Abstract
Burden and risk factors for wasting in the first 6 months of life among Indian children are not well documented. We used data from India's National Family Health Survey 4 to estimate the prevalence of severe wasting (weight for length < ‐3 SD) among 18,898 infants under 6 months of age. We also examined the association of severe wasting with household, maternal, and child‐related factors using multivariable logistic regression analysis. Prevalence of severe wasting among infants less than 6 months of age was 14.8%, ranging from 3.5 to 21% across states. Low birth weight (<2,500 g; adjusted odds ratio [AOR] 1.40, 95% CI [1.19, 1.65]), nonutilization of supplementary nutrition by mother during lactation (AOR 1.23, 95% CI [1.05, 1.43]), and anthropometric assessment during summer (AOR 1.37, 95% CI [1.13, 1.65]) and monsoon months (AOR 1.53, 95% CI [1.20, 1.95]) were associated with higher odds of severe wasting. Infants aged 2 to 3 months (AOR 0.78, 95% CI [0.66, 0.93]) and 4 to 5 months (AOR 0.65, 95% CI [0.55, 0.73]) had lower odds of severe wasting as compared with the 0‐ to 1‐month category. This analysis reveals a high burden of severe wasting in infants less than 6 months in India. Preventive interventions must be targeted at reducing low birth weight due to fatal growth restriction and prematurity. Appropriate care practices at facilities and postdischarge with extra attention to those born small and sick can prevent further deterioration in nutritional status.
Keywords: India, infant, malnutrition, NFHS, wasting
Key messages.
Prevalence of severe wasting among infants aged less than 6 months was 14.8% in India and ranged from 3.5 to 21% across different states.
Key predictors of severe wasting among infants aged less than 6 months are younger infant age, low birth weight, anthropometric assessments in summer and monsoon, and nonutilization of supplementary nutrition provided during lactation through the public system.
Measures to reduce the factors that cause low birth weight such as prematurity and small for gestational age and appropriate care practices at facilities and post‐discharge need to be prioritized.
1. INTRODUCTION
Severe wasting, defined as weight‐for‐length z‐score (WLZ) < −3 standard deviation (SD) of the World Health Organization (WHO) child growth standards (World Health Organization [WHO], 2006) accounts for 0.5 million under‐five deaths annually (Black et al., 2013). South Asia alone accounts for more than half of the estimated 16.4 million under‐five children with severe wasting (United Nations Children's Fund [UNICEF], 2017). The risk of death among under‐five children with severe wasting is approximately 10 times higher as compared with those with weight‐for‐height z‐score (WHZ) > −1 SD (Black et al., 2008). Severe wasting increases the incidence and severity of common childhood illnesses like diarrhoea and pneumonia (Chisti et al., 2013; Jones & Berkley, 2014; Talbert et al., 2012). Severe wasting in children aged less than 6 months of postnatal life may also affect long‐term developmental outcomes (Lelijveld et al., 2016).
Data from developing countries on the burden of severe wasting, its risk factors, the guidelines for diagnosis, and treatment are well described for children 6‐ to 59‐month‐old (Ambadekar & Zodpey, 2017; Singh et al., 2014; WHO, 2013). Severe wasting in the first 6 months of life has received less attention (Kerac et al., 2011; Kerac, Mwangome, McGrath, Haider, & Berkley, 2015). Infants up to 6 months of age are reported to account for 12–25% of inpatient cases of severe wasting (Grijalva‐Eternod et al., 2017; Singh et al., 2014). According to available estimates, around half of infants aged less than 6 months with wasting (WLZ < ‐2 SD) are severely (WLZ < −3 SD) wasted (Kerac et al., 2015). The risk of mortality among such infants is particularly high (Kerac et al., 2014).
Information on the magnitude of severe malnutrition (SAM) in this age group, its risk factors, and guidelines for treatment are lacking. We conducted a secondary analysis using the National Family Health Survey‐4 (NFHS‐4) data for India to estimate the prevalence of early severe wasting in the country and in different states. We have also examined the association of early severe wasting with household, maternal, and child‐related factors, particularly birth weight. Data such as these will contribute to policy and strategy for addressing early severe wasting.
2. METHODS
2.1. Data source
We analysed individual‐level data from India's NFHS‐4, which was conducted, under the stewardship of the Ministry of Health and Family Welfare, Government of India, during 2015–2016. The NFHS‐4 provides estimates on fertility, mortality, reproductive health, child health, and other health and demographic indicators at national, state, and district level (IIPS & ICF, 2017). Around 600,000 households in 29 states and seven union territories in India were interviewed with a response rate of 98%. A two‐stage stratified sampling design was used. In the first stage, villages in rural areas and Census Enumeration Blocks in urban areas were the primary sampling units. In the second stage, within each primary sampling unit, households were selected using systematic random sampling and data on clinical, anthropometric, and biochemical parameters were collected. The sampling design and instruments used in the survey are described in detail elsewhere (IIPS & ICF, 2017) .
Stata file format of the children recode file (IAKR73FL) was used for this analysis. The children recode file contains information on health and immunization status, details of pregnancy and postnatal period for the mother as well as socioeconomic and demographic data at household level for every child of the interviewed women born during 5 years preceding the survey in a standard, easy to analyse format with common variable names and coding categories for easy comparability between countries and includes summary variables and indices which were calculated post data collection.
Data on children's nutritional status is provided as length‐for‐age, weight‐for‐length, and weight‐for‐age z‐scores as per the WHO child growth standards (WHO, 2006). The current analysis was restricted to 18,898 of out of the 23,754 infants aged less than 6 months for whom WLZ was within plausible limits, that is, between −5 SD and +5 SD of the WHO child growth standards (WHO, 2006; WHO, 2015), aiming to exclude obvious data entry or measurement errors. The reasons for exclusion from the analysis are depicted in Figure 1.
Figure 1.
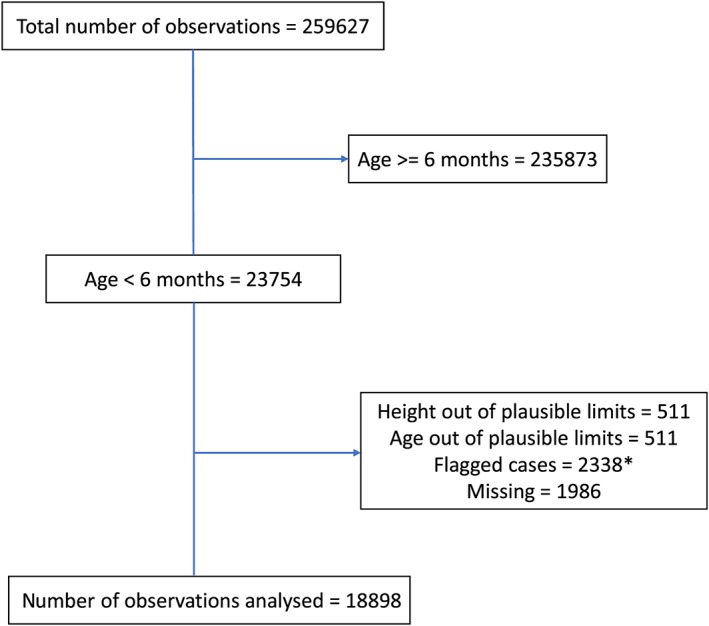
Flowchart showing number of observations analysed and reasons for exclusion (IAKR73FL.dta, National Family Health Survey‐4, 2015–2016)
2.2. Analysis
Severe wasting was defined as a WLZ < ‐3 SD of the WHO child growth standards (WHO, 2006). Prevalence and 95% confidence intervals (95% CIs) were estimated for infants aged less than 6 months at national level and for states and union territories with ≥50 unweighted records. Age less than 6 months indicated that the child's age in completed months was less than 6 months on the day of the survey. The proportion of infants with severe wasting within each subcategory of household, maternal, and child variables (listed below) was also calculated.
Based on the literature, we identified household, maternal, and child‐level variables likely to be associated with severe wasting in the first 6 months of life. We then examined these characteristics, when available in the dataset, as possible explanatory variables in this analysis. Household variables included place of residence, wealth quintile, access to improved sanitation, access to improved drinking water, religion, and caste. Maternal variables included education, employment status, mass media exposure, height, body mass index, anaemia status at time of survey, number of antenatal care visits, birth interval, utilization of supplementary nutrition from Integrated Child Development Services (ICDS; MWCD, 2019) during pregnancy and during lactation, place of delivery, and mode of delivery. Child variables included age of the infant, gender, birth weight, season of anthropometric measurement, birth order, breastfeeding status, early initiation of breastfeeding (within an hour of birth), prelacteal feeding, and diarrhoea during the 2 weeks preceding the survey. The categorization of these variables is shown in Table S1 in the Supporting Information.
Birth weight data were available for 14,981 of the 18,898 infants; in 8,318 infants, it was based on documentation in the maternal and child health card and on maternal recall for the remaining 6,663 infants. In the multivariable analysis, association of birth weight with severe wasting overall and restricted to those with documented birth weight was examined.
Variables of known clinical or contextual importance were used in the multivariable logistic regression model with adjustment for the child's state of residence (Harrell, 2015). We also examined possible interaction (multiplicative scale) of breastfeeding and utilization of supplementary nutrition during lactation with birth weight. STATA© 15.1 (StataCorp, College Station, TX) was used for all analysis and adjustment for sampling weight, cluster and strata was done using “svyset” command.
2.3. Ethical considerations
As this analysis was based on secondary data available in the public domain, ethical clearance was not needed. The guidelines for data use as required by the DHS program were strictly followed.
3. RESULTS
3.1. Prevalence of severe wasting
Prevalence of severe wasting among infants aged less than 6 months at national level averaged 14.8% (95% CI [14.0%, 15.7%]; Figure 2). The median prevalence among the states was 12.9% (interquartile range 10.8%, 16.1%). States in the north‐eastern part of India (Mizoram, Manipur, Nagaland, Sikkim, and Assam) and Jammu and Kashmir had prevalence below 10%, and Gujarat and Maharashtra had prevalence above 20%. In the national‐level analysis, prevalence of severe wasting declined from 18.7% at less than 1 month of age to 12.6% in the fifth month of life (Figure 3). The prevalence of severe wasting among low birth weight (LBW) infants was higher than those born with normal birth weight (birth weight ≥ 2,500 g) at all ages until 6 months of age (Figure 3).
Figure 2.
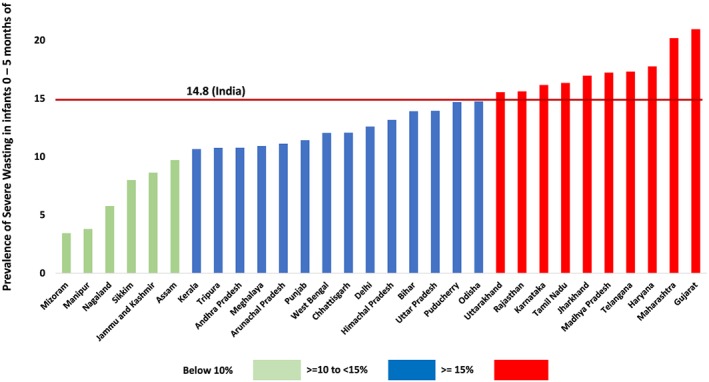
Prevalence of severe wasting (weight for length score < −3 standard deviation) among infants aged less than 6 months of age in India and states/union territories* (National Family Health Survey‐4, 2015–2016)
Figure 3.
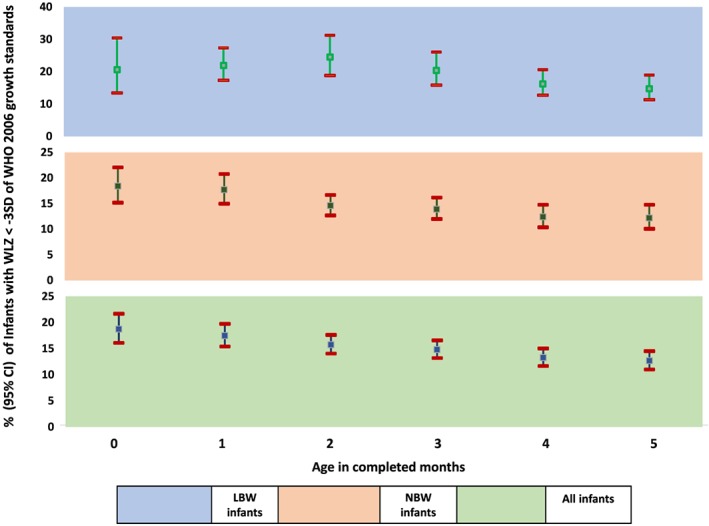
Age‐wise prevalence (95% confidence interval [CI]) of severe wasting (weight for length score < −3 standard deviation) among infants aged less than 6 months of age in India overall and by birth weight categories (National Family Health Survey‐4, 2015–2016)
3.2. Factors associated with severe wasting
The association of household, maternal, and child‐level characteristics with severe wasting in the univariable analysis is shown in Tables 1, 2, 3. None of the household‐level variables examined were significantly associated with severe wasting. Among the maternal variables, utilization of supplementary nutrition from ICDS by mother during lactation showed an inverse, significant association with severe wasting. Among the child‐level variables, older infant age, normal birth weight (≥2,500 g), and anthropometric measurement obtained in winter were significantly, inversely associated with severe wasting (Table 2). We did not find significant interaction (p = .051) between the effect of exclusive breastfeeding status and birth weight on severe acute malnutrition. Also, we did not find significant interaction (p = .850) between the effect of utilization of supplementary nutrition by mother during lactation and birth weight on severe acute malnutrition.
Table 1.
Percentage of infants aged less than 6 months with severe wasting (WLZ < ‐3 SD) in India and its association with household characteristics (NFHS‐4, 2015–2016)
| Variable | Prevalence of severe wasting weighted n (%) | Unadjusted OR (95% CI) | p‐value |
|---|---|---|---|
| Place of residence | |||
| Urban (4,763) | 787 (16.52) | Reference | ‐‐ |
| Rural (14,135) | 2,017 (14.27) | 0.84 (0.70–1.01) | .064 |
| Wealth index quintiles | |||
| Highest (2,712) | 439 (16.20) | Reference | ‐‐ |
| Fourth (3,286) | 490 (14.91) | 0.91 (0.70–1.17) | .458 |
| Middle (3,737) | 536 (14.35) | 0.87 (0.71–1.06) | .166 |
| Second (4,475) | 622 (13.91) | 0.84 (0.68–1.03) | .090 |
| Lowest (4,689) | 716 (15.27) | 0.93 (0.76–1.14) | .502 |
| Improved sanitation | |||
| Yes (6,668) | 1,015 (15.23) | Reference | ‐‐ |
| No (10,286) | 1,478 (14.37) | 0.93 (0.82–1.07) | .325 |
| Improved drinking water access | |||
| Yes (15,261) | 2,256 (14.79) | Reference | ‐‐ |
| No (1,693) | 237 (14.02) | 0.94 (0.78–1.13) | .509 |
| Religion | |||
| Hindu (14,911) | 2,254 (15.12) | Reference | ‐‐ |
| Muslim (3,145) | 446 (14.19) | 0.93 (0.79–1.10) | .381 |
| Others (843) | 104 (12.31) | 0.79 (0.59–1.06) | .112 |
| Caste | |||
| Others (3,574) | 501 (14.01) | Reference | ‐‐ |
| Scheduled caste/tribes (6,194) | 940 (15.18) | 1.10 (0.87–1.38) | .427 |
| Other backward class (8,345) | 1,242 (14.88) | 1.07 (0.86–1.34) | .542 |
Abbreviations: 95% CI: 95% confidence interval; NFHS‐4: National Family Health Survey‐4; OR: odds ratio; SD: standard deviation; WLZ: weight for length.
Table 2.
Percentage of infants aged less than 6 months with severe wasting (WLZ < ‐3SD) in India and its association with maternal characteristics (NFHS‐4, 2015–2016)
| Variable | Prevalence of severe wasting weighted n (%) | Unadjusted OR (95% CI) | p‐value |
|---|---|---|---|
| Maternal age at first birth (mean [SD]) | 20.8 (3.3) | ||
| <19 (n = 3,756) | 497 (13.22) | 0.84 (0.59–1.19) | .332 |
| 19–30 (n = 14,859) | 2,262 (15.22) | Reference | ‐‐ |
| >30 (n = 271) | 46 (16.90) | 1.11 (0.93–1.32) | .259 |
| Maternal current age (mean [SD]) | 26.1 (4.4) | ||
| <19 (n = 823) | 105 (12.73) | 0.85 (0.73–0.99) | .034 |
| 19–30 (n = 16,111) | 2,383 (14.79) | Reference | ‐‐ |
| >30 (n = 1,964) | 316 (16.10) | 1.13 (0.76–1.69) | .541 |
| Education | |||
| No education/primary (n = 7,413) | 1110 (14.97) | 1.02 (0.90–1.15) | .779 |
| Secondary/higher (n = 11,485) | 1,694 (14.75) | Reference | ‐‐ |
| Maternal employment status | |||
| Homemaker (n = 2,852) | 471 (16.50) | Reference | ‐‐ |
| Employed outside house (n = 391) | 50 (12.70) | 0.74 (0.49–1.12) | .149 |
| Exposure to mass media | |||
| No exposure (n = 5,032) | 764 (15.19) | Reference | ‐‐ |
| Any exposure (n = 13,866) | 2,039 (14.71) | 0.96 (0.84–1.11) | .595 |
| Height (mean [SD]) | 151.4 (6.0) | ||
| Not short (≥150 cm) (n = 11,350) | 1668 (14.69) | Reference | ‐‐ |
| Short (<150 cm) (n = 7,505) | 1124 (14.98) | 1.02 (0.90–1.17) | .737 |
| BMI (mean [SD]) | 21.4 (3.7) | ||
| Normal weight (n = 12,618) | 1,866 (14.79) | Reference | ‐‐ |
| Undernourished (n = 3,872) | 614 (15.85) | 1.09 (0.93–1.26) | .29 |
| Overweight and obese (n = 2,344) | 307 (13.11) | 0.87 (0.71–1.07) | .189 |
| Anaemia (mean [SD]) | 11.5 (1.6) | ||
| Non‐anaemic (n = 7,505) | 1,079 (14.38) | Reference | ‐‐ |
| Anaemic (n = 11,252) | 1,713 (15.22) | 1.07 (0.95–1.20) | .266 |
| ANC visits (mean [SD]) | 4.8 (4.7) | ||
| ≥4 ANC visits (n = 9,421) | 13,85 (14.71) | Reference | ‐‐ |
| 1–3 ANC visits (n = 6,449) | 993 (15.40) | 1.06 (0.92–1.21) | .434 |
| No ANC (n = 2,735) | 392 (14.33) | 0.97 (0.82–1.16) | .735 |
| Birth interval (mean [SD]) | 38.4 (22.0) | ||
| 12–23 months (n = 2,785) | 425 (15.28) | Reference | ‐‐ |
| <12 months (n = 116) | 13 (10.85) | 0.67 (0.33–1.36) | .272 |
| ≥24 months (n = 8,903) | 1,311 (14.73) | 0.96 (0.77–1.19) | .696 |
| Received supplementary nutrition during pregnancy | |||
| Yes (n = 10,859) | 1,585 (14.60) | Reference | ‐‐ |
| No (n = 7,903) | 1,202 (15.21) | 1.05 (0.94–1.18) | .409 |
| Place of delivery | |||
| Institution (n = 15,616) | 2,349 (15.04) | Reference | ‐‐ |
| Home birth (n = 3282) | 455 (13.86) | 0.91 (0.79–1.05) | .187 |
| Mode of delivery | |||
| Caesarean section (n = 3,650) | 523 (14.32) | Reference | ‐‐ |
| Normal vaginal delivery (n = 15,248) | 2,281 (14.96) | 1.05 (0.89–1.25) | .546 |
| Nutrition benefits from ICDS during lactation | |||
| Yes (n = 8,430) | 1,165 (13.82) | Reference | ‐‐ |
| No (n = 10,441) | 1,624 (15.55) | 1.15 (1.02–1.30) | .028 |
Abbreviations: 95% CI: 95% confidence interval; ICDS: Integrated Child Development Services; NFHS‐4: National Family Health Survey–4; OR: odds ratio; SD: standard deviation; WLZ: weight for length.
Table 3.
Percentage of infants aged less than 6 months with severe wasting (WLZ < −3 SD) in India and its association with child characteristics (NFHS‐4, 2015–16)
| Variable | Prevalence of severe wasting weighted n (%) | Unadjusted OR (95% CI) | p‐value |
|---|---|---|---|
| Age of child (in months) | |||
| 0–1 (n = 4,041) | 720 (17.81) | Reference | ‐‐ |
| 2–3 (n = 6,968) | 1,062 (15.25) | 0.83 (0.72–0.96) | .013 |
| 4–5 (n = 7,889) | 1,022 (12.95) | 0.69 (0.59–0.79) | <.000 |
| Gender | |||
| Male (n = 9,730) | 1,483 (15.24) | Reference | |
| Female (n = 9,168) | 1,321 (14.41) | 0.94 (0.84–1.05) | 0.251 |
| Birth weight [mean (SD)] | 2,840.7 (555.7) | ||
| ≥2500 g (n = 12,413) | 1,766 (14.20) | Reference | ‐‐ |
| < 2500 g (n = 2,562) | 495 (19.30) | 1.44 (1.23–1.70) | <0.00 |
| Season of measurement | |||
| Winter (n = 2,750) | 333 (12.11) | Reference | ‐‐ |
| Summer (n = 11,128) | 1,633 (14.68) | 1.25 (1.07–1.46) | 0.006 |
| Monsoon (n = 5,020) | 837 (16.68) | 1.45 (1.23–1.72) | <0.000 |
| Birth order | |||
| 1 (n = 7,232) | 1,076 (14.88) | Reference | ‐‐ |
| 2 (n = 6,237) | 880 (14.12) | 0.94 (0.81–1.09) | 0.415 |
| 3 (n = 2,920) | 454 (15.55) | 1.05 (0.89–1.25) | 0.549 |
| ≥4 (n = 2,510) | 393 (15.66) | 1.06 (0.90–1.25) | 0.469 |
| Breastfeeding status | |||
| Any breastfeeding (n = 18,034) | 2,649 (14.69) | Reference | ‐‐ |
| No breastfeeding (n = 725) | 137 (18.85) | 1.35 (1.00–1.82) | 0.052 |
| Initiation of breastfeeding | |||
| Within 1 hr (n = 7681) | 1,111 (14.47) | Reference | ‐‐ |
| More than 1 hr (n = 10,684) | 1,596 (14.94) | 1.04 (0.91–1.18) | 0.561 |
| Pre‐lacteal feed | |||
| No (n = 14,489) | 2,123 (14.65) | Reference | ‐‐ |
| Yes (n = 3,876) | 584 (15.07) | 1.03 (0.89–1.20) | 0.653 |
| Had diarrhoea in 2 weeks preceding the survey | |||
| No (n = 16,667) | 2,484 (14.90) | Reference | ‐‐ |
| Yes (n = 2,218) | 320 (14.41) | 0.96 (0.81–1.14) | 0.653 |
Abbreviations: 95% CI: 95% confidence interval; NFHS‐4: National Family Health Survey–4; OR: odds ratio; SD: standard deviation; WLZ: weight for length.
The significant predictors of severe wasting in the final multivariable logistic regression model based on children with documented birth weight only and in all children are shown in Table 4. Infants aged 2–3 months and 4–5 months had 0.78 (95% CI [0.66, 0.93]) and 0.65 (95% CI [0.55, 0.73]) times lower odds of being severely malnourished compared with infants aged 0–1 months, respectively. LBW infants had 1.40 times higher odds (95% CI [1.19, 1.65]) of severe wasting compared with non‐LBW infants. Nonutilization of ICDS supplementary nutrition by the mother during lactation was associated with 1.23 times higher odds (95% CI [1.05, 1.43]) of severe wasting compared with having utilized the supplementary food. Season of anthropometric measurement was also significantly associated with severe wasting. Infants assessed during summer and monsoon had 1.37 (95% CI [1.13, 1.65]) and 1.53 (95% CI [1.20, 1.95]) times higher odds, respectively of severe wasting compared with those assessed in winter. Restricting the analysis to only those with documented birth weight yielded odds that were very similar to estimates in all infants with either documented or reported birth weight (Table 4).
Table 4.
Predictors of severe wasting (WLZ < −3 SD) among infants aged less than 6 months in India for all birth weight and documented birth weight (NFHS‐4, 2015–2016)
| Variable | All birth weight (weighted N = 14, 012) | Documented birth weight (weighted N = 7, 705) |
|---|---|---|
| AOR (95%CI) | AOR (95% CI) | |
| Place of residence | ||
| Urban | Reference | Reference |
| Rural | 0.90 (0.74–1.09) | 1.09 (0.85–1.40) |
| Caste | ||
| Others | Reference | Reference |
| Scheduled caste/tribes | 1.07 (0.84–1.37) | 1.18 (0.88–1.59) |
| Other backward class | 1.09 (0.86–1.37) | 1.24 (0.93–1.66) |
| Age | ||
| <19 | 0.87 (0.57–1.33) | 0.90 (0.55–1.48) |
| 19–30 | Reference | Reference |
| >30 | 1.13 (0.91–1.41) | 1.02 (0.78–1.33) |
| Maternal education | ||
| Secondary/higher | Reference | Reference |
| No education/primary education | 1.12 (0.98–1.29) | 1.17 (0.97–1.41) |
| BMI | ||
| Normal weight | Reference | Reference |
| Undernourished | 1.03 (0.86–1.24) | 1.01 (0.80–1.28) |
| Overweight and obese | 0.93 (0.72–1.19) | 0.97 (0.72–1.30) |
| Nutrition benefits from ICDS during lactation | ||
| Yes | Reference | Reference |
| No | 1.23 (1.05–1.43) | 1.36 (1.13–1.65) |
| Age of child (in months) | ||
| 0–1 | Reference | Reference |
| 2–3 | 0.78 (0.66–0.93) | 0.89 (0.72–1.09) |
| 4–5 | 0.65 (0.55–0.78) | 0.67 (0.53–0.84) |
| Gender | ||
| Male | Reference | Reference |
| Female | 0.94(0.82, 1.07) | 0.89 (0.74–1.06) |
| Birth weight | ||
| ≥2,500 g | Reference | Reference |
| <2,500 g | 1.40(1.19, 1.65) | 1.41 (1.12–1.77) |
| Breastfeeding status | ||
| Any breastfeeding | Reference | Reference |
| No breastfeeding | 1.23 (0.86–1.75) | 1.27 (0.79–2.03) |
| Had diarrhoea in 2 weeks preceding the survey | ||
| No | Reference | Reference |
| Yes | 0.98(0.80, 1.21) | 0.94(0.71, 1.24) |
| Season of measurement | ||
| Winter | Reference | Reference |
| Summer | 1.37(1.13, 1.65) | 1.49(1.14, 1.95) |
| Monsoon | 1.53(1.20,1.95) | 1.71(1.23,2.37) |
Note. Model adjusted for state of residence (estimates not shown).
Abbreviations: 95% CI: 95% confidence interval; AOR: adjusted odds ratio; BMI: body mass index; ICDS: Integrated Child Development Services; NFHS‐4: National Family Health Survey–4; OR: odds ratio; SD: standard deviation; WLZ: weight for length.
All birth weight includes infants for whom birth weight was available on health card or based on mother's recall.
Documented birth weight includes only those infants for whom birth weight was available on health card.
Place of residence, caste, maternal age, maternal education, maternal body mass index, child's gender, breastfeeding status, and diarrhoea in the last 2 weeks preceding the survey were not associated with severe wasting in the adjusted analysis (Table 4).
4. DISCUSSION
The secondary analysis of the NFHS‐4 data reveals a high burden of severe wasting in the first 6 months of life. The prevalence peaked in the first month itself with a steady decline thereafter until 6 months of age. The prevalence was high in all the states except for the north eastern states of Mizoram, Manipur, Nagaland, Sikkim, Assam, and in Jammu and Kashmir. Factors independently associated with severe wasting in young infants included LBW, younger infant age within the first 6 months window, and nonutilization of supplementary nutrition by the mothers during lactation that are provided by the nationwide Integrated Child Development Scheme. Anthropometric assessment during summer or monsoon was associated with higher likelihood of being severe wasting compared with winter season.
It is of interest that the North Eastern states have lower prevalence of early life severe wasting. A review of the state wise NFHS‐4 report suggests that as against the national average, these states have higher proportion of mothers having secondary or higher education, lower proportion of mothers being severely undernourished or anaemic, higher mean maternal age at child birth, and higher average birth weight of children. Further exploration of this issue would be of interest (IIPS & ICF, 2017).
The high burden of severe wasting in first 6 months of life in India is consistent with 2018 estimates by WHO, United Nations Children's Fund, and the World Bank (Blake et al., 2016; UNICEF, 2017). The prevalence of severe wasting in infants aged less than 6 months in countries ranged from 0.8 to 10.6% in Sub Saharan Africa, 1.2 to 9.6% in Middle East and North Africa, 0.2 to 3.9% in Latin America and Caribbean, and 5.3 to 14.1% in South Asia. Within South East Asia, compared with 14.1% in India, Bangladesh (5.9%) and Nepal (5.3%) have reported lower prevalence of severe wasting. As these country‐based estimates are based on periodic national surveys using methodology similar to NFHS‐4, they are likely to share the same methodological strengths and weakness.
Significant contribution of LBW to severe wasting observed in this analysis has been reported by others as well (Blake et al., 2016). The steady decline in the prevalence of severe wasting after the first 2 months may reflect improved growth as breastfeeding practices are better established. This, however, should be interpreted with caution as different groups of infants were assessed in each age category and the composition of the age‐category sample may have been affected by the higher mortality among the LBW neonates in the first few months of life. Given that the proportion of children born with LBW did not differ significantly across the different age category points in our analysis, we believe that this is unlikely to have affected the decline in severe wasting with age (Figure 3).
A possible protective effect of the utilization of supplementary nutrition from the government program is of interest. It is plausible that utilization of supplementary nutrition may be higher among women who also follow appropriate child caring practices. On the other hand, improved utilization may lead to better maternal nutrition and in this way favourably influence breast milk output or quality, and infant nutrition. The evidence for the impact of maternal nutrition on breast milk quality and quantity is not yet conclusive (Nikniaz, Mahdavi, Arefhoesseini, & Khiabani, 2009).
The higher risk of severe wasting among children for whom anthropometry was obtained in summer or monsoon season maybe on account of seasonal differences in food and nutrient availability and access, and possibly diarrhoea and fluid loss (Hillbruner, Egan, & Bulletin, 2008; Talbert et al., 2012). Children also tend to be weighed with some clothes on in winter months. Further studies are needed to assess if there is an association between season and severe wasting.
The lack of association with expected risk factors like place of residence, caste, maternal age, and maternal education raises the possibility that the aetiology of early severe wasting is different from what is observed at later ages. In early severe wasting birth weight is likely to be more important. The quality of care for small and sick babies in early postnatal life may also modify the risks and pathways towards early severe wasting.
Potential limitations of this analysis need to be noted. As the survey was done on a nationwide basis, errors in measuring length and weight during the survey may have occurred and we are unable to comment on the magnitude of misclassification on this account. Recording of age is also error prone. We used the WHO child growth standards as reference for defining severe wasting (WHO, 2006). These may not be appropriate for preterm infants particularly in the first 6 months of life and maybe even later and will overestimate the prevalence of severe wasting. Information on only a limited number of potential covariates of severe wasting was available. Further, documented evidence of birth weight was only available in around 55% of births. Analysis of only those with documented birth weight did not change the findings of its association with severe wasting substantially when all infants were considered.
Our findings confirm the high prevalence of severe wasting among infants aged less than 6 months in India. In addition, they reveal a significant variability across states, reaching over 20% in Gujarat and Maharashtra. Given short term and plausible long‐term consequences of severe wasting (Chisti et al., 2013; Jones & Berkley, 2014; Lelijveld et al., 2016; Talbert et al., 2012), it is imperative that actions be considered for its prevention and management. Focusing on increasing birth weight would be an appealing first choice for action—but we still lack interventions of demonstrated high impact. Interventions delivered during the antenatal period have shown modest effects on birth size. More ambitious life course approaches to achieve improved health and nutrition among women during the reproductive age should be explored when searching for more effective interventions (Christian, 2014). Such preventive interventions should be complemented by improved postnatal care of LBW babies. These include early initiation of breastfeeding, Kangaroo Mother Care and community‐based care with extra visitations for LBW babies (WHO & UNICEF, 2014).
The programs for care of LBW in India are restricted to those weighing less than 1,800 or 2,000 g based on greater concerns about their survival. However, this excludes many LBW infants at increased risk of early severe wasting. From the thriving perspective all LBW need improved care and not only a subset of them. High burden of LBW and suboptimal postnatal care often coexist. Ensuring equitable access to appropriate health care and nutrition for women, particularly from preconception through pregnancy and the postnatal period, will contribute not only to their own health and ability to care for their babies but will assist in increasing birth weights, improved breastfeeding, and nutrition in early infancy and prevention of early severe wasting.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTORS
TSC developed the analytical strategy, performed the statistical analysis, interpreted the results, and wrote the first draft and revised the manuscript. AS and RC contributed to obtaining data, statistical analysis, interpretation of the results, and revision of the manuscript. NB, JM, RB, ST, and MKB contributed to the conceptualization of the study, analytical strategy, and interpretation of results, and performed critical revisions of the manuscript.
Supporting information
Table S1: Details of the explanatory variables and sub groups used in the analysis
ACKNOWLEDGMENTS
The Society for Applied Studies acknowledges the core support provided by the Department of Maternal, Newborn, Child and Adolescent Health, World Health Organization, Geneva (WHO Collaborating Centre IND‐096); the Centre for Intervention Science in Maternal and Child Health (RCN Project No. 223269), Centre for International Health, University of Bergen (Norway); and Knowledge Integration and Translational Platform (KnIT), a Grand Challenges Initiative of the Biotechnology Industry Research Assistance Council (BIRAC), Department of Biotechnology, Government of India and Bill & Melinda Gates Foundation (USA).
Choudhary TS, Srivastava A, Chowdhury R, et al. Severe wasting among Indian infants < 6 months: Findings from the National Family Health Survey 4. Matern Child Nutr. 2019;15:e12866 10.1111/mcn.12866
REFERENCES
- Ambadekar, N. N. , & Zodpey, S. P. (2017). Risk factors for severe acute malnutrition in under‐five children: A case‐control study in a rural part of India. Public Health, 142, 136–143. 10.1016/j.puhe.2016.07.018 [DOI] [PubMed] [Google Scholar]
- Black, R. E. , Allen, L. H. , Bhutta, Z. A. , Caulfield, L. E. , de Onis, M. , Ezzati, M. , … Rivera, J. (2008). Maternal and child undernutrition: Global and regional exposures and health consequences. Lancet, 371, 243–260. 10.1016/S0140-6736(07)61690-0 [DOI] [PubMed] [Google Scholar]
- Black, R. E. , Victora, C. G. , Walker, S. P. , Bhutta, Z. A. , Christian, P. , de Onis, M. , … Uauy, R. (2013). Maternal and child undernutrition and overweight in low‐income and middle‐income countries. The Lancet, 382(9890), 427–451. 10.1016/s0140-6736(13)60937-x [DOI] [PubMed] [Google Scholar]
- Blake, R. A. , Park, S. , Baltazar, P. , Ayaso, E. B. , Monterde, D. B. S. , Acosta, L. P. , … Friedman, J. F. J. P. O. (2016). LBW and SGA impact longitudinal growth and nutritional status of Filipino infants. PLoS ONE, 11(7), e0159461 10.1371/journal.pone.0159461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisti, M. J. , Salam, M. A. , Ashraf, H. , Faruque, A. S. , Bardhan, P. K. , Hossain, M. I. , … Ahmed, T. (2013). Clinical risk factors of death from pneumonia in children with severe acute malnutrition in an urban critical care ward of Bangladesh. PLoS ONE, 8(9), e73728 10.1371/journal.pone.0073728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian, P. (2014). Fetal growth restriction and preterm as determinants of child growth in the first two years and potential interventions In International Nutrition: Achieving Millennium Goals and Beyond (Vol. 78) (pp. 81–91). Karger Publishers. [DOI] [PubMed] [Google Scholar]
- Grijalva‐Eternod, C. S. , Kerac, M. , McGrath, M. , Wilkinson, C. , Hirsch, J. C. , Delchevalerie, P. , & Seal, A. J. (2017). Admission profile and discharge outcomes for infants aged less than 6 months admitted to inpatient therapeutic care in 10 countries. A secondary data analysis. Maternal & Child Nutrition, 13(3). 10.1111/mcn.12345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell, F. E. (2015). Binary logistic regression In Regression modeling strategies (pp. 219–274). New York. Springer. [Google Scholar]
- Hillbruner, C. , Egan, R. J. F. , & Bulletin, N. (2008). Seasonality, household food security, and nutritional status in Dinajpur, Bangladesh. Food and Nutrition Bulletin, 29(3), 221–231. [DOI] [PubMed] [Google Scholar]
- ICF, IIPS (2017). India National Family Health Survey NFHS‐4 2015–16. Mumbai, India: IIPS and ICF. 2017 [Google Scholar]
- Jones, K. D. , & Berkley, J. A. (2014). Severe acute malnutrition and infection. Paediatrics and International Child Health, 34(1), S1–S29. 10.1179/2046904714Z.000000000218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerac, M. , Blencowe, H. , Grijalva‐Eternod, C. , McGrath, M. , Shoham, J. , Cole, T. J. , & Seal, A. (2011). Prevalence of wasting among under 6‐month‐old infants in developing countries and implications of new case definitions using WHO growth standards: a secondary data analysis. Archives of Disease in Childhood, 96(11), 1008–1013. 10.1136/adc.2010.191882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerac, M. , Bunn, J. , Chagaluka, G. , Bahwere, P. , Tomkins, A. , Collins, S. , & Seal, A. (2014). Follow‐up of post‐discharge growth and mortality after treatment for severe acute malnutrition (FuSAM study): a prospective cohort study. PLoS ONE, 9(6), e96030 10.1371/journal.pone.0096030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerac, M. , Mwangome, M. , McGrath, M. , Haider, R. , & Berkley, J. A. (2015). Management of acute malnutrition in infants aged under 6 months (MAMI): Current issues and future directions in policy and research. Food and Nutrition Bulletin, 36(1), S30–S34. 10.1177/15648265150361s105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelijveld, N. , Seal, A. , Wells, J. C. , Kirkby, J. , Opondo, C. , Chimwezi, E. , … Kerac, M. (2016). Chronic disease outcomes after severe acute malnutrition in Malawian children (ChroSAM): A cohort study. The Lancet Global Health, 4(9), e654–e662. 10.1016/s2214-109x(16)30133-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MWCD (2019), Government of India . Integrated Child Development Services (ICDS) scheme. Available from: https://www.icds-wcd.nic.in/icds.aspx (Last accessed: 09/02/2019).
- Nikniaz, L. , Mahdavi, R. , Arefhoesseini, S. , & Khiabani, M. S. (2009). Association between fat content of breast milk and maternal nutritional status and infants' weight in tabriz, iran. Malaysian Journal of Nutrition, 15(1). [PubMed] [Google Scholar]
- Singh, D. K. , Rai, R. , Mishra, P. C. , Maurya, M. , & Srivastava, A. J. T. I. (2014). Nutritional rehabilitation of children<6 mo with severe acute malnutrition. The Indian Journal of Pediatrics, 81(8), 805–807. 10.1007/s12098-013-1285-3 [DOI] [PubMed] [Google Scholar]
- Singh, K. , Badgaiyan, N. , Ranjan, A. , Dixit, H. O. , Kaushik, A. , Kushwaha, K. P. , & Aguayo, V. M. (2014). Management of children with severe acute malnutrition: Experience of Nutrition Rehabilitation Centers in Uttar Pradesh, India. Indian Pediatrics, 51(1), 21–25. 10.1007/s13312-014-0328-9 [DOI] [PubMed] [Google Scholar]
- Talbert, A. , Thuo, N. , Karisa, J. , Chesaro, C. , Ohuma, E. , Ignas, J. , … Maitland, K. (2012). Diarrhoea complicating severe acute malnutrition in Kenyan children: A prospective descriptive study of risk factors and outcome. PLoS ONE, 7(6), e38321 10.1371/journal.pone.0038321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF (2017). WHO, the World Bank. Joint child malnutrition estimates—Levels and trends (2017th ed.). Geneva: World Health Organization. 2017 [Google Scholar]
- WHO (2006). WHO child growth standards: Length/height for age, weight‐for‐age, weight‐for‐length, weight‐for‐height and body mass index‐for‐age, methods and development: World Health Organization.
- WHO (2013). Guideline: updates on the management of severe acute malnutrition in infants and children: World Health Organization. [PubMed]
- WHO . WHO Anthro and macros Geneva: WHO; 2015. [Internet]. [Cited 29 Novemeber 2018]. Available from: http://www.who.int/childgrowth/software/en/.
- WHO, UNICEF (2014). Every newborn: An action plan to end preventable deaths. Geneva: World Health Organization. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1: Details of the explanatory variables and sub groups used in the analysis


