Abstract
Antibody Panel Based N-glycan imaging is a novel platform for N-glycan analysis of immunocaptured proteins. N-glycosylation is a post-translational modification of pathophysiological importance and is often studied in the context of disease biomarkers. Determination of protein-specific N-glycosylation changes in patient samples has traditionally been laborious or limited to study of a single protein per analysis. This novel technique allows for the multiplexed analysis of N-glycoproteins from biofluids. Briefly, this platform consists of antibodies spotted in an array panel to a microscope slide, specific capture of glycoproteins from a biological sample, and then enzymatic release of N-glycans for analysis by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry (MS). N-glycans are detected at each individual spot, allowing N-glycan information to easily be linked back to its protein carrier. Using this protocol, multiplexed analysis of N-glycosylation on serum glycoproteins can be performed. Human serum is discussed here, but this method has potential to be applied to other biofluids and to any glycoprotein that can be captured by a validated antibody.
Basic Protocol 1: Antibody Panel Based N-glycan Imaging by MALDI MS
Keywords: Biomarker, N-glycan, Antibody Panel, Mass Spectrometry Imaging
INTRODUCTION:
Alterations in N-glycosylation have been associated with a number of diseases. Generally, these changes are observed through glycan analysis of complex protein mixtures or through the analysis of a few specific individual proteins. Additionally, in most cases, glycan analysis is done on released glycan pools without any connection to the protein carrier. When analysis is done with the protein carrier included, this generally requires large amounts of protein and is only feasible for a small number of proteins (Kailemia et al., 2017; Kuzmanov et al., 2013; Ruhaak et al., 2013; Novokmet et al., 2014).
Array based platforms allow for analysis of many targets simultaneously, with applications ranging from nucleic acids to proteins. The array panel presented here consists of immunocaptured glycoproteins and is conceptually similar to other N-glycan detection microarrays such as lectin binding (Chen et al., 2007; Reatini et al., 2016). Antibodies to target glycoproteins (specific for the protein component, not glycan) are spotted to a microscope slide as discrete spots and incubated with serum samples. Glycoproteins are specifically captured and localized to a unique position along the array panel. N-glycans are then enzymatically released by PNGaseF and MALDI mass spectrometry imaging is used instead of lectins to detect N-glycans localized to their position on the array. This MS detection method reports compositional N-glycan information rather than simply motifs, which is valuable for identifying specific N-glycan changes occurring on captured proteins. The data acquired from this technique is a profile of the N-glycans present on each glycoprotein target captured by the antibody panel. N-glycans can be easily identified based on their theoretical mass value and disease-related changes in protein glycosylation could lead to the discovery of clinically relevant biomarkers.
Antibody Panel Based (APB) N-glycan imaging was developed to address the need for more high-throughput methods of protein-specific N-glycan analysis from biological samples (Black et al., 2019). In this unit, we describe the preparation and application of slide-based antibody panels for N-glycoprotein analysis from human serum, Basic Protocol 1. An orthogonal method for validating antibodies is described in Support Protocol 1.
STRATEGIC PLANNING
Validation of Capture Antibodies
The choice of antibodies used for capture of glycoproteins is foundational to the success of this methodology. Nonspecific binding of glycoproteins will lead to confounding results, as the location of an N-glycan signal is linked to the capture spot. Prior to the start of this protocol, it is important that antibodies be validated in affinity and specificity for the target capture protein. This can be accomplished through a standard ELISA assay or by adding labelled proteins to the panel of spotted antibodies and visualizing specific capture, see Support Protocol 1. It is also important to use antibodies that only contain mass-spectrometry compatible detergents or stabilizers (not tween or glycerol) to prevent ion suppression and spectral contamination (Olaf Bornsen et al., 1997). Deglycosylated antibodies are beneficial to prevent background antibody signal, but not required.
Solvents
All solvents used should be of the grade specified in order to achieve optimal performance of the MALDI MS for N-glycan detection.
Method development on MALDI FT-ICR MS
In order to successfully detect and localize N-glycans to their protein carriers, the instrument method should be optimized on the MALDI FT-ICR mass spectrometer used for this technique. This can be performed by spotting N-glycan or glycoprotein standards to a microscope slide or target plate and optimizing signal obtained from these standards. Key parameters to optimize include laser power, number of laser shots, sweep power, and raster. Our optimization of parameters has been done exclusively on a 7.0 Tesla solariX Legacy MALDI FT-ICR MS (Bruker). Laser power used will vary with instrument age and usage, but is generally close to 16% (Drake et al., 2018a). The number of laser shots can be optimized on-slide by setting the laser to 25 shots and doing sequential shots at the same spot. When N-glycan signal diminishes, you have reached the limit for laser shots. A typical number of laser shots for N-glycan imaging is 200 (Black et al., 2019; Drake et al., 2018a). Sweep power ranges between 20–24% for N-glycan imaging on this instrument. In optimizing these parameters, it is important to monitor the ratio of m/z 1976 to m/z 1809 to ensure that excess sialic acids are not lost. Raster size is dependent on the available instrument time for acquisition as a smaller raster will increase the acquisition time, but lead to more pixels per capture spot. We have chosen a 250μm raster for an instrument runtime of 3–4 hours per slide and believe this leads to sufficient coverage of the individual capture spots (Black et al., 2019). More information on MALDI FT-ICR N-glycan methodology can be found in previous publications (Drake et al., 2018a; Powers et al., 2015, 2013; Drake et al., 2018b).
BASIC PROTOCOL 1
Antibody Panel Based N-glycan Imaging by MALDI MS
Introductory paragraph:
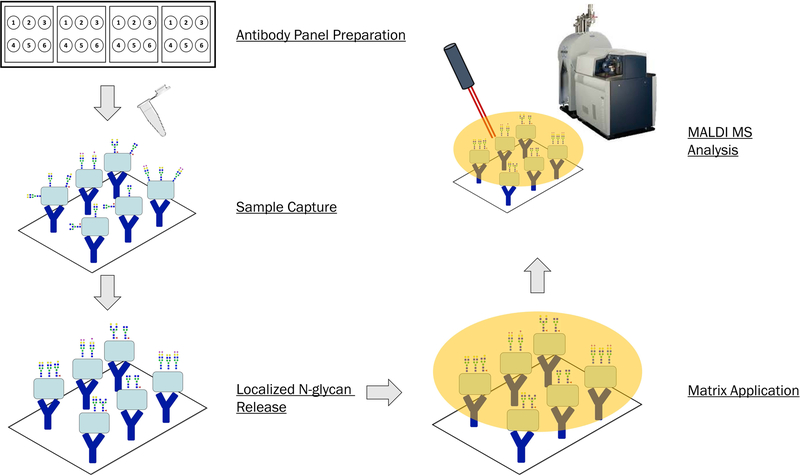
This protocol describes the creation of a slide-based antibody panel and analysis of N-glycans from immunocaptured proteins by MALDI Mass Spectrometry Imaging. The antibody panel is created manually and incubated with human serum samples. N-glycans are enzymatically released from captured glycoproteins and slide prepped for MALDI MSI analysis. This methodology can be extended to incorporate additional antibodies; discussed here is a general method for a 6-antibody panel. Results obtained from this protocol are N-glycan profiles of each of the 6 target proteins captured out of serum. Safety considerations such as PPE and proper biohazard disposal should be observed when handling human serum samples. A generic workflow is shown in Figure 1.
Figure 1.
Overall workflow for Antibody Panel Based N-glycan imaging. The major steps of this protocol consist of antibody panel preparation, sample capture, localized N-glycan release by PNGaseF, matrix application, and MALDI MS analysis.
Materials:
Phosphate buffered saline (PBS)
Detergent wash solution (see recipe in Reagents and Solutions)
Octyl-β-D-glucopyranoside (Sigma-Aldrich, cat. no. O8001)
BSA Blocking buffer (see recipe in Reagents and Solutions)
Bovine serum albumin (Fisher Scientific, cat. no. BP9706)
Double distilled water
Human serum
Silver Sharpie marker
PNGaseF solution (see recipe in Reagents and Solutions)
Peptide N-glycosidase F Prime (N-Zyme Scientifics)
HPLC grade water (Fisher Scientific, cat. no. W5–1)
HPLC grade methanol (Fisher Scientific, cat. no. A454–1)
Methanol solvent solution (see recipe in Reagents and Solutions)
CHCA matrix solution (see recipe in Reagents and Solutions)
Trifluoroacetic acid (>99% purity) (Fisher Scientific, cat. no. BP618)
α-cyano-4-hydroxycinnamic acid (Sigma-Aldrich, cat. no. 70990)
HPLC grade acetonitrile (Fisher Scientific, cat. no. A998SK)
PATH Protein Microarray Slides (Grace Biolabs, cat. no. 805025)
ProPlate 4 Well Slide Modules (Grace Biolabs, cat. no 248864)
Humidity chamber 1: 12×9×3.5cm western blot incubation box
Humidity chamber 2: 10-cm cell culture dish
Kim Wipes (Kimberly-Clark, cat. no. 34155)
Wypall X 60 paper towel (Kimberly-Clark, cat. no. 34790)
HTX M3 or M5 TM-Sprayer™ (HTX Imaging)
Ultra-pure nitrogen gas
Oven, operating at 37.5°C ± 1.5°C
Vortex
Benchtop plate shaker
Sonicator
Syringe pump, capable of 25μL/min within 0.05% accuracy
Isocratic pump, capable of 100μL/min within 0.05% accuracy
6mL plastic syringe
1mL plastic or glass syringe
Syringe filter 0.2μm PTFE (Millex, cat. no. SLLGH13NL)
Glass 5mL Luer lock syringe (Sigma-Aldrich, cat. no. 21965-U)
Document scanner
Desiccator attached to laboratory vacuum
SolariX FT-ICR MALDI MS (Bruker Scientific)
flexImaging software (Bruker Scientific)
SCiLS Lab software (Bruker Scientific)
Protocol steps:
Antibody Panel Preparation
-
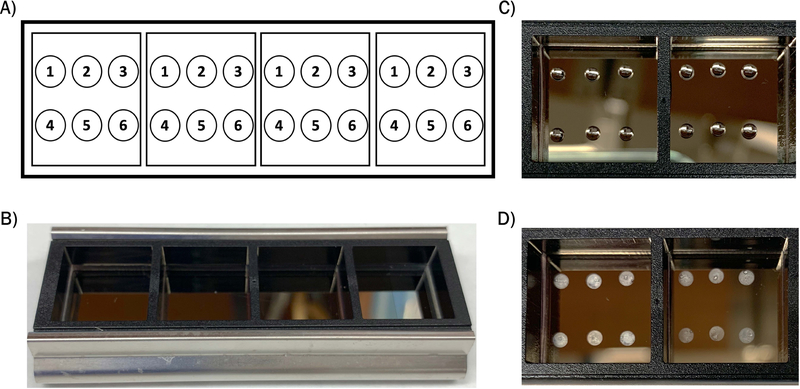
1Obtain a nitrocellulose coated slide and attach a well module to the slide using the stainless steel clips.Ensure that the nitrocellulose-coated side is facing up. Prior to attaching the module, mark the well locations on the slide using a lab quality permanent marker. An example template for 6 antibody panels within the well module is shown in Figure 2A, and the slide with module attached is shown in Figure 2B.
-
2Spot antibodies within each well at 133.3ng/μL in a volume of 1.5μL spots using a pipette.A manual or electronic pipette may be used to create spots, as shown in Figure 2C. Each well can fit up to 9 antibody spots in a 3×3 grid pattern, ensure that the spots do not touch the edges of the well or other spots.
-
3Prepare Humidity Chamber 1 using a plastic 1×9×3.5cm western blot incubation box. Place a single layer paper towel (Wypall x60) at the bottom of the dish. Fold and roll two 4 × 6 Kim Wipes and place at opposite ends of the box. Saturate Kim Wipes and paper towel with double distilled water, but do not allow water to pool inside dish.The Kim wipes inside the chamber should be thoroughly soaked with water, but not enough for water to pool inside the chamber. For specified towel and Kim wipes, this is about 3 mL of water.
-
4Place slide inside Humidity Chamber 1 and let incubate overnight at 4°C to allow the antibodies to adhere to slide.Slide should sit on the bottom of the box on the saturated Wypall towel. Up to 3 slides can fit in specified chamber at one time. This step is important to maintain spot morphology.
-
5Remove the slide from the humidity chamber and let air dry at room temperature.This generally takes 30 minutes to 1 hour. Slide may be placed inside a flow hood to speed drying. Antibody spots will become opaque when completely dry, shown in Figure 2C.
-
6Wash each well with 800μL of Detergent Wash Solution for 1 minute to remove any unbound proteins from the slide.It is recommended to use a multi-channel pipette for washing steps. Liquid is slowly pipetted on the edge of the wells. This wash is done as a static wash with no shaking or agitating.
-
7
Dump out washing solution and gently tap slide module against bench to remove residual liquid.
-
8Add 800μL BSA Blocking Buffer to each well and place in Humidity Chamber 1 on a benchtop shaker for 1 hour with gentle shaking at room temperature.This blocking step is important to prevent sample proteins from binding non-specifically to antibodies or to the surrounding slide.
-
9Wash each well with 800μL PBS for 3 minutes, repeating once for a total of two times.These wash steps are done as static washes with no shaking or agitating.
-
10Wash each well with 800μL double distilled water for 1 minute.This wash is done as a static wash with no shaking or agitating.
-
11
Let slide airdry.
Figure 2.
Antibody panel setup. A) Example template of 6-antibody panel set-up within well modules. B) Image of nitrocellulose coated slide with 4-well module attached. C) Image of antibody spots within wells. D) Image of dried antibody spots.
Sample Addition
-
12
Store serum samples at −80°C prior to use. Let thaw to room temperature and mix by pipetting up and down. Dilute samples in PBS to desired concentration.
Serum samples are collected following the Early Detection Research Network SOP for serum collection, which can be found at https://edrn.nci.nih.gov/resources/standard-operating-procedures/standard-operating-procedures/serum-sop.pdf. This SOP does not include preservatives in the serum samples. The concentration of serum samples added to the antibody panel will vary based on application and glycoprotein targets. We recommend trying a 1:100 or 1:1000 dilution in PBS.
-
13Add samples to each well in 700μL volumes and place in Humidity Chamber 1. Place on a benchtop shaker for 2 hours with gentle shaking at room temperature.Shaking speed should be such that the box is slowly moving, but not fast enough for any liquid to spill out of wells.
-
14Wash each well with 800μL Detergent Wash Solution for 1 minute, repeating once for a total of two times.These wash steps are done as static washes with no shaking or agitating.
-
15Wash each well with 800μL PBS for 3 minutes, repeating once for a total of two times.These wash steps are done as static washes with no shaking or agitating.
-
16Wash each well with 800μL double distilled water for 1 minute.This wash is done as a static wash with no shaking or agitating.
-
17
Gently remove the well module from the slide.
-
18Submerge the slide in 50mL double distilled water for 1 minute and then let airdry.This wash is done as a static wash with no shaking or agitating. This final water wash is important to remove any residual salt from the slide, as this would suppress the signal on the MALDI MS. Slide should be completely dry before proceeding to enzyme application steps.
-
19Mark the slide at each corner with fiducials, which are reference points drawn on the slide for imaging analysis.It is recommended to use a reflective metallic marker, such as a silver Sharpie, to draw circles and then a black marker to draw an X mark on top. The metallic marker provides a background for easier visualization of the black fiducial mark.
Enzyme Application by TM-Sprayer™
-
20
Use a syringe pump capable of pumping 25μL/min within 0.05% accuracy for delivering the PNGaseF enzyme to the TM-Sprayer™. A Luer lock glass or plastic 1mL syringe can be used with this pump.
-
21Fill the syringe with 0.5mL PNGaseF solution, removing any bubbles.If spraying multiple slides at once, prepare 1mL of PNGaseF solution. This 1mL volume can spray up to 4 slides on the TM-Sprayer™. If bubbles are present in the syringe, draw a small volume of air into the syringe and slowly dispense the air back out.
-
22
Place syringe into the syringe pump and check that syringe head is securely seated in the pump.
-
23
Place slide on the TM-Sprayer™ area and fasten with tape.
-
24
Turn on the TM-Sprayer™ and attached computer.
-
25
Open the nitrogen gas tank valve until the TM-Sprayer™ gas gauge reads 10psi.
-
26
In the TM-Sprayer™ software, set the temperature to 45°C. Nitrogen gas must be flowing for temperature to begin rising.
-
27Program the TM-Sprayer™ spray area to cover the entire slide with an additional 5mm overspray margin.5mm overspray margin is necessary to prevent delocalization during sprayhead turnaround.
-
28
Program the TM-Sprayer™ method to use 15 passes, crisscross pattern, 1200mm/min velocity, 3.0mm track spacing, and a dry time of zero. The nozzle height should be 40mm from slide surface.
-
29
Set the inner diameter of the syringe pump to match the used syringe and set the pump flow rate to 25μL/min. Start the syringe pump.
-
30Place a dummy slide underneath the nozzle of the spray head to monitor the start of enzyme solution spraying.This generally takes 1–3 minutes after starting the pump.
-
31
Once moisture is observed, start the TM-Sprayer™ method.
PNGaseF solution will be sprayed in a thin layer across the slide.
Incubation for Enzyme Digest
-
32Prepare Humidity Chamber 2 using a plastic 100 × 15mm cell culture dish. Place a single layer paper towel (Wypall x60) at the bottom of the dish. Fold and roll two 4 × 6 Kim Wipes and place at opposite ends of the dish. Saturate Kim Wipes and paper towel with double distilled water, but do not allow water to pool inside dish.For specified towel and Kim Wipes, this is about 2.5–3.0 mL of water.
-
33Preheat Humidity Chamber 2 in a 37°C oven for 15 minutes prior to starting slide incubation.A thin layer of condensation should be observed on the lid of the dish. No tape or sealant is necessary along the lid.
-
34
Incubate the slide immediately after PNGaseF application to slide.
-
35Place the slide in Humidity Chamber 2, with the sample side facing upward. Slide should rest on top of the 2 Kim Wipes, and the lid should not touch the surface of the slide.Only 1 slide can be placed inside Humidity Chamber 2. If working with multiple slides, multiple chambers must be prepared.
-
36
Incubate overnight at 37°C.
-
37
After incubation, remove slide gently and hold parallel. Wipe off moisture present on the underside of the slide. Slide is ready to be sprayed with MALDI matrix, but it may also be stored in a slide mailer at −20°C overnight.
It is recommended to immediately spray matrix on the slide. Storage of slides at −20°C has only been tested overnight, but longer durations may be possible.
MALDI Matrix Application by TM-Sprayer™
-
38Use an isocratic pump for delivering the matrix to the TM-Sprayer™. Use Methanol Solvent Solution as the push solvent for the pump. Set the pump to flow at 100μL/min.Solvent should be degassed to prevent flow variations.
-
39
Use a glass 5mL Luer lock syringe to load matrix into the sprayer.
-
40
Place slide on the TM-Sprayer™ area and fasten with tape.
-
41
Turn on the TM-Sprayer™ and attached computer.
-
42
Open the nitrogen gas tank valve until the TM-Sprayer™ gas gauge reads 10psi.
-
43
In the TM-Sprayer™ software, set the temperature to 79°C. Nitrogen gas must be flowing for temperature to begin rising.
-
44Program the TM-Sprayer™ spray area to cover the entire slide with an additional 5mm overspray margin.5mm overspray margin is necessary to prevent delocalization and excess matrix deposition during sprayhead turnaround.
-
45
Program the TM-Sprayer™ method to use 2 passes, crisscross pattern, 1300mm/min velocity, 2.5mm track spacing, and a dry time of zero. The nozzle height should be 40mm from slide surface.
-
46
Fill glass 5mL syringe with filtered CHCA Matrix Solution, ensure there are no bubbles in the syringe.
-
47Attach the syringe to the TM-Sprayer™ line going to the 6-port valve. Inject the CHCA Matrix Solution with the valve switch in the “Load” position.This injects the CHCA Matrix Solution into the instrument’s 5mL loop.
-
48
Ensure the pump is flowing at 100μL/min with no variations in pump pressure.
-
49
Turn the 6-port valve switch to “Spray.”
-
50
Place a dummy slide underneath the TM-Sprayer™ nozzle. Once opaque matrix solution is observed depositing on the dummy slide, press “Start” on the TM-Sprayer™ software.
-
51
CHCA Matrix Solution will be applied to the slide in a thin layer.
-
52After matrix application, scan the slide on a document scanner at a 1200ppi resolution. Image can be saved as a .jpg, .bmp. or .tiff format.This provides an optical image of the slide needing for selecting target regions in MALDI imaging analysis. Fiducials are used to “teach” the instrument where the antibodies are located on the slide. Scanning the slide after matrix application rather than before makes the slide easier to visualize due to the nitrocellulose reflective surface.
-
53Image slide by MALDI MS; this may be done immediately or slide stored in a desiccator until imaging. A discussion on MALDI MS instrument parameters for N-glycan imaging can be found in the Strategic Planning section as well as prior publications (Drake et al., 2018a; Powers et al., 2013, 2015; Drake et al., 2018b).It is recommended to image the slide within 4 weeks of preparation.
Data Analysis
-
54
Visualize N-glycan abundances using flexImaging software, which will allow for individual N-glycan masses to be selected as mass filters and observed as shown in Figure 3. Normalize the data to total ion count.
This creates a heat map image of the selected mass filter’s intensity across the slide, illustrating which captured proteins contain the particular N-glycan. We recommend setting the mass filter interval to 0.005%.
-
55
To quantify relative N-glycan abundances from captured proteins, import the acquired data into SCiLS Lab software. Normalize the data to total ion count.
-
56
Create regions for each individual capture spot by using the “create new polygonal region tool.” Draw region around each capture spot.
Care must be taken to draw the region around the entirety of each spot.
-
57
Create an N-glycan peak list from masses of interest and save as a m/z interval.
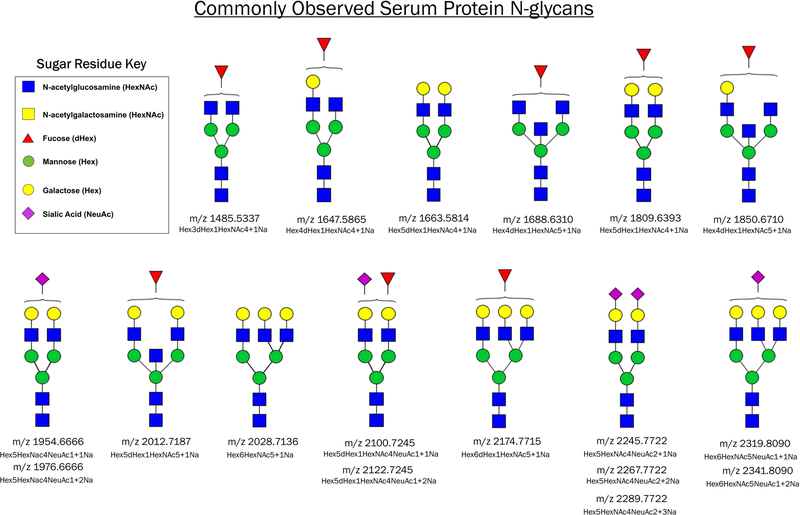
This peak list can contain a few target N-glycans or encompass a range of mass values such as shown in Figure 4. Additional N-glycan masses can be predicted using GlycoWorkbench (Ceroni et al., 2008).
-
58
Generate a Report Table using the “m/z report table” tab and select the created N-glycan m/z interval list.
-
59
Click the red plus sign and select “add mean intensities.” In this dialog box, ensure that data is still normalized to total ion count. Select regions drawn for the capture spots and export the column “area under curve.”
This generates a table of AUC values for each capture region and each mass interval in the N-glycan peak list.
-
60
Export numerical data to Microsoft Excel or other data processing software using the copy function. This allows for comparison of relative signal intensities across spots or samples.
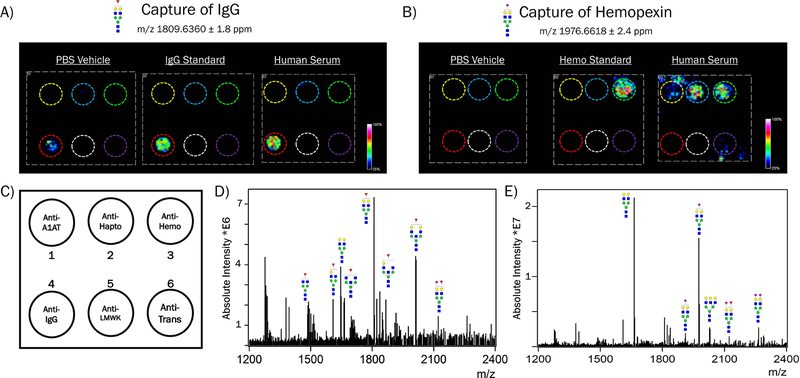
Figure 3.
Representative data obtained from Antibody Panel Based N-glycan imaging. The experiments shown here used a 6-panel setup containing antibodies for alpha-1-antitrypsin, haptoglobin, hemopexin, immunoglobulin G (IgG), low molecular weight kininogen, and transferrin. A) Data shown is from an IgG-associated N-glycan with proposed structure shown. PBS vehicle, IgG standard solution (500ng), or human serum (1:70) was added to the antibody panel. This N-glycan is observed localized to the anti-IgG spot (red circle). Some background signal from this N-glycan is observed on the antibody itself in the vehicle-treated well. B) Data shown is from a sialylated N-glycan found on hemopexin and other serum proteins. PBS vehicle, hemopexin standard solution (500ng), or human serum (1:70 in PBS) was added to the antibody panel. N-glycan signal is observed at the anti-hemopexin spot (green circle), as well as from other serum proteins. C) Template of the 6-antibody panel used in these experiments. D) Mass spectrum obtained from IgG showing the major N-glycan species observed. E) Mass spectrum obtained from hemopexin showing the major N-glycan species observed.
Figure 4.
Common N-glycans observed on serum glycoproteins. The proposed structures and theoretical mass values of N-glycans are shown.
SUPPORT PROTOCOL 1
Confirmation of Antibody Capture by IR-labelled Proteins
Introductory paragraph:
This support protocol is for initial testing to confirm the capture of target proteins by their antibodies. IR-labelled proteins can be visualized on an imager so that capture can be tracked to individual antibody spots. This may be performed using only one antibody or with a panel of antibodies to confirm the specificity of capture to the target antibody.
Materials:
IRDye 800 CW Protein Labeling Kit (Li-cor Biosciences, cat. no. 928–38040)
Target glycoprotein
Target antibody or antibodies
Phosphate buffered saline (PBS)
Detergent wash solution (see recipe in Reagents and Solutions)
Octyl-β-D-glucopyranoside (Sigma-Aldrich, cat. no. O8001)
BSA Blocking buffer (see recipe in Reagents and Solutions)
Bovine serum albumin (Fisher Scientific, cat. no. BP9706)
Double distilled water
PATH Protein Microarray Slides (Grace Biolabs, cat. no. 805025)
ProPlate 4 Well Slide Modules (Grace Biolabs, cat. no 248864)
Humidity chamber 1: 12×9×3.5cm western blot incubation box
Kim Wipes (Kimberly-Clark, cat. no. 34155)
Wypall X 60 paper towel (Kimberly-Clark, cat. no. 34790)
Benchtop plate shaker
Odyssey CLx Imager (Li-cor Biosciences)
Image Studio software (Li-cor Biosciences)
Protocol steps:
-
Obtain desired protein target and IR-label as specified in Li-Cor Labeling Kit.
We recommend labelling a minimum of 10μg of protein. This kit can also be used with 680LT NHS Ester dyes purchased from Li-Cor if another wavelength is desired.
-
Follow steps 1–11 of Basic Protocol 1 using the target antibody or antibodies.
These steps prepare the antibody panel for protein capture.
-
Add 700μL of IR-labelled target protein to well at 0.71ng/μL and place in Humidity Chamber 1. Follow steps 13–18 of Basic Protocol 1.
These steps will allow for capture of target protein.
-
Place the slide facedown on the Odyssey CLx Imager.
Ensure that the slide is dry before placing on Imager.
On the Imager software, set the scan area to that of the slide.
-
Select the 800 channel and set intensity to Auto.
These parameters can be changed if using a different dye. The intensity can also be adjusted manually if too much or too little signal is acquired.
-
Set Scan Controls to 169μm, low quality, and 0.0mm offset.
These are the default settings and may be changed if higher quality images are desired.
-
Ensure the instrument status is “connected,” and select Start.
Image will begin acquiring, this generally takes less than 3 minutes for one slide.
-
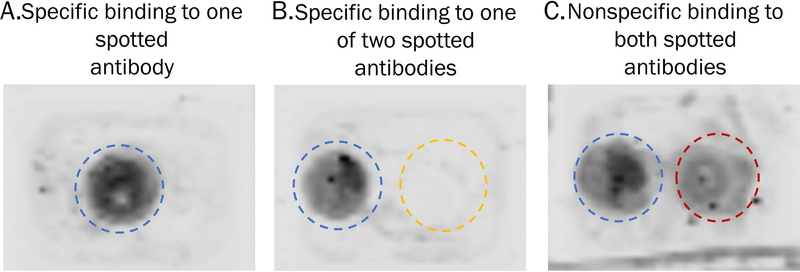
View the final image by clicking the Image tab. Adjust the visualized intensity of the dye using the Curves tab on the right of the screen. Slide the intensity dots to the left or the right to alter the signal intensity of the detected IR label. Labelled protein will appear as dark spots localized to the antibody capture spots, see Figure 5.
Nonspecific binding of the protein to the slide or other antibodies will be apparent if there is dark residue apart from the target antibody spot, this is observed in Figure 5C.
Figure 5.
Visualization of IR-labelled proteins captured to antibody spots. A) A single antibody was spotted and signal is localized to a circle, indicating capture of IR-labelled protein to its antibody spot. B) Two antibodies were spotted side-by side and signal is only present in one circle, indicating specific capture of the IR-labelled protein by its antibody. C) Two antibodies were spotted side-by-side and protein signal is observed on both spots, indicating non-specific capture of IR-labelled protein.
Sample Data
Results from Support Protocol 1 show localization of IR-labelled target protein across the slide. Antibodies suitable for this technique would result in one discrete circle of protein signal, as seen in Figure 5A–B. Nonspecific binding of the target protein to other antibodies would be visualized by signal present on more than one spot, as shown in Figure 5C. This protocol can be performed with just one antibody to confirm protein capture, or multiple antibodies to ensure specificity of capture. It is not recommended to subsequently take slides containing IR-labelled proteins through the rest of Basic Protocol 1 for MALDI MS analysis, as the IR dye interferes with the CHCA matrix.
REAGENTS AND SOLUTIONS:
Acetonitrile, 50%, trifluoroacetic acid (v/v), 0.1% (v/v), solution
Add 25mL HPLC grade water to a 100mL bottle. Add 0.1mL trifluoroacetic acid (>99% purity) to the water
Add 50mL HPLC grade acetonitrile and mix
Add 24.9mL HPLC grade water and mix
Store at room temperature for up to 2 months in a tightly sealed container
BSA Blocking buffer
Dissolve 0.05g bovine serum albumin (BSA) in 5mL of prepared Detergent wash solution (see recipe) and mix
Prepare fresh each day
Final concentration: 1% (w/v) bovine serum albumin in PBS/0.1% octyl-β-D-glucopyranoside
1 batch (5mL) allows for blocking of one slide
CHCA matrix solution
Dissolve 0.042g α-cyano-4-hydroxycinnamic acid (CHCA) in 6mL of prepared 50% acetonitrile/0.1% trifluoroacetic acid solution (see recipe)
Vortex vigorously and then sonicate for 10 minutes
Filter by passing through a 0.2μm syringe filter using a 6mL plastic syringe with Luer lock
Prepare fresh each day
Final concentration: 7mg/mL CHCA in 50% acetonitrile/0.1% TFA
Detergent wash solution
Add 0.1g octyl-β-D-glucopyranoside to 100mL of 1X PBS
Store up to 2 weeks at 4°C
Final concentration: 0.1% (w/v) octyl-β-D-glucopyranoside
Methanol solvent solution
50% (v/v) HPLC grade methanol prepared in HPLC grade water
Degas solvent to remove any bubbles
Solvent can be stored at room temperature
PNGaseF solution
50μg Peptide N-glycosidaseF Prime™ (frozen or lyophilized, N-Zyme Scientifics)
Add HPLC grade water to bring to final volume of 500μL
Prepare fresh each day
1 batch (500μL) allows spraying of 1–2 slides on the TM-Sprayer™.
COMMENTARY
BACKGROUND INFORMATION:
Previous work in our laboratory led to the development of a tissue-based N-glycan imaging protocol on both frozen and FFPE tissues (Powers et al., 2013, 2014; Drake et al., 2018a). This technique uses PNGaseF for localized release of N-glycans, which can be detected across tissue sections using a MALDI FT-ICR MS (Drake et al., 2017, 2018b; West et al., 2018). These tissue-based analyses are powerful methods of increasing our knowledge of N-glycosylation in disease states, yet this methodology does not report on target glycoproteins carrying the N-glycan biomarkers. Protein biomarkers and non-invasive approaches are continuously sought for early detection of disease.
Serum N-glycan analysis has grown in recent decades due to the clinical accessibility of this biofluid. The discovery of N-glycan biomarkers in serum could lead to clinical implementation for disease detection. The majority of serum N-glycan methods focus on the analysis of a pool of N-glycans released from all proteins in the serum (Ruhaak et al., 2008; Novokmet et al., 2014; Kirmiz et al., 2007; Ruhaak et al., 2018; Reiding et al., 2019; Gornik et al., 2007), or the analysis of one target protein’s N-glycan profile (Comunale et al., 2010; Pompach et al., 2016; Ruhaak et al., 2013; Šimurina et al., 2018; Zhang et al., 2016; Shubhakar et al., 2016; Ruhaak et al., 2018; Theodoratou et al., 2016; Simunovic et al., 2019). Pooled serum analyses have shown trends in overall N-glycan changes in the presence of cancer, such as increased fucosylation, branching, and bisects (Gebrehiwot et al., 2019; Snyder et al., 2016; Hecht et al., 2015; Vučković et al., 2016).
Array-based platforms for the multiplexed analysis of protein N-glycosylation have primarily been used by lectin-based techniques. An antibody microarray platform for glycans analysis of serum glycoproteins was developed that captured target glycoproteins from serum for N-glycan profiling (Chen et al., 2007). This technique used antibody microspots printed on nitrocellulose-coated microscopes slides that were then incubated with patient serum. Lectins were used to profile changing glycan motifs across more than 35 target glycoproteins. This sandwich N-glycan detection assay is a powerful tool for elucidating disease-related markers. However, it is confined to the biology of lectins, which bind to glycan motifs with varying affinities and do not report structural composition of an N-glycan.
To extend the capabilities of multiplexed glycoprotein analysis, we developed a novel antibody panel based platform to profile discrete N-glycans on proteins captured from biological fluids. This method was reported in 2019 using the proteins alpha-1-antitrypsin and immunoglobulin G (Black et al., 2019). A key advantage of this protocol is its overall simplicity. The immunocapture steps involved follow typical procedures and are not difficult to perform. Data analysis from this protocol is also relatively simple in nature, often focusing on a small list of N-glycans (see examples in Figure 4) and the protein information is obtained from the location along the array. N-glycan structures from detected m/z values can be predicted in free software GlycoWorkbench (Ceroni et al., 2008).
Disadvantages to this technique are the necessity of high quality antibodies as well as previously reported MS limitations including lack of structural information to distinguish isomers, sialic acid instability, and instrument run time (Drake et al., 2018a; Black et al., 2019; Powers et al., 2013). Additionally, the N-glycans present on capture antibodies can cause background signal, however strategies for deglycosylation of antibodies prior to sample capture are emerging.
CRITICAL PARAMETERS and TROUBLESHOOTING:
Troubleshooting is likely to occur in the creation of the antibody panel itself. The first parameter to notice is the selection of nitrocellulose coated slides. We recommend the use of microarray quality slides listed here in this protocol to improve antibody adherence and spot morphology. Standard microscope slides may be used with the MALDI FT-ICR instrument, but will likely lead to spot bleeding and loss of antibodies from the slide. Slides must be 75mm x 25mm x 1mm to fit in the instrument slide holder. Another important parameter to consider from the start is the individual antibody storage buffers. Antibodies that are stored in stabilizers such as glycerol will be slow to dry as a spot and lead to spectral interference and ion suppression. Therefore, it is important to select antibodies that are preserved without stabilizer. Minimal amounts of sodium azide in the buffer do not appear to present a problem. Choosing high quality antibodies for specific protein capture is essential to the success of this method. Antibody capture quality can be assessed using Support Protocol 1. Spot morphology can also be an area of troubleshooting in the initial steps. Allowing the antibodies to adhere to the slide overnight at 4°C is key for spot uniformity. If non-uniform spots or donut-like rings are observed, slowing the spot drying time may improve the morphology of the individual spots. Smearing of spots is an indication that the spots were not completely dry before proceeding to the initial wash step or that the nitrocellulose coating on the slide is not of high quality. Selection of detergent used for wash steps is also important, as the more commonly used detergent Tween will lead to ion suppression and spectral contamination. Collection of serum samples was performed following the Early Detection Research Network Standard Operating Procedures for serum collection with no preservatives or additives. Other methods of collection may lead to contamination or interference in this protocol, but this has not been tested.
After the capture of glycoproteins along the antibody panel, care must be taken to gently remove the well module. Slides may crack if the module is removed forcefully. Additionally, the nitrocellulose coating of the slide can peel off at the location of the module seals, so gentle removal prevents this peel from extending to the sample portion of the slide. The presence of PBS salt in the initial stages of this protocol (Antibody Panel Preparation and Sample Addition) may present problems for MALDI analysis due to ion suppression. The water wash steps are therefore critical to remove residual salt at each step of the antibody panel preparation and sample incubation, followed by the final immersion step in water after removing the well module. The presence of residual salt would be indicated by non-uniform matrix coating to the slide or across spots as well as low signal in the MS analysis. This could be remedied by additional or prolonged water washes.
Choice of PNGaseF enzyme is important to minimize additives present in the buffer. Detergents and stabilizers found in commercially available enzyme may significantly affect N-glycan signal obtained. We have success with PNGaseF Prime™; other enzymes may also be suitable, but we have not tested any others for this protocol. It is also noted that the incubation of PNGaseF enzyme takes place overnight in a heated humidity chamber. Therefore, the addition of too much water into Humidity Chamber 2 could lead to excessive condensation during enzyme incubation. Large droplets may form on the top of the slide, which would delocalize released N-glycans from their captured protein. This problem can be avoided by adding 3 mL or less of water to the cell culture dish. However, enough water should be present to produce a thin film of condensation at the top of the dish before placing the sample inside.
The MALDI matrix is essential for co-crystallization with N-glycan analytes. We recommend the use of CHCA matrix, but other matrices such as 2,5-dihydroxybenzoic acid can also be used for N-glycan analysis. When preparing the matrix solution, adequate sonication of CHCA matrix (10 minutes) is required for the matrix to saturate the solution. During CHCA matrix applications, variations in pump pressure associated with the TM-Sprayer™ may occur. A high pressure that remains steady likely indicates a clog in the sprayer line. This can be addressed by cleaning the line with an acid/base wash or temporarily increasing the pump pressure to push out a blockage. Routine maintenance and cleaning of the sprayer line after each use is essential to keep the instrument at optimal performance. If pump pressure fluctuates drastically, this likely indicates that there are bubbles in the line. Degassing the solvent prior to pushing through the pump can help prevent this problem. Additionally, the pump can be purged to remove bubbles that are present in the solvent line.
Poor detection of N-glycans from captured glycoproteins could be due to i) lack of protein capture ii) improper PNGaseF incubation conditions or iii) instrumental problems. Low glycoprotein capture is likely to be caused by choice of antibodies or concentration of sample added. Antibodies should be validated before using in this platform. We recommend selecting monoclonal antibodies. If capturing glycoproteins out of human serum, the concentration of sample may need to be increased for lower abundance target proteins. PNGaseF activity can be tested in-solution using glycoprotein standards such as ribonucleaseB or fetuin. Additionally, it is important to ensure the enzyme incubation occurs at proper temperature and with sufficient humidity. MALDI FT-ICR performance can be assessed by spotting N-glycan or glycoprotein standards. Low signal could be improved by increasing the laser power while monitoring signal to noise and ensuring the instrument is appropriately tuned for the array-based analyses. Discussion of MALDI FT-ICR parameters can be found in the Strategic Planning section as well as prior publications (Drake et al., 2018a, 2018b; Powers et al., 2013, 2015).
UNDERSTANDING RESULTS:
Anticipated results from this protocol include the detection of common serum protein N-glycans listed in Figure 4. N-glycans can be visualized in flexImaging as heatmaps of the glycan’s abundance across the slide, which should show signal localized to the antibody capture spots. Representative images of N-glycans released from captured protein spots are shown in Figure 3A–B. The experiments shown here were conducted with glycoprotein standard solutions and 10μL of normal human serum diluted into PBS. The 6-panel shown contained antibodies for the following glycoproteins: alpha-1-antitrypsin, haptoglobin, hemopexin, immunoglobulin G (IgG), low molecular weight kininogen, and transferrin; their locations are denoted by colored circles. The most abundant N-glycan observed on IgG was m/z 1809.6360 ± 1.8 ppm (Hex5dHex1HexNAc4+Na), which is seen in capture of the glycoprotein standard as well as from human serum, Figure 3A. The location of IgG capture is indicated by a red circle. The antibody itself produces some background signal for this glycan, as evidenced by the addition of just PBS vehicle to the panel. An N-glycan of m/z 1976.6618 ± 2.4 ppm (Hex5HexNAc4NeuAc1+2Na) was observed on hemopexin in capture of the glycoprotein standard as well as human serum, Figure 3B. The location of hemopexin capture is indicated by a green circle. This N-glycan can also be observed on other glycoproteins captured from the serum sample, alpha-1-antitrypsin and haptoglobin (yellow and blue circles, respectively). The appearance of an N-glycan signal on multiple spots is to be expected as many serum glycoproteins have similar N-glycan profiles, again see the list of common serum N-glycans shown in Figure 4.
An example mass spectrum is shown in Figure 3D, which is an individual spectrum of the captured IgG standard showing major detected N-glycans. The presence of significant matrix peaks is noted in the overall spectrum, thus it is recommended to manually pick peaks in the spectra using a list of N-glycan theoretical mass values that can be created using GlycoWorkbench (Ceroni et al., 2008). The high mass resolution of the FT-ICR instrument allows for N-glycan peaks to be easily distinguished from matrix peaks. N-glycans will generally be detected as single sodiated species, but sialylated N-glycans often appear in multiple sodiated forms (+22 Da possible for each sialic acid present), as listed in Figure 4 with multiple mass values possible for the sialylated N-glycans. This is also seen in the spectrum of the hemopexin standard, Figure 3E.
TIME CONSIDERATIONS:
The process of fabricating the antibody panel slide and capturing sample can be completed in under 24 hours. Antibodies can be spotted to the slide at the end of a day; and then the following day consisting of blocking, sample capture, and PNGaseF application can be completed in 5–6 working hours. CHCA matrix application is performed in less than an hour on the third day. The total hands-on time to complete this protocol is approximately 8 hours, spanning across 3 days.
ACKNOWLEDGEMENTS:
The authors wish to thank Grace Grimsley for her assistance with sample preparation. This work was supported in part by the NIH National Center for Advancing Translational Sciences (NCATS) through grant numbers TL1 TR001451 & UL1 TR001450 (APB); as well as funding from R21 CA225474 (ASM), U01CA242096 (ASM, PMA, RRD); U01CA226052 (ASM, RRD) and the South Carolina Centers of Economic Excellence (ASM and RRD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
CONFLICT OF INTEREST:
Anand Mehta is an inventor of PNGaseF Prime™ and has ownership stake in the company N-Zyme Scientifics, LLC, which has commercialized this enzyme.
LITERATURE CITED:
- Black AP, Liang H, West CA, Wang M, Herrera HP, Haab BB, Angel PM, Drake RR, and Mehta AS 2019. A Novel Mass Spectrometry Platform for Multiplexed N-Glycoprotein Biomarker Discovery from Patient Biofluids by Antibody Panel Based N-Glycan Imaging. Analytical Chemistry 91:8429–8435.This paper reports the first use of Antibody Panel Based N-glycan imaging by MALDI FT-ICR. This work was the initial development of the protocol described here in this unit.
- Ceroni A, Maass K, Geyer H, Geyer R, Dell A, and Haslam SM 2008. GlycoWorkbench: A Tool for the Computer-Assisted Annotation of Mass Spectra of Glycans. Journal of Proteome Research 7:1650–1659. [DOI] [PubMed] [Google Scholar]
- Chen S, LaRoche T, Hamelinck D, Bergsma D, Brenner D, Simeone D, Brand RE, and Haab BB 2007. Multiplexed analysis of glycan variation on native proteins captured by antibody microarrays. Nature Methods 4:437–444.This paper describes an antibody microarray platform that uses lectins for detection of glycan changes. This work inspired the development of Antibody Panel Based N-glycan imaging by MALDI MS.
- Comunale MA, Rodemich-Betesh L, Hafner J, Wang M, Norton P, di Bisceglie AM, Block T, and Mehta A 2010. Linkage specific fucosylation of alpha-1-antitrypsin in liver cirrhosis and cancer patients: Implications for a biomarker of hepatocellular carcinoma. PLoS ONE 5:e12419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake RR, Powers TW, Jones EE, Bruner E, Mehta AS, and Angel PM 2017. MALDI Mass Spectrometry Imaging of N-Linked Glycans in Cancer Tissues. Advances in cancer research 134:85–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake RR, Powers TW, Norris-Caneda K, Mehta AS, and Angel PM 2018a. In Situ Imaging of N-Glycans by MALDI Imaging Mass Spectrometry of Fresh or Formalin-Fixed Paraffin-Embedded Tissue. Current Protocols in Protein Science 94:e68.This paper reports a detailed protocol for MALDI MSI of N-glycans on tissue sections. This referenced protocol was the foundation for the protocol steps described here in this unit.
- Drake RR, West CA, Mehta AS, and Angel PM 2018b. MALDI Mass Spectrometry Imaging of N-Linked Glycans in Tissues In Glycobiophsics. Advances in Experimental Medicine and Biology (Yamaguchi Y and Kato K, eds.) pp. 59–76. Springer, Singapore. [DOI] [PubMed] [Google Scholar]
- Gebrehiwot AG, Melka DS, Kassaye YM, Gemechu T, Lako W, Hinou H, and Nishimura S-I 2019. Exploring serum and immunoglobulin G N-glycome as diagnostic biomarkers for early detection of breast cancer in Ethiopian women. BMC Cancer 19:588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gornik O, Royle L, Harvey DJ, Radcliffe CM, Saldova R, Dwek RA, Rudd P, and Lauc G 2007. Changes of serum glycans during sepsis and acute pancreatitis. Glycobiology 17:1321–1332. [DOI] [PubMed] [Google Scholar]
- Hecht ES, Scholl EH, Walker SH, Taylor AD, Cliby WA, Motsinger-Reif AA, and Muddiman DC 2015. Relative Quantification and Higher-Order Modeling of the Plasma Glycan Cancer Burden Ratio in Ovarian Cancer Case-Control Samples. Journal of Proteome Research 14:4394–4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kailemia MJ, Park D, and Lebrilla CB 2017. Glycans and glycoproteins as specific biomarkers for cancer. Analytical and Bioanalytical Chemistry 409:395–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirmiz C, Li B, An HJ, Clowers BH, Chew HK, Lam KS, Ferrige A, Alecio R, Borowsky AD, Sulaimon S, et al. 2007. A serum glycomics approach to breast cancer biomarkers. Molecular & Cellular Proteomics 6:43–55. [DOI] [PubMed] [Google Scholar]
- Kuzmanov U, Kosanam H, and Diamandis EP 2013. The sweet and sour of serological glycoprotein tumor biomarker quantification. BMC Medicine 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novokmet M, Lukić E, Vučković F, Durić Ž, Keser T, Rajšl K, Remondini D, Castellani G, Gašparović H, Gornik O, et al. 2014. Changes in IgG and total plasma protein glycomes in acute systemic inflammation. Scientific Reports 4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olaf Bornsen K, Gass MAS, Bruin GJM, Von Adrichem JHM, Biro MC, Kresbach GM, and Ehrat M 1997. Influence of Solvents and Detergents on Matrix-assisted Laser Desorption/Ionization Mass Spectrometry Measurements of Proteins and Oligonucleotides. [DOI] [PubMed]
- Pompach P, Nováková J, Kavan D, Benada O, Růžička V, Volný M, and Novák P 2016. Planar Functionalized Surfaces for Direct Immunoaffinity Desorption/Ionization Mass Spectrometry. Clinical chemistry 62:270–8. [DOI] [PubMed] [Google Scholar]
- Powers T, Holst S, Wuhrer M, Mehta A, Drake R, Powers TW, Holst S, Wuhrer M, Mehta AS, and Drake RR 2015. Two-Dimensional N-Glycan Distribution Mapping of Hepatocellular Carcinoma Tissues by MALDI-Imaging Mass Spectrometry. Biomolecules 5:2554–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers TW, Jones EE, Betesh LR, Romano PR, Gao P, Copland JA, Mehta AS, and Drake RR 2013. Matrix assisted laser desorption ionization imaging mass spectrometry workflow for spatial profiling analysis of N-linked Glycan expression in tissues. Analytical Chemistry 85:9799–9806.This paper reports the first use of MALDI FT-ICR MS to image N-glycans on tissue sections placed on microscope slides. This work provided the conceptual groundwork for the protocol described here in this unit.
- Powers TW, Neely BA, Shao Y, Tang H, Troyer DA, Mehta AS, Haab BB, Drake RR, and Batra SK 2014. MALDI Imaging Mass Spectrometry Profiling of N-Glycans in Formalin-Fixed Paraffin Embedded Clinical Tissue Blocks and Tissue Microarrays. PLoS ONE 9:e106255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reatini BS, Ensink E, Liau B, Sinha JY, Powers TW, Partyka K, Bern M, Brand RE, Rudd PM, Kletter D, et al. 2016. Characterizing Protein Glycosylation through On-Chip Glycan Modification and Probing. Analytical Chemistry 88:11584–11592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiding KR, Bondt A, Hennig R, Gardner RA, O’Flaherty R, Trbojević-Akmačić I, Shubhakar A, Hazes JMW, Reichl U, Fernandes DL, et al. 2019. High-throughput Serum N-Glycomics: Method Comparison and Application to Study Rheumatoid Arthritis and Pregnancy-associated Changes. Molecular & Cellular Proteomics 18:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhaak LR, Huhn C, Waterreus W, Boer A. R. De Neusu C, Hokke CH, Deelder AM, Wuhrer M, and Deelder M 2008. Hydrophilic Interaction Chromatography-Based High-Throughput Sample Preparation Method for N-Glycan Analysis from Total Human Plasma Glycoproteins Hydrophilic Interaction Chromatography-Based High-Throughput Sample Preparation Method for N-Glycan Analysis. Analytical Chemistry 80:6119–6126. [DOI] [PubMed] [Google Scholar]
- Ruhaak LR, Koeleman CAM, Uh HW, Stam JC, van Heemst D, Maier AB, Houwing-Duistermaat JJ, Hensbergen PJ, Slagboom PE, Deelder AM, et al. 2013. Targeted Biomarker Discovery by High Throughput Glycosylation Profiling of Human Plasma Alpha1-Antitrypsin and Immunoglobulin A. PLoS ONE 8:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhaak LR, Xu G, Li Q, Goonatilleke E, and Lebrilla CB 2018. Mass Spectrometry Approaches to Glycomic and Glycoproteomic Analyses. Chemical Reviews 118:7886–7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shubhakar A, Kozak RP, Reiding KR, Royle L, Spencer DIR, Fernandes DL, and Wuhrer M 2016. Automated High-Throughput Permethylation for Glycosylation Analysis of Biologics Using MALDI-TOF-MS. Analytical Chemistry 88:8562–8569. [DOI] [PubMed] [Google Scholar]
- Simunovic J, Vilaj M, Trbojevic-Akmacic I, Momcilovic A, Vuckovic F, Gudelj I, Juric J, Nakic N, Lauc G, and Pezer M 2019. Comprehensive N-glycosylation analysis of immunoglobulin G from dried blood spots. Glycobiology. [DOI] [PubMed] [Google Scholar]
- Šimurina M, de Haan N, Vučković F, Kennedy NA, Štambuk J, Falck D, Trbojević-Akmačić I, Clerc F, Razdorov G, Khon A, et al. 2018. Glycosylation of Immunoglobulin G Associates With Clinical Features of Inflammatory Bowel Diseases. Gastroenterology 154:1320–1333.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder CM, Alley WR, Campos MI, Svoboda M, Goetz JA, Vasseur JA, Jacobson SC, and Novotny MV 2016. Complementary Glycomic Analyses of Sera Derived from Colorectal Cancer Patients by MALDI-TOF-MS and Microchip Electrophoresis. Analytical Chemistry 88:9597–9605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodoratou E, Thaçi K, Agakov F, Timofeeva MN, Štambuk J, Pučić-Baković M, Vučković F, Orchard P, Agakova A, Din FVN, et al. 2016. Glycosylation of plasma IgG in colorectal cancer prognosis. Scientific reports 6:28098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vučković F, Theodoratou E, Thaçi K, Timofeeva M, Vojta A, Štambuk J, Pučić-Baković M, Rudd PM, Đerek L, Servis D, et al. 2016. IgG Glycome in Colorectal Cancer. Clinical Cancer Research 22:3078–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West CA, Wang M, Herrera H, Liang H, Black A, Angel PM, Drake RR, and Mehta AS 2018. N-Linked Glycan Branching and Fucosylation Are Increased Directly in Hcc Tissue As Determined through in Situ Glycan Imaging. Journal of Proteome Research 17:3454–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Chen B, Wang Y, Xia P, He C, Liu Y, Zhang R, Zhang M, and Li Z 2016. Disease-specific IgG Fc N-glycosylation as personalized biomarkers to differentiate gastric cancer from benign gastric diseases. Scientific Reports 6:25957. [DOI] [PMC free article] [PubMed] [Google Scholar]