Abstract
Observed associations between breastfeeding and reduced risk of type 2 diabetes in adulthood may be confounded. We examined if the duration of breastfeeding in infancy was associated with the risk of type 2 diabetes in adulthood after adjustment for a range of prenatal and postnatal risk factors. We prospectively followed 6,044 individuals from the Copenhagen Perinatal Cohort born 1959–1961. Duration of any breastfeeding (≤0.5, >0.5–1, >1–2, >2–4, >4 months) was assessed at the infant's 1‐year health examination. We estimated hazard ratios (HRs) with 95% confidence intervals (CIs) for type 2 diabetes (at age ≥30 years, 237 persons) by breastfeeding duration without and with adjustment for parental social status and education, maternal pre‐pregnancy body mass index (BMI), maternal diabetes and smoking during pregnancy, gestational weight gain, parity, preterm birth, birth weight, sex, and BMI at ages 7 and 41–43 years. In the unadjusted analysis, compared with infants breastfed for ≤0.5 month, those breastfed for >4 months had a 51% reduced risk of type 2 diabetes (HR = 0.49; 95% CI [0.32, 0.75]). After the stepwise adjustment for putative early life confounders, this was attenuated to a nonsignificant 31% reduced risk (HR = 0.69; 95% CI [0.44, 1.07]). Adjustment for childhood and adulthood BMI minimally changed the results. We found that the inverse association between the duration of breastfeeding and risk of type 2 diabetes in adulthood is considerably weakened and no longer significant after adjustment for prenatal and postnatal factors in the infant and mother.
Keywords: breastfeeding, breastfeeding and diabetes, breastfeeding duration, cohort study, confounding variables, epidemiology, type 2 diabetes
Key messages.
The duration of any breastfeeding was inversely associated with the risk of type 2 diabetes in analyses not accounting for other risk factors.
Adjustment for prenatal and postnatal type 2 diabetes risk factors weakened the association, and it was no longer significant, thus indicating that it is confounded.
Adjustment for childhood or adulthood BMI, however, only minimally changed the associations.
1. INTRODUCTION
In recent decades, the prevalence of type 2 diabetes mellitus has increased dramatically in Denmark and globally (Carstensen, Kristensen, Ottosen, & Borch‐Johnsen, 2008; World Health Organization, 2014). Diabetes is a well‐known cause of premature death and disability with severe economic and personal implications (Christensen, Doblhammer, Rau, & Vaupel, 2009; Suzman & Beard, 2011), and prevention is more urgent than ever. The increase is likely related to the obesity epidemic that is already evident in early childhood (Cattaneo et al., 2010; Lee et al., 2010).
Potential prevention strategies that begin during infancy have received increasing attention (Blake‐Lamb et al., 2016; Redsell et al., 2016). Infancy is of particular interest because the demands of nutritional intake are at their highest, adipogenesis is still ongoing, and the organs are still in developmentally plastic stages (Gillman, 2008). For instance, breastfeeding has numerous health benefits for the mother and child (Baker, 2015). Breastfeeding may have protective effects against later obesity in the child, and more so the longer the duration of breastfeeding although it remains controversial (Brion et al., 2011; Harder, Bergmann, Kallischnigg, & Plagemann, 2005; Horta, Loret de Mola, & Victora, 2015). Given clear links between body mass index (BMI) in childhood and adulthood and type 2 diabetes (Whitlock et al., 2009; Zimmermann et al., 2017), it is possible that breastfeeding reduces the risk of type 2 diabetes in adulthood through an inverse association with BMI.
Although a number of observational studies have provided evidence that breastfeeding modestly protects the infant against the later development of type 2 diabetes in adulthood (Pettitt, Forman, Hanson, Knowler, & Bennett, 1997; Ravelli, van der Meulen, Osmond, Barker, & Bleker, 2000; Rich‐Edwards et al., 2004), some provide only limited evidence (Fall et al., 1995; Parikh et al., 2009) and others do not support an association (Fall et al., 2011; Martin et al., 2005; Martin et al., 2005). A meta‐analysis based on 11 studies reported that breastfeeding significantly decreased the odds of type 2 diabetes, but when socio‐economic status (SES) and birthweight were adjusted for the association attenuated (Horta et al., 2015). The populations of the studies differ in ethnicity, era, and setting and many studies of adult type 2 diabetes have been limited to comparing breastfed versus bottle‐fed infants (Fall et al., 1995; Martin, Ebrahim, et al., 2005; Parikh et al., 2009; Ravelli et al., 2000) and therefore were unable to evaluate potential effects of duration of breastfeeding. Maternal and infant factors such as parental social status, maternal pre‐pregnancy BMI, maternal diabetes, maternal smoking, preterm birth, and birth weight are all associated with breastfeeding and with type 2 diabetes (Baker, 2015; Mortensen, Michaelsen, Sanders, & Reinisch, 2002; Parikh et al., 2009). Differences in these factors may plausibly explain the associations between breastfeeding and type 2 diabetes, which several studies did not fully address (Martin, Ben‐Shlomo, et al., 2005; Martin, Ebrahim, et al., 2005; Parikh et al., 2009; Pettitt et al., 1997; Rich‐Edwards et al., 2004). The meta‐analysis was unable to examine these associations due to a lack of high‐quality studies when the relevant criteria of including more than 500 participants, having a breastfeeding recall of less than 3 years, and adjusting for SES and birthweight as a minimum were applied (Horta et al., 2015). In fact, only one study fulfilled these criteria, and it was based on data from low‐ and middle‐income countries (Fall et al., 2011).
We examined whether the duration of breastfeeding in infancy is independently associated with the risk of type 2 diabetes in adulthood, when adjusting for putative confounding factors and for child and adult BMI.
2. MATERIALS AND METHODS
2.1. Study population
2.1.1. Copenhagen Perinatal Cohort
The Copenhagen Perinatal Cohort consists of 9,125 individuals who were born at the Copenhagen State University Hospital from September 1959 to December 1961 (Willumsen, 1970; Zachau‐Christiansen & Ross, 1975). The Copenhagen State University Hospital preferentially admitted mothers who previous had complications of pregnancy, those expected to undergo a complicated delivery, and single mothers for whom home delivery was impractical. However, the majority of the admitted women were residents in Copenhagen and were without pregnancy complications. Interviews conducted before delivery when the mother attended the antenatal clinic at the hospital and again during the first few days after birth provided information about social, general medical and obstetrical history. The mothers and their children were invited to a follow‐up examination of the children at 1 year of age carried out at the hospital (Zachau‐Christiansen & Ross, 1975). Beginning in 1968, a personal identification number was assigned to all Danish residents (Pedersen, 2011), including 8,112 children of this cohort, corresponding to 96.6% of those who survived infancy (Figure 1). Using this number, information on vital status was obtained by linkage to the Danish Civil Registration System (Pedersen, 2011). Among the study participants surviving infancy, 98% were still alive and traceable at the age of 41 years, and 54% of those responded to a questionnaire on lifestyle risk factors and health issues (Schack‐Nielsen, Sørensen, Mortensen, & Michaelsen, 2010).
Figure 1.
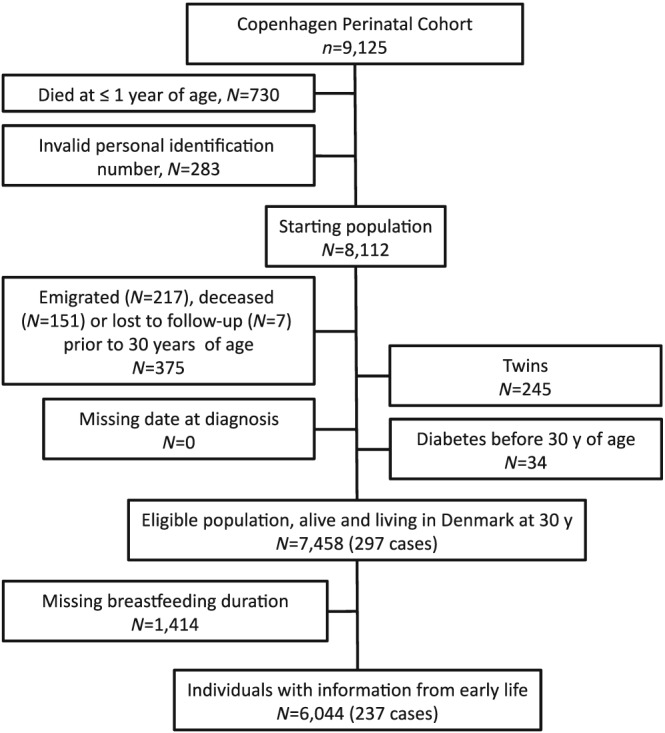
Flow chart of eligible individuals and those included in the study
2.1.2. Copenhagen School Health Records Register
Information on child BMI was obtained by linkage to the Copenhagen School Health Records Register using the personal identification number. This register contains computerized information on 372,636 children who were born in 1930 through 1989 and who ever attended school in the Copenhagen municipality (Baker et al., 2009).
2.1.3. Breastfeeding
The duration of exclusive and any breastfeeding (in days, weeks, and/or months) was assessed by a physician interviewing the mothers at the infant's 1‐year examination (Zachau‐Christiansen & Ross, 1975). Many mothers reported the same durations for exclusive and any breastfeeding. Consequently, we chose to focus on the duration of any breastfeeding in these analyses to attain the longest span of duration of breastfeeding. The duration of any breastfeeding was divided into five categories (≤0.5, >0.5–1, >1–2, >2–4, >4 months).
2.1.4. Covariates
The Copenhagen Perinatal Cohort provides information on a series of putative confounders: parental social status (low, middle, high), the breadwinner's education (4‐level categorical scale), marital status (yes/no), maternal age (years), attitude towards pregnancy (wanted/unwanted), maternal pre‐pregnancy BMI (grouped into <18.5, ≥18.5 to <25, ≥25 to <30, ≥30 kg/m2), maternal diabetes during pregnancy (yes/no), maternal smoking during the third trimester (none, <3, 3–10, 11–20, >20 cigarettes per day), gestational weight gain (<6, 6–8, 9–10, 11–12, 13–15, >16 kg), parity, preterm birth (yes/no, defined as a gestational age <38 weeks due to the original categorization of the variable), birth weight (≤2,750, >2,750–3,250, >3,250–3,750, >3,750–4,250, >4,250–4,750, >4,750 grams), sex, and age at introduction of complementary foods (i.e., spoon feeding; 0–2, 3–5, 6–8, or 9–11 months; Zachau‐Christiansen & Ross, 1975).
The school health records included measurements of height and weight routinely performed by school doctors or nurses at mandatory school health examinations (Baker et al., 2009). From these measures, BMI was calculated and the value closest to 7 years from ages ≥6.5 to <7.5 years was included as a 7‐year measurement. At ages 41–43 years, weight and height measures were self‐reported.
2.1.5. Type 2 diabetes
Information on inpatient and outpatient diagnoses of type 2 diabetes was obtained by linking the personal identification number to the National Patient Register (NPR; Andersen, Madsen, Jørgensen, Mellemkjær, & Olsen, 1999) until December 31, 2015. This register contains complete hospital discharge diagnosis histories for general hospital departments in Denmark since 1977 and for outpatient and emergency departments since 1995 (Andersen et al., 1999). Age at hospital admission defined the age at diagnosis. Type 2 diabetes was defined by the International Classification of Diseases (ICD) 8th revision until 1994 (250) and ICD‐10 thereafter (E11‐E14). In 1987, code 249 (insulin‐dependent diabetes mellitus) was introduced in Denmark; prior to this, code 250 included all forms of diabetes. To reduce potential misclassification, we excluded individuals diagnosed with code 250 before 30 years, as most individuals with type 1 diabetes are diagnosed before this age (Diabetesforeningen, 2015).
2.1.6. Analytic sample
Major reasons for not being included in the study were death during infancy, an invalid identification number, emigration, or being a twin (Figure 1). Among individuals eligible for analysis, follow‐up started at age 30 years and ended at the date of a type 2 diabetes diagnosis, death, emigration, loss to follow‐up, or December 31, 2015, whichever came first. Individuals with missing information on duration of breastfeeding were excluded. In total, 6,044 individuals were included in the analyses, corresponding to 74.5% of the starting population for this study (Figure 1). Of these individuals, 2,426 had information on BMI at age 7 years and 3,391 had information on BMI at 41–43 years of age.
2.1.7. Ethics
The Danish Data Protection Agency approved the project. According to Danish law, ethical approval is not required for purely register‐based studies.
2.2. Statistical methods
Using t tests and chi‐square statistics, we assessed potential impact of missing data by comparing key characteristics between (a) included and excluded individuals from among eligible individuals, (b) individuals with BMI at 7 years available and those without from among the included individuals, and (c) individuals who completed the questionnaire at age 41–43 and those who did not from among included individuals. Key characteristics (percentages within a given category or mean and standard deviation [SD]) were described for included individuals.
In a time‐to‐event analysis, hazard ratios (HRs) and 95% confidence intervals (CIs) for the association between the duration of breastfeeding and type 2 diabetes were estimated with Cox proportional hazards regression models. The model included each covariate one at a time, and covariates were included as potential confounders in the final models if they changed the estimates of breastfeeding on type 2 diabetes by more than 10% or were significantly associated with type 2 diabetes. The following covariates were selected a priori on the basis of established associations and/or plausible biological relations and tested: parental social status, breadwinner's education, marital status, maternal age at birth, attitude towards pregnancy, maternal pre‐pregnancy BMI, maternal diabetes during pregnancy, smoking during the third trimester, number of cigarettes during the third trimester, gestational weight gain, parity, preterm birth, birth weight, sex, and age at introduction of complementary foods.
For most covariates, the missing data proportion was less than 3%, but for parental social status, breadwinner's education, gestational weight gain, gestational age, and age at introduction of complementary feeding, they were 12%, 13%, 41%, 20%, and 12%, respectively. Multiple imputations were performed using chained equations as implemented in Stata through the mi command (n = 7,458) due to the missing data on breastfeeding and covariates. Using this method with 40 imputations of missing values on the basis of all a priori chosen covariates, breastfeeding and type 2 diabetes, essentially the same results were obtained as without imputations of missing values and there was no gain in efficiency (Table S1). Therefore, the sample of 6,044 individuals with information on breastfeeding available was the basis for all subsequent analyses. A missing category was created for all covariates, which were included as class variables, so that all individuals could be retained in the analyses. Four regression models were used to investigate the impact of stepwise adjustment for social‐, pregnancy‐, birth‐, and infancy‐related factors.
To assess the extent to which the association between breastfeeding and risk of diabetes could be attributed to child or adult BMI, we investigated if adjustment for these factors influenced the associations in the subsamples with information available. BMI at 7 years was categorized into tertiles and BMI at 41–43 years was categorized as normal weight (<25 kg/m2), overweight (≥25 to <30 kg/m2), or obese (≥30 kg/m2; World Health Organization, 1995).
Potential interactions between breastfeeding and selected risk factors for type 2 diabetes (parental social status, pre‐pregnancy BMI, smoking during the third trimester, and sex) were tested in nested models with and without cross‐product terms using a likelihood ratio test. With a similar test, to assess the proportional hazard assumption, an interaction by categories of age at diagnosis (30–48 or >48–56 years; divided at the median age at diagnosis) was tested. Analyses were conducted in STATA version 14.1 (StataCorp LP, College Station, TX, USA).
3. RESULTS
During a mean of 23.9 years of follow‐up (144,732 person‐years), 237 type 2 diabetes diagnoses (n = 136 men, n = 101 women) were recorded in the NPR. The majority of the mothers were married and smoked during the third trimester (Table 1). Few were obese before becoming pregnant, and 1% had diabetes during pregnancy. Approximately 29% of the infants were breastfed for 0.5 months or less, and 18.6% were breastfed for more than 4 months (Table 2). Eighteen percent of the children had complementary feeding introduced during the first 3 months after birth. Compared with individuals who were included in the study, individuals who were excluded due to missing information on breastfeeding were from homes of lower SES. However, they were not different in maternal or child anthropometrics or risk of type 2 diabetes from those who were included in the study (Table S2).
Table 1.
Characteristics of the study population, parental characteristics
| Parental characteristics | Data available, n | % or mean (SD) | Min, max |
|---|---|---|---|
| Parental social statusa | 5,372 | ||
| Low: 0–6 points | 23.1% | ||
| Medium: 7–10 points | 42.6% | ||
| High: 11–20 points | 34.3% | ||
| Breadwinner's education | 5,259 | ||
| Remedial education | 1.3% | ||
| Grade school | 67.1% | ||
| High school | 21.1% | ||
| College | 10.6% | ||
| Maternal marital status, % married | 5,955 | 63.4% | |
| Maternal age at birth (years) | 6,044 | 25.4 (6.5) | 14, 48 |
| Attitude towards pregnancy, % wanted | 5,828 | 43.9 | |
| Maternal pre‐pregnancy BMI (kg/m2) | 5,483 | 21.8 (3.0) | 14.7, 47.8 |
| Categories of maternal pre‐pregnancy BMI (kg/m2) | 5,483 | ||
| <18.5 | 8.7% | ||
| ≥18.5 & <25 | 79.9% | ||
| ≥25 & <30 | 9.7% | ||
| ≥30 | 1.7% | ||
| Maternal diabetes during pregnancy, % yes | 6,043 | 1.0% | |
| Smoking during the third trimester (cigarettes/day) | 5,952 | ||
| None | 48.2% | ||
| <3 daily | 7.0% | ||
| 2–10 daily | 30.1% | ||
| 11–20 daily | 13.1% | ||
| >20 daily | 1.6% | ||
| Gestational weight gain (kg) | 3,594 | ||
| <6 | 7.7% | ||
| 6–8 | 16.6% | ||
| 9–10 | 18.3% | ||
| 11–12 | 17.2% | ||
| 13–15 | 20.5% | ||
| >16 | 19.6% |
Note. The table provides the number of subjects with information available and percentages within a given category of the characteristics or mean (SD) and minimum and maximum value.
Points for social status was given 0–5 according to occupation of the breadwinner, the way in which the breadwinner earn his/her wages, the training of the breadwinner, and the character of the living accommodation (the highest class gives the fewest points; Zachau‐Christiansen & Ross, 1975).
Table 2.
Characteristics of the study population, infant characteristics
| Infant characteristics | Data available, n | % or mean (SD) | Min, max |
|---|---|---|---|
| Sex, % boys | 6,044 | 50.6% | |
| Parity | 6,044 | 1.9 (1.2) | 1, 9 |
| Preterm birtha, % yes | 4,867 | 18.5% | |
| Birth weight (g) | 5,995 | ||
| ≤2750 | 19.2% | ||
| >2750–3250 | 32.9% | ||
| >3250–3750 | 32.3% | ||
| >3750–4250 | 12.4% | ||
| >4250–4750 | 2.3% | ||
| >4750 | 0.4% | ||
| Breastfeeding (months) | 6,044 | ||
| ≤0.5 | 28.8% | ||
| >0.5–1 | 13.7% | ||
| >1–2 | 18.4% | ||
| >2–4 | 20.5% | ||
| >4 | 18.6% | ||
| Age at introduction of spoon‐feeding (months) | 5,341 | ||
| 0–2 | 17.9% | ||
| 3–5 | 68.2% | ||
| 6–8 | 12.7% | ||
| 9–11 | 1.2% | ||
| BMI at 7 years | 2,426 | 15.5 (1.4) | 11.1, 24.3 |
| 41–43 years BMI (kg/m2/years) | 3,391 | 25.1 (4.5) | 14.1, 54.9 |
| 41–43 years BMI ≥ 25 kg/m2 | 43.0% | ||
| 41–43 years BMI ≥ 30 kg/m2 | 12.2% |
Note. The table provides the number of subjects with information available and percentages within a given category of the characteristics or mean (SD) and minimum and maximum value.
Defined as <38 weeks of gestational age.
Parental social status, breadwinner's education, mother's age, gestational weight gain, and birth weight were positively associated with the duration of any breastfeeding (Table S3). In addition, the duration of any breastfeeding was positively associated with age at introduction of complementary feeding. Single mother status, attitude towards pregnancy (wanted), maternal pre‐pregnancy BMI, diabetes and smoking during pregnancy, preterm birth, and parity were inversely associated with the duration of breastfeeding. Sex was not associated with breastfeeding duration.
The following covariates changed the estimates of breastfeeding on type 2 diabetes by more than 10%: parental social status, breadwinner's education, maternal pre‐pregnancy BMI, maternal diabetes during pregnancy, number of cigarettes during the third trimester, gestational weight gain, parity, preterm birth, and birth weight (Table S4). Sex did not, but was significantly associated with type 2 diabetes (Table S5). Marital status, maternal age at birth, attitude towards pregnancy, and age at introduction of complementary foods did not change the association between breastfeeding and type 2 diabetes by at least 10% (Table S4) nor were they associated with type 2 diabetes (Table S5). We found no indications of interactions between breastfeeding and potential interaction variables (all p values >.17).
The proportion of individuals with type 2 diabetes decreased with increasing duration of any breastfeeding (Table 3). The unadjusted Cox regressions analysis showed that infants breastfed for >2–4 months had a 33% reduced risk of type 2 diabetes (HR = 0.67; 95% CI [0.46, 0.96]; Figure 2) and infants breastfed for >4 months had a 51% reduced risk of type 2 diabetes (HR = 0.49; 95% CI [0.32, 0.75]) compared with infants breastfed for 0.5 months or less. The overall association was significant (p = .01) and using the duration of breastfeeding as an ordinal variable, there was a significant and inverse trend between the duration of breastfeeding and risk of type 2 diabetes in adulthood (p = .001), indicating a dose–response effect of the duration of breastfeeding.
Table 3.
Distribution of type 2 diabetes among individuals by the duration of any breastfeeding
| Type 2 diabetes, n (%) | ||
|---|---|---|
| Duration of any breastfeeding (months) | No | Yes |
| ≤0.5 | 1,655 (95.01) | 87 (4.99) |
| >0.5–1 | 796 (96.25) | 31 (3.75) |
| >1–2 | 1,060 (95.58) | 49 (4.42) |
| >2–4 | 1,199 (96.62) | 42 (3.38) |
| >4 | 1,097 (97.51) | 28 (2.49) |
Figure 2.
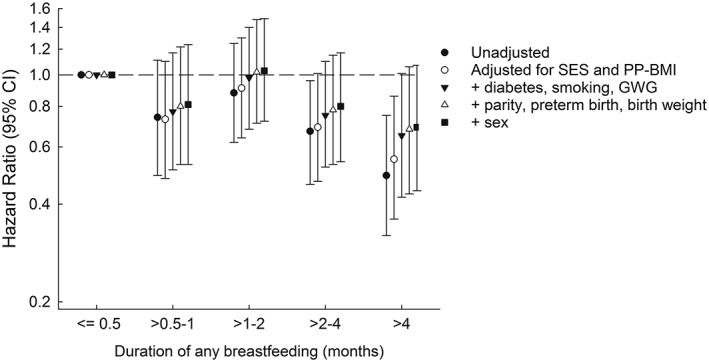
Duration of any breastfeeding and risk of type 2 diabetes unadjusted and adjusted for factors early in life. The estimates are adjusted for parental social status, breadwinner's education (socio‐economic status [SES]), and maternal body mass index (PP‐BMI); maternal diabetes during pregnancy, smoking quantity during the third trimester, and gestational weight gain (GWG); parity, preterm birth, and birth weight; sex
After adjustment for potential confounding factors, the results were no longer statistically significant (p = .32). Adjustment for each group of additional potential confounding factors attenuated the associations (Figure 2). Adjustment for the pregnancy‐related factors (maternal diabetes during pregnancy, smoking during the third trimester, and gestational weight gain) changed the HR for breastfeeding for >4 months by 28%, from 0.55 (95% CI [0.36, 0.86]) to 0.65 (95% CI [0.42, 1.01]). Adjustment for social factors changed the HR for breastfeeding for >4 months by 16%, from 0.49 (95% CI [0.32, 0.75]) to 0.55 (95% CI [0.36, 0.86]). In the fully adjusted model, there was a HR of 0.80 (95% CI [0.54, 1.17]) and a HR of 0.69 (95% CI [0.44, 1.07]) associated with breastfeeding for >2–4 months and >4 months, respectively. Although there was still an indication of a duration‐response effect, the inverse trend was no longer significant (p = .12). An analysis adjusted only for factors that were significantly associated with breastfeeding and type 2 diabetes in the present data showed similar associations (Table S6).
The unadjusted Cox regression analysis showed that infants who were exclusively breastfed for >4 months had a 49% reduced risk of type 2 diabetes compared with infants breastfed for 0.5 months or less (Table S7). This attenuated to a 32% nonsignificantly reduced risk after adjustment for potential confounding factors (Table S7).
Children who did not have information on BMI at 7 years were from homes of lower SES. They were breastfed for shorter durations, and they had a slightly higher BMI in adulthood, but there were no differences in maternal pre‐pregnancy BMI, smoking or diabetes during pregnancy, or in risk of type 2 diabetes from those who had information on child BMI (Table S8). Individuals who did not answer the adult questionnaire were more often men from homes of lower SES, born to smoking mothers; they were breastfed for shorter periods of time and had higher risk of type 2 diabetes than those who returned the questionnaire. Nonetheless, they were born to mothers with similar pre‐pregnancy BMI, similar frequency of diabetes during pregnancy and gestational weight gain (Table S9).
Duration of breastfeeding was not associated with BMI at 7 years (p overall = .60), but BMI at 41–43 years decreased from 25.2 kg/m2 among infants breastfed for 0.5 months or less to 24.6 kg/m2 among infants breastfed for >4 months (p overall = .009). Despite BMI in the upper tertile at 7 years having a significant and positive association with type 2 diabetes, even in the adjusted model (HR = 2.35; 95% CI [1.41, 3.94], p overall = .0006), adding BMI at 7 years to the fully adjusted model did not change the association between breastfeeding and type 2 diabetes (Table S10). Similarly, overweight or obesity at 41–43 years were each associated with significantly increased risks of type 2 diabetes (HR = 2.24; 95% CI [1.24, 4.05] and HR = 8.69; 95% CI [4.94, 15.29], respectively, p overall < .0001), yet adjustment for BMI at 41–43 years only slightly attenuated the association between breastfeeding and type 2 diabetes in the subsample with adult BMI (Table S10).
4. DISCUSSION
This study showed that the inverse association between the duration of any breastfeeding and the risk of type 2 diabetes in adulthood is considerably weakened by adjustment for other well‐known risk factors in the infant and mother, thus indicating that these factors may confound the association. When we did not include putative confounding factors, infants breastfed for 2 months or more had lower risks of type 2 diabetes than infants breastfed for 0.5 month or less. However, adjustment for each additional group of factors gradually attenuated the association. Adjustment for the pregnancy‐related factors resulted in the largest attenuation, whereas additional adjustment for birth‐related factors and sex resulted in minimal changes. Confounding by unknown or not included confounders, from, for example, family history of diabetes, cannot be excluded. After adjustment for all putative confounders, our results suggest a 31% reduced risk, although this is not statistically significant as the lower and upper limits of the CI were 0.44 and 1.07, respectively. Based on these results, a risk reduction as low as 56% or a small positive effect cannot be precluded. However, our results need to be confirmed in a sample with more type 2 diabetes events.
It has been suggested that breastfeeding reduces the risks of metabolic disease only in high‐income countries, due to confounding from SES (Brion et al., 2011). Our results are consistent with this, because there was a positive association between parental social status and duration of breastfeeding, and the association between breastfeeding and type 2 diabetes was no longer significant after adjustment for putative confounders. One other study of individuals born in the second half of the 20th century came from America when breastfeeding was more prevalent among mothers with a higher education (Parikh et al., 2009). After adjustment for a range of participant and maternal risk factors, they found an inverse although nonsignificant association between ever versus never breastfeeding and a fasting plasma glucose >126 mg/dl (odds ratio = 0.40 [0.09, 1.70]), which is in line with our findings. However, the power of the study was relatively low (N = 24 cases; Parikh et al., 2009). In contrast, in different eras and settings where the association between SES and breastfeeding is less likely to be positive, three studies provide some evidence of an inverse association between breastfeeding and type 2 diabetes in adulthood (Fall et al., 1995; Pettitt et al., 1997; Ravelli et al., 2000), whereas three do not (Fall et al., 2011; Martin, Ben‐Shlomo, et al., 2005; Martin, Ebrahim, et al., 2005). Notably, in the latter three studies, there was little difference in the infant feeding modes by SES (Fall et al., 2011; Martin, Ben‐Shlomo, et al., 2005; Martin, Ebrahim, et al., 2005).
Despite the strong positive associations between child or adult BMI and type 2 diabetes, adjustment for these factors only minimally changed the associations between duration of breastfeeding and type 2 diabetes in our study. Although some misreporting of adult BMI is possible, other studies have reported that associations between breastfeeding and type 2 diabetes changed only slightly with adjustment for current BMI in early adulthood (Fall et al., 1995; Fall et al., 2011; Pettitt et al., 1997). Thus, contrary to what might be expected, the association of breastfeeding with later body size does not appear to explain the association with type 2 diabetes.
Pathophysiological mechanisms underlying an association between breastfeeding and type 2 diabetes are speculative. It has been proposed that breastfeeding confers protection against type 2 diabetes through learned capability in the infant to self‐regulate the intake, through ghrelin and leptin in the milk as these hormones may be important for appetite regulation, or through metabolic programming by the provision of bioactive factors that regulate energy intake and energy expenditure (Bartok & Ventura, 2009; Savino, Liguori, Fissore, & Oggero, 2009). Another possibility is that bacteria from breast milk affect the susceptibility to type 2 diabetes through an influence on the gut microbiota (Pannaraj et al., 2017). Moreover, the feeding of the nonbreastfed infant may influence the association between breastfeeding and type 2 diabetes. At the time our cohort was born, it was recommended that nonbreastfed infants received cow's milk mixtures from glass bottles (Indenrigsministeriets helbredsudvalg, 1960). Early introduction of complementary feeding has been suggested to be associated with later BMI (Schack‐Nielsen et al., 2010). Nevertheless, in our study, it was not associated with type 2 diabetes nor did it change the association between breastfeeding and type 2 diabetes.
Compared with other studies on the association between breastfeeding in infancy and adult type 2 diabetes, the major strengths of our study are the short recall on the duration of breastfeeding, the relatively large study sample in an ethnically homogenous population. Only one study reported a higher number of cases (n > 3,000) than in our study (Rich‐Edwards et al., 2004); however, type 2 diabetes was not the focus, and the results were only reported in a meta‐analysis (Owen, Martin, Whincup, Smith, & Cook, 2006, 2012). In addition, we were able to adjust for a wide range of potential confounding factors. Many studies did not adjust for birth weight and socio‐economic indicators as a minimum and had long‐term recall of the breastfeeding information, which could introduce bias and result in overestimation of the association (Martin, Ben‐Shlomo, et al., 2005; Martin, Ebrahim, et al., 2005; Parikh et al., 2009; Pettitt et al., 1997; Rich‐Edwards et al., 2004). Moreover, several studies did not have the power to estimate these associations with sufficient precision (Fall et al., 1995; Martin, Ben‐Shlomo, et al., 2005; Martin, Ebrahim, et al., 2005; Parikh et al., 2009). Finally, the similarity of the sensitivity analysis using multiple imputation of missing data, in combination with the minimal loss to follow‐up due to the register linkage, means that the potential effects of selection bias in our study were likely limited.
The admission criteria of the hospital were reflected in a high still birth rate and infant mortality (Figure 1), but this was explained by a higher rate of low birth weight infants. The infant mortality rate was 33% among those with low birth weight and only 1.6% among those with normal birth weight (Zachau‐Christiansen & Ross, 1975). Even though excluded individuals were of lower SES, the included individuals represented a broad range of SES, and among these, we found no interactions of breastfeeding with SES in the association with type 2 diabetes risk. Therefore, we find it unlikely that our analysis for this reason underestimates the effect of breastfeeding. The duration of breastfeeding in our cohort was relatively short when compared with current and study specific recommendations of 5–6 months of exclusive breastfeeding (Indenrigsministeriets helbredsudvalg, 1960). The proportion of infants that were breastfed for >4 months (19%) was about one third of that in a Danish cohort born in 2012 (70%; Bruun et al., 2016). Nevertheless, it was in the range of observed proportions among infants born in (32%) and out of wedlock (18%) in the Copenhagen Municipality in the years 1958–1962 (Biering‐Sørensen, Hilden, & Biering‐Sørensen, 1980). Due to the nature of our data, it was not possible to distinguish between those who were breastfed for a very short period, or not breastfed at all. It is nevertheless likely that the majority of women tried initiating breastfeeding, as another study showed that 80% of women initiated breastfeeding in Denmark at that time period (Biering‐Sørensen et al., 1980). Due to the similarity in duration of any and exclusive breastfeeding in this cohort, we cannot preclude that our results may to a large degree represent effects of exclusive breastfeeding. We followed the individuals only until middle age, that is, before the incidence of type 2 diabetes increases greatly. However, few other studies followed individuals to >60 years (Fall et al., 1995; Martin, Ebrahim, et al., 2005). In our study, just like the majority of previous studies, information on the exact age at onset of type 2 diabetes was unavailable. The register‐based information on type 2 diabetes means that patients who were treated exclusively in primary care and undiagnosed individuals were not included. Whereas the completeness of the NPR over a 5‐year period is moderate (sensitivity: 64%), the positive predictive value of a diagnosis of diabetes in the NPR is high (97%; Kristensen, Drivsholm, Carstensen, Steding‐Jensen, & Green, 2007). In the context of the Danish system, the long follow‐up period (24 years) increases the probability of capturing cases as most individuals with type 2 diabetes will eventually appear in the hospital register. However, by using age at first hospital discharge as a proxy for age at onset, it may be delayed to an unknown extent.
In conclusion, the duration of any breastfeeding is in this high‐income population inversely associated with the risk of type 2 diabetes in crude analyses, but when a range of type 2 diabetes risk factors are accounted for, the association is considerably weakened and is no longer significant in this study. The association is not altered in a subsample by adjustment for BMI in childhood or adulthood.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
LGB and JLB conceived and designed the study. ELM, TIAS, and JLB provided data material. LGB performed the statistical analysis. LGB and JLB wrote the paper. All authors were involved in the data interpretation. LGB and JLB had primary responsibility for final content. All authors critically revised the manuscript for important intellectual content and approved the final version of this manuscript.
Supporting information
Table S1. Associations between duration of any breastfeeding and type 2 diabetes in the included population and after imputation of missing values
Table S2. Characteristics of included study participants (n = 6,044) and study participants who were excluded due to missing information on breastfeeding (n = 1,414) from among eligible individuals).1
Table S3. Relationship between covariates and duration of any breastfeeding
Table S4. Associations between duration of any breastfeeding and type 2 diabetes with and without adjustment for covariates, one at a time.
Table S5. Associations between covariates and type 2 diabetes from bivariate analyses.a
Table S6. Duration of any breastfeeding and risk of type 2 diabetes adjusted for factors adjusted for factors that were significantly associated with breastfeeding and type 2 diabetes in the present data.
Table S7. Duration of exclusive breastfeeding and risk of type 2 diabetes unadjusted and adjusted for factors early in life
Table S8. Characteristics of individuals with information on BMI at 7 years (n = 2,426) and those without BMI at 7 years (n = 3,618) from among included individuals.1
Table S9. Characteristics of those who answered the adult questionnaire (n = 3,443) and those who did not (n = 2,601) from among included individuals.1
Table S10. Associations between duration of any breastfeeding and type 2 diabetes adjusted for factors early in life and for BMI in childhood (N = 2,426) and adulthood (N = 3,353) respectively.
ACKNOWLEDGMENTS
We are grateful for the collection of the Copenhagen Perinatal Cohort data led by the late Drs Aage Willumsen and Bengt Zachau‐Christiansen.
Bjerregaard LG, Pedersen DC, Mortensen EL, Sørensen TIA, Baker JL. Breastfeeding duration in infancy and adult risks of type 2 diabetes in a high‐income country. Matern Child Nutr. 2019;15:e12869 10.1111/mcn.12869
REFERENCES
- Andersen, T. F. , Madsen, M. , Jørgensen, J. , Mellemkjær, L. , & Olsen, J. H. (1999). The Danish National Hospital Register. A valuable source of data for modern health sciences. Danish Medical Bulletin, 46(3), 263–268. [PubMed] [Google Scholar]
- Baker, J. L. (2015). Breastfeeding and weight in mothers and infants In Gill T. (Ed.), Managing and preventing obesity: Behavioural factors and dietary interventions (1 ed. (pp. 123–134). Waltham, MA: Woodhead Publishing; 10.1533/9781782420996.2.123 [DOI] [Google Scholar]
- Baker, J. L. , Olsen, L. W. , Andersen, I. , Pearson, S. , Hansen, B. , & Sørensen, T. I. A. (2009). Cohort profile: The Copenhagen School Health Records Register. International Journal of Epidemiology, 38(3), 656–662. 10.1093/ije/dyn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartok, C. J. , & Ventura, A. K. (2009). Mechanisms underlying the association between breastfeeding and obesity. International Journal of Pediatric Obesity, 4(4), 196–204. 10.3109/17477160902763309 [DOI] [PubMed] [Google Scholar]
- Biering‐Sørensen, F. , Hilden, J. , & Biering‐Sørensen, K. (1980). Breast‐feeding in Copenhagen, 1938‐1977. Data on more than 365,000 infants. Danish Medical Bulletin, 27(1), 42–48. [PubMed] [Google Scholar]
- Blake‐Lamb, T. L. , Locks, L. M. , Perkins, M. E. , Woo Baidal, J. A. , Cheng, E. R. , & Taveras, E. M. (2016). Interventions for childhood obesity in the first 1,000 days a systematic review. American Journal of Preventive Medicine, 50(6), 780–789. 10.1016/j.amepre.2015.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion, M. J. , Lawlor, D. A. , Matijasevich, A. , Horta, B. , Anselmi, L. , Araujo, C. L. , … Smith, G. D. (2011). What are the causal effects of breastfeeding on IQ, obesity and blood pressure? Evidence from comparing high‐income with middle‐income cohorts. International Journal of Epidemiology, 40(3), 670–680. 10.1093/ije/dyr020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruun, S. , Wedderkopp, N. , Mølgaard, C. , Kyhl, H. B. , Zachariassen, G. , & Husby, S. (2016). Using text messaging to obtain weekly data on infant feeding in a Danish birth cohort resulted in high participation rates. Acta Paediatrica, 105(6), 648–654. 10.1111/apa.13382 [DOI] [PubMed] [Google Scholar]
- Carstensen, B. , Kristensen, J. K. , Ottosen, P. , & Borch‐Johnsen, K. (2008). The Danish National Diabetes Register: Trends in incidence, prevalence and mortality. Diabetologia, 51(12), 2187–2196. 10.1007/s00125-008-1156-z [DOI] [PubMed] [Google Scholar]
- Cattaneo, A. , Monasta, L. , Stamatakis, E. , Lioret, S. , Castetbon, K. , Frenken, F. , … Brug, J. (2010). Overweight and obesity in infants and pre‐school children in the European Union: A review of existing data. Obesity Reviews, 11(5), 389–398. 10.1111/j.1467-789X.2009.00639.x [DOI] [PubMed] [Google Scholar]
- Christensen, K. , Doblhammer, G. , Rau, R. , & Vaupel, J. W. (2009). Ageing populations: The challenges ahead. Lancet, 374(9696), 1196–1208. doi: S0140–6736(09)61460–4 [pii]. 10.1016/S0140-6736(09)61460-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetesforeningen. (16 July 2015). Diabetes in Denmark. Retrieved from http://diabetes.dk/presse/diabetes-i-tal/diabetes-i-danmark.aspx
- Fall, C. H. , Borja, J. B. , Osmond, C. , Richter, L. , Bhargava, S. K. , Martorell, R. , … Group, C. (2011). Infant‐feeding patterns and cardiovascular risk factors in young adulthood: Data from five cohorts in low‐ and middle‐income countries. International Journal of Epidemiology, 40(1), 47–62. 10.1093/ije/dyq155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fall, C. H. , Osmond, C. , Barker, D. J. , Clark, P. M. , Hales, C. N. , Stirling, Y. , & Meade, T. W. (1995). Fetal and infant growth and cardiovascular risk factors in women. BMJ, 310(6977), 428–432. 10.1136/bmj.310.6977.428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillman, M. W. (2008). The first months of life: A critical period for development of obesity. The American Journal of Clinical Nutrition, 87(6), 1587–1589. 10.1093/ajcn/87.6.1587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder, T. , Bergmann, R. , Kallischnigg, G. , & Plagemann, A. (2005). Duration of breastfeeding and risk of overweight: A meta‐analysis. American Journal of Epidemiology, 162(5), 397–403. 10.1093/aje/kwi222 [DOI] [PubMed] [Google Scholar]
- Horta, B. L. , Loret de Mola, C. , & Victora, C. G. (2015). Long‐term consequences of breastfeeding on cholesterol, obesity, systolic blood pressure and type 2 diabetes: A systematic review and meta‐analysis. Acta Paediatrica, 104(467), 30–37. 10.1111/apa.13133 [DOI] [PubMed] [Google Scholar]
- Indenrigsministeriets helbredsudvalg, S . (1960). Den Danske Almindelige Lægeforenings Hygiejnekomité. Lille Ny Vejledning for Vordende Mødre [Little New Guideline for Mothers to be]. Retrieved from Copenhagen:
- Kristensen, J. K. , Drivsholm, T. B. , Carstensen, B. , Steding‐Jensen, M. , & Green, A. (2007). Validering af metoder til identifikation af erkendt diabetes pa basis af administrative sundhedsregistre [Validation of methods to identify known diabetes on the basis of health registers]. Ugeskrift for Laeger, 169(18), 1687–1692. [PubMed] [Google Scholar]
- Lee, J. M. , Pilli, S. , Gebremariam, A. , Keirns, C. C. , Davis, M. M. , Vijan, S. , … Gurney, J. G. (2010). Getting heavier, younger: trajectories of obesity over the life course. International Journal of Obesity, 34(4), 614–623. doi:ijo2009235 [pii];. 10.1038/ijo.2009.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, R. M. , Ben‐Shlomo, Y. , Gunnell, D. , Elwood, P. , Yarnell, J. W. , & Davey Smith, G. (2005). Breast feeding and cardiovascular disease risk factors, incidence, and mortality: The Caerphilly study. Journal of Epidemiology and Community Health, 59(2), 121–129. 10.1136/jech.2003.018952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, R. M. , Ebrahim, S. , Griffin, M. , Davey Smith, G. , Nicolaides, A. N. , Georgiou, N. , … Gunnell, D. (2005). Breastfeeding and atherosclerosis: Intima‐media thickness and plaques at 65‐year follow‐up of the Boyd Orr cohort. Arteriosclerosis, Thrombosis, and Vascular Biology, 25(7), 1482–1488. 10.1161/01.ATV.0000170129.20609.49 [DOI] [PubMed] [Google Scholar]
- Mortensen, E. L. , Michaelsen, K. F. , Sanders, S. A. , & Reinisch, J. M. (2002). The association between duration of breastfeeding and adult intelligence. JAMA, 287(18), 2365–2371. 10.1001/jama.287.18.2365 [DOI] [PubMed] [Google Scholar]
- Owen, C. G. , Martin, R. M. , Whincup, P. H. , Smith, G. D. , & Cook, D. G. (2006). Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. The American Journal of Clinical Nutrition, 84(5), 1043–1054. 10.1093/ajcn/84.5.1043 [DOI] [PubMed] [Google Scholar]
- Owen, C. G. , Martin, R. M. , Whincup, P. H. , Smith, G. D. , & Cook, D. G. (2012). Erratum. The American Journal of Clinical Nutrition, 95(3), 779 10.3945/ajcn.111.033035 [DOI] [Google Scholar]
- Pannaraj, P. S. , Li, F. , Cerini, C. , Bender, J. M. , Yang, S. , Rollie, A. , … Aldrovandi, G. M. (2017). Association between breast milk bacterial communities and establishment and development of the infant gut microbiome. JAMA Pediatrics, 171(7), 647–654. 10.1001/jamapediatrics.2017.0378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh, N. I. , Hwang, S. J. , Ingelsson, E. , Benjamin, E. J. , Fox, C. S. , Vasan, R. S. , & Murabito, J. M. (2009). Breastfeeding in infancy and adult cardiovascular disease risk factors. The American Journal of Medicine, 122(7), 656–663.e651. 10.1016/j.amjmed.2008.11.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, C. B. (2011). The Danish Civil Registration System. Scandinavian Journal of Public Health, 39(7 Suppl), 22–25. 10.1177/1403494810387965 [DOI] [PubMed] [Google Scholar]
- Pettitt, D. J. , Forman, M. R. , Hanson, R. L. , Knowler, W. C. , & Bennett, P. H. (1997). Breastfeeding and incidence of non‐insulin‐dependent diabetes mellitus in Pima Indians. Lancet, 350(9072), 166–168. 10.1016/S0140-6736(96)12103-6 [DOI] [PubMed] [Google Scholar]
- Ravelli, A. C. , van der Meulen, J. H. , Osmond, C. , Barker, D. J. , & Bleker, O. P. (2000). Infant feeding and adult glucose tolerance, lipid profile, blood pressure, and obesity. Archives of Disease in Childhood, 82(3), 248–252. 10.1136/adc.82.3.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redsell, S. A. , Edmonds, B. , Swift, J. A. , Siriwardena, A. N. , Weng, S. , Nathan, D. , & Glazebrook, C. (2016). Systematic review of randomised controlled trials of interventions that aim to reduce the risk, either directly or indirectly, of overweight and obesity in infancy and early childhood. Maternal & Child Nutrition, 12(1), 24–38. 10.1111/mcn.12184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich‐Edwards, J. W. , Stampfer, M. J. , Manson, J. E. , Rosner, B. , Hu, F. B. , Michels, K. B. , & Willett, W. C. (2004). Breastfeeding during infancy and the risk of cardiovascular disease in adulthood. Epidemiology, 15(5), 550–556. 10.1097/01.ede.0000129513.69321.ba [DOI] [PubMed] [Google Scholar]
- Savino, F. , Liguori, S. A. , Fissore, M. F. , & Oggero, R. (2009). Breast milk hormones and their protective effect on obesity. International Journal of Pediatric Endocrinology , 2009, 327505 10.1155/2009/327505, 1, 8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schack‐Nielsen, L. , Sørensen, T. I. A. , Mortensen, E. L. , & Michaelsen, K. F. (2010). Late introduction of complementary feeding, rather than duration of breastfeeding, may protect against adult overweight. The American Journal of Clinical Nutrition, 91(3), 619–627. 10.3945/ajcn.2008.27078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzman, R. , & Beard, J. (2011). Global Health and Aging. Retrieved from http://who.int/ageing/publications/global_health.pdf
- Whitlock, G. , Lewington, S. , Sherliker, P. , Clarke, R. , Emberson, J. , Halsey, J. , … Peto, R. (2009). Body‐mass index and cause‐specific mortality in 900 000 adults: Collaborative analyses of 57 prospective studies. Lancet, 373(9669), 1083–1096. doi: S0140–6736(09)60318–4 [pii]. 10.1016/S0140-6736(09)60318-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . (1995). Physical status: The use and interpretation of anthropometry . Report of a WHO Expert Committee. Retrieved from Geneva: [PubMed]
- World Health Organization . (2014). Global status report on noncommunicable diseases 2014. Retrieved from Switzerland: http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf?ua=1
- Willumsen, A. (1970). Environmental factors in congenital malformations: A prospective study of 9006 human pregnancies. Copenhagen: F.A.D.L.'s Forlag. [Google Scholar]
- Zachau‐Christiansen, B. , & Ross, E. M. (1975). Babies: Human development during the first year. London: John Wiley & Sons Ltd. [Google Scholar]
- Zimmermann, E. , Bjerregaard, L. G. , Gamborg, M. , Vaag, A. A. , Sørensen, T. I. A. , & Baker, J. L. (2017). Childhood body mass index and development of type 2 diabetes throughout adult life—A large‐scale Danish cohort study. Obesity, 25(5), 965–971. 10.1002/oby.21820 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Associations between duration of any breastfeeding and type 2 diabetes in the included population and after imputation of missing values
Table S2. Characteristics of included study participants (n = 6,044) and study participants who were excluded due to missing information on breastfeeding (n = 1,414) from among eligible individuals).1
Table S3. Relationship between covariates and duration of any breastfeeding
Table S4. Associations between duration of any breastfeeding and type 2 diabetes with and without adjustment for covariates, one at a time.
Table S5. Associations between covariates and type 2 diabetes from bivariate analyses.a
Table S6. Duration of any breastfeeding and risk of type 2 diabetes adjusted for factors adjusted for factors that were significantly associated with breastfeeding and type 2 diabetes in the present data.
Table S7. Duration of exclusive breastfeeding and risk of type 2 diabetes unadjusted and adjusted for factors early in life
Table S8. Characteristics of individuals with information on BMI at 7 years (n = 2,426) and those without BMI at 7 years (n = 3,618) from among included individuals.1
Table S9. Characteristics of those who answered the adult questionnaire (n = 3,443) and those who did not (n = 2,601) from among included individuals.1
Table S10. Associations between duration of any breastfeeding and type 2 diabetes adjusted for factors early in life and for BMI in childhood (N = 2,426) and adulthood (N = 3,353) respectively.


