Abstract
Background:
Older patients with heart failure with preserved ejection fraction have severe exercise intolerance. Vitamin D may play a role in cardiovascular and skeletal muscle function, and may therefore be implicated in exercise intolerance in heart failure with preserved ejection fraction. However, there are few data on vitamin D status and its relationship to exercise capacity in heart failure with preserved ejection fraction patients.
Methods:
Plasma 25-hydroxyvitamin D (25[OH]D) and exercise capacity (peak oxygen consumption, [VO2], 6-minute walk distance) were measured in 112 older heart failure with preserved ejection fraction patients (mean ± SD age = 70 ± 8 years) and 37 healthy age-matched controls. General linear models were used to compare 25(OH)D between heart failure with preserved ejection fraction patients and healthy controls, and to determine the cross-sectional association between 25(OH)D and exercise capacity. The association between 25(OH)D and left ventricular function was evaluated secondarily in heart failure with preserved ejection fraction patients.
Results:
25(OH)D concentrations were significantly lower in heart failure with preserved ejection fraction vs healthy controls (11.4 ± 0.6 ng/mL vs 19.1 ± 2.1 ng/mL; P = .001, adjusted for age, race, sex, body mass index, season). More than 90% of heart failure with preserved ejection fraction patients had 25(OH) D insufficiency (<20 ng/mL) and 30% had frank 25(OH)D deficiency (<10 ng/mL). In heart failure with preserved ejection fraction patients, but not healthy controls, 25(OH)D was significantly correlated with peak VO2 (r = 0.26; P = 0.007) and 6-minute walk distance (r = 0.34; P < .001).
Conclusions:
More than 90% of heart failure with preserved ejection fraction patients had 25(OH)D insufficiency, and 30% were frankly deficient. Lower 25(OH)D was associated with lower peak VO2 and 6minute walk distance in heart failure with preserved ejection fraction, suggesting that 25(OH)D insufficiency could contribute to exercise intolerance in this patient population. These findings provide the data and rationale for a future randomized trial designed to test the potential for vitamin D supplementation to improve exercise intolerance in heart failure with preserved ejection fraction.
Keywords: Aging, Exercise capacity, Heart failure with preserved ejection fraction, Skeletal muscle, Vitamin D
INTRODUCTION
The predominant form of heart failure in older age is heart failure with preserved ejection fraction.1,2 Exercise intolerance, characterized by dyspnea and exertional fatigue, is the primary symptom of chronic heart failure with preserved ejection fraction.3 However, its pathophysiology is not well understood, and to date, few treatments have proven effective.1 Cardiac output and arteriovenous oxygen difference (AVO2-Diff), the 2 components of peak VO2 according to the Fick equation, are both reduced during peak exercise and contribute equally to the reduced peak VO2 in heart failure with preserved ejection fraction.4–8 The reduction in peak AVO2-Diff indicates that peripheral factors such as abnormal skeletal muscle function or perfusion play an important role in limiting exercise capacity in heart failure with preserved ejection fraction.8 This is supported by the results of randomized trials of exercise training in this population, which demonstrated that improvements in physical function following exercise training in heart failure with preserved ejection fraction patients were independent of changes in cardiac output.9–11
Several lines of evidence suggest a potential role for vitamin D insufficiency in exercise intolerance in older patients with heart failure.12,13 Vitamin D receptors are present in several cell types, including skeletal muscle, cardiac muscle, and vascular smooth muscle. Vitamin D insufficiency is also implicated in skeletal muscle weakness and is associated with arterial thickening, myocardial hypertrophy, and hypertension, all of which have particular relevance to heart failure with preserved ejection fraction.14–17 Vitamin D insufficiency is highly prevalent in chronic heart failure patients in general,18–21 but the prevalence of vitamin D insufficiency specifically in heart failure with preserved ejection fraction patients has not been previously reported, and there is little information regarding the relationship between vitamin D status and exercise intolerance in heart failure with preserved ejection fraction. Therefore, the purpose of this study was to determine the vitamin D status (plasma 25-hydroxyvitamin D [25(OH)D]) of patients with heart failure with preserved ejection fraction compared with healthy age-matched controls, and to examine the association between plasma 25(OH) D and exercise performance and left ventricular function.
METHODS
Participants
Heart failure with preserved ejection fraction patients (n = 116) were studied at baseline, prior to randomization into 1 of 2 randomized, placebo-controlled trials designed to determine the effect of enalapril or spironolactone on exercise capacity and quality of life. Patient selection and methods have been reported in detail, including the primary outcomes, which were negative.22,23 Heart failure with preserved ejection fraction patients were interviewed and examined by a board-certified cardiologist, and met the following inclusion criteria: signs and symptoms of heart failure defined by National Health and Nutrition Examination Survey score ≥3,24 the criteria by Rich et al,25 or both; left ventricular ejection fraction ≥50%; no segmental wall motion abnormalities; and no significant ischemic or valvular heart disease, pulmonary disease, anemia, or other disorder that could explain the patients’ symptoms.3,6,10 Thirty-seven healthy community-dwelling men (n = 17) and women (n = 20) from the same area (Forsyth County, NC) served as age-matched healthy controls. Healthy controls were recruited from the community and excluded if they had any chronic medical illness, were on any chronic medication, had current complaints or an abnormal physical examination (including blood pressure ≥140/90 mm Hg), had abnormal results on the screening tests (echocardiogram, electrocardiogram, cardiopulmonary exercise testing), or regularly undertook vigorous exercise.3,6 All participants provided written informed consent; all protocols were approved by the Wake Forest School of Medicine institutional review board.
Vitamin D Status
Plasma 25(OH)D was measured from stored plasma samples in 116 heart failure with preserved ejection fraction patients and the 37 healthy controls using the DiaSorin (Stillwater, MN) radioimmunoassay, following extraction with acetonitrile as recommended by the manufacturer. Blood samples from heart failure with preserved ejection fraction patients were obtained at the screening visit and blood samples from all participants were drawn prior to exercise testing, and immediately chilled and centrifuged. Plasma was separated and stored at −80˚C until time of analysis. Plasma 25(OH)D assays were completed without knowledge of patient/control status. The sensitivity of the assay is 1.5 ng/mL. The intra-assay and inter-assay coefficients of variation were 7.3% and 10%, respectively.
Exercise Performance
Cardiopulmonary exercise testing was performed using an electronically braked cycle ergometer in the upright position as previously described in detail.3,22,23 The initial output was set at 12.5W, increased to 25W for 3 minutes, and increased by 25W increments every 3 minutes thereafter.3,22 Expired gases were analyzed continuously during exercise using the MedGraphics CPX metabolic system (Medical Graphics Corp., St. Paul, Minn) and averaged >15-second intervals. Peak values were defined as the highest value obtained during the final 30 seconds of the test. Six-minute walk distance test was assessed according to Guyatt.26
Left Ventricular Structure and Function
Cardiac magnetic resonance imaging (MRI) scans were obtained on heart failure with preserved ejection fraction patients and healthy controls using a whole body imaging system (1.5T Siemens Avanto scanner; Siemens Medical Solutions USA, Malvern, Pa) with a phased-array cardiac surface coil on the chest, as previously described.22,23 Left ventricular mass, stroke volume, and ejection fraction were calculated using Simpson’s rule formula. Doppler echocardiography was performed in the supine position using a Sonos 5500 ultrasound system (Phillips Ultrasound, Andover, Mass) with a multiple frequency transducer, and analyzed as previously described.3,10,22,23
Neurohormones
B-type natriuretic peptide was measured from stored plasma using a commercially available radioimmunoassay (Phoenix Pharmaceuticals, Mountain View, Calif) as previously described.3,22,23,27
Statistical Analyses
The primary outcome was the relationships of season-adjusted plasma 25(OH)D with peak VO2 (mL/kg/min) and 6-minute walk distance; relationships with other measures of physical function and left ventricular function were secondary outcomes. Comparisons between heart failure with preserved ejection fraction patients and healthy controls were made by independent samples t tests for continuous measures and chi-squared tests for categorical measures. For this comparison, plasma 25(OH)D was natural log-transformed because the 25(OH)D distribution of healthy controls and heart failure with preserved ejection fraction patients combined was skewed. A natural log transformation was also applied to B-type natriuretic peptide. Univariate and multivariate general linear models were used to evaluate the association of 25(OH)D with exercise capacity in heart failure with preserved ejection fraction patients and healthy controls separately. Multivariate models were adjusted for age, sex, race, body mass index (BMI), and season of blood draw. In accord with precedent for reporting vitamin D data, the unadjusted and adjusted unstandardized beta coefficients and standard errors are presented, indicating the estimated change in outcome per ng/mL increment in 25(OH)D. Based on the results of this analysis, we subsequently analyzed the association between plasma 25(OH)D and ventricular function in heart failure with preserved ejection fraction patients using a similar approach. To examine if the association of 25(OH)D with exercise capacity and ventricular function was nonlinear, we fit a quadratic term (25[OH]D × 25[OH] D) into the multivariate regression models, but none were significant (all quadratic term P > .14). Of the 116 heart failure with preserved ejection fraction patients with plasma 25(OH)D measures, 4 were missing data on exercise performance, leaving 112 available for the analyses of exercise tolerance. Ninety-seven of these 112 heart failure with preserved ejection fraction patients had usable MRI measures, so were included in the analyses of left ventricular structure and function. All analyses were carried out using SAS v.9.3 (SAS Institute, Cary, NC), and outcomes were assessed at the 5% 2-sided level of significance.
RESULTS
Participant characteristics are shown in Table 1. HFpEF patients were consistent with those reported in population-based studies, including predominately older women with history of systolic hypertension and overweight/obesity.28
Table 1.
Baseline Characteristics
| Variable | HFpEF(n=112) | Healthy Controls(n=37) | P Value |
|---|---|---|---|
| Age (y) | 70 ± 8 | 69 ± 7 | .462 |
| Women, n (%) | 88 (81) | 24 (58) | .004 |
| White, n (%) | 85 (76) | 37 (100) | .001 |
| Weight (kg) | 83.0 ± 17.5 | 75.6 ± 14.7 | .022 |
| BMI (kg/m2) | 30.8 ± 5.3 | 26.0 ± 4.5 | <.001 |
| Resting SBP (mm Hg) | 143 ± 18 | 123 ± 13 | <.001 |
| Resting DBP (mm Hg) | 81 ± 9 | 73 ± 7 | .001 |
| Resting HR(beats per min) | 68 ± 11 | 66 ± 13 | .80 |
| 25(OH)D (ng/mL) | |||
| Unadjusted* | 11.8 ± 6.3 | 17.8 ± 11.0 | <.001 |
| Adjusted† | 11.4 ± 0.6 | 19.1 ± 2.1 | <.001 |
| Season of measurement, n (%) | |||
| Winter (Dec-Feb) | 29 (26) | 4 (11) | <.001 |
| Spring (Mar-May) | 39 (35) | 33 (89) | |
| Summer (Jun-Aug) | 22 (20) | 0 (0) | |
| Fall (Sept-Nov) | 21 (19) | 0 (0) | |
| Medications | |||
| Beta-blocker | 42 (38) | — | — |
| Ca channel blocker | 38 (34) | — | |
| Diuretic | 68 (61) | — | |
| NYHA Class | |||
| II | 85 (76) | — | — |
| III | 27 (24) | — | |
| Exercise capacity | |||
| Peak VO2 (mL/kg/min) | 14.2 ± 3.0 | 22.9 ± 6.7 | <.001 |
| Peak VO2 (mL/min) | 1296 ± 381 | 1716 ± 552 | <.001 |
| Exercise Time (min) | 9.0 ± 3.0 | 14.6 ± 5.0 | <.001 |
| Peak workload (W)* | 67 ± 25 | 114 ± 40 | <.001 |
| Peak RER | 1.11 ± 0.09 | 1.16 ± 0.09 | .005 |
| Peak SBP (mm Hg) | 191 ± 24 | 177 ± 22 | <.001 |
| Peak DBP (mm Hg) | 86 ± 11 | 77 ± 7.5 | <.001 |
| Peak HR (beats/min) | 127 ± 20 | 148 ± 16 | .005 |
| VAT (mL/min)* | 715 ± 178 | 917 ± 361 | <.001 |
| 6-min walk distance (m) | 433 ± 83 | 574 ± 71 | <.001 |
| Left ventricle measures | n = 97 | n = 35 | |
| Mass (g) | 127 ± 35 | 90 ± 5 | <.001 |
| End diastolic volume (mL) | 73 ± 19 | 114 ± 4 | <.001 |
| End systolic volume (mL) | 26 ± 8 | 47 ± 2 | <.001 |
| Ejection fraction (%) | 64 ± 8 | 59 ± 5 | .002 |
| E/A ratio* | 0.95 ± 0.29 | 0.94 ± 0.36 | .753 |
| BNP (pg/dL)* | 67.6 ± 61.4* | 33.9 ± 12.1 | <.001 |
Data represented as mean ± SD or count (%). P value represents between-group comparison based on Student’s t test (continuous measures) or chi-squared (categorical measures) unless indicated otherwise.
BMI = body mass index; BNP = brain natriuretic peptide; DBP = diastolic blood pressure; E/A = ratio of early to late diastolic filling; HFpEF = heart failure with preserved ejection fraction; HR = heart rate; IQR = interquartile range; NYHA = New York Heart Association; RER = respiratory exchange ratio; SBP = systolic blood pressure; VAT = ventilatory anaerobic threshold; VO2 = volume of oxygen consumption; 25(OH)D = 25-hydroxyvitamin D.
Between-group comparison analyzed using a general linear model of natural-log transformed outcome; means ± SDs presented in original scale.
Presented as least-square geometric mean ± SEM, adjusted for age, sex, race, BMI, and season; between-group comparison analyzed using a general linear model of natural-log transformed 25(OH)D. SEMs were estimated using the delta method: [(geometric mean) × (SEM of ln-transformed 25(OH)D)].
Vitamin D Status
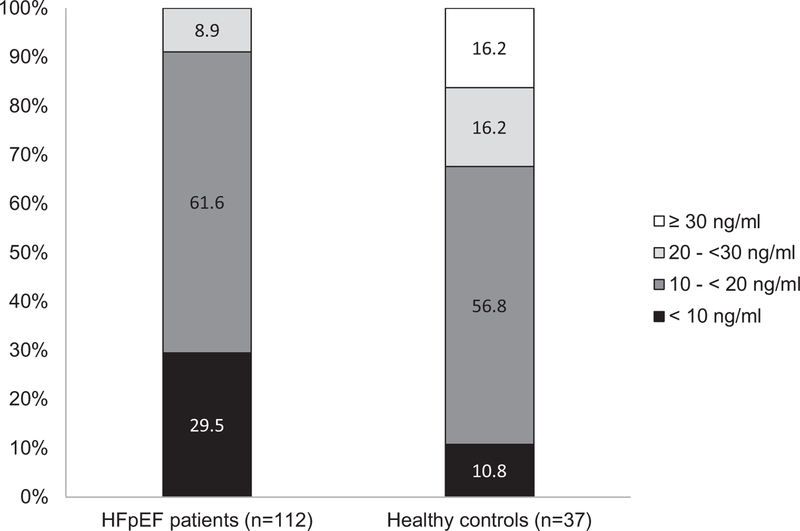
The distributions of vitamin D status in heart failure with preserved ejection fraction patients and healthy controls are shown in Figure 1. Among heart failure with preserved ejection fraction patients, 91% had insufficient plasma 25(OH)D (defined as <20 ng/mL29) vs only 68% of healthy controls. No heart failure with preserved ejection fraction patients had plasma 25(OH)D >30 ng/mL compared with 16% of healthy controls. Because no healthy controls were recruited in the summer or fall, we also evaluated the prevalence of vitamin D insufficiency in heart failure with preserved ejection fraction patients whose 25(OH)D was measured in the winter or spring (n = 68) and found 93% had insufficient plasma 25(OH)D (40% <10 ng/mL 25[OH]D and 53% 10–<20 ng/mL 25[OH]D). When (ln)25(OH)D was compared as a continuous measure and adjusted for age, sex, race, BMI, and season, heart failure with preserved ejection fraction patients had significantly lower 25(OH) D than healthy controls (adjusted geometric least-square mean ± SEM = 11.4 ± 0.6 ng/mL vs 19.1 ± 2.1 ng/mL, P < .001). We also conducted a sensitivity analysis comparing the (ln)25(OH)D concentrations between healthy controls and the heart failure with preserved ejection fraction patients only measured in the winter or spring. Heart failure with preserved ejection fraction patients had significantly lower plasma 25(OH)D compared with healthy controls in winter and spring (adjusted geometric least-square mean ± SEM 25(OH) D = 10.0 ± 0.7 ng/mL vs 16.3 ± 1.7 ng/mL; P < .001).
Figure 1.
Distribution of plasma 25(OH)D in heart failure with preserved ejection fraction patients (n =112) and healthy controls (n = 37). Grayscale bar graphs divided into 4 concentrations: <10 ng/mL, 10 to <20 ng/mL, 20 to <30 ng/mL, and ≥30 ng/mL. All heart failure with preserved ejection fraction patients had 25(OH)D concentrations <30 ng/mL, indicating insufficiency, and about 30% had 25(OH)D concentrations <10 ng/mL, indicating frank deficiency.
Vitamin D Status and Exercise Capacity
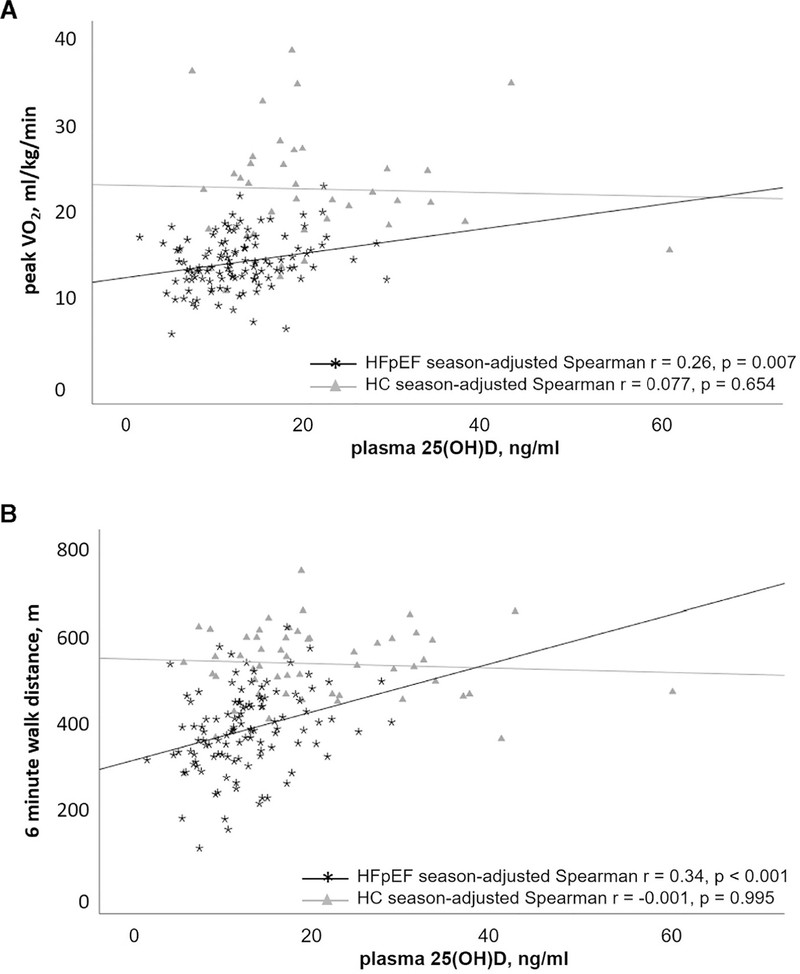
In heart failure with preserved ejection fraction patients, but not in healthy controls, plasma 25(OH)D was positively correlated with peak VO2 (mL/kg/min) and 6-minute walk distance (Figure 2). In heart failure with preserved ejection fraction patients, plasma 25(OH)D was positively associated with 6-minute walk distance, peak exercise workload, and exercise time, including after adjustment for age, sex, race, season, and BMI (Table 2). The results of our adjusted models of heart failure with preserved ejection fraction patients suggest that a 1-ng/mL increment in plasma 25(OH)D was associated with a 4.8-m increment in 6-minute walk distance and with a 0.11-mL/kg/min increment in peak VO2.
Figure 2.
Regression plots for plasma 25(OH)D concentrations vs peak VO2 (mL/kg/min) (A) and 6-minute walk distance (m) (B) for heart failure with preserved ejection fraction patients and healthy controls. Season-adjusted Spearman r and P values additionally included for reference.
Table 2.
Relationship between 25(OH)D and Measures of Exercise Capacity in HFpEF Patients and Healthy Controls
| Outcome | HFpEF Patients (n = 112) |
Healthy Controls(n=37) |
||||||
|---|---|---|---|---|---|---|---|---|
| Univariate* |
Multivariate† |
Univariate* |
Multivariate† |
|||||
| Beta (SE) | P Value | Beta (SE) | P Value | Beta (SE) | P Value | Beta (SE) | P Value | |
| 6-min walk distance (m) | 4.83 (1.49) | .002 | 4.01 (1.46) | .007 | −0.32 (1.11) | .777 | −0.19 (0.90) | .837 |
| Peak VO2 (mL/kg/min) | 0.11 (0.06) | .047 | 0.09 (0.04) | .029 | −0.02 (0.10) | .827 | −0.01 (0.06) | .851 |
| Peak workload(W) | 0.52(0.48) | .283 | 1.01(0.39) | .011 | −0.61 (0.61) | .330 | −0.36 (0.37) | .337 |
| Peak RER | 0.003(0.002) | .121 | 0.002 (0.002) | .280 | 0.002 (0.001) | .213 | 0.002 (0.001) | .189 |
| Exercise time (min) | 0.07 (0.06) | .243 | 0.13 (0.04) | .006 | −0.07 (0.07) | .390 | −0.04 (0.05) | .394 |
| Peak HR (beats/min) | 0.15 (0.37) | .688 | 0.26 (0.36) | .459 | 0.10 (0.25) | .690 | 0.06 (0.21) | .785 |
| Peak SBP (mm Hg) | 0.50 (0.44) | .263 | 0.52 (0.47) | .266 | 0.26 (0.34) | .450 | 0.48 (0.30) | .118 |
| Peak DBP (mm Hg) | 0.01 (0.20) | .961 | 0.24 (0.20) | .224 | −0.06 (0.12) | .586 | −0.10 (0.12) | .418 |
| (ln) VAT (mL/min) | −0.004 (0.005) | .344 | 0.003 (0.004) | .497 | −0.007 (0.005) | .170 | −0.005 (0.004) | .195 |
DBP = diastolic blood pressure; HR = heart rate; E/A = ratio of early to late diastolic filling; HFpEF = heart failure with preserved ejection fraction; RER = respiratory exchange ratio; SBP = systolic blood pressure; VAT = ventilatory anaerobic threshold; VO2 = volume of oxygen consumption; 25(OH)D = 25-hydroxyvitamin D.
Data analyzed using general linear regression and are presented as estimated change in outcome per ng/mL increment in 25(OH)D [Beta].
Adjusted for age, sex, race, body mass index, and season.
Vitamin D Status and Left Ventricular Structure/Function
Usable measures were noted in 97 of the 112 heart failure with preserved ejection fraction patients, and therefore these patients were included in the analysis of left ventricular structure and function. In these patients, plasma 25(OH) D was not significantly associated with any measure of ventricular function, including ejection fraction, following adjustment for age, sex, race, BMI, and season (Table 3).
Table 3.
Relationship between 25(OH)D and Measures of Ventricular Function in HFpEF Patients (n = 97)
| Outcome | Univariate* |
Multivariate† |
||
|---|---|---|---|---|
| Beta (SE) | P Value | Beta (SE) | P Value | |
| Left ventricular mass (g) | −0.84 (0.70) | .233 | 0.28 (0.59) | .632 |
| End diastolic volume (mL) | −0.32 (0.39) | .407 | −0.05 (0.41) | .904 |
| End systolic volume (mL) | −0.24 (0.17) | .151 | −0.22 (0.18) | .227 |
| Ejection fraction (%) | 0.18 (0.16) | .274 | 0.27 (0.18) | .134 |
| E/A ratio | 0.003 (0.008) | .656 | 0.005 (0.008) | .563 |
| (ln) BNP (pg/dL) | 0.003 (0.014) | .813 | 0.003 (0.015) | .843 |
BNP = brain natriuretic peptide; E/A = ratio of early to late diastolic filling; HFpEF = heart failure with preserved ejection fraction; 25(OH)D = 25-hydroxyvitamin D.
Data analyzed using general linear regression and are presented as estimated change in outcome per ng/mL increment in 25(OH)D [Beta].
Adjusted for age, sex, race, body mass index, and season.
DISCUSSION
We observed several important findings in this study. First, in this cohort of patients with chronic stable heart failure with preserved ejection fraction, >90% of patients had 25(OH)D concentrations <20 ng/mL, and no heart failure with preserved ejection fraction patients had 25(OH)D concentrations >30 ng/mL. Second, lower 25(OH)D concentrations were associated with reduced exercise capacity, measured as peak VO2 and the 6-minute walk distance, in heart failure with preserved ejection fraction patients but not in healthy controls. Finally, vitamin D status was not associated with measures of resting left ventricular function in our heart failure with preserved ejection fraction patients. Taken together, these findings suggest that low 25(OH)D concentrations may play a role in exercise intolerance among patients with heart failure with preserved ejection fraction through mechanisms that may be independent of cardiac structure and function, such as skeletal muscle function.
Multiple lines of evidence suggest that vitamin D significantly influences skeletal muscle strength and function, including the distribution pattern of vitamin D receptors in skeletal muscle and the fact that vitamin D metabolites affect muscle metabolism through both genomic and nongenomic pathways to influence calcium uptake; phosphate transport across the cell membrane; phospholipid metabolism; initiation of myogenesis and muscle cell proliferation, differentiation and apoptosis; and mitochondrial function.16,17,30,31 Furthermore, the results of 2 meta-analyses of randomized trials that tested the effect of vitamin D supplementation on muscle strength and function suggested a beneficial effect in older adults with lower vitamin D status.16,17 Furthermore, several studies also have demonstrated significant positive associations between 25(OH)D concentrations and peak exercise VO2, an objective measure of cardiorespiratory fitness.32 Among patients with established heart failure with reduced ejection fraction, several observational studies have demonstrated a significant association between vitamin D deficiency and low exercise capacity. In a recent study of 200 patients with heart failure with reduced ejection fraction, patients with 25(OH)D concentrations <10 ng/mL had significantly lower peak VO2, with a significant positive correlation between the 2 (r = 0.16, P = .008).33 Similarly, Boxer et al34 reported a positive association between 25(OH)D and 6-minute walk distance and peak VO2 in heart failure with reduced ejection fraction patients. Consistent with these observations in heart failure with reduced ejection fraction, our study findings demonstrated a significant positive association between 25(OH)D concentrations and multiple measures of exercise performance in heart failure with preserved ejection fraction patients.
Several mechanisms may underlie the observed association between 25(OH)D concentrations and exercise capacity among heart failure with preserved ejection fraction patients. First, it is plausible that patients with heart failure with preserved ejection fraction that have higher 25(OH)D concentrations are more physically active and spend more of their leisure time engaging in outdoor activities. Second, the observed association between 25(OH)D concentrations and exercise capacity may be related to its direct favorable effect on peripheral skeletal muscle function. Vitamin D deficiency has been associated with fatty infiltration and fibrosis of muscle fibers, particularly type II muscle fibers.1 This may lead to muscle fiber atrophy, slow muscle contraction, and delayed relaxation, and contribute to suboptimal exercise performance.35 This is particularly relevant in heart failure with preserved ejection fraction where impaired peripheral skeletal muscle efficiency contributes significantly to exercise intolerance.4,5 Finally, higher 25 (OH)D concentrations may also be a surrogate marker of better nutritional status and less frailty, particularly among older patients with heart failure with preserved ejection fraction.
Although findings from our study and others suggest that vitamin D deficiency is associated with poor exercise tolerance, the efficacy of vitamin D supplementation in improving outcomes among patients with heart failure is not well established. In the recent EVITA (Effect of VItamin D on all-cause morTAlity in heart failure) trial, vitamin D supplementation among 400 patients with both heart failure with reduced ejection fraction and vitamin D deficiency was not associated with lower risk of mortality.36 However, there was a trend toward higher readmission rates and significantly higher rates of mechanical circulatory support in the vitamin D treatment arm. Prior small-scale randomized controlled trials have also failed to demonstrate a significant improvement in exercise capacity and physical function. Witham et al37 tested the effect of 100,000 IU vitamin D taken twice over 20 weeks (at baseline and 10 weeks) on physical function in a randomized controlled trial of vitamin D-deficient older adults with chronic heart failure with reduced ejection fraction but found no significant effect of vitamin D on any outcomes. Boxer et al34 also did not observe a significant improvement in exercise capacity with vitamin D supplementation in a randomized controlled trial of weekly vitamin D supplementation for 6 months among 64 patients with heart failure with reduced ejection fraction. More recently, in the VINDICATE (VitamIN D treating patIents with Chronic heArT failurE) trial, 1 year of vitamin D supplementation among heart failure with reduced ejection fraction patients with vitamin D deficiency was not associated with improved 6-minute walk distance.38 However, the authors did observe a significant improvement in cardiac function and reverse left ventricular remodeling with vitamin D supplementation.38
However, the effect of vitamin D supplementation on improving exercise capacity, left ventricular structure and function, and clinical outcomes has not been evaluated in patients with heart failure with preserved ejection fraction. Because the pathology and clinical characteristics of heart failure with preserved ejection fraction and heart failure with reduced ejection fraction differ, it is plausible that the therapeutic approaches for the 2 forms of heart failure may also differ.3 We and others have recently shown that exercise intolerance in heart failure with preserved ejection fraction is partially attributable to peripheral factors, such as arterial or skeletal muscle function, so treatments targeting muscle function may be more relevant to patients with heart failure with preserved ejection fraction than heart failure with reduced ejection fraction.4–8 In addition, heart failure with preserved ejection fraction is more prevalent in older age groups (≥70 years); in women; in hypertensives; and in sedentary, obese individuals, groups who are also more likely to have lower vitamin D status.39
We did not observe a significant association between 25(OH)D concentrations and measures of cardiac structure and function in the heart failure with preserved ejection fraction patients. This is consistent with findings from some studies among older community-dwelling adults free of CVD.15,40 In contrast, others have demonstrated that lower 25(OH)D concentrations are associated with higher prevalence of adverse left ventricular remodeling in older adults with and without CVD.18,41 These discrepant findings may be related to the differences in cohort characteristics as well as diverse effects of vitamin D on the cardiovascular system. Among randomized controlled trials evaluating the efficacy of vitamin D supplementation in patients with heart failure with reduced ejection fraction, some studies have demonstrated a significant improvement in cardiac structure and function with vitamin D supplementation38,42 while others have not.43
Our results indicate that heart failure with preserved ejection fraction patients represent a group with significant vitamin D insufficiency.29 The prevalence we found is in agreement with earlier studies of heart failure patients reporting that >90% had 25(OH)D <30 ng/mL.18,20 However, none of these studies differentiated between heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. About 60% of the healthy age-matched controls had 25(OH)D <20 ng/mL. This proportion is higher than typically seen in healthy older adults.44 However, this is likely owing to all healthy controls being assessed in the winter and spring, when circulating 25(OH) D concentrations are generally lower. We adjusted all analyses for season, so the associations we found are independent of any seasonal differences.
Limitations
The cross-sectional design does not allow us to prove a causal link between 25(OH)D and exercise capacity. We lack measures of some key factors that influence 25(OH)D concentrations, including sun exposure, dietary intake, or supplement use. Whatever the cause, heart failure with preserved ejection fraction patients appear to be at significantly higher risk for vitamin D insufficiency and deficiency, and this is associated with the exercise intolerance. Left ventricular function was measured only at rest; there may be relationships between vitamin D and exercise left ventricular function.
Impact
Because most medication trials to date aimed at improving clinical outcomes and exercise capacity in heart failure with preserved ejection fraction have been negative,1 there is a need for considering novel, nonpharmacological approaches. Indeed, recent studies have demonstrated that other dietary strategies, including caloric restriction weight loss, were effective in improving exercise capacity in heart failure with preserved ejection fraction and appeared to do so via noncardiac mechanisms.27 Another study suggested that a sodium-restricted diet improved diastolic function and ventricular-arterial coupling in heart failure with preserved ejection fraction.45 These recent encouraging successes further support the potential for a future trial to determine if vitamin D supplementation may be effective in improving physical function, quality of life, and clinical outcomes in this important patient population. The data in the present study provide valuable information for designing such a trial.
CONCLUSIONS
More than 90% of older heart failure with preserved ejection fraction patients have vitamin D insufficiency, and this may contribute to their severely reduced exercise tolerance and worse physical function. These data provide the rationale and preliminary data to support an adequately powered, randomized controlled trial to determine if vitamin D supplementation can improve exercise intolerance and other clinically meaningful outcomes in heart failure with preserved ejection fraction, particularly in those with vitamin D insufficiency.
CLINICAL SIGNIFICANCE.
More than 90% of patients with heart failure with preserved ejection fraction had vitamin D insufficiency, and 30% had frank deficiency.
In patients with heart failure with preserved ejection fraction, lower plasma 25-hydroxyvitamin D (25[OH]D) was associated with worse exercise intolerance, measured objectively as peak oxygen consumption and 6-minute walk distance. However, plasma 25 (OH)D was not associated with left ventricular structure or function.
Acknowledgments
Funding: Supported in part by the following grants from the National Institutes of Health: R01AG18917, R01AG045551, R01AG042411, P30-AG21331, UL1TR0014, R37AG18915, P30-AG21332, and R01AG042411. However, the funding sources were not involved in data collection, analysis, or interpretation or in manuscript preparation.
Footnotes
Conflict of Interest: None.
References
- 1.Upadhya B, Pisani B, Kitzman DW. Evolution of geriatric syndrome: pathophysiology and treatment of heart failure with preserved ejection fraction. J Am Geriatr Soc 2017;65:2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med 2006;355(3):251–259. [DOI] [PubMed] [Google Scholar]
- 3.Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288(17):2144–2150. [DOI] [PubMed] [Google Scholar]
- 4.Haykowsky MJ, Tomczak CR, Scott JM, Paterson DI, Kitzman DW. Determinants of exercise intolerance in patients with heart failure and reduced or preserved ejection fraction. J Appl Physiol (1985) 2015;119(6):739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pandey A, Khera R, Park B, et al. Relative impairments in hemodynamic exercise reserve parameters in heart failure with preserved ejection fraction: a study-level pooled analysis. JACC Heart Fail 2018;6 (2):117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol 2011;58(3):265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhakal BP, Malhotra R, Murphy RM, et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circ Heart Fail 2015;8:286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houstis N, Eisman A, Pappagianopoulos P, et al. Exercise intolerance in heart failure with preserved ejection fraction: diagnosing and ranking its causes using personalized O2 pathway analysis. Circulation. 2018;137:148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol 2012;60(2):120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail 2010;3(6):659–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey A, Parashar A, Kumbhani D, et al. Exercise training in patients with heart failure and preserved ejection fraction: meta-analysis of randomized control trials. Circ Heart Fail 2015;8(1):33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal M, Phan A, Willix R Jr, Barber M, Schwarz ER. Is vitamin D deficiency associated with heart failure? A review of current evidence. J Cardiovasc Pharmacol Ther 2011;16(3–4):354–363. [DOI] [PubMed] [Google Scholar]
- 13.Meems LM, van der Harst P, van Gilst WH, de Boer RA. Vitamin D biology in heart failure: molecular mechanisms and systematic review. Curr Drug Targets. 2011;12(1):29–41. [DOI] [PubMed] [Google Scholar]
- 14.Houston DK, Cesari M, Ferrucci L, et al. Association between vitamin D status and physical performance: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2007;62(4):440–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pilz S, Henry RM, Snijder MB, et al. Vitamin D deficiency and myocardial structure and function in older men and women: the Hoorn study. J Endocrinol Invest 2010;33(9):612–617. [DOI] [PubMed] [Google Scholar]
- 16.Stockton KA, Mengersen K, Paratz JD, Kandiah D, Bennell KL. Effect of vitamin D supplementation on muscle strength: a systematic review and meta-analysis. Osteoporos Int 2011;22(3):859–871. [DOI] [PubMed] [Google Scholar]
- 17.Beaudart C, Buckinx F, Rabenda V, et al. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J Clin Endocrinol Metab 2014;99(11):4336–4345. [DOI] [PubMed] [Google Scholar]
- 18.Ameri P, Ronco D, Casu M, et al. High prevalence of vitamin D deficiency and its association with left ventricular dilation: an echocardiography study in elderly patients with chronic heart failure. Nutr Metab Cardiovasc Dis 2010;20(9):633–640. [DOI] [PubMed] [Google Scholar]
- 19.Anderson JL, May HT, Horne BD, et al. Relation of vitamin D deficiency to cardiovascular risk factors, disease status, and incident events in a general healthcare population. Am J Cardiol 2010;106 (7):963–968. [DOI] [PubMed] [Google Scholar]
- 20.Gotsman I, Shauer A, Zwas DR, et al. Vitamin D deficiency is a predictor of reduced survival in patients with heart failure; vitamin D supplementation improves outcome. Eur J Heart Fail 2012;14(4): 357–366. [DOI] [PubMed] [Google Scholar]
- 21.Liu LC, Voors AA, van Veldhuisen DJ, et al. Vitamin D status and outcomes in heart failure patients. Eur J Heart Fail 2011;13(6): 619–625. [DOI] [PubMed] [Google Scholar]
- 22.Kitzman DW, Hundley WG, Brubaker PH, et al. A randomized double-blind trial of enalapril in older patients with heart failure and preserved ejection fraction: effects on exercise tolerance and arterial distensibility. Circ Heart Fail 2010;3(4):477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Upadhya B, Hundley W, Brubaker P, Morgan T, Stewart K, Kitzman D. Effect of Spironolactone on exercise tolerance and arterial function in older adults with heart failure with preserved ejection fraction. J Am Geriatr Soc 2017;65:2374–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol 1992;20:301–306. [DOI] [PubMed] [Google Scholar]
- 25.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney R. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med 1995;333:1190–1195. [DOI] [PubMed] [Google Scholar]
- 26.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132(8):919–923. [PMC free article] [PubMed] [Google Scholar]
- 27.Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2016;315(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Senni M, Tribouilloy CM, Rodeheffer RJ, et al. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98(21):2282–2289. [DOI] [PubMed] [Google Scholar]
- 29.Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: National Academies Press; 2010. [PubMed] [Google Scholar]
- 30.Dirks-Naylor AJ, Lennon-Edwards S. The effects of vitamin D on skeletal muscle function and cellular signaling. J Steroid Biochem Mol Biol 2011;125(3–5):159–168. [DOI] [PubMed] [Google Scholar]
- 31.Sinha A, Hollingsworth KG, Ball S, Cheetham T. Improving the vitamin D status of vitamin D deficient adults is associated with improved mitochondrial oxidative function in skeletal muscle. J Clin Endocrinol Metab 2013;98(3):E509–E513. [DOI] [PubMed] [Google Scholar]
- 32.Ardestani A, Parker B, Mathur S, et al. Relation of vitamin D level to maximal oxygen uptake in adults. Am J Cardiol 2011;107(8):1246–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saponaro F, Marcocci C, Zucchi R, et al. Hypovitaminosis D in patients with heart failure: effects on functional capacity and patients’ survival. Endocrine. 2017;58(3):574–581. [DOI] [PubMed] [Google Scholar]
- 34.Boxer RS, Kenny AM, Schmotzer BJ, Vest M, Fiutem JJ, Pina IL. A randomized controlled trial of high dose vitamin D3 in patients with heart failure. JACC Heart Fail 2013;1(1):84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ceglia L. Vitamin D and its role in skeletal muscle. Curr Opin Clin Nutr Metab Care. 2009;12(6):628–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zittermann A, Ernst JB, Prokop S, et al. Effect of vitamin D on allcause mortality in heart failure (EVITA): a 3-year randomized clinical trial with 4000 IU vitamin D daily. Eur Heart J. 2017;38(29):2279–2286. [DOI] [PubMed] [Google Scholar]
- 37.Witham MD, Crighton LJ, Gillespie ND, Struthers AD, McMurdo ME. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: a randomized controlled trial. Circ Heart Fail 2010;3(2):195–201. [DOI] [PubMed] [Google Scholar]
- 38.Witte KK, Byrom R, Gierula J, et al. Effects of vitamin D on cardiac function in patients with chronic HF: the VINDICATE Study. J Am Coll Cardiol 2016;67(22):2593–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pandey A, Omar W, Ayers C, et al. Sex and race differences in lifetime risk of heart failure with preserved ejection fraction and heart failure with reduced ejection fraction. Circulation. 2018;137(17): 1814–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Ballegooijen AJ, Visser M, Kestenbaum B, et al. Relation of vitamin D and parathyroid hormone to cardiac biomarkers and to left ventricular mass (from the Cardiovascular Health Study). Am J Cardiol 2013;111(3):418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ameri P, Canepa M, Milaneschi Y, et al. Relationship between vitamin D status and left ventricular geometry in a healthy population: results from the Baltimore Longitudinal Study of Aging. J Intern Med 2013;273(3):253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dalbeni A, Scaturro G, Degan M, Minuz P, Delva P. Effects of six months of vitamin D supplementation in patients with heart failure: a randomized double-blind controlled trial. Nutr Metab Cardiovasc Dis 2014;24(8):861–868. [DOI] [PubMed] [Google Scholar]
- 43.Jiang WL, Gu HB, Zhang YF, Xia QQ, Qi J, Chen JC. Vitamin D supplementation in the treatment of chronic heart failure: a meta-analysis of randomized controlled trials. Clin Cardiol 2016;39(1):56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shea MK, Houston DK, Tooze JA, et al. Correlates and prevalence of insufficient 25-hydroxyvitamin D status in black and white older adults: the health, aging and body composition study. J Am Geriatr Soc 2011;59(7):1165–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hummel S, Seymour E, Brook R, et al. Low-sodium DASH diet improves diastolic function and ventricular-arterial coupling in hypertensive heart failure with preserved ejection fraction. Circ Heart Fail 2013;6:1165–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]