Abstract
Hypovitaminosis D during pregnancy is suggested to have a link with complications in both mother and infant. We aimed to evaluate the efficacy of two doses of vitamin D3 supplementation during pregnancy on maternal and cord blood vitamin D status, inflammatory biomarkers, and maternal and neonatal outcomes. A total of 84 pregnant women (gestational age of <12 weeks) were randomly allocated to one of two groups: (a) 1,000‐IU/d vitamin D and (b) 2,000 IU/d. Biochemical assessments (25‐hydroxycalciferol (25(OH)D), hs‐CRP, and cell‐culture supernatant concentrations of IL‐1β, IL‐6, and TNF‐α) of mothers were performed at the beginning and 34 weeks of gestation. Assessments of infants at delivery comprised cord blood serum concentrations of 25(OH)D, hs‐CRP, IL‐1β, IL‐6, TNF‐α, birth sizes, and Apgar score. Circulating concentrations of 25(OH)D increased in both intervention groups with more increment in 2,000 IU/d than in 1,000 IU/d (46.7 ± 30.7 vs. 24.0 ± 21.07 nmol L−1, P = .001). Concentrations of TNF‐α decreased significantly in group 2,000 (−913.1 ± 1261.3 ng L−1, P = .01). The cord blood concentration of IL‐6 in group 2,000 IU/d, compared with 1,000 IU/d, was significantly lower (25.9 ± 32.0 vs. 4.6 ± 1.4 ng L−1, P = .03). The birth sizes including weight, length, and head circumference of the infants of group 2,000 IU/d were significantly higher than the infants' of group 1,000 IU/d. Supplementation with 2,000‐IU/d vitamin D3 is more effective than 1,000 IU/d in pregnant women in terms of increasing circulating 25(OH)D, ameliorating pro‐inflammatory markers notably TNF‐α in mother and IL‐6 in cord blood, and improving neonatal outcomes including the birth sizes.
Keywords: micronutrient malnutrition, neonate, policy, pregnancy and nutrition, public health, vitamin D
Abbreviations
- 25 (OH) D
25‐hydroxycalciferol
- CBMCs
Cord blood mononuclear cells
- CV
Coefficient of variation
- Cr
Creatinine
- GDM
Gestational diabetes mellitus
- HPLC
High‐‐performance liquid chromatography
- hs‐CRP
High sensitivity C‐reactive protein
- IL‐1β
Interleukin 1 beta
- IL‐6
Interleukin 6
- IADPSG
International Association of the Diabetes and Pregnancy Study Groups
- IFN‐γ
Interferon gamma
- IOM
Institute of Medicine
- LBW
Low birth weight
- LMP
Last menstrual period
- MS
Multiple sclerosis
- PBMC
Peripheral blood mononuclear cells
- SD
Standard deviation
- SEM
Standard error of mean
- SGA
Small for gestational age
- TNF‐α
Tumour necrosis factor alpha
Key Messages.
Undesirable vitamin D status in pregnant women is prevalent and may adversely affect both infant and maternal outcomes.
Different doses of vitamin D supplements, including 1000 and 2000 IU/d, have been suggested during pregnancy.
We demonstrated that supplementation with 2000 IU/d vitamin D3 from the first trimester of pregnancy is more effective than 1000 IU/d in terms of increasing circulating 25(OH)D, ameliorating pro‐inflammatory markers notably TNF‐α in mother and IL‐6 in cord blood and improving neonatal outcomes including the birth sizes.
1. INTRODUCTION
Poor vitamin D status in pregnant women reported from many countries has been associated with inauspicious outcomes in mother, her newborn, and the birth itself (Palacios & González, 2014). Some of these are gestational diabetes mellitus and preeclampsia in mother (Abedi, Mohaghegh, Afshary, & Latifi, 2014), small for gestational age (SGA), low birth weight, and respiratory problems like wheezing and asthma in child (Newhook et al., 2009). Of the adverse birth outcomes associated with vitamin D deficiency (VDD), preterm delivery, abortion, and caesarean section can be mentioned (Agarwal, Kovilam, & Agrawal, 2018; Wei, 2014).
High prevalence of VDD in pregnant women reported from Iran (~60–80%; Abbasian et al., 2016; Pirdehghan, Vakili, Dehghan, & Zare, 2016), and even in sunny regions (Cashman et al., 2016; Kelishadi et al., 2013; Olmos‐Ortiz, Avila, Durand‐Carbajal, & Diaz, 2015; Schneuer et al., 2014) was alarming for the policy makers in the Iran Ministry of Health. As a result, supplementation with 1,000 IU/d of vitamin D3 from the second trimester of pregnancy was recommended to all hospitals and maternal care centres in Iran. This is somehow in accord with the opinion of the American College of Obstetricians and Gynaecologists, who has considered 1,000–2,000 IU/d during pregnancy safe (https://www.acog.org/Clinical‐Guidance‐and‐Publications/Committee‐Opinions/Committee‐on‐Obstetric‐Practice/Vitamin‐D‐Screening‐and‐Supplementation‐During‐Pregnancy, 2011). World Health Organization, on the other hand, has recommended only 200 IU/d just for those pregnant women diagnosed as vitamin D deficient (WHO, 2012). The effectiveness of these dosages of vitamin D3 supplementation in Iranian pregnant women has not been documented yet. In most efficacy trials, changes of circulating 25(OH)D, pregnancy, and birth outcomes have been considered as the main outcomes (Curtis, Moon, Harvey, & Cooper, 2018; Roth et al., 2017). However, vitamin D can affect several health aspects including metabolism, inflammatory response, oxidative stress, and immunity (Hollis & Wagner, 2017a). To make a better evaluation of the possible effects of vitamin D supplementation during pregnancy, a clinical trial was designed to assess a wide spectrum of maternal, birth, and neonatal outcomes to compare two doses of vitamin D3 supplementation. In this study, the efficacy of two doses of vitamin D3 supplementation (1,000 vs. 2,000 IU/d) on vitamin D status, some selected inflammatory biomarkers in both mother and her infant as well as maternal and neonatal outcomes was evaluated.
2. SUBJECTS AND METHODS
2.1. Study design
The study protocol has been comprehensively described elsewhere (Motamed, Nikooyeh, & Neyestani, 2018). This was an open‐label randomized clinical trial performed among pregnant women attending the outpatient obstetric clinics of three hospitals in Tehran between February 2017 and January 2018. It was calculated that at least 37 subjects in each group would have 90% power with assuming an effect size of 0.75. Figure 1 shows the study protocol at a glance.
Figure 1.
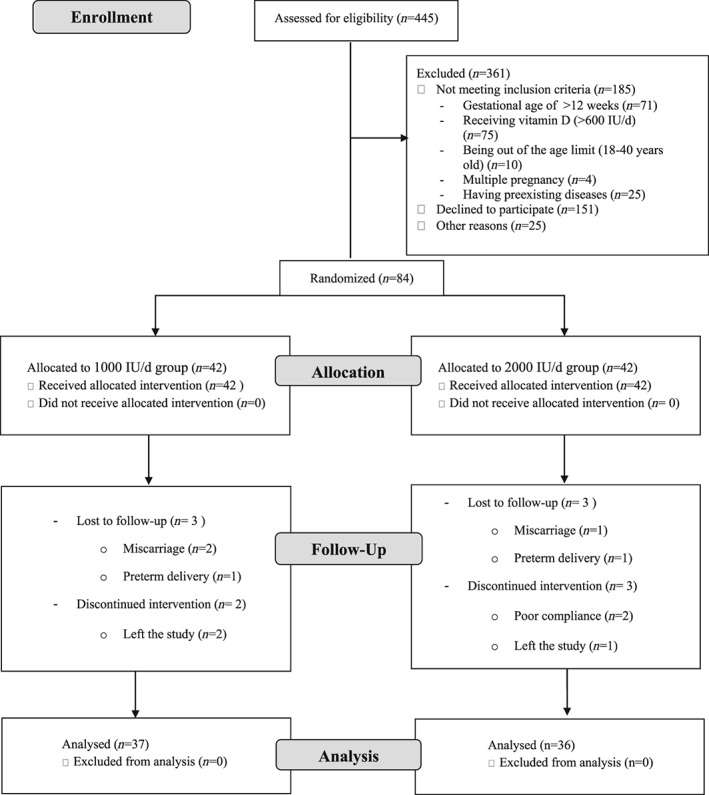
Flow diagram of the study
During the recruitment process, pregnant women referred to the obstetric clinics for prenatal care during the first trimester of gestation were evaluated for eligibility. All eligible subjects were then invited to the study, but from those who were willing to participate, written informed consents were obtained. The participants were then randomly allocated to one of the two vitamin D3 supplementation groups: (a) 1,000 IU/d and (b) 2,000 IU/d. In order to do this, six blocked sizes of four were used to generate 21 randomized block allocations.
The vitamin D3 tablets were manufactured by Jalinous pharmaceutical company, Tehran, Iran.
The inclusion criteria were gestational age of ≤12 weeks, age of 18–40 years, singleton pregnancy, having no clinical disease at the time of enrollment including diabetes (type1 and 2), liver, cardiac, renal, autoimmune, parathyroid, and thyroid disorders, receiving no vitamin D (>600 IU/d), omega‐3 supplements, steroids, and anticoagulants since 3 months prior to the intervention and willingness to participate in the study. The exclusion criteria were the absence of inclusion criteria, having fasting blood sugar >92 at first blood sampling, blood pressure > 140/90 mmHg at the first visit, foetal anomaly, and poor adherence to the study protocol.
All participants were visited at the first trimester and 34–36 weeks of gestation. Demographic and sun exposure behaviour data were collected at the first visit using face to face interview. Physical activity levels were assessed by using Iranian version of international physical activity questionnaire (Vasheghani‐Farahani et al., 2011) at first and last visit. A 24 hr, dietary recall questionnaire was completed for all participants in two non‐consecutive days of a week to assess dietary intakes. To assess energy and nutrient intakes, Nutritionist IV (First Databank, San Bruno, CA, USA) modified for Iranian foods was used. The adherence to the study protocol was assessed using the following equation as described elsewhere (Motamed et al., 2018):(Number of pills dispensed − number of pills remained)/(prescribed number of pills per day × number of days between two visits.
The protocol of this study was approved by the Ethics Committee of Shahid Beheshti University of Medical Sciences. This trial is registered at http://clinicaltrials.gov (NCT03308487).
2.2. Primary and secondary outcomes
The primary outcome was serum concentration of 25(OH)D, and the secondary outcomes were maternal and cord blood inflammatory biomarkers (IL‐1β, IL‐6, TNF‐α, and hs‐CRP) as well as neonatal outcomes including newborn weight, height, head circumference, and Apgar score and pregnancy outcomes including preeclampsia, gestational diabetes, preterm birth, SGA, and caesarean section miscarriage.
2.3. Anthropometric measurements
For pregnant women, weight was measured with light clothing and no shoes using a digital scale to the nearest of 0.1 kg. A stadiometer was applied to measure height of the participants to the nearest of 0.1 cm. To calculate body mass index, weight (kg) was divided by square of height (m).
For infants, birth weight (g), length (cm), and head circumferences (cm) were measured using standard methods.
2.4. Laboratory investigations
2.4.1. Blood and urine sampling and handling
After 12–14 hr of fasting, 10 ml of blood sample taken from all pregnant women was divided in two tubes, one heparinized and the other without anticoagulant. The heparinized blood was used for cell separation and culture, whereas the clot blood samples were kept at room temperature for 30–60 min and then centrifuged at 800 g at room temperature for 30 min. The separated sera were aliquoted in fresh microtubes and kept at −80°C for further analyses. Sera from cord blood samples were handled similarly.
Maternal spot urine samples were collected at first trimester and 34–36 weeks of gestation. Urine samples were also aliquoted and kept at −80°C until the day of analysis. All laboratory bench works were done at the Laboratory of Nutrition Research, NNFTRI.
2.4.2. Serum and urine analyses
Maternal and cord blood serum concentrations of 25(OH)D were determined using a commercial enzyme immunoassay (EIA) kit (EUROIMMUN, Lubeck, Germany). According to the manufacturer's data, the intra‐ and inter‐assay coefficient of variation ranged from 3.2–6.9% to 7–8.6%, respectively. Deficiency is defined as <50 nmol L−1, insufficiency as 50–75 nmol L−1, and sufficiency as >75 nmol L−1 (Cunningham, 2018).
Serum hs‐CRP was assayed using an EIAkit (Pars‐Azmoon, Tehran, Iran) with intra‐ and inter‐assay coefficient of variations of 2.5% and 4.5%, respectively.
Urinary concentrations of calcium and creatinine were determined using commercial kits (Pars‐Azmoon, Tehran, Iran) and an auto‐analyser (Selecta E; Vitalab, Holliston, Netherlands).
2.4.3. Cell separation and culture for cytokine assays
Peripheral blood mononuclear cells were separated and cultured with the method described elsewhere (Neyestani, Gharavi, & Kalayi, 2009), with some minor modifications. Peripheral blood mononuclear cells plated at a density of 2 × 106 cells/well in 12‐well plates were incubated at 37°C in a 5% humidified CO2 incubator for 24 hr. The supernatants were used for cytokine assays by EIA kits (all from Diaclone, Vienna, Austria) and a microplate reader (Stat Fax 3200; Awareness Technology, Inc., Palm City, FL, USA).
2.5. Statistical analyses
Normality of the distribution of continuous variables was checked using the Shapiro–Wilk test. Data were presented as frequency distribution tables and numeric indices. Continuous data (normal in distribution) were presented as mean and standard deviation (SD) or geometric mean (standard error, SE) in the case of skewed distribution, and categorical data were presented as frequency or percentage (%). Paired Student's t test was used to compare baseline and final measures (within group comparison). Independent‐samples Student's t test was applied to compare normally distributed data between groups. Pearson's correlation was applied to assess the possible correlation between variables. Categorical variables between treatment groups (intergroup comparisons) were compared using Chi‐square test or Fisher's exact test.
3. RESULTS
Between February and June 2017, 445 pregnant women at the first trimester of gestation attended three hospitals were interviewed and assessed for eligibility. Finally, 84 eligible pregnant women accepted to participate in this study. The follow‐up duration lasted for 28.7 ± 2.5 weeks.
Of all participants, five women from 1,000‐IU/d group and six women from 2,000‐IU/d group did not complete the study because of some reasons including miscarriage, preterm delivery, and lost to follow‐up (leaving the study and inaccessibility). Among those who completed the study, the rate of adherence to the vitamin D supplementation was higher than 90% in both groups.
Baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics of subjects by vitamin D supplementation group
| Characteristic | 1,000 IU/day n = 37 | 2,000 IU/day n = 36 |
|---|---|---|
| Gestational age at enrolment, week | 10.39 (1.69) | 10.08 (2.17) |
| Gestational age at last visit (week) | 34.65 (0.86) | 34.58 (0.87) |
| Gestational age at delivery (week) | 38.79 (1.65) | 39.91 (5.78) |
| Season at enrolment, n (%) | ||
| Winter | 14 (38.2) | 16 (46.3) |
| Spring | 23 (61.8) | 20 (53.7) |
| Season at last visit, n (%) | ||
| Summer | 17 (44.4) | 14 (38.9) |
| Fall | 20 (55.6) | 22 (61.1) |
| Weight at enrollment (kg) | 60.91 (12.99) | 62.80 (12.61) |
| BMI at enrollment (kg/m2) | 24.70 (4.69) | 25.35 (5.00) |
| Weight at the end of pregnancy (kg) | 71.74 (11.89) | 74.82 (10.98) |
| Weight gain (kg) | 10.82 (5.21) | 12.01 (5.94) |
| Physical activity level at enrollment, n (%) | ||
| Low | 29 (78.4) | 28 (77.8) |
| Moderate | 8 (21.6) | 6 (16.7) |
| High | 0 (0.0) | 2 (5.6) |
| Physical activity level at last visit, n (%) | ||
| Low | 32 (86.5) | 29 (80.6) |
| Moderate | 5 (13.5) | 6 (16.7) |
| High | 0 (0.0) | 1 (2.8) |
Note. Data are expressed as mean (SD, standard deviation) and the distribution between groups expressed as n (%). Missing data were n = 2–6.
Abbreviation: BMI, body mass index.
Analysis of 24‐hr recalls obtained from participants at the first and last visit did not show any between‐group significant changes in energy and nutrient intakes (data not shown).
Low vitamin D status (25(OH)D < 75 nmol L−1) was found in 83.8% and 86.2% of the subjects in 1,000‐IU/d group and 2,000‐IU/d group, respectively, which decreased to 62.2% and 33.4%, respectively, by the end of the intervention (Table 2). Circulating concentrations of 25(OH)D increased in both intervention groups with significantly more increment in 2,000 IU/d than in 1,000 IU/d (46.7 ± 30.7 vs. 24.0 ± 21.07 nmol L−1, P = .001; Table 3).
Table 2.
Comparison of vitamin D status based on serum concentration of 25(OH)D between groups
| Groups | Before intervention | After intervention | P valuea | ||||
|---|---|---|---|---|---|---|---|
| Deficient | Insufficient | Sufficient | Deficient | Insufficient | Sufficient | ||
| 1,000 IU/day, n (%) | 26 (70.3) | 5 (13.5) | 6 (16.2) | 3 (8.1) | 20 (54.1) | 14 (37.8) | .04 |
| 2,000 IU/day, n (%) | 20 (55.6) | 11 (30.6) | 5 (13.9) | 2 (5.6) | 10 (27.8) | 24 (66.7) | |
| Total, n (%) | 46 (63) | 16 (21.9) | 11 (15.1) | 5 (6.8) | 30 (41.1) | 38 (52.1) | |
Note. Deficiency is defined as <50 nmol L−1, insufficiency as 50–75 nmol L−1, and sufficiency as >75 nmol L−1.
Denotes the significance of differences in the distribution of vitamin D categories between the two groups (chi‐square test).
Table 3.
Comparison of changes in variables within and between groups after the intervention
| Variable | 1,000 IU/day | 2,000 IU/day | Between group differences (95% CI) | P a | ||
|---|---|---|---|---|---|---|
| Before | After | Before | After | |||
| 25(OH)D3 (nmol L−1) | 45.32 (29.70) | 71.19 (23.65) | 47.03 (31.70) | 91.82 (28.81) | −22.69 [−35.08, −10.3] | .001 |
| hs‐CRP (mg L−1) | 4.30 (5.04) | 4.71 (4.87) | 5.52 (6.25) | 6.41 (5.58) | −0.48 [−3.75, 2.77] | .76 |
| IL‐1 (ng L−1) | 3,213.93 (1,534.05) | 2,859.36 (2,037.75) | 3,502.30 (1,337.87) | 2,521.12 (1,857.86) | 626.60 [−1,042.68, 2,295.89] | .44 |
| IL‐6 (ng L−1) | 75.00 (13.64) | 68.53 (16.17) | 81.23 (12.42) | 76.04 (19.61) | −1.28 [−17.12, 14.55] | .87 |
| TNF‐α (ng L−1) | 2,186.86 (990.9) | 1,741.59 (1,077.4) | 2,528.31 (715.4) | 1,615.21 (1,050.9) | 467.82 [−660.82 to 1,596.47] | .40 |
Note. All values are expressed as means (SD, standard deviation). The missing data were n = 4–7.
Abbreviations: hs‐CRP, highly sensitive‐C reactive protein; IL_1, interleukin 1; IL_6 interleukin; TNF‐α, tumour necrosis factor alpha; 25(OH)D3, 25‐hydroxyvitamin D3.
Denotes the significance between‐group differences (independent t test).
The concentrations of 25(OH)D in cord blood did not show a significant between‐group difference (84.0 ± 35.1 vs. 89.0 ± 25.3 nmol L−1 in 1,000‐ and 2,000‐IU/d groups, P = .64, respectively). Interestingly, a significant positive correlation between cord blood and final maternal serum concentrations of 25(OH)D was observed (r = 0.38, P = .02).
Maternal serum concentrations of hs‐CRP did not show any significant within‐ or between‐group changes. Similarly, initial and final concentrations of the pro‐inflammatory cytokines IL‐1β, IL‐6, and TNF‐α did not differ significantly between the two interventional groups (Table 3).
No significant difference in cord blood hs‐CRP was observed (Table 4). As for cord blood, though concentrations of IL‐1β and TNF‐α were not significantly different between two groups, concentrations of IL‐6 was in order of magnitude higher in 1,000 IU/d than in 2,000‐IU/d group (25.9 ± 32.0 vs. 4.6 ± 1.4 ng L−1, P = .03).
Table 4.
Cord blood biomarkers and neonatal outcomes
| Variables | 1,000 IU/day | 2,000 IU/day | P a |
|---|---|---|---|
| 25 (OH)D (nmol L−1) | 84.04 (35.14) | 89 (25.27) | .64 |
| Cord blood 25(OH)D, n (%) | .13 | ||
| <50 nmol L−1 | 3 (20) | 0 (0.0) | |
| 50–75 nmol L−1 | 4 (26.7) | 7 (38.9) | |
| >75 nmol L−1 | 8 (53.3) | 11 (61.1) | |
| Weight (g) | 3,103.2 (440.26) | 3,369.80 (450.26) | .018 |
| Length (cm) | 49.85 (2.21) | 51.48 (3.02) | .017 |
| Head circumference (cm) | 34.19 (1.49) | 35.01 (1.62) | .040 |
| Apgar (minute 1) | 8.83 (0.37) | 8.91 (0.28) | .37 |
| Apgar (minute 5) | 9.83 (0.37) | 9.91 (0.28) | .37 |
| TNF‐α (ng L−1) | 37.54 (22.46) | 35.47 (12.44) | .78 |
| IL‐6 (ng L−1) | 25.95 (32.02) | 4.61 (1.44) | 0.03 |
| IL‐1 (ng L−1) | 90.92 (142.69) | 115.55 (165.94) | 0.68 |
| hs‐CRP (mg L−1) | 0.38 (0.32) | 0.35 (0.35) | 0.85 |
Note. All values are expressed as means (SD, standard deviation).
Abbreviations: Hs‐CRP, highly sensitive‐C reactive protein; IL‐1, interleukin 1; IL‐6 interleukin; TNF‐α, tumour necrosis factor alpha; 25(OH)D3, 25‐hydroxyvitamin D3.
Denotes the significance between groups differences (independent t test).
Maternal outcomes and duration of gestation did not show any significant between groups difference. However, infants born to mothers in the 2,000 IU/d treatment group, compared with 1,000 IU/d group, had a significantly greater birth weight (3,369.80 ± 450.26 vs. 3,103.2 ± 440.26 g, P = .018), length (51.48 ± 3.02 vs. 49.85 ± 2.21 cm, P = .01) and head circumference (35.01 ± 1.62 vs. 34.19 ± 1.49 cm, P = .04), but the difference in Apgar score and the proportion of low birth weight were not statistically significant between groups. Gender proportion was not different by treatment group either (P = .30).
4. DISCUSSION
4.1. Vitamin D (maternal and cord blood)
We found a high occurrence of low vitamin D status (63% deficiency and 21.9% insufficiency) in our subjects at the first trimester of pregnancy. This finding is in accord with the reported high prevalence (60–80%) of VDD among Iranian pregnant women (Pirdehghan et al., 2016) and also in the whole community (Nikooyeh et al., 2017a; Nikooyeh et al., 2017b).
In the present study, taking 2,000‐IU/d vitamin D3 for almost 29 weeks was more effective than 1,000 IU/d in providing sufficient levels of vitamin D (25(OH)D > 75 nmol L−1). At 34 weeks of gestation, frequency of vitamin D sufficiency in the 2,000‐IU/d group was 1.8 times higher than in the 1,000‐IU/d group. This finding was similar to that of other trials that examined the effectiveness of the same doses of vitamin D supplementation (Cooper et al., 2016; De‐Regil, Palacios, Lombardo, & Pena‐Rosas, 2016; Grant et al., 2014; Hollis, Johnson, Hulsey, Ebeling, & Wagner, 2011; Roth et al., 2018; Sahoo, Katam, Das, Agarwal, & Bhatia, 2017; Zerofsky, Jacoby, Pedersen, & Stephensen, 2016). Pregnancy‐induced increased body fat mass and hemodilution may explain to some extent the increased vitamin D need of a pregnant woman. Several studies have shown the inverse relationship of body fat mass and vitamin D status (Golzarand, Hollis, Mirmiran, Wagner, & Shab‐Bidar, 2018; McAree, 2013). On the other hand, circulating 25(OH)D may decline from the first to the third trimester of pregnancy due to the physiologic hemodilution (Cunningham, 2018).
In the present study, we found a significant positive correlation between the maternal and cord blood serum concentration of 25(OH)D like other studies (Miliku et al., 2019). We observed that the cord blood serum concentration of 25(OH)D was in the range of sufficient level (>75 nmol L−1) in both groups, and a non‐significant difference was found between groups. Consistent with our findings, an open‐label randomized controlled trial showed that maternal vitamin D supplementation (2,000–4,000‐IU cholecalciferol) among women with VDD/insufficiency from 12 to 16 weeks of gestation until delivery prevents neonatal VDD (Rodda et al., 2015). It is likely that placental transportal sites, notably megalin–cubilin system, are saturated with taking a certain dose of vitamin D a day (Hollis & Wagner, 2017a). Though vitamin D is not considered a teratogen, this may be a protective mechanism to regulate foetal vitamin D status. Further studies should address this issue.
4.2. Inflammatory markers
In the present study, serum concentration of hs‐CRP was not affected by vitamin D3 supplementation with any of two doses: 1,000 IU/d or 2,000 IU/d for 24 weeks. There are some other interventional studies among women with gestational diabetes mellitus or preeclampsia that support our study data (Miroliaee, Salamzadeh, Shokouhi, & Sahraei, 2018; Samimi et al., 2016; Yazdchi, Gargari, Asghari‐Jafarabadi, & Sahhaf, 2016). Conversely, other studies have shown a negative correlation between 25(OH)D and hs‐CRP levels among pregnant women (Asemi, Hashemi, Karamali, Samimi, & Esmaillzadeh, 2013; Haidari, Jalali, Shahbazian, Haghighizadeh, & Azadegan, 2016). However, an indirect association between vitamin D status and CRP concentrations in neonates with serum 25(OH)D concentrations below 50 nmol L−1 has been observed by other researchers (Asemi et al., 2013; Haidari et al., 2016). The initial values of both hs‐CRP and 25(OH)D dose and duration of supplementation seem to be the main factors affecting the outcome.
A large preeclampsia cohort study has shown no association between VDD and the level of IL‐6. Indeed, the association between third trimester IL‐6 elevation and preeclampsia was not mediated by VDD. Therefore, according to this study, the role of VDD in the pathogenesis of preeclampsia could not be through activation of inflammation (Xu, Lee, Jeyabalan, & Roberts, 2014). In contrast, it has been asserted that low levels of calcitriol in maternal serum and placenta via increased pro‐inflammatory cytokine levels could be considered as a reason for adverse pregnancy outcomes (Barrera, Diaz, Noyola‐Martinez, & Halhali, 2015; Noyola‐Martinez et al., 2013). A randomized clinical trial reported a significant inverse association between plasma 25(OH)D concentrations in late pregnancy and plasma levels of TNF‐α. The authors suggested that immune changes could happen in greater magnitude among women with baseline serum 25(OH)D concentrations <50 nmol L−1 in response to 2,000‐IU/d vitamin D supplementation (Zerofsky et al., 2016).
Though in cord blood initial concentrations of IL‐1β and TNF‐α were not significantly different between groups, final levels of IL‐6 in 2,000 IU/d, as compared with 1,000‐IU/d group, were significantly lower. This finding may have a very noticeable clinical implication. Increased umbilical cord blood concentrations of IL‐6 have been associated with many neonatal complications including histological chorioamnionitis (Andrys et al., 2010) and neonatal sepsis in preterm ruptures of the membranes (Cobo et al., 2013). Furthermore, umbilical cord blood IL‐6 concentration has been proposed as a biomarker for early detection of neonatal sepsis with group B streptococcus (Nakstad, Sonerud, & Solevag, 2016), bronchopulmonary dysplasia in preterm newborns (Rocha et al., 2012), and neonatal brain damage due to non‐asphyxia foetal distress (Tian, Cheng, & Gu, 2017). Altogether, decreased cord blood concentrations of IL‐6 due to supplementation with 2,000 IU/d from the first trimester of pregnancy may have protective effect against several low neonatal outcomes. Conducting future interventional studies with larger sample size and different doses of vitamin D3 and evaluation of various kinds of inflammatory markers of both Th1 and Th2 arms in each trimester of pregnancy could potentially reveal the effect of vitamin D supplementation and vitamin D status on immune modulation and inflammation during pregnancy.
4.3. Birth outcomes
The present study observed significantly higher anthropometric values (weight, length, and head circumference) in infants of 2,000‐IU/d group compared with those belonged to 1,000‐IU/d group. It is worth mentioning that confounding factors including diet, seasonality, maternal weight gain during pregnancy, and birth spacing were not significantly different between groups. In accord with our findings, other trials suggested that vitamin D supplementation during pregnancy resulted in improved birth outcomes (Chen et al., 2015; De‐Regil et al., 2016; Hollis & Wagner, 2017b; Miliku et al., 2019; Sablok et al., 2015; Weernink et al., [Link]; Zerofsky et al., 2016). It has been reported that maternal vitamin D status was positively and significantly associated with birth size (weight and length; Nandal et al., 2016; Perez‐Lopez et al., 2015; Rostami, Ramezani Tehrani, Simbar, Hosseinpanah, & Alavi Majd, 2017). On the other hand, some studies showed no significant associations between vitamin D supplementation and birth size (Asemi, Karamali, & Esmaillzadeh, 2014a; Gale et al., 2008). Interestingly, the results of a meta‐analysis showed the effectiveness of vitamin D supplementation only in interventional studies, which used both vitamin D and calcium supplementation (Moon, Harvey, & Cooper, 2015). In a large randomized, double‐blind, placebo‐controlled trial among pregnant women in Bangladesh, the effectiveness of different doses of vitamin D (from zero to 28,000 IU/week) on the anthropometric measures of infants at birth and 1 year of age was evaluated. Supplementation started from 17 to 24 weeks of gestation until birth or 6‐month postpartum. In this study, despite remarkable increase in circulating calcidiol (from initially 23 nmol/L to finally 69–114 nmol L−1, depending on the supplementation dose), birth sizes did not differ significantly among different supplementation groups (Roth et al., 2018). Late commencement of supplementation in this study may, at least in part, explain the unresponsiveness of birth sizes to vitamin D supplementation. Further investigations are necessary to determine the mechanism of this effect with considering the other factors affected foetal growth including genetic background, trophoblast implantation, and placental development.
4.4. Pregnancy outcomes
The present investigation found that the occurrence of preeclampsia, gestational diabetes, preterm birth, SGA, and caesarean section were not different between groups. The effect of vitamin D supplementation on the pregnancy outcomes including preeclampsia, gestational diabetes, and spontaneous abortion has not been confirmed by several RCTs (Asemi, Karamali, & Esmaillzadeh, 2014b; Karamali, Asemi, Ahmadi‐Dastjerdi, & Esmaillzadeh, 2016; Perez‐Lopez et al., 2015; Roth et al., 2018). Nonetheless, there are few interventional studies with large sample size that support the positive effect of vitamin D supplementation during pregnancy on pregnancy outcomes (De‐Regil et al., 2016).
In addition, observational studies with large sample size have shown the association between maternal VDD during pregnancy and increased risk of pregnancy outcomes (Amegah, Klevor, & Wagner, 2017; Chen et al., 2015; Wei, Qi, Luo, & Fraser, 2013), albeit there are still inconsistencies in this regard (Bodnar et al., 2014; Bomba‐Opon et al., 2014).
It has been stated that the potential effect of vitamin D supplementation on these outcomes mediated by its impact on placental gene expression and inflammation. Therefore, it seems crucial to initiating vitamin D supplementation earlier and eliminating VDD before pregnancy (Hollis & Wagner, 2017b). Moreover, it has been suggested that achieving serum 25(OH)D concentration of at least 100 nmol L−1 is required for optimizing the conversion of 25(OH)D to 1,25(OH)2D and reducing the adverse pregnancy outcomes (Hollis & Wagner, 2017a).
Future investigations with greater dose of vitamin D supplementation in larger population of pregnant women are needed to determine the optimal dose of vitamin D supplementation for pregnant women with taking into consideration the mechanism of the effects of vitamin D status during pregnancy on immune function, inflammatory markers, and clinical outcomes.
The advantages of the present study were the randomized clinical design, initiating vitamin D supplementation early in pregnancy until delivery, evaluating the confounding factors (diet, physical activity level and sun exposure) and assessing inflammatory markers in both maternal and cord blood samples. However, some limitations are acknowledged. One of them was lack of control group (no vitamin D supplement intake), which was ethically impossible. The concentrations of calcitriol (1, 25(OH)2D) were not assayed, either.
5. CONCLUSION
Supplementation with 2,000‐IU/d vitamin D3 instead of 1,000 IU/d from the first trimester of pregnancy could be more effective in improving vitamin D status of the pregnant women and decreasing pro‐inflammatory cytokines including TNF‐α in mother and IL‐6 in cord blood. Also, supplementation with 2,000‐IU/d vitamin D3 helps improve neonatal outcomes including the birth sizes (weight, length, and head circumference) compared with 1,000‐IU/d group.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
TN and BN designed and supervised the study. BN involved in estimation of sample size and statistical analyses. All field works and most of laboratory analyses were performed by SM. TN and BN also helped in laboratory investigations. Criteria for subject selection were set with the aid of MK who also made all arrangements in Akbarabadi Hospital. The preliminary manuscript was written by SM. The final manuscript was written by TN with the intellectual aid and comments of BH.
ACKNOWLEDGMENTS
This work represents part of data obtained from Soudabe Motamed's PhD thesis in nutrition science. All laboratory bench works were done at Laboratory of Nutrition Research, NNFTRI. We hereby thank all women who heartily participated in this research.
Motamed S, Nikooyeh B, Kashanian M, Hollis BW, Neyestani TR. Efficacy of two different doses of oral vitamin D supplementation on inflammatory biomarkers and maternal and neonatal outcomes. Matern Child Nutr. 2019;15:e12867 10.1111/mcn.12867
REFERENCES
- Abbasian, M. , Chaman, R. , Amiri, M. , Ajami, M. E. , Jafari‐Koshki, T. , … Raei, M. (2016). Vitamin D deficiency in pregnant women and their neonates. Global Journal of Health Science, 8(9), 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abedi, P. , Mohaghegh, Z. , Afshary, P. , & Latifi, M. (2014). The relationship of serum vitamin D with pre‐eclampsia in the Iranian women. Maternal & Child Nutrition, 10(2), 206–212. 10.1111/mcn.12058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal, S. , Kovilam, O. , & Agrawal, D. K. (2018). Vitamin D and its impact on maternal‐fetal outcomes in pregnancy: A critical review. Critical Reviews in Food Science and Nutrition, 58(5), 755–769. 10.1080/10408398.2016.1220915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amegah, A. K. , Klevor, M. K. , & Wagner, C. L. (2017). Maternal Vitamin D insufficiency and risk of adverse pregnancy and birth outcomes: A systematic review and meta‐analysis of longitudinal studies. PLoS ONE, 12(3). 10.1371/journal.pone.0173605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrys, C. , Drahosova, M. , Hornychova, H. , Tambor, V. , Musilova, I. , Tosner, J. , … Kacerovsky, M. (2010). Umbilical cord blood concentrations of IL‐6, IL‐8, and MMP‐8 in pregnancy complicated by preterm premature rupture of the membranes and histological chorioamnionitis. Neuro Endocrinology Letters, 31(6), 857–863. [PubMed] [Google Scholar]
- Asemi, Z. , Hashemi, T. , Karamali, M. , Samimi, M. , & Esmaillzadeh, A. (2013). Effects of vitamin D supplementation on glucose metabolism, lipid concentrations, inflammation, and oxidative stress in gestational diabetes: A double‐blind randomized controlled clinical trial. The American Journal of Clinical Nutrition, 98(6), 1425–1432. 10.3945/ajcn.113.072785 [DOI] [PubMed] [Google Scholar]
- Asemi, Z. , Karamali, M. , & Esmaillzadeh, A. (2014a). Effects of calcium‐vitamin D co‐supplementation on glycaemic control, inflammation and oxidative stress in gestational diabetes: A randomised placebo‐controlled trial. Diabetologia, 57(9), 1798–1806. 10.1007/s00125-014-3293-x [DOI] [PubMed] [Google Scholar]
- Asemi, Z. , Karamali, M. , & Esmaillzadeh, A. (2014b). Favorable effects of vitamin D supplementation on pregnancy outcomes in gestational diabetes: A double blind randomized controlled clinical trial. Hormone and Metabolic Research, 47(8), 565–570. [DOI] [PubMed] [Google Scholar]
- Barrera, D. , Diaz, L. , Noyola‐Martinez, N. , & Halhali, A. (2015). Vitamin D and inflammatory cytokines in healthy and preeclamptic pregnancies. Nutrients, 7(8), 6465–6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar, L. M. , Simhan, H. N. , Catov, J. M. , Roberts, J. M. , Platt, R. W. , Diesel, J. C. , & Klebanoff, M. A. (2014). Maternal vitamin D status and the risk of mild and severe preeclampsia. Epidemiology, 25(2), 207–214. 10.1097/EDE.0000000000000039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomba‐Opon, D. A. , Brawura‐Biskupski‐Samaha, R. , Kozlowski, S. , Kosinski, P. , Bartoszewicz, Z. , Bednarczuk, T. , & Wielgos, M. (2014). First trimester maternal serum vitamin D and markers of preeclampsia. The Journal of Maternal‐Fetal & Neonatal Medicine, 27(10), 1078–1079. 10.3109/14767058.2013.846318 [DOI] [PubMed] [Google Scholar]
- Cashman, K. D. , Dowling, K. G. , Skrabakova, Z. , Gonzalez‐Gross, M. , Valtuena, J. , De Henauw, S. , et al. (2016. Apr). Vitamin D deficiency in Europe: Pandemic? The American Journal of Clinical Nutrition, 103(4), 1033–1044. PubMed PMID: 26864360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. H. , Fu, L. , Hao, J. H. , Yu, Z. , Zhu, P. , Wang, H. , … Xu, D. X. (2015). Maternal vitamin D deficiency during pregnancy elevates the risks of small for gestational age and low birth weight infants in Chinese population. The Journal of Clinical Endocrinology and Metabolism, 100(5), 1912–1919. 10.1210/jc.2014-4407 [DOI] [PubMed] [Google Scholar]
- Cobo, T. , Kacerovsky, M. , Andrys, C. , Drahosova, M. , Musilova, I. , Hornychova, H. , & Jacobsson, B. (2013). Umbilical cord blood IL‐6 as predictor of early‐onset neonatal sepsis in women with preterm prelabour rupture of membranes. PLoS ONE, 8(7), e69341. PubMed PMID: 23894452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, C. , Harvey, N. C. , Bishop, N. J. , Kennedy, S. , Papageorghiou, A. T. , Schoenmakers, I. , … MAVIDOS Study Group . (2016). Maternal gestational vitamin D supplementation and off spring bone health (MAVIDOS): a multicentre, double‐blind, randomised placebo‐controlled trial. The Lancet Diabetes and Endocrinology, 4(5), 393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham, F . (2018) Normal reference ranges for laboratory values in pregnancy. https://www.uptodate.com/contents/normal-reference-ranges-for-laboratory-values-in-pregnancy.
- Curtis, E. M. , Moon, R. J. , Harvey, N. C. , & Cooper, C. (2018). Maternal vitamin D supplementation during pregnancy. British Medical Bulletin, 126(1), 57–77. PubMed PMID: 29684104. 10.1093/bmb/ldy010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De‐Regil, L. M. , Palacios, C. , Lombardo, L. K. , & Pena‐Rosas, J. P. (2016. May‐Jun). Vitamin D supplementation for women during pregnancy. Sao Paulo Medical Journal = Revista Paulista de Medicina, 134(3), 274–275. 10.1590/1516-3180.20161343T2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale, C. R. , Robinson, S. M. , Harvey, N. C. , Javaid, M. K. , Jiang, B. , Martyn, C. N. , … Princess Anne Hospital Study Group . (2008). Maternal vitamin D status during pregnancy and child outcomes. European Journal of Clinical Nutrition, 62(1), 68–77. 10.1038/sj.ejcn.1602680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golzarand, M. , Hollis, B. W. , Mirmiran, P. , Wagner, C. L. , & Shab‐Bidar, S. (2018). Vitamin D supplementation and body fat mass: A systematic review and meta‐analysis. European Journal of Clinical Nutrition, 72, 1345–1357. 10.1038/s41430-018-0132-z [DOI] [PubMed] [Google Scholar]
- Grant, C. C. , Stewart, A. W. , Scragg, R. , Milne, T. , Rowden, J. , Ekeroma, A. , … Camargo, C. A. Jr. (2014). Vitamin D during pregnancy and infancy and infant serum 25‐hydroxyvitamin D concentration. Pediatrics, 133(1), e143–e153. 10.1542/peds.2013-2602 [DOI] [PubMed] [Google Scholar]
- Haidari, F. , Jalali, M. T. , Shahbazian, N. , Haghighizadeh, M. H. , & Azadegan, E. (2016). Comparison of serum levels of vitamin D and inflammatory markers between women with gestational diabetes mellitus and healthy pregnant control. Journal of Family & Reproductive Health, 10(1), 1–8. [PMC free article] [PubMed] [Google Scholar]
- Hollis, B. W. , Johnson, D. , Hulsey, T. C. , Ebeling, M. , & Wagner, C. L. (2011). Vitamin D supplementation during pregnancy: Double‐blind, randomized clinical trial of safety and effectiveness. Journal of Bone and Mineral Research, 26(10), 2341–2357. 10.1002/jbmr.463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis, B. W. , & Wagner, C. L. (2017a). New insights into the vitamin D requirements during pregnancy. Bone Research, 5 10.1038/boneres.2017.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollis, B. W. , & Wagner, C. L. (2017b). Vitamin D supplementation during pregnancy: Improvements in birth outcomes and complications through direct genomic alteration. Molecular and Cellular Endocrinology, 453, 113–130. 10.1016/j.mce.2017.01.039 [DOI] [PubMed] [Google Scholar]
- Karamali, M. , Asemi, Z. , Ahmadi‐Dastjerdi, M. , & Esmaillzadeh, A. (2016). Calcium plus Vitamin D supplementation affects pregnancy outcomes in gestational diabetes: Randomized, double‐blind, placebo‐controlled trial. Public Health Nutrition, 19(1), 156–163. 10.1017/S1368980015000609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelishadi, R. , Sharifi‐Ghazvini, F. , Poursafa, P. , Mehrabian, F. , Farajian, S. , Yousefy, H. , … Sharifi‐Ghazvini, S. (2013). Determinants of hypovitaminosis D in pregnant women and their newborns in a sunny region. International Journal of Endocrinology, 2013, 1–6. 10.1155/2013/460970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAree, T. (2013). Obesity and vitamin D deficiency‐current concepts on their impact on pregnancy. European Endocrinology, 9(2), 125–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miliku, K. , Felix, J. F. , Voortman, T. , Tiemeier, H. , Eyles, D. W. , Burne, T. H. , … Jaddoe, V. W. V. , (2019). Associations of maternal and fetal vitamin D status with childhood body composition and cardiovascular risk factors. Maternal & Child Nutrition, 15, e12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miroliaee, A. E. , Salamzadeh, J. , Shokouhi, S. , & Sahraei, Z. (2018). The study of vitamin D administration effect on CRP and Interleukin‐6 as prognostic biomarkers of ventilator associated pneumonia. Journal of Critical Care, 44, 300–305. 10.1016/j.jcrc.2017.08.040 [DOI] [PubMed] [Google Scholar]
- Moon, R. J. , Harvey, N. C. , & Cooper, C. (2015). Endocrinology in pregnancy: Influence of maternal vitamin D status on obstetric outcomes and the fetal skeleton. European Journal of Endocrinology, 173(2), R69–R83. 10.1530/EJE-14-0826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motamed, S. , Nikooyeh, B. , & Neyestani, T. R. (2018). Evaluation of the Efficacy of Vitamin D Supplementation With Two Different Doses During Pregnancy on Maternal and Cord Blood Vitamin D Status, Metabolic, Inflammatory and Oxidative Stress Biomarkers, and Maternal and Neonatal Outcomes: a Study Protocol. Nutrition and Food Sciences Research, 5(3), 3–10. [Google Scholar]
- Nakstad, B. , Sonerud, T. , & Solevag, A. L. (2016). Early detection of neonatal group B streptococcus sepsis and the possible diagnostic utility of IL‐6, IL‐8, and CD11b in a human umbilical cord blood in vitro model. Infection and Drug Resistance, 9, 171–179. 10.2147/IDR.S106181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nandal, R. , Chhabra, R. , Sharma, D. , Lallar, M. , Rathee, U. , & Maheshwari, P. (2016). Comparison of cord blood Vitamin D levels in newborns of Vitamin D supplemented and unsupplemented pregnant women: A prospective, comparative study. The Journal of Maternal‐Fetal & Neonatal Medicine, 29(11), 1812–1816. [DOI] [PubMed] [Google Scholar]
- Newhook, L. A. , Sloka, S. , Grant, M. , Randell, E. , Kovacs, C. S. , & Twells, L. K. (2009). Vitamin D insufficiency common in newborns, children and pregnant women living in newfoundland and labrador, Canada. Maternal & Child Nutrition, 5(2), 186–191. 10.1111/j.1740-8709.2008.00157.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyestani, T. R. , Gharavi, A. , & Kalayi, A. (2009). Selective effects of tea extract and its phenolic compounds on human peripheral blood mononuclear cell cytokine secretions. International Journal of Food Sciences and Nutrition, 60(Suppl 1), 79–88. 10.1080/09637480802158184 [DOI] [PubMed] [Google Scholar]
- Nikooyeh, B. , Abdollahi, Z. , Hajifaraji, M. , Alavi‐Majd, H. , Salehi, F. , Yarparvar, A. H. , & Neyestan, T. R. (2017a). Vitamin D status and cardiometabolic risk factors across latitudinal gradient in Iranian adults: National food and nutrition surveillance. Nutrition and Health, 23(2), 87–94. 10.1177/0260106017702918 [DOI] [PubMed] [Google Scholar]
- Nikooyeh, B. , Abdollahi, Z. , Hajifaraji, M. , Alavi‐Majd, H. , Salehi, F. , Yarparvar, A. H. , & Neyestani, T. R. (2017b). Vitamin D status, latitude and their associations with some health parameters in children: National Food and nutrition surveillance. Journal of Tropical Pediatrics, 63(1), 57–64. 10.1093/tropej/fmw057 [DOI] [PubMed] [Google Scholar]
- Noyola‐Martinez, N. , Diaz, L. , Avila, E. , Halhali, A. , Larrea, F. , & Barrera, D. (2013). Calcitriol downregulates TNF‐alpha and IL‐6 expression in cultured placental cells from preeclamptic women. Cytokine, 61(1), 245–250. 10.1016/j.cyto.2012.10.001 [DOI] [PubMed] [Google Scholar]
- Olmos‐Ortiz, A. , Avila, E. , Durand‐Carbajal, M. , & Diaz, L. (2015). Regulation of calcitriol biosynthesis and activity: Focus on gestational vitamin D deficiency and adverse pregnancy outcomes. Nutrients, 7(1), 443–480. PubMed PMID: 25584965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios, C. , & González, L. (2014). Vitamin d deficiency is a major global public health problem. Anales Venezolanos de Nutricion, 27(1), 57–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez‐Lopez, F. R. , Pasupuleti, V. , Mezones‐Holguin, E. , Benites‐Zapata, V. A. , Thota, P. , Deshpande, A. , &. Hernandez, A.V. (2015). Effect of vitamin D supplementation during pregnancy on maternal and neonatal outcomes: A systematic review and meta‐analysis of randomized controlled trials. Fertility and Sterility, 103(5), 1278–88 e4. 10.1016/j.fertnstert.2015.02.019 [DOI] [PubMed] [Google Scholar]
- Pirdehghan, A. , Vakili, M. , Dehghan, R. , & Zare, F. (2016). High prevalence of vitamin D deficiency and adverse pregnancy outcomes in Yazd, a central province of Iran. Journal of Reproduction & Infertility, 17(1), 34–38. [PMC free article] [PubMed] [Google Scholar]
- Rocha, G. , Proenca, E. , Guedes, A. , Carvalho, C. , Areias, A. , Ramos, J. P. , … Guimarães, H. , (2012). Cord blood levels of IL‐6, IL‐8 and IL‐10 may be early predictors of bronchopulmonary dysplasia in preterm newborns small for gestational age. Disease Markers, 33(1), 51–60. PubMed PMID: 22710869. 10.1155/2012/925632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodda, C. P. , Benson, J. E. , Vincent, A. J. , Whitehead, C. L. , Polykov, A. , & Vollenhoven, B. (2015). Maternal vitamin D supplementation during pregnancy prevents vitamin D deficiency in the newborn: An open‐label randomized controlled trial. Clinical Endocrinology, 83(3), 363–368. 10.1111/cen.12762 [DOI] [PubMed] [Google Scholar]
- Rostami, M. , Ramezani Tehrani, F. , Simbar, M. , Hosseinpanah, F. , & Alavi Majd, H. (2017). Rationale and design of Khuzestan Vitamin D deficiency screening program in pregnancy: A stratified randomized vitamin D supplementation controlled trial. JMIR Research Protocols, 6(4), e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth, D. E. , Leung, M. , Mesfin, E. , Qamar, H. , Watterworth, J. , & Papp, E. (2017). Vitamin D supplementation during pregnancy: State of the evidence from a systematic review of randomised trials. BMJ, 359, j5237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth, D. E. , Morris, S. K. , Zlotkin, S. , Gernand, A. D. , Ahmed, T. , Shanta, S. S. , … Al Mahmud, A. , (2018). Vitamin D supplementation in pregnancy and lactation and infant growth. The New England Journal of Medicine, 379(6), 535–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablok, A. , Batra, A. , Thariani, K. , Batra, A. , Bharti, R. , Aggarwal, A. R. , … Chellani, H. (2015). Supplementation of Vitamin D in pregnancy and its correlation with feto‐maternal outcome. Clinical Endocrinology, 83(4), 536–541. 10.1111/cen.12751 [DOI] [PubMed] [Google Scholar]
- Sahoo, S. K. , Katam, K. K. , Das, V. , Agarwal, A. , & Bhatia, V. (2017). Maternal vitamin D supplementation in pregnancy and offspring outcomes: A double‐blind randomized placebo‐controlled trial. Journal of Bone and Mineral Metabolism, 35(4), 464–471. 10.1007/s00774-016-0777-4 [DOI] [PubMed] [Google Scholar]
- Samimi, M. , Kashi, M. , Foroozanfard, F. , Karamali, M. , Bahmani, F. , Asemi, Z. , … Esmaillzadeh, A. (2016). The effects of vitamin D plus calcium supplementation on metabolic profiles, biomarkers of inflammation, oxidative stress and pregnancy outcomes in pregnant women at risk for pre‐eclampsia. Journal of Human Nutrition and Dietetics, 29(4), 505–515. 10.1111/jhn.12339 [DOI] [PubMed] [Google Scholar]
- Schneuer, F. J. , Roberts, C. L. , Guilbert, C. , Simpson, J. M. , Algert, C. S. , Khambalia, A. Z. , … Nassar, N. (2014). Effects of maternal serum 25‐hydroxyvitamin D concentrations in the first trimester on subsequent pregnancy outcomes in an Australian population. The American Journal of Clinical Nutrition, 99(2), 287–295. 10.3945/ajcn.113.065672 [DOI] [PubMed] [Google Scholar]
- The American College of Obstetricians and Gynecologists: Vitamin D: Screening and supplementation during pregnancy: Committee opinion. 2011. https://www.acog.org/Clinical-Guidance-and-Publications/Committee-Opinions/Committee-on-Obstetric-Practice/Vitamin-D-Screening-and-Supplementation-During-Pregnancy.
- Tian, C. , Cheng, L. , & Gu, X. (2017). Cord blood TNF‐alpha and IL‐6 levels as diagnostic indicators of brain damage in neonates with non‐asphyxia fetal distress. Archives of Gynecology and Obstetrics, 295(2), 337–342. 10.1007/s00404-016-4241-4 [DOI] [PubMed] [Google Scholar]
- Vasheghani‐Farahani, A. , Tahmasbi, M. , Asheri, H. , Ashraf, H. , Nedjat, S. , & Kordi, R. (2011). The Persian, last 7‐day, long form of the International Physical Activity Questionnaire: translation and validation study. Asian Journal of Sports Medicine, 2(2), 106–116. PubMed PMID: 22375226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weernink, M. G. , van Wijk, R. M. , Groothuis‐Oudshoorn, C. G. , Lanting, C. I. , Grant, C. C. , van Vlimmeren, L. A. , &. Boere‐Boonekamp, M. M. , Insufficient vitamin D supplement use during pregnancy and early childhood: a risk factor for positional skull deformation. Maternal & Child Nutrition, 12(1), 177–188. 10.1111/mcn.12153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, S. Q. (2014). Vitamin D and pregnancy outcomes. Current Opinion in Obstetrics and Gynecology, 26(6), 438–447. 10.1097/GCO.0000000000000117 [DOI] [PubMed] [Google Scholar]
- Wei, S. Q. , Qi, H. P. , Luo, Z. C. , & Fraser, W. D. (2013). Maternal vitamin D status and adverse pregnancy outcomes: A systematic review and meta‐analysis. The Journal of Maternal‐Fetal & Neonatal Medicine, 26(9), 889–899. 10.3109/14767058.2013.765849 [DOI] [PubMed] [Google Scholar]
- WHO Guideline: Vitamin D Supplementation in Pregnant Women. Geneva: World Health Organization 2012; 2012. [PubMed]
- Xu, L. , Lee, M. , Jeyabalan, A. , & Roberts, J. M. (2014). The relationship of hypovitaminosis D and IL‐6 in preeclampsia. American Journal of Obstetrics and Gynecology, 210(2), 149.e1–e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazdchi, R. , Gargari, B. P. , Asghari‐Jafarabadi, M. , & Sahhaf, F. (2016). Effects of vitamin D supplementation on metabolic indices and hs‐CRP levels in gestational diabetes mellitus patients: A randomized, double‐blinded, placebo‐controlled clinical trial. Nutrition Research and Practice, 10(3), 328–335. 10.4162/nrp.2016.10.3.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerofsky M. S., Jacoby B.N., Pedersen T.L., & Stephensen C.B. (2016) Daily Cholecalciferol Supplementation during Pregnancy Alters Markers of Regulatory Immunity, Inflammation, and Clinical Outcomes in a Randomized Controlled Trial. Journal of Nutrition. ,146(11), 2388–2397. [DOI] [PubMed] [Google Scholar]


