Abstract
The impact of quality complementary food products on infant growth and body composition has not been adequately investigated. This study evaluated the effect on fat‐free mass (FFM) accrual, linear growth, and iron status of locally produced complementary food products comparing to a standard product. In a randomized, double‐blind trial, 499 infants at 6 months received nine monthly rations of (a) WinFood Classic (WFC) comprising germinated amaranth (71%), maize (10.4%), small fish (3%), and edible termites (10%); (b) WinFood Lite (WFL) comprising germinated amaranth (82.5%), maize (10.2%), and multimicronutrient premix; or (c) fortified corn–soy blend plus (CSB+). Primary outcomes were changes in FFM, length, and plasma ferritin and transferrin receptors (TfR). FFM was determined using deuterium dilution. Analysis was by intention to treat, based on available cases. Compared with CSB+, there were no differences in change from 6 to 15 months in FFM for WFC 0.0 kg (95% CI [−0.30, 0.29]) and WFL 0.03 kg (95% CI [−0.25, 0.32]) and length change for WFC −0.3 cm (95% CI [−0.9, 0.4]) and WFL −0.3 cm (95% CI [−0.9, 0.3]). TfR increased in WFC group 3.3 mg L−1 (95% CI [1.7, 4.9]) and WFL group 1.7 mg L−1 (95% CI [0.1, 3.4]) compared with CSB+. Compared with the increase in Hb in CSB+ group, there was a reduction in Hb in WFC of −0.9 g dl−1 (95% CI [−1.3, −0.5]) and a lower increase in WFL −0.4 g dl−1 (95% CI [−0.8, 0.0]). In conclusion, the tested WinFoods had the same effect on FFM and length as CSB+, whereas Hb and iron status decreased, suggesting inhibited iron bioavailability from the amaranth‐based WinFoods.
Keywords: animal‐source foods, body composition, complementary feeding, deuterium dilution technique, edible termites, iron status
Key messages.
To develop effective interventions targeting linear growth, it is important to explore using locally available foods to enhance their intake in infants and young children diet.
Understanding the body composition in terms of fat free mass in complementary feeding interventions so as to link them to growth outcomes later in life is important.
Engaging local food resources like grain amaranth, termites and fish has a potential to the utilization and sustainability of stunting and anaemia reduction interventions.
Germinated amaranth with ASFs or fortificant need optimizing to improve nutritional status compared to CSB+ in resource limited settings.
Abbreviations
- ASF
animal‐source food
- CSB
corn–soy blend
- WFC
WinFood Classic
- WFL
WinFood Lite
- CSB+
corn–soy blend plus
- CSB++
corn–soy blend plus
- FFM
fat‐free mass
- FM
fat mass
- LAZ
length‐for‐age z‐score
- MUAC
mid‐upper arm circumference
- WAZ
weight‐for‐age z‐score
- WFP
World Food Programme
- WLZ
weight‐for‐length z‐score
1. INTRODUCTION
Growth faltering begins and rapidly accelerates in the first 1,000 days of life with lifelong consequences (UNICEF‐WHO‐The World Bank Group, 2017). Linear growth failure is a strong marker of a complex of pathological disorders that in sum lead to increased morbidity and mortality, loss of physical growth potential, reduced neurodevelopment and cognitive functions and decreased human potential (UNICEF‐WHO‐The World Bank Group, 2017). Attempts to address linear growth faltering through a number of interventions including high‐energy plant‐based foods fortified with a mix of multiple micronutrients, improved water, hygiene and sanitation, behaviour change communication to improve infant and young child feeding practices have had limited effects on linear growth (Byrd et al., 2018; Lin et al., 2018; Nair et al., 2017; Null et al., 2018).
However, a number of studies have shown that animal‐source foods (ASFs) are beneficial to child growth, cognitive functions and reduced morbidity and mortality as they provide high‐quality protein and micronutrients that are difficult to obtain in adequate quantities from a diet based on plant‐source foods alone (Allen, 2008; Dror & Allen, 2011). ASFs are not easily accessible and often unaffordable to many poor households and therefore lacking or scarce in the diets of children in low‐ and middle‐income countries (LMICs; Dror & Allen, 2011). However, communities in LMICs may have access to other sources of relatively affordable ASFs such as small fish species with low market value (Roos, Wahab, Chamnan, & Thilsted, 2007) and edible insects (or other arthropods such as spiders) collected from the wild (FAO, 2013).
The WinFoods study aimed to develop nutritionally improved foods for infants in LMIC based on improved utilization of locally available foods, together with improved traditional food technologies (e.g., fermentation and malting). These foods were dubbed “WinFoods.” The WinFood project was carried out in parallel in Kenya and Cambodia from 2009 to 2012. In each site, processed complementary food products were formulated and produced on the basis of locally available foods and optimized for nutrient composition with emphasis on iron and zinc (Kinyuru et al., 2012; Kinyuru et al., 2015; Skau et al., 2015). Prior to the final decision on formulations, the acceptability of the products was assessed among mothers and infants (Konyole et al., 2012). The efficacy of the developed complementary food products was then tested in randomized trials to assess the impacts of a daily supplement over a 9‐month period on growth, nutritional status, and development. A Cambodian study found no difference in the primary outcomes of increment in fat‐free mass (FFM) and iron status after 9‐month intervention, between either of the two versions of WinFood products tested (one fortified and one not fortified with micronutrients) in comparison with either of two corn–soy blend (CSB+ and CSB++) products (Skau et al., 2015). It was concluded that micronutrient fortification may be necessary, and small fish may be an affordable alternative to milk to improve complementary foods. The Cambodian trial also showed that, despite the daily complementary food supplement, the children became increasingly stunted over the intervention period (Skau et al., 2015).
The aim of the current study was to evaluate the effect on FFM accrual, linear growth, and iron status of improved cereal‐legume‐based WinFood products, one product prepared with ASFs (small fish and white‐winged termites) and one product prepared without ASFs but fortified with a multimicronutrient premix. The products were compared with the fortified standard product CSB+.
2. METHODS
2.1. Study setting
The study was conducted in a malaria‐prone and food‐insecure rural area of Mumias Sub‐County in Kakamega County, Western Kenya (Desai et al., 2005) from January 2012 to January 2013. It was based at Makunga, Khaunga, and Lusheya health centres. About 26% of children aged below 5 years in the region are stunted (Kenya National Bureau of Statistics et al., 2015).
2.2. Study participant recruitment, inclusion, and exclusion criteria
Mothers and infants pairs were invited to the study at 5 months of age and randomized at 6 to receive one of the three study foods from the health facilities they visited for routine monthly growth monitoring. Trained health workers screened the infants for severe acute malnutrition (≤3 weight‐for‐length z‐score [WLZ]), pitting oedema, clinical signs of vitamin A deficiency, severe anaemia (haemoglobin <80 g L−1), and mid‐upper arm circumference (MUAC) <11.5 cm. If any of these symptoms were detected, the infant was excluded and referred for treatment as per the Kenya Ministry of Health guidelines. An additional inclusion criterion was that caregivers had to consent to participate and accept to prepare and feed their infants with the assigned complementary foods. Exclusion criteria were lack of consent, severe malnutrition, or anaemia as defined above or chronic illness requiring medication or genetic disorders interfering with normal growth. Twins were recruited into the study if both were healthy and met the inclusion criteria and randomized to receive the same intervention to avoid cross‐contamination due to confusing the foods or sharing during feeding.
2.3. Study design
This was a randomized, double‐blind, controlled trial in which infants aged 6 months received a monthly ration for 9 months of one of the three study foods: (a) WinFood Classic (WFC), (b) WinFood Lite (WFL), or (c) CSB+ as the comparison group. Changes in FFM, length, plasma ferritin, plasma transferrin receptors, and haemoglobin from 6 to 15 months of age were the main outcomes. Secondary outcomes were change in weight, MUAC, head circumference, skinfolds, and weight.
2.4. Intervention foods
Food ingredients used for the complementary foods are already widely available and consumed in the locality (Kinyuru et al., 2012). Details of recipe formulation, nutrient composition, processing technology, and safety are described elsewhere (Kinyuru et al., 2015), and the foods were found to be acceptable prior to the intervention in the study population (Konyole et al., 2012).
All the foods were centrally processed by extrusion cooking at AllGrain Co. Limited in Nairobi Kenya and packed in opaque food grade white plastic containers, weighed at 500 g, and labelled with computer‐generated random numbers corresponding to WFL, WFC, and CSB+ under close supervision of the study team. Two complementary foods WFC and WFL based on germinated grain amaranth (Amaranthus cruentus) and maize (Zea mays) had been developed. WFC had (as percentage of dry w/w) 71% grain amaranth, 10.4% maize, 0.6% soybean oil, 5% sugar, 10% edible termites (Macrotermes subhylanus), and 3% small fish (Rastrineobola argentea). These fish are the silver cyprinid, a species of ray‐finned fish in the family Cyprinidae found in Lake Victoria and locally called omena (Kenya), dagaa (Tanzania), and mukene (Uganda). Omena is a rich source of iron and zinc (Capinera et al., 2008; FAO, 2013; Kinyuru et al., 2013; Rumpold & Schlüter, 2013). The grain amaranth was germinated to reduce phytates and potentially enhance micronutrient bioavailability (Kinyuru et al., 2015), especially the bioavailability of iron and zinc. WFL had 82.5% grain amaranth, 10.2% maize, 0.6% soybean oil, 5% sugar, and no ASFs but fortified with micronutrients (vitamins and minerals premix) at 0.2% of mineral/vitamin premix and 1.56% mono‐calcium phosphate and sodium chloride, which are the same rate as CSB+ (World Food Program, 2015). According to World Food Program (2015), CSB+ also called a supercereal plus is made of corn (74%) and soya (19%), sugar (5%), oil (0.5%), Premix (1.5%), and contains no other ASF not even milk powder and is meant for children 6–23 months. The micronutrients were added to the blends (where applicable) after extrusion cooking to avoid vitamin losses at high processing temperatures (World Food Program, 2015). WFC provided, per 100 g dry weight, 423.6 kcal, 19 g protein, 12.2 mg Fe, and 6.3 mg Zn; WFL provided 407.2 kcal, 14.6 g protein, 12.5 mg Fe, and 5.5 mg Zn; and CSB+ provided 391.7 kcal, 15.1 g protein, 7.7 mg Fe, and 5.1 mg Zn. All the study foods were given in daily rations adjusted to the age of the child with children in the age group 6–8, 9–11, and 12–15 months receiving 50, 75, and 125 g day−1 of flour, respectively, based on the WHO recommendations for complementary feeding of breastfed infants to supply 200, 300, or 550 kcal day−1 (Dewey & Brown, 2003; Pan American Health Organization, 2001).
The food ration was a complement to breast milk and other foods. The daily rations were delivered in monthly rations packages with instructions to caregivers not to share the food with other young children not in the study and how to correctly measure quantities for daily use. Compliance was assessed by asking the caregivers how much of the study foods the child consumed while checking for any spoilage, spillages, and the frequency of feeding (results not shown). A regimen compliance study was also done once midway through the intervention in a subsample of 254 participants to confirm compliance with the prescribed feeding regime; adequate compliance, defined as consuming at least 60% of the amount provided, was achieved by 65% of the group (results not shown). The degree of sharing with other household members was also assessed by occasional home visits and caregiver's recall at the health facility during the monthly visits. The caregivers were to keep all the distributed packets, including those used, to be counted on a monthly basis.
2.5. Randomization and blinding
Individual randomization stratified by sex was done by labels generated using Microsoft Excel™ for the infants to receive WFC, WFL, or CSB+ at 6 months old. Packaging was similar for all three foods. Barcodes were assigned to the foods for complete blinding with two different codes for each food, resulting in a total of six codes. The randomization key was kept in a sealed envelope, not available to the study team or participants, until preliminary analyses were completed.
2.6. Participant visits
Health personnel then examined the child for different symptoms, which could be related to diseases and malnutrition. Additional data were collected on breastfeeding, introduction of complementary foods (dietary assessment using the 24 hr recall), morbidity, and sociodemographic and socio‐economic variables at baseline. Defaulting participants were followed up and accommodated during the next session.
2.7. Body composition measurement
FFM and fat mass (FM) were assessed at 6 and 15 months using the deuterium dilution technique. Briefly, a predose sample of about 2 ml of saliva was taken from the child's mouth using a cotton ball. Each child was given a standardized oral dose of deuterium labelled water (15 ml of 2H2O solution comprising 3 g deuterium [99.8% 2H2O—Cambridge Isotope Laboratories Inc.] and 12 ml of mineral water) that had been accurately weighed at the Kenya Medical Research Institute (KEMRI) laboratories in Nairobi, Kenya, and transported refrigerated at 4°C to the field following guidelines from the International Atomic Energy Agency (IAEA; IAEA, 2011). Postdose saliva samples were taken at 2 and 3 hr. Saliva samples were collected into a tightly capped 1.5 ml cryogenic tube by squeezing the saliva from the wet cotton ball removed from the child's mouth using a syringe. Samples were kept in a cooler box with ice packs and were transported the same day to a central collection point at Lusheya health centre where they were stored in a chest freezer at −20°C pending transfer in dry ice package to KEMRI in Nairobi for analysis. Enrichment of deuterium in saliva samples was determined using Fourier transform infrared spectrophotometer (Shimadzu model 8400s, Shimadzu Corporation, Kyoto, Japan). Enrichment of the predose sample from the child was used for background correction of postdose samples. Our study was based on a previous protocol assuming that deuterium equilibration takes less than 3 hr in infants and children when saliva is the primary specimen (Colley, Byrne, & Hills, 2007). Using the mean of deuterium enrichment based on the two postdose samples (Colley et al., 2007) as per the protocol at the time (IAEA, 2011), the dilution space and total body water (TBW) were calculated accordingly. FFM was calculated by dividing TBW by an age‐specific hydration factor as TBW/0.79 for both sexes. FM was calculated as body weight minus FFM (IAEA, 2011).
2.8. Iron status
Three‐millilitre blood samples were drawn by venepucture from nonfasting subjects both at 6 and 15 months. Haemoglobin concentration was measured on blood drop aliquots using a HemoCue HB301 photometer (HemoCue Sheffield, UK). Blood left in the syringe was put into a plain Vacutainer (Becton Dickinson), kept chilled at 4°C, and separated within 4 hr by centrifugation (1,300 g, 10 min at 4°C). Plasma samples were kept frozen at −20°C for 3 months at Lusheya health centre until they were sent to the University of Nairobi Institute of Tropical and Infectious Diseases, Nairobi, Kenya, for further storage at −80°C. Plasma samples were subsequently transported by air to the VitMin Lab (Willstaett, Germany) for analysis of plasma ferritin, plasma transferrin receptors, alpha‐1‐acid glycoprotein (AGP), and C‐reactive protein (CRP) concentrations using enzyme immunoassays using commercial ELISA test kits (Ramco Laboratories) as described by Erhardt, Estes, Pfeiffer, Biesalski, and Craft (2004).
2.9. Anthropometry
Measurements were carried out monthly by trained assistants who had previous experience in growth monitoring at the clinics' maternal and child health department. Measurements (nude weight, recumbent length, subscapular skinfolds, head circumference, and MUAC) were made in triplicate using standardized anthropometric techniques and calibrated equipment (Lohman, Roche, & Martorell, 1988). Length was measured to the nearest 0.1 cm using calibrated length board. Weight was measured to the nearest 0.01 kg using a hanging Seca scale (UniScale). Triceps, biceps, subscapular, and suprailiac skinfolds were measured to the nearest 0.1 mm using Harpenden skinfold callipers (Crymych, UK), whereas the head circumference and MUAC were measured to the nearest 0.1 cm using nonstretchable measuring tape (Harlow Printing Limited). To minimize interobserver variation in measurements, each assistant took daily measurements of weight, height, MUAC, and skinfolds (triceps, biceps, subscapular, and suprailiac) of the same volunteer until these agreed within the allowable error margin during the training period (Lohman et al., 1988).
2.10. Morbidity
Morbidity data were collected on the basis of caregivers' recall about specific symptoms and clinic visits in the past 7 days especially for the upper respiratory tract infection and diarrhoea as defined by WHO, respectively (WHO, 2001a, 2001b; WHO, 2017). General assessment of overall morbidity in the last month (scored as healthy; mild, self‐limited illness; moderate illness requiring symptomatic treatment at the clinic; severe illness requiring antibiotics or other medical intervention) for the child during their monthly visits to clinic using questionnaires was also done. Caregivers were encouraged to bring their children to the clinic in the event of severe illness prior to the next visit.
2.11. Sample size consideration
Sample size was based on expected increase length‐for‐age of at least 0.1 ± 1.2 standard deviations (SDs; Ashworth, 2006; Lartey, Manu, Brown, Peerson, & Dewey, 1999), an SD of 4.6 (Eichler, Wieser, Rüthemann, & Brügger, 2012; Faber, Kvalsvig, Lombard, & Benadé, 2005), and change in FFM. Based on the expected increase length‐for‐age, a sample size of at least 165 children per group (total of 499) was needed at 80% power and 5% level of significance allowing for 10% loss to follow up as observed in previous studies (Admassu et al., 2017; Owino et al., 2007; Bauserman et al., 2015).
2.12. Data analysis
Primary outcomes were changes in FFM, length, plasma ferritin, and plasma transferrin receptors. Analysis was by intention to treat, based on available cases. Case record forms were checked daily and entered within 2 weeks. Quantitative data were double entered in Microsoft Excel™ with length and weight measurements converted to z‐scores using WHO Anthro™ v3.2.2 based on the WHO's 2006 Child Growth Standards (WHO, 2014). Frequencies, means, and median values were calculated using STATA® version 12 (A Stata Press Publication, 2011). Analysis of variance was used to determine differences between groups in change in parameters from 6 to 15 months. Plasma ferritin concentrations were log‐transformed after correction for inflammation using CRP and AGP concentrations and the correction factors as published elsewhere (Thurnham et al., 2010). The means from the log‐transformed plasma ferritin values were then back‐transformed to get a geometric mean. The same was done for the differences and back‐transformed to give a ratio.
Selected pair‐wise comparisons were considered with CSB+ used as the reference group. Stunting, underweight and wasting were defined as length‐for‐age, weight‐for‐age and weight‐for‐length, respectively, ≤2 SDs of the WHO reference standards, whereas moderate‐to‐severe and severe acute malnutrition in infants were defined as MUAC <12.5 cm and MUAC <11.5 cm, respectively (WHO, 2014). Before the study was unblinded, all infants with negative percentage of FM were considered implausible and removed because negative values occur when the deuterium dose has not had sufficient time to fully equilibrate with body water, or the dose was not completely consumed (IAEA, 2011). We also reviewed and checked with field notes regarding problems administering the 2H2O to the child. Any uncertainty of how much 2H2O the child consumed led to his or her exclusion from the deuterium analyses. Furthermore, in cases of poor agreement between pairs of enrichment values, we discarded all of those where the two values differed by >50 ppm based on expert opinion of what is plausible but these variations could be due to spillages of deuterium during dosing. We also rejected outliers that fitted very poorly with the general association of body water with weight and height as described by other workers (IAEA, 2011; Colley et al., 2007). More samples were removed at 15 than 6 months because more had poor agreement due to longer equilibration times in older children as observed previously by Colley et al. (2007).
2.13. Ethical considerations
Mothers and caretakers gave written informed consent after explanations in the local language and Kiswahili with an option to discontinue from the study at anytime while still receiving the monthly food ration and other health facility services. All data obtained in the study were kept anonymous. The study was approved by the Kenyatta National Hospital University of Nairobi Ethics Review Committee (KNH‐UON ERC‐P436/12/2010) with a consultative approval also obtained from the Danish National Committee on Biomedical Research Ethics. Permission to implement the study was obtained from relevant government line ministries and local authorities.
The trial was registered at http://Controlled-trials.com (No: ISRCTN30012997).
3. RESULTS
We screened 527 infants of whom 499 (94.6%) met the inclusion criteria and were randomized to one of the three food groups (Figure 1). Four hundred twenty‐eight children (86%) completed the study. Of the 71 (14%) children lost to follow up, 63 (89%) relocated from the study area, whereas eight (11%) died. The dropout rate did not differ between groups. Of the 499 included, we obtained body composition data from 442 (89%) at 6 months and 288 (58%) at 15 months. The numbers at 15 months being lower compared with 6 months due to longer equilibration time as explained by Colley et al. (2007).
Figure 1.
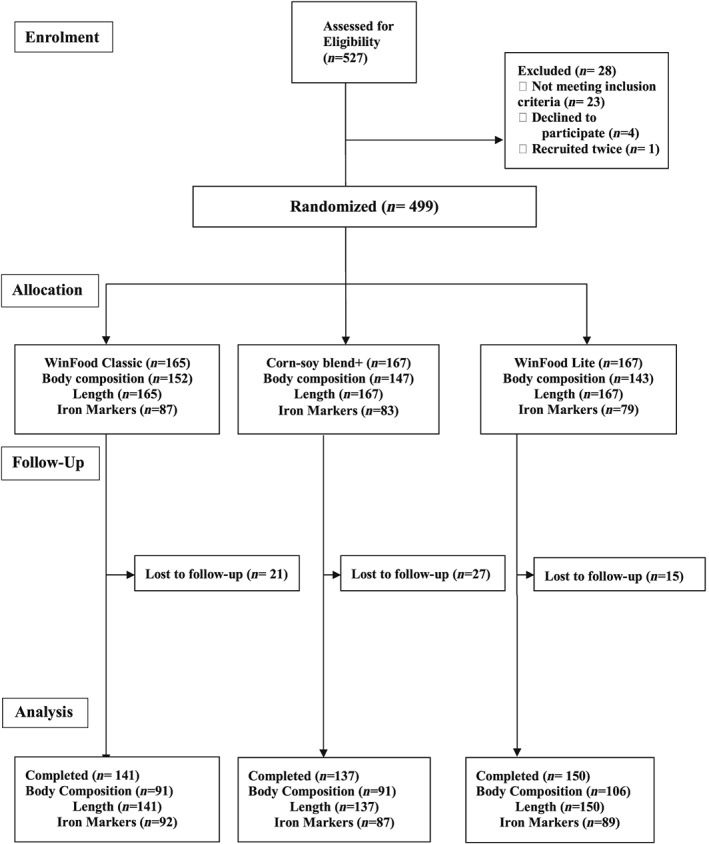
Participant screening, recruitment, and follow‐up. Five hundred twenty‐seven infant mother pairs were eligible at enrolment. Twenty‐eight were however excluded for various reasons. The remaining 499 were randomized into the three arms of the study to either receive WinFood Classic (165), corn–soy blend plus (167), or WinFood Lite (167). At 6 months, body composition was taken from WFC = 152, CSB+ = 147, and WFL = 143 participants, whereas blood samples for iron biomarkers were obtained from 87, 83, and 79 participants, respectively. During the follow‐up, WFC, CSB+, and WFL lost 21, 27, and 15 participants, respectively; the main reasons being relocation from the study area. WFC and CSB+ arms each recorded three deaths, whereas WFL recorded two deaths. For analysis therefore, 141,137, and 150 completed the study and were included in the analysis with an intention to treat for WFC, CSB+, and WFL, respectively. Body composition had 91 each for WFC and CSB+ with 106 being in the WFL group as detailed in Figure 1. Analysis was by intention to treat, based on available cases
Randomization resulted in baseline equivalence with respect to breastfeeding status, means age of introduction of complementary foods, weight at 6 months and household characteristics, although the LAZ scores at baseline was slightly higher among children receiving WFC (Table 1). Infants lost to follow up did not differ from those who remained in the study during the 9 months intervention. All caregivers reported the intervention foods were not shared. Breastfeeding at end line was 87%.
Table 1.
Baseline characteristics of study participants randomized to supplementation with WinFood Classic (WFC), corn–soy blend+ (CSB+), and WinFood Lite
| Number of children by food | WinFood Classic = 165 | Corn–soy blend+ = 167 | WinFood Lite = 167 |
|---|---|---|---|
| Child characteristics | |||
| Sex, boys, n (%) | 76 (46.1) | 83 (49.7) | 81 (48.5) |
| Infant birth order | 3.5 ± 2.2 | 3.1 ± 1.9 | 3.3 ± 1.9 |
| Child age (months) | 6.0 ± 0.2 | 6.1 ± 0.2 | 6.0 ± 0.2 |
| Currently breastfeed, n (%) | 162 (98.2) | 166 (99.4) | 166 (99.2) |
| Age infant introduced to other foods (months) | 3.1 ± 2.1 | 3.1 ± 2.0 | 3.4 ± 2.0 |
| Weight (kg) | 7.6 ± 1.0 | 7.4 ± 1.0 | 7.3 ± 1.1 |
| Length (cm) | 65.9 ± 2.9 | 65.3 ± 2.8 | 65.1 ± 3.0 |
| Haemoglobin (g dl−1) | 10.8 ± 1.5 | 10.7 ± 1.3 | 10.7 ± 1.4 |
| Weight‐for‐length z‐score (WLZ) | 0.25 ± 1.19 | 0.24 ± 1.19 | 0.16 ± 1.17 |
| Length‐for‐age z‐score (LAZ) | −0.47 ± 1.26 | −0.76 ± 1.13 | −0.85 ± 1.33 |
| Caregiver characteristics | |||
| Age of main caregiver (years) | 26.4 ± 6.9 | 25.6 ± 6.7 | 25.3 ± 5.6 |
| Education level | |||
| Unable to read and write, n (%) | 14 (8.5) | 15 (9.0) | 7 (4.2) |
| Primary incomplete, n (%) | 62 (37.6) | 73 (43.7) | 83 (49.7) |
| Primary completed or higher, n (%) | 89 (53.9) | 79 (47.3) | 77 (46.1) |
| Marital status | |||
| Married | 154 (93.3) | 150 (89.8) | 153 (91.6) |
| Single | 9 (5.5) | 13 (7.8) | 8 (4.8) |
| Widowed | 2 (1.2) | 0 (0.0) | 2 (1.2) |
| Household characteristics | |||
| Total household members | 5.9 ± 2.2 | 5.6 ± 2.2 | 5.4 ± 2.1 |
| Children <5 years | 1.8 ± 0.7 | 1.9 ± 0.8 | 1.9 ± 0.7 |
| Use of insecticide treated net, n (%) | 161 (97.6) | 159 (95.2) | 161 (96.4) |
| Access to water | |||
| Protected well/borehole, n (%) | 77 (48.4) | 75 (46.6) | 68 (41.2) |
| Primary income | |||
| Farming, n (%) | 90 (55.6) | 76 (47) | 72 (44) |
Note. Values are means ± SDs, unless stated otherwise.
No differences in body composition were observed among the three intervention groups over the 9 month intervention (Table 2). There were no differences in FFM gain in WFC 0.0 kg (95% CI [−0.30, 0.29]) or WFL 0.03 kg (95% CI [−0.25, 0.32]), compared with the CSB+ group. Similarly, length gain in either WFC 0.3 cm (95% CI [−0.9, 0.4]) or WFL −0.3 cm (95% CI [−0.9, 0.3]) groups was not different compared with CSB+. The weight gained in all three groups was mainly FFM, whereas FM remained unchanged.
Table 2.
Effects on body composition and length of WinFood Classic and WinFood Lite compared with the corn–soy blend+ after a 9‐month intervention from 6 to 15 months
| Body composition (kg) | Weight (kg) | Length (cm) | ||
|---|---|---|---|---|
| Fat‐free mass | Fat mass | |||
| Age 6 months | ||||
| WinFood Classic | 5.98 (5.85, 6.11) (152) | 1.58 (1.48, 1.67) (152) | 7.5 (7.3, 7.7) (152) | 65.7 (65.1, 66.4) (152) |
| WinFood Lite | 5.79 (5.65, 5.94) (143) | 1.54 (1.44, 1.65) (143) | 7.3 (7.1, 7.5) (143) | 64.8 (64.3, 65.4) (143) |
| Corn–soy blend+a | 5.89 (5.76, 6.02) (147) | 1.57 (1.46, 1.68) (147) | 7.3 (7.1, 7.5) (147) | 65.2 (64.6, 65.8) (147) |
| Age 15 months | ||||
| WinFood Classic | 8.24 (8.01, 8.48) (89) | 1.42 (1.25, 1.59) (89) | 9.6 (9.4, 9.9) (89) | 75.5 (74.9, 76.1) (89) |
| WinFood Lite | 8.16 (7.97, 8.35) (102) | 1.31 (1.17, 1.45) (102) | 9.4 (9.2, 9.6) (102) | 75.2 (74.6, 75.8) (102) |
| Corn–soy blend+a | 8.14 (7.90, 8.38) (88) | 1.42 (1.25, 1.60) (88) | 9.5 (9.3, 9.7) (88) | 74.6 (74.0, 75.2) (88) |
| Difference (15–6 months) compared with corn–soy blend+a | ||||
| WinFood Classic | 0.00 (−0.30, 0.29) | −0.05 (−0.32, 0.22) | −0.1 (−0.3, 0.2) | −0.3 (−0.9, 0.4) |
| WinFood Lite | 0.03 (−0.25, 0.32) | −0.10 (−0.37, 0.15) | −0.1 (−0.3, 0.1) | −0.3 (−0.9, 0.3) |
Note. Analysis was by intention to treat based on available cases and are presented as mean differences; 95% CIs in parentheses (n).
Standard corn–soy blend+.
There was a decrease in plasma ferritin in the WFC group ratio of geometric means: 0.6 μg L−1 (95% CI [0.4, 0.8]) and the WFL group 0.6 μg L−1 (95% CI [0.5, 0.9]) compared with CSB+ (Table 3). There was also an increase in plasma transferrin receptor in the WFC group 3.3 mg L−1 (95% CI [1.7, 4.9]) and the WFL group 1.7 mg L−1 (95% CI [0.1, 3.4]). As seen in Table 3, compared with the increase in haemoglobin over time in the CSB+ group, there was a reduction in haemoglobin in the WFC group −0.9 g dl−1 (95% CI [−1.3, −0.5]) and a lower increase in the WFL group −0.4 g dl−1 (95% CI [−0.8, 0.0]). Despite all these findings, the low follow‐up was a limitation.
Table 3.
Effects on iron status and haemoglobin of WinFood Classic and WinFood Lite compared with the corn–soy blend+ after a 9‐month intervention from 6 to 15 months
| Characteristic | Plasma ferritin (μg L−1) | Plasma transferrin receptor (mg L−1) | Haemoglobin (g dl−1) |
|---|---|---|---|
| Age 6 months | |||
| WinFood Classic | 29.9 (24.5, 36.2) (87) | 11.8 (11.0, 12.5) (87) | 10.8 (10.6, 11.0) (164) |
| WinFood Lite | 33.9 (27.0, 39.9) (79) | 11.6 (10.6, 12.5) (79) | 10.8 (10.5, 11.0) (166) |
| Corn–soy blend+a | 32.8 (28.4, 40.4) (83) | 11.9 (11.0, 12.7) (83) | 10.7 (10.5, 11.0) (166) |
| Age 15 months | |||
| WinFood Classic | 16.3 (13.6, 19.5) (92) | 13.5 (12.5, 14.6) (92) | 10.5 (10.3, 10.8) (142) |
| WinFood Lite | 22.5 (18.5, 27.2) (89) | 11.6 (10.7, 12.5) (89) | 11.0 (10.7, 11.2) (152) |
| Corn–soy blend+a | 25.8 (21.8, 30.4) (87) | 11.2 (10.2, 12.2) (87) | 11.3 (11.1, 11.5) (137) |
| Difference (15–6 months) compared with corn–soy blend+a | |||
| WinFood Classic | 0.6 (0.4, 0.8)b | 3.3 (1.7, 4.9) | −0.9 (−1.3, −0.5) |
| WinFood Lite | 0.6 (0.5, 0.9)b | 1.7 (0.1, 3.4) | −0.4 (−0.8, 0.0) |
Note. Analysis was by intention to treat based on available cases and are presented as means or mean differences; 95% CIs in parentheses (n).
Standard Corn–soy blend+.
The means from the log‐transformed plasma ferritin values, back‐transformed to get a ratio of means.
As defined by WHO cut‐offs (WHO, 2001a, 2001b), 67% of all children were anaemic at 6 months, whereas 73%, 64%, and 63%, respectively, were anaemic at 15 months for WFC, WFL, and CSB+, respectively; the WFC group was different from the CSB+ group (P = .01). A similar trend was observed for mild anaemia (Hb between 10 and 10.9 g dl−1) where a greater proportion (P = .04) of children (41.8%) in the WFC group had mild anaemia at 15 months compared with children in the CSB+ group (22.4%).
CRP was slightly elevated at 6 months 6.9 (SD 12.4) mg L−1 and at 15 months 6.2 (SD 11.3) mg L−1 but did not differ among the study groups. AGP was 1.1 (SD 0.4) g L−1 at 6 months and 1.2 (SD 0.5) g L−1 at 15 months.
There were no differences between the WinFoods and the CSB+ in the relative changes in MUAC and biceps triceps, subscapular and suprailiac skinfolds. In contrast, there was a slight positive change in head circumference for WFC 0.2 cm (95% CI [−0.3, 0.8]) and WFL 0.5 cm (95% CI [−0.0, 1.1]) relative to CSB+ (Table 4).
Table 4.
Effects on mid‐upper arm circumference, head circumference, and skin folds thickness of WinFood Classic and WinFood Lite compared with the corn–soy blend+ after a 9‐month intervention from 6 to 15 months
| Mid‐upper arm circumference (cm) | Head circumference (cm) | Skinfolds (mm) | ||||
|---|---|---|---|---|---|---|
| Biceps | Triceps | Subscapular | Suprailiac | |||
| Age 6 months | ||||||
| WinFood Classic | 14.4 (14.2, 14.5) (165) | 43.4 (43.2, 43.7) (165) | 7.0 (6.7, 7.3) (165) | 8.5 (8.2, 8.8) (165) | 8.1 (7.8, 8.4) (165) | 8.7 (8.3, 9.1) (165) |
| WinFood Lite | 14.0 (13.8, 14.2) (167) | 43.1 (42.9, 43.3) (167) | 6.9 (6.6, 7.1) (166) | 8.5 (8.2, 8.7) (166) | 7.8 (7.5, 8.1) (166) | 8.7 (8.3, 9.2) (166) |
| CSB+a | 14.2 (14.0, 14.4) (167) | 43.5 (43.3, 43.8) (167) | 7.0 (6.8, 7.3) (167) | 8.4 (8.2, 8.7) (167) | 8.0 (7.7, 8.3) (167) | 8.6 (8.2, 9.1) (167) |
| Age 15 months | ||||||
| WinFood Classic | 14.8 (14.6, 15.0) (141) | 47.0 (46.7, 47.3) (141) | 6.0 (5.8, 6.3) (140) | 7.6 (7.3, 7.9) (140) | 7.0 (6.8, 7.3) (140) | 6.7 (6.3, 7.1) (139) |
| WinFood Lite | 14.7 (14.5, 14.9) (150) | 46.8 (46.5, 47.0) (150) | 6.0 (5.8, 6.2) (150) | 7.6 (7.3, 7.9) (151) | 7.1 (6.8, 7.4) (150) | 6.9 (6.5, 7.3) (149) |
| Corn–soy blend+a | 14.8 (14.6, 15.0) (137) | 46.8 (46.1, 47.5) (138) | 6.0 (5.8, 6.3) (137) | 7.6 (7.3, 7.9) (137) | 6.9 (6.6, 7.2) (137) | 6.8 (6.4, 7.1) (137) |
| Difference (15–6 months) compared with corn–soy blend+a | ||||||
| WinFood Classic | −0.1 (−0.3, 0.1) (141) | 0.2 (−0.3, 0.8) (141) | 0.0 (−0.5, 0.4) (140) | 0.1(−0.4, 0.5) (140) | 0.2 (−0.2, 0.6) (140) | −0.1 (−0.7, 0.6) (139) |
| WinFood Lite | 0.1 (−0.2, 0.3) (150) | 0.5 (0.0, 1.1) (150) | 0.2 (−0.3, 0.6) (150) | 0.1(−0.4, 0.5) (150) | 0.4 (0.0, 0.8) (149) | 0.1 (−0.05, 0.7) (148) |
Note. Analysis was by intention to treat based on available cases and are presented as mean differences; 95% CIs in parentheses (n).
Standard corn–soy blend+ product.
4. DISCUSSION
The current study compared with CSB+ the effects of two locally produced, centrally processed complementary food products, one made with germinated grain amaranth, maize and small fish and edible termites and the other without the ASFs but fortified with micronutrients. The results show no differences in 9‐month changes in FFM, length gain and weight gain in the WFC and WFL groups, compared with infants receiving CSB+. Weight gained in all three groups over the 9 months was mainly FFM, whereas FM remained unchanged. Plasma ferritin decreased, whereas plasma transferrin receptors increased in all three groups over the 9 months indicating an overall deterioration in iron status. However, the deterioration in the indicators of iron status was more pronounced among children in the ASF‐fortified food (WFC) compared with children in the two groups (WFL and CSB+) fortified with multimicronutrient premix. Haemoglobin concentration also dropped in the WFC group relative to CSB+.
The inclusion of ASFs (small fish and termites) in the nonfortified WFC product did not promote growth or impact body composition differently than the fortified products without ASFs. This is similar to the findings in the WinFood trial in Cambodia in which the two WinFoods tested both contained ASFs, whereas one of the products was fortified with micronutrients and the other product was nonfortified (Skau et al., 2015). The CSB products used as references in the Cambodia study included the milk‐enriched CSB++. The results in Cambodia supported a tendency for better linear growth and gain of FFM in children receiving foods, which were both fortified and contained ASFs (fish or milk; Skau et al., 2015). Because the present study did not include foods, which contained ASFs and were also fortified with micronutrients, it is not possible to make similar comparison, but the findings are consistent with the Cambodian trial. The present study supports that adding ASFs to a nonfortified food supplement may not be sufficient to compensate for generally nutritionally insufficient complementary foods.
The fact that all three foods had similar results in terms of impact on nutritional status could reflect a more systemic nonfood‐related phenomenon in the environment. For example, exposure to environmental hazards may limit the benefits of ASFs through effects on gut integrity (Hetherington, Wiethoelter, Negin, & Mor, 2017; Kaur, Graham, & Eisenberg, 2017). Children may suffer from environmental enteric dysfunction, which has been associated with stunting by inflammation‐mediated interference with the insulin‐growth factor synthesis pathway and through negative impacts on absorption of nutrients (Owino et al., 2016). Another possible reason for no effect of animal foods could be breastfeeding which remained high during the intervention period.
Comparing the body composition data with reference data based on healthy infants from Ethiopia (Admassu et al., 2017) and the United States (Butte, Hopkinson, Wong, Smith, & Ellis, 2000), Kenyan children's body fat is low and an intervention with a daily supplement of nutritious complementary food was not able to increase fat deposition, as was observed in the Cambodian population (Skau et al., 2015). The lack of differences in FFM and anthropometric measures among the groups may be explained by the fact that all the groups had comparable nutrient intakes. Furthermore, the lack of differences on length and weight seen were possibly because a majority of the children's weight and length z‐scores were in the normal range for all the groups.
The overall deterioration of iron status among all food groups, including those receiving foods fortified with micronutrients including iron, may also be caused by nonfood factors. Although we did not assess malaria infection, the study area is vulnerable to malaria (Desai et al., 2005). Malaria is known to be strongly associated with iron deficiency anaemia (Friedman, Kurtis, Kabyemela, Fried, & Duffy, 2009; Spottiswoode, Duffy, & Drakesmith, 2014). Environmental enteric dysfunction is also linked to bacterial overgrowth in the gut epithelia, which may lead to increased iron requirements (Owino et al., 2016). The deterioration of iron status and haemoglobin concentration in the nonfortified food (WFC) could be caused by the presence of phytic acids in maize and amaranth grains (Albarracín, De Greef, González, & Drago, 2015; Azeke, Egielewa, Eigbogbo, & Ihimire, 2011). Although partial germination of grain amaranth was done to reduce phytic acid, the efficiency in the reduction may not have been adequate to remove the inhibition of mineral absorption by phytic acid. Phytic acid content was assessed during the development of the product where it was concluded that the amaranth grain should be germinated for 72 hr to reduce phytic acid to an acceptable level. However, for the scaled up production of the intervention foods, the lengthy germination duration was found to increase the risk of growth of pathogenic bacteria, and the germination time was limited to a standardized 48 hr (Kinyuru et al., 2015). The fortification of WFL and CSB+ with multiple micronutrient premix had no benefit on iron status.
For secondary anthropometric measures, a higher head circumference was found in the WFL group compared with CSB+. The circumference was 0.5 cm larger, for mean head circumference close to 47 cm. This is equal to a difference in the radius of about 0.8 mm.
The results from previous studies examining the effects of micronutrient supplementation on growth have been mixed (Admassu et al., 2017; Labbé & Dewanji, 2004) with some demonstrating a beneficial role, particularly in resource‐limited settings, where fortified foods have improved growth (Admassu et al., 2017), haemoglobin (Owino et al., 2007), micronutrients (Faber et al., 2005), and others showing no difference in linear growth among infants supplemented with micronutrients from 6 to 18 months (Bauserman et al., 2015; Lartey et al., 1999). Linear growth is not only as a result of dietary improvement during complementary feeding and therefore other measures beyond increasing the nutrient content of complementary foods needs to be explored (Bauserman et al., 2015). Some of these studies, however, have heterogeneous baseline participant populations, varying measures of anthropometry, various timing of interventions and some lack appropriate control groups emanating from diverse intervention products and study designs (Admassu et al., 2017; Arnold et al., 2013; Bauserman et al., 2015; Owino et al., 2007; Skau et al., 2015) unlike the present study and a similar parallel one in Cambodia (Skau et al., 2015) which recruited children of similar age, had a control(s), and were conducted in food‐insecure settings. Another strength of the current study is that we evaluated locally available food sources for infant feeding with the developed products being acceptable to mothers and infants (Konyole et al., 2012).
Although in our study we did not determine malaria parasitaemia, two acute phase protein biomarkers, CRP and AGP, were measured. CRP was slightly high at but did not differ among the study groups indicating a possibility of infection masking the benefits of the study foods (Shinoda et al., 2012; Thurnham & Mccabe, 2010). Clearly, the low follow‐up was a limitation to the findings reporting the effects on iron status. Another limitation of the current study could have been that unlike in the Burkina Faso study which refined procedures for administration of isotope doses and collection of saliva where equilibration time in local context has been found to be 3 hr (Fabiansen et al., 2017), in our study, we used the average of 2 and 3 hr as per the protocol then postdose (IAEA, 2011; Colley et al., 2007). We thus acknowledge the recent study showing that 3 hr were the most optimum (Fabiansen et al., 2017); however, our study was based on a previous protocol assuming that deuterium equilibration takes less than 3 hr in infants and children (Colley et al., 2007) when saliva is the primary specimen.
Although we did not demonstrate a clear beneficial effect from supplementing with WFC on FFM, linear growth and Fe status, we cannot exclude insects as a potentially promising food source for people in food‐insecure areas. The lack of impact of the insect‐based WFC product on improving iron status need further investigations to clarify iron absorption from different blends to isolate the specific impact of the termites. It is also possible that improving the quality of complementary foods is beneficial if other growth‐limiting pathologies are prevented. Given the high disease burden among infants in this resource‐limited rural area (Friedman et al., 2009; Spottiswoode et al., 2014), the role of conditions that impair nutrient absorption and utilization is a potential area of further research.
The WinFoods did not differ from the CSB+ in FFM, length gain and weight gain. There was overall deterioration of iron status among all food groups with a significant drop in the nonfortified food group. This study did not include products which were fortified and also contained ASF and could therefore not confirm the finding in a similar study in Cambodia concluding that complementary food supplements distributed in food‐insecure populations can benefit from combined fortification and inclusion of ASF (Skau et al., 2015). Infants in all food groups gained FFM and, unlike the Cambodian infants, the FM was preserved during the 9 months intervention. The long‐term implications for health and development of these differences in growth patterns in early childhood between populations need further investigations.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
The authors' responsibilities were as follows: NR, HF, and KFM conceived the idea and designed the study. BBE, SMF, JKHS, and VOO reviewed the study design. SOK, SAO, JNK, and BOO trained field workers and implemented all aspects of data collection and quality assurance under supervison by BBE and VOO. SOK, SAO, JNK, BOO, BBE, NR, and VOO developed the WinFood products; SOK, HR, and NR analysed and interpreted the data; SOK drafted the manuscript. NR, HF, and VOO offered overall editorial oversight. All authors contributed to manuscript development and read and approved the final version prior to submission.
ACKNOWLEDGMENTS
This work is indebted to the caregivers and their children who participated in the study. We acknowledge the staff of the WinFood project Kenya, Mumias District Health Management Team, Dr Juergen Erhardt for iron status samples analysis, KEMRI Centre of Public Health Research Nairobi for the Body composition determination using FTIR, and the Danish International Development Assistance (DANIDA) for financial support through the WINFOOD Project. Men and Traditions Against AIDS (MTAA) Non‐Governmental Organization with the fieldwork support is also appreciated as well as AllGrain Co. Ltd for the food production.
Konyole SO, Omollo SA, Kinyuru JN, et al. Effect of locally produced complementary foods on fat‐free mass, linear growth, and iron status among Kenyan infants: A randomized controlled trial. Matern Child Nutr. 2019;15:e12836 10.1111/mcn.12836
Registered randomized trial: ISRCTN30012997 (http://www.isrctn.com/ISRCTN30012997)
REFERENCES
- A Stata Press Publication , StataCorp L.P ., and T. College Station . (2011). STATA user's guide release 13, 12th ed. Texas: Stata Press, 4905 Lakeway Drive, College Station, TX 77845.
- Admassu, B. , Wells, J. , Girma, T. , Andersen, G. , Owino, V. , Belachew, T. , … Kæstel, P. (2017). Body composition at birth and height at 2 years: A prospective cohort study among children in Jimma, Ethiopia. Pediatric Research, 82, 209–214. 10.1038/pr.2017.59 [DOI] [PubMed] [Google Scholar]
- Albarracín, M. , De Greef, D. , González, R. , & Drago, S. (2015). Germination and extrusion as combined processes for reducing phytates and increasing phenolics content and antioxidant capacity of Oryza sativa L. whole grain flours. International Journal of Food Science and Nutrition, 66, 904–911. 10.3109/09637486.2015.1110689 [DOI] [PubMed] [Google Scholar]
- Allen, L. H. (2008). To what extent can food‐based approaches improve micronutrient status? Asia Pacific Journal of Clinical Nutrition, 17, 103–105. [PubMed] [Google Scholar]
- Arnold, B. F. , Null, C. , Luby, S. P. , Unicomb, L. , Stewart, C. P. , Dewey, K. G. , … Colford, J. M. Jr. (2013). Cluster‐randomised controlled trials of individual and combined water, sanitation, hygiene and nutritional interventions in rural Bangladesh and Kenya: The WASH benefits study design and rationale. BMJ Open, 3(8), e003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth, A. (2006). Efficacy and effectiveness of community‐based treatment of severe malnutrition. Food and Nutrition Bulletin, 27, S24–S48. 10.1177/15648265060273S303 [DOI] [PubMed] [Google Scholar]
- Azeke, M. A. , Egielewa, S. J. , Eigbogbo, M. U. , & Ihimire, I. G. (2011). Effect of germination on the phytase activity, phytate and total phosphorus contents of rice (Oryza sativa), maize (Zea mays), millet (Panicum miliaceum), sorghum (Sorghum bicolor) and wheat (Triticum aestivum). Journal of Food Science and Technology, 48, 724–729. 10.1007/s13197-010-0186-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauserman, M. , Lokangaka, A. , Gado, J. , Close, K. , Wallace, D. , Kodondi, K. K. , … Bose, C. (2015). A cluster‐randomized trial determining the efficacy of caterpillar cereal as a locally available and sustainable complementary food to prevent stunting and anaemia. Public Health Nutrition, 18, 1785–1792. 10.1017/S1368980014003334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte, N. F. , Hopkinson, J. M. , Wong, W. W. , Smith, E. O. , & Ellis, K. J. (2000). Body composition during the first 2 years of life: An updated reference. Pediatric Research, 47, 578–585. 10.1203/00006450-200005000-00004 [DOI] [PubMed] [Google Scholar]
- Byrd, K. , Dentz, H. N. , Williams, A. , Kiprotich, M. , Pickering, A. J. , Omondi, R. , … Stewart, C. P. (2018). A behaviour change intervention with lipid‐based nutrient supplements had little impact on young child feeding indicators in rural Kenya. Maternal & Child Nutrition, 12, e12660 [Epub ahead of print]. 10.1111/mcn.12660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capinera, J. L. , Hoy, M. A. , Paré, P. W. , Farag, M. A. , Trumble, J. T. , Isman, M. B. , … Sánchez, N. E. (2008). Nutrient content of insects In Encyclopedia of entomology (pp. 2623–2646). Dordrecht: Springer Netherlands. [Google Scholar]
- Colley, R. C. , Byrne, N. M. , & Hills, A. P. (2007). Implications of the variability in time to isotopic equilibrium in the deuterium dilution technique. European Journal of Clinical Nutrition, 61, 1250–1255. 10.1038/sj.ejcn.1602653 [DOI] [PubMed] [Google Scholar]
- Desai, M. R. , Terlouw, D. J. , Kwena, A. M. , Phillips‐Howard, P. A. , Kariuki, S. K. , Wannemuehler, K. A. , … Ter Kuile, F. O. (2005). Factors associated with hemoglobin concentrations in pre‐school children in western Kenya: Cross‐sectional studies. American Journal of Tropical Medicine and Hygiene, 72, 47–59. 10.4269/ajtmh.2005.72.47 [DOI] [PubMed] [Google Scholar]
- Dewey, K. G. , & Brown, K. H. (2003). Update on technical issues concerning complementary feeding of young children in developing countries and implications for intervention programs. Food and Nutrition Bulletin, 24, 5–28. 10.1177/156482650302400102 [DOI] [PubMed] [Google Scholar]
- Dror, D. K. , & Allen, L. H. (2011). The importance of milk and other animal‐source foods for children in low‐income countries. Food and Nutrition Bulletin, 32, 227–243. 10.1177/156482651103200307 [DOI] [PubMed] [Google Scholar]
- Eichler, K. , Wieser, S. , Rüthemann, I. , & Brügger, U. (2012). Effects of micronutrient fortified milk and cereal food for infants and children: A systematic review. BMC Public Health, 12(1), 506 10.1186/1471-2458-12-506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt, J. G. , Estes, J. E. , Pfeiffer, C. M. , Biesalski, H. K. , & Craft, N. E. (2004). Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C‐reactive protein by an inexpensive, sensitive, and simple sandwich enzyme‐linked immunosorbent assay technique. The Journal of Nutrition, 134, 3127–3132. 10.1093/jn/134.11.3127 [DOI] [PubMed] [Google Scholar]
- Faber, M. , Kvalsvig, J. D. , Lombard, C. J. , & Benadé, A. J. S. (2005). Effect of a fortified maize‐meal porridge on anemia, micronutrient status, and motor development of infants. The American Journal of Clinical Nutrition, 82, 1032–1039. 10.1093/ajcn/82.5.1032 [DOI] [PubMed] [Google Scholar]
- Fabiansen, C. , Yaméogo, C. W. , Devi, S. , Friis, H. , Kurpad, A. , & Wells, J. C. (2017). Deuterium dilution technique for body composition assessment: Resolving methodological issues in children with moderate acute malnutrition. Isotopes in Environmental and Health Studies, 53, 344–355. 10.1080/10256016.2017.1295043 [DOI] [PubMed] [Google Scholar]
- FAO (2013). Edible insects future prospects for food and feed security. Rome: Food and Agriculture Organization of the United Nations. [Google Scholar]
- Friedman, J. F. , Kurtis, J. D. , Kabyemela, E. R. , Fried, M. , & Duffy, P. E. (2009). The iron trap: iron, malaria and anemia at the mother‐child interface. Microbes and Infection, 11, 460–466. 10.1016/j.micinf.2009.02.006 [DOI] [PubMed] [Google Scholar]
- Hetherington, J. B. , Wiethoelter, A. K. , Negin, J. , & Mor, S. M. (2017). Livestock ownership, animal source foods and child nutritional outcomes in seven rural village clusters in Sub‐Saharan Africa. Agriculture & Food Security, 6(1), 9 10.1186/s40066-016-0079-z [DOI] [Google Scholar]
- International Atomic Energy Agency (2011). Introduction to body composition assessment using the deuterium dilution technique with analysis of urine samples by isotope ratio mass spectrometry. IAEA Human Health. Series no. 13 (p. 84). Vienna, Austria: International Atomic Energy Agency. [Google Scholar]
- Kaur, M. , Graham, J. P. , & Eisenberg, J. N. S. (2017). Livestock ownership among rural households and child morbidity and mortality: An analysis of demographic health survey data from 30 sub‐Saharan African countries (2005–2015). The American Journal of Tropical Medicine and Hygiene, 96, 741–748. 10.4269/ajtmh.16-0664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenya National Bureau of Statistics , Ministry of Health/Kenya , National Aids Control Council/Kenya and Development/Kenya . (2015). Kenya demographic and health survey.
- Kinyuru, J. N. , Konyole, S. O. , Kenji, G. M. , Onyango, C. A. , Owino, V. O. , Owuor, B. O. , … Roos, N. (2012). Identification of traditional foods with public health potential for complementary feeding in Western Kenya. Journal of Food Research, 1(2), 148–158. [Google Scholar]
- Kinyuru, J. N. , Konyole, S. O. , Onyango‐Omolo, S. A. , Kenji, G. M. , Onyango, C. A. , Owino, V. O. , … Roos, N. (2015). Nutrients, functional properties, storage stability and costing of complementary foods enriched with either termites and fish or commercial micronutrients. Journal of Insects as Food and Feed., 1, 149–158. 10.3920/JIFF2014.0011 [DOI] [Google Scholar]
- Kinyuru, J. N. , Konyole, S. O. , Roos, N. , Onyango, C. A. , Owino, V. O. , Owuor, B. O. , … Kenji, G. M. (2013). Nutrient composition of four species of winged termites consumed in Western Kenya. Journal of Food Composition and Analysis, 30, 120–124. 10.1016/j.jfca.2013.02.008 [DOI] [Google Scholar]
- Konyole, S. O. , Kinyuru, J. N. , Owuor, B. O. , Kenji, G. M. , Onyango, C. A. , Estambale, B. B. , … Owino, V. O. (2012). Acceptability of amaranth grain‐based nutritious complementary foods with dagaa fish (Rastrineobola argentea) and edible termites (Macrotermes subhylanus) compared to corn soy blend plus among young children/mothers dyads in Western Kenya. Journal of Food Research, 1, 111–120. 10.5539/jfr.v1n3p111 [DOI] [Google Scholar]
- Labbé, R. F. , & Dewanji, A. (2004). Iron assessment tests: Transferrin receptor vis‐à‐vis zinc protoporphyrin. Clinical Biochemistry, 37, 165–174. 10.1016/j.clinbiochem.2003.10.006 [DOI] [PubMed] [Google Scholar]
- Lartey, A. , Manu, A. , Brown, K. H. , Peerson, J. M. , & Dewey, K. G. (1999). A randomized, community‐based trial of the effects of improved, centrally processed complementary foods on growth and micronutrient status of Ghanaian infants from 6 to 12 mo of age. The American Journal of Clinical Nutrition, 70, 391–404. 10.1093/ajcn/70.3.391 [DOI] [PubMed] [Google Scholar]
- Lin, A. , Ercumen, A. , Benjamin‐Chung, J. , Arnold, B. F. , Das, S. , Haque, R. , … Luby, S. P. (2018). Effects of water, sanitation, handwashing, and nutritional interventions on child enteric protozoan infections in rural Bangladesh: A cluster‐randomized controlled trial. Clinical Infectious Diseases, 13 [Epub ahead of print]. 10.1093/cid/ciy320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohman, T. G. , Roche, A. F. , & Martorell, R. (1988). Anthropometric standardization reference manual. Champaign IL: Human Kinetics Books. [Google Scholar]
- Nair, N. , Tripathy, P. , Sachdev, H. S. , Pradhan, H. , Bhattacharyya, S. , Gope, R. , … Prost, A. (2017). Effect of participatory women's groups and counseling through home visits on children's linear growth in rural eastern India (CARING trial): A cluster‐randomised controlled trial. The Lancet Global Health, 5, e1004–e1016. 10.1016/S2214-109X(17)30339-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Null, C. , Stewart, C. P. , Pickering, A. J. , Dentz, H. N. , Arnold, B. F. , Arnold, C. D. , … Colford, J. M. Jr. (2018). Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: A cluster‐randomised controlled trial. The Lancet Global Health, 6, e316–e329. 10.1016/S2214-109X(18)30005-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owino, V. , Ahmed, T. , Freemark, M. , Kelly, P. , Loy, A. , Manary, M. , & Loechl, C. (2016). Environmental enteric dysfunction and growth failure/stunting in global child health. Pediatrics, 138(6: pii:), e20160641. [DOI] [PubMed] [Google Scholar]
- Owino, V. O. , Kasonka, L. M. , Sinkala, M. M. , Wells, J. K. , Eaton, S. , Darch, T. , … Filteau, S. M. (2007). Fortified complementary foods with or without alpha‐amylase treatment increase hemoglobin but do not reduce breast milk intake of 9‐mo‐old Zambian infants. The American Journal of Clinical Nutrition, 86, 1094–1103. 10.1093/ajcn/86.4.1094 [DOI] [PubMed] [Google Scholar]
- Pan American Health Organization . (2001). Guiding principles for complementary feeding of the breastfed child. Washington (DC). PAHO.
- Roos, N. , Wahab, M. A. , Chamnan, C. , & Thilsted, S. H. (2007). The role of fish in food‐based strategies to combat vitamin A and mineral deficiencies in developing countries. The Journal of Nutrition., 137, 1106–1109. 10.1093/jn/137.4.1106 [DOI] [PubMed] [Google Scholar]
- Rumpold, B. A. , & Schlüter, O. K. (2013). Nutritional composition and safety aspects of edible insects. Molecular Nutrition & Food Research, 57, 802–823. 10.1002/mnfr.201200735 [DOI] [PubMed] [Google Scholar]
- Shinoda, N. , Sullivan, K. M. , Tripp, K. , Erhardt, J. G. , Haynes, B. M. , Temple, V. J. , & Woodruff, B. (2012). Relationship between markers of inflammation and anaemia in children of Papua New Guinea. Public Health Nutrition, 16, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skau, J. K. H. , Touch, B. , Chhoun, C. , Chea, M. , Unni, U. S. , Makurat, J. , … Roos, N. (2015). Effects of animal source food and micronutrient fortification in complementary food products on body composition, iron status, and linear growth: A randomized trial in Cambodia. The American Journal of Clinical Nutrition, 101, 742–751. 10.3945/ajcn.114.084889 [DOI] [PubMed] [Google Scholar]
- Spottiswoode, N. , Duffy, P. E. , & Drakesmith, H. (2014). Iron, anemia and hepcidin in malaria. Frontiers in Pharmacology, 5, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurnham, D. I. & Mccabe, G. P. (2010). Influence of infection and inflammation on biomarkers of nutritional status with an emphasis on vitamin A and iron. World Health Organisation; Geneva, 15–17.
- Thurnham, D. I. , McCabe, L. D. , Haldar, S. , Wieringa, F. T. , Northrop‐Clewes, C. A. , & McCabe, G. P. (2010). Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: A meta‐analysis. The American Journal of Clinical Nutrition, 92, 546–555. 10.3945/ajcn.2010.29284 [DOI] [PubMed] [Google Scholar]
- UNICEF‐WHO‐The World Bank Group . (2017). Joint child malnutrition estimates—Levels and trends http://www.who.int/nutgrowthdb/estimates2016/en/Accessed 20/05/2018
- World Food Program . (2015). WFP specialized nutritious foods sheet programme treating moderate acute malnutrition (MAM) 1, p.1.
- World Health Organisation (2001a). WHO model prescribing information—Drugs used in bacterial infections. In Essential medicines and health products information portal, a WHO resource. http://apps.who.int/medicinedocs/pdf/s5406e/s5406e.pdf. accessed 15/10/2017.
- World Health Organisation . (2001b). Iron deficiency anaemia: Assessment, prevention, and control. A guide for programme managers. p.114.
- World Health Organization . (2014). WHO child growth standards and the identification of severe acute malnutrition in infants and children. [PubMed]
- World Health Organization . (2017). Diarrheal disease fact sheets. http://www.who.int/mediacentre/factsheets/fs330/en/accessed 15/06/2018.


