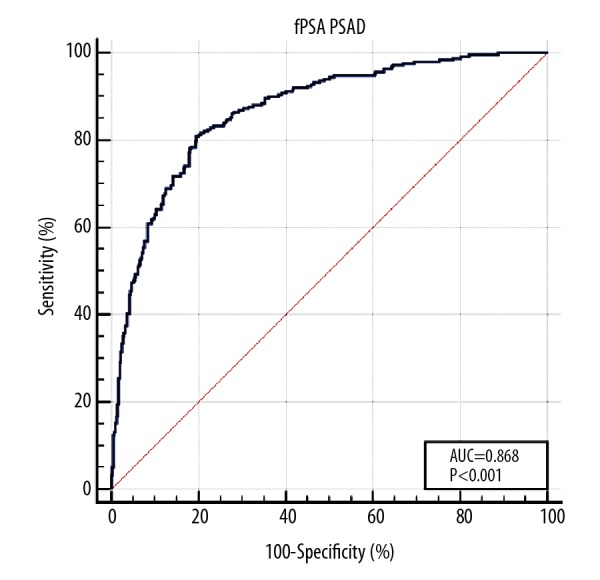
ROC of logistic regression model for prostate cancer diagnosis. ROC – receiver operating characteristic curve.
Table 5.
Comparison results of ROC curves between the regression model and tPSA, fPSA, PV, F/T, PSAD and (F/T)/PSAD (P-values).
| Parameter | tPSA | fPSA | PV | F/T | PSAD | fPSA/PSAD | (F/T)/PSAD |
|---|---|---|---|---|---|---|---|
| Model | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
ROC – receiver operating characteristic curves; PSA – prostate specific antigen; tPSA – total PSA; fPSA – free PSA; PV – prostate volume; F/T – the ratio of fPSA to tPSA; PSAD – prostate specific antigen density.
Discussion
Results from this study share similarity with an earlier study by Yan et al. (2007) [15], which showed significantly higher specificity of (F/T)/PSAD than F/T. Although the specificity of (F/T)/PSAD appeared higher than that of PSAD, it was not statistically significant. Moreover, the sensitivity of (F/T)/PSAD was significantly higher than F/T and PSAD. An earlier study showed different diagnostic accuracy of PSAD in patients with different PSA values [18], and these conflicting results could be due to different PSA values and other confounding factors (e.g., region, sample size, and age). Further studies are needed to investigate the factors affecting the diagnostic accuracy of PSA parameters for prostate cancer detection.
In addition, compared to an earlier study, this study has some advantages. Firstly, we analyzed the diagnostic accuracy of PV for prostate cancer. Based on the results of this study, we conclude that patients with PV ≤39.226 cm3 had a higher risk of prostate cancer. Moreover, the diagnostic accuracy of PV for prostate cancer was significantly higher than TPSA, FPSA, and F/T. Earlier studies showed that PV might influence the diagnostic accuracy of PSA [16,19], and an increase in the detection rate of prostate cancer along with a decrease in PV [20]. No study to date reported the diagnostic accuracy of PV for prostate cancer previously, hence, this is the first study comparing the diagnostic accuracy of PV with the PSA parameters (TPSA, FPSA, F/T, PSAD, and (F/T)/PSAD). Secondly, age is a prominent factor leading to an increase in prostate cancer risk [21–23], and multiple regression analysis performed in this study showed PV, PSAD, and (F/T)/PSAD as independent predictors of prostate cancer regardless of age. Thirdly, the sample size in this study was higher than in the study of Yan et al. [15]. In addition, a newly calculated parameter FPSA/PSAD analyzed in this study showed similar diagnostic accuracy with (F/T)/PSAD. Further studies are necessary to validate this new parameter.
In this study, the optimal cutoff value for PSAD was 0.198, similar to the one reported (0.20) by Xie et al. (2016) [24], and Matsuyama et al. [11]. Xie et al. (2016) [24] analyzed the PSAD variation rate (PSADVR) and showed significantly higher diagnostic accuracy than PSAD. PSADVR may also be a potential parameter that increases the predictive accuracy of PSA. Further studies are required to compare the diagnostic accuracy of PSADVR and (F/T)/PSAD for prostate cancer detection. Furthermore, some limitations of this study should be noted. Firstly, due to the CUA guidance in China, men with PSA between 4 and 10 ng/mL received biopsy only if F/T <0.16 or PSAD >0.15, so there was bias in this study. Secondly, being a retrospective study, some data (such as voiding symptoms, family history, and cancer grades) could not be analyzed due to lack of availability.
Conclusions
Within a PSA range of 2.5–20 ng/mL, the diagnostic value of (F/T)/PSAD, FPSA/PSAD, PV, or PSAD is significantly higher compared than F/T, FPSA, and TPSA. Moreover, (F/T)/PSAD has higher sensitivity than PV, F/T, or PSAD alone, with the cutoff values for (F/T)/PSAD ≤0.731. Being a new parameter, FPSA/PSAD has similar diagnostic accuracy of (F/T)/PSAD. The combined diagnostic value using age, FPSA, PV, PSAD, FPSA/PSAD, and (F/T)/PSAD needs further investigation, as it had a significantly higher AUC and increased NPV when analyzed using a logistic regression model.
Abbreviations
- PSA
prostate-specific antigen
- FPSA
free-PSA
- TPSA
total-PSA
- F/T
FPSA/TPSA
- PSAD
prostate-specific antigen density
- PV
prostate volume
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Abubakar SO, Amoako YA, Tag N, et al. False-positive prostate cancer bone metastases on magnetic resonance imaging correctly classified on gallium-68-prostate-specific membrane antigen positron emission tomography computed tomography. World J Nucl Med. 2018;17:305–7. doi: 10.4103/wjnm.WJNM_89_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aminsharifi A, Howard L, Wu Y, et al. Prostate specific antigen density as a predictor of clinically significant prostate cancer when the prostate specific antigen is in the diagnostic gray zone: Defining the optimum cutoff point stratified by race and body mass index. J Urol. 2018;200(4):758–66. doi: 10.1016/j.juro.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Ilic D, Djulbegovic M, Jung JH, et al. Prostate cancer screening with prostate-specific antigen (PSA) test a systematic review and meta-analysis. BMJ. 2018;362:k3519. doi: 10.1136/bmj.k3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verma P, Malhotra G, Agrawal R, et al. Evidence of prostate-specific membrane antigen expression in metastatic differentiated thyroid cancer using 68Ga-PSMA-HBED-CC PET/CT. Clin Nucl Med. 2018;43(8):e265–68. doi: 10.1097/RLU.0000000000002161. [DOI] [PubMed] [Google Scholar]
- 5.Okada K, Kojima M, Naya Y, et al. Correlation of histological inflammation in needle biopsy specimens with serum prostate- specific antigen levels in men with negative biopsy for prostate cancer. Urology. 2000;55(6):892–98. doi: 10.1016/s0090-4295(00)00519-7. [DOI] [PubMed] [Google Scholar]
- 6.Lee SE, Chung JS, Han BK, et al. Relationship of prostate-specific antigen and prostate volume in Korean men with biopsy-proven benign prostatic hyperplasia. Urology. 2008;71(3):395–98. doi: 10.1016/j.urology.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Mao Q, Zheng X, Jia X, et al. Relationships between total/free prostate-specific antigen and prostate volume in Chinese men with biopsy-proven benign prostatic hyperplasia. Int Urol Nephrol. 2009;41(4):761–66. doi: 10.1007/s11255-009-9533-1. [DOI] [PubMed] [Google Scholar]
- 8.Roehrborn CG, Mcconnell J, Bonilla J, et al. Serum prostate specific antigen is a strong predictor of future prostate growth in men with benign prostatic hyperplasia. PROSCAR long-term efficacy and safety study. J Urol. 2000;163(1):13–20. [PubMed] [Google Scholar]
- 9.Gilbert SM, Cavallo CB, Kahane H, et al. Evidence suggesting PSA cutpoint of 2.5 ng/mL for prompting prostate biopsy: Review of 36,316 biopsies. Urology. 2005;65(3):549–53. doi: 10.1016/j.urology.2004.10.064. [DOI] [PubMed] [Google Scholar]
- 10.Saito S. Prostate-specific antigen cut-off point of 2.5 ng/mL and increasing the number of prostate biopsies results in the detection of curable prostate cancer even in Japanese population. Int J Urol. 2007;14(8):709–12. doi: 10.1111/j.1442-2042.2007.01800.x. [DOI] [PubMed] [Google Scholar]
- 11.Matsuyama H, Baba Y, Yamakawa G, et al. Diagnostic value of prostate-specific antigen-related parameters in discriminating prostate cancer. Int J Urol. 2000;7(11):409–14. doi: 10.1046/j.1442-2042.2000.00221.x. [DOI] [PubMed] [Google Scholar]
- 12.Udeh EI, Nnabugwu II, Ozoemena FO, et al. Prostate-specific antigen density values among patients with symptomatic prostatic enlargement in Nigeria. World J Surg Oncol. 2016;14(1):174. doi: 10.1186/s12957-016-0921-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yilmaz H, Ciftci S, Yavuz U, et al. Percentage of free prostate-specific antigen (PSA) is a useful method in deciding to perform prostate biopsy with higher core numbers in patients with low PSA cut-off values. Kaohsiung J Med Sci. 2015;31(6):315–19. doi: 10.1016/j.kjms.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Veneziano S, Pavlica P, Compagnone G, et al. Usefulness of the (F/T)/PSA density ratio to detect prostate cancer. Urol Int. 2005;74(1):13–18. doi: 10.1159/000082702. [DOI] [PubMed] [Google Scholar]
- 15.Yan D, Hu E, Zhang H, et al. [The significance of PSA modified parameters (F/T)/PSAD for diagnosing prostatic cancer in the grey zone of 4~10 ng/mL]. Chinese Journal of Clinical Oncology. 2007;4(5):347–50. [in Chinese] [Google Scholar]
- 16.Lee A, Lim J, Gao X, et al. A nomogram for prediction of prostate cancer on multi-core biopsy using age, serum prostate-specific antigen, prostate volume and digital rectal examination in Singapore. Asia Pac J Clin Oncol. 2016;13(5):e348–55. doi: 10.1111/ajco.12596. [DOI] [PubMed] [Google Scholar]
- 17.DeLong E, DeLong D, Clarke-Pearson D. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 18.Hoshii T, Nishiyama T, Toyabe S, et al. Evaluation of magnetic resonance imaging-based prostate-specific antigen density of the prostate in the diagnosis of prostate cancer. Int J Urol. 2007;14(4):305–10. doi: 10.1111/j.1442-2042.2007.01686.x. [DOI] [PubMed] [Google Scholar]
- 19.Partin AW, Catalona WJ, Southwick PC, et al. Analysis of percent free prostate-specific antigen (PSA) for prostate cancer detection: Influence of total PSA, prostate volume, and age. Urology. 1996;48(6A Suppl):55–61. doi: 10.1016/s0090-4295(96)00611-5. [DOI] [PubMed] [Google Scholar]
- 20.Özden E, Turgut AT, Talas H, et al. Effect of dimensions and volume of the prostate on cancer detection rate of 12 core prostate biopsy. Int Urol Nephrol. 2007;39(2):525–29. doi: 10.1007/s11255-006-9078-5. [DOI] [PubMed] [Google Scholar]
- 21.Peng P, Gong YM, Bao PP, et al. [Estimates and prediction of prostate cancer incidence, mortality and prevalence in China 2008]. Zhonghua Liu Xing Bing Xue Za Zhi. 2012;33(10):1056–59. [in Chinese] [PubMed] [Google Scholar]
- 22.Braga SFM, Souza MCD, Cherchiglia ML. Time trends for prostate cancer mortality in Brazil and its geographic regions: An age-period-cohort analysis. Cancer Epidemiol. 2017;50(Pt A):53–59. doi: 10.1016/j.canep.2017.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Pettersson A, Robinson D, Garmo H, et al. Age at diagnosis and prostate cancer treatment and prognosis: A population-based cohort study. Ann Oncol. 2018;29(2):377–85. doi: 10.1093/annonc/mdx742. [DOI] [PubMed] [Google Scholar]
- 24.Xie GS, Lyv JX, Li G, et al. Prostate-specific antigen density variation rate as a potential guideline parameter for second prostate cancer detection biopsy. Chin Med J (Engl) 2016;129(15):1800–4. doi: 10.4103/0366-6999.186635. [DOI] [PMC free article] [PubMed] [Google Scholar]


