Abstract
Folate insufficiency during the periconceptional period increases the risk of neural tube defects (NTDs) in offspring, and folic acid supplementation substantially reduces the risk. Widespread large‐scale folic acid supplementation (0.4‐mg folic acid tablet) has been adopted as a main strategy to prevent NTDs in China since 2009. We examined folate concentrations in plasma and red blood cells (RBCs) of pregnant women and the factors associated with blood folate concentrations in a population with a high prevalence of NTDs in northern China. A cross‐sectional survey was conducted in 2014, and 1,107 pregnant women were recruited from 11 county or city maternal and child health centres across Shanxi province. Microbiological assays were used to determine folate concentrations. Factors associated with blood folate insufficiency were identified. The median (25th and 75th percentiles) folate concentrations were 28.4 (17.6, 45.2) nmol L−1 and 1,001.2 (658.7, 1,402.5) nmol L−1 in plasma and RBCs, respectively. According to the proposed RBC (906 nmol L−1) concentrations for optimal NTD prevention, 42.4% participants had RBC folate insufficiency. Rural women had a higher proportion of folate insufficiency than urban women. Folic acid supplementation was the only factor associated with RBC folate insufficiency. A large proportion of women had RBC folate concentrations that are not optimal for the prevention of NTDs despite free access to folic acid supplements. Actions that aim to improve folic acid supplementation compliance are needed to reach the full potential of the nationwide folic acid supplementation programme in terms of NTD prevention.
Keywords: folic acid supplementation, plasma folate, pregnant women, red blood cell folate
Key messages.
A large proportion of women failed to reach the optimal blood folate concentrations that are considered effective for NTD prevention 5 years after implementation of the nationwide folic acid supplementation program in China.
More than 90% of the women reported having taken a folic acid supplement during the current pregnancy. However, almost two thirds began to take the supplement after last menstrual period.
Low educational level and not taking folic acid supplements were correlated with RBC folate insufficiency.
The challenge is to ensure folic acid supplementation from before pregnancy and ensure adherence to the supplementation regimen.
1. INTRODUCTION
Folate insufficiency during the periconceptional period increases the risk of neural tube defects (NTDs) in offspring, and maternal folic acid supplementation can effectively reduce the risk of NTDs and possibly other congenital abnormalities (Czeizel & Dudas, 1992; MRC Vitamin Study Research Group, 1991; van Rooij et al., 2004). Public health measures aim to increase folate intake by recommending a daily folic acid supplement during the periconceptional period and fortifying staple foods with folic acid. Because nearly 40% of pregnancies are unplanned (Sedgh, Singh, & Hussain, 2014) and merely recommending a folic acid supplement seems ineffective for reducing the NTD rate (Zaganjor et al., 2016), many countries have turned to fortifying staple foods with folic acid (Bower, 2007; Busby et al., 2005; Martorell & de Romana, 2017; Viswanathan et al., 2017). To decrease the NTD burden in rural populations, which have a higher NTD prevalence rate than urban populations, the Ministry of Health of China launched a nationwide programme in 2009 to increase folic acid intake in reproductive‐aged women in rural areas; the programme provides folic acid supplements (0.4‐mg folic acid per tablet) free of charge to women with a rural household registry and who intend to get pregnant (Liu, Jin, et al., 2015; Ren, 2015). In the subsequent 2–3 years, women with an urban household registry were covered as well. Today, all child‐bearing aged women who plan to become pregnant have access to free folic acid supplements (0.4‐mg folic acid per tablet). No data on changes in red blood cell (RBC) folate concentrations, which are more relevant to NTD risk, were available thus far in the literature from China.
Although the prevalence of NTDs in Shanxi province decreased from 78.8/10,000 in 2009 to 31.5/10,000 in 2014, the prevalence of NTDs in 2014 is among the highest in the world (Li et al., 2006; Liu et al., 2016). A previous study indicated that plasma folate concentrations are significantly influenced by diet, folic acid supplementation, gestational weeks, and residence, which showed folic acid supplementation was positively associated with plasma folate concentration, whereas women with higher gestational weeks and residing in high prevalence area had lower folate concentrations; however, the study was conducted in a limited number of counties and on only plasma folate concentration, and the results only demonstrate the short‐term folate condition (Liu et al., 2015). Other studies revealed that younger age, lower fibre, and higher carbohydrate intakes were associated with lower blood folate (Obeid, Schon, Wilhelm, Pietrzik, & Pilz, 2018a). To more comprehensively measure effects of the national supplementation programme, we assessed folate concentrations in blood plasma and RBCs from a larger number of pregnant women from all cities in the province. We also investigated the factors associated with a high risk of blood folate insufficiency.
2. METHODS
2.1. Participants
Pregnant women in their early second trimester (12–20 weeks of gestation) who sought prenatal check‐ups at local maternal and child health care centres in 11 cities/counties across the province were invited. We estimated that about 100 women in each city/county would be adequate to obtain a stable mean or median measurement of blood folate concentrations and to compare differences between major associated factors based on our previous survey (Ren, Zhang, Hao, Li, & Tian, 2007); Considering seasonal changes in diet patterns and effects on folate concentrations, we enrolled pregnant women in summer and winter; the first 50 eligible women from each centre were enrolled in June 2014 and December 2014. Women with severe heart, liver, kidney, blood, or cancer conditions were excluded. A 5‐ml nonfasting blood sample was collected and drawn into vacutainer tubes (Becton Dickinson) containing potassium ethylene diamine tetraacetic acid (K3EDTA). Aliquots of plasma and hemolysate (100 μl of whole blood added to 900 μl 1% ascorbic acid solution) were prepared and stored temporarily at −20°C at local centres and then transferred to our laboratory on dry ice and stored at −80°C until assay. The study was approved by the institutional review board of Shanxi Provincial Hospital for Maternal and Children Health. Written consent was received from all participants.
2.2. Data collection
Face‐to‐face interviews were conducted by local trained health care workers to collect demographic characteristics, information on the use of folic acid supplements, history of pregnancy, and other exposure or confounding factors. Self‐reported information on height and weight were collected, and BMI was calculated as weight (kg)/height2(m2). Folic acid supplementation was defined as a self‐report of having ever taken a folic acid supplement or multivitamin containing folic acid in the periconceptual period of the current pregnancy (i.e., 3 months before to 3 months after the last menstrual period [LMP]). Information on the timing of supplementation and frequency and the total number of pills of taken was also collected. Timing of supplementation refers to the time when a woman began to take a folic acid supplement (e.g., before or after the LMP). We conducted a follow‐up study by telephone to verify the birth outcomes, focusing mainly on birth defects, in January 2019.
At the same time, another data source on NTDs prevalence was used. During the study, birth defects surveillance was undertaken in Shanxi Province as previously reported (Liu, Xie, Li, Greene, & Ren, 2018). The surveillance system was developed to monitor the prevalence of major structural birth defects, including NTDs. All live births (28 or more complete gestational weeks), all stillbirths of at least 20 weeks' gestational age, and pregnancy terminations at any gestational age following the prenatal diagnosis of NTDs were included. Because blood samples for the analysis of folate concentrations in this study were collected in June and December 2014 and the pregnancy outcomes of these women were available in 2015, we used data on NTD prevalence from the birth defects surveillance conducted in 2015–2016 for a better estimation.
2.3. Blood folate assessment
Plasma and whole‐blood folate concentrations were determined using microbiological assay with 96‐well microtitre plates as reported previously (Liu, Gao, et al., 2015; O'Broin & Kelleher, 1992). The microbiological assay used a chloramphenicol‐resistant microorganism with folic acid as the calibrator (Pfeiffer et al., 2016). In short, the reconstituted Lactobacillus casei (NCIB 10463) was added to the assay medium (7.05‐g folate assay broth [BD, 282210], 3‐mg chloramphenicol [Sigma, C‐0378], 75‐mg ascorbic acid, to 100‐ml distilled water.) as well as diluted plasma in the plate. All plates were incubated at 37°C in the dark for 42 hr. A multifunctional microplate tester (Biotek, Winooski, VT) was used to read at 590 nm. A calibration curve was prepared, representing different concentrations folic acid from 0 to 0.50 μg L−1. RBC folate concentrations in nmol L−1 were calculated as follows: whole‐blood hemolysate folate × 11 (dilution factor of whole blood) – (plasma folate values × (1.0 − haematocrit [Hct] expressed as a decimal))/Hct. Dilutions were done as necessary, and the respective dilution factors were considered in calculations of plasma and RBC folate concentrations. The intra‐ and inter‐assay coefficients of variation were lower than 10% across the full range of folate concentrations. Folate insufficiency was defined as RBC folate concentrations below 906 nmol L−1 in this study, as recommended by WHO (World Health Organization, 2015; Pfeiffer et al., 2016).
2.4. Statistical analyses
Because folate concentrations were not normally distributed, median and 25th and 75th percentiles were used to describe the distribution. Median concentrations of plasma and RBC folate were compared, stratified by residence (urban or rural) using nonparametric analyses (Mann–Whitney U test). Differences in demographic characteristics between groups were examined using the Student's t test. Comparisons of categorical variables were done using chi‐square tests. Binary logistic regression analyses were used to identify variables related to folate insufficiency while controlling for potential confounding variables. The results of logistic analyses are presented as odds ratios (ORs) and their 95% confidence intervals (95% CIs). All statistical analyses were performed using SPSS package version 20.0 (SPSS Inc.). A two‐tailed P ≤ .05 was considered statistically significant.
3. RESULTS
Total 1,107 pregnant women were enrolled in the study, and the mean age of the subjects was 27.3 (±4.44) years old, the mean gestational weeks was 16.8 (±3.83), and the mean BMI was 22.7 (±3.28) kg m−2. More than half of the women were living in cities (51.4%) and completed education at a community college or above (52.2%), and 12.5% were farmers and workers. Nearly a third of them were white‐collar workers, including teachers, doctors, technicians, and administrative staff. Approximately 60% of them were primigravidas (Table 1).
Table 1.
Characteristics of pregnant women and blood folate concentrations (nmol L−1) in a province with high prevalence of neural tube defects in northern China, 2014
| Variable | N | % | Plasma folate | RBC folate |
|---|---|---|---|---|
| Age (years) | ||||
| <25 | 266 | 24.0 | 25.9 (16.3, 40.6) | 1,022.0 (651.3, 1,370.6) |
| 25–29 | 573 | 51.8 | 28.4 (17.7, 46.3) | 993.9 (658.7, 1,403.8) |
| ≥30 | 260 | 23.5 | 32.9 (18.4, 47.1) | 1,001.3 (658.4, 1,503.6) |
| Missing | 8 | 0.7 | ||
| P | 0.019 | 0.605 | ||
| Gestational weeks | ||||
| <12 | 67 | 6.1 | 42.1 (21.2, 55.9) | 904.6 (501.3, 1,214.3) |
| 13–20 | 967 | 87.4 | 28.5 (17.9, 44.5) | 1,017.4 (691.3, 1,417.0) |
| ≥21 | 68 | 6.1 | 19.3 (8.5, 30.9) | 852.4 (433.7, 1,352.1) |
| Missing | 5 | 0.5 | ||
| P | 0.001 | 0.013 | ||
| BMI (kg m−2) | ||||
| <25 | 870 | 78.6 | 28.4 (17.5, 46.1) | 1,007.4 (666.3, 1,410.1) |
| ≥25 | 236 | 21.3 | 28.2 (18.1, 42.5) | 977.9 (651.0, 1,380.5) |
| Missing | 1 | 0.1 | ||
| P | 0.250 | 0.237 | ||
| Education (%) | ||||
| Junior high school or less | 268 | 24.2 | 27.9 (15.3, 44.6) | 939.3 (617.1, 1,356.4) |
| Senior high school | 261 | 23.6 | 26.2 (17.3, 40.7) | 1,012.2 (688.3, 1,394.2) |
| College and above | 578 | 52.2 | 29.7 (18.9, 47.3) | 1,029.7 (686.0, 1,446.1) |
| P | 0.148 | 0.235 | ||
| Occupation (%) | ||||
| Blue collar | 138 | 12.5 | 27.3 (16.7,46.9) | 1,042.9 (695.7, 1,412.4) |
| White collar | 351 | 31.7 | 29.1 (18.3, 48.1) | 973.8 (645.8, 1,418.9) |
| Others | 617 | 55.7 | 28.0 (17.4, 44.3) | 998.9 (658.7, 998.9) |
| Missing | 1 | 0.1 | ||
| P | 0.392 | 0.557 | ||
| Residence | ||||
| Urban | 569 | 51.4 | 29.5 (18.6, 48.7) | 1,003.4 (657.1, 1,419.6) |
| Rural | 538 | 48.6 | 27.3 (16.3, 42.4) | 994.3 (660.3, 1,393.4) |
| P | 0.006 | 0.691 | ||
| Gravidity | ||||
| Primigravidas | 647 | 58.4 | 27.3 (17.2, 43.5) | 992.3 (652.2, 1,394.2) |
| Multigravidas | 460 | 41.6 | 30.6 (18.2, 47.4) | 1,001.9 (670.6, 1,446.4) |
| P | 0.061 | 0.170 | ||
| Folic acid supplementation | ||||
| Yes | 1,002 | 90.5 | 29.3 (18.5, 45.8) | 1,017.4 (673.7, 1,413.1) |
| No | 104 | 9.4 | 18.4 (11.6, 37.9) | 844.3 (518.5, 1,270.4) |
| Missing | 1 | 0.1 | ||
| P | 0.016 | 0.018 | ||
Abbreviation: RBC, red blood cell.
3.1. Folic acid supplementation
More than 90% of the women reported having taken a folic acid supplement during the current pregnancy. However, almost two thirds (64.7%) began to take the supplement after LMP (Table 2). Rural women were more likely to take a supplement after LMP (69.3%) than urban women (60.7%). Regarding adherence to folic acid intake, 80% of 44 reported using folic acid almost every day, and 5% missed almost half of the days. The proportion of women who took more than 120 pills was lower among rural women than urban women (9.8% vs. 14.8%), and rural women took fewer pills than urban women (Table 3).
Table 2.
Folate insufficiency associated with supplementation behaviour among pregnant Chinese women who reported taking a folic acid supplement
| Plasma folate (nmol L−1) | RBC folate (nmol L−1) | Folate insufficiency (%) | ||
|---|---|---|---|---|
| Variables | N (%) | Median (25th and 75th percentiles) | Median (25th and 75th percentiles) | RBC < 906 nmol L−1 |
| Timing of supplementation | ||||
| Before LMP | 354 (35.3) | 29.4 (18.3, 49.3) | 1,031.2 (677.9, 1,450.1)* | 39.8 |
| After LMP | 649 (64.7) | 29.2 (18.7, 44.7) | 1,001.3 (671.5, 1,397.1) | 41.4 |
| Supplementation frequency | ||||
| ≥8 days/10 days | 799 (79.7) | 31.0 (19.7, 47.8) | 1,031.2 (690.4, 1,433.2)* | 39.2 |
| 5–7 days/10 days | 149 (14.9) | 25.4 (16.9, 40.1) | 947.7 (650.8, 1,316.7) | 46.9 |
| ≤5 days/10 days | 54 (5.4) | 17.9 (12.4, 29.0) | 934.2 (624.2, 1,293.9) | 48.1 |
| Total pills of folic acid | ||||
| ≥120 pills | 507 (50.5) | 37.2 (21.8, 56.2) | 1,113.5 (696.8, 1,608.7)* | 36.1 |
| 60–120 pills | 371 (37.0) | 29.4 (17.9, 45.7) | 1,018.8 (703.2, 1,466.9) | 38.8 |
| <60 pills | 125 (12.5) | 27.9 (17.9, 43.9) | 984.8 (646.4, 1,367.6) | 43.5 |
Note. There are missing values for some samples.
Abbreviation: LM, last menstrual period.
P < .05 compared between the subgroup.
Table 3.
Plasma and RBC folate concentration associated with supplementation behaviour among pregnant Chinese women: median (25th and 75th percentiles), nmol L−1
| Variables | Urban | Rural | ||||
|---|---|---|---|---|---|---|
| N (%) | Plasma | RBC | N (%) | Plasma | RBC | |
| Timing of supplementation | ||||||
| Before LMP | 210 (39.3) | 30.4 (18.3, 54.2) | 1,043.4 (675.0, 1,439.6) | 144 (30.7) | 27.8 (17.9, 43.6) | 1,026.4 (677.9, 1,466.5)* |
| After LMP | 324 (60.7) | 30.4 (20.9, 45.8) | 1,004.8 (670.6, 1,424.8) | 325 (69.3) | 27.9 (17.5, 42.5) | 994.3 (670.9, 1,382.7) |
| Supplementation frequency | ||||||
| ≥8 days/10 days | 431 (80.9) | 32.5 (20.9, 51.2) | 1,011.5 (667.7, 1,441.0) | 368 (78.5) | 29.6 (18.7, 44.7)* | 1,045.1 (710.8, 1,433.2)* |
| 5–7 days/10 days | 74 (13.9) | 25.3 (19.5, 42.6) | 1,045.4 (732.3, 1,451.7) | 75 (16.0) | 25.5 (15.5, 36.5) | 793.1 (608.6, 1,221.9) |
| ≤5 days/10 days | 28 (5.3) | 17.6 (12.4, 30.0) | 995.9 (596.1, 1,215.4) | 26 (5.5) | 18.0 (12.6, 28.9) | 877.6 (626.7, 1,446.7) |
| Total pills of folic acid | ||||||
| ≥120 pills | 79 (14.8) | 41.5 (21.6, 61.9) | 1,105.6 (639.1, 1,590.5) | 46 (9.8) | 32.1 (22.2, 42.4) | 1,161.7 (787.6, 1,640.5)* |
| 60–120 pills | 217 (40.6) | 29.5 (17.9, 48.6) | 1,003.0 (707.8, 1,421.9) | 154 (32.8) | 28.8 (17.6, 43.6) | 1,046.1 (690.8, 1,731.9) |
| <60 pills | 238 (44.6) | 29.2 (19.9, 44.9) | 1,027.4 (639.4, 1,430.3) | 269 (57.4) | 27.7 (16.9, 43.1) | 967.0 (646.7, 1,331.3) |
Note. There are missing values for some samples.
Abbreviations: LM, last menstrual period; RBC, red blood cell.
P < .05 compared between urban and rural.
3.2. Blood folate concentration
The median (25th and 75th percentiles) folate concentrations were 28.4 nmol L−1 (17.6, 45.2 nmol L−1) in plasma and 1,001.2 nmol L−1 (658.7, 1,402.5 nmol L−1) in RBCs. Overall, 42.4% of women had RBC folate concentrations below the cut‐off for insufficiency (≤906 nmol L−1). The prevalence of NTDs tended to decrease with an increase in RBC folate concentrations, with a Pearson correlation of −0.677 (Figure 1).
Figure 1.
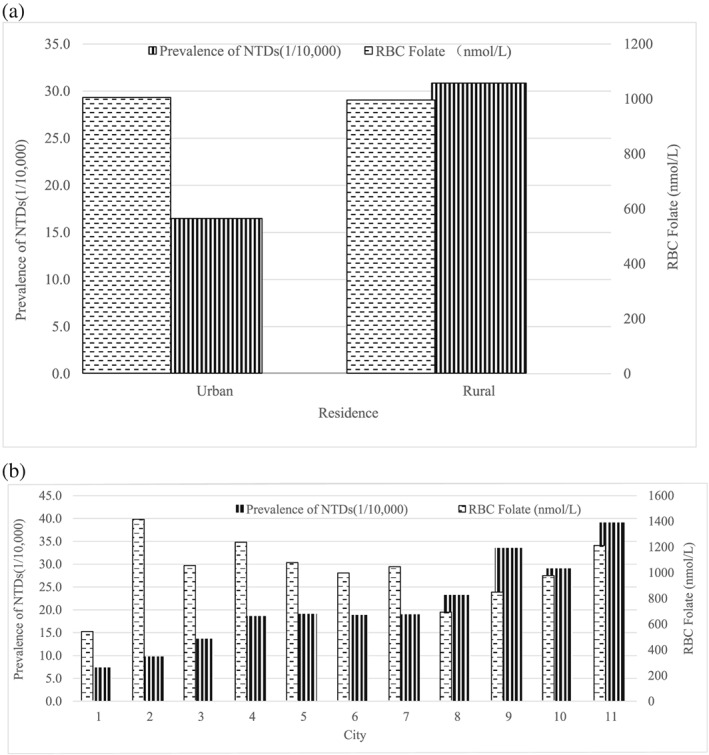
Prevalence of NTDs and blood folate in 2015–2016 in Shanxi province by (a) residence and (b) city. Data on prevalence of NTDs were from birth defect surveillance in Shanxi Province. In (b), the horizontal axis refers to 11 cities in Shanxi Province
Folic acid supplementation was correlated with RBCs folate concentrations. Plasma folate was 29.3 (18.5, 45.8) nmol L−1 in women who reported taking supplements compared with 18.4 (11.6, 37.9) nmol L−1 in women who reported not taking supplements. Regarding RBC folate, supplement users had median concentration of 1,017.4 (637.7, 1,413.1) nmol L−1 compared with 844.3 (518.5, 1,270.4) nmol L−1 in non‐users. Users had a lower proportion of RBC folate insufficiency than non‐users (40.9% vs. 57%, χ 2 = 9.672, 0.002; Table 2). There was no statistical significance between urban and rural residents (42.1% vs. 42.7, χ 2 = 0.034, 0.854). In addition, women who took more than 120 pills had a much lower rate of plasma folate insufficiency (13.6%) than women who took fewer than 60 pills (25%).
3.3. Follow‐up of birth outcomes
In the follow‐up study, 73.8% (817/1,107) women were included. There were no children with a reported NTD, four had congenital heart disease, two had cleft lip and palate, and one had hypospadias. Women with RBC folate insufficiency had a higher proportion of children with birth defects compared with the group with RBC folate concentrations > 906 nmol L−1 (1.3% vs. 0.2%, 0.02).
3.4. Factors associated with blood folate insufficiency
We further analysed the factors potentially associated with the risk of folate insufficiency while adjusting for maternal age, education, gestational weeks, residence, and folic acid supplementation. Low educational level and not taking folic acid supplements were correlated with RBC folate insufficiency (Table 4).
Table 4.
Factors associated with blood folate insufficiency by binary logistic regression
| Variable | RBC folate <906 nmol L−1 |
|---|---|
| OR (95% CI) | |
| Age (>30 years) | |
| <25 | 0.95 (0.66–1.37) |
| 25–29 | 1.03 (0.76–1.40) |
| Education (college and above) | |
| Junior high school or less | 1.43 (1.01–2.04) |
| Senior high school or more | 1.11 (0.79–1.56) |
| Gestational week (<12 weeksa) | |
| 13–20 | 0.63 (0.38–1.04) |
| >21 | 0.93 (0.47–1.86) |
| Residence (rural vs. urban) | 0.88 (0.66–1.78) |
| Take folic acid (no vs. yes) | 1.81 (1.17–2.79) |
Note. Adjusted for maternal age, education, gestational weeks, residence, take folic acid or not.
Abbreviation: OR, odds ratio; 95% CI, 95% confidence interval.
Reference group.
4. DISCUSSION
In a Chinese population with a high prevalence of NTDs, we reported plasma and RBC folate concentrations in women 5 years after the implementation of a nationwide folic acid supplementation programme. The median concentrations of folate in both plasma (28.4 nmol L−1) and RBCs (1,001.2 nmol L−1) were much lower than the concentration in women of childbearing age in the United States (plasma: 43.5 nmol L−1; RBCs: 1,379 nmol L−1; Marchetta & Hamner, 2016; McDowell et al., 2008), where mandatory flour fortification has been implemented since 1998 (Viswanathan et al., 2017). WHO recommends an RBC folate concentration threshold of 906 nmol L−1 for defining optimal NTD prevention (World Health Organization, 2015; Pfeiffer et al., 2016). In our population, 42% of the women had RBC folate concentration ≤ 906 nmol L−1, a much higher than that reported in the United States (McDowell et al., 2008) and Canada (Shere, Kapur, & Koren, 2016). Although the national folic acid supplementation programme has been implemented for 5 years at the time of conducting this research in China, the folate concentrations of pregnant women have failed to progressively increase. In fact, plasma concentrations were slightly lower in 2014 than in 2011–2012 (33.4 nmol L−1; Liu, Gao, et al., 2015), which indicates that additional measures are needed to further increase the blood folate concentrations in this population.
Because the neural tube closes by the 28th day of pregnancy, the use of RBC folate around that period could best estimate NTD risk, whereas the blood was collected from women in their 12th–20th weeks of gestation. Blood folate concentrations are expected to vary throughout pregnancy. Plasma folate levels significantly increased from preconception to 12 gestational weeks and were significantly lower in 24 gestational weeks (Looman et al., 2019). However, this study aimed to evaluate RBC folate concentrations when women had completed the recommended supplementation, that is, periconceptual period of the current pregnancy (i.e., 3 months before to 3 months after the last menstrual period). This information is valuable in determining whether the recommended daily 400‐μg folic acid is sufficient in such a population with very low dietary folate intake or staple fortification would be necessary in the setting that massive supplementation with daily 400‐μg folic acid is in place. This is why we chose women at 12th–20th gestational weeks. Although blood folate concentrations may decrease with gestation, we assume the RBC concentrations we obtained during 12th–20th gestational weeks could essentially reflect RBC concentrations around 12 weeks.
The persistently low concentrations of folate in this population may be attributable to two main reasons. First, dietary folate intake is very low. In this population, the diet is characterized by high levels of sucrose and few vegetables and fruits. The mean (standard deviation) and median (25th and 75th percentiles) daily dietary folate intake levels in women who plan to become pregnant are about 114.3 (59.7) and 102.8 (69.3–146.8) μg day−1, respectively (Meng et al., 2015), much lower than the recommended intake of 320 μg day−1 for nonpregnant women (Institute of Medicine, 2000). In fact, the U.S. Preventive Services Task Force recommends that all women who are planning or capable of pregnancy take a daily supplement containing 0.4 to 0.8 mg of folic acid (Force et al., 2017). The results of the present study support an increase in the daily dose of folic acid to 0.8 mg instead of the currently recommended dose of 0.4 mg in this population. The recently issued Guideline for the Prevention of Neural Tube Defects by Periconceptional Folic Acid Supplementation (2017) by the China Association of Maternal and Child Health Care recommends a higher dose of folic acid for women who live in northern provinces, particularly in rural areas of northern provinces (Ren et al., 2017). Besides, a very recent randomized trial in Germany also revealed that supplementation recommendations are not sufficient in countries that do not have a fortification programme (Obeid, Schon, Wilhelm, Pietrzik, & Pilz, 2018b); hence, food fortification may be another option, which is an effective way to raise blood folate concentrations (Ren, 2015).
Second, compliance with supplementation was inadequate. In the present survey, more than 90% of women reported taking a folic acid supplement, whereas about two thirds of women began taking supplements after they knew that they were pregnant, 20% took supplements in fewer than 8 days out of 10 days, and about half of the women took fewer than 120 pills total. These indicators of poor compliance are associated with low folate concentrations. These findings should prompt health care workers to devise innovative measures to ensure initiation of folic acid supplementation from before pregnancy and continued supplementation every day.
Consistent with a recent report from Guatemala (Rosenthal et al., 2016), gaps were present in folate concentrations between rural and urban women: Urban women had higher folate concentrations than rural women. Moreover, rural women had a higher proportion of folate insufficiency than urban women, replicating findings from our previous study (Liu, Gao, et al., 2015). In accordance with the gaps in blood folate concentrations, disparities in folic acid supplementation remained between rural women and urban women. Urban women have advantages in terms of socio‐economic status and higher education, and they may have more knowledge of folic acid (2008), which may then positively affect the use of folic acid supplements. Future educational campaigns on folic acid supplementation should focus on women living in rural areas.
We identified several other factors associated with a high risk of blood folate insufficiency. No folic acid supplementation during the periconceptional period was associated with an increased risk of RBC folate insufficiency. Studies have repeatedly demonstrated that folic acid supplementation is an important factor associated with folic acid deficiency or insufficiency (Liu, Gao, et al., 2015), further emphasizing the importance of supplementation during the periconceptional period in raising folate concentrations for the prevention of NTDs. With the low acceptance of free folic acid pills, the folate‐biofortified rice would be a possible complementary micronutrient intervention as suggested (De Steur, Feng, Xiaoping, & Gellynck, 2014). The amount of folic acid should be added to a women's diet depends on the additional source(s) of folic acid. The staple food in this population was wheat flour instead of rice, given the truth that wheat flour was the ideal food for fortification. There is no rigorous study that has evaluated the amount of folic acid to be added to flour targeted to this population. However, study from the United States evaluated the mandated fortification of enriched cereal grain products with 140 μg of folic acid per 100 g, and the intervention seemed effective, which birth prevalence of NTD decreased 35% (Crider, Qi, Devine, Tinker, & Berry, 2018). Therefore, the widely used fortification with 140 μg of folic acid per 100 g could be used at the initial stage, and fortification dose may be adjusted according the effectiveness.
The present study had several strengths. First, it covered a wider graphical areas of the province than previous studies, which facilitates the generalization of the findings to the whole province and perhaps to neighbouring provinces. Second, the microbiological assay used to determine plasma folate concentrations is reliable as evidenced by the cross‐validation results in the VITAL‐External Quality Assurance (VITAL‐EQA) programme (Liu, Gao, et al., 2015). Third, not only plasma folate but also RBC folate, which reflects long‐term folate intake, were analysed.
The main limitation of the present study was that subjects were not randomly selected. However, the first 100 women who came to seek prenatal care (and thus were recruited into this study) should not be different from those who came later because all pregnant women are covered in the prenatal care programme. In addition, data on folic acid supplementation and adherence were based on self‐reports. However, reporting is generally reliable because folic acid users have significantly higher blood folate concentrations than non‐users (Ren et al., 2007). Another limitation of the study was information on dietary folate intake was not collected. However, as there is traditional northern dietary habit characterized by high levels of sucrose and few vegetables and fruits in Shanxi province, the dietary style is not expected to change much between woman who are pregnant and women who are not, whose daily dietary folate intake levels was low (Meng et al., 2015; Institute of Medicine, 2000).
In conclusion, a large proportion of women failed to reach the optimal blood folate concentrations that are considered effective for NTD prevention 5 years after implementation of the nationwide folic acid supplementation programme (0.4 mg folic acid per tablet) in China. Most importantly, the majority of the women initiated supplementation after they know they were pregnant. Supplementation was associated with a lower risk of folate insufficiency. The challenge for governmental departments and maternal and child health workers is to ensure folic acid supplementation from before pregnancy and ensure adherence to the supplementation regimen. The findings of this study support the implementation of folic acid fortification of staple foods in this population with a low dietary folate intake and a persistently high prevalence rate of NTDs.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
XZ designed and conducted research, JL analysed the data and wrote the first draft of the manuscript, YJ supervised and coordinated the study, SY and ZS collected the data and analysed the data, LJ and LW participated in the blood folate assessment, AR provided critical suggestions on the study plan, supervised data collection, and revised the draft. All authors read, reviewed, and approved the final manuscript.
Zhang X, Liu J, Jin Y, et al. Folate of pregnant women after a nationwide folic acid supplementation in China. Matern Child Nutr. 2019;15:e12828 10.1111/mcn.12828
Xuejuan Zhang and Jufen Liu have equal contribution.
REFERENCES
- Bower, C. (2007). Mandatory fortification of flour with folic acid to prevent neural‐tube defects. Womens Health (Lond), 3, 309–314. 10.2217/17455057.3.3.309 [DOI] [PubMed] [Google Scholar]
- Busby, A. , Abramsky, L. , Dolk, H. , Armstrong, B. , Addor, M. C. , Anneren, G. , … Steinbicker, V. (2005). Preventing neural tube defects in Europe: A missed opportunity. Reproductive Toxicology, 20, 393–402. 10.1016/j.reprotox.2005.03.009 [DOI] [PubMed] [Google Scholar]
- Crider, K. S. , Qi, Y. P. , Devine, O. , Tinker, S. C. , & Berry, R. J. (2018). Modeling the impact of folic acid fortification and supplementation on red blood cell folate concentrations and predicted neural tube defect risk in the United States: havewe reached optimal prevention? Am J Clin Nutr, 107, 1027–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeizel, A. E. , & Dudas, I. (1992). Prevention of the first occurrence of neural‐tube defects by periconceptional vitamin supplementation. The New England Journal of Medicine, 327, 1832–1835. 10.1056/NEJM199212243272602 [DOI] [PubMed] [Google Scholar]
- De Steur, H. , Feng, S. , Xiaoping, S. , & Gellynck, X. (2014). Consumer preferences for micronutrient strategies in China. A comparison between folic acid supplementation and folate biofortification. Public Health Nutrition, 17, 1410–1420. 10.1017/S1368980013000682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Force, U. S. P. S. T. , Bibbins‐Domingo, K. , Grossman, D. C. , Curry, S. J. , Davidson, K. W. , Epling, J. W. Jr. , … Tseng, C. W. (2017). Folic acid supplementation for the prevention of neural tube defects: US Preventive Services Task Force ecommendation statement. Jama, 317, 183–189. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (2000). Dietary reference intakes for thiamin, riboflavin, niacin, vitamin B6, folate, vitamin B12, pantothenic acid, biotin, and choline. Washington, D.C.: National Academy Press. [PubMed] [Google Scholar]
- Li, Z. , Ren, A. , Zhang, L. , Ye, R. , Li, S. , Zheng, J. , … Li, Z. (2006). Extremely high prevalence of neural tube defects in a 4‐county area in Shanxi Province, China. Birth Defects Research. Part a, Clinical and Molecular Teratology, 76, 237–240. 10.1002/bdra.20248 [DOI] [PubMed] [Google Scholar]
- Liu, J. , Gao, L. , Zhang, Y. , Jin, L. , Li, Z. , Zhang, L. , … Ren, A. (2015). Plasma folate levels in early to mid pregnancy after a nation‐wide folic acid supplementation program in areas with high and low prevalence of neural tube defects in China. Birth Defects Research. Part a, Clinical and Molecular Teratology, 103, 501–508. 10.1002/bdra.23368 [DOI] [PubMed] [Google Scholar]
- Liu, J. , Jin, L. , Meng, Q. , Gao, L. , Zhang, L. , Li, Z. , & Ren, A. (2015). Changes in folic acid supplementation behaviour among women of reproductive age after the implementation of a massive supplementation programme in China. Public Health Nutrition, 18, 582–588. 10.1017/S1368980014000950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Xie, J. , Li, Z. , Greene, N. D. E. , & Ren, A. (2018). Sex differences in the prevalence of neural tube defects and preventive effects of folic acid (FA) supplementation among five counties in northern China: Results from a population‐based birth defect surveillance programme. BMJ Open, 8, e022565 10.1136/bmjopen-2018-022565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, J. , Zhang, L. , Li, Z. , Jin, L. , Zhang, Y. , Ye, R. , … Ren, A. (2016). Prevalence and trend of neural tube defects in five counties in Shanxi province of Northern China, 2000 to 2014. Birth Defects Research. Part a, Clinical and Molecular Teratology, 106, 267–274. 10.1002/bdra.23486 [DOI] [PubMed] [Google Scholar]
- Looman, M. , Geelen, A. , Samlal, R. A. K. , Heijligenberg, R. , Klein Gunnewiek, J. M. T. , Balvers, M. G. J. , … Feskens, E. J. M. (2019). Changes in micronutrient intake and status, diet quality and glucose tolerance from preconception to the second trimester of pregnancy. Nutrients, 11 10.3390/nu11020460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetta, C. M. , & Hamner, H. C. (2016). Blood folate concentrations among women of childbearing age by race/ethnicity and acculturation, NHANES 2001‐2010. Maternal & Child Nutrition, 12, 39–50. 10.1111/mcn.12134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorell, R. , & de Romana, D. L. (2017). Components of successful staple food fortification programs: Lessons from Latin America. Food and Nutrition Bulletin, 38, 384–404. 10.1177/0379572117707890 [DOI] [PubMed] [Google Scholar]
- McDowell, M. A. , Lacher, D. A. , Pfeiffer, C. M. , Mulinare, J. , Picciano, M. F. , Rader, J. I. , … Johnson, C. L. (2008). Blood folate levels: The latest NHANES results. NCHS Data Brief, 1–8. [PubMed] [Google Scholar]
- Meng, Q. , Zhang, L. , Liu, J. , Li, Z. , Jin, L. , Zhang, Y. , … Ren, A. (2015). Dietary folate intake levels in rural women immediately before pregnancy in Northern China. Birth Defects Research. Part a, Clinical and Molecular Teratology, 103, 27–36. 10.1002/bdra.23280 [DOI] [PubMed] [Google Scholar]
- MRC Vitamin Study Research Group (1991). Prevention of neural tube defects: Results of the Medical Research Council Vitamin Study. Lancet, 338, 131–137. [PubMed] [Google Scholar]
- Obeid, R. , Schon, C. , Wilhelm, M. , Pietrzik, K. , & Pilz, S. (2018a). Dietary and lifestyle predictors of folate insufficiency in non‐supplemented German women. Int J Food Sci Nutr, 1–10. [DOI] [PubMed] [Google Scholar]
- Obeid, R. , Schon, C. , Wilhelm, M. , Pietrzik, K. , & Pilz, S. (2018b). The effectiveness of daily supplementation with 400 or 800 microg/day folate in reaching protective red blood folate concentrations in non‐pregnant women: A randomized trial. European Journal of Nutrition, 57, 1771–1780. 10.1007/s00394-017-1461-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Broin, S. , & Kelleher, B. (1992). Microbiological assay on microtitre plates of folate in serum and red cells. Journal of Clinical Pathology, 45, 344–347. 10.1136/jcp.45.4.344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer, C. M. , Sternberg, M. R. , Hamner, H. C. , Crider, K. S. , Lacher, D. A. , Rogers, L. M. , … Yetley, E. A. (2016). Applying inappropriate cutoffs leads to misinterpretation of folate status in the US population. The American Journal of Clinical Nutrition, 104, 1607–1615. 10.3945/ajcn.116.138529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, A. (2015). Prevention of neural tube defects with folic acid: The Chinese experience. World Journal of Clinical Pediatrics, 4, 41–44. 10.5409/wjcp.v4.i3.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, A. , Zhang, L. , Hao, L. , Li, Z. , & Tian, Y. (2007). Comparison of blood folate levels among pregnant Chinese women in areas with high and low prevalence of neural tube defects. Public Health Nutrition, 10, 762–768. 10.1017/S1368980007246786 [DOI] [PubMed] [Google Scholar]
- Ren, A. , Zhang, X. , Liu, H. , Zhu, L. , Liu, K. , Jia, Y. , & for the Working Group of Neural Tube Defects Prevention with Folic Acid Supplementation (2017). Guideline for the prevention of neural tube defects by periconceptional folic acid supplementation. Zhongguo Shengyu Jiankang Zazhi, 28, 401–410. [Google Scholar]
- Rosenthal, J. , Reeve, M. E. , Ramirez, N. , Crider, K. S. , Sniezek, J. , Vellozzi, C. , … Lopez‐Pazos, E. (2016). Red blood cell folate insufficiency among nonpregnant women of childbearing age in Guatemala 2009 to 2010: Prevalence and predicted neural tube defects risk. Birth Defects Research. Part a, Clinical and Molecular Teratology, 106, 587–595. 10.1002/bdra.23499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgh, G. , Singh, S. , & Hussain, R. (2014). Intended and unintended pregnancies worldwide in 2012 and recent trends. Studies in Family Planning, 45, 301–314. 10.1111/j.1728-4465.2014.00393.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shere, M. , Kapur, B. M. , & Koren, G. (2016). Folate status of women in Toronto: Implications of folate fortification and supplementation. Canadian Journal of Public Health, 106, e509–e513. 10.17269/cjph.106.5109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooij, I. A. , Ocke, M. C. , Straatman, H. , Zielhuis, G. A. , Merkus, H. M. , & Steegers‐Theunissen, R. P. (2004). Periconceptional folate intake by supplement and food reduces the risk of nonsyndromic cleft lip with or without cleft palate. Preventive Medicine, 39, 689–694. [DOI] [PubMed] [Google Scholar]
- Viswanathan, M. , Treiman, K. A. , Kish‐Doto, J. , Middleton, J. C. , Coker‐Schwimmer, E. J. , & Nicholson, W. K. (2017). Folic acid supplementation for the prevention of neural tube defects: An updated evidence report and systematic review for the US Preventive Services Task Force. Jama, 317, 190–203. 10.1001/jama.2016.19193 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2015). WHO guidelines approved by the guidelines review committee guideline: Optimal serum and red blood cell folate concentrations in women of reproductive age for prevention of neural tube defects. Geneva: World Health Organization. [PubMed] [Google Scholar]
- Zaganjor, I. , Sekkarie, A. , Tsang, B. L. , Williams, J. , Razzaghi, H. , Mulinare, J. , … Rosenthal, J. (2016). Describing the prevalence of neural tube defects worldwide: A systematic literature review. PLoS ONE, 11, e0151586 10.1371/journal.pone.0151586 [DOI] [PMC free article] [PubMed] [Google Scholar]


