Abstract
Purpose:
We investigated the safety, pharmacokinetics, and efficacy of gemcitabine administered via bronchial artery infusion (BAI) and IV infusion in advanced NSCLC patients.
Methods:
Patients were eligible if they had received at least two prior cytotoxic chemotherapy regimens. Gemcitabine was administered via BAI as 600 mg/m2 on day one of cycle one, followed by IV as 1000 mg/m2 on day eight of cycle one, and IV on days one and eight of all subsequent cycles. Pharmacokinetics for gemcitabine and dFdU metabolite in plasma, and dFdCTP active metabolite in peripheral blood mononuclear cells (PBMC) were evaluated. Intensive pharmacokinetic sampling was performed after BAI and IV infusions during cycle one.
Results:
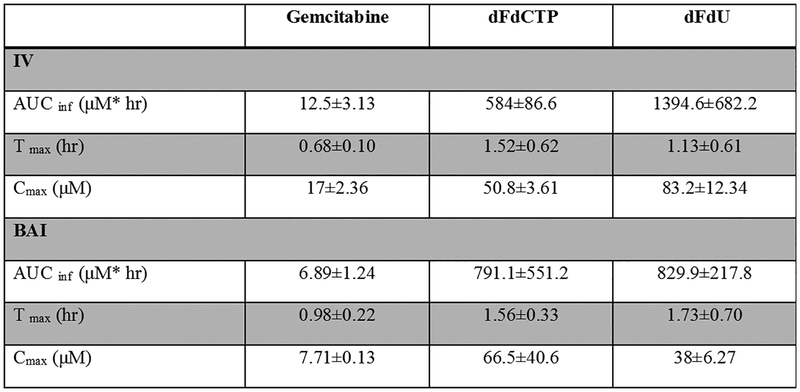
Three male patients (age range 59–68 years) were evaluated. All patients responded with stable disease or better. One PR was observed after cycle three, and the remaining had SD. Cmax (Mean±SD) following BAI for gemcitabine, dFdCTP and dFdU were 7.71±0.13, 66.5±40.6 and 38±6.27 μM and following IV infusion, 17±2.36, 50.8±3.61 and 83.2±12.3 μM, respectively. The AUCinf (Mean±SD) following BAI for gemcitabine, dFdCTP, and dFdU were 6.89±1.2, 791.1±551.2 and 829.9±217.8 μM*hr and following IV infusion, 12.5±3.13, 584±86.6 and 1394.64±682.2 μM*hr, respectively. The AUC and Cmax of dFdCTP after BAI were higher than IV. The median OS was 6.27 months. No grade 3 or 4 toxicity was observed. The most common side effects were all grade ≤ 2 involving nausea, vomiting, rigor, thrombocytopenia, and anemia.
Conclusions:
Systemic exposure to dFdCTP was higher after BAI than IV in two out of three patients.
Keywords: BAI, IV infusion, Gemcitabine, NSCLC, PK
1. Introduction
Systemic chemotherapy has made notable advances in the treatment of NSCLC, particularly in patients with recurrent or advanced NSCLC who are not suitable candidates for surgery or radiotherapy. Systemic chemotherapy leads to enhanced quality of life, palliated symptoms, and prolonged overall survival (OS). In contrast, these therapies are associated with severe side effects. Chemotherapy administered locally near the tumor site may be advantageous because it is possible to deliver higher concentrations of cytotoxic agents to the tumor relative to systemic circulation; such chemotherapy would be expected to result in a greater antitumor response than when given intravenously. Local administration can be combined with more traditional routes, such as intravenous, to achieve high concentrations systemically which can improve possible undetected micro metastatic disease elsewhere.
Gemcitabine, an antimetabolite prodrug analog of nucleoside deoxycytidine, is a cell cycle-specific chemotherapeutic agent. It interferes with DNA replication and repair [1]. Gemcitabine is phosphorylated intracellularly to the active triphosphate (dFdCTP) nucleoside and leads to the termination of DNA replication and apoptosis. Gemcitabine is also metabolized systemically and hepatically by cytidine deaminase to form the much less potent 2′,2′difluorodeoxyuridine (dFdU) [1].Gemcitabine is used to treat NSCLC, and its use is associated with myelosuppression when administered intravenously. To improve delivery to centrally located lung tumors, bronchial artery infusion (BAI) can be used to deliver chemotherapy closer to the site of the tumor when it is mainly supplied by the bronchial and subclavian arteries. This method is beneficial in delivering high concentrations into tumor tissues locally, thereby maximizing the antineoplastic effect, reducing the tumor size, and providing a therapeutic modality for patients with advanced NSCLC. Zhu et al. (2017) concluded that, compared to neoadjuvant chemotherapy, BAI chemotherapy significantly enhances surgical debulking, prolongs progression-free survival, and improves OS rates. In addition, BAI chemotherapy improves the quality of life for patients with unresectable stage III squamous cell carcinoma (SCC) of the lung [2]. However, there is a paucity of published clinical data on the safety, pharmacokinetics, and efficacy of gemcitabine administered through the BAI and intravenous infusion in NSCLC patients. It is especially important to characterize systemic exposure to its active intracellular metabolite, dFdCTP. To address this, a phase 1 clinical trial was conducted on patients with advanced NSCLC refractory who had had at least two prior regimens of cytotoxic chemotherapy.
2. Materials and methods
2.1. Patients and study design
This was a phase I dose-escalation study of gemcitabine administration by both IV and BAI. The objectives were to assess tolerability and estimate pharmacokinetic parameters of gemcitabine, dFdU, and dFdCTP. Patient accrual was stopped after four patients because of futility. Patients with cytologically or histologically proven stage IIIB and IV NSCLC following at least two lines of therapy were recruited in the phase 1 clinical trial () at the University of Minnesota hospital. The inclusion criteria were as follows: patients who had an Eastern Cooperative Oncology Group performance status between zero and 1; age ≥ 18 years; estimated life expectancy ≥ 3 months; hemoglobin ≥ 9.0 gm%; absolute neutrophil count ≥ 1,500; platelet count ≥ 100,000/mm; INR ≤ 1.3, creatinine ≤ 3.0 mg/dL, and total bilirubin < 1.5 times the upper limit of the institutional normal. This study was approved by the University of Minnesota’s institutional review board.
2.2. Treatment plan
Gemcitabine, 600 mg/m2, was administered by BAI on day one of cycle one followed by intravenous infusion as 1,000 mg/m2 on day eight of cycle one, and days one and eight of all subsequent three-week cycles. Optional BAI of gemcitabine on day one of each odd-numbered cycle was offered.
2.3. Pharmacokinetic study
The pharmacokinetics for gemcitabine and dFdU in plasma, and dFdCTP in peripheral blood mononuclear cells were investigated following BAI and intravenous infusion during the first cycle. Pharmacokinetic samples were collected prior to infusion, and 0.5, 0.75, 1, 1.25, 1.5, 2, 24, 48, and 72 hr after end of infusion. After sample processing, the three analytes were measured with validated assays using LC-MS/MS as described in our previous publication at the clinical pharmacology shared resource of the Masonic Cancer Center [3]. Non-compartmental PK analysis was performed using R and PKNCA package for Windows (version 3.4.1) to estimate pharmacokinetic parameters of gemcitabine, dFdU, and dFdCTP [4].
2.4. Response and toxicity analyses
Disease response was evaluated using response evaluation criteria in solid tumors after cycles one and two and then every two cycles until disease progression. Toxicity and adverse events were evaluated and graded according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events version 3.0.
2.5. Statistical analysis
OS was estimated using the Kaplan–Meier method. Results are presented as basic descriptive statistics, including proportion, mean ± SD, median, and range. Data analysis was performed using R for Windows (version 3.4.1).
3. Results
3.1. Patient characteristics
Four patients were enrolled in this trial. One patient discontinued due to the inability to cannulate the bronchial artery. The characteristics of these patients are listed in Table 1.
Table 1:
Patient Characteristics (n=3)
| Median (Range) or Number | |
|---|---|
| Demographic factors | |
| Age (years) | 62(59–68) |
| Sex (Male) | 3 |
| Race (Caucasian) | 3 |
| Weight (kg) | 84.4(79–89.8) |
| Height (cm) | 176.5 (172.7–180.3) |
| BSA (m2) | 2.1(1.92–2.5) |
| Clinical factors | |
| Smoking history | |
| Former smoker | 3 |
| Histologic subtype | |
| Squamous | 1 |
| Not Otherwise Specified | 2 |
| Previous chemotherapy treatments | |
| Cisplatin | 3 |
| Carboplatin | 2 |
| Etoposide | 2 |
| Docetaxel | 2 |
| Previous surgery | 1 |
| Previous radiation therapy | 3 |
| INR | 0.99 (0.95–1.02) |
| Blood pressure (Mean±SD) | 153 / 93 (16.5/12.12) |
| GFR (mL/min) | 89(83–91) |
| Creatinine (mg/dL) | 0.9 (0.71– 0.98) |
| Time of infusion (hr) | |
| IV | 0.55 (0.53–0.72) |
| BAI | 0.75 (0.78–1.15) |
NOS (not otherwise specified)
3.2. Treatment received
A total of four gemcitabine infusions via BAI were administered, with one patient receiving an additional BAI on day one of cycle five. A median of four cycles (range: 4–6 cycles) was delivered to these patients.
3.3. Pharmacokinetics
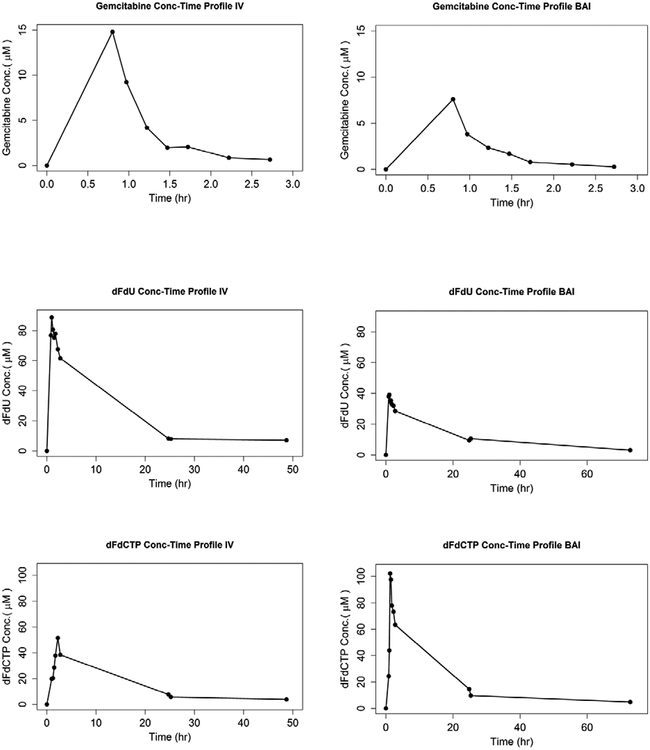
The pharmacokinetic parameter estimates are presented in figure 1.A. The CL (L/hr) and Vd (L) estimates of gemcitabine were slightly higher after BAI (744.5 ± 216.6 L/hr and 444.5 ± 236.8 L, respectively) than after intravenous infusion (702.6 ± 272.7 L/hr and 327.7 ± 190.7 L, respectively). Interestingly, the AUCinf and Cmax of dFdCTP in BAI were observed to be higher after BAI than after intravenous infusion even though the dose of BAI was 60% of the dose given intravenously. Representative concentration-time plots are shown in figure 1.B.
Figure 1.
Pharmacokinetics. (A) Pharmacokinetic parameters of gemcitabine and dFdU metabolite in plasma, and dFdCTP active metabolite in peripheral blood mononuclear cells (PBMC). Data are presented as Mean±SD. (B) Plasma concentration-time plots for subject 1.
3.4. Tolerability and overall safety
Toxicity and side effects of gemcitabine after intravenous and BAI infusions were mild, and no grade 3 or 4 toxicity was observed. The most common side effects were all grade ≤ two including nausea, vomiting, rigor, thrombocytopenia, lymphopenia, and anemia. BAI chemotherapy was well tolerated. No BAI-related adverse effects, such as bleeding and paraplegia, were reported.
3.5. Efficacy
All patients responded to treatment with a stable disease or better. One patient achieved a partial response after cycle three, and the other two, stable disease. The median OS was 6.27 months.
4. Discussion
The current study demonstrated that the delivery of BAI of gemcitabine is safe and well tolerated when followed by IV eight days later in patients with advanced NSCLC. This finding is consistent with other research in which BAI chemotherapy was well tolerated in patients with stage III lung SCC [2]. The mean peak plasma concentration of gemcitabine observed in this investigation was far lower than that found by Ciccolini et al. (17 μM vs 32 μM) and was within the range of reported data (from 16.7 to 81.7 μM) by Derissen and colleagues[5,6].The exposures of dFdU were approximately similar and consistent with the previously reported data [6]. Surprisingly, in two out of three patients, the systemic exposure of dFdCTP was found to be higher after low-dose BAI than intravenous infusion. A possible explanation for this is that prolonged infusion led to increased levels of dFdCTP. This finding was also reported by Tempero et al. (2003) [7]. The mean time of intravenous infusion was 36 minutes, versus 54 minutes for BAI. It can therefore be suggested that prolonged infusion might enhance the therapeutic effect and that delivering it directly to the tumor site could improve survival rates and reduce systemic side effects. Contrary to expectations, the infusion length of BAI was longer in subject 3, but the gemcitabine triphosphate exposure was lower than observed in the other patients. In contrast to the first two patients, this patient had a genetic variant (rs1130902) in the DCTD (deoxycytidylate deaminase) gene responsible for intracellular gemcitabine metabolic deamination after phosphorylation. It has been reported that this AG variant is associated with increased formation clearance of dFdCTP compared to the GG genotype in people with cancer[8,9].The presence of this and other genetic variants that may contribute to dFdCTP variability requires further studies to assess therapeutic relevance. The median peak plasma concentration of dFdCTP observed in this investigation was lower than that found by Derissen (51.6 versus 497 μM), and the t-max presented within the published range (from 0.5 to 2 h) [6].Moreover, the dose-normalized AUCinf and Cmax were similar after administration via IV or BAI, and no significant differences were identified (paired sample t-test, P > 0.05). Whilst this study did not intend to assess the efficacy, the overall survival data is affected by subsequent therapies for two patients. This is the first study to characterize the pharmacokinetic parameters of both intravenous gemcitabine infusion and BAI in patients with advanced NSCLC. The results of this study suggest that BAI might be a suitable delivery technique in NSCLC patients, particularly in those who are not suitable candidates for systemic chemotherapy or radiotherapy. However, BAI chemotherapy is expensive and requires a specific route of administration. Further investigation through a larger study is warranted.
Acknowledgements
We thank all the patients for their participation in this study. We would like to acknowledge the assistance of the Clinical Pharmacology Shared Resource of the Masonic Cancer Center, a comprehensive cancer center designated by the National Cancer Institute, supported in part by P30 CA77598.
Funding: This study was funded by Eli Lilly.
Footnotes
Conflict of interest: The authors have no conflict of interest to report.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Ethical approval: This article does not contain any studies with animals performed by any of the authors.
Informed consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.de Sousa Cavalcante L, Monteiro G. Gemcitabine: metabolism and molecular mechanisms of action, sensitivity and chemo resistance in pancreatic cancer. European Journal of Pharmacology. 2014;741:8–16. [DOI] [PubMed] [Google Scholar]
- 2.Zhu J, Zhang H, Jiang S, Ni J. Neoadjuvant chemotherapy by bronchial arterial infusion in patients with unresectable stage III squamous cell lung cancer. Therapeutic Advances in Respiratory Disease. 2017;11(8):301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khatri A, Williams B, Fisher J, Brundage R, Gurvich V, Lis L et al. SLC28A3 genotype and gemcitabine rate of infusion affect dFdCTP metabolite disposition in patients with solid tumours. British Journal of Cancer. 2013;110(2):304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.R Development Core Team. A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2018).
- 5.Ciccolini J, Serdjebi C, Peters G, Giovannetti E. Pharmacokinetics and pharmacogenetics of gemcitabine as a mainstay in adult and pediatric oncology: an EORTC-PAMM perspective. Cancer Chemotherapy and Pharmacology. 2016;78(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Derissen E, Huitema A, Rosing H, Schellens J, Beijnen J. Intracellular pharmacokinetics of gemcitabine, its deaminated metabolite 2′,2′-difluorodeoxyuridine and their nucleotides. British Journal of Clinical Pharmacology. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tempero M, Plunkett W, Ruiz van Haperen V, Hainsworth J, Hochster H, Lenzi R, et al. Randomized phase II comparison of dose-intense gemcitabine: Thirty-minute infusion and fixed dose rate infusion in patients with pancreatic adenocarcinoma. Journal of Clinical Oncology. 2003;21(18):3402–8. [DOI] [PubMed] [Google Scholar]
- 8.Alvarellos M, Lamba J, Sangkuhl K, Thorn C, Wang L, Klein D et al. PharmGKB summary. Pharmacogenetics and Genomics. 2014;24(11):564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mitra A, Kirstein M, Khatri A, Skubitz K, Dudek A, Greeno E et al. Pathway-based pharmacogenomics of gemcitabine pharmacokinetics in patients with solid tumors. Pharmacogenomics. 2012;13(9):1009–1021. [DOI] [PubMed] [Google Scholar]