Abstract
Micronutrients powder (MNP) can prevent anaemia amongst children 6–23 months old. However, evidence of an effect on growth is limited and concerns about the safety of iron‐containing MNP interventions limits their applicability. In a cluster randomized controlled intervention, we evaluated the effectiveness of a nutritional package including counselling and provision of MNP to improve the nutritional status of children aged 6–23 months and the effect of sustained use of MNP on morbidity in a malaria‐endemic area. Child feeding practises and nutritional status were assessed through cross‐sectional surveys. Biweekly morbidity surveillance and anthropometry measurements were carried out in a nested cohort study. No significant differences in the prevalence of wasting (−0.7% [−6.8, 5.3] points; p = .805), stunting (+4.6% [−2.9, 12.0] points; p = .201), or mean length‐for‐age z‐score and weight‐for‐length z‐score scores were found between study groups. The proportion of children with a minimum dietary diversity score and those with a minimum acceptable diet significantly increased in the intervention group compared with the control by 6.5% points (p = .043) and 5.8% points (p = .037), respectively. There were no significant differences in the risk of diarrhoea (RR: 1.68, 95% CI [0.94, 3.08]), fever (RR: 1.20 [0.82, 1.77]), and malaria (RR: 0.68 [0.37, 1.26]) between study groups. In the nested study, the rate of linear growth was higher in the intervention than in the control group by 0.013 SD/month (p = .027). In a programmatic intervention, MNP and nutrition education marginally improved child feeding practises and growth, without increasing morbidity from malaria or fever.
Keywords: feeding practises, growth, micronutrients powder, morbidity, nutrition counselling
Key messages.
MNP supplementation coupled with nutrition education yielded marginally significant benefit on child growth and feeding practises of the mothers.
Evaluation of MNP programmes using longitudinal follow‐up designs may be appropriate to detect the effects of such interventions on child growth.
A sustained iron‐contained supplement distribution in a malaria‐endemic rural area did not have significant effects on malaria and fever amongst children aged 6–23 months.
List of abbreviations
- CSPS
centre de santé et de promotion sociale
- GMP
growth monitoring and promotion
- IYCN
infant and young child nutrition
- LAZ
length‐for‐age z‐score
- MNP
micronutrients powder
- IYCF
infant and young child feeding
- RDT
rapid diagnostic test
- SEM
standard error of the mean
1. INTRODUCTION
Micronutrient deficiencies affect billions of people worldwide and contribute, together with foetal growth restriction, stunting, wasting, and suboptimum breastfeeding, to over 3.1 million (45%) child deaths annually (Black et al., 2013). Young children and women of reproductive age in low‐ and middle‐income countries are the most at risk of developing these deficiencies due to their increased needs and inadequate nutrient intakes. It has been shown that interventions promoting optimal breastfeeding and appropriate complementary feeding practises can prevent 7.1% of child deaths and micronutrient supplementation in children at‐risk could prevent an additional 4.7% of child deaths (Bhutta et al., 2013). Micronutrients powder (MNP) containing multiple vitamins and minerals for home food fortification has been recommended for infants and young children between 6 and 23 months of age (World Health Organization, 2011) and is widely used as a promising strategy to combat micronutrient deficiencies (Jefferds, Irizarry, Timmer, & Tripp, 2013). Despite the increasing implementation of this intervention, the effects of MNP on growth of children at risk is limited (De‐Regil, Suchdev, Vist, Walleser, & Peña‐Rosas, 2013). MNP interventions have been shown to reduce overall anaemia and iron deficiency prevalence in children (Locks et al., 2017; Salam, MacPhail, Das, & Bhutta, 2013). However, concerns have been raised about the safety of iron supplementation amongst iron‐replete children, as a daily provision of supplemental iron may exacerbate the occurrence and severity of infections including malaria and diarrhoea (Sazawal et al., 2006; Soofi et al., 2013). Systematic reviews on iron supplementation trials concluded no significant increase in the risk for malaria morbidity or mortality when there is adequate malaria surveillance and treatment service (Ojukwu, Okebe, Yahav, & Paul, 2009; Okebe, Yahav, Shbita, & Paul, 2011), but more evidence from programme settings is lacking.
The use of the MNP requires their addition to foods suitable for young children, offering an opportunity to promote healthy complementary feeding (Cardoso et al., 2016). Studies have suggest that educational interventions on infant and young child feeding (IYCF) practises are effective in improving children's dietary intake and growth (Penny et al., 2005; Salehi, Kimiagar, Shahbazi, Mehrabi, & Kolahi, 2004; Shi, Zhang, Wang, Caulfield, & Guyer, 2010) but should be culturally sensitive, accessible, and integrated with local resources (Dewey & Adu‐Afarwuah, 2008; Shi & Zhang, 2011). Whereas educational interventions can improve diet quality, the net increase on micronutrient intakes to meet the daily requirements could be limited by the availability of nutrient‐rich animal source foods (Dewey & Adu‐Afarwuah, 2008). It is hypothesized that nutrition education could be beneficial for better compliance and diet quality and could contribute to better impact of MNP on malnutrition. Therefore, evidence is required from programmes implementing integrated infant and young child nutrition (IYCN) strategies balancing potential benefits of filling nutrient gaps and promoting healthy growth (Bhutta et al., 2013; Dewey & Arimond, 2012) with possible adverse effects on morbidity. The objectives of this study were first to evaluate the effectiveness of this promotional package on improving nutritional status and feeding practises of Burkinabe children aged 6–23 months and second to evaluate the safety of sustained MNP supplementation on morbidity in a malaria‐endemic setting.
2. METHODS
2.1. Study setting
From November 2014 to October 2015, a programme integrating the promotion of adequate IYCF practises, provision of MNP, and community‐based management of acute malnutrition was implemented by Terre des Hommes (TdH) in collaboration with the Micronutrient Initiative (MI). The programme was implemented in the rural area of the health district of Tougan, in Northwest Burkina Faso. The district covers eight municipalities with an estimated population of 240,474 in 2014. Twenty‐seven health and social promotion centers (Centre de santé et de promotion sociale, CSPS) constitute the primary operational care units in the national health care system and provide a minimum package of curative, promotional, and preventive services in the Tougan health district. The area has a holoendemic malaria transmission reaching its peak during the rainy season between June and October. All children under five in the programme area were exempted from health service fees, and intermittent malaria treatment was provided for children and women of reproductive age (Konaté et al., 2011).
2.2. Study design and randomization
Four out of eight municipalities (Di, Gomboro, Kassoum, and Lanfiera) in the Tougan health district were selected for programme implementation. For the purpose of randomization, all eligible villages that were listed and villages within a municipality were paired according to their population size and distance from the nearest health centre. Four villages where there was an on‐going nutrition intervention programme were excluded from the list. Two additional villages were also excluded due to the seasonal migration of the population to the neighbouring country (Mali). For random allocation of villages to study groups, a letter was blindly selected from a text, in this case it was the letter “i.” Village names with their first letter closest to “i” were placed in the intervention group (Intervention). The other village of the pair was then allocated to the control group (Control). In cases where the first letter was identical and/or equidistant from “i,” the second letter was used and so forth. In total, 31 villages (clusters) were allocated to each of the Intervention and Control groups (Figure 1).
Figure 1.
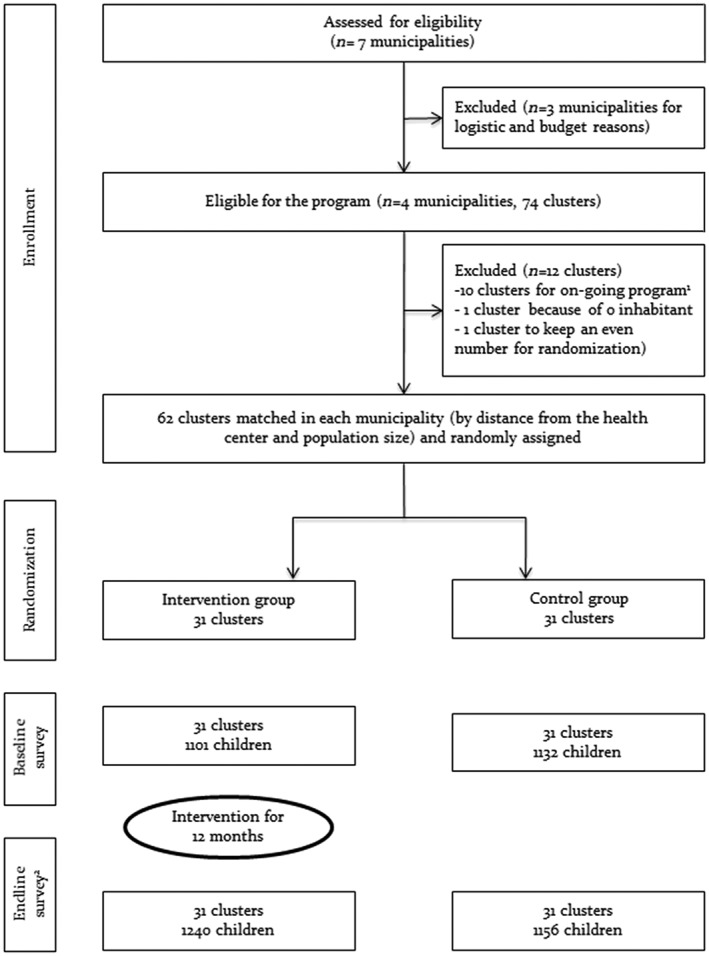
Trial profile. 1These clusters (villages) were already benefiting from an on‐going nutrition intervention at the time of the study. 2The children surveyed at the endline are different from those at the baseline.
Effectiveness of the intervention on selected study outcomes was assessed using repeated cross‐sectional surveys (baseline and endline including all 62 clusters). Children aged 6–23 months and their mothers, who were living in the study villages during the preintervention and postintervention survey periods, were eligible for inclusion. The number of mother–child pairs to be included from each village was determined proportionally to the population size of each village. In each village, households were sampled for data collection using a random walk approach. Starting from a central place in a village, an arbitrary direction was chosen by spinning a bottle. Thereafter, all households with an eligible child that were located in the same direction were recruited for data collection. Once we reached the border of the village, we repeated the same procedure until the required number of subjects per village was achieved. In cases where there was more than one eligible child within a household, a lottery method was applied to select one. In certain villages, there were fewer children aged 6–23 months than expected based on the health district records. Therefore, we modified our sampling accordingly by enrolling additional mother–child pairs from larger adjacent villages.
The primary outcomes were the prevalence of stunting (length‐for‐age z score < −2 SD) and the proportion of children with minimum dietary diversity (Dietary Diversity Score [DDS] ≥ 4). For stunting, sample size calculation was based on an estimated 30% reduction in stunting prevalence, from 22.0% to 15.4% (Direction de La Nutrition, 2014), which was considered to be of public health importance. A sample size of 806 children was required for each study group using an α error of 5%, a β error of 20%, and the coefficient of variation assumed at 0.25. For the dietary diversity outcome, a sample size of 1,106 children per study group was required to detect a 100% (from 10% to 20%) increase in the proportion of children with DDS ≥ 4 (Direction de La Nutrition, 2014) using an α error of 5%, β error of 20%, and a coefficient of variation (k) estimated at 0.25. The intervention effect on growth was assessed in children aged 12–23 months to allow for a sufficient exposure to the intervention (Menon, Rawat, & Ruel, 2013), whereas the effect on feeding practises was evaluated for children aged 6–23 months. Thus, the overall sample size required was 1,106 children aged 6–23 months per study group, including 806 children aged 12–23 months.
In addition, a longitudinal cohort of children aged 6–11 months was established to evaluate the impact of sustained MNP supplementation on morbidity and anthropometric outcomes. Children for this cohort were sampled from the largest villages in each group, using the same sampling procedure described above and followed for a duration of 12 months via biweekly home visits for morbidity surveillance and anthropometric measurements. The sample size calculation was based on the baseline survey (July 2014), where around 20% of mothers reported that their child had suffered at least one episode of either fever, diarrhoea, or cough during the 7 days preceding the survey. To be able to detect a 10% increase in morbidity prevalence between groups (from 20% to 22%, i.e., 2 percentage points), with an α error of 5%, a β error of 20%, and a coefficient of variation of the true proportion between clusters within each trial group (k) of 0.15 (accounting not only for the clustering per village but also for the repeated measurements on a proportion of children), 330 children aged 6–11 months were needed in each study group.
2.3. Ethics
The study was approved by the National Ethics Committee of Burkina Faso, the Institutional Review Board of the Institute of Tropical Medicine (ITM), and the Ethics Committee of the University Hospital of Antwerp. Written informed consent was obtained from all mothers included in the study for the participation of their child and their own participation in the study. The trial was registered at ClinicalTrials.gov with identification number NCT02136966.
2.4. Intervention
The intervention package was developed for the prevention and treatment of malnutrition amongst children aged 6–23 months and was delivered through monthly community‐based growth monitoring and promotion (GMP) sessions run by the TdH staff and the community health workers (CHWs). The intervention package comprises (a) child growth monitoring, (b) provision of MNP for home fortification of complementary foods, (c) child‐centred counselling, and (d) cooking demonstrations. The GMP consisted of screening children under 24 months of age for malnutrition based on anthropometric measurements. Children with no malnutrition and those with moderate acute malnutrition (MAM) benefited from the programme. Mothers of children with MAM were invited for intensive counselling and biweekly cooking demonstrations in addition to their child receiving the same dosage of MNP as children with no malnutrition. Children with severe acute malnutrition (SAM) were referred to clinics where therapeutic doses of PlumpyNut were provided for cases with uncomplicated SAM and clinic‐based therapeutic feeding and medical care for cases with complicated SAM according to the national guidelines. During the course of the treatment, SAM children did not receive MNP to avoid risk of over dosage. Once discharged from SAM treatment, they were enrolled into the MAM group with intensive follow‐up and MNP treatment. The group counselling, organized with mothers, was focused on selected topics including texture and quantity of complementary foods, feeding frequency, hygienic methods of food preparation and storage, feeding during and after illness, MNP fortification of complementary foods, and the importance of growth monitoring. During the cooking demonstrations, CHWs instructed mothers on how to prepare complementary foods from locally available ingredients and how to use the MNP to fortify these recipes. Mothers were encouraged to prepare the same meals at home and were provided with a monthly supply of 15 MNP servings for fortification of complementary foods on alternate days. The MNP contained 15 micronutrients, including 10 mg of elemental iron per serving, packaged in individual dose sachets (Table 1). Child‐centred counselling was provided to the mothers individually, using improved behavioural change intervention (BCI) messages that were developed based on a rapid assessment of practises and foods available in the community and adaptation of the World Health Organization (WHO) manual (World Health Organization, 2012).
Table 1.
Composition of the micronutrients powder
| Vitamin or mineral | Amount per gram (one serving) |
|---|---|
| Vitamin A (RE) | 400 μg |
| Vitamin D | 5 μg |
| Vitamin E | 5 mg |
| Vitamin C | 30 mg |
| Vitamin B1 | 0.5 mg |
| Vitamin B2 | 0.5 mg |
| Vitamin B3 | 6 mg |
| Vitamin B6 | 0.5 mg |
| Vitamin B12 | 0.9 μg |
| Folic acid | 150.0 μg |
| Iron | 10.0 mg |
| Zinc | 4.1 mg |
| Copper | 0.56 mg |
| Selenium | 17.0 μg |
| Iodine | 90 μg |
In the Control villages, the routine GMP activities and the screening and referral of MAM/SAM cases according to the recommendation by the Ministry of Health were continued. The routine treatment in these clinics included therapeutic dosages of PlumpyNut for children with SAM and PlumpySup for children with MAM.
2.5. Data collection and outcome measures
Feeding practises were evaluated by assessing WHO IYCF indicators (World Health Organization, 2010), based on information collected through a questionnaire on the diet of the child during the 24 hr preceding the survey. The following indicators were assessed: continued breastfeeding at 1 year; introduction of solid, semi‐solid, or soft foods; minimum dietary diversity; minimum meal frequency; and minimum acceptable diet. Caregiver and CHW knowledge on IYCF practises was assessed through open‐ended questions on what nutritional advise they would give to mothers of children aged 6–23 months, based on the essential ideal behaviours of the UNICEF programming guide on IYCN (UNICEF, 2011). The correct answers of the respondent were ticked from a list of pre‐coded possible answers. Duplicate measurements of weight, length, and mid‐upper‐arm circumference (MUAC) were taken. Child length was measured to the nearest 1 mm using a SECA 207 scale and weight to the nearest 10 g using a SECA 725 scale. MUAC was measured to the nearest 1 mm using a SECA Girth Measuring Tape or a SECA 212 tape. All weighing scales were calibrated on a daily basis. The accuracy and precision of measurements were evaluated through monthly standardization sessions. Haemoglobin concentration was determined by spectrophotometry using a HemoCue device (Dronfield, United Kingdom). Calibration was done daily using HemoCue Control Cuvettes.
Data collection in the nested cohort study was done through home visits using a pretested questionnaire. This comprises a summary of the symptoms that the child was experiencing the day of the visit, a review of symptoms/illnesses presented by the child in the last 7 days (or diagnoses in a health centre or by a health‐care professional), a clinical examination, and where applicable, confirmation of malaria when the child had fever through a rapid diagnostic test (RDT, SD BIOLINE Malaria Ag Pf). A diagnosis (or diagnoses) was assigned using the case definitions given below. In cases where a child was admitted to the CSPS or the main hospital, the main symptoms, final diagnosis, and treatments, as noted on the child's health booklet by the health personnel, were reported. If there was no record of an admission/visit to the health facility (or no health booklet at all), then the mother was asked to report the main symptoms, and the assessment of the illness was conducted by the study nurse. The same procedure was carried out for any outpatient consultations to the CSPS in the last 7 days. Anthropometric measurements were collected during each visit, according to the procedures described above. Children who needed further medical care or those who had a positive malaria test were referred to the CSPS.
Case definitions: Malaria was defined as the presence and/or history of fever (axillary temperature above 37.5° centigrade) and a positive RDT. To conclude recovery from an episode of malaria, a 28‐day malaria‐free period must have been observed. Diarrhoea was defined as having three or more liquid stools per day. An episode of diarrhoea was defined as having diarrhoea for at least 2 days followed by a diarrhoea‐free period of at least 2 days. Dysentery was defined as diarrhoea with presence of blood or mucus in more than one stool. Lower respiratory tract infection (LRTI) was defined as rapid respiration (>50 breaths per minute for infants and ≥40 for older children) and/or inter/subcostal recession, fever, and/or cough in the absence of malaria. The symptoms must have been observed by the study nurse. An episode of LRTI was defined as having symptoms for at least 2 days followed by a period of at least 7 days without any symptoms.
2.6. Statistical analysis
Anthropometric indices (weight‐for‐age, length‐for‐age, and weight‐for‐length z scores) were calculated using the zscore06 command in Stata based on the 2006 WHO child growth standards (Leroy, 2011). The difference‐in‐differences (DID) approach was employed for the analysis of the preintervention and postintervention survey data. We fitted general linear models to estimate the effect of intervention on both the continuous and the binary (linear probability modelling in this case) outcomes with standard robust variance estimation to consider the clustering effect by village. The use of linear probability models for binary outcomes is well established and allows for a straightforward interpretation of average intervention effects with risk differences expressed as a difference in percentage points (Wooldridge, 2002). Covariates in the model included the study group (Intervention vs Control), period (preintervention vs. postintervention), and the interaction between study group and period. The intervention effect was estimated using the interaction term between study group and period indicating the mean intervention effect in the case of the continuous outcomes and percentage point differences in the case of binary outcomes.
For the nested cohort study, longitudinal prevalence of disease was calculated using the total number of days with the disease outcome as the numerator and the total number of days at‐risk as the denominator (Morris, Cousens, Kirkwood, Arthur, & Ross, 1996). Three‐level mixed‐effects models were used with a level two random intercept for child and a level three random intercept for village. The decision for considering three‐level versus two‐level models was made using likelihood ratio tests comparing two‐level models (random intercept for child or village only) versus three‐level model (random intercept for both child and intervention village). Weekly morbidity counts were fitted using generalized linear latent and mixed models (GLLAMM) and risk ratios comparing Intervention versus Control groups were calculated using the Poisson link function and the robust variance estimator adjusted for child age as a fixed effect (Cummings, 2009). Linear mixed effects models were fitted for the anthropometric data from the nested cohort study. The growth models additionally considered a random slope for intervention time to account for the variations in individual growth trajectories. Fixed‐effects included child sex, study group, intervention time, quadratic term of intervention time, and the interaction between study group and time as an estimate for the intervention effect on monthly changes in z scores. All analyses were performed using Stata 12 (StataCorp, Texas, USA), and all tests were two sided with statistical significance set at α < 5%.
3. RESULTS
3.1. General characteristics
A total of 4,629 children aged 6–23 months were surveyed in the main study representing 2,233 children at baseline (Control = 1,132, Intervention = 1,101) and 2396 at endline survey (Control = 1,156, Intervention = 1,240). In the sample at baseline, the mean (SD) age of mothers was 27.5 (6.8) years, 77.8% did not attend school and 19.7% were primiparous. The mean (SD) age of children was 14.9 (5.0) months and 50.5% were female. The prevalence of stunting, wasting, and underweight was 20.4%, 17.1%, and 24.7% respectively. The characteristics of children, mothers, and households were similar between study groups and between survey periods except for the percentage of children in the age group “6–11 months,” which was lower at baseline compared with endline (27.9% vs. 34.0%) in the Intervention group (Table 2). However, these differences were not statistically significant. Similarly, there were no significant differences in the baseline characteristics of infants between study groups in the nested cohort study. The mean (SEM) differences were 0.18 (0.13), −0.038 (0.100), and 0.070 (0.098) for child age, length‐for‐age (LAZ) and weight‐for‐length (WLZ) z scores, respectively.
Table 2.
Characteristics of children and mothers by study groups and survey periods
| Baseline | Endline | |||
|---|---|---|---|---|
| Characteristics | Control (n = 1,132) | Intervention (n = 1,101) | Control (n = 1,156) | Intervention (n = 1,240) |
| Child characteristics | ||||
| Child age, mo | 15.0 ± 5.0a | 15.2 ± 5.0 | 14.8 ± 5.0 | 14.8 ± 5.2 |
| Age group 6–11 months, (%) | 340 (30.0)b | 308 (28.0) | 369 (31.9) | 421 (34.0) |
| Female, (%) | 575 (50.8) | 547 (49.7) | 576 (49.8) | 636 (51.3) |
| Weight, kg | 8.7 ± 1.4 | 8.6 ± 1.4 | 8.5 ± 1.4 | 8.5 ± 1.4 |
| Height, cm | 75.0 ± 5.7 | 75.1 ± 6.0 | 74.8 ± 5.8 | 74.7 ± 5.8 |
| MUAC, cm | 140.0 ± 9.9 | 140.5 ± 10.2 | 137.7 ± 10.8 | 138.2 ± 11.1 |
| LAZ, SD | −1.10 ± 1.31 | −1.15 ± 1.20 | −1.10 ± 1.35 | −1.06 ± 1.32 |
| WLZ, SD | −1.02 ± 1.10 | −1.04 ± 1.05 | −1.03 ± 1.16 | −0.99 ± 1.15 |
| Mothers characteristics | ||||
| Maternal age, year | 28.1 ± 7.0 | 28.0 ± 6.9 | 27.0 ± 6.9 | 27.0 ± 6.7 |
| No school attendance, (%) | 868 (76.7) | 860(78.1) | 898 (77.7) | 974 (78.5) |
| Primiparity, (%) | 209 (18.5) | 219 (19.9) | 250 (21.6) | 236 (19.0) |
| Household characteristics | ||||
| Household size | 8.1 ± 4.8 | 7.8 ± 6.5 | 7.5 ± 4.2 | 7.2 ± 3.9 |
| Caregiver‐mother, (%) | 1,125 (99.4) | 1,093 (99.3) | 1,150 (99.5) | 1,231 (99.3) |
Abbreviations: LAZ, length‐for‐age z score; MUAC, mid‐upper‐arm circumference; WLZ, weight‐for‐a‐length z score.
Mean ± SD, all such values.
Number (%), all such values.
3.2. Nutritional status
There was no significant difference between the Control and Intervention group with respect to the prevalence of stunting, wasting and anaemia, and mean LAZ and WLZ (Table 3). However, in the nested cohort study of children aged 6–11 months, the children in the intervention group showed significantly greater length gain (0.04 cm/month; p = .005) compared with children in the Control group. Similarly, children in the Intervention group showed significantly higher LAZ gain, 0.013 SD per month (p = .027), compared with children in the Control group (Table 4). There was no significant difference in the risk of stunting at the end of the follow‐up between study groups in the nested cohort study.
Table 3.
Effects of the intervention on haemoglobin and growth outcomesa
| Outcomes | Baseline | Endline | Endline‐baseline | Intervention‐control | ||
|---|---|---|---|---|---|---|
| Differencea | p | DIDb | p | |||
| LAZ | ||||||
| Control | −1.26 ± 0.04c | −1.26 ± 0.05 | 0.007 (−0.180, 0.181) | .938 | −0.13 (−0.33, 0.08) | .218 |
| Intervention | −1.19 ± 0.04 | −1.32 ± 0.04 | −0.12 (−0.23, −0.02) | .018 | ||
| WLZ | ||||||
| Control | −1.01 ± 0.04 | −1.05 ± 0.04 | −0.05 (−0.19; 0.09) | .493 | 0.06 (−0.15, 0.26) | .532 |
| Intervention | −1.01 ± 0.04 | −0.99 ± 0.04 | 0.01 (−0.14; 0.16) | .860 | ||
| WAZ | ||||||
| Control | −1.34 ± 0.04 | −1.37 ± 0.04 | −0.03 (−0.20, 0.13) | .687 | −0.02 (−0.22, 0.19) | .871 |
| Intervention | −1.32 ± 0.04 | −1.36 ± 0.04 | −0.05 (−0.17, 0.07) | .420 | ||
| Haemoglobin (g/dL) | ||||||
| Control | 9.96 ± 0.04 | 9.12 ± 0.05 | −0.84 (−1.05, −0.63) | <.001 | 0.12 (−0.14, 0.40) | .372 |
| Intervention | 10.09 ± 0.05 | 9.38 ± 0.05 | −0.72 (−0.89, −0.54) | <.001 | ||
| Stunting (LAZ < −2) | ||||||
| Control | 187 (23.3)d | 200 (25.5) | 1.9 (−3.4, 7.2) | .481 | 4.6 (−2.9, 12.0) | .201 |
| Intervention | 156 (19.7) | 214 (26.3) | 6.5 (1.3, 11.6) | .015 | ||
| Severe stunting (LAZ < −3) | ||||||
| Control | 50 (6.3) | 71 (9.0) | 2.6 (−1.3, 6.) | .180 | −2.0 (−6.9, 2.6) | .388 |
| Intervention | 47 (5.9) | 52 (6.4) | 0.6 (−1.9, 3.1) | .630 | ||
| Wasting (WLZ < −2) | ||||||
| Control | 124 (15.7) | 137 (17.4) | 2.0 (−1.5, 5.6) | .259 | −0.7 (−6.8, 5.3) | .805 |
| Intervention | 122 (15.4) | 135 (16.6) | 1.3 (−3.6, 6.2) | .595 | ||
| Severe wasting (WLZ < −3) | ||||||
| Control | 21 (2.6) | 27 (3.4) | 0.8 (−0.8, 2.4) | .311 | 0.5 (−2.0, 3.0) | .679 |
| Intervention | 22 (2.8) | 34 (4.2) | 1.3 (−0.4, 3.3) | .182 | ||
| Anaemia (Hb < 11 g/dL) | ||||||
| Control | 642 (81.2) | 716 (91.0) | 9.6 (6.2, 12.9) | <.001 | 3.2 (−2.4, 8.9) | .251 |
| Intervention | 597 (75.3) | 721 (88.0) | 12.8 (8.3, 17.4) | <.001 | ||
| Severe anaemia (Hb < 7 g/dL) | ||||||
| Control | 5 (0.6) | 57 (7.2) | 6.9 (4.4, 9.3) | <.001 | −1.3 (−5.0, 2.6) | .513 |
| Intervention | 9 (1.1) | 54 (6.6) | 5.6 (2.7, 8.6) | <.001 | ||
Abbreviations: DID, difference‐in‐differences; Hb, haemoglobin; LAZ, length‐for‐age z score; WLZ, weight‐for‐length z score; WAZ, Weight‐for‐age z score.
Estimated using linear regression models, including the outcome as dependent variables and the intervention (Intervention vs. Control), the period of survey (preintervention vs. postintervention), the interaction Intervention*period, and a variable for the pairwise allocation as predictors. Difference in mean (95% CI) for continuous and percentage points (95% CI) for binary variables, between the endline and the baseline surveys in each study group.
Difference‐indifferences: Coefficient of the interaction Intervention*period from the regression models.
Mean ± SD, all such values.
Number (%), all such values.
Table 4.
Effect of intervention on infant growth outcomes in the nested cohort study
| Outcomes | Control | Intervention | Adjusted model | |||
|---|---|---|---|---|---|---|
| N a | Mean ± SE | N | Mean ± SE | β (95% CI)b | p | |
| LAZ | 6858 | −0.066 ± 0.009 | 7,117 | −0.049 ± 0.009 | .013 (0.0012, 0.025) | .027 |
| WLZ | 6858 | −0.084 ± 0.013 | 7,117 | −0.088 ± 0.013 | −.009 (−0.023, 0.004) | .165 |
| WAZ | 6858 | −0.061 ± 0.010 | 7,117 | −0.054 ± 0.009 | −.001 (−0.011, 0.010) | .917 |
| Length, cm | 6858 | 1.145 ± 0.026 | 7,117 | 1.208 ± 0.024 | .044 (0.013, 0.075) | .005 |
| Weight, kg | 6858 | 0.171 ± .009 | 7,117 | 0.187 ± 0.008 | .003 (−0.008, 0.014) | .585 |
| MUAC, cm | 6837 | 0.174 ± 0.112 | 7,115 | 0.120 ± 0.112 | −.049 (−0.174, 0.081) | .471 |
Abbreviations: LAZ, length‐for‐age z score; MUAC, mid‐upper‐arm circumference; WAZ, weight‐for‐age z sore; WLZ, weight‐for‐a‐length z score.
Total number of measurements during the follow‐up period.
Interaction coefficients between intervention allocation (Intervention vs. Control) and child age, estimated from quadratic mixed models and expressed as monthly rate. Fixed effects for growth models included intervention, child sex, child age, and child age squared. Random effects for growth models included village identifier, child identifier, child age, and child age squared.
The mean haemoglobin concentration decreased in both Intervention and Control group with no statistical difference observed between groups. The DID was 0.13 g/dL; (95% CI [−0.13, 0.40]; p = .324; Table 3). There was a non‐significant 2.9% points (95% CI [−2.46, 8.4]; p = .294) increase in the proportion of children with anaemia and 1.1% point (95% CI [−4.9, 2.6]; p = .549) decrease in the proportion of children with severe anaemia in the Intervention compared with the Control group (Table 3).
3.3. Mothers' knowledge and feeding practises
The intervention resulted in a significant 6.5% percentage points (95% CI [0.2, 12.7]; p = .043) increase in the proportion of children with minimum dietary diversity and a 5.8% percentage points (95% CI [0.2, 11.9]; p = .037) increase in the proportion of breastfed children with minimum acceptable diet in the Intervention compared with the Control group. However, the proportion of children with timely introduction of solid and semi‐solid foods decreased in the Intervention group compared with the Control by 17.9% points (95%CI [−34.0, −1.5]; p = .032). There was a significant increase in the proportion of mothers who were able to describe at least three optimal IYCF practises in the Intervention compared with the Control group (Table 5).
Table 5.
Effects of the intervention on IYCF and nutritional indicatorsa
| Outcome | Baseline | Endline | Endline‐baseline | Intervention‐control | ||
|---|---|---|---|---|---|---|
| Difference | Difference‐in‐differencesb | |||||
| n (%) | n (%) | % (95% CI) | p | % (95% CI) | p | |
| Children 6–23 months breastfed | ||||||
| Control | 1,048 (92.6) | 1,108 (95.9) | 3.3 (1.3, 5.2) | .001 | −2.2 (−5.6, 1.2) | .205 |
| Intervention | 1,034 (93.9) | 1,178 (95.1) | 1.1 (−1.8, 3.1) | .461 | ||
| Children breastfed at 1 year | ||||||
| Control | 249 (98.4) | 218 (99.1) | 0.6 (−1.0, 2.1) | .460 | −1.8 (−4.2, 0.5) | .122 |
| Intervention | 261 (99.6) | 192 (98.5) | −1.2 (−3.0, 0.5) | .167 | ||
| Timely introduction of solid, semi‐solid, or soft foods | ||||||
| Control | 31 (31.3) | 24 (18.5) | 12.9 (1.8, 23.9) | .023 | −17.9 (−34.2, −1.5) | .032 |
| Intervention | 28 (29.5) | 60 (34.5) | −5.0 (−17.0, 7.0) | .409 | ||
| Minimum meal frequency, 6–8 months breastfed | ||||||
| Control | 91 (93.8) | 124 (96.1) | 3.2 (−4.0, 10.5) | .377 | −4.2 (−13.5, 5.1) | .371 |
| Intervention | 91 (95.8) | 164 (94.8) | −1.0 (−6.8, 4.8) | .742 | ||
| Minimum meal frequency, 9–23 months breastfed | ||||||
| Control | 653 (68.7) | 823 (84.1) | 15.2 (9.9, 20.6) | <.001 | −5.6 (−13.1, 1.9) | .143 |
| Intervention | 717 (76.4) | 868 (86.4) | 9.7 (4.3, 15.0) | .001 | ||
| Minimum meal frequency, 6–23 months nonbreastfed | ||||||
| Control | 60 (71.4) | 36 (76.6) | 6.5 (−9.4, 22.5) | .416 | 5.8 (−13.3, 24.8) | .546 |
| Intervention | 54 (80.6) | 56 (91.8) | 12.3 (−2.5, 22.0) | .015 | ||
| Minimum dietary diversity, 6–23 months (diversity score ≥ 4) | ||||||
| Control | 133 (11.8) | 63 (5.5) | −6.4 (11.1, −1.6) | .009 | 6.5 (0.2, 12.7) | .043 |
| Intervention | 90 (8.2) | 104 (8.4) | 0.1 (−3.9, 4.1) | .961 | ||
| Minimum acceptable diet 6–23 months breastfed | ||||||
| Control | 114 (10.9) | 58 (5.2) | −5.7 (−10.0, −1.4) | .011 | 5.8 (0.2, 11.9) | .037 |
| Intervention | 79 (7.6) | 93 (7.9) | 0.1 (−3.9, 4.2) | .943 | ||
| Minimum acceptable diet 6–23 months nonbreastfed | ||||||
| Control | 16 (19.1) | 3 (6.4) | −10.2 (−27.0, 6.7) | .233 | 7.2 (−13.8, 28.1) | 0497 |
| Intervention | 10 (14.9) | 6 (9.8) | −3.0 (−16.2, 10.2) | .647 | ||
| Caregivers who can describe at least three optimal IYCF practises | ||||||
| Control | 383 (33.8) | 515 (44.6) | 10.7 (−2.7, 24.1) | .114 | 9.5 (7.4, 26.4) | 0.264 |
| Intervention | 380 (34.5) | 679 (54.8) | 20.2 (9.9, 30.2) | <.001 | ||
| CHWs who can describe at least three optimal IYCF practisesc | ||||||
| Control | 25 (80.7) | 28 (90.3) | 9.7 (−7.4, 26.7) | .261 | 6.4 (−17.2, 30.1) | 0.587 |
| Intervention | 25 (80.7) | 30 (96.8) | 16.1 (−0.3, 32.5) | .054 | ||
Abbreviations: CHW, community health workers; IYCF, infant and young child feeding practises.
Estimated using linear regression models, including the outcome as dependent variables and the intervention (Intervention vs Control), the period of survey (preintervention vs postintervention), the interaction Intervention*period, and a variable for the pairwise allocation as predictors.
The coefficient of the interaction term is interpreted as the percentage difference between the Intervention and Control groups.
Thirty‐one CHWs were surveyed in each study group.
3.4. Morbidity
A higher longitudinal prevalence of diarrhoea (risk ratio [RR] 95% CI = 1.68 [0.94, 3.08]) and cough (RR = 2.21 [0.96, 5.13]) was observed amongst children in the Intervention compared with the Control group. Similarly, the occurrence of morbidity from any illnesses was higher in children in the Intervention compared with the Control group, although the difference was not statistically significant [RR = 1.46 [0.83, 2.58]). We did not find any significant difference for other morbidity risks, including fever, tachypnea, and malaria, between Intervention and Control groups (Table 6 ).
Table 6.
Effects of the intervention on morbidity outcomes in the nested cohort study
| Outcome | Control | Intervention | RR (95% CI) | p | ||
|---|---|---|---|---|---|---|
| n/N | LPa | n/N | LP | |||
| Diarrhoea | 96/6786 | 1.42 | 146/7,481 | 1.95 | 1.68 (0.94, 3.08) | 0.082 |
| Cough | 139/6786 | 2.05 | 187/7,481 | 2.50 | 2.21 (0.96, 5.13) | 0.063 |
| Fever | 42/927 | 4.50 | 58/1,024 | 5.65 | 1.20 (0.82, 1.77) | 0.341 |
| Tachypnea | 60/927 | 6.42 | 38/1,024 | 3.74 | 0.13 (0.00, 3.78) | 0.233 |
| Malariab | 167/286 | 58.4 | 98/210 | 46.7 | 0.68 (0.37, 1.26) | 0.224 |
| Any illness during the day of visit | 100/927 | 10.80 | 125/1,024 | 12.16 | 1.46 (0.83, 2.58) | 0.191 |
| Health problem referred to health centre | 86/927 | 9.24 | 102/1,024 | 9.96 | 1.31 (0.82, 2.12) | 0.259 |
Abbreviation: LP, longitudinal prevalence.
Estimated by the total number of days with the outcome over the total number of days at risk, weighted for the number of follow‐up days of each child.
Defined as the presence of fever or a history of fever and a positive rapid diagnostic test.
4. DISCUSSION
We evaluated a nutritional promotion intervention package combining home fortification of complementary foods with MNP and individual nutritional counselling in a rural setting of Burkina Faso. The results suggest that the intervention had no effect on stunting but led to improved dietary diversity. The results also suggest a non‐significant increase in risk of reported cough and diarrhoea in children receiving the intervention. No effects were observed on confirmed fever and malaria.
4.1. Anthropometry
We found no difference in stunting and wasting prevalence or mean LAZ and WLZ between children at baseline and the follow‐up surveys. Several studies with randomized controlled design, which combined home fortification of foods with MNP and nutritional counselling sessions, found similar results (Inayati et al., 2012; Osei et al., 2015; Suchdev et al., 2012; Yousafzai et al., 2016). The results of studies using only MNP as intervention are mixed. A positive effect on linear growth was found in some trials (Adu‐Afarwuah et al., 2007; Shafique et al., 2016; Soofi et al., 2013), whereas no difference between intervention and control children was observed in other studies (Bilukha, Howard, Wilkinson, Bamrah, & Husain, 2011; Giovannini et al., 2006; Lemaire et al., 2011; Macharia‐Mutie et al., 2012; Osei et al., 2010). Moreover, two systematics reviews conducted in 2013 concluded no significant effects on growth assessed by mean z scores (De‐Regil et al., 2013; Salam et al., 2013) and prevalence of stunting and wasting (Salam et al., 2013). However, complementary feeding education alone has been shown to improve child linear growth and reduce rates of stunting in both food secure and insecure populations (Lassi, Das, Zahid, Imdad, & Bhutta, 2013). There are several possible explanations for our overall null results. First, coverage and duration issues of the programme may have hampered the effect of the intervention on linear growth. Monitoring data from TdH showed that about one quarter of the villages in the Intervention only started implementation after 3 months of the programme initiation. In addition, mothers only attended on average 6.9 GMP sessions of the 12 scheduled. The effect on growth would likely be more pronounced with higher coverage and/or longer programme duration. Second, the lack of effect may suggest that the potent effect of the multifactorial and complex determinants of early child growth faltering negated the effect of improved dietary diversity. Therefore, improved feeding practises were not be translated into linear growth outcomes as suggested by findings of several other studies that provided nutrition education on complementary feeding (Negash et al., 2014; Owais et al., 2016; Schroeder et al., 2002; Zhang et al., 2016). On the other hand, a positive effect on growth has been reported in two randomized controlled trials with prospective follow‐up of young children in Pakistan (Soofi et al., 2013) and Bangladesh (Shafique et al., 2016). The latter trial was similar to ours with regard to the intervention (MNP + IYCF education) but included full‐term low birth weight infants only and used a 22‐micronutrient formulation. The results of our nested cohort study of infants aged 6–11 months also showed a significant, although small effect, on linear growth rate (0.013 LAZ/month) in the Intervention group. These findings indicate that early intervention may lead to a greater impact on linear growth or that the effects of an MNP intervention on child growth are subtle and may not be detected by cross‐sectional study designs. Longitudinal follow‐up studies may therefore be more appropriate for impact evaluation of integrated IYCN/MNP programmes, especially because subtle effects on average linear growth may result in shifts in the population distribution leading to meaningful changes in stunting prevalence.
Although the differences between study groups were not significant, the mean LAZ, WAZ, and the prevalence of stunting increased more in the Intervention than the Control group at endline. In parallel, breastfeeding practises, timely introduction of solid, semi‐solid or soft foods, and minimum meal frequency declined. The reasons for these trends are unknown, but effects of other interventions in the study areas are unlikely as all health interventions were suspended by the Ministry of Health in the Health District area during the implementation of the TdH pilot programme. Nonetheless, the same trend of increased prevalence of stunting, wasting, and underweight was reported in nationally representative cross‐sectional nutritional surveys between 2014 (Ministère de la Santé, 2015) and 2015 (Ministère de la Santé, 2016) in Burkina Faso. MNP programmes require context‐specific and strategic BCI in order to influence child‐feeding practises in the target population (Siekmans, Bégin, Situma, & Kupka, 2017). It can be hypothesized that, in a challenging food and nutrition context and in the absence of a specific BCI strategy, mothers in the intervention group prematurely introduced semi‐solid foods into the diet of their children, which negatively impacted on certain IYCF practises and nutritional outcomes.
4.2. Haemoglobin
This study was not powered to detect an effect on anaemia status. However, our results contrast with the reported increase in haemoglobin levels and reduced anaemia prevalence in a review of MNP supplementation randomized controlled trials (Salam et al., 2013). Although there was no significant difference in the mean haemoglobin change between the study groups, the haemoglobin concentrations decreased significantly between baseline and endline in both study groups. The large decline in haemoglobin concentrations in both groups may have masked any possible effects of the intervention. In addition, anaemia is multifactorial, and haemoglobin levels may not reflect changes in iron status in areas where infectious diseases and malaria are endemic (Crawley, 2004; Sumbele, Samje, & Nkuo‐Akenji, 2013).
4.3. Feeding practises
The evaluation of the IYCF practises showed an improved dietary diversity amongst children in the Intervention group. The observed improvement in child feeding practises is comparable with that of a similar study in Nepal that evaluated an integrated IYCF/MNP pilot programme (Mirkovic et al., 2016). This study also combined consumption of MNP by children aged 6–23 months and a behavioural change component supporting the adoption of improved IYCF practises by mothers. Improvements in feeding practises have also been reported in studies evaluating the impact of nutrition education alone (Shi & Zhang, 2011). In addition, MNP interventions are reported to increase the delivery of IYCF counselling and messages (De Pee, Irizarry, Kraemer, & Jefferds, 2013). Moreover, this was also suggested by the findings of a qualitative study (unpublished data) of this evaluation, where MNPs were seen as an incentive for mothers to attend the GMP sessions.
4.4. Morbidity
The longitudinal prevalence of malaria was lower, albeit not significant, in the Intervention compared with the Control group. The results on malaria and fever were objectively measured by rapid diagnostic test and temperature measurements, respectively. Recall bias due to underreporting and overreporting of morbidity events, has been shown in morbidity studies using recalled symptoms (Feikin et al., 2010). Our results are in line with the findings of reviews of oral iron supplementation trials that did not show an increased risk of malaria parasitemia (Pasricha, Hayes, Kalumba, & Biggs, 2013) and clinical malaria (Neuberger, Okebe, Yahav, & Paul, 2016) when regular malaria prevention or management services are provided. The associated risks of malaria infection found in other trials were shown for subgroups of children older than 5 years old (Richard et al., 2006), those with no anaemia (Sazawal et al., 2006), and children with iron deficiency (Veenemans et al., 2011). We could not investigate the effects on such subgroups as our trial only included children aged 6–24 months and did not assess other indicators of iron status (serum ferritin or transferrin receptors). Further investigations are needed to clarify the role of age and iron status in the physiological processes of iron supplements in the presence of malaria infection.
No significant differences in reported diarrhoea and cough were found between the study groups. This result contrasts with the findings of a large trial in Pakistan, which found an increased incidence of diarrhoea and chest indrawing morbidity in MNP‐supplemented children (Soofi et al., 2013) but supports the results of other trials that evaluated the impact of MNP on morbidity outcomes (Lemaire et al., 2011; Osei et al., 2015; Sharieff, Bhutta, Schauer, Tomlinson, & Zlotkin, 2006; Suchdev et al., 2016). Most of the latter studies even reported lower risk of diarrhoea (Lemaire et al., 2011; Osei et al., 2015; Suchdev et al., 2016). In our study, the increase in diarrhoea prevalence may be an indication of side‐effects of the supplements, which seemed to be mitigated due to the intensive counselling on the use of MNP and appropriate complementary feeding. The causative mechanisms for increased morbidity during iron supplementation are thought to be due, in part, to adverse effects on the gut microbiome (Jaeggi et al., 2015). Some evidence also suggests that iron present in human blood, after supplementation, enhances the rate of replication of a number of pathogenic enterobacteria (Cross et al., 2015). A higher preference of Plasmodium falciparum for young red blood cells following the increased erythropoiesis in response to iron supplementation has been suggested as possible mechanism for observed increases in malaria (Clark et al., 2014; Goheen et al., 2016).
Our study has certain limitations. First, whereas the assignment of the villages to the two study groups was done in keeping with a cluster randomized control evaluation, the analytical strategy was designed as a DID between preintervention and postintervention on independent cross‐sectional samples of the two groups. Thus, the analyses compared children present in the communities at the time of baseline and endline surveys, whether their mothers participated in the community‐based GMP sessions or not, rather than comparing longitudinal changes in the same individuals in each study group. However, the evaluation design and the DID analysis approach minimize the risk of selection bias. The inclusion of a clustering effect at the village‐level can address possible risk factors related to villages that may contribute to the explanation of differences in outcomes. Second, the coverage and duration of the programme in some villages were suboptimal, and this might have led to an underestimation of the effect of the intervention. Not all villages initiated the implementation of the intervention at the same time, and not all infants in each village had the same duration of exposure to the intervention. On the other hand, establishing the results of MNP supplementation in the context of a programme setting, with all its limitations, provides us with a more realistic indication of the real‐life risks and benefits of such interventions in a field setting.
In conclusion, the findings of this study suggest that in the context of a programme intervention, a multiple micronutrients powder associated with nutritional education, marginally improved growth and feeding practises without significant increase in morbidity from confirmed malaria and fever in a high malaria transmission setting. The results from the nested longitudinal cohort study suggest that different study designs may be able to detect more subtle effects of such programme interventions on linear growth. More studies using these designs are required to confirm these findings.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
The authors' responsibilities were as follows: PK, HBL, and SO designed the research; HBL implemented the study, analysed and interpreted the data, and drafted the manuscript; AA helped with the data analysis; KDP, SK, and CO made substantial contributions to the execution and supervision of the study; all authors contributed substantially to the manuscript and approved the final version.
ACKNOWLEDGMENTS
The authors are grateful to the “Micronutrient Initiative” for funding the study. They would like to thank the families of the Tougan Health District who participated in the study, Faroukou Garba from the NGO “Terre des Hommes” for his valued contribution in the implementation of the intervention.
Lanou HB, Osendarp SJM, Argaw A, et al. Micronutrient powder supplements combined with nutrition education marginally improve growth amongst children aged 6–23 months in rural Burkina Faso: A cluster randomized controlled trial. Matern Child Nutr. 2019; 15 e12820 10.1111/mcn.12820
Address requests for reprints to
Patrick Kolsteren, Department of Food Technology, Safety and Health. Ghent University, Ghent, Belgium, Coupure Links 653, 9000 Gent. Tel: 0032 9 2649377.
E‐mail: patrick.kolsteren@ugent.be
REFERENCES
- Adu‐Afarwuah, S. , Lartey, A. , Brown, K. H. , Zlotkin, S. , Briend, A. , & Dewey, K. G. (2007). Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: Effects on growth and motor development. American Journal of Clinical Nutrition, 86, 412–420. 10.1093/ajcn/86.2.412 [DOI] [PubMed] [Google Scholar]
- Bhutta, Z. A. , Das, J. K. , Rizvi, A. , Gaffey, M. F. , Walker, N. , Horton, S. , … Black, R. E. (2013). Evidence‐based interventions for improvement of maternal and child nutrition: What can be done and at what cost? The Lancet, 382, 452–477. 10.1016/S0140-6736(13)60996-4 [DOI] [PubMed] [Google Scholar]
- Bilukha, O. , Howard, C. , Wilkinson, C. , Bamrah, S. , & Husain, F. (2011). Effects of multimicronutrient home fortification on anemia and growth in Bhutanese refugee children. Food and Nutrition Bulletin, 32, 264–276. 10.1177/156482651103200312 [DOI] [PubMed] [Google Scholar]
- Black, R. E. , Victora, C. G. , Walker, S. P. , Bhutta, Z. A. , Christian, P. , De Onis, M. , … Uauy, R. (2013). Maternal and child undernutrition and overweight in low‐income and middle‐income countries. The Lancet, 382, 427–451. 10.1016/S0140-6736(13)60937-X [DOI] [PubMed] [Google Scholar]
- Cardoso, M. A. , Augusto, R. A. , Bortolini, G. A. , Oliveira, C. S. M. , Tietzman, D. C. , Sequeira, L. A. S. , … ENFAC Working Group (2016). Effect of providing multiple micronutrients in powder through primary healthcare on anemia in young Brazilian children: A multicentre pragmatic controlled trial. PLoS ONE, 63, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, M. A. , Goheen, M. M. , Fulford, A. , Prentice, A. M. , Elnagheeb, M. A. , Patel, J. , … Cerami, C. (2014). Host iron status and iron supplementation mediate susceptibility to erythrocytic stage Plasmodium falciparum . Nature Communications, 5, 4446, 1–25. 10.1038/ncomms5446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley, J. (2004). Reducing the burden of anemia in infants and young children in malaria‐endemic countries of Africa: From evidence to action. The American Journal of Tropical Medicine and Hygiene, 71, 25–34. 10.4269/ajtmh.2004.71.25 [DOI] [PubMed] [Google Scholar]
- Cross, J. H. , Bradbury, R. S. , Fulford, A. J. , Jallow, A. T. , Wegmüller, R. , Prentice, A. M. , & Cerami, C. (2015). Oral iron acutely elevates bacterial growth in human serum. Scientific Reports, 5, 16670 10.1038/srep16670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings, P. (2009). Methods for estimating adjusted risk ratios. The Stata Journal, 9, 175–196. 10.1177/1536867X0900900201 [DOI] [Google Scholar]
- De Pee, S. , Irizarry, L. , Kraemer, K. , & Jefferds, M. (2013). Micronutrient powder interventions: the basis for current programming guidance and needs for additional knowledge and experience, In de Pee S, Flores‐Ayala R, Van Hees J, et al (eds), Home Fortification with Micronutrient Powders (MNP). Basel, Sight and Life, pp. 51–56. [Google Scholar]
- De‐Regil, L. M. , Suchdev, P. S. , Vist, G. E. , Walleser, S. , & Peña‐Rosas, J. P. (2013). Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age. Evidence‐Based Child Health: A Cochrane Review Journal, 8, 112–201. 10.1002/ebch.1895 [DOI] [PubMed] [Google Scholar]
- Dewey, K. G. , & Adu‐Afarwuah, S. (2008). Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Maternal & Child Nutrition, 4, 24–85. 10.1111/j.1740-8709.2007.00124.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey, K. G. , & Arimond, M. (2012). Lipid‐based nutrient supplements: How can they combat child malnutrition? PLoS Medicine, 9, e1001314 10.1371/journal.pmed.1001314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Direction de La Nutrition . (2014). Enquête SMART, Burkina Faso.
- Feikin, D. R. , Audi, A. , Olack, B. , Bigogo, G. M. , Polyak, C. , Burke, H. , … Breiman, R. F. (2010). Evaluation of the optimal recall period for disease symptoms in home‐based morbidity surveillance in rural and urban Kenya. International Journal of Epidemiology, 39, 450–458. 10.1093/ije/dyp374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannini, M. , Sala, D. , Usuelli, M. , Livio, L. , Francescato, G. , Braga, M. , … Riva, E. (2006). Double‐blind, placebo‐controlled trial comparing effects of supplementation with two different combinations of micronutrients delivered as sprinkles on growth, anemia, and iron deficiency in cambodian infants. Journal of Pediatric Gastroenterology and Nutrition, 42, 306–312. 10.1097/01.mpg.0000189363.07040.4b [DOI] [PubMed] [Google Scholar]
- Goheen, M. M. , Wegmüller, R. , Bah, A. , Darboe, B. , Danso, E. , Affara, M. , … Cerami, C. (2016). Anemia offers stronger protection than sickle cell trait against the erythrocytic stage of falciparum malaria and this protection is reversed by iron supplementation. eBioMedicine, 14, 123–130. 10.1016/j.ebiom.2016.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inayati, D. A. , Scherbaum, V. , Purwestri, R. C. , Wirawan, N. N. , Suryantan, J. , Hartono, S. , … Bellows, A. C. (2012). Combined intensive nutrition education and micronutrient powder supplementation improved nutritional status of mildly wasted children on Nias Island, Indonesia. Asia Pacific Journal of Clinical Nutrition, 21, 361–373. [PubMed] [Google Scholar]
- Jaeggi, T. , Kortman, G. A. , Moretti, D. , Chassard, C. , Holding, P. , Dostal, A. , … Zimmermann, M. B. (2015). Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut, 64, 731–742. 10.1136/gutjnl-2014-307720 [DOI] [PubMed] [Google Scholar]
- Jefferds, M. E. , Irizarry, L. , Timmer, A. , & Tripp, K. (2013). Unicef‐cdc global assessment of home fortification interventions 2011: Current status, new directions, and implications for policy and programmatic guidance. Food and Nutrition Bulletin, 34, 434–443. 10.1177/156482651303400409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konaté, A. T. , Yaro, J. B. , Ouédraogo, A. Z. , Diarra, A. , Gansané, A. , Soulama, I. , … Diallo, D. A. (2011). Intermittent preventive treatment of malaria provides substantial protection against malaria in children already protected by an insecticide‐treated bednet in Burkina Faso: A randomised, double‐blind, placebo‐controlled trial. PLoS Medicine, 8 10.1371/journal.pmed.1000408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassi, Z. S. , Das, J. K. , Zahid, G. , Imdad, A. , & Bhutta, Z. A. (2013). Impact of education and provision of complementary feeding on growth and morbidity in children less than 2 years of age in developing countries: A systematic review. BMC Public Health, 13, S13 10.1186/1471-2458-13-S3-S13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire, M. , Islam, Q. S. , Shen, H. , Khan, M. A. , Parveen, M. , Abedin, F. , … Zlotkin, S. H. (2011). Iron‐containing micronutrient powder provided to children with moderate‐to‐severe malnutrition increases hemoglobin concentrations but not the risk of infectious morbidity: A randomized, double‐blind,,placebo‐controlled, noninferiority safety trial. American Journal of Clinical Nutrition, 94, 585–593. 10.3945/ajcn.110.009316 [DOI] [PubMed] [Google Scholar]
- Leroy, J. L. (2011). ZSCORE06: Stata module to calculate anthropometric z‐scores using the 2006 WHO child growth standards. Statistical Software Components. Boston College Department of economics.
- Locks, L. M. , Reerink, I. , Tucker Brown, A. , Gnegne, S. , Ramalanjaona, N. , Nanama, S. , … Garg, A. (2017). The impact of integrated infant and young child feeding and micronutrient powder intervention on feeding practices and anemia in children aged 6‐23 months in Madagascar. Nutrients, 9, 581, 1–17. 10.3390/nu9060581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macharia‐Mutie, C. W. , Moretti, D. , Van den Briel, N. , Omusundi, a. M. , Mwangi, a. M. , Kok, F. J. , … Brouwer, I. D. (2012). Maize porridge enriched with a micronutrient powder containing low‐dose iron as NaFeEDTA but not amaranth grain flour reduces anemia and iron deficiency in Kenyan preschool children. Journal of Nutrition, 142, 1756–1763. 10.3945/jn.112.157578 [DOI] [PubMed] [Google Scholar]
- Menon, P. , Rawat, R. , & Ruel, M. (2013). Bringing rigor to evaluations of large‐scale programs to improve infant and young child feeding and nutrition: The evaluation designs for the Alive & Thrive initiative. Food and Nutrition Bulletin, 34, S195–S211. 10.1177/15648265130343S206 [DOI] [PubMed] [Google Scholar]
- Ministère de la Santé . (2015). Enquete Nutritionnelle Nationale 2014.
- Ministère de la Santé . (2016). Enquete Nutritionnelle Nationale 2015.
- Mirkovic, K. R. , Perrine, C. G. , Subedi, G. R. , Mebrahtu, S. , Dahal, P. , & Jefferds, M. E. D. (2016). Micronutrient powder use and infant and young child feeding practices in an integrated program. Asia Pacific Journal of Clinical Nutrition, 25, 350–355. 10.6133/apjcn.2016.25.2.19 [DOI] [PubMed] [Google Scholar]
- Morris, S. S. , Cousens, S. N. , Kirkwood, B. R. , Arthur, P. , & Ross, D. A. (1996). Is prevalence of diarrhea a better predictor of subsequent mortality and weight gain than diarrhea incidence? American Journal of Epidemiology, 144, 582–588. 10.1093/oxfordjournals.aje.a008968 [DOI] [PubMed] [Google Scholar]
- Negash, C. , Belachew, T. , Henry, C. J. , Kebebu, A. , Abegaz, K. , & Whiting, S. J. (2014). Nutrition education and introduction of broad bean‐based complementary food improves knowledge and dietary practices of caregivers and nutritional status of their young children in Hula, Ethiopia. Food and Nutrition Bulletin, 35, 480–486. 10.1177/156482651403500409 [DOI] [PubMed] [Google Scholar]
- Neuberger, A. , Okebe, J. , Yahav, D. , & Paul, M. (2016). Oral iron supplements for children in malaria-endemic areas. The Cochrane database of systematic reviews, 2(2), CD006589 10.1002/14651858.CD006589.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojukwu, J. U. , Okebe, J. U. , Yahav, D. , & Paul, M. (2009). Oral iron supplementation for preventing or treating anaemia among children in malaria‐endemic areas In Ojukwu J. U. (Ed.), Cochrane database of systematic reviews (p. CD006589). Chichester, UK, UK: John Wiley & Sons, Ltd. [DOI] [PubMed] [Google Scholar]
- Okebe, J. U. , Yahav, D. , Shbita, R. , & Paul, M. (2011). Oral iron supplements for children in malaria‐endemic areas. The Cochrane Library‐Okebe ‐ Wiley Online Library; 10.1002/14651858.CD006589.pub4.www.cochranelibrary.com [DOI] [PubMed] [Google Scholar]
- Osei, A. K. , Pandey, P. , Spiro, D. , Adhikari, D. , Haselow, N. , De Morais, C. , & Davis, D. (2015). Adding multiple micronutrient powders to a homestead food production programme yields marginally significant benefit on anaemia reduction among young children in Nepal. Maternal & Child Nutrition, 11, 188–202. 10.1111/mcn.12173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osei, A. K. , Rosenberg, I. H. , Houser, R. F. , Bulusu, S. , Mathews, M. , & Hamer, D. H. (2010). Community‐level micronutrient fortification of school lunch meals improved vitamin A, folate, and iron status of schoolchildren in Himalayan villages of India. The Journal of Nutrition, 140, 1146–1154. 10.3945/jn.109.114751 [DOI] [PubMed] [Google Scholar]
- Owais, A. , Schwartz, B. , Kleinbaum, D. G. , Suchdev, P. S. , Faruque, A. S. G. , Das, S. K. , & Stein, A. D. (2016). Minimum acceptable diet at 9 months but not exclusive breastfeeding at 3 months or timely complementary feeding initiation is predictive of infant growth in rural Bangladesh. PLoS ONE, 11, e0165128 10.1371/journal.pone.0165128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasricha, S. R. , Hayes, E. , Kalumba, K. , & Biggs, B. A. (2013). Effect of daily iron supplementation on health in children aged 4‐23 months: A systematic review and meta‐analysis of randomised controlled trials. The Lancet Global Health, 1, e77–e86. 10.1016/S2214-109X(13)70046-9 [DOI] [PubMed] [Google Scholar]
- Penny, M. E. , Creed‐Kanashiro, H. M. , Robert, R. C. , Narro, M. R. , Caulfield, L. E. , & Black, R. E. (2005). Effectiveness of an educational intervention delivered through the health services to improve nutrition in young children: A cluster‐randomised controlled trial. The Lancet, 365, 1863–1872. 10.1016/S0140-6736(05)66426-4 [DOI] [PubMed] [Google Scholar]
- Richard, S. A. , Zavaleta, N. , Caulfield, L. E. , Black, R. E. , Witzig, R. S. , & Shankar, A. H. (2006). Zinc and iron supplementation and malaria, diarrhea, and respiratory infections in children in the Peruvian Amazon. The American Journal of Tropical Medicine and Hygiene, 75, 126–132. 10.4269/ajtmh.2006.75.1.0750126 [DOI] [PubMed] [Google Scholar]
- Salam, R. A. , MacPhail, C. , Das, J. K. , & Bhutta, Z. A. (2013). Effectiveness of micronutrient powders (MNP) in women and children. BMC Public Health, 13(Suppl 3), S22: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehi, M. , Kimiagar, S. M. , Shahbazi, M. , Mehrabi, Y. , & Kolahi, A. A. (2004). Assessing the impact of nutrition education on growth indices of Iranian nomadic children: An application of a modified beliefs, attitudes, subjective‐norms and enabling‐factors model. The British Journal of Nutrition, 91, 779–787. 10.1079/BJN20041099 [DOI] [PubMed] [Google Scholar]
- Sazawal, S. , Black, R. E. , Ramsan, M. , Chwaya, H. M. , Stoltzfus, R. J. , Dutta, A. , … Kabole, F. M. (2006). Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: Community‐based, randomised, placebo‐controlled trial. Lancet, 367, 133–143. 10.1016/S0140-6736(06)67962-2 [DOI] [PubMed] [Google Scholar]
- Schroeder, D. G. , Pachón, H. , Dearden, K. A. , Ha, T. T. , Lang, T. T. , & Marsh, D. R. (2002). An integrated child nutrition intervention improved growth of younger, more malnourished children in Northern Viet Nam. Food and Nutrition Bulletin, 23, 50–58. 10.1177/15648265020234S108 [DOI] [PubMed] [Google Scholar]
- Shafique, S. , Sellen, D. W. , Lou, W. , Jalal, C. S. , Jolly, S. P. , & Zlotkin, S. H. (2016). Mineral‐ and vitamin‐enhanced micronutrient powder reduces stunting in full‐term low‐birth‐weight infants receiving nutrition, health, and hygiene education: A 2 x 2 factorial, cluster‐randomized trial in Bangladesh. American Journal of Clinical Nutrition, 103, 1357–1369. 10.3945/ajcn.115.117770 [DOI] [PubMed] [Google Scholar]
- Sharieff, W. , Bhutta, Z. , Schauer, C. , Tomlinson, G. , & Zlotkin, S. (2006). Micronutrients (including zinc) reduce diarrhoea in children: The Pakistan Sprinkles Diarrhoea Study. Archives of Disease in Childhood, 91, 573–579. 10.1136/adc.2005.086199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, L. , & Zhang, J. (2011). Recent evidence of the effectiveness of educational interventions for improving complementary feeding practices in developing countries. Journal of Tropical Pediatrics, 57, 91–98. 10.1093/tropej/fmq053 [DOI] [PubMed] [Google Scholar]
- Shi, L. , Zhang, J. , Wang, Y. , Caulfield, L. E. , & Guyer, B. (2010). Effectiveness of an educational intervention on complementary feeding practices and growth in rural China: A cluster randomised controlled trial. Public Health Nutrition, 13, 556–565. 10.1017/S1368980009991364 [DOI] [PubMed] [Google Scholar]
- Siekmans, K. , Bégin, F. , Situma, R. , & Kupka, R. (2017). The potential role of micronutrient powders to improve complementary feeding practices. Maternal & Child Nutrition, 13, e12464 10.1111/mcn.12464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soofi, S. , Cousens, S. , Iqbal, S. P. , Akhund, T. , Khan, J. , Ahmed, I. , … Bhutta, Z. A. (2013). Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: A cluster‐randomised trial. The Lancet, 382, 29–40. 10.1016/S0140-6736(13)60437-7 [DOI] [PubMed] [Google Scholar]
- Suchdev, P. S. , Addo, O. Y. , Martorell, R. , Grant, F. K. E. , Ruth, L. J. , Patel, M. K. , … Flores‐Ayala, R. (2016). Effects of community‐based sales of micronutrient powders on morbidity episodes in preschool children in Western Kenya. American Journal of Clinical Nutrition, 103, 934–941. 10.3945/ajcn.115.118000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suchdev, P. S. , Ruth, L. J. , Woodruff, B. A. , Mbakaya, C. , Mandava, U. , Flores‐Ayala, R. , … Quick, R. (2012). Selling sprinkles micronutrient powder reduces anemia, iron deficiency, and vitamin A deficiency in young children in Western Kenya: A cluster‐randomized controlled trial. American Journal of Clinical Nutrition, 95, 1223–1230. 10.3945/ajcn.111.030072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumbele, I. U. N. , Samje, M. , & Nkuo‐Akenji, T. (2013). A longitudinal study on anaemia in children with Plasmodium falciparum infection in the Mount Cameroon region: prevalence, risk factors and perceptions by caregivers. BMC Infectious Diseases, 13, 123 10.1186/1471-2334-13-123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF . (2011). Programming guide infant and young child feeding. Nutrition Section, UNICEF, 173.
- Veenemans, J. , Milligan, P. , Prentice, A. M. , Schouten, L. R. A. , Inja, N. , van der Heijden, A. C. , … Verhoef, H. (2011). Effect of supplementation with zinc and other micronutrients on malaria in Tanzanian children: A randomised trial. PLoS Medicine, 8, e1001125 10.1371/journal.pmed.1001125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2010). Indicators for assessing infant and young child feeding practices. Part 3‐Country Profiles, 1–52.
- WHO (2011). Guideline: Use of multiple micronutrient powders for home fortification of foods consumed by infants and children 6–23 months of age. Who. Geneva: World Health Organization. [PubMed] [Google Scholar]
- Wooldridge, J. M. (2002). Econometric analysis of cross section and panel data. The MIT Press (Vol. 58). Cambridge: Massachussetts, USA; 10.1515/humr.2003.021 [DOI] [Google Scholar]
- World Health Organization . (2012). Combined course on growth assessment and IYCF counselling, 1–524.
- Yousafzai, A. K. , Obradović, J. , Rasheed, M. A. , Rizvi, A. , Portilla, X. A. , Tirado‐Strayer, N. , … Memon, U. (2016). Effects of responsive stimulation and nutrition interventions on children's development and growth at age 4 years in a disadvantaged population in Pakistan: A longitudinal follow‐up of a cluster‐randomised factorial effectiveness trial. The Lancet Global Health, 4, e548–e558. 10.1016/S2214-109X(16)30100-0 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Wu, Q. , Wang, W. , van Velthoven, M. H. , Chang, S. , Han, H. , … Scherpbier, R. W. (2016). Effectiveness of complementary food supplements and dietary counselling on anaemia and stunting in children aged 6‐23 months in poor areas of Qinghai Province, China: A controlled interventional study. BMJ Open, 6, e011234 10.1136/bmjopen-2016-011234 [DOI] [PMC free article] [PubMed] [Google Scholar]


