Abstract
Although several studies have shown a positive association between socio‐economic position and size at birth, not enough is known about the modifiable factors that may be involved. We aimed to investigate whether maternal prepregnancy body mass index (BMI), smoking, diet, and depression during pregnancy mediate the positive association between maternal education and birth size. Weight and length z‐scores specific for gestational age and sex were calculated for 1,500 children from the EDEN mother–child cohort. A mediation analysis of the associations between maternal education and birth size was conducted with a counterfactual method, adjusted for recruitment centre, parity, maternal height, and age. In the comparison of children of mothers with low versus intermediate education levels, maternal smoking during pregnancy explained 52% of the total effect of education on birth weight. Similar findings were observed with birth length z‐score (37%). The comparison of children of mothers with high versus intermediate education levels yielded a non‐significant total effect, which masked opposite mediating effects by maternal BMI and smoking during pregnancy on both birth weight and length. Prepregnancy BMI and maternal smoking during pregnancy mediate the positive association between maternal education and birth weight and length z‐scores. These mediators, however, act in opposite directions, thereby masking the extent to which healthy prenatal growth is socially differentiated.
Keywords: birth length, birth weight, BMI, maternal education, mediation analysis, smoking
Key messages.
A positive association exists between socio‐economic position and birth weight, but less research has been done on the prenatal factors involved.
Prepregnancy BMI and smoking during pregnancy were shown to be important mediators of the positive associations between maternal education and both birth weight and birth length.
Other modifiable factors like dietary patterns and depressive symptoms were not shown to mediate these associations.
These results suggest that promoting a healthy prepregnancy weight and preventing smoking during pregnancy are keys to addressing socio‐economic inequalities in healthy fetal growth.
1. INTRODUCTION
The existence of social inequalities in growth and childhood overweight in high‐income countries has been highlighted in recent years (Ballon et al., 2018; Barriuso et al., 2015; Howe et al., 2012; McCrory et al., 2017). These inequalities are observed as early as the first day of life, since positive associations have been described between socio‐economic position and both weight and length at birth (Ballon et al., 2018; Howe et al., 2012; Jansen et al., 2009; Mortensen, Helweg‐Larsen, & Andersen, 2011). It is also well known that a lower birth weight, a marker of suboptimal fetal growth, is associated with a range of short‐ and long‐term health issues, such as cardiovascular disease, diabetes, and obesity (Barker, Osmond, Forsen, Kajantie, & Eriksson, 2005; Jornayvaz et al., 2016; Shenkin, Starr, & Deary, 2004; Zanetti et al., 2018). These findings suggest intergenerational transmission of health inequalities between the mother and her offspring (Aizer & Currie, 2014; Barker, Barker, Fleming, & Lampl, 2013). The development of interventions to prevent or reduce these social inequalities in health programming requires that the modifiable factors mediating the positive association between socio‐economic position and birth weight be identified.
Some studies have shown that prepregnancy body mass index (BMI) and smoking during pregnancy mediate the association between socio‐economic position and birth weight (Gissler, Merilainen, Vuori, & Hemminki, 2003; Jansen et al., 2009; van den Berg, van Eijsden, Vrijkotte, & Gemke, 2012), but only one study has investigated the joint effects of both factors on this birth outcome (Mortensen, Diderichsen, Smith, & Andersen, 2009). To our knowledge, only one study has examined factors involved in the association between socio‐economic position and offspring length or height (Galobardes et al., 2012). The authors described social inequalities for both prenatal and postnatal growth within a single longitudinal model. However, they did not seek to identify mediators of the association between socio‐economic position and birth length specifically. Although weight and length at birth are strongly correlated, their separate associations with socio‐economic position may differ by age and sex (Ballon et al., 2018) and thus involve different mediators. Moreover, other prenatal factors remain to be studied. Indeed, healthy dietary patterns during pregnancy have been shown to be positively associated with birth weight (Chia et al., 2019; Emmett, Jones, & Northstone, 2015), whereas negative associations were observed between both unhealthy dietary patterns and maternal depression and birth weight (Chia et al., 2019; Field, 2011). One review also reported a birth length decrease in offspring of mothers with depression during pregnancy (Field, 2011). Given that social inequalities have been described for these three factors (Emmett et al., 2015; Hein et al., 2014), they can be considered as potential mediators of the relation between socio‐economic position and birth size (i.e., weight and length).
Recently, advanced methods for mediation analyses have been developed. In particular, counterfactual approaches allow for causal inference based on observational data by estimating direct and indirect effects with more power than allowed by traditional approaches (Lange, Rasmussen, & Thygesen, 2013; VanderWeele & Vansteelandt, 2014). Among the various existing approaches, the counterfactual method proposed by Lange et al. (2013) enables the simultaneous assessment of the mediating effect of several factors as well as the consideration of an exposure variable in more than two categories.
The objective of this paper was to investigate whether any of maternal prepregnancy BMI, smoking, diet, and depression during pregnancy mediate the positive association between maternal education level and offspring birth size, that is, weight and length.
2. METHODS
2.1. Study design and participants
The EDEN mother–child cohort was designed to assess the prenatal and postnatal determinants of children's growth, health, and development. This cohort includes 2,002 pregnant women recruited in two French maternity hospitals (in Poitiers and Nancy) between 2003 and 2006. Exclusion criteria were multiple pregnancies, known diabetes, illiteracy, and intention to give birth elsewhere than these two university hospitals or to move outside the region within 3 years. Due to the mode of recruitment and the selective acceptance of participation, urban and well‐educated mothers were overrepresented in the EDEN study compared with the national population (Heude et al., 2016). Details of the study protocol have been published elsewhere (Heude et al., 2016). Both parents provided written consent. The ethics committee of the Kremlin‐Bicêtre Hospital approved this study, which was also submitted to the national commission for data protection and liberties.
2.2. Measurements
Data come from obstetric and paediatric records at birth, as well as from self‐reported questionnaires completed by the mothers and clinical examinations undertaken at different stages of follow‐up.
2.2.1. Socio‐economic position
Maternal education level, commonly studied in relation to child birth size (Jansen et al., 2009; McCrory et al., 2017; Mortensen et al., 2009) and less likely to be affected by childbearing than income and occupation, was used as a proxy for socio‐economic position. Mothers were asked to self‐report their highest educational attainment at study inclusion. Education level was categorized as low (did not complete high school), intermediate (high school diploma to 2‐year university degree, reference category), and high (3‐year university degree or more). Intermediate category was chosen as the reference in order to better disentangle the mediation process when comparing one category of education level to its adjacent one.
2.2.2. Child weight and length
At birth, weight and length were measured by midwives with an electronic scale (Seca Ltd) and a somatometer (Testut). As measurement errors are common for birth length, we used predicted length at birth, obtained from growth modelling of an average of 16 measurements of length/height between birth and 5 years (Botton et al., 2014; Carles et al., 2016). For children without predicted data available, measured length was used instead (7%). Birth weight and length z‐scores specific for gestational age and sex were calculated according to Audipog references (Faculté de médecine RTH Laennec Lyon, 2008).
2.2.3. Candidate mediators
Women self‐reported their prepregnancy weight at inclusion. Maternal height was measured by research midwives with a wall stadiometer (Seca 206) during clinical examinations conducted between 24 and 28 weeks of gestation. Prepregnancy BMI was calculated as weight (kg) divided by height squared (m2) and categorized as underweight (<18.5 kg/m2), normal (≥18.5 and <25 kg/m2), overweight (≥25 and <30 kg/m2), or obese (≥30 kg/m2; WHO). During the first visit with research midwives (between 24 and 28 weeks of gestation), mothers reported their daily cigarette consumption and smoking habits at the beginning of pregnancy (smoking in the first trimester) and their smoking status at the time of the visit (smoking in the second trimester). After delivery, research midwives collected similar information for smoking at the end of the third trimester of pregnancy (third trimester smoking status). All this information was combined into one variable categorized as nonsmokers, smokers only in the first trimester, and smokers throughout pregnancy. Depressive symptoms during pregnancy were assessed with the 20‐item Center for Epidemiology Studies Depression Scale (Radloff, 1977). Each response was coded between 0 and 3 points and then summed into a depressive symptoms score (ranging from 0 to 60). Different cut‐offs, ranging from 16 to 23 (Henry, Grant, & Cropsey, 2018; Radloff, 1977; Vilagut, Forero, Barbaglia, & Alonso, 2016), have been proposed to detect individuals with probable depression. We chose the threshold of 23 to define women with depressive symptoms, as suggested by a validation study for the French population (Fuhrer & Rouillon, 1989). Maternal diet in the last trimester of pregnancy was assessed during the maternity ward stay after delivery by a validated food frequency questionnaire (Deschamps et al., 2009). The 137 items from the food frequency questionnaire have previously been synthesized by principal component analysis into two dietary patterns (Yuan et al., 2017): the so‐called Healthy dietary pattern, characterized by a high intake of fruit, vegetables, fish, and whole grains, and the Western dietary pattern, characterized by a high intake of processed and snacking foods. These variables were considered to be continuous scores reflecting adherence to each dietary pattern.
2.2.4. Other variables
Gestational age, maternal age, and parity were collected at birth from medical records. Preterm birth (yes/no) was defined by a gestational age <37 weeks of gestation.
2.3. Population studied
Of the 1,907 children included in the EDEN cohort, 407 were excluded because of missing values for any of the variables of interest, (i.e., outcomes, exposure, mediators, and confounders). The final sample thus included 1,500 children.
2.4. Statistical methods
Participants included in the analysis were compared with those who were not included. Characteristics of the study population were described at birth according to maternal education level. The bivariate statistical analyses used chi‐square tests, correlations, and analysis of variance as appropriate.
The counterfactual method developed by Lange et al. (2013), based on marginal structural models, was used to conduct the mediation analysis. This allowed us to break down the total effect of maternal education level on birth size (i.e., weight and length z‐scores) into natural direct and indirect effects through the candidate mediators. Based on this method, the total effect can be considered as the change in z‐score that would be observed if, for example, maternal education level could change (e.g., from intermediate to high). The natural direct effect can be considered the difference in birth z‐scores for a given change in education level (e.g., from intermediate to high), keeping mediators at the value they naturally take when maternal education is unchanged (e.g., at the intermediate level). The natural indirect effect is the difference in birth z‐scores when maternal education remains unmodified, but the mediators change to the value they would naturally take if maternal education were to change (e.g., from intermediate to high). The validity of this statistical method depends on whether or not it satisfies specific hypotheses. First, for a given mediator, there should be no unmeasured confounders in associations between (a) exposure and outcome, (b) mediator and outcome, (c) exposure and mediator, and (d) mediator and outcome conditional on the exposure. Second, no causal associations should exist between the mediators.
To select candidate mediators of the association between maternal education and birth outcomes, we first conducted separate mediation analyses for each of the following potential mediators, selected a priori as most likely to explain this relation: maternal prepregnancy BMI, smoking, depressive symptoms, and maternal dietary patterns during pregnancy. We checked that they were independent of each other, conditional on the exposure and the confounders in our sample, by running a multiple regression model of one mediator on the others, adjusting for maternal education and the confounders. When a statistically significant association was observed between candidate mediators, we used the residual method to obtain independent variables and verify the model's assumptions, by generating new variables as the residuals of the regression of one mediator on the others. Simple mediation analyses were then conducted with these new variables. All individually significant mediators were next included in two multivariable marginal structural models to assess how they jointly mediated the association between maternal education and each of the birth weight and length z‐scores. To obtain robust 95% confidence intervals, we used a bootstrap approach with 5,000 replications. Analyses were adjusted for centre (i.e., Nancy or Poitiers), parity, maternal height, and age.
Because analyses were conducted on the database with no missing data for any of the five mediators (Population B, Figure 1), the first sensitivity analysis used the database with no missing data for the mediators selected for the final multivariable model (Population A, Figure 1). A second sensitivity analysis excluded 84 preterm infants from the sample (Population C, Figure 1). SAS v9.3 (SAS Institute, Cary, NC, USA) was used for all but the mediation analyses, which were run under R v3.4.2 as proposed by Lange et al. (2013). Graphs were also plotted with R v3.4.2. Statistical significance was defined at P ≤ .05.
Figure 1.
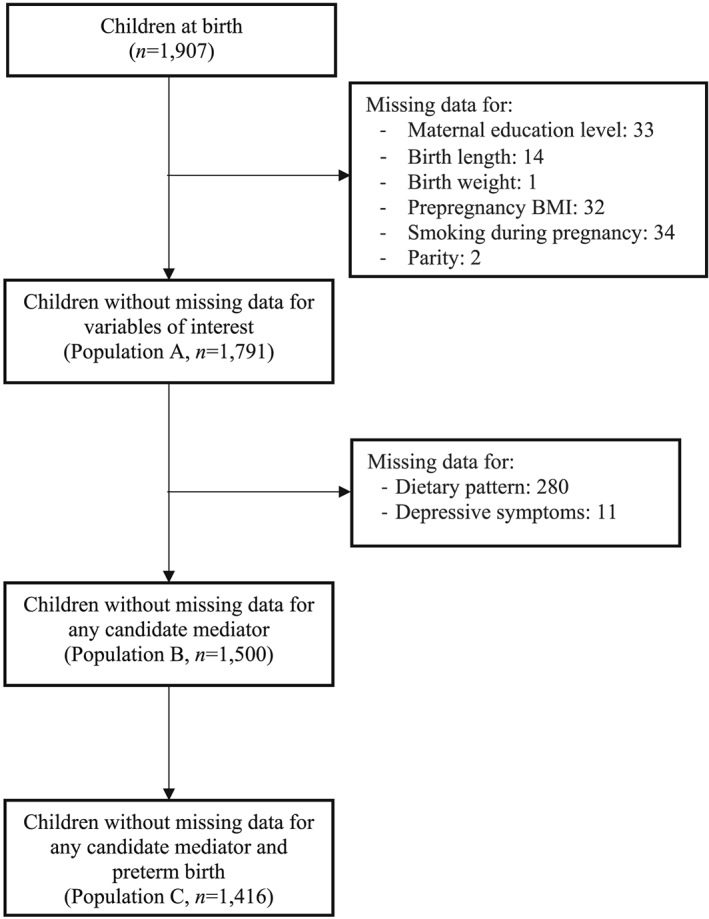
Flow chart of the population included in the study. EDEN mother–child cohort
2.5. Ethics statement
The ethics committee of the Kremlin‐Bicêtre Hospital approved this study, which was also submitted to the national commission for data protection and liberties (CNIL).
3. RESULTS
3.1. Population characteristics
Mothers excluded from the analysis (n = 407) had lower education levels and were more likely to have experienced depressive symptoms or to be underweight or obese than those who were included (low education level, 42.0% vs. 25.5%; depressive symptoms, 13.8% vs. 7.7%; underweight, 11.2% vs. 8.0%; obesity, 12.3% vs. 7.9%). No statistically significant differences were observed in birth weight and length z‐scores between those included and excluded.
There was a positive gradient between maternal education level and both the mother's age and height and the z‐scores for the child's birth weight and length (Table 1). All candidate mediators except depressive symptoms were strongly associated with both maternal education level and birth size z‐scores (Tables 1 and 2).
Table 1.
Characteristics of the population at birth
| Maternal education levela | ||||
|---|---|---|---|---|
| Low | Intermediate | High | P | |
| (n = 383) | (n = 626) | (n = 491) | ||
| n (%) or mean ± SD | ||||
| Centre, Poitiers | 218 (56.9) | 302 (48.2) | 201 (40.9) | <.0001 |
| Birth weight z‐score | −0.15 ± 1.0 | −0.03 ± 1.0 | 0.06 ± 0.9 | .0076 |
| Birth length z‐score | 0.10 ± 1.0 | 0.30 ± 0.9 | 0.41 ± 0.9 | <.0001 |
| Sex, girls | 171 (44.7) | 298 (47.6) | 232 (47.3) | .63 |
| Preterm birth, yes | 26 (6.8) | 28 (4.5) | 30 (6.1) | .25 |
| Gestational age (week) | 39.0 ± 1.9 | 39.4 ± 1.5 | 39.3 ± 1.5 | .0004 |
| Mother's height (cm) | 162.3 ± 5.8 | 163.4 ± 6.4 | 164.8 ± 5.8 | <.0001 |
| Mother's age (year) | 28.1 ± 5.7 | 29.4 ± 4.6 | 30.8 ± 4.0 | <.0001 |
| Parity, yes | 151 (39.4) | 301 (48.1) | 216 (44.0) | .03 |
| Depressive symptoms, yes | 41 (10.7) | 49 (7.8) | 25 (5.1) | .01 |
| Smoking during pregnancy | <.0001 | |||
| No | 218 (56.9) | 471 (75.2) | 416 (84.7) | |
| Only during the 1st trimester | 30 (7.8) | 56 (9.0) | 33 (6.7) | |
| During pregnancy | 135 (35.3) | 99 (15.8) | 42 (8.6) | |
| Prepregnancy BMIb | <.0001 | |||
| Underweight | 37 (9.6) | 47 (7.5) | 36 (7.4) | |
| Normal | 227 (59.3) | 396 (63.2) | 374 (76.2) | |
| Overweight | 83 (21.7) | 125 (20.0) | 57 (11.6) | |
| Obesity | 36 (9.4) | 58 (9.3) | 24 (4.8) | |
| Healthy dietary pattern | −0.2 ± 1.0 | −0.1 ± 0.9 | 0.2 ± 1.0 | <.0001 |
| Western dietary pattern | 0.4 ± 1.2 | −0.0 ± 0.9 | −0.3 ± 0.8 | <.0001 |
Note. The EDEN mother–child cohort (N = 1,500).
Low, less than high school; intermediate, high school diploma to 2‐year university degree, reference category; high, 3‐year university degree or more.
Underweight, <18.5 kg/m2; normal, ≥18.5 and <25 kg/m2; overweight, ≥25 and <30 kg/m2; obesity, ≥30 kg/m2.
Table 2.
Unadjusted associations between birth size z‐scores and candidate mediators
| Birth weight z‐score | Birth length z‐score | |||
|---|---|---|---|---|
| mean ± SD | P | mean ± SD | P | |
| Depressive symptoms | .86 | .24 | ||
| No | −0.03 ± 0.95 | 0.30 ± 0.91 | ||
| Yes | −0.02 ± 1.08 | 0.19 ± 1.03 | ||
| Smoking during pregnancy | <.0001 | <.0001 | ||
| No | 0.05 ± 0.93 | 0.39 ± 0.88 | ||
| Only during the 1st trimester | −0.06 ± 0.91 | 0.19 ± 0.87 | ||
| During pregnancy | −0.35 ± 1.01 | −0.06 ± 0.97 | ||
| Prepregnancy BMI | <.0001 | <.0001 | ||
| Underweight | −0.41 ± 0.92 | −0.01 ± 1.00 | ||
| Normal | −0.06 ± 0.93 | 0.28 ± 0.90 | ||
| Overweight | 0.09 ± 1.01 | 0.45 ± 0.95 | ||
| Obesity | 0.27 ± 0.96 | 0.41 ± 0.84 | ||
| Corra | P | Corra | P | |
| Healthy dietary pattern | 0.06 | .013 | 0.05 | .006 |
| Western dietary pattern | −0.07 | .056 | −0.11 | <.0001 |
Note. EDEN mother–child cohort (N = 1,500).
Pearson's correlation.
3.2. Simple mediation analysis
In children from low educated mothers, compared with those from mothers of intermediate education level, natural indirect effect of smoking during pregnancy was significant for birth weight and length, which suggests that smoking during pregnancy was a mediator of the association between maternal education and birth size (Table 3). Likewise, prepregnancy BMI was also a mediator of these associations, whereas the Western dietary pattern mediated the association with birth length only. Because this dietary pattern was strongly associated with prepregnancy BMI and smoking during pregnancy, we performed an additional simple mediation analysis by using the residuals of its regression on BMI, smoking during pregnancy, and confounders, as a new mediator. This new variable assessed variability in the Western dietary pattern independent of other variables and was not significant in the simple mediation analysis. Therefore, the multiple mediation analyses used only prepregnancy BMI and smoking during pregnancy.
Table 3.
Natural indirect effect (β and 95% CI) of candidate mediators of the associations between maternal education and birth z‐scores
| Birth weight z‐score | Birth length z‐score | |||
|---|---|---|---|---|
| Maternal education level | Maternal education level | |||
| Low vs. intermediate | High vs. intermediate | Low vs. intermediate | High vs. intermediate | |
| Smoking during pregnancy | −0.07 [−0.11; −0.04] | 0.03 [0.01; 0.05] | −0.08 [−0.12; −0.05] | 0.03 [0.02; 0.06] |
| Prepregnancy BMI | −0.00 [−0.03; 0.02] | −0.03 [−0.06; −0.01] | −0.00 [−0.02; 0.02] | −0.03 [−0.05; −0.01] |
| Depressive symptoms | 0.00 [−0.01; 0.01] | −0.00 [−0.01; 0.01] | −0.00 [−0.01; 0.00] | 0.00 [−0.00; 0.01] |
| Healthy dietary pattern | −0.00 [−0.01; 0.00] | 0.01 [−0.01; 0.02] | 0.00 [−0.00; 0.01] | 0.00 [−0.01; 0.01] |
| Western dietary pattern | −0.02 [−0.04; 0.00] | 0.01 [−0.00; 0.02] | −0.02 [−0.05; −0.01] | 0.01 [0.00; 0.02] |
| Western dietary pattern residualsa | — | — | −0.01 [−0.02; 0.00] | 0.00 [−0.00; 0.01] |
Note. Adjustment for centre, mother's height, parity, and mother's age at delivery.
Residuals of the regression of the Western dietary pattern on BMI, smoking during pregnancy, and confounders.
3.3. Multiple mediation analyses of birth weight and length
The multiple mediation analysis of birth weight showed that the total effect of maternal education level on birth weight in children of mothers with a low, compared with an intermediate, education level was negative, with a −0.14 difference in z‐scores (Figure 2). The natural indirect effect through smoking was also negative, with a −0.07 difference. There was no significant natural direct or indirect effect through prepregnancy BMI. Smoking during pregnancy mediated 52% of this relation. Similar findings were observed with birth length, although the percentage of mediation was lower (37%; Figure 2).
Figure 2.
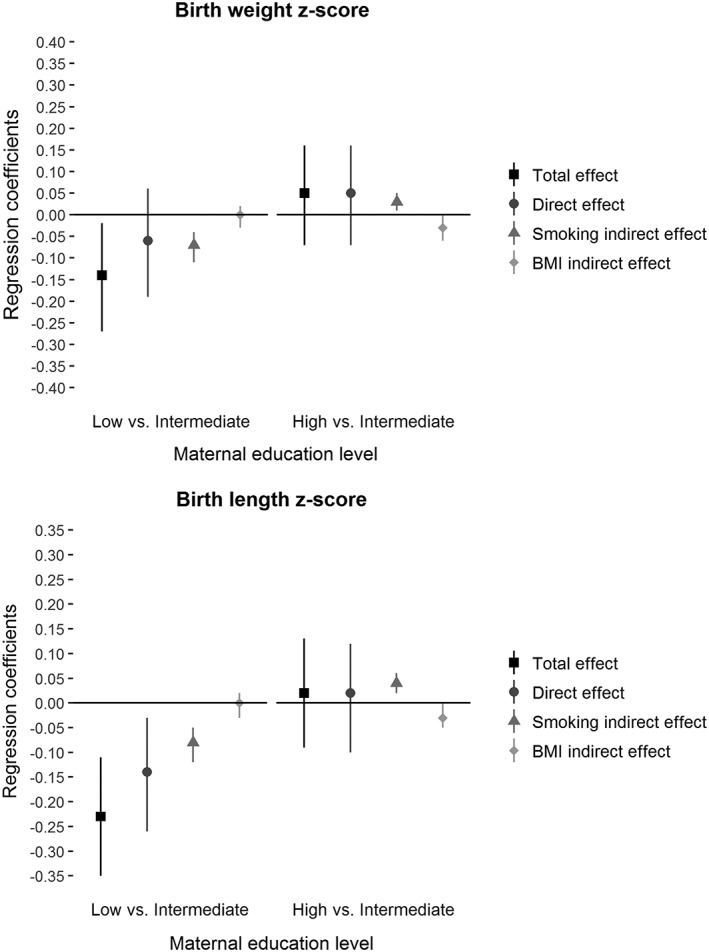
Total, direct, and mediated effects (β and 95% CI) for association between maternal education level and birth weight and length z‐scores, mediated by smoking during pregnancy and prepregnancy BMI, adjusted for centre, mother's height, parity, and mother's age at delivery
The comparison of children from mothers with high and with intermediate education levels showed no total or direct effect of maternal education level on birth weight or length. There were, however, significant natural indirect effects through smoking and BMI, and they worked in opposite directions for both birth weight and length (0.03 difference in z‐score: positive and negative, respectively); this difference explains the absence of a total effect.
3.4. Sensitivity analyses
Results were on the whole consistent when analyses were performed for the complete database (Population B) for all mediators or repeated for the database with no missing items on smoking and BMI only (Population A, results not shown but available on request). Results remained consistent after the exclusion of children born preterm.
4. DISCUSSION
This study, using a validated method to assess multiple mediation, provides new and comprehensive insights into modifiable prenatal mediators of the social gradient in birth size.
4.1. Mediators of birth weight and length
When using the residuals, the mediating effect of the Western dietary pattern in the association between maternal education and birth length disappeared, suggesting that there was no mediation of dietary pattern not attributable to BMI and/or smoking on birth size. Moreover, the Healthy dietary patterns and depressive symptoms during pregnancy did not explain the association between maternal education level and birth size in this study. To our knowledge, no others have investigated these two candidate mediators, although this apparent lack of literature may reflect publication bias. Moreover, dietary patterns and depressive symptoms may be subject to stronger measurement errors than prepregnancy BMI and smoking, which might have reduced statistical power for these two candidate mediators. Indeed, Center for Epidemiology Studies Depression Scale measure is a screening tool rather than a diagnostic one, and dietary patterns were assessed retrospectively focusing on dietary intake during the last trimester of pregnancy, which could have led to a recall bias. Further research is needed to confirm our findings. Further research is needed to confirm our findings. Perhaps stress during pregnancy, rather than depression, and energy intake, rather than diet during pregnancy, explain the association between maternal education and birth size.
Consistent with other studies (Jansen et al., 2009; Mortensen et al., 2009; van den Berg et al., 2012), smoking during pregnancy mediated about 52% of the association between maternal education and birth weight in the comparison of children of mothers with low, compared with intermediate, education levels. The pattern of mediation was different when we compared high versus intermediate education levels: prepregnancy BMI and smoking during pregnancy mediated the association of interest, but their indirect effects were in opposite directions and thus cancelled each other out. Mortensen et al. also found that prepregnancy BMI and smoking during pregnancy mediated in opposite directions across the entire gradient (and did not differ whether low or high education levels were compared with the intermediate level; Mortensen et al., 2009). Consistent findings for mediation observed with birth length are noteworthy in our study, which adds a novel and more comprehensive perspective into the social patterning of birth size.
The method proposed by Lange et al. (2013) enabled us to investigate maternal education in three categories and observe differential effects in these three groups. However, the lack of any mediating effect by prepregnancy BMI between the groups with low and intermediate maternal education must be confirmed in cohorts with greater social variability. The women in these two subgroups of the Eden cohort were very similar in terms of prepregnancy BMI.
The fact that prepregnancy BMI and smoking during pregnancy cancelled each other out should not be taken lightly. It means that a baby born to an overweight mother who smokes has the same birth weight and length as another whose non‐smoking mother has a normal weight. In the first case, birth size would be the result of growth restriction due to exposure to smoking, masked by excess growth due to maternal obesity (6% of the EDEN women combined these two characteristics) and misleadingly suggests that their children's growth is optimal, when it is not. For any given birth size, the distinct causes producing them are expected to affect later growth, development, and health differentially. Future studies, investigating metabolic biomarkers in cord blood, might provide evidence supporting (or contradicting) this hypothesis.
4.2. Potential explanation of mechanisms involved
Smoking during pregnancy may affect birth size through different mechanisms. It could lead to vasoconstriction and to higher maternal and fetal bloods levels of carboxyhaemoglobin (Wickstrom, 2007), which are responsible for fetal hypoxia. Exposure to smoking during pregnancy is also suspected of modifying regulation of fetal gene expression, by altering DNA methylation and microRNA expression (Knopik, Maccani, Francazio, & McGeary, 2012). Maternal overweight or obesity is associated with higher birth size of her offspring (Gaudet, Ferraro, Wen, & Walker, 2014). Mothers with higher BMI have higher levels of circulating blood glucose (Harmon et al., 2011) and lipids, so that more of these nutrients are available for the fetus. Increased insulin secretion by the fetal pancreas in response to glucose in turn accelerates fetal growth (Hapo Study Cooperative Research Group, 2009).
It is noteworthy that prepregnancy BMI and smoking during pregnancy are modifiable factors. As two reviews have shown, effective strategies already exist to reduce maternal BMI and smoking (Johnson et al., 2016; Lancaster, Stead, Silagy, & Sowden, 2000), and the consistency of our findings across birth weight and length suggests that promoting healthy weight or preventing smoking during pregnancy in future mothers is likely to favourably and simultaneously affect the offspring's birth weight and length.
4.3. Limitations and strengths
Study limitations include our inability to consider all potential mediators; thus, we did not fully explain the relation between maternal education and birth size. For example, we did not take health care utilization into account, although it is hypothesized to improve fetal monitoring and help prevent fetal growth anomalies. Mothers from the EDEN cohort were, however, very homogeneous in this respect given the mode of inclusion at the maternity ward before 24 weeks of gestation. Maternal stress during pregnancy and passive smoking (due to paternal smoking), unmeasured in the current study, would be other relevant mediators to explore. Classification of prepregnancy BMI relies on self‐reported weight. Although women are likely to underestimate their prepregnancy weight, and more importantly when overweighed or obese (Bannon et al., 2017), previous studies have suggested that classification of prepregnancy BMI remains usually unchanged (Bannon et al., 2017; Holland, Moore Simas, Doyle Curiale, Liao, & Waring, 2013). Moreover, validity of prepregnancy weight was previously validated within the EDEN study using multiple pregnancy weights collected all over pregnancy (Diouf et al., 2014). The presence of selection bias at inclusion, as is often the case in cohort studies, has implications for the generalization of our findings. We can hypothesize that a better representation of disadvantaged families at baseline would have provided more variability and therefore more power to address the study objectives. Further research is needed to confirm our findings on more datasets with greater social variability. The method we used to conduct mediation analyses is validated and relevant. To our knowledge, no dedicated codes to conduct multiple imputation have however been developed to address missing data. Although there were about 20% missing data in the principal analysis, sensitivity analysis on Population B, which includes only 6% missing data, led to the same results. Furthermore, the Lange method relies on several assumptions. Residual confounding not taken into account cannot be ruled out; prepregnancy BMI and smoking during pregnancy might be proxies for other factors related to unhealthy lifestyles. Our data nonetheless met the principal assumption necessary for using this method: maternal smoking and prepregnancy BMI were conditionally independent of maternal education level. Examining the stability of estimates across the one‐by‐one mediation analysis, multiple mediation, and sensitivity analyses shows that the results appear robust. Moreover, a clear strength of our study is that data are from a prospective birth cohort and can thus identify mediators of the longitudinal association between maternal education level and birth parameters. To our knowledge, this study, as the first to investigate birth length, provides new insights into the existing literature. Finally, unlike most mediation analyses, we investigated maternal education level in three categories to examine the social gradient in birth size in more detail.
5. CONCLUSION
Among the modifiable factors examined, dietary patterns and depressive symptoms did not mediate the positive association between maternal education and birth weight and length, whereas prepregnancy BMI and maternal smoking during pregnancy did so. The latter two factors, however, act in opposite directions and thus mask the extent to which prenatal growth is socially differentiated. Although these original findings need to be replicated in more socially diverse samples, they suggest that promoting a healthy prepregnancy weight and preventing smoking during pregnancy are keys to addressing socio‐economic inequalities in healthy fetal growth and thereby attenuating the intergenerational transmission of socio‐economic health inequalities.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
MB, SL, BH, and JB conceived and designed the work, with advice from MAC. MB analysed the data with advice from BH, JB, and SL. MB, BH, and SL drafted and revised the manuscript. All authors interpreted the data and criticized the manuscript for important intellectual content. MAC and BH designed and led the EDEN mother–child cohort. AF is responsible for the EDEN data management. All authors have read and approved the final version of the manuscript. This article is the work of the authors. MB serves as guarantor for the contents of this article. All authors had full access to all of the data (including statistical reports and tables) in the study and take the responsibility for the integrity of the data and the accuracy of the data analysis. All researchers are independent of the funding bodies. All members in the EDEN mother–child cohort study group designed the study.
Abbreviation
- BMI
body mass index
ACKNOWLEDGMENTS
We are extremely grateful to all the families who took part in this study; the midwives and psychologists who recruited and followed them; the whole EDEN team, including research scientists, engineers, technicians, and managers; and especially Josiane Sahuquillo and Edith Lesieux for their commitment and their role in the success of the study. We also acknowledge the commitment of the members of the EDEN Mother–Child Cohort Study Group: I. Annesi‐Maesano, J.Y. Bernard, J. Botton, M.A. Charles, P. Dargent‐Molina, B. de Lauzon‐Guillain, P. Ducimetière, M. de Agostini, B. Foliguet, A. Forhan, X. Fritel, A. Germa, V. Goua, R. Hankard, B. Heude, M. Kaminski, B. Larroque, N. Lelong, J. Lepeule, G. Magnin, L. Marchand, C. Nabet, F Pierre, R. Slama, M.J. Saurel‐Cubizolles, M. Schweitzer, and O. Thiebaugeorges. We thank Jo Ann Cahn for her help in preparing the manuscript. This work was conducted within the framework of the LIFE‐CYCLE project, which has received funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 733206. This publication reflects only the author's views and the European Commission is not liable for any use that may be made of the information contained therein.
Ballon M, Botton J, Forhan A, et al. Which modifiable prenatal factors mediate the relation between socio‐economic position and a child's weight and length at birth? Matern Child Nutr. 2019;15:e12878 10.1111/mcn.12878
REFERENCES
- Aizer, A. , & Currie, J. (2014). The intergenerational transmission of inequality: maternal disadvantage and health at birth. Science, 344(6186), 856–861. 10.1126/science.1251872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballon, M. , Botton, J. , Charles, M. A. , Carles, S. , de Lauzon‐Guillain, B. , Forhan, A. , … on behalf of the EDEN Mother–Child Cohort Study Group (2018). Socioeconomic inequalities in weight, height and body mass index from birth to 5 years. International Journal of Obesity, 42(9), 1671–1679. 10.1038/s41366-018-0180-4 [DOI] [PubMed] [Google Scholar]
- Bannon, A. L. , Waring, M. E. , Leung, K. , Masiero, J. V. , Stone, J. M. , Scannell, E. C. , & Moore Simas, T. A. (2017). Comparison of Self‐reported and measured pre‐pregnancy weight: Implications for gestational weight gain counseling. Maternal and Child Health Journal, 21(7), 1469–1478. 10.1007/s10995-017-2266-3 [DOI] [PubMed] [Google Scholar]
- Barker, D. , Barker, M. , Fleming, T. , & Lampl, M. (2013). Developmental biology: Support mothers to secure future public health. Nature, 504(7479), 209–211. 10.1038/504209a [DOI] [PubMed] [Google Scholar]
- Barker, D. J. , Osmond, C. , Forsen, T. J. , Kajantie, E. , & Eriksson, J. G. (2005). Trajectories of growth among children who have coronary events as adults. The New England Journal of Medicine, 353(17), 1802–1809. 10.1056/NEJMoa044160 [DOI] [PubMed] [Google Scholar]
- Barriuso, L. , Miqueleiz, E. , Albaladejo, R. , Villanueva, R. , Santos, J. M. , & Regidor, E. (2015). Socioeconomic position and childhood‐adolescent weight status in rich countries: a systematic review, 1990‐2013. BMC Pediatrics, 15, 129 10.1186/s12887-015-0443-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Berg, G. , van Eijsden, M. , Vrijkotte, T. G. , & Gemke, R. J. (2012). Educational inequalities in perinatal outcomes: the mediating effect of smoking and environmental tobacco exposure. PLoS ONE, 7(5), e37002 10.1371/journal.pone.0037002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botton, J. , Scherdel, P. , Regnault, N. , Heude, B. , Charles, M. A. , & Group, E. M.‐C. C. S (2014). Postnatal weight and height growth modeling and prediction of body mass index as a function of time for the study of growth determinants. Annals of Nutrition & Metabolism, 65(2–3), 156–166. 10.1159/000362203 [DOI] [PubMed] [Google Scholar]
- Carles, S. , Charles, M. A. , Forhan, A. , Slama, R. , Heude, B. , Botton, J. , & group, E. m. c. s (2016). A novel method to describe early offspring body mass index (bmi) trajectories and to study its determinants. PLoS ONE, 11(6), e0157766 10.1371/journal.pone.0157766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia, A. R. , Chen, L. W. , Lai, J. S. , Wong, C. H. , Neelakantan, N. , van Dam, R. M. , & Chong, M. F. (2019). Maternal dietary patterns and birth outcomes: A systematic review and meta‐analysis. Adv Nutr., 10, 685–695. 10.1093/advances/nmy123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschamps, V. , de Lauzon‐Guillain, B. , Lafay, L. , Borys, J. M. , Charles, M. A. , & Romon, M. (2009). Reproducibility and relative validity of a food‐frequency questionnaire among French adults and adolescents. European Journal of Clinical Nutrition, 63(2), 282–291. 10.1038/sj.ejcn.1602914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diouf, I. , Botton, J. , Charles, M. A. , Morel, O. , Forhan, A. , Kaminski, M. , … The EDEN Study Group (2014). Specific role of maternal weight change in the first trimester of pregnancy on birth size. Maternal & Child Nutrition, 10(3), 315–326. 10.1111/j.1740-8709.2012.00423.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett, P. M. , Jones, L. R. , & Northstone, K. (2015). Dietary patterns in the Avon longitudinal study of parents and children. Nutrition Reviews, 73(Suppl 3), 207–230. 10.1093/nutrit/nuv055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faculté de médecine RTH Laennec Lyon . (2008). AUDIPOG.
- Field, T. (2011). Prenatal depression effects on early development: A review. Infant Behavior & Development, 34(1), 1–14. 10.1016/j.infbeh.2010.09.008 [DOI] [PubMed] [Google Scholar]
- Fuhrer, R. , & Rouillon, F. (1989). La version française de l'échelle CES‐D (Center for Epidemiologic Studies‐Depression Scale). Description et traduction de l'échelle d'auto‐évaluaion. Psychiatry Psychobiol, 4, 163–166. [Google Scholar]
- Galobardes, B. , McCormack, V. A. , McCarron, P. , Howe, L. D. , Lynch, J. , Lawlor, D. A. , & Smith, G. D. (2012). Social inequalities in height: Persisting differences today depend upon height of the parents. PLoS ONE, 7(1), e29118 10.1371/journal.pone.0029118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudet, L. , Ferraro, Z. M. , Wen, S. W. , & Walker, M. (2014). Maternal obesity and occurrence of fetal macrosomia: A systematic review and meta‐analysis. BioMed Research International, 2014, 640291–22. 10.1155/2014/640291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gissler, M. , Merilainen, J. , Vuori, E. , & Hemminki, E. (2003). Register based monitoring shows decreasing socioeconomic differences in Finnish perinatal health. Journal of Epidemiology and Community Health, 57(6), 433–439. 10.1136/jech.57.6.433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hapo Study Cooperative Research Group (2009). Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study: Associations with neonatal anthropometrics. Diabetes, 58(2), 453–459. 10.2337/db08-1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon, K. A. , Gerard, L. , Jensen, D. R. , Kealey, E. H. , Hernandez, T. L. , Reece, M. S. , … Bessesen, D. H. (2011). Continuous glucose profiles in obese and normal‐weight pregnant women on a controlled diet: Metabolic determinants of fetal growth. Diabetes Care, 34(10), 2198–2204. 10.2337/dc11-0723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein, A. , Rauh, C. , Engel, A. , Haberle, L. , Dammer, U. , Voigt, F. , … Goecke, T. W. (2014). Socioeconomic status and depression during and after pregnancy in the Franconian Maternal Health Evaluation Studies (FRAMES). Archives of Gynecology and Obstetrics, 289(4), 755–763. 10.1007/s00404-013-3046-y [DOI] [PubMed] [Google Scholar]
- Henry, S. K. , Grant, M. M. , & Cropsey, K. L. (2018). Determining the optimal clinical cutoff on the CES‐D for depression in a community corrections sample. Journal of Affective Disorders, 234, 270–275. 10.1016/j.jad.2018.02.071 [DOI] [PubMed] [Google Scholar]
- Heude, B. , Forhan, A. , Slama, R. , Douhaud, L. , Bedel, S. , Saurel‐Cubizolles, M. J. , … group, E. m.‐c. c. s (2016). Cohort Profile: The EDEN mother‐child cohort on the prenatal and early postnatal determinants of child health and development. International Journal of Epidemiology, 45(2), 353–363. 10.1093/ije/dyv151 [DOI] [PubMed] [Google Scholar]
- Holland, E. , Moore Simas, T. A. , Doyle Curiale, D. K. , Liao, X. , & Waring, M. E. (2013). Self‐reported pre‐pregnancy weight versus weight measured at first prenatal visit: Effects on categorization of pre‐pregnancy body mass index. Maternal and Child Health Journal, 17(10), 1872–1878. 10.1007/s10995-012-1210-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe, L. D. , Tilling, K. , Galobardes, B. , Smith, G. D. , Gunnell, D. , & Lawlor, D. A. (2012). Socioeconomic differences in childhood growth trajectories: At what age do height inequalities emerge? Journal of Epidemiology and Community Health, 66(2), 143–148. 10.1136/jech.2010.113068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen, P. W. , Tiemeier, H. , Looman, C. W. , Jaddoe, V. W. , Hofman, A. , Moll, H. A. , … Raat, H. (2009). Explaining educational inequalities in birthweight: The Generation R Study. Paediatric and Perinatal Epidemiology, 23(3), 216–228. 10.1111/j.1365-3016.2009.01023.x [DOI] [PubMed] [Google Scholar]
- Johnson, M. , Backman, D. , Kohatsu, N. , Stewart, O. , Abbott, R. , Yu, Z. , & Lee, P. (2016). Interventions for reducing body mass index and other weight‐related indicators: A review of systematic reviews. Institute for population health improvement, 1–10. [Google Scholar]
- Jornayvaz, F. R. , Vollenweider, P. , Bochud, M. , Mooser, V. , Waeber, G. , & Marques‐Vidal, P. (2016). Low birth weight leads to obesity, diabetes and increased leptin levels in adults: The CoLaus study. Cardiovascular Diabetology, 15, 73 10.1186/s12933-016-0389-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopik, V. S. , Maccani, M. A. , Francazio, S. , & McGeary, J. E. (2012). The epigenetics of maternal cigarette smoking during pregnancy and effects on child development. Development and Psychopathology, 24(4), 1377–1390. 10.1017/S0954579412000776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster, T. , Stead, L. , Silagy, C. , & Sowden, A. (2000). Effectiveness of interventions to help people stop smoking: Findings from the Cochrane Library. BMJ, 321(7257), 355–358. 10.1136/bmj.321.7257.355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange, T. , Rasmussen, M. , & Thygesen, L. C. (2013). Assessing natural direct and indirect effects through multiple pathways. American Journal of Epidemiology, 179(4), 513–518. 10.1093/aje/kwt270 [DOI] [PubMed] [Google Scholar]
- McCrory, C. , O'Leary, N. , Fraga, S. , Ribeiro, A. I. , Barros, H. , Kartiosuo, N. , … Lifepath, C. (2017). Socioeconomic differences in children's growth trajectories from infancy to early adulthood: Evidence from four European countries. Journal of Epidemiology and Community Health, 71(10), 981–989. 10.1136/jech-2016-208556 [DOI] [PubMed] [Google Scholar]
- Mortensen, L. H. , Diderichsen, F. , Smith, G. D. , & Andersen, A. M. (2009). The social gradient in birthweight at term: Quantification of the mediating role of maternal smoking and body mass index. Human Reproduction, 24(10), 2629–2635. 10.1093/humrep/dep211 [DOI] [PubMed] [Google Scholar]
- Mortensen, L. H. , Helweg‐Larsen, K. , & Andersen, A. M. (2011). Socioeconomic differences in perinatal health and disease. Scandinavian Journal of Public Health, 39(7 Suppl), 110–114. 10.1177/1403494811405096 [DOI] [PubMed] [Google Scholar]
- Radloff, L. S. (1977). The CES‐D scale: A self‐report depression scale for research in the general population. Applied Psychological Measurement, 1, 385–401. 10.1177/014662167700100306 [DOI] [Google Scholar]
- Shenkin, S. D. , Starr, J. M. , & Deary, I. J. (2004). Birth weight and cognitive ability in childhood: A systematic review. Psychological Bulletin, 130(6), 989–1013. 10.1037/0033-2909.130.6.989 [DOI] [PubMed] [Google Scholar]
- VanderWeele, T. J. , & Vansteelandt, S. (2014). Mediation analysis with multiple mediators. Epidemiological Methods, 2(1), 95–115. 10.1515/em-2012-0010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilagut, G. , Forero, C. G. , Barbaglia, G. , & Alonso, J. (2016). Screening for depression in the general population with the Center for Epidemiologic Studies Depression (CES‐D): A systematic review with meta‐analysis. PLoS ONE, 11(5), e0155431 10.1371/journal.pone.0155431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . Body mass index. http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi.
- Wickstrom, R. (2007). Effects of nicotine during pregnancy: Human and experimental evidence. Current Neuropharmacology, 5(3), 213–222. 10.2174/157015907781695955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan, W. L. , Nicklaus, S. , Lioret, S. , Lange, C. , Forhan, A. , Heude, B. , … de Lauzon‐Guillain, B. (2017). Early factors related to carbohydrate and fat intake at 8 and 12 months: Results from the EDEN mother‐child cohort. European Journal of Clinical Nutrition, 71(2), 219–226. 10.1038/ejcn.2016.216 [DOI] [PubMed] [Google Scholar]
- Zanetti, D. , Tikkanen, E. , Gustafsson, S. , Priest, J. R. , Burgess, S. , & Ingelsson, E. (2018). Birthweight, type 2 diabetes mellitus, and cardiovascular disease: Addressing the Barker hypothesis with mendelian randomization. Circ Genom Precis Med, 11(6), e002054 10.1161/circgen.117.002054 [DOI] [PMC free article] [PubMed] [Google Scholar]


