Abstract
Background:
Incidence and survival rates of nonserous epithelial ovarian cancer in racial/ethnic minorities remain relatively unknown in the United States. We examined the trends in incidence and survival rates for epithelial ovarian cancer by histologic subtypes and race/ethnicity.
Methods:
Ovarian cancer incidence and mortality data from 2000 to 2013 were obtained from the Surveillance, Epidemiology, and End Results database. Age-adjusted incidence rate, incidence rate ratio, and annual percentage changes (APC) were calculated by histology and race/ethnicity subgroups and stratified by age at diagnosis. Five-year relative survival rates were calculated by stage and race/ethnicity.
Results:
A small but significant decrease in incidence rates was seen in non-Hispanic white (NHW), non-Hispanic black (NHB), and Hispanic women (APC −1.58, −0.84, and −1.31, respectively), while incidence rates remained relatively stable in Asian women (APC – 0.37). With exception of significant increase in the incidence rate of clear cell carcinoma among Asian woman (APC 1.85), an overall trend toward decreasing incidence rates was seen across histologic subtypes and age-strata, although not all results were statistically significant. Compared with NHW women, NHB women experienced poorer 5-year survival at every stage across histologic subtypes, while Hispanic and Asian women had equivalent or better survival.
Conclusions:
Over the last decade, incidence rates of epithelial ovarian cancer in the United States have decreased or remained stable across race/ethnic and histologic subgroups, except for clear cell carcinoma. Survival remains poorest among NHB women.
Impact:
Comparative histologic subtype distribution and incidence trends do not explain the ovarian cancer survival disparity disproportionately affecting NHB women
Introduction
With an estimated 22,000 new cases and 14,000 deaths in 2016, ovarian cancer accounts for 3% of all new cancer cases and 5% of all cancer-related deaths among women in the United States (1). The overall ovarian cancer incidence in the United States has steadily declined since the mid-1970s (2, 3). The declining incidence of ovarian cancer has been attributed to increased exposure to oral contraceptives (3, 4), whose protective effect has been well-established (5). Based on a survey of 2,000 North American women without ovarian cancer, Sopik and colleagues estimated that the proportion of 70-year-old women who ever used oral contraceptives increased from 20% to 85% between 1990 and 2015 (3). Rates of oral contraceptive use are lower among non-Hispanic black (NHB), Hispanic, and Asian women compared with non-Hispanic white (NHW) women (6, 7). The prevalence of other reproductive factors associated with ovarian cancer risk such as parity (8), breastfeeding (9), and tubal ligation (6) has also been reported to differ by race and ethnicity. Additionally, the association between ovarian cancer and known risk factors appears to differ by histologic subtypes (10–12).
Incidence of ovarian cancer varies by race and ethnicity with the highest rates reported among NHW women (13), while lower survival rates disproportionately affect NHB women compared with other race/ethnicity groups (14). The underlying etiology for these disparities is not well understood. The predominance of serous histology, in what is already a relatively rare disease, makes it challenging to study epidemiologic and survival patterns of nonserous histologic subtypes of ovarian cancer in racial and ethnic minorities. Consequently, reports on population-based incidence rates, temporal trends, and survival data specific to histologic subtypes and race/ethnic subgroups in the United States are sparse.
We utilized large U.S. population-based cancer registry data to examine the incidence and survival rates of epithelial ovarian cancer by histologic subtypes and race/ethnic groups. To reduce bias from changes in histologic subtype categorizations that occur over time, we examined epidemiologic trends using data only from the most recent decade.
Materials and Methods
Study population
Incidence and mortality data for ovarian cancer were obtained from the Surveillance, Epidemiology, and End Results (SEER) database (15). The SEER program contains data from 18 population-based registries, covering 28% of the U.S. population (16). We analyzed cases diagnosed between 2000 and 2013 to reflect the most current data and to include the Greater California registry, which has the largest Hispanic and Asian populations in the database (16). SEER collects data on patient demographics, tumor characteristics, first course of treatment, and actively follows cases for vital status. This study was exempt from institutional review board approval as all data are de-identified and coded for public use.
SEER data
Cases were identified using International Classification of Diseases for Oncology, Third Edition (17) codes. Tumor site and histology codes included in the analysis are as follows: primary site (C56.9, C57.0) classified as malignant tumors (behavior code,3); serous carcinoma (8050, 8120, 8122, 8130, 8140, 8201, 8260, 8440–8442, 8450, 8452, 8460–8463, 9014); clear cell carcinoma (8005, 8310, 8313, 8443, 8444); endometrioid carcinoma (8290, 8380–8383); carcinosarcoma (8575, 8950, 8951, 8980, 8981); mucinous carcinoma (8144, 8384, 8470–8472, 8480–8482, 9015); mixed, other, undifferentiated, unspecified carcinoma (other/NOS; 8000–8004, 8010, 8020–8022, 8030–8033, 8046, 8052, 8070–8072, 8074, 8084, 8230, 8255, 8261–8263, 8323, 8560, 8562, 8570, 8574, 8940, 9000). Nonepithelial histologic types such as germ cell tumors or sex cord-stromal tumors were excluded. A total of 3,024 cases diagnosed within 6 months of an endometrial cancer diagnosis were considered a synchronous diagnosis and were excluded.
Statistical analysis
Age-adjusted incidence rates (per 100,000) and corresponding 95% confidence intervals (CI) were calculated for NHW, NHB, Hispanic, and Asian women by histologic subtype. Because of insufficient sample size, American Indian/Native American women and women of unknown race were excluded from analysis. Rates were adjusted to the 2,000 U.S. Standard Population. Incidence rate ratio (iRR) and 95% CI by histologic subtype were calculated for NHB, Hispanic, and Asian women referent to NHW women. Annual percentage change (APC) of age-adjusted incidence rates between 2000 and 2013 was calculated by race/ethnicity, histologic subtype, and age groups (<50, 50–59, 60–69, and ≥70). To test if the APC was not equal to 1, a weighted least squares regression was used with a two-sided P value. All CI estimates for rates were calculated using the method described by Tiwari and colleagues (18). Five-year relative survival rates were calculated for cases diagnosed between 2000 and 2009 by race/ethnicity, histologic subtype, and SEER summary stage. Relative survival is the observed survival adjusted for the expected survival in the general U.S. population based on the age, race, sex, and year of diagnosis of the cases. Observed survival and expected survival were calculated using the actuarial and the Ederer-II methods (19), respectively, and a Z test was used to compare survival rates with NHW women. Statistically significant P values were considered <0.05, and analyses were performed using SEER-Stat software.
Results
A total of 76,241 cases of epithelial ovarian and fallopian tube cancer diagnosed between 2000 and 2013 were identified from SEER registries. NHW women (n = 57,366) accounted for the majority (75%) of cases, followed by Hispanic (10%), NHB (8%), and Asian (7%) women (Table 1). Among NHW and NHB women, the distribution of ovarian cancer was similar across the age groups, while Hispanic and Asian women were comparatively younger at the time of diagnosis than NHW or NHB women. Serous histology was most common regardless of race/ethnicity (61%). The histologic distribution was similar across race/ethnicity, but notably, clear cell tumor was markedly more prevalent among Asian women (12%) than among any other race/ethnicity where clear cell carcinoma accounted for less than 5% of epithelial ovarian cancer. Forty-five percent of all women had high-grade tumors, but tumor grade was unknown or missing in 34% of cases. Not surprisingly, 67% of women had distant metastatic disease at time of diagnosis. Similar distribution of SEER summary stage was observed across race/ethnicity, but the prevalence of localized disease was highest among Asian women.
Table 1.
Distribution of clinical features of epithelial ovarian and fallopian tube cancers, SEER, 2000–2013
| Characteristics | NHW N (%) | NHB N (%) | Hispanic N (%) | Asian N (%) |
|---|---|---|---|---|
| Total | 57,366 (75.2) | 5,814 (7.6) | 7,901 (10.4) | 5,160 (6.8) |
| Age at diagnosis | ||||
| <50 | 7,907 (13.8) | 1,083 (18.6) | 2,201 (27.9) | 1,422 (27.6) |
| 50–59 | 12,299 (21.4) | 1,293 (22.2) | 1,967 (24.9) | 1,424 (27.6) |
| 60–69 | 13,817 (24.1) | 1,448 (24.9) | 1,649 (20.9) | 1,021 (19.8) |
| 70–79 | 12,677 (22.1) | 1,143 (19.7) | 1,304 (16.5) | 801 (15.5) |
| 80+ | 10,666 (18.6) | 847 (14.6) | 780 (9.9) | 492 (9.5) |
| Histology | ||||
| Serous | 35,593 (62.0) | 3,563 (61.3) | 4,594 (58.1) | 2,601 (50.4) |
| Clear cell | 2,570 (4.5) | 141 (2.4) | 354 (4.5) | 602 (11.7) |
| Endometrioid | 4,718 (8.2) | 339 (5.8) | 761 (9.6) | 566 (11.0) |
| Carcinosarcoma | 1,715 (3.0) | 174 (3.0) | 203 (2.6) | 120 (2.3) |
| Mucinious | 2,899 (5.1) | 364 (6.3) | 580 (7.3) | 423 (8.2) |
| Other/NOSa | 9,871 (17.2) | 1,233 (21.2) | 1,409 (17.8) | 848 (16.4) |
| Grade | ||||
| Lowb | 10,076 (17.6) | 842 (14.5) | 1,525 (19.3) | 986 (19.1) |
| Highc | 26,141 (45.6) | 2,116 (36.4) | 3,308 (41.9) | 2,464 (47.8) |
| Unknown | 21,149 (36.9) | 2,856 (49.1) | 3,068 (38.8) | 1,710 (33.1) |
| SEER summary stage | ||||
| Local | 9,143 (15.9) | 724 (12.5) | 1,416 (17.9) | 1,239 (24.0) |
| Regional | 4,974 (8.7) | 492 (8.5) | 697 (8.8) | 515 (10.0) |
| Distant | 38,755 (67.6) | 3,994 (68.7) | 5,134 (65.0) | 3,090 (59.9) |
| Unstaged | 4,494 (7.8) | 604 (10.4) | 654 (8.3) | 316 (6.1) |
Abbreviation: NOS, not otherwise specified.
Include mixed, other, undifferentiated, unspecified carcinoma.
Well to moderately differentiated.
Poor to undifferentiated.
Table 2 shows histology-specific age-adjusted incidence rates and iRR by race/ethnicity referent to NHW women. The incidence rate of epithelial ovarian cancer was highest among NHW women (13.12; 95% CI, 13.01–13.01) followed by Hispanic (10.35; 95% CI, 10.12–10.59), NHB (9.30; 95% CI, 9.06–9.54), and Asian women (9.11; 95% CI, 8.86–9.36). The incidence rates were significantly higher in NHW women across nearly all histologic subtypes except for clear cell carcinoma. Asian women were1.65 times more likely to be diagnosed with clear cell carcinoma compared with NHW women (iRR 1.65; 95% CI, 1.50–1.80; P < 0.001).
Table 2.
Age-adjusted incidence rates of ovarian and fallopian tube cancer by histology subtype and race, SEER, 2000–2013
| Histology | Race | N | Ratea | Ratea 95% CI | Ratea ratio | Ratea ratio 95% CI | Ratea ratio P value |
|---|---|---|---|---|---|---|---|
| All histologies | NHW | 57,366 | 13.12 | 13.01–13.23 | 1.00 | Referent | |
| NHB | 5,814 | 9.30 | 9.06–9.54 | 0.71 | 0.69–0.73 | <0.0001 | |
| Hispanic | 7,901 | 10.35 | 10.12–10.59 | 0.79 | 0.77–0.81 | <0.0001 | |
| Asian | 5,160 | 9.11 | 8.86–9.36 | 0.69 | 0.67–0.71 | <0.0001 | |
| Serous | NHW | 35,593 | 8.11 | 8.03–8.20 | 1.00 | Referent | |
| NHB | 3,563 | 5.69 | 5.50–5.88 | 0.70 | 0.68–0.73 | <0.0001 | |
| Hispanic | 4,594 | 6.09 | 5.90–6.27 | 0.75 | 0.73–0.77 | <0.0001 | |
| Asian | 2,601 | 4.62 | 4.44–4.8O | 0.57 | 0.55–0.59 | <0.0001 | |
| Clear Cell | NHW | 2,570 | 0.62 | 0.59–0.64 | 1.00 | Referent | |
| NHB | 141 | 0.21 | 0.18–0.25 | 0.35 | 0.29–0.41 | <0.0001 | |
| Hispanic | 354 | 0.41 | 0.37–0.46 | 0.67 | 0.59–0.75 | <0.0001 | |
| Asian | 602 | 1.02 | 0.94–1.10 | 1.65 | 1.50–1.80 | <0.0001 | |
| Endometrioid | NHW | 4,718 | 1.15 | 1.11–1.18 | 1.00 | Referent | |
| NHB | 339 | 0.52 | 0.47–0.58 | 0.45 | 0.40–0.51 | <0.0001 | |
| Hispanic | 761 | 0.91 | 0.84–0.98 | 0.79 | 0.73–0.86 | <0.0001 | |
| Asian | 566 | 0.97 | 0.89–1.05 | 0.84 | 0.77–0.92 | 0.0001 | |
| Carcinosarcoma | NHW | 1,715 | 0.39 | 0.37–0.41 | 1.00 | Referent | |
| NHB | 174 | 0.28 | 0.24–0.33 | 0.73 | 0.62–0.86 | 0.0001 | |
| Hispanic | 203 | 0.29 | 0.25–0.33 | 0.74 | 0.63–0.86 | <0.0001 | |
| Asian | 120 | 0.21 | 0.18–0.25 | 0.55 | 0.45–0.66 | <0.0001 | |
| Mucinious | NHW | 2,899 | 0.73 | 0.70–0.75 | 1.00 | Referent | |
| NHB | 364 | 0.56 | 0.50–0.62 | 0.77 | 0.69–0.86 | <0.0001 | |
| Hispanic | 580 | 0.68 | 0.62–0.74 | 0.93 | 0.85–1.02 | 0.1445 | |
| Asian | 423 | 0.73 | 0.66–0.80 | 1.01 | 0.90–1.11 | 0.9383 | |
| Other/NOSb | NHW | 9,871 | 2.13 | 2.08–2.17 | 1.00 | Referent | |
| NHB | 1,233 | 2.03 | 1.92–2.15 | 0.96 | 0.90–1.01 | 0.1364 | |
| Hispanic | 1,409 | 1.99 | 1.88–2.10 | 0.93 | 0.88–0.99 | 0.0213 | |
| Asian | 848 | 1.56 | 1.46–1.67 | 0.74 | 0.68–0.79 | <0.0001 |
Abbreviation: NOS, not otherwise specified.
Rates are per 100,000 and age-adjusted to the 2000 U.S. standard population (19 age groups - Census P25–1130) standard; CIs (Tiwari mod) are 95% for rates and ratios.
Include mixed, other, undifferentiated, unspecified carcinoma.
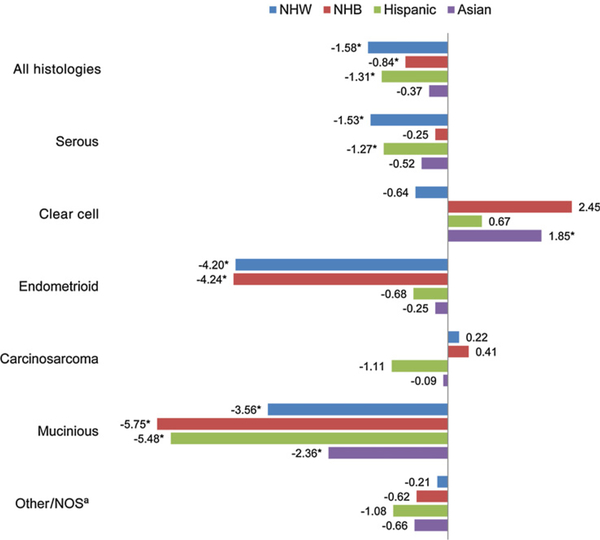
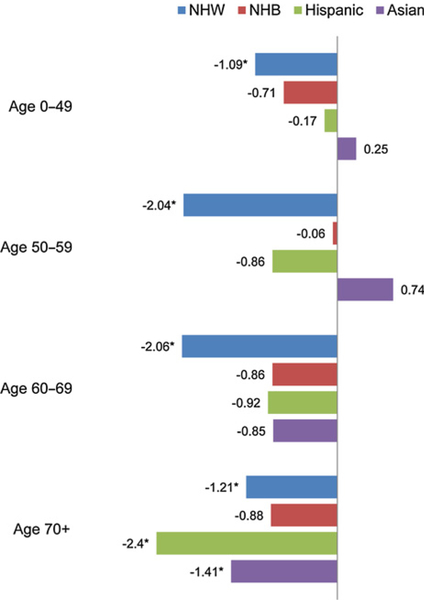
Figure 1 illustrates the APC in age-adjusted incidence rates from 2000 to 2013 by histologic subtypes and race/ethnicity sub-groups. The overall incidence rate has decreased across race/ethnicity although the result was not significant for Asian women. The largest decreases in incidence rates were seen among NHW (APC–1.58; 95% CI, −1.80 to −1.35) and Hispanic (APC–1.31; 95% CI, −2.18 to −0.43) women. Decreases in incidence rates were also seen across most histologic subtypes including serous, endometrioid, and mucinous carcinoma. The largest decreases were seen for endometrioid and mucinous carcinoma, especially among NHW and NHB women, as well as among Hispanic women for mucinous carcinoma. Notably, increases in incidence rates were seen for clear cell carcinoma, especially among Asian women (APC 1.85; 95% CI, 0.39 – 3.33). Incidence rates of carcinosarcoma fluctuated across the study period due to small sample size and limited observation of meaningful trends. Over-all, ovarian cancer incidence rates have decreased across all age groups, although most decreases were not statistically significant. Among women under 70 years old, the only significant decreases were seen among NHW women (Fig. 2). Among women 70 years or older, statistically significant decreases were seen among NHW, Hispanic, and Asian women. Largest decreases for age groups 0 to 49, 50 to 59, and 60 to 69 were seen among NHW women.
Figure 1.
APC of age-adjusted incidence rates of ovarian and fallopian tube cancer by histology subtype and race, SEER, 2000 to 2013. *, Statistically significant APC (P < 0.05). aInclude mixed, other, undifferentiated, unspecified carcinoma. Abbreviation: NOS, not otherwise specified.
Figure 2.
APC of age-adjusted incidence rates of ovarian and fallopian tube cancer by age-group and race, SEER, 2000 to 2013. *, Statistically significant APC (P < 0.05).
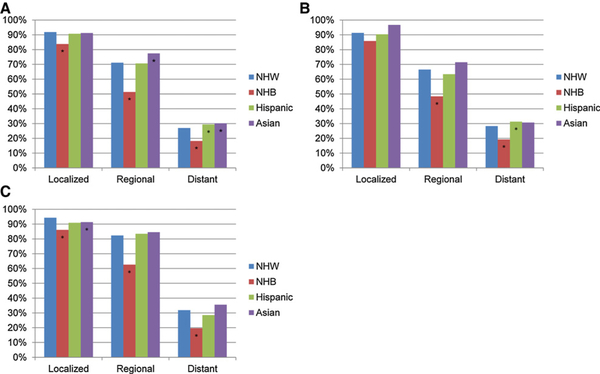
NHB women had poorer 5-year survival at every stage compared with NHW women (Fig. 3A). The relative survival difference between NHB and NHW women was greatest for distant disease (18% vs. 27%, P < 0.001). The relative poor survival among NHB women persisted across both serous and nonserous (clear cell, endometrioid, mucinous) carcinoma (Fig. 3B and C). Hispanic and Asian women had similar or improved survival compare with NHW women.
Figure 3.
Five-year survival by histology subtype, race, and stage for ovarian and fallopian tube cancer diagnosed from 2000 to 2009, SEER. A, All types. B, Serous. C, Clear cell, endometrioid, and mucinous. *, Statistically significant difference (P < 0.05) in relative survival compared with NHW women.
Discussion
Our analysis of over 75,000 cases of epithelial ovarian cancer diagnosed between 2000 to 2013 revealed that incidence rates have decreased over the last decade among NHW, NHB, and Hispanic women, while remaining relatively stable among Asian women. Previous SEER registry reports analyzing trends over the 3 decades preceding our study period have reported similar trends of decreasing incidence rates of epithelial ovarian cancer in the United States (2, 3). However, race/ethnicity-specific estimates, if reported, were limited to NHW and NHB women (13) due to relative small numbers of Hispanic and Asian women in the SEER database prior to 2000. Expansion of the SEER database to include the Greater California registry in 2000 significantly increased the total number of cancer cases, and the number of cases representing Hispanic and Asian groups. This presented an opportunity to examine the recent trends in histology-specific incidence rates among these other race and ethnic minority populations.
Overall changes in incidence rates of epithelial ovarian cancer were modest across race/ethnic groups; however, histology-specific incidence trends over time differed considerably. Among NHW women, we observed declines in incidence rates of both serous and nonserous carcinoma while incidence rates only declined for nonserous subtypes among NHB women. Among Hispanic women, sharpest decline was seen for mucinous carcinoma and to a lesser degree for serous carcinoma with no change in incidence of endometrioid carcinoma. Although the overall incidence rate did not change over the study period among Asian women, a significant decrease in incidence rate of mucinous carcinoma was observed with a concurrent increase in incidence rate of clear cell carcinoma.
Declining incidence of epithelial ovarian cancer over the past decades has been attributed to the protective effect of increased rates of oral contraceptives exposure between 1960 and 1990 (3, 4). However, etiology of racial/ethnic differences in incidence patterns of histologic subtypes are not well understood. The reported rate of oral contraceptive use among NHB is lower than among NHW counterparts (6, 7) while other protective factors such as parity, hysterectomy, and tubal ligation are reported at higher rates (7, 8, 20). These differences in incidence of reproductive risk factors between NHW and NHB women may partially explain the differences in histology-specific incidence trends. Oral contraceptive use has been reported to reduce the risk of all major epithelial ovarian cancer subtypes with exception of mucinous carcinoma (21). In contrast, tubal ligation and to a lesser degree hysterectomy appears to have the greatest impact on risk reduction of nonserous carcinoma (12, 22, 23).
Curiously, Hispanic women, who report similar patterns of reproductive risk factors as NHB women, did not mirror the incidence trends seen among NHB women in our study. In an analysis of 28,000 epithelial ovarian cancer cases from the California Cancer Registry, Morris and colleagues reported no change in age-adjusted incidence among Hispanic women between 1990 and 2003 while incidence declined significantly among NHB women (24). Decline in incidence rate observed in our study among Hispanic women in more recent periods may reflect alterations in reproductive patterns over time that more closely mirrors patterns seen among NHW women, especially among Hispanic women born in the United States or foreign-born women with longer duration of residence in the United States(7). The decreasing incidence rate of ovarian cancer may also be attributable to the large decrease in incidence rate of mucinous carcinoma. Improving accuracy of classifying metastatic mucinous carcinoma of nonmullerian origin (25) has led to a steady decline in incidence rate of ovarian mucinous carcinoma across all race/ethnicity groups.
In the report by Morris and colleagues, no change in the incidence rate of ovarian cancer was observed among Asian women (24). The incidence rate remained unchanged in our examination of the contiguous time period as the increase in incidence of clear cell carcinoma was countered by decrease in incidence of mucinous carcinoma. No other published report has examined the incidence trends of epithelial ovarian cancer in the United States among Asian women over the recent decade. In a recent report, Kim and colleagues examined the incidence of epithelial ovarian cancer according to histologic subtypes in Korea between 1999 and 2012 using national cancer registry data and found increased age-adjusted incidence rate of not only clear cell carcinoma (APC 8.13), but also serous (APC 4.34) and endometrioid carcinoma (APC 1.48; ref. 26). Despite this observed increase, the incidence rate estimates among Asian women in the current study were twice the reported estimates from Korea. Recent reports from Taiwan, China, and Singapore also found similar increases in incidence of epithelial ovarian cancer (27–29).
Decreasing parity, low rate of oral contraceptive use, and westernization of dietary and behavioral risk factors among women in Asia have been suggested as explanations for the increasing trend in epithelial ovarian cancer across multiple subtypes (26, 28). However, reasons for the disproportionate increase in the incidence of clear cell carcinoma in East Asia as well as among Asian women in the United States are poorly understood. Clear cell carcinoma is a rare subtype accounting for approximately 5% of epithelial ovarian cancer in the United States (13, 30). However, clear cell carcinoma makes up 10% to 20% of epithelial ovarian cancer in Asian countries with highest rates reported in Japan (26, 27, 29, 31).
Endometriosis is a well-established risk factor associated with nonserous epithelial ovarian cancer, in particular clear cell and endometrioid carcinoma (32). Available literature does not suggest higher prevalence of endometriosis among Asian women compared with other race/ethnicity (20). Additionally, lack of concurrent increase in endometrioid carcinoma among Asian women in our study argues against attributing the rise in clear cell carcinoma to endometriosis. However, higher prevalence of endometriosis at the time of surgery has been reported in Japanese women with ovarian clear cell carcinoma compared with reports from Europe and North America, suggesting a possible underlying genetic susceptibility for carcinogenesis mediated by endometriosis (31, 33). A recent pooled genetic analysis of over 46,000 ovarian cancer patients from 41 studies in Europe, North America, and Australia demonstrated alterations in 4 regions containing endometriosis-associated single-nucleotide polymorphisms that were linked to increased risk of clear cell carcinoma (34). Comparative analysis of the frequency and patterns of genetic alterations associated with endometriosis and clear cell carcinoma by race/ethnicity, in addition to fine mapping and functional analyses of shared regions could shed light on the shared etiologic pathways and genetic susceptibility for ovarian clear cell carcinoma among Asian women.
Ovarian cancer survival in the United States has improved across all stages over the past 4 decades, but NHB women continue to experience higher mortality compared with NHW women (14). In the current study, lowest survival rates were observed for NHB compared with other race/ethnic groups across all histology subtypes. This survival disparity persisted at every disease stage and was most pronounced for distant disease. NHB women in the United States have lower socioeconomic status, higher rate of comorbidities, and are less likely to receive guideline-recommended therapy from a high-volume surgeon specializing in gynecologic oncology for treatment of early or advanced stage ovarian cancer compared with their NHW counterparts as summarized by Collins and colleagues (35). Clinical trials and retrospective studies have observed similar outcomes between NHB and NHW women who have received similar treatments (36, 37). Low household income, having Medicaid or being uninsured, suboptimal care settings (nonspecialist or non–high-volume surgeon, non–high-volume center), older age, and greater comorbidities have been reported as predictors for non-guideline–recommended treatment (38–40). Further research on the role of physician bias and patient’s psychosocial factors (health belief, trust in physician and health care system, perceived barriers, etc.) associated with race/ethnicity in shared decision making and treatment tolerance or adherence may help improve targeted interventions to reduce the racial disparity in treatment of ovarian cancer.
Hispanic women have been reported to face similar socioeconomic, health, and treatment challenges as NHB women (41, 42), but had similar or better survival compared with NHW women, suggesting a possible role of racial differences in somatic mutations on survival. Whether the frequency and prognostic value of genetic and epigenetic alterations in ovarian cancer tumor types differ by race/ethnicity remains to be elucidated and may help predict racial differences in tumor biology, drug response, and novel drug targets. For example, The Cancer Genome Atlas (TCGA) analysis has revealed that approximately 50% of serous ovarian cancer carry mutations in various genes leading to defects in the homologous recombination repair, which may be exploited for targeted therapy such as poly(ADP-ribose) Polymerase inhibitors (43, 44). However, race/ethnic minority women are under-represented in the TCGA analysis and patterns of genetic alteration remain to be validated in non-white patients.
We present an analysis of histology-specific incidence trends and survival of epithelial ovarian cancer by major race and ethnicity groups from a large population-based dataset. However, this study has limitations to be considered when interpreting the findings. The SEER program does not perform a central pathologic review and, therefore, misclassification may influence the histology-specific incidence rates. Misclassifications may occur not only due to inter-observer variability, but also due to changes in histologic classifications and advances in molecular techniques allowing for more accurate diagnosis over time. In a SEER analysis, Mink and colleagues observed an increase in age-adjusted incidence rates of serous carcinoma between 1978 and 1998, while the overall incidence rate of epithelial ovarian cancer remained stable and the rate of unknown histology declined (13). This likely reflects increasing diagnostic accuracy evidenced by concurrent and proportional decline in the number of unknown cases. Limiting our analysis to the most recent decade reduces spurious observation of histology-specific incidence trends over time resulting from shifts in histologic classifications since 1970s, but our data may not be directly comparable with incidence rates or trends observed in previous decades. Our analysis also did not adjust for the age-adjusted prevalence of bilateral oophorectomy, which has steadily declined between 1975 and 2005 in an analysis of National Hospital Discharge Survey database by Sopik and colleagues (3). Therefore, our data underestimate the true incidence rate among at-risk women with intact ovaries, and the degree of this underestimation may vary over time. Additionally, despite the large sample size in our study, interpretation of incidence trends and survival of certain rare histologic subtypes such as carcinosarcoma was limited. Lastly, examination of potential causes of survival disparity is limited in this report as SEER does not collect information on many potential prognostic indicators such as detailed individual socioeconomic variables, comorbidities, and receipt of chemotherapy.
The incidence rates of epithelial ovarian cancer for major race and ethnic subgroups in the United States declined or remained stable over the past decade across histologic subtypes and age groups, with the exception of clear cell carcinoma. The incidence of clear cell carcinoma has continued to rise among Asian women from 2000 to 2013, mirroring trends reported from Asia, but the underlying etiology remains unclear. Continued investigations of incidence patterns by histologic subtypes will be valuable in understanding the racial influences in carcinogenesis and risk modification of epithelial ovarian cancer. Furthermore, our report highlights the survival disparity primarily affecting NHB women for every histology and at every stage. In addition to highlighting the existing white–black disparity in outcome, understanding the relative differences in genetic and clinical determinants between NHB and Hispanic women who often face similar socioeconomic and health challenges may shed light on opportunities to reduce the burden of poor cancer outcome among NHB women.
Grant Support
This work was supported, in part, by the Epidemiology Core and National Institute of Health Center Grant P30 CA022453 to the Karmanos Cancer Institute at Wayne State University.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, et al. SEER Cancer Statistics Review, 1975–2013, National Cancer Institute; Bethesda, MD, http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER website, April 2016. [Google Scholar]
- 3.Sopik V, Iqbal J, Rosen B, Narod SA. Why have ovarian cancer mortality rates declined? Part I. Incidence. Gynecol Oncol 2015;138:741–9. [DOI] [PubMed] [Google Scholar]
- 4.Gnagy S, Ming EE, Devesa SS, Hartge P, Wittemore AS. Declining ovarian cancer rates in U.S. women in relation to parity and oral contraceptive use. Epidemiology 2000;11:102–5. [DOI] [PubMed] [Google Scholar]
- 5.Beral V, Doll R, Hermon C, Peto R, Reeves G. Ovarian cancer and oral contraceptives: collaborative reanalysis of data from 45 epidemiological studies including 23,257 women with ovarian cancer and 87,303 controls. Lancet 2008;371;303–14. [DOI] [PubMed] [Google Scholar]
- 6.Mosher WD, Bachrach CA. Understanding U.S. fertility: continuity and change in the National Survey of Family Growth, 1988–1995. Fam Plann Perspect 1996;28:4–12. [PubMed] [Google Scholar]
- 7.Jones J, Mosher W, Daniels K. Current contraceptive use in the United States, 2006–2010, and changes in patterns of use since 1995. Natl Health Stat Report 2012;60:1–25. [PubMed] [Google Scholar]
- 8.Hamilton BE, Ventura SJ. Fertility and abortion rates in the United States, 1960–2002. Int J Androl 2006;29:34–45. [DOI] [PubMed] [Google Scholar]
- 9.Li R, Grummer-Strawn L. Racial and ethnic disparities in breastfeeding among United States infants: Third National Health and Nutritional Examination Survey, 1988–1994. Birth 2002;29:251–7. [DOI] [PubMed] [Google Scholar]
- 10.Kurian AW, Balise RR, McGuire V, Wittemore AS. Histologic types of epithelial ovarian cancer: have they different risk factors? Gynecol Oncol 2005;96:520–30. [DOI] [PubMed] [Google Scholar]
- 11.Modugno F, Ness RB, Wheeler JE. Reproductive risk factors for epithelial ovarian cancer according to histologic type and invasiveness. Ann Epidemiol 2001;11:568–74. [DOI] [PubMed] [Google Scholar]
- 12.Wentzensen N, Poole EM, Trabert B, White E, Arsian AA, Patel AV, et al. Ovarian cancer risk factors by histologic subtype: an analysis from the ovarian cancer cohort consortium. J Clin Oncol 2016;34;2888–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mink PJ, Sherman ME, Devesa SS. Incidence patterns of invasive and borderline ovarian tumors among White women and Black women in the United States: results from the SEER Program, 1978–1998. Cancer 2002; 95:2380–9. [DOI] [PubMed] [Google Scholar]
- 14.Terplan M, Schluterman N, McNamara EJ, Tracy JK, Temkin SM. Have racial disparities in ovarian cancer increased over time? An analysis of SEER data. Gynecol Oncol 2012;125:19–24. [DOI] [PubMed] [Google Scholar]
- 15.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 18 Regs Research Data+Hurricane Katrina Impacted Louisiana Cases, Nov 2015 Sub (2000–2013) <Katrina/Rita Population Adjustment>- Linked To County Attributes - Total U.S., 1969–2014 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016, based on the November 2015 submission.
- 16.National Cancer Institute. Surveillance Epidemiology and End Results Program. About the SEER program. Available from: https://seer.cancer.gov/about/[accessed November 8, 2016].
- 17.Fritz APC, Jack A, Shanmugaratnam K, Sobin L, Parkin DM, Whelan S. International classification of diseases for oncology. et al. Geneva: World Health Organization; 2000. [Google Scholar]
- 18.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res 2006;15:547–69. [DOI] [PubMed] [Google Scholar]
- 19.Cho H, Howlader N, Mariotto AB, Cronin KA. Estimating relative survival for cancer patients from the SEER Program using expected rates based on Ederer I versus Ederer II method. In: Surveillance Research Program NCI, editor. 2011. [Google Scholar]
- 20.Jacoby VL, Fujimoto VY, Guidice LC, Kuppermann M, Washington AE. Racial and ethnic disparities in benign gynecologic conditions and associated surgeries. Am J Obstet Gynecol 2010;202:514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Risch H, Marrett L, Jain M, Howe GR. Differences in risk factors for epithelial ovarian cancer by histologic type: results of a case control study. Am J Epidemiol 1996;144:363–72. [DOI] [PubMed] [Google Scholar]
- 22.Sieh W, Salvador S, McGuire V, Weber RP, Terry KL, Rossing MA, et al. Tubal ligation and risk of ovarian cancer subtypes: a pooled analysis of case-control studies. Int J Epidemiol 2013;41:579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice MS, Hankinson SE, Tworoger SS. Tubal ligation, hysterectomy, unilateral oophorectomy, and risk of ovarian cancer in the Nurses’ Health Studies. Fertil Steril 2014;102:192–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris CR, Rodriguez AO, Epstein J, Cress RD. Declining trends of epithelial ovarian cancer in California. Gynecol Oncol 2008;108: 207–13. [DOI] [PubMed] [Google Scholar]
- 25.Seidman JD, Kurman RJ, Ronnett BM. Primary and metastatic mucinous adenocarcinomas in the ovaries: incidence in routine practice with a new approach to improve intraoperative diagnosis. Am J Surg Pathol 2003; 27:985–93. [DOI] [PubMed] [Google Scholar]
- 26.Kim SI, Lim MC, Lim J, Won YJ, Seo SS, Kang S, et al. Incidence of epithelial ovarian cancer according to histologic subtypes in Korea, 1999 to 2012. J Gynecol Oncol 2016;27:e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiang YC, Cheng CA, Chiang CJ, Hsu TH, Lin MC, You SL, et al. Trends in incidence and survival outcome of epithelial ovarian cancer: 30-year national population-based registry in Taiwan. J Gynecol Oncol 2013;24: 342–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teng Z, Han R, Huang X, Zhou J, Yang J, Luo P, et al. Increase of incidence and mortality of ovarian cancer during 2003–2012 in Jiangsu Province, China Front Public Health 2016;4:e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tay SK, Cheong MA. Evidence for ethnic and environmental contributions to frequency of ovarian clear cell carcinoma. Aust N Z J Obstet Gynaecol 2014;54:225–30. [DOI] [PubMed] [Google Scholar]
- 30.Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancer. Gynecol Oncol 2008;109:370–6. [DOI] [PubMed] [Google Scholar]
- 31.Jimbo H, Yoshikawa H, Onda T, Yasugi T, Sakamoto A, Taketani Y. Prevalence of ovarian endometriosis in epithelial ovarian cancer. Int J Gynaecol Obstet 1997;59:245–50. [DOI] [PubMed] [Google Scholar]
- 32.Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol 2012;13:385–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim MC, Lee DO, Kang S, Seo SS, Lee BY, Park SY. Clinical manifestations in patients with ovarian clear cell carcinoma with or without co-existing endometriosis. Gynecol Endocrinol 2009;25:435–40. [DOI] [PubMed] [Google Scholar]
- 34.Lee AW, Templeman C, Stram DA, Beesley J, Tyrer J, Berchuck A, et al. Evidence of a genetic link between endometriosis and ovarian cancer. Fertil Steril 2016;105:35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Collins Y, Holcomb K, Chapman-Davis E, Khabele D, Farley JH. Gynecologic cancer disparities: a report from the Health Disparities Taskforce of the Society of Gynecologic Oncology. Gynecol Oncol 2014;133:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Farley JH, Tian C, Rose GS, Brown CL, Birrer M, Maxwell GL, et al. Race does not impact outcome for advanced ovarian cancer patients treated with cisplatin/paclitaxel: an analysis of Gynecologic Oncology Group trials. Cancer 2009;115:4210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Terplan M, Temkin S, Tergas A, Lengyel E. Does equal treatment yield equal outcomes? The impact of race on survival in epithelial ovarian cancer. Gynecol Oncol 2008;111:173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howell EA, Egorova N, Hayes M, Wisnivesky J, Franco R, Bickell N. Racial disparities in the treatment of advanced epithelial ovarian cancer. Obstet Gynecol 2013;122:1025–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bristow RE, Powell MA, Al-Hammadi N, Chen L, Miller JP, Roland PY, et al. J Natl Cancer Inst 2013;105:823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bristow RE, Chang J, Ziogas A, Campos B, Chavez LR, Anton-Culver H. Sociodemographic disparities in advanced ovarian cancer survival and adherence to treatment guidelines. Obstet Gynecol 2015;125:833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morales LS, Lara M, Kington RS, Valdez RO, Escarce JJ. Socioeconomic, cultural, and behavioral factors affecting Hispanic health outcomes. J Health Care Poor Underserved 2002;13:477–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goff BA, Matthews BJ, Larson EH, Andrilla CH, Wynn M, Lishner DM, et al. Predictors of comprehensive surgical treatment in patients in with ovarian cancer. Cancer 2007;109:2031–42. [DOI] [PubMed] [Google Scholar]
- 43.Cancer Genome Atlas Research N. Integrated genomic analyses of ovarian carcinoma. Nature 2011;474:609–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crafton SM, Bixel K, Hays JL. PARP inhibition and gynecologic malignancies: a review of literature and on-going trials. Gynecol Oncol 2016;142: 588–96. [DOI] [PubMed] [Google Scholar]