Abstract
Child stunting in Vietnam has reduced substantially since the turn of the century but has remained relatively high for several years. We analysed data on children 6–59 months (n = 85,932) from the Vietnam Nutritional Surveillance System, a nationally representative cross‐sectional survey. Multivariable Poisson regression models were used to estimate relative risk (RR) of stunting, stratified by child age and ecological region. Covariates at the child, maternal, household, and environmental levels were included based on available data and the World Health Organization conceptual framework on child stunting. Among children 6–23 months, the strongest associations with child stunting were child age in years (RR: 2.49; 95% CI [2.26, 2.73]), maternal height < 145 cm compared with ≥150 cm (RR: 2.04; 95% CI [1.85, 2.26]), living in the Northeast compared with the Southeast (RR: 2.01; 95% CI [1.69, 2.39]), no maternal education compared with a graduate education (RR: 1.77; 95% CI, [1.44, 2.16]), and birthweight < 2,500 g (RR: 1.75; 95% CI [1.55, 1.98]). For children 24–59 months, the strongest associations with child stunting were no maternal education compared with a graduate education (RR: 2.07; 95% CI [1.79, 2.40]), living in the Northeast compared with the Southeast (RR: 1.94; 95% CI [1.74, 2.16]), and maternal height < 145 cm compared with ≥150 cm (RR: 1.81; 95% CI [1.69, 1.94]). Targeted approaches that address the strongest stunting determinants among vulnerable populations are needed and discussed. Multifaceted approaches outside the health sector are also needed to reduce inequalities in socioeconomic status.
Keywords: causes, child stunting, determinants, aetiology, linear growth faltering, Vietnam
Key messages.
Among children 6–59 months, child stunting in Vietnam is strongly associated with maternal, child, and environmental factors, particularly short maternal height, low maternal education, and living in a northern region.
Among children 6–23 months, child age and birthweight are also strongly associated with child stunting.
There are striking differences in stunting risk factors between regions, which suggests policies and programmes aimed at reducing stunting would have more impact if they were tailored geographically.
Multifaceted approaches outside of the health sector are needed to reduce inequalities in socio‐economic status, which are inextricably linked with malnutrition in Vietnam.
1. INTRODUCTION
Child stunting has immediate and long‐term consequences including increased morbidity and mortality as well as detrimental impacts on child development and adult health, and it contributes to the intergenerational cycle of malnutrition, hindering economic development (Black et al., 2013; Stewart, Iannotti, Dewey, Michaelsen, & Onyango, 2013). Reducing child stunting is a high priority on the global nutrition and development agenda, a key indicator in the Global Nutrition Targets for 2025 (World Health Organization[WHO], 2012) and the Sustainable Development Goal of Zero Hunger (United Nations, Department of Economic and Social Affairs, 2016). Stunting determinants are complex and extensive and vary substantially by local context. To optimally reduce stunting through appropriate policies and programmes, there must be an understanding of which stunting determinants are most important nationally and for targetable subpopulations.
The prevalence of child stunting in Vietnam has reduced from 43% in 2000 to 25% in 2015 (Ministry of Health, National Institute of Nutrition, 2012a; Vietnam National Institute of Nutrition, 2018). Vietnam is one of the few countries in Southeast Asia to have reduced malnutrition at nearly the rates set in the Millennium Development Goals (Ministry of Health, National Institute of Nutrition, 2012b). Rapid economic development has helped to improve the nutritional status of the population along with specific nutritional efforts by the Vietnamese government (Ministry of Health, National Institute of Nutrition, 2012b; Trinh Thi, Simioni, & Thomas‐Agnan, 2018). For example, the national nutrition strategy outlines goals to reduce hunger and improve dietary quality and recommends the development of nutrition interventions that target vulnerable populations, including women and children, the poor, and ethnic minorities (Ministry of Health, National Institute of Nutrition, 2012b). Political will to improve nutrition and reduce stunting is strong in Vietnam, but more evidence is needed on effective interventions, especially because stunting reduction appears to be stagnating (Vietnam National Institute of Nutrition, 2018).
There is little nationally representative evidence of specific stunting determinants in Vietnam and how they vary by subpopulation. Stunting prevalence varies substantially by region (Viet Nam National Institute of Nutrition, UNICEF, Alive, & Thrive, 2014), and regional disparities in socio‐economic conditions and access to education and other services may be driving these differences. A recent analysis of children from select Vietnamese provinces found the following determinants accounted for 90% of the change in height‐for‐age z‐scores (HAZs) between 2010 and 2014: socio‐economic status (26%), prenatal visits (25%), hygiene (19%), child birth weight (10%), maternal education (7%), and household food insecurity (3%; P. H. Nguyen et al., 2017). To our knowledge, no multivariable analyses of stunting determinants at the national level have been conducted in Vietnam. We aimed to improve understanding of the determinants of child stunting in Vietnam nationally and among vulnerable subpopulations by assessing associations of hypothesized child‐, maternal‐, household‐ and environment‐level determinants of stunting in children 6–59 months using nationally representative data from the 2015 Vietnam Nutrition Surveillance System. We used a modified version of the WHO conceptual framework on child stunting (Beal, Tumilowicz, Sutrisna, Izwardy, & Neufeld, 2018) and hypothesized that relationships with stunting would exist at all levels among young Vietnamese children, but child‐ and maternal‐level characteristics would have the strongest relationships, especially among children 6–23 months.
2. METHODS
2.1. Sampling design and participants
The 2015 Vietnam Nutrition Surveillance System surveyed 99,450 children 0–59 months, following a three‐stage cluster sampling design to obtain a nationally representative sample. In the first stage, 30 clusters (communes/wards) were selected from each of 63 provinces using probability proportion to size. Ha Noi and Ho Chi Minh were first stratified by urban and rural before selecting 30 clusters in each locality for both cities. In the second stage, three villages were randomly selected from each cluster using a lottery system or table of random numbers. In the third stage, two children 0–5 months, five children 6–23 months, and 10 children 24–59 months were selected from each village using simple random sampling (Viet Nam National Institute of Nutrition, UNICEF, Alive, & Thrive, 2014). Sample weights were calculated for each cluster and province using population data from the 2009 Census (General Statistics Office [Vietnam] & United Nations Population Fund [UNFPA], 2009). No sample weights were calculated at the village level because administrative codes to facilitate the match between the census population and survey sample were unavailable. Figure 1 shows the participant flow chart. Our analysis included 85,932 children 6–59 months. Execution of the National Nutrition Surveillance System in Vietnam has been approved as an integral part of the 2001–2010 National Nutrition Strategy, which was approved by the Prime Minister of Vietnam on February, 22, 2001 (Document No. 21/2011/QD TTG). The National Institute of Nutrition's Scientific and Ethical Committee and the Ministry of Health's Scientific and Ethical Committee approved the current tools and methodology in 2009.
Figure 1.
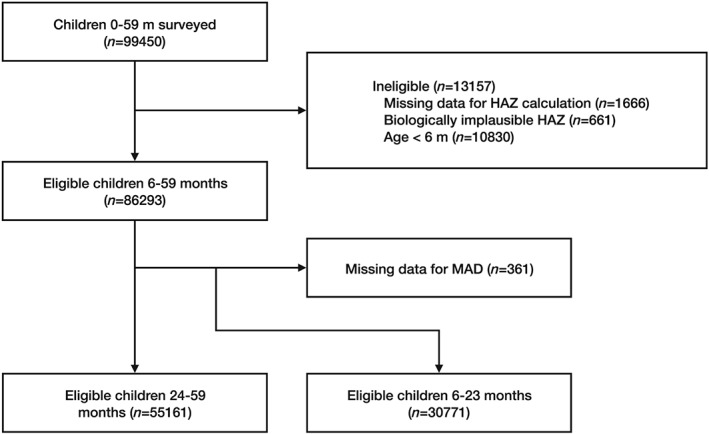
Participant flow chart. HAZ, height‐for‐age z‐score; MAD, minimum acceptable diet
2.2. Variables
2.2.1. Outcome
Child stunting (HAZ < −2) was the outcome variable in this analysis. Length of children 6–23 months was measured using an infantometer and height of children 24–59 months with a stadiometer to the nearest 0.1 cm. HAZs were calculated using the 2006 WHO child growth standards (WHO, 2006).
2.2.2. Covariates
An expanded version of the WHO conceptual framework on child stunting (Beal et al., 2018) was used to identify potential covariates from the available data. A total of 28 variables were categorized at the child, maternal, household, and environmental levels. Child characteristics included age; sex; ethnicity; birthweight; if the child had a fever or diarrhoea, respectively, in past 2 weeks; and if the child was given deworming medicine or Vitamin A supplements, respectively, in the past 6 months. Child feeding variables were included for children 6–23 months including currently breastfed; consumed a minimum acceptable diet (MAD) in the past 24 hr; consumed meat, fish, eggs, or milk in the past 24 hr; and consumed Vitamin A‐rich fruits and vegetables in the past 24 hr. MAD was calculated based on the 2010 WHO guidelines (WHO, 2010) but counting breast milk as a dairy food in the 7‐point score for minimum dietary diversity to eliminate the advantage of nonbreastfed children (UNICEF, FANTA, USAID, & WHO, 2017). Maternal characteristics included age, height, BMI, education, occupation (farmer or not), washed hands before preparing foods and feeding child, took iron‐folic acids tablets in the past 6 months, and met with health staff in the past 3 months. Household‐level variables included number of children in the home (>2) and whether iodized salt was currently used. Environmental variables included locality (urban/rural), poor commune, mountainous commune, and ecological region.
2.3. Statistical analysis
All analyses accounted for the study design and sample weights so that results are representative of the national population of children 6–59 months in Vietnam. We excluded biologically implausible values (HAZ > +6 or <−6) according to WHO guidelines (Organization, 2009). Children 6–23 months were analysed separately from children 24–59 months (except for in regional analyses that were limited by sample size) to allow for inclusion of child feeding variables for the younger age group and for a different maternal age categorical cutoff for the two child age groups. All continuous independent variables except for child age were coded into commonly reported categorical levels. Four variables had relatively high levels of missingness but all less than 10%: consumption of Vitamin A‐rich fruits and vegetables, maternal education, poor commune, and mountainous commune. To include observations with missing values for these categorical variables, we created a new factor level for answers that were not applicable (NAs) but did not report results for this level.
We conducted relative risk (RR) regression with a quasi‐Poisson log link function and model‐robust standard error estimates (Lumley, Kronmal, & Ma, 2006) to calculate RRs with corresponding 95% confidence intervals (CIs) and p values, using the “survey” package (Lumley & Lumley, 2018) in R (Version 3.5.1). We first conducted bivariate analyses for all available variables identified in the conceptual framework (Beal et al., 2018) and then built full multivariable models using these variables. We selected the variables for the final models using a combination of approaches; most of which considered sample weights and complex survey design. Approaches that considered sample weights and complex survey design included bivariate analyses, likelihood ratio tests from the “survey” package (Lumley & Lumley, 2018), and stepwise (forwards/backwards) model selection from the “mass” package (Ripley et al., 2013) in R (v 3.5.1) using both Akaike information criterion and Bayesian information criterion (Zhang, 2016). We also used the feature selection approach with the random forest classifier, which did not consider sample weights and complex survey designs (Kursa & Rudnicki, 2010; Liaw & Wiener, 2002). For the random forest‐based feature selection, we obtained an optimum value of “mtry” by tuning the full model with 1,000 trees, with the improvement parameter set at 1e‐05. We partitioned the dataset into 60% training and 40% for model validation using the “caret” package (Max Kuhn et al., 2018) in R (v 3.5.1) and deployed the random forest classifier on the training dataset to calculate a “variable importance” metric for each variable in the full model. Variables obtained from these multiple approaches were assessed and compared with prior knowledge of stunting determinants globally (Beal et al., 2018; Stewart et al., 2013), based on which we selected the final set of variables. For children 6–23 months, we included the interaction between breastfeeding status and child age to account for the influence of reverse causality of breastfeeding and stunting (Kramer, Moodie, Dahhou, & Platt, 2011; Marquis, Habicht, Lanata, Black, & Rasmussen, 1997).
Because the final models differed between the two child age groups, we also built an alternative model with the same variables for each age group. This allowed us to compare effect sizes of included covariates between age groups and conduct stratified regional analyses. This model dropped variables that were not available for both age groups (child feeding variables) and retained variables that were included in the final model of either age group. Using this alternative model, we conducted stratified analyses of children 6–59 months for each of the eight regions in Vietnam to understand how stunting determinants varied regionally.
3. RESULTS
Among 30,771 children 6–23 months, mean HAZ was −0.73 (95% CI [–0.77, −0.70]) and prevalence of stunting 17.5% (95% CI [16.8, 18.2]; Table 1). About half (53%) of children 6–23 months were male, 18% ethnic minorities, 63% from rural areas, and 44% currently breastfed. Among 55,161 children 24–59 months, mean HAZ was −1.05 (95% CI [–1.08, −1.03]) and prevalence of stunting 21.4% (95% CI [20.7, 22.1]). Background characteristics were similar between children 6–23 months and 24–59 months, with the exception of the child experiencing fever in the past 2 weeks (15% vs. 10%, respectively), not being given deworming medication within the past 6 months (97% vs. 56%, respectively) and not being given Vitamin A supplements in the past 6 months (24% vs. 30%, respectively).
Table 1.
Background characteristics of Vietnamese children by age
| Children 6–23 months | Children 24–59 months | |||
|---|---|---|---|---|
| Variable | N | Mean/prevalence (95% CI) | N | Mean/prevalence (95% CI) |
| Child characteristics | ||||
| Height‐for‐age z‐score | 30,771 | −0.73 (−0.77, −0.70) | 55,161 | −1.05 (−1.08, −1.03) |
| Child stunting (HAZ < −2) | 5,823 | 17.5% (16.8, 18.2) | 12,432 | 21.4% (20.7, 22.1) |
| Child age (month) | ||||
| 6–11 | 9,136 | 29.4% (28.6, 30.0) | — | — |
| 12–23 | 21,635 | 70.6% (69.8, 71.0) | — | — |
| 24–35 | — | — | 24,023 | 44.3% (43.6, 45.0) |
| 36–59 | — | — | 31,138 | 55.7% (55.0, 56.4) |
| Child sex, male | 16,307 | 53.0% (52.2, 54.0) | 29,216 | 52.6% (52.0, 53.2) |
| Ethnic minority | 6,912 | 18.3% (17.0, 20.0) | 12,975 | 19.2% (18.0, 20.5) |
| Birthweight < 2,500 g | 1,421 | 4.5% (4.2, 5.0) | 2,798 | 4.8% (4.6, 5.1) |
| Child fever in past 2 weeks | 4,502 | 15.3% (14.6, 16.0) | 5,348 | 10.0% (9.6, 10.5) |
| Child not given deworming medication in past 6 months | 29,758 | 96.7% (96.4, 97.0) | 30,215 | 55.9% (54.9, 56.8) |
| Child not given Vitamin A supplements in past 6 months | 7,118 | 23.5% (22.4, 25.0) | 16,468 | 30.0% (29.0, 31.0) |
| Child diarrhoea in past 2 weeks | 1,226 | 4.3% (3.9, 5.0) | 1,285 | 2.3% (2.1, 2.5) |
| Currently breastfed | 13,275 | 43.6% (42.7, 44.0) | — | — |
| Child did not consume a minimum acceptable diet in past 24 hr | 17,017 | 53.1% (51.9, 54.0) | — | — |
| Child did not consume meat, fish, eggs, or milk products in past 24 hr | 3,296 | 10.2% (9.3, 11.0) | — | — |
| Child did not consume Vitamin A‐rich fruits and vegetables in past 24 hr | 7,047 | 24.4% (23.5, 25.0) | — | — |
| Maternal characteristics | ||||
| Maternal age < 18 years | 4,814 | 14.3% (13.7, 15.0) | — | — |
| Maternal age < 20 years | — | — | 9,316 | 15.8% (15.2, 16.4) |
| Maternal height (cm) | ||||
| <145 | 1,347 | 4.1% (3.8, 4.0) | 2,118 | 3.6% (3.3, 3.9) |
| 145–149.9 | 4,454 | 13.8% (13.2, 14.0) | 7,246 | 12.5% (12.0, 13.0) |
| ≥150 | 24,970 | 82.1% (81.3, 83.0) | 45,797 | 83.9% (83.2, 84.5) |
| Maternal body mass index (BMI) < 18.5 | 4,541 | 14.4% (13.9, 15.0) | 6,519 | 11.6% (11.2, 12.1) |
| Maternal education (year) | ||||
| None | 1,149 | 3.3% (2.9, 4.0) | 2,525 | 4.1% (3.7, 4.5) |
| Primary (Grades 1–5) | 3,278 | 9.8% (9.2, 10.0) | 6,836 | 11.4% (10.9, 12.0) |
| Secondary (Grades 6–9) | 11,435 | 35.7% (34.6, 37.0) | 21,326 | 37.4% (36.4, 38.4) |
| High school (Grades 10–12) | 9,079 | 30.5% (29.5, 31.0) | 15,533 | 29.3% (28.5, 30.1) |
| College or university | 3,360 | 12.2% (11.5, 13.0) | 3,372 | 10.3% (9.7, 11.0) |
| Graduate school | 2,266 | 8.6% (7.9, 9.0) | 5,083 | 7.5% (6.9, 8.1) |
| Mother's occupation, farmer | 11,398 | 31.9% (30.3, 34.0) | 22,200 | 35.2% (33.7, 36.7) |
| Mother did not wash hands both before preparing foods and feeding child | 17,065 | 54.7% (53.2, 56.0) | 32,161 | 56.8% (55.4, 58.2) |
| Mother did not take iron or iron‐folate tablets in 3 months before or after birth | 5,846 | 18.1% (17.2, 19.0) | — | — |
| Caregiver did not meet with health staff in past 3 months | 5,425 | 18.9% (17.7, 20.0) | 12,144 | 22.9% (21.9, 24.0) |
| Household characteristics | ||||
| More than two children in the household | 4,102 | 13.5% (12.9, 14.0) | 8,373 | 15.5% (14.9, 16.0) |
| Household does not use iodized salt | 3,516 | 11.7% (10.9, 13.0) | 6,113 | 11.9% (11.0, 12.7) |
| Environmental characteristics | ||||
| Rural | 21,978 | 63.2% (60.3, 66.0) | 40,477 | 66.1% (63.5, 68.6) |
| Poor commune | 5,371 | 14.7% (13.1, 16.0) | 9,982 | 15.2% (13.7, 16.8) |
| Mountainous commune | 9,497 | 26.9% (25.0, 29.0) | 17,364 | 28.1% (26.3, 29.9) |
| Region | ||||
| Red River Delta | 4,152 | 15.6% (14.6, 17.0) | 7,820 | 16.9% (15.8, 18.1) |
| Northeast | 6,066 | 16.0% (15.0, 17.0) | 11,009 | 16.5% (15.6, 17.5) |
| Northwest | 1,717 | 4.6% (4.1, 5.0) | 3,410 | 5.1% (4.6, 5.6) |
| Central North | 2,800 | 13.0% (11.8, 14.0) | 5,023 | 12.8% (11.7, 13.9) |
| Central South | 2,853 | 8.6% (8.0, 9.0) | 5,195 | 9.0% (8.4, 9.7) |
| Central Highlands | 1,933 | 6.4% (5.8, 7.0) | 3,483 | 6.4% (5.8, 7.0) |
| Southeast | 5,461 | 16.0% (14.6, 17.0) | 8,167 | 16.0% (14.6, 17.4) |
| Mekong River Delta | 5,789 | 16.2% (15.3, 17.0) | 11,054 | 17.3% (16.4, 18.2) |
3.1. Risk factors of stunting among children 6–23 months
In the multivariable analysis, variables with the strongest association with stunting in children 6–23 months included child age, birthweight, maternal height, maternal education, and region (Figure 2). An increase in child age of 1 year was associated with a more than two‐fold increase in stunting risk (RR: 2.49; 95% CI [2.26, 2.73]). Having a birthweight below 2,500 g was associated with a 75% increase in stunting risk (RR: 1.75; 95% CI [1.55, 1.98]). Children of mothers with a height < 145 cm had twice the stunting risk (RR: 2.04; 95% CI [1.85, 2.26]) and height 145–149 cm a 62% increase in risk (RR: 1.62; 95% CI [1.50, 1.74]) compared with children of mothers with a height ≥ 150 cm. The risk of stunting increased with decreasing maternal education. Children of mothers with no education (RR: 1.77; 95% CI [1.44, 2.16]) or only primary education (RR: 1.77; 95% CI [1.48, 2.11]) had a 77% increase in stunting risk compared with children of mothers with a graduate education. Compared with children from the Southeast, children from all other regions had significantly increased stunting risk. Risk of stunting in the Northeast was double that in the Southeast (RR: 2.01; 95% CI [1.69, 2.39]), 83% higher in the Northwest (RR: 1.83; 95% CI [1.49, 2.26]), and ~75% higher in the Central North (RR: 1.75; 95% CI [1.46, 2.10]) and Central Highlands (RR: 1.73; 95% CI [1.43, 2.08]). Other variables with moderately strong associations with child stunting included not currently breastfeeding (RR: 1.44; 95% CI [1.14, 1.82]), sex (male; RR: 1.35; 95% CI [1.27, 1.43]), ethnic minority (RR: 1.31; 95% CI [1.19, 1.44]), and maternal BMI < 18.5 (RR: 1.20; 95% CI [1.12, 1.30]; Figure 2). The interaction of child age (in years) and not currently breastfeeding was associated with a reduced risk of stunting (RR: 0.65; 95% CI [0.55, 0.75]).
Figure 2.
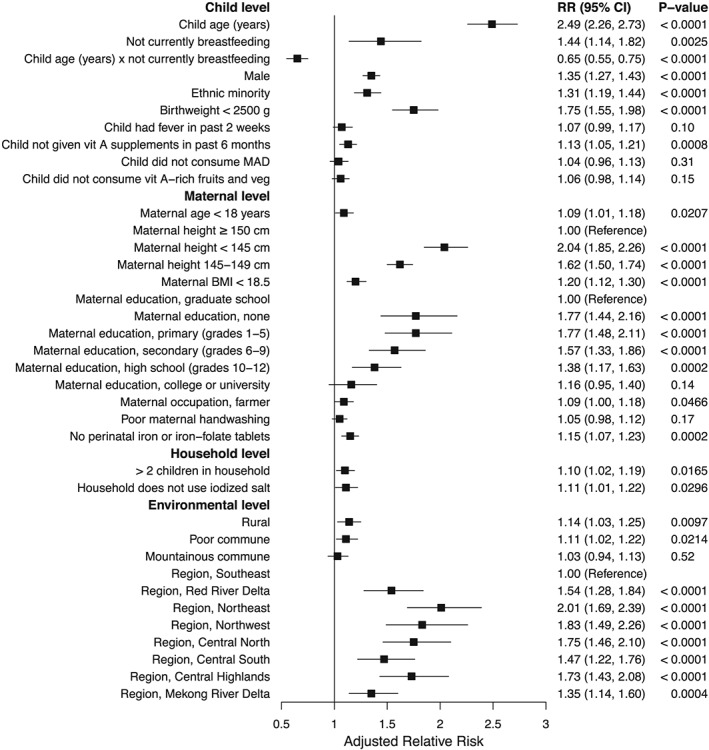
Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 30,771 Vietnamese children 6–23 months. Error bars show 95% confidence intervals. The multivariable model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; MAD, minimum acceptable diet; RR, relative risk; veg, vegetables; vit, vitamin
3.2. Risk factors of stunting among children 24–59 months
Variables with the strongest association with stunting in children 24–59 months in the multivariable analysis included maternal height, maternal education, and region (Figure 3). Children of mothers with a height < 145 cm had an 81% increase in stunting risk (RR: 1.81; 95% CI [1.69, 1.94]) and height 145–149 cm a 49% increase in risk (RR: 1.49; 95% CI [1.41, 1.57]) compared with children of mothers with a height ≥ 150 cm. Compared with children of mothers with a graduate education, mothers with all other levels of schooling, including a college or university education, had a significantly higher risk of stunting, and risk increased with decreasing education. Risk of stunting for children of mothers with no schooling was double that of children from mothers with a graduate education (RR: 2.07; 95% CI [1.79, 2.40]). Children from the Northeast had twice the stunting risk (RR: 1.94; 95% CI [1.74, 2.16]), Central North a 78% increase (RR: 1.78, 95% CI [1.56, 2.03]), Northwest a 72% increase (RR: 1.72; 95% CI [1.49, 1.98]), and Central Highlands a 68% increase (RR: 1.68; 95% CI [1.47, 1.91]) in risk compared with children from the Southeast. Variables with moderately strong associations with child stunting included ethnic minority (RR: 1.30; 95% CI [1.21, 1.39]), birthweight < 2,500 g (RR: 1.43; 95% CI [1.33, 1.53]), maternal age < 20 years (RR: 1.20; 95% CI [1.15, 1.26]), maternal BMI < 18.5 (RR: 1.17; 95% CI [1.11, 1.25]), and >2 children in the household (RR: 1.18; 95% CI [1.12, 1.24]; Figure 3).
Figure 3.
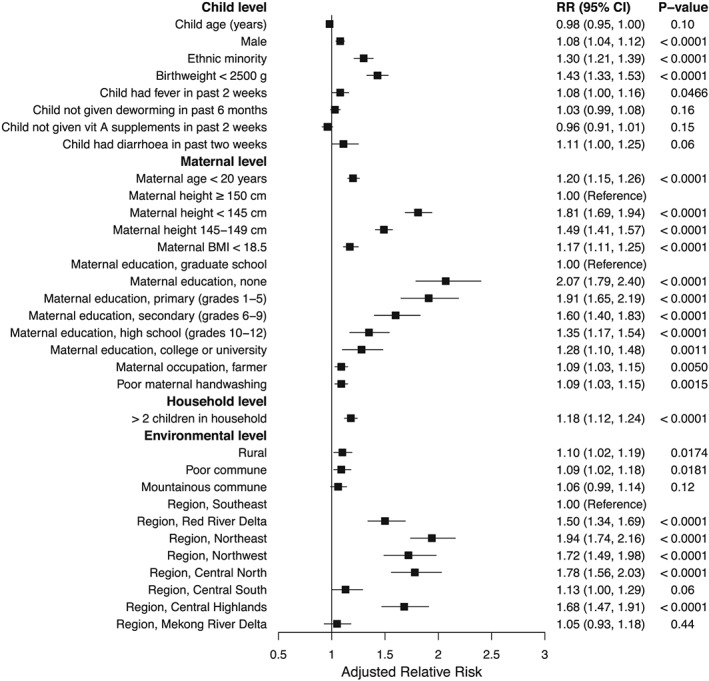
Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 55,161 Vietnamese children 24–59 months. Error bars show 95% confidence intervals. The multivariable model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin
3.3. Comparing risk factors of stunting between younger and older children
The alternative models, which used the same variables for both age groups, allowed us to compare stunting risks between children 6–23 months and 24–59 months (Figures S1 and S2). We do not report exact risk values because the models included a suboptimal set of covariates, but they were suitable for comparing risks between age groups. Child age, sex (male), and birthweight < 2,500 g were much more strongly associated with stunting among children 6–23 months than 24–59 months—child age was not meaningfully associated with stunting in the older age group. Sex differences in stunting risk disappeared by age ~3 years (RR in children 36–59 months: 1.02; 95% CI [0.97, 1.08]). Short maternal height was a larger risk factor and maternal education a smaller risk factor for children 6–23 months than 24–59 months. Risk of stunting regionally maintained a similar pattern between children 6–23 months and children 24–59 months, but risk was lower relative to the Southeast among the older age group for all regions except the Central North and substantially lower for the Mekong River Delta and Central South, whose RRs became insignificant in children 24–59 months.
3.4. Comparing risk factors of stunting across regions
Stratified analyses by region of the combined age group of children 6–59 months using the alternative models revealed substantial heterogeneity in stunting risk factors between regions (Figures S3–S10). Ethnic minorities had double the stunting risk (RR: 1.99; 95% CI [1.72, 2.31]) in the Central Highlands but no increased risk in the Mekong River Delta (RR: 1.00; 95% CI [0.86, 1.17]). Birthweight < 2,500 g was associated with a two‐fold increase in stunting risk in the Southeast (RR: 2.06; 95% CI [1.61, 2.63]) and only a 17% increase in the Northeast (RR: 1.17; 95% CI [1.04, 1.31]). Stunting risk among children of mothers with only a primary school education compared with mothers with a graduate education increased by three‐fold in the Southeast (RR: 3.01; 95% CI [1.77, 5.12]) and only ~55% in the Northwest (RR: 1.55; 95% CI [0.77, 3.11]) and Central North (RR: 1.54; 95% CI [1.18, 2.01]). Interestingly, living in a mountainous commune was associated with a 25% decreased risk of stunting in the Central Highlands (RR: 0.75; 95% CI [0.67, 0.85]), a 63% increased risk in the Southeast (RR: 1.63; 95% CI [1.38, 1.93]), and a two‐fold increased risk in the Northwest (RR: 1.91; 95% CI [1.04, 3.49]).
4. DISCUSSION
Our multivariable analysis of child stunting and its determinants adjusted for a range of covariates at the child, maternal, household, and environmental levels. Although correlations in cross‐sectional data do not prove causal relationships, we included variables that have a strong theoretical basis as determinants of stunting (Stewart et al., 2013). The variables with the strongest relationships with stunting among children 6–59 months were maternal height and education, and region—child stunting risk was roughly double among children of stunted mothers (height < 145 cm) compared with those not stunted (height ≥ 150 cm), mothers with no education or primary school only compared with those with a graduate education, and living in the Northeast compared with the Southeast. Maternal height and education have been shown to be strongly associated with child growth in Vietnam (P. H. Nguyen et al., 2017; Young et al., 2018) and other countries in South and Southeast Asia (Beal et al., 2018; Ikeda, Irie, & Shibuya, 2013; P. H. Nguyen et al., 2017) as well as globally (Black et al., 2013; Danaei et al., 2016). About 15% of Vietnamese mothers had no education or primary school only, highlighting the need to improve access to education for women, which has potential to reduce stunting and provides other benefits (Black et al., 2013). The strong relationship between maternal short stature and child stunting in Vietnam indicates the importance of nutrition interventions before and during pregnancy, particularly because maternal short stature is clearly linked to fetal growth restriction and newborn stunting (Black et al., 2013; Victora et al., 2015). Research is needed to determine which nutrition interventions among mothers with short stature can most effectively improve birth outcomes and prevent children from following the same path as their mothers. Unsurprisingly, the child‐level variables age, sex, and birthweight were more strongly associated with stunting among younger than older children. Our findings correspond with recent global evidence that sex differences in child stunting diminish with age and disappear by about age 40 months (Alderman & Headey, 2018). Among children 6–23 months, discontinuing breastfeeding in older child age was associated with reduced risk of stunting, but this was likely due to children's poor growth leading to increased breastfeeding, not the other way around (Kramer et al., 2011; Marquis et al., 1997).
Risk of stunting was highest in the Northeast, Northwest, Central North, and Central Highlands. This geographic pattern clearly represents variations in socio‐economic status, proportion of ethnic minority groups, and access to food, health services, and clean water (Dearden et al., 2017). The small size of landholdings, consequent carrying capacity of farming systems to provide sufficient quality food, level of inclusion in profitable agricultural value chains, and opportunities for off‐farm employment pose a challenge for the socio‐economic development in these regions (Minot & Baulch, 2005; Phat, 2012; Pimhidzai, 2018). Stunting reduction efforts should target regions at the highest risk. Regional variations in stunting risk factors were striking and suggest that tailoring policies and programmes by region will more effectively address the most relevant stunting determinants. For example, ethnic minorities in the Central Highlands, children with low birthweight and of poorly educated mothers in the Southeast, and mountainous communes in the Northwest could be prioritized. Risk factors even varied substantially between regions with similar stunting risk. For example, in the Northwest, living in a mountainous commune was the strongest risk factor, whereas in the Central North, maternal height and education were the strongest risk factors, and living in a mountainous commune was not a risk factor.
We found little evidence that dietary factors such as consumption of animal‐source foods, Vitamin A‐rich fruits and vegetables, and a MAD, measured by recent dietary intake or feeding practices, influenced stunting risk. It is unlikely that dietary factors are unimportant for child growth in Vietnam, however, given the dietary inadequacies of child diets (21). Methodological limitations may explain why dietary factors appeared unimportant. Recent intake in the past 24 hr does not necessarily reflect usual consumption in earlier childhood, and nutritional insults must have occurred over a period of time to influence stunting. Additionally, quantities of foods consumed were not measured, so the dose of the foods may not have been sufficient to demonstrate a change in stunting. Living in a poor commune was associated with only a small increase in stunting risk. Socio‐economic status is clearly a strong driver of child stunting (P. H. Nguyen et al., 2017), but our analysis only included poverty at the commune level, and household wealth can vary substantially within communes of various socio‐economic status. Furthermore, stunting and other indicators of malnutrition have improved, whereas socio‐economic and ethnic inequalities have increased, highlighting the need for policies and comprehensive measures beyond the health sector that aim to reduce socio‐economic disparities (Benjamin, Brandt, & McCaig, 2017; Kien et al., 2016).
Our study has many strengths. Variables were selected from a rigorous conceptual framework and thus have a strong theoretical basis for being stunting determinants in Vietnam. Model selection was informed by this framework along with a combination of sophisticated statistical model selection approaches. The large, recent nationally representative sample of Vietnamese children 6–59 months allows for the generalization of findings across Vietnam and inference that is applicable to the current context. Analysing children 6–23 months separately from 24–59 months allowed for richer models for each age group and the ability to compare determinants between younger and older children. Stratified analyses by region provides insight into how to best tailor interventions geographically to be most effective.
There are also important limitations. Although variables were selected from a strong conceptual framework, it is impossible to know with certainty the direction of the relationships, and there were likely important confounding factors omitted from the analysis. Many of the determinants listed in the conceptual framework were unavailable in the dataset. Poverty was only included at the commune level and would have likely had a much stronger relationship with child stunting if it were included at the household level (P. H. Nguyen et al., 2017). Sample weights were unavailable at the village level, so any differences in population sizes between villages were not reflected in weighting. Lastly, child stunting is an imperfect proxy indicator for child growth failure (Perumal, Bassani, & Roth, 2018), and an unknown proportion of Vietnamese children with a HAZ < −2 are growing normally and HAZ ≥ −2 experiencing growth faltering. This suggests that our findings underestimated associations between child growth failure and its determinants.
In conclusion, our cross‐sectional analysis of children 6–59 months found strong relationships between variables at the maternal and environmental levels with child stunting, especially short maternal height, low maternal education, and living in a northern region. Among children 6–23 months, child‐level variables also had strong relationships with stunting, particularly age and birthweight. Regional analyses revealed strong differences in stunting risk factors between regions, which suggests that policies and programmes aimed at reducing stunting would have more impact if they were tailored geographically. Important progress has been made to reduce stunting since 2000, which has largely been due to economic development, but more specialized approaches may be required to reach the most vulnerable populations, where prevalence remains much higher than the national average. At the same time, multifaceted approaches outside of the health sector are needed to reduce inequalities in socio‐economicstatus, which are inextricably linked with malnutrition.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
TB conceptualized the study, drafted the initial manuscript, and had primary responsibility for the final content of the manuscript; LDT, TTM, and NDS designed and facilitated data collection; TB, HTT, and DB analysed the data; and all authors read and approved the final manuscript.
Supporting information
FIGURE S1 Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 30771 Vietnamese children 6–23 m. Error bars show 95% confidence intervals. The multivariate model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin.
FIGURE S2 Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 55161 Vietnamese children 24–59 m. Error bars show 95% confidence intervals. The multivariate model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin.
FIGURE S3 Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 13505 children 6–59 m living in Southeast Vietnam. Error bars show 95% confidence intervals. The multivariate model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin.
Figure S4 Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 12068 Vietnamese children 6–59 m living in the Red River Delta. Error bars show 95% confidence intervals. The multivariate model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin.
Figure S5 Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 17160 children 6–59 m living in the Northeast Vietnam. Error bars show 95% confidence intervals. The multivariate model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin.
Figure S6 Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 5169 children 6–59 m living in the Northwest Vietnam. Error bars show 95% confidence intervals. The multivariate model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin.
Figure S7 Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 7880 Vietnamese children 6–59 m living in the Central North. Error bars show 95% confidence intervals. The multivariate model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin.
Figure S8 Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 8090 Vietnamese children 6–59 m living in the Central South. Error bars show 95% confidence intervals. The multivariate model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin.
Figure S9 Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 5464 Vietnamese children 6–59 m living in the Central Highlands. Error bars show 95% confidence intervals. The multivariate model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin.
Figure S10 Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 16957 Vietnamese children 6–59 m living in the Mekong River Delta. Error bars show 95% confidence intervals. The multivariate model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin.
Table S1 Bivariate relative risks of stunting determinants at the child, maternal, household, and environmental levels in Vietnamese children by age
ACKNOWLEDGMENTS
Authors Le Danh Tuyen, Truong Tuyet Mai, and Nguyen Duy Son acknowledge Dr. Tran Thanh Do, former head of the Nutrition Surveillance and Policy Department, NIN, for his devoted work and contribution to developing and completing the NSS database.
Beal T, Le DT, Trinh TH, et al. Child stunting is associated with child, maternal, and environmental factors in Vietnam, Matern Child Nutr. 2019; 15:e12826 10.1111/mcn.12826.
REFERENCES
- Alderman, H. , & Headey, D. (2018). The timing of growth faltering has important implications for observational analyses of the underlying determinants of nutrition outcomes. PLoS ONE, 13(4), e0195904 10.1371/journal.pone.0195904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal, T. , Tumilowicz, A. , Sutrisna, A. , Izwardy, D. , & Neufeld, L. M. (2018). A review of child stunting determinants in Indonesia. Maternal & Child Nutrition, 14(4), e12617 10.1111/mcn.12617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin, D. , Brandt, L. , & McCaig, B. (2017). Growth with equity: Income inequality in Vietnam, 2002–14. The Journal of Economic Inequality, 15(1), 25–46. 10.1007/s10888-016-9341-7 [DOI] [Google Scholar]
- Black, R. E. , Victora, C. G. , Walker, S. P. , Bhutta, Z. A. , Christian, P. , de Onis, M. , … Uauy, R. (2013). Maternal and child undernutrition and overweight in low‐income and middle‐income countries. The Lancet, 382(9890), 427–451. 10.1016/S0140-6736(13)60937-X [DOI] [PubMed] [Google Scholar]
- Danaei, G. , Andrews, K. G. , Sudfeld, C. R. , Fink, G. , McCoy, D. C. , Peet, E. , … Fawzi, W. W. (2016). Risk factors for childhood stunting in 137 developing countries: A comparative risk assessment analysis at global, regional, and country levels. PLoS Medicine, 13(11), e1002164 10.1371/journal.pmed.1002164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dearden, K. A. , Schott, W. , Crookston, B. T. , Humphries, D. L. , Penny, M. E. , Behrman, J. R. , … The Young Lives Determinants and Consequences of Child Growth Project Team (2017). Children with access to improved sanitation but not improved water are at lower risk of stunting compared to children without access: A cohort study in Ethiopia, India, Peru, and Vietnam. BMC Public Health, 17(1), 110 10.1186/s12889-017-4033-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- General statistics office (Vietnam), & United Nations Population Fund (UNFPA) . (2009). Vietnam Population and Housing Census 2009.
- Ikeda, N. , Irie, Y. , & Shibuya, K. (2013). Determinants of reduced child stunting in Cambodia: analysis of pooled data from three Demographic and Health Surveys. Bulletin of the World Health Organization, 91(5), 341–349. 10.2471/BLT.12.113381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kien, V. D. , Lee, H.‐Y. , Nam, Y.‐S. , Oh, J. , Giang, K. B. , & Minh, H. V. (2016). Trends in socioeconomic inequalities in child malnutrition in Vietnam: Findings from the Multiple Indicator Cluster Surveys, 2000–2011. Global Health Action, 9(s1), 29263 10.3402/gha.v9.29263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, M. S. , Moodie, E. E. M. , Dahhou, M. , & Platt, R. W. (2011). Breastfeeding and infant size: Evidence of reverse causality. American Journal of Epidemiology, 173(9), 978–983. 10.1093/aje/kwq495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn, M. Contributions from Wing, J. , Weston, S. , Williams, A. , Keefer, C. , Engelhardt, A. , Cooper, T. , Mayer, Z. , Kenkel, B. , the R Core Team , Benestym, M. , Lescarbeau, R. , Ziem, A. , Scrucca, L. , Tang, Y. , Candan, C. , and Hunt, T. (2018). Package “caret”.
- Kursa, M. B. , & Rudnicki, W. R. (2010). Feature selection with the Boruta package. Journal of Statistical Software, 36(11), 1–13. [Google Scholar]
- Liaw, A. , & Wiener, M. (2002). Classification and regression by randomForest. R News, 2(3), 18–22. [Google Scholar]
- Lumley, T. , Kronmal, R. , & Ma, S. (2006). Relative risk regression in medical research: Models, contrasts, estimators, and algorithms, 25.
- Lumley, T , & Lumley, M. T . (2018). Package ‘survey’.
- Marquis, G. S. , Habicht, J. P. , Lanata, C. F. , Black, R. E. , & Rasmussen, K. M. (1997). Association of breastfeeding and stunting in Peruvian toddlers: An example of reverse causality. International Journal of Epidemiology, 26(2), 349–356. 10.1093/ije/26.2.349 [DOI] [PubMed] [Google Scholar]
- Minot, N. , & Baulch, B. (2005). Spatial patterns of poverty in Vietnam and their implications for policy. Food Policy, 30(5), 461–475. 10.1016/j.foodpol.2005.09.002 [DOI] [Google Scholar]
- Ministry of Health, National Institute of Nutrition (2012a). General nutrition survey 2009–2010. Hanoi, Vietnam: Medical Publishing House. [Google Scholar]
- Ministry of Health, National Institute of Nutrition (2012b). National nutrition strategy for 2011–2020, with a vision towards 2030. Hanoi, Vietnam: Medical Publishing House. [Google Scholar]
- Nguyen, P. H. , Headey, D. , Frongillo, E. A. , Tran, L. M. , Rawat, R. , Ruel, M. T. , & Menon, P. (2017). Changes in underlying determinants explain rapid increases in child linear growth in alive & thrive study areas between 2010 and 2014 in Bangladesh and Vietnam. The Journal of Nutrition, jn243949 10.3945/jn.116.243949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Organization, W. H (2009). WHO AnthroPlus for personal computers manual: Software for assessing growth of the world's children and adolescents. Geneva: WHO. [Google Scholar]
- Perumal, N. , Bassani, D. G. , & Roth, D. E. (2018). Use and misuse of stunting as a measure of child health. The Journal of Nutrition, 148(3), 311–315. 10.1093/jn/nxx064 [DOI] [PubMed] [Google Scholar]
- Phat, N. T. (2012). Geographical barriers impacts on the efficiency of poverty alleviation policy in Vietnam. Journal of Research in International Business Management, 2(3), 073–076. [Google Scholar]
- Pimhidzai, O. (2018). Climbing the ladder: Poverty reduction and shared prosperity in Vietnam (No. 124916) (pp. 1–49)The World Bank; Retrieved from http://documents.worldbank.org/curated/en/206981522843253122/Climbing-the-ladder-poverty-reduction-and-shared-prosperity-in-Vietnam [Google Scholar]
- Ripley, B. , Venables, B. , Bates, D. M. , Hornik, K. , Gebhardt, A. , Firth, D. , & Maintainer Brian Ripley . (2013). Package ‘mass.’ CRAN Repos. Httpcran R‐Proj. OrgwebpackagesMASSMASS Pdf .
- Stewart, C. P. , Iannotti, L. , Dewey, K. G. , Michaelsen, K. F. , & Onyango, A. W. (2013). Contextualising complementary feeding in a broader framework for stunting prevention. Maternal & Child Nutrition, 9, 27–45. 10.1111/mcn.12088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh Thi, H. , Simioni, M. , & Thomas‐Agnan, C. (2018). Assessing the nonlinearity of the calorie‐income relationship: An estimation strategy—With new insights on nutritional transition in Vietnam. World Development, 110, 192–204. 10.1016/j.worlddev.2018.05.030 [DOI] [Google Scholar]
- UNICEF , FANTA , USAID , & WHO . (2017). Meeting report: Reconsidering, refining, and extending the World Health Organization infant and Young child feeding indicators (June 20–22, 2017). New York, NY. Retrieved from https://reliefweb.int/report/world/meeting-report-reconsidering-refining-and-extending-world-health-organization-infant
- United Nations, Department of Economic and Social Affairs . (2016). Goal 2: End hunger, achieve food security and improved nutrition and promote sustainable agriculture (Sustainable Development Knowledge Platform). Retrieved from http://sustainabledevelopment.un.org/sdg2
- Victora, C. G. , Villar, J. , Barros, F. C. , Ismail, L. C. , Chumlea, C. , Papageorghiou, A. T. , … Kennedy, S. H. (2015). Anthropometric characterization of impaired fetal growth: Risk factors for and prognosis of newborns with stunting or wasting. JAMA Pediatrics, 169(7), e151431–e151431. 10.1001/jamapediatrics.2015.1431 [DOI] [PubMed] [Google Scholar]
- Viet Nam National Institute of Nutrition, UNICEF, Alive & Thrive . (2014). Nutrition Surveillance Profiles 2013. Ha Noi, Viet Nam.
- Vietnam National Institute of Nutrition . (2018). Statistical data on child nutritional status over the years. Retrieved October 16, 2018, from http://viendinhduong.vn/vi/suy-dinh-duong-tre-em/so-lieu-thong-ke-ve-tinh-trang-dinh-duong-tre-em-qua-cac-nam-106.html
- WHO . (2006). The WHO Child Growth Standards. Retrieved March 31, 2017, from http://www.who.int/childgrowth/standards/en/
- WHO . (2012). Maternal, infant and young child nutrition. ( WHO , Ed.). Geneva, Switzerland: The sixty‐fifth world health assembly WHA65.6. [Google Scholar]
- World Health Organization . (2010). Indicators for assessing infant and young child feeding practices: part 2: measurement.
- Young, M. F. , Nguyen, P. H. , Casanova, I. G. , Addo, O. Y. , Tran, L. M. , Nguyen, S. , … Ramakrishnan, U. (2018). Role of maternal preconception nutrition on offspring growth and risk of stunting across the first 1000 days in Vietnam: A prospective cohort study. PLoS ONE, 13(8), e0203201 10.1371/journal.pone.0203201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. (2016). Variable selection with stepwise and best subset approaches. Annals of Translational Medicine, 4(7), 136–136. 10.21037/atm.2016.03.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FIGURE S1 Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 30771 Vietnamese children 6–23 m. Error bars show 95% confidence intervals. The multivariate model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin.
FIGURE S2 Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 55161 Vietnamese children 24–59 m. Error bars show 95% confidence intervals. The multivariate model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin.
FIGURE S3 Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 13505 children 6–59 m living in Southeast Vietnam. Error bars show 95% confidence intervals. The multivariate model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin.
Figure S4 Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 12068 Vietnamese children 6–59 m living in the Red River Delta. Error bars show 95% confidence intervals. The multivariate model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin.
Figure S5 Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 17160 children 6–59 m living in the Northeast Vietnam. Error bars show 95% confidence intervals. The multivariate model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin.
Figure S6 Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 5169 children 6–59 m living in the Northwest Vietnam. Error bars show 95% confidence intervals. The multivariate model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin.
Figure S7 Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 7880 Vietnamese children 6–59 m living in the Central North. Error bars show 95% confidence intervals. The multivariate model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin.
Figure S8 Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 8090 Vietnamese children 6–59 m living in the Central South. Error bars show 95% confidence intervals. The multivariate model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin.
Figure S9 Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 5464 Vietnamese children 6–59 m living in the Central Highlands. Error bars show 95% confidence intervals. The multivariate model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin.
Figure S10 Adjusted relative risks of stunting determinants at the child, maternal, household, and environmental levels among 16957 Vietnamese children 6–59 m living in the Mekong River Delta. Error bars show 95% confidence intervals. The multivariate model adjusted for all variables displayed. BMI, body mass index; CI, confidence interval; RR, relative risk; vit, vitamin.
Table S1 Bivariate relative risks of stunting determinants at the child, maternal, household, and environmental levels in Vietnamese children by age


