Surveillance studies have shown that OXA-48-like carbapenemases are the most common carbapenemases in Enterobacterales in certain regions of the world and are being introduced on a regular basis into regions of nonendemicity, where they are responsible for nosocomial outbreaks. OXA-48, OXA-181, OXA-232, OXA-204, OXA-162, and OXA-244, in that order, are the most common enzymes identified among the OXA-48-like carbapenemase group.
KEYWORDS: carbapenemases, Enterobacteriaceae, OXA-48-like
SUMMARY
Surveillance studies have shown that OXA-48-like carbapenemases are the most common carbapenemases in Enterobacterales in certain regions of the world and are being introduced on a regular basis into regions of nonendemicity, where they are responsible for nosocomial outbreaks. OXA-48, OXA-181, OXA-232, OXA-204, OXA-162, and OXA-244, in that order, are the most common enzymes identified among the OXA-48-like carbapenemase group. OXA-48 is associated with different Tn1999 variants on IncL plasmids and is endemic in North Africa and the Middle East. OXA-162 and OXA-244 are derivatives of OXA-48 and are present in Europe. OXA-181 and OXA-232 are associated with ISEcp1, Tn2013 on ColE2, and IncX3 types of plasmids and are endemic in the Indian subcontinent (e.g., India, Bangladesh, Pakistan, and Sri Lanka) and certain sub-Saharan African countries. Overall, clonal dissemination plays a minor role in the spread of OXA-48-like carbapenemases, but certain high-risk clones (e.g., Klebsiella pneumoniae sequence type 147 [ST147], ST307, ST15, and ST14 and Escherichia coli ST38 and ST410) have been associated with the global dispersion of OXA-48, OXA-181, OXA-232, and OXA-204. Chromosomal integration of blaOXA-48 within Tn6237 occurred among E. coli ST38 isolates, especially in the United Kingdom. The detection of Enterobacterales with OXA-48-like enzymes using phenotypic methods has improved recently but remains challenging for clinical laboratories in regions of nonendemicity. Identification of the specific type of OXA-48-like enzyme requires sequencing of the corresponding genes. Bacteria (especially K. pneumoniae and E. coli) with blaOXA-48, blaOXA-181, and blaOXA-232 are emerging in different parts of the world and are most likely underreported due to problems with the laboratory detection of these enzymes. The medical community should be aware of the looming threat that is posed by bacteria with OXA-48-like carbapenemases.
INTRODUCTION
The global spread of antimicrobial-resistant organisms (AROs) was recently identified by the World Health Organization, the European Union, the U.S. Government, and the Centers for Disease Control and Prevention (USA) as one of the most significant threats to human health (1). The spread of AROs in general is troublesome for medical practitioners at large, since infections due to such bacteria are often responsible for increased patient mortality and morbidity due to the delayed administration of suitable antibiotics (2, 3).
β-Lactam antibiotics, such as penicillins, cephalosporins, monobactams, and carbapenems, are among the most frequently prescribed antibiotics worldwide. These agents bind to and inhibit bacterial enzymes (referred to as penicillin binding proteins [PBPs]) responsible for cell wall synthesis (4).
Resistance to β-lactam antibiotics involves different mechanisms, summarized as follows (4). (i) Mutational changes in the active site of PBPs lower the affinity for β-lactams and lead to decreased binding between antibiotic and enzymes. This is a common mechanism among Gram-positive organisms, and penicillin resistance in Streptococcus pneumoniae due to mutations in PBPs is a good example of decreased affinity. (ii) Alterations in outer membrane permeability are mediated by decreased expression and subsequent loss of outer membrane proteins. The loss of OprD in Pseudomonas aeruginosa leads to the decreased entry of imipenem into the periplasmic space, with subsequent resistance to this carbapenem. (iii) Membrane efflux pumps that are capable of removing antibiotics from the periplasmic space to the external environment are upregulated. The increased expression of the MexA-MexB-OprD pump system in P. aeruginosa can decrease the periplasm levels of several drugs, including the cephalosporins and carbapenems. (iv) Enzymes, such as β-lactamases, that bind to and inactivate different β-lactam antibiotics are produced.
The aim of this article is to provide a brief overview on β-lactamases (including a section on the OXA-type β-lactamases) and then to review in detail the characteristics, evolution, molecular epidemiology, and laboratory diagnosis of bacteria with acquired OXA-48-like β-lactamases.
β-Lactamases
β-Lactamases are bacterial enzymes that inactivate β-lactam antibiotics by hydrolysis, which results in ineffective compounds (5). The first enzyme with the ability to hydrolyze penicillin was described nearly 70 years ago for Escherichia coli (6).
In Gram-negative bacteria, β-lactamase production remains the most important contributing factor to β-lactam resistance, and their increasing frequency and their continuous evolution are directly linked to selection by the use of different β-lactam agents (7). β-Lactamases differ from each other in their substrate profiles (i.e., the different types of β-lactam antibiotics they inactivate), inhibitor profiles (i.e., which compounds inactivate them), and sequence homologies (i.e., amino acid compositions of these enzymes) (5). Using these different characteristics, two classification systems have been created to divide β-lactamases into Ambler classes (i.e., classes A, B, C, and D, based on amino acid sequence homology) and the Bush-Jacoby-Medeiros groups (i.e., groups 1, 2, 3, and 4, based on substrate and inhibitor profiles) (8, 9). The Ambler classification system is more commonly used in published literature.
Enzymes that belong to classes A, C, and D share the amino acid serine as part of their respective active sites, while the class B β-lactamases (also known as metallo-β-lactamases [MBLs]) contain zinc ions in their active sites (10).
One of the most pressing current ARO concerns is the expeditious growth and global spread of carbapenem-resistant Gram-negative bacteria (11). Carbapenems are some of the last efficacious antibiotic therapies available for treating serious infections due to Gram-negative AROs. β-Lactamases that specifically target the carbapenems are known as the carbapenemases, and they are the most important causes of carbapenem resistance among Gram-negative bacteria (12). The main reason is because genes encoding carbapenemases are typically part of mobile genetic elements (MGEs) that have the ability to move between different Gram-negative species, especially among members of the Enterobacterales (13).
Carbapenemases belong to Ambler class A (i.e., KPC types), class B (i.e., MBLs: VIM, IMP, and NDM types), and class D (OXA β-lactamases) (11). The KPC, NDM, IMP, and VIM and certain OXA-type enzymes are the most common global carbapenemases among Gram-negative bacteria.
OXA β-Lactamases
The class D β-lactamases are also referred to as oxacillinases or OXA β-lactamases due to their capacity for hydrolyzing oxacillin more efficiently than benzylpenicillin (14). These enzymes were first recognized in the 1960s and 1970s and showed hydrolytic activity against the penicillins and oxacillin (15, 16). Later on, certain OXA β-lactamases were described that also inactivate the cephalosporins and carbapenems (17). Currently, more than 750 types of OXA β-lactamases have been reported. It is important to remember that the OXA β-lactamases, as a group, show heterogeneous substrate profiles that are confusing to medical practitioners, even those with a special interest in infectious disease and microbiology. A general principle does exist: OXA enzymes are resistant to inhibition by β-lactam inhibitors such as clavulanate, sulbactam, and tazobactam, and they confer resistance to the amino-, carboxy-, and ureidopenicillins (18).
The OXA β-lactamases hydrolyze β-lactam antibiotics by a unique method that features a carbamylated lysine (5). The OXA enzymes use this carbamylated lysine to activate and anchor the serine active site, removing the steric hindrances from the deacylating water molecule pathway and leading to tighter binding and more efficient hydrolysis of β-lactam agents.
According to the class D β-lactamase numbering scheme, OXA β-lactamases possess a serine residue at position 70 and a carbamoylated lysine at position 73 (5). The Tyr-Gly-Asn motif at positions 144 to 146 and Lys-Thr-Gly motif at positions 216 to 218 are mostly conserved among the class D β-lactamases. In some instances, the Tyr-Gly-Asn motif can be replaced by a Phe-Gly-Asn motif. Mutations in the omega loop of the β-lactamases can change an enzyme’s specific function and substrate profile and are responsible for enhanced activity to the cephalosporins and carbapenems.
In 2010, Poirel and colleagues divided the OXA β-lactamases into the following 4 groups (19). Group I is the acquired narrow-spectrum class D β-lactamases that do not have significant activity against the cephalosporins or the carbapenems. This group consists of the OXA-1, OXA-2, and OXA-101 subgroups. Group II is the acquired extended spectrum class D β-lactamases that are able to hydrolyze certain extended-spectrum cephalosporins (especially ceftriaxone and cefepime). Group II members are often point mutants of the narrow-spectrum class D β-lactamases, while certain extended-spectrum OXA enzymes are not structurally related to the narrow-spectrum group I OXA β-lactamases. Group III is acquired carbapenem-hydrolyzing class D β-lactamases with weak activity against the carbapenems that do not significantly hydrolyze the extend-spectrum cephalosporins. One subgroup of the acquired carbapenem-hydrolyzing class D β-lactamases, named the OXA-23-like enzymes, is a very important cause of carbapenem resistance among Acinetobacter spp. (17). Another subgroup, named the OXA-48-like enzymes, has found a niche among the Enterobacterales (20) and is the main topic of this review. Group IV is naturally occurring class D β-lactamases that form parts of the chromosomes of various non-fermenting Gram-negative bacteria and includes the OXA-51-like subgroup in the Acinetobacter baumannii complex.
OXA Carbapenemases
The carbapenem-hydrolyzing class D carbapenemases are principal causes of carbapenem resistance among A. baumannii isolates and the Enterobacterales (21). These enzymes have activity against the penicillins (especially the amino-, carboxy- and ureidopenicillins) and narrow-spectrum cephalosporins (e.g., cephalothin), weakly hydrolyze the carbapenems, and have limited activities against the broad-spectrum cephalosporins (especially ceftazidime) and most β-lactam inhibitors (e.g., clavulanate, sulbactam, and tazobactam).
They are divided phylogenetically into two groups (21). Group I is present mainly in A. baumannii and includes 4 subgroups. Subgroup Ia is named the OXA-23-like β-lactamases (examples include OXA-23, -27, -49, -73, -102, -103, -133, -146, -165, -166, -167, -168, -169, -170, -171, -225, -239, -366, -398, -422, -423, -435, -440, -481, -482, -483, and -565). Subgroup Ib is named the OXA-24/40-like β-lactamases, and examples include OXA-24, -25, -26, -72, -139, -160, -207, and -437. Subgroup Ic is named the OXA-51-like β-lactamases, with numerous examples, and subgroup Id is named the OXA-58-like β-lactamases (examples include OXA-58, -96, -97, -164, -397, -420, and -512).
The group II OXA carbapenemases consist of the OXA-48-related variants and is named OXA-48-like β-lactamases. The encoded OXA-48-like enzymes are weakly related to other class D β-lactamases, sharing less than 50% amino acid identity to the other OXA members (e.g., 46%, 36%, and 21% amino acid identity exists with OXA-10, OXA-23, and OXA-1, respectively). OXA-48-like enzymes share 87% amino acid identity within the OXA-48-like group (the outlier being OXA-436; please refer to “Enzyme Characteristics for OXA-48-Like Carbapenemases” below for details).
Global genomic surveillance and clinical studies as well as several case reports suggest that Enterobacterales with OXA-48-like carbapenemases are endemic in certain parts of the world and are being introduced on a regular basis into regions of nonendemicity where they are responsible for nosocomial outbreaks. The laboratory detection of Enterobacterales with OXA-48-like carbapenemases is challenging for some clinical laboratories, especially for those that are situated in regions of nonendemicity. The medical community at large, especially individuals interested in antimicrobial resistance, should be aware of the looming threat that is posed by OXA-48-like carbapenemases.
THE OXA-48-LIKE β-LACTAMASES
Acquired OXA-48-type carbapenemases are important causes of nonsusceptibility to the carbapenems among the Enterobacterales and include the following enzymes: OXA-48 (22), OXA-162 (23), OXA-181 (24), OXA-204 (25), OXA-232 (26), OXA-244 (27), OXA-245 (27), OXA-247 (28), OXA-436 (29), OXA-484 (30) and OXA-519 (31). Other OXA-48 variants, such as OXA-163 (32), OXA-252 (listed on GenBank only), and OXA-405 (33), do not have sufficient activity against the carbapenems to be considered carbapenemases (34). Klebsiella pneumoniae and Enterobacter spp. with blaOXA-163 were first obtained during 2008 in Argentina and are presently widely dispersed in that country (20). Serratia marcescens with blaOXA-405 was reported in France in 2012. OXA-163 and OXA-405 hydrolyze the extended-spectrum cephalosporins and show limited activities against the carbapenems. These enzymes are not addressed in detail in this article.
Enterobacter hormaechei with OXA-370 (35) showed high MICs to ertapenem and meropenem, but it is unclear if this enzyme is a true carbapenemase since its hydrolytic profile has not yet been determined (36). The sequences of certain OXA-48-variants (e.g., OXA-438, OXA-439, OXA-505, OXA-517, OXA-566, and OXA-567) are listed only in GenBank, and it is currently uncertain if these enzymes have carbapenemase activities.
OXA-54, OXA-199, OXA-252, OXA-416, and various others listed in GenBank and on the β-Lactamase DataBase (BLDB; http://bldb.eu) (37) form parts of the Shewanella oneidensis and Shewanella xiamenensis chromosomes and shares more than 90% amino acid identity with OXA-48-like β-lactamases, indicating that aquatic Shewanella spp. are likely the ancestors of OXA-48-like enzymes (please refer to “Origin and Evolution of OXA-48-Like Carbapenemases” below for more details on the origin and evolution of OXA-48 β-lactamases) (38–40). Chromosomal OXA-48-like β-lactamases are also not specifically addressed in this review.
OXA-48, OXA-181, OXA-232, OXA-204, OXA-162, and OXA-244, in that order, are the most common enzymes identified among the OXA-48-like carbapenemases. OXA-436, OXA-245, OXA-484, and OXA-519 are less often reported group (please see below for more details).
Enzyme Characteristics for OXA-48-Like Carbapenemases
OXA-48-like β-lactamases consist of 261 to 265 amino acids encoded by the blaOXA open reading frame (ORF), consisting of 798-bp nucleotides (22). Overall, the carbapenemase activities of OXA-48-like enzymes are low, and the production of extended-spectrum β-lactamases (ESBLs) and/or porin mutations are often required to provide the high levels of carbapenem resistance (41, 42).
The crystal structure of OXA-48 and hydrolysis assays showed that the carbapenem hydrolysis is different from that of other non-48 OXA carbapenemases. Hydrolytic efficiency of OXA-48 against imipenem is approximately 10-fold higher than that of the OXA carbapenemases (OXA-23-like, etc.) from Acinetobacter spp. (17). OXA-48 has the highest kcat value for imipenem, 2 s−1, which represented the highest hydrolysis rate of all of the published kinetic parameters among the OXA-48-like enzymes (22). For OXA-48, hydrolysis relies on the rotation of the carbapenem-hydroxyethyl group within the active site of the enzyme in a manner that allows movement of the deacylating water molecules toward the acylated serine residue (43). Consequently, OXA-48-producing K. pneumoniae isolates exhibit elevated MICs to the carbapenems (especially to imipenem). OXA-48 efficiently hydrolyzes the amino-, carboxy-, and ureidopenicillins and narrow-spectrum cephalosporins (e.g., cephalothin), but not the extended-spectrum cephalosporins (e.g., ceftriaxone, ceftazidime, and cefepime) (22).
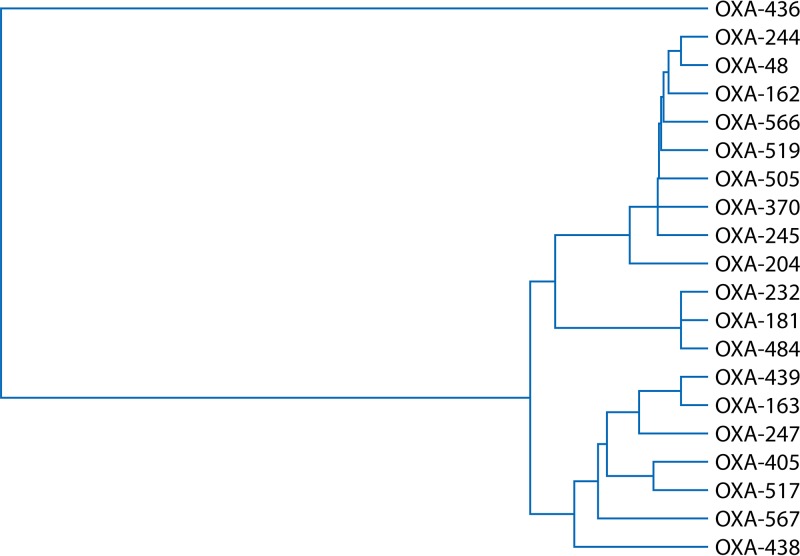
Figure 1 shows a phylogenetic tree of the amino acid alignment of the different OXA-48-like enzymes using the ClustalW2 sequence alignment program. OXA-48-like carbapenemases can be broadly divided into 3 clusters, namely, the OXA-48 cluster (which shows more than 95% amino acid homology and consists of OXA-48, -162, -204, -244, -245, and -519), the OXA-181 cluster (which shows more than 98% amino acid homology and consists of OXA-181, -232 and -484), and OXA-436, which has significantly different amino acid alignment than other OXA-48-like carbapenemases (showing 76% amino acid homology with the OXA-48 and OXA-181 clusters). Please note that the OXA-48-like noncarbapenemases (e.g., OXA-163 and OXA-204) formed a separate cluster from the OXA-48, OXA-181, and OXA-436 clusters (Fig. 1).
FIG 1.
UPGMA (unweighted pair group method using average linkages) phylogenetic tree of OXA-48-producing Enterobacterales using amino acid alignments. The tree calculation was performed with the ClustalW2 multiple-sequence alignment program.
Tables 1 and 2 show the amino acid differences and kinetic parameters of certain OXA-48-like β-lactamases. Most of the differences are located within the β5-β6 loop, which is important for the substrate specificity of OXA-48-like enzymes. Some OXA-48 variants have hydrolytic activities similar to that of OXA-48 (e.g., OXA-204 and OXA-181); others have slightly increased carbapenem-hydrolyzing activities (e.g., OXA-162) or slightly reduced carbapenem- and temocillin-hydrolyzing activities (e.g., OXA-232 and OXA-244). In contrast, OXA-48 variants with a 4-amino-acid deletion within the β5-β6 loop (e.g., OXA-163 and OXA-405) have lost their carbapenem-hydrolytic activity and gained the capacity to hydrolyze extended-spectrum cephalosporins (44).
TABLE 1.
Amino acid divergences among OXA-48-like β-lactamases
| OXA-48-like enzyme | Species | NCBI protein accession number | Divergence from OXA-48 |
|---|---|---|---|
| OXA-162 | K. pneumoniae | ACZ73269 | Single substitution: Thr213Ala |
| OXA-163 | E. cloacae | ADY06444 | Single substitution: Ser212Asp |
| Four deletions: Arg214, Ile215, Glu216, Pro217 | |||
| OXA-181 | K. pneumoniae | AEP16366 | Four substitutions: Thr104Ala, Asn110Asp, Glu168Gln, Ser171Ala |
| OXA-204 | E. coli | AJF39128 | Two substitutions: Gln98His, Thr99Arg |
| OXA-232 | E. coli | AGD91915 | Five substitutions: Thr104Ala, Asn110Asp, Glu168Gln, Ser171Ala, Arg214Ser |
| OXA-244 | E. coli | AKJ18768 | Single substitution: Arg214Gly |
| OXA-245 | K. pneumoniae | AGC60013 | Single substitution: Glu125Tyr |
| OXA-247 | K. pneumoniae | AGC70814 | Two substitutions: Tyr211Ser, Asp212Asn |
| OXA-370 | E. hormaechei | AHW47891 | Single substitution: Gly220Glu |
| OXA-405 | S. marcescens | AJA30430 | Four deletions: Thr213, Arg214, Ile215, Glu216 |
| OXA-436 | C. freundii | ALN39145 | 23 substitutions: Val3Ala, Phe10Leu, Leu11Met, Ala13Thr, Ser14Thr, Ile15Met, Thr36Ser, Ser40Thr, Lys51Thr, Asn58Asp, Thr104Ala, Asn110Asp, Val153Leu, Glu168Gln, Ser171Ala, Gly201Ala, Thr213Val, Val226Ile, Met237Thr, Ser244Ala, Asp245Glu, Ala252Thr, Glu256Ala |
| OXA-438 | E. coli | AKL59521 | Four substitutions: Ser212Gly, Ile215Tyr, Glu216Asp, Pro217Thr |
| Two deletions: Thr213, Arg214 | |||
| OXA-439 | E. coli | AKR53961 | Two substitutions: Tyr123His, Ser212Asp |
| Four deletions: Arg214, Ile215, Glu216, Pro217 | |||
| OXA-484 | K. pneumoniae | ALI16502 | Five substitutions: Thr104Ala, Asn110Asp, Glu168Gln, Ser171Ala, Arg214Gly |
| OXA-505 | K. pneumoniae | ALZ40809 | Single substitution: Ala8Thr |
| OXA-517 | K. pneumoniae | AMO66558 | Single substitution: Arg214Lys |
| Two deletions: Ile215, Glu216 | |||
| OXA-519 | K. pneumoniae | ANI25017 | Single substitution: Val120Leu |
| OXA-566 | E. coli | ACS55290 | Single substitution: Ala141Asp |
| OXA-567 | K. pneumoniae | ATJ25944 | Three substitutions: Ser212Asp, Arg214Lys, Glu216Gly |
| Single deletion: Pro217 |
TABLE 2.
Kinetic parameters of certain OXA-48-like β-lactamasesa
| Antibiotic and parameter | Value for indicated β-lactamase |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| OXA-48 | OXA-162 | OXA-163 | OXA-181 | OXA-204 | OXA-232 | OXA-245 | OXA-436 | OXA-519 | |
| Benzylpenicillin | |||||||||
| kcat (s−1) | 245 | 123 | 23 | 444 | 353 | 125 | NE | 900 | NE |
| Km (μM) | 40 | 35 | 13 | 90 | 90 | 60 | NE | 200 | NE |
| kcat/Km (mM−1/s−1) | 6,100 | 3,400 | 1,800 | 5,000 | 4,100 | 2,100 | NE | 4,500 | NE |
| Ampicillin | |||||||||
| kcat (s−1) | 955 | 269 | 23 | 218 | 389 | 132 | 1,200 | 600 | 131 |
| Km (μM) | 400 | 315 | 315 | 170 | 450 | 220 | 35 | 5 | 776 |
| kcat/Km (mM−1/s−1) | 2,400 | 830 | 70 | 1,300 | 860 | 600 | 3,428 | 120,000 | 169 |
| Oxacillin | |||||||||
| kcat (s−1) | 130 | 3 | 34 | 90 | 56 | 156 | NE | NE | 8 |
| Km (μM) | 95 | 75 | 90 | 80 | 100 | 130 | NE | NE | 338 |
| kcat/Km (mM−1/s−1) | 1,400 | 40 | 370 | 1,100 | 540 | 1,200 | NE | NE | 23 |
| Piperacillin | |||||||||
| kcat (s−1) | 75 | NE | 8 | NE | NE | 380 | NE | NE | 36 |
| Km (μM) | 410 | NE | 70 | NE | NE | 540 | NE | NE | 109 |
| kcat/Km (mM−1/s−1) | 180 | NE | 110 | NE | NE | 700 | NE | NE | 330 |
| Temocillin | |||||||||
| kcat (s−1) | 0.3 | 0.7 | NH | 0.3 | 0.5 | 0.03 | NE | 3 | >12 |
| Km (μM) | 45 | 170 | NH | 60 | 75 | 60 | NE | 200 | >1,000 |
| kcat/Km (mM−1/s−1) | 6 | 4 | ND | 5 | 7 | 0.5 | NE | 15 | 8.8 |
| Imipenem | |||||||||
| kcat (s−1) | 5 | 11 | 0.03 | 7.5 | 4 | 0.2 | 4.4 | 6 | 2.1 |
| Km (μM) | 13 | 25 | 530 | 13 | 9 | 9 | 11 | 20 | 982 |
| kcat/Km (mM−1/s−1) | 370 | 420 | 0.06 | 550 | 420 | 20 | 400 | 300 | 2.138 |
| Meropenem | |||||||||
| kcat (s−1) | 0.07 | 0.1 | >0.1 | 0.1 | 0.05 | 0.03 | 0.11 | 0.14 | 3.4 |
| Km (μM) | 10 | 80 | >2,000 | 70 | 60 | 100 | 2 | 3 | 358 |
| kcat/Km (mM−1/s−1) | 6 | 1.3 | 0.03 | 1.5 | 0.8 | 0.3 | 55 | 4,666 | 9.497 |
| Ertapenem | |||||||||
| kcat (s−1) | 0.13 | 0.3 | 0.05 | 0.2 | 0.1 | 0.04 | 0.29 | 0.4 | 1.1 |
| Km (μM) | 100 | 30 | 130 | 100 | 90 | 110 | 80 | 160 | 83 |
| kcat/Km (mM−1/s−1) | 1 | 9 | 0.3 | 2 | 1 | 0.4 | 3,625 | 2.5 | 13.25 |
| Cefotaxime | |||||||||
| kcat (s−1) | >9 | 3 | 10 | >62 | 12 | >6.5 | NE | 6 | >1.3 |
| Km (μM) | >900 | 310 | 45 | >1,000 | 990 | >1,000 | NE | 130 | >1,000 |
| kcat/Km (mM−1/s−1) | 10 | 10 | 230 | 13 | 12 | 6 | NE | 46.15 | 0.39 |
| Ceftazidime | |||||||||
| kcat (s−1) | NH | ND | 8 | ND | ND | >0.6 | 0.3 | 7 | 0.02 |
| Km (μM) | NH | NH | >1,000 | NH | NH | >1,000 | 110 | 150 | 373 |
| kcat/Km (mM−1/s−1) | NH | ND | 3 | ND | ND | 0.1 | 2,727 | 4,666 | 0.05 |
| Cefepime | |||||||||
| kcat (s−1) | 1 | NE | 2 | NE | NE | 13 | NE | NE | >1.3 |
| Km (μM) | 160 | NE | 350 | NE | NE | 1,200 | NE | NE | >1,000 |
| kcat/Km (mM−1/s−1) | 6 | NE | 6 | NE | NE | 10 | NE | NE | 0.31 |
| Cephalothin | |||||||||
| kcat (s−1) | 44 | 12 | 3 | 13 | 12 | 13 | NE | NE | >13 |
| Km (μM) | 195 | 180 | 10 | 250 | 270 | 125 | NE | NE | >1,000 |
| kcat/Km (mM−1/s−1) | 255 | 70 | 300 | 50 | 45 | 105 | NE | NE | 3.5 |
Kinetic parameter values for OXA-48 are from reference 44, except those for benzylpenicillin, piperacillin, and cefepime, which are from reference 22. Values for OXA-163 are from reference 44, except those for piperacillin and cefepime, which are from reference 32. Values for OXA-232 are from reference 44, except those for piperacillin and cefepime, which are from reference 26. All values for OXA-162, OXA-181, and OXA-204 are from reference 44. All values for OXA-245 are from reference 307. All values for OXA-436 are from reference 214. All values for OXA-519 are from reference 31. kcat, number of times each enzyme site converts substrate to product per unit time; Km, inverse measure of affinity; NE, not evaluated; ND, not determined; NH, not detectable.
Origin and Evolution of OXA-48-Like Carbapenemases
The OXA-54 gene is present on the chromosome of S. oneidensis and was reported around the same time as OXA-48. It shares 92% amino acid identity with OXA-48, suggesting that this genus is most likely the progenitors of OXA-48 (39). S. xiamenensis type strain S4, isolated in China, harbored an OXA-48 gene variant, namely, blaOXA-48b, on its chromosome that differs by four nucleotides but results in the same amino acids as blaOXA-48 (40). S. xiamenensis with blaOXA-48b was also found in Portugal (45).
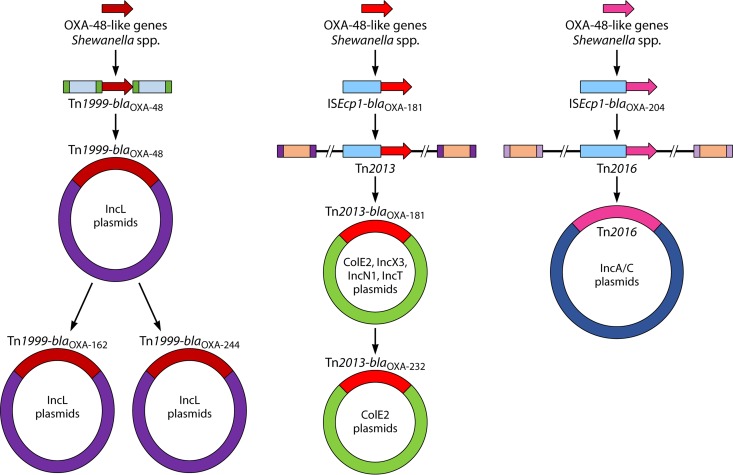
Mobile genetic elements, especially the composite transposon Tn1999, were likely responsible for the capture and mobilization of OXA-48-like β-lactamases from the chromosome of Shewanella spp. onto conjugative plasmids and then spread to members of the Enterobacterales. The blaOXA-48-like genes on the chromosomes of several Shewanella species are flanked by a gene encoding the peptidase C15 and lysR-acc (lysR is a transcriptional regulator and acc encodes acetyl coenzyme A [acetyl-CoA] carboxylase). The lysR sequence in S. xiamenensis S4 showed 99% nucleotide identity and the acc sequences were identical to that of Tn1999 (GenBank accession numbers JX644945 and AY236073, respectively). It is thus more than likely that two copies of IS1999 mobilized blaOXA-48–lysR–Δacc from the chromosome of Shewanella spp. to an IncL plasmid that was then transferred to Enterobacterales (Fig. 2).
FIG 2.
Origin and evolution of OXA-48-like carbapenemases, namely, OXA-48, OXA-162, OXA-181, OXA-204, OXA-232, and OXA-244.
S. xiamenensis, obtained from Indian seepage water during 2010, was identified as the progenitor of the OXA-181 gene, with chromosomal genetic structures similar to those described for the ColE-type OXA-181-containing plasmid (46). The blaOXA-181 gene and its flanking regions (i.e., a 128-bp fragment downstream of ISEcp1 and a 378-bp fragment upstream of ΔlysR and partial ere) are identical to regions on the chromosome of S. xiamenensis strains S12 and Sh5 (46). The initial capture of the OXA-181 gene was mediated via ISEcp1 and the gene was then incorporated into Tn2013, which found its way onto ColE2, IncX3, IncN1, and IncT plasmids (Fig. 2) (46).
OXA-232 differs from OXA-181 by a single amino acid substitution, and the genetic environment surrounding the blaOXA-232 is very similar to the environment surrounding blaOXA-181 (Table 1 and Fig. 2; see “Molecular Epidemiology of Enterobacterales with blaOXA-232” below for details). This suggests that OXA-232 is a derivative of OXA-181.
The OXA-204 gene has a genetic environment similar to that of blaOXA-181, but OXA-204 differs from OXA-181 by 6 amino acid substitutions (Table 1 and Fig. 2; see “Molecular Epidemiology of Enterobacterales with blaOXA-204” below for details). The origin of blaOXA-204 is most likely the chromosome of S. xiamenensis (45), and the capture of blaOXA-204 was most likely due to distinct transposition processes independently from blaOXA-181 (Fig. 2).
OXA-162 and OXA-244 differ from OXA-48 by a single amino acid substitution and the genetic environments surrounding the blaOXA-162 and blaOXA-244 are very similar to the environment surrounding blaOXA-48. (Table 1 and Fig. 2; see “Molecular Epidemiology of Enterobacterales with blaOXA-162” and “Molecular Epidemiology of Enterobacterales with blaOXA-244” below for details). This suggests that OXA-162 and OXA-244 are derivatives of OXA-48.
In summary, the nucleotide similarities and genetic structures flanking the carbapenemase genes suggest that blaOXA-48, blaOXA-181, and blaOXA-204 were derived from different S. xiamenensis isolates through distinct transposition processes and that OXA-232, OXA-162, and OXA-244 are derivatives of OXA-181 (i.e., OXA-232) and OXA-48 (i.e., OXA-162 and OXA-244) (Fig. 2).
Global Surveillance for OXA-48-Like Carbapenemases
Surveillance studies that use molecular methodologies to identify carbapenemases have shown that OXA-48-like β-lactamases are the 2nd or 3rd most common carbapenemases among global Enterobacterales (47, 48). Data from the SMART (2008 to 2014) and INFORM (2012 to 2014) global surveillance programs show that 27% of carbapenemase-producing Enterobacterales (CPE; n = 1,615) were positive for OXA-48-like carbapenemases (compared to 55% KPCs and 26% NDMs). In certain areas (e.g., the Middle East, North Africa, and European countries such as Belgium and Spain), OXA-48-like enzymes were the most common carbapenemases among the Enterobacterales. OXA-48-like carbapenemases was mainly present in K. pneumoniae isolates submitted from hospitals sites and were increasing toward the end of the surveillance periods (47, 48).
OXA-48
OXA-48 is currently the most common global OXA-48-like enzyme and was first reported in 2004 from a clinical isolate obtained in Istanbul, Turkey (22). K. pneumoniae 11978 was responsible for lower urinary tract infection and tested nonsusceptible to all β-lactam antibiotics (including imipenem and ertapenem), the aminoglycosides, chloramphenicol, nalidixic acid, ciprofloxacin, rifampin, sulfonamides, and tetracycline. K. pneumoniae 11978 produced five different β-lactamases, and the enzyme with a pI of 7.2 turned out to be OXA-48. OXA-48 was distantly related to other oxacillinases and hydrolyzed the penicillins, cephalothin, and imipenem. The insertion sequence element IS1999 was found up- and downstream of the blaOXA-48 gene harbored on a 70-kb plasmid named pA-1 (22). K. pneumoniae with blaOXA-48 quickly became endemic in Istanbul, causing several nosocomial outbreaks at the University Hospital of Istanbul (49), while K. pneumoniae and E. coli with this enzyme gradually spread to other parts of Turkey (50).
Geographical Distribution of OXA-48
The 1st report of K. pneumoniae with OXA-48 outside Turkey occurred in Brussels, Belgium, in 2010 when a patient, with no apparent connection with Turkey, underwent chemotherapy for lymphoma (51). This was followed by a study that described different Enterobacterales (i.e., K. pneumoniae, Enterobacter cloacae, Providencia rettgeri, Citrobacter freundii, and E. coli) with the OXA-48 gene situated on similar 70-kb plasmids, recovered from Turkey and Lebanon (52) and Egypt, France, and Belgium (53) and described in reports from France (54) and North Africa, namely, Tunisia (55, 56) and Morocco (57, 58).
In 2011, reports of OXA-48-producing Enterobacterales appeared from Israel (59), Senegal (60), the Netherlands (61), Spain (62), Ireland (63), and Slovenia (64). The reports from France (65, 66), Slovenia (64), Spain (62), and Israel (59) were due to patients that had previously visited or were transferred from hospitals in Turkey or North Africa (especially Morocco). Nosocomial outbreaks with OXA-48-producing Enterobacterales (especially due to K. pneumoniae) occurred early after their introduction into France (67), Belgium (68), Ireland (63), and Spain (62).
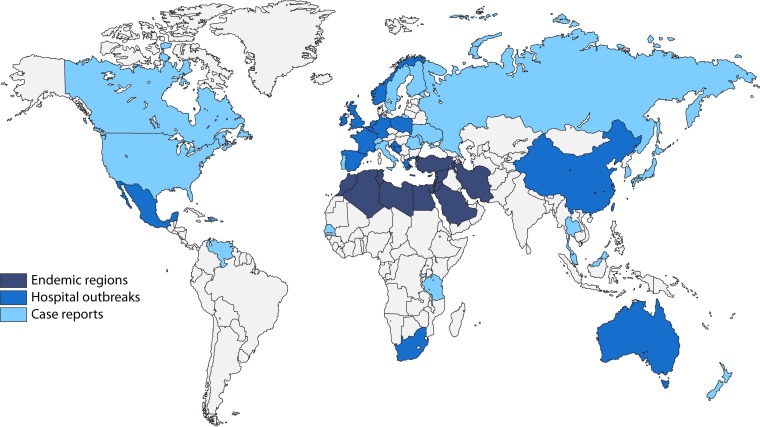
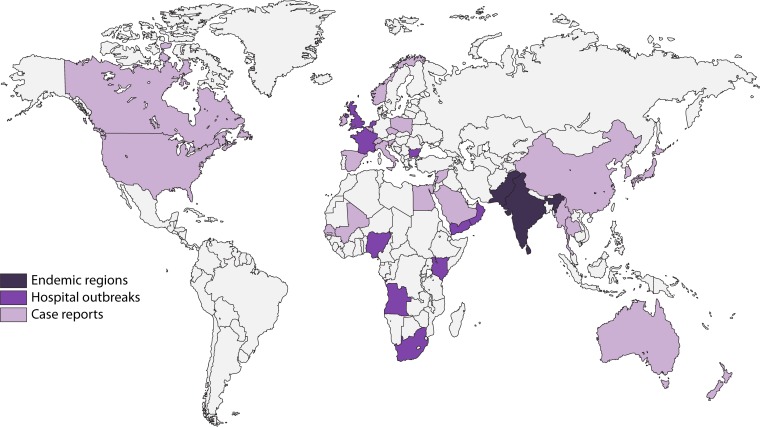
Enterobacterales with blaOXA-48 are currently endemic in Turkey, other Middle Eastern countries (i.e., Lebanon, Jordan, Oman, Iran, and Saudi Arabia), and North Africa (Morocco, Algeria, Tunisia, and Egypt) and are important causes of nosocomial outbreaks in these regions and countries (69) (Fig. 3). Health care-associated outbreaks have also been described in various other countries, including Greece (70, 71), Australia (72), Israel (73, 74), the United Kingdom (75, 76), the Netherlands (77, 78), Ireland (79, 80), Spain (81–85), France (86–88), Germany (89, 90), Mexico (91), China (92), Belgium (93), Norway (94), Poland (95), Taiwan (96), South Africa (97), Croatia (98), and Slovenia (99).
FIG 3.
Global distribution of Enterobacterales with OXA-48.
Currently, Enterobacterales with blaOXA-48 have a truly global distribution, and reports of imported and nonimported cases have described from all continents except Antarctica, including the Americas (e.g., Colombia [100] and Mexico [91]), various European and North American countries (69), Sub-Saharan Africa (e.g., Tanzania [101], South Africa [97], and Senegal [60]), Asia (e.g., Japan [102], Singapore [103], Taiwan [104], and Malaysia [105]), Oceania (e.g., New Zealand [106]), and the Caribbean islands (107).
Bacteria Producing OXA-48
OXA-48 was first described for K. pneumoniae, and it remains the most common global bacterium associated with health care-associated infections due to organisms with blaOXA-48 (47, 48). Subsequently OXA-48 was reported from several members of the Enterobacterales, especially E. coli (108), the E. cloacae complex (109), Citrobacter freundii (98), Serratia marcescens (74), Proteus mirabilis (110), Kluyvera spp. (111), Klebsiella oxytoca (112), and Salmonella spp. (113). Klebsiella spp. and Enterobacter spp. tend to be responsible for health care-associated infections, while E. coli with blaOXA-48 is an important cause of community-acquired infections due to carbapenemase-producing bacteria (114). The wide distribution of OXA-48 among different species in hospitals and especially in community settings remains one of the reasons why it is so difficult to limit and control the spread of bacteria with these enzymes. Plasmids containing blaOXA-48 have the ability to easily and widely disperse between various bacterial species via horizontal transmission. Different bacteria obtained from the same patient (either as colonizers or as clinical isolates) often contain identical plasmids harboring OXA-48 (115).
Enterobacterales with OXA-48 have been obtained from humans, animals (companion, other domestic, farm, and wildlife) and various types of environmental specimens from different parts of the world (69).
Molecular Epidemiology of Enterobacterales with blaOXA-48
The current international and interspecies spread of blaOXA-48 is mainly driven by the composite transposon Tn1999 and its variants situated on pOXA-48a-like IncL conjugative plasmids (116).
Genetic Environments Surrounding blaOXA-48
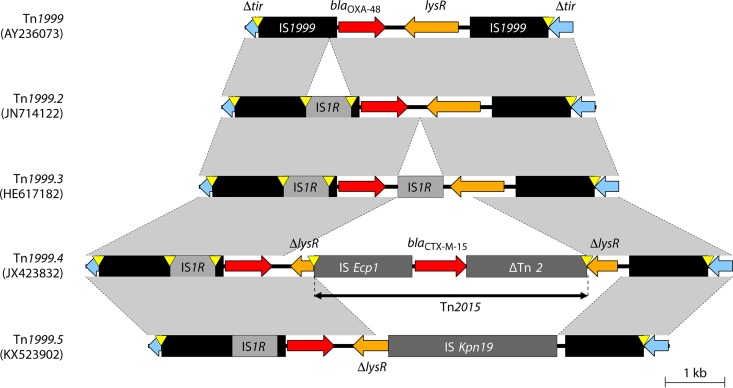
Tn1999 variants.
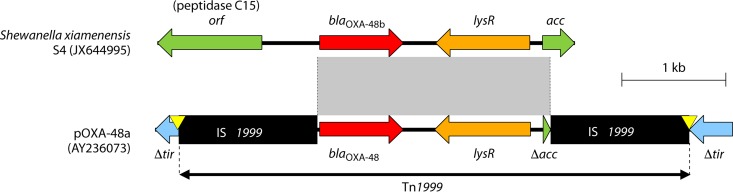
Tn1999, also named Tn1999.1, consists of two copies of the insertion sequence IS1999; one copy is inserted 26 bp upstream of blaOXA-48 and another copy is inserted downstream of blaOXA-48–lysR–Δacc (Fig. 4) (116). IS1999 was first identified in Pseudomonas aeruginosa from Thailand and was inserted into the integron-specific recombination site, attI1, upstream of blaVEB-1 (117). The blaOXA-48-like genes on the chromosomes of several Shewanella species are flanked by a gene encoding the peptidase C15 and lysR-acc (lysR is a transcriptional regulator and acc encodes acetyl-CoA carboxylase), which is similar to regions surrounding IS1999 (Fig. 4) (40).
FIG 4.
Similarities between Tn1999 with blaOXA-48 within the pOXA-48a plasmid and the Shewanella xiamenensis chromosome. Genes from background plasmids are indicated with cyan, and chromosomal genes of Shewanella are indicated with green. Yellow triangles indicate target site duplications.
Tn1999.2 and Tn1999.3 are Tn1999 variants with an IS1R insertion into IS1999 upstream of blaOXA-48 creating a strong hybrid promoter (−35 box from IS1R and −10 box from IS1999), leading to a 2-fold-higher enzymatic activity than with Tn1999 (Fig. 5) (49). Tn1999.2 was first described in Turkey in 2006 to 2007 (49) and then among clinical isolates from France, Turkey, Lebanon, Belgium, and Egypt situated on pOXA-48a-like plasmids (53, 68). Inverted copies of Tn1999.2 were found on pOXA-48a-like plasmids from K. pneumoniae, Klebsiella aerogenes, E. cloacae, Citrobacter koseri, and Raoultella planticola obtained in Lebanon (118). Tn1999.3 differs from Tn1999.2, with a second copy of IS1R located just downstream of blaOXA-48 (Fig. 5), and has been identified in an E. coli isolate from Italy (119).
FIG 5.
Genetic environments of Tn1999 and its variants within pOXA-48a-like plasmids. Mobile elements are indicated with black (e.g., IS1999) or gray (others). Genes from background plasmids are indicated with cyan. Yellow triangles indicate target site duplications.
Tn1999.4 is a Tn1999.2 variant with Tn2015 inserted downstream of blaOXA-48 within lysR and was described for E. coli and E. cloacae from France situated on a pOXA-48a-like plasmid (Fig. 5) (120). Tn2015 included ISEcp1 with the blaCTX-M-15 ESBL gene, which contributes to reduced susceptibility to the cephalosporins and carbapenems (120). Tn1999.5 is another variant of Tn1999.2 in which lysR is truncated by ISKpn19 (Fig. 5) found in K. pneumoniae sequence type 891 (ST891) from the Czech Republic, also situated on a pOXA-48a-like plasmid (121).
All the Tn1999 variants were integrated into the tir site of pOXA-48a-like plasmids, suggesting that they originated from Tn1999 by stepwise insertion of different MGEs.
Tn2016-like element.
Izdebski et al. in 2018 described a blaOXA-48 gene among K. pneumoniae ST15 and ST11 isolates obtained from Poland that was located downstream of ISEcp1, situated within Tn2016, that had previously been reported for blaOXA-204 (95) (please refer to the OXA-204 section for details). The Tn2016-like element was situated on nontypeable plasmids ranging in size from 90 to 160 kb, suggesting a different event for mobilization of blaOXA-48 from Shewanella spp.
Plasmids harboring blaOXA-48.
The most common plasmids that harbor Tn1999 variants associated with blaOXA-48 belong to the pOXA-48a-like IncL (IncL/M) replicon types (122). The IncL/M replicon type is currently one of the seven major plasmid families together with IncF, A/C type (IncA/C), IncI, IncHI, IncX, and IncN associated with antimicrobial resistance (AMR) among Enterobacterales (123). Recent molecular analyses have divided IncL/M plasmids into IncL, IncM1, and IncM2 subgroups based on incompatibility, differences in lncRNA (long noncoding RNA), and entry exclusion systems (122). IncL plasmids (e.g., pOXA-48a) and its variants have been mainly associated with blaOXA-48 (122).
pOXA-48a-like IncL plasmids do not contain additional AMR genes other than blaOXA-48, which differs from IncM plasmids (116, 121, 122, 124). pOXA-48a is highly similar to the IncM2 plasmid named pCTX-M3 (122), except for ISEcp1–blaCTX-M-3, which was inserted close to Tn1999 and a 27-kb AMR island near the trb transfer locus (116). The pOXA-48a-like IncL plasmids are conjugative and have been described for E. coli, E. cloacae, K. pneumoniae, Klebsiella oxytoca, Citrobacter freundii, and R. planticola (53, 116, 118). Transformation assays were unsuccessful for transferring pOXA-48a into A. baumannii and P. aeruginosa (116), and it seems that the host range is limited to the Enterobacterales (125).
pOXA-48a is highly transmissible, exhibiting a transfer frequency 50 times higher than those of the similar pNDM-OM IncL plasmids with blaNDM-1. This is likely due to the insertion and disruption of Tn1999 variants into the tir regions of pOXA-48a (the tir gene encodes a protein that inhibits transfer) (126). The insertion of Tn1999 variants into tir in pOXA-48a-like plasmids plays an important role in the mobility of these plasmids.
The PCR-based replicon typing scheme developed by Carattoli et al. was unable to detect pOXA-48a-like plasmids (127). Poirel et al. designed three primer pairs for the detection of pOXA-48a-like plasmids that amplified the repA, traU, and parA genes (116). The presence of Tn1999 variants and these three genes suggests the presence of pOXA-48a-like plasmids, but these genes are not specific markers, since they are also present in other IncL/M group plasmids.
Non-IncL plasmids have rarely been associated with blaOXA-48, but Tn1999 (without blaOXA-48) has been translocated to other plasmids, e.g., a nontypeable 29-kb plasmid in K. pneumoniae ST530 from Bulgaria (128), an A/C-type 150-kb plasmid in Providencia rettgeri from Turkey (129), and a 160-kb IncF plasmid in E. coli ST963 from France (129).
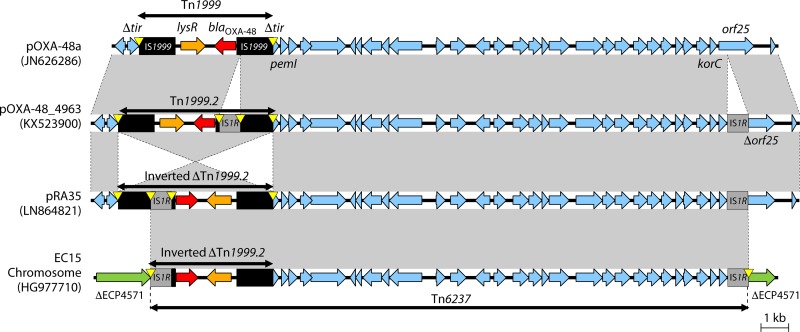
Chromosomal insertion of blaOXA-48.
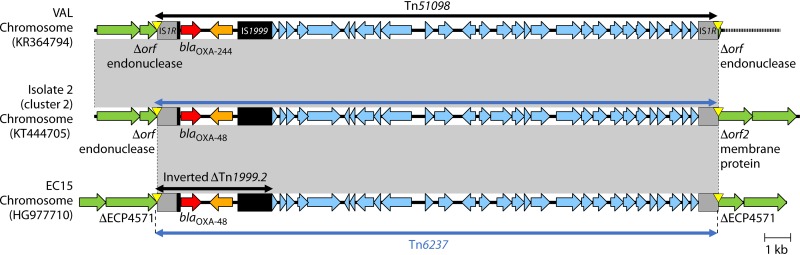
Tn6237 is a 21.9-kb IS1R-based composite transposon consisting of an inverted ΔTn1999.2 and a fragment from pOXA-48a (stretching from Δtir-pemI to korC-orf25) (Fig. 6). Tn6237 has been integrated into different chromosomal insertion sites among several E. coli and K. pneumoniae STs obtained from Lebanon and the Czech Republic (118, 121). Tn6237 most likely originated when the pOXA-48_4963 plasmid (with Tn1999.2) and the pRA35 plasmid (with inverted Tn1999.2 and IS1R insertion in orf25) underwent IS1R-mediated transposition and recombination and was then inserted into the chromosome of E. coli and K. pneumoniae STs (Fig. 6) (118).
FIG 6.
Genetic environments of Tn1999 within pOXA-48a, Tn1999.2 in pOXA-48_4963, inverted Tn1999.2 in pRA35, and Tn6237 containing inverted ΔTn1999.2 in the E. coli chromosome. In the E. coli EC15 chromosome, Tn6237 was inserted into a gene coding histidine kinase-like ATPase (ECP4571 in E. coli 536 [GenBank accession number CP000247]). Mobile elements are indicated with black (e.g., IS1999) or gray (others). Genes from background plasmids are indicated with cyan, and those from the chromosome are indicated with green. Yellow triangles indicate target site duplications.
E. coli ST38 without OXA-48-containing plasmids emerged in different hospitals throughout the United Kingdom in 2014 to 2015, as a common host of blaOXA-48 (130). Tn6237 variants with different plasmid-derived sequences (9.7 kb or 11.2 kb) and different insertion sites were identified among sequenced isolates. The sequences and insertion sites identified in the UK E. coli ST38 were different than those characterized from Lebanon (118). The Lebanese study included 3 E. coli ST38 isolates with chromosomal insertion of blaOXA-48 and was obtained from hospital patients and the stool of a healthy child. The chromosomal integration of blaOXA-48 into Tn6237 among E. coli ST38 isolates and the subsequent clonal dissemination across different UK sites have contributed to the increase of OXA-48-producing E. coli.
Tn6237 has also been described on an IncC plasmid of 117 kb obtained from K. pneumoniae ST147 (131), suggesting that IS1R-mediated transposition from pOXA-48a-like plasmids played an important role in the mobilization of blaOXA-48 among various plasmid and chromosomal genetic backgrounds.
Clonal dissemination of Enterobacterales with blaOXA-48.
K. pneumoniae, followed by E. coli and E. cloacae, is a common vehicle for blaOXA-48 (129, 132). Potron et al. conducted a molecular epidemiological study characterizing 107 OXA-48-producing Enterobacterales obtained mainly from Europe and North Africa between 2001 and 2011 (129). The blaOXA-48 gene was situated on pOXA-48a-like IncL plasmids among 93% of isolates. Different Tn1999 variants were associated with blaOXA-48 in all isolates, with Tn1999.2 being the most common (79%), followed by Tn1999 (20%) (129). Coproduction of ESBLs (most often CTX-M-15) was observed in 75% of isolates.
Among K. pneumoniae isolates, diverse sequence types have been reported, with global AMR high-risk clones such as ST101, ST395, ST15, and ST147 being the most common (129). The most common STs identified in nationwide surveillances were ST101 in the United Kingdom (30) and ST395 in Poland (95). Regional nosocomial outbreaks of different K. pneumoniae STs have been reported from various countries, including the following: ST11, ST101, and ST405 in Spain (84), ST147 in Germany (131) and Slovenia (99), and ST11 in Croatia (98) and Spain (133). The analysis of the K. pneumoniae ST147 outbreak in Germany showed that blaOXA-48 was harbored on pOXA-48a-like IncL plasmids, while blaCTX-M-15 and other antimicrobial resistance genes were situated within IncR and IncC plasmids (131). Tn1999.2 on pOXA-48a-like plasmids was common among the ST147 isolates from the United Kingdom (134). Colistin-resistant K. pneumoniae ST11 with blaOXA-48 and blaCTX-M-15 caused nosocomial outbreaks in France (135), while pan-drug-resistant K. pneumoniae ST147 and extensively-drug resistant K. pneumoniae ST101 with blaOXA-48 and blaCTX-M-15 caused nosocomial outbreaks in Spain (70).
Among E. coli isolates, diverse STs, including international AMR high-risk clones (e.g., ST38 [130], ST131 [136], ST410 [137], and ST648 [95]), have been associated with OXA-48. E. coli ST38 is characteristic for the chromosomal location of blaOXA-48 within Tn6237 and has been reported from Canada, the Czech Republic, Egypt, Finland, France, Lebanon, Switzerland, and the United Kingdom (118, 121, 129, 130, 134). A single ST38 isolate with blaOXA-48 on a plasmid (pOXA-48a-like) has also been described (129). In the United Kingdom, 16% of OXA-48-producing Enterobacterales referred to the national reference laboratory between 2013 and 2015 were E. coli ST38 with Tn6237 (130). E. coli ST38 isolates were collected across the country, often without clear epidemiological links. E. coli ST38 isolates from the Czech Republic and Lebanon also contained chromosomal insertions of Tn6237 (118, 121). These epidemiological data show that E. coli ST38 with chromosomal Tn6237 harboring blaOXA-48 was responsible for international spread of OXA-48-producing E. coli.
Enterobacter species isolates with OXA-48 were collected by two global surveillance programs between 2008 and 2014 (109). Three global clones (i.e., Enterobacter xiangfangensis ST114, Enterobacter hormaechei subsp. steigerwaltii ST93, and E. cloacae Hoffmann cluster III ST78) from Europe, Africa, the Middle East, and Asia were associated with blaOXA-48 situated within Tn1999.2 (109).
OXA-48-producing Enterobacterales have also been identified in nonhuman sources. For example, E. coli ST38 with Tn1999.2 was isolated from poultry in Lebanon (138). K. pneumoniae ST15 and E. coli ST1431 and ST1196 with Tn1999.2 on pOXA-48a-like plasmids were isolated from dogs from veterinary clinics in Germany (139).
In summary, pOXA-48a-like IncL plasmids with Tn1999.2 and Tn1999 have been the main sources of the current global distribution of blaOXA-48 into multiple Enterobacterales members. Clonal outbreaks caused by specific AMR high-risk clones such as K. pneumoniae STs (e.g., ST101, ST395, ST15, and ST147) with pOXA-48a-like plasmids and E. coli ST38 with chromosomally carried blaOXA-48 within Tn623 also have contributed to the international spread of blaOXA-48 into various hospitals, regions, and countries.
OXA-181
OXA-181 is currently the 2nd most common global OXA-48-like derivative and was first described in Indian hospitals in 2006 to 2007 (140), was followed by reports from France (141), the Netherlands (61), Oman (24), and New Zealand (142) in 2011. The Indian publication was from the Sentry Antimicrobial Surveillance program and described 10 K. pneumoniae isolates and 1 E. cloacae complex isolate with OXA-181 obtained from blood and respiratory specimens from hospitals in Kolkata, New Delhi, and Mumbai (140). The French, Dutch, New Zealand, and Oman studies characterized Citrobacter freundii and K. pneumoniae isolates, resistant to all β-lactams (including the carbapenems), isolated from patients that had been transferred from India or Tanzania to France, the Netherlands, New Zealand, and the Sultanate of Oman. C. freundii STE from France was isolated from urine, was resistant to the aminoglycosides, sulfonamides, tetracycline, tigecycline, nitrofurantoin, and fluoroquinolones, and coproduced NDM-1, VIM-4, CTX-M-15, OXA-1, OXA-9, and TEM-1 (141). blaOXA-181 was situated downstream of the insertion element ISEcp1 harbored within Tn2013 located on an 84-kb mobile IncT-type plasmid (143). K. pneumoniae KP3 from Oman had a resistance profile similar to that of C. freundii STE and coproduced CTX-M-15, OXA-1, and TEM-1, and the blaOXA-181 gene was also incorporated within Tn2013 but located on a 7.6-kb ColE-type plasmid (24).
Geographical Distribution of OXA-181
Over the next 3 years (i.e., 2012 to 2014), OXA-181 was reported for Enterobacterales obtained from the United Kingdom (134), Singapore (144), South Africa (97), Norway (145), Romania (146), Canada (147), and Sri Lanka (148). The majority of these isolates were associated with coproduction of NDM-1 and previous travel to the Indian subcontinent.
Since 2014, the description of OXA-181 has escalated on a global scale, with reports from Asia (e.g., Japan [149], South Korea [150], China [151], Thailand [152], Pakistan [153], and Myanmar [154]), North America (e.g., the United States [155–157] and Canada [158]), Europe (e.g., Denmark [159], Austria [160], Switzerland [161], the Czech Republic [121], France [162], Poland [95], Italy [163], and Spain [164]), Africa (Burkina Faso [165], Egypt [166], Angola [167], Mali [168], Nigeria [169], and São Tomé and Príncipe [170]), the Middle East (e.g., Lebanon [171] and Saudi Arabia [172]), and Oceania (e.g., Australia [173]). Previous travel to India (147), Nigeria (174), and the Middle East (150) was identified as a risk factor of infection with OXA-181-producing bacteria.
Currently, Enterobacterales with blaOXA-181 are endemic in the Indian subcontinent (e.g., India, Pakistan, Bangladesh, and Sri Lanka) and are important causes of nosocomial outbreaks in India (175, 176) and Bangladesh (177). Nosocomial outbreaks have also been described in Angola (167), South Africa (178), São Tomé and Príncipe (170), and the United Arab Emirates (179) (Fig. 7).
FIG 7.
Global distribution of Enterobacterales with OXA-181.
Bacteria Producing OXA-181
OXA-181 was first described for K. pneumoniae, and K. pneumoniae remains the most common global bacterium associated with blaOXA-181 (153, 158, 164, 180). OXA-181 has also been reported among E. coli isolates (151, 155, 158, 167), followed by other members of the Enterobacterales such Morganella morganii (156), Proteus mirabilis (160), Citrobacter freundii (141), and the E. cloacae complex (30). OXA-181 has also been detected in Aeromonas caviae (181) and P. aeruginosa (182).
Enterobacterales with OXA-181 have been obtained from humans and animals (69) and from fresh Swiss vegetables imported from Asia (161).
Molecular Epidemiology of Enterobacterales with blaOXA-181
The current international and interspecies spread of blaOXA-181 among Enterobacterales is mainly driven by the insertion element ISEcp1, which is situated within Tn2013 located on various plasmid backbones.
Genetic environments surrounding blaOXA-181.
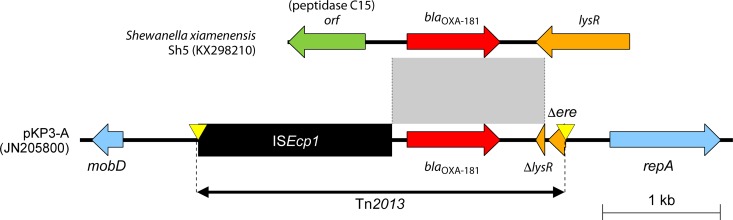
Tn2013 is composed of blaOXA-181, ISEcp1 (located upstream), and ΔlysR-Δere (located downstream) (Fig. 8) (24). ISEcp1 includes promoter sequences near the inverted repeat right (IRR) (at positions −35 and −10), uses one-sided transposition for mobilizing AMR genes, and is often associated with other β-lactamase genes, including blaCTX-M-15 (117). In addition to the original inverted repeat left (IRL) and the IRR of ISEcp1, Tn2013 also contains a second IRR named IRR2 (24). Tn2013 was initially inserted into a noncoding region of ColE2-type plasmid pKP3-A, with a target site duplication of 5 bp (ATATA) (Fig. 8). In vitro experiments showed that Tn2013 can be integrated into the E. coli chromosome (24).
FIG 8.
Similarities between S. xiamenensis chromosome with blaOXA-181 and Tn2013 within the pKP3-A plasmid. Genes from background plasmids are indicated with cyan, and chromosomal genes of Shewanella are indicated with green. Yellow triangles indicate target site duplications.
The origin of blaOXA-181 is most likely the chromosome of S. xiamenensis (Fig. 2) (46). The blaOXA-181 gene and its flanking regions (i.e., a 128-bp fragment downstream of ISEcp1 and a 378-bp fragment upstream of ΔlysR and partial ere) are identical to regions on the chromosome of S. xiamenensis strains S12 and Sh5 (Fig. 8) (46).
Plasmids harboring blaOXA-181.
The OXA-181 gene is harbored on mainly on 4 different plasmid types belonging to the ColE2, IncX3, IncN1, and IncT replicon types. K. pneumoniae KP3 from Oman belonged to ST11 and contained pKP3-A, which is a 7.6-kb ColE2-type (also referred to as ColKP3-type) plasmid harboring Tn2013 with blaOXA-181. The backbone of pKP3-A consisted of the repA replicase and mob mobilization genes (Fig. 9), and this plasmid is not self-transmissible but is mobilizable with the aid of other plasmids (24, 134). Electrotransformation assays have shown that pKP3-A can be introduced into and maintained within P. aeruginosa (24).
FIG 9.
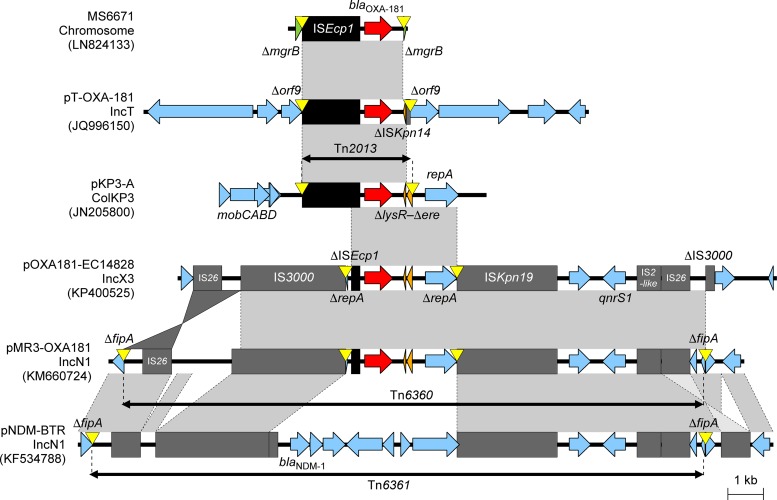
Genetic environments of blaOXA-181 in the K. pneumoniae chromosome, IncT plasmid, Tn2013 (pKP3-A plasmid), IncX3 plasmid, and IncN1 plasmid. Tn6361 with blaNDM-1 in the IncN1 plasmid pNDM-BTR was also shown for comparison with Tn6360 in the IncN1 plasmid pMR3-OXA181. Mobile elements are indicated with black (ISEcp1) or gray (others). Genes from background plasmids are indicated with cyan, and those from the chromosome are indicated with green. Yellow triangles indicate target site duplications.
E. coli ST410 from China contained pOXA181_EC14828, a 51-kb self-transmissible, mobile IncX3 plasmid with blaOXA-181 and qnrS1 (151). IncX plasmids are narrow-host-range Enterobacterales plasmids (183) that are difficult to detect using PCR-based replicon typing schemes (127). IncX3 plasmids are associated with several types of blaNDM genes (184), and the 26-kb backbone of pOXA181_EC14828 was similar to other IncX3 backbones with blaNDM-1 previously described in China (151). The immediate genetic environment of blaOXA-181 (i.e., ΔISEcp1–blaOXA-181–ΔlysR–Δere–ΔrepA) situated within pOXA181_EC14828 is similar to the structure described for pKP3-A (Fig. 9). The repA gene from pKP3-A is interrupted and two parts are located upstream of ISEcp1 and downstream of ΔlysR-Δere, which are flanked by a 5-bp AT-rich duplicate sequence (ATCTT). These events might explain the insertion of an additional ISEcp1 with the subsequent homologous recombination between two copies of ISEcp1 (151). pOXA181_EC14828 contains ISKpn19 and qnrS1 downstream of blaOXA-181 and IS3000 upstream of blaOXA-181 and is flanked by a composite transposon consisting of two copies of the insertion sequence IS26 (Fig. 9). Similar IncX3 plasmids with identical genetic environments surrounding blaOXA-181 have also been described for K. pneumoniae isolates from the Czech Republic (121), Angola (167), and South Africa (185).
The 58-kb conjugative IncN1 plasmid named pMR3-OXA181 with blaOXA-181 was identified in Morganella morganii MRSN 22709 and E. coli ST131 obtained from a U.S. patient with a history of travel to Germany and Afghanistan (Fig. 9) (156). pMR3-OXA181 contained a pKP3-A-like fragment with blaOXA-181, flanked by ISKpn19, IS3000, and two copies of IS26, showing high similarity to pOXA181_EC14828 (151), the IncX3 plasmid obtained in China (Fig. 9). The main difference between plasmids pMR3-OXA181 and pOXA181_EC14828 is situated in the target duplication site that flanks the pKP3-A-like fragment. The pMR3-OXA181-like plasmids, obtained from the different U.S. E. coli ST131 isolates mentioned before, contained a second copy of ISKpn19 that was inserted upstream of Δrep–ΔISEcp1–blaOXA-181 and was flanked by a 9-bp target repeat region (156).
pMR3-OXA181 is also similar to an IncN1 plasmid with blaNDM-1, named pNDM-BTR, obtained from a Chinese E. coli isolate (186). The main difference between these two plasmids occurs in the blaNDM-1 flanking region (i.e., Tn6360 with blaOXA-181 in pMR3-OXA181 and Tn6361 with blaNDM-1 in pNDM-BTR) (Fig. 9). This is most likely due to IS3000-mediated transposition.
C. freundii isolate STE harbored an 84-kb IncT plasmid with a Tn2013 variant, named pT-OXA-181 (Fig. 9). This plasmid is non-self-transmissible but is mobilizable with the help of two other plasmids (143). pT-OXA-181 is highly similar to a 217-kb IncT-type plasmid named Rts1 (187), and a large portion of the IncT plasmid backbone (including the transfer system) is deleted in pT-OXA-181. The Tn2013-like element of pT-OXA-181 lacked IS26, IS3000, the ere gene and IRR2 (24) and contained ISKpn14 (as opposed to ISKpn19, found in pMR3-OXA181 and pOXA181_EC14828) (Fig. 9). The presence of a 5-bp target site duplication (ATTTA) flanking the Tn2013-like element suggested that this mobile genetic element was also acquired through transposition (143).
Chromosomal insertion of blaOXA-181.
The integration of blaOXA-181 into the chromosome of K. pneumoniae ST147 was reported from several U.S. patients that had previously visited India (157), the United Arab Emirates (179, 188), and Pakistan (153). Three copies of a partial Tn2013 element (ISEcp1–blaOXA-181) were described for the isolates obtained from the patient that visited the United Arab Emirates (179, 188). The insertion of one of these copies into mgrB conferred colistin resistance (179). Among K. pneumoniae ST14 isolates from Canada, the chromosomal integration of Tn2013 with blaOXA-181 (or blaOXA-232) occurred in 39% of OXA-48-like isolates, while the remaining isolates harbored pKP3-A-like plasmids (158). In this Canadian study, the most common site of integration was an intergenic region between tRNA and lysR. Tn2013 was also integrated into a different chromosomal position on K. pneumoniae ST43 obtained from Japan (149).
Clonal dissemination of Enterobacterales with blaOXA-181.
The National Microbiology Reference in Canada reported on 35 OXA-181-producing Enterobacterales obtained from 2011 to 2014. The K. pneumoniae isolates belonged to ST14, ST147, and ST1847 and harbored blaOXA-181 on ColE2-type plasmids, whereas the E. coli isolates belonged to ST410, ST940, and ST1284 and harbored blaOXA-181 on IncX3 plasmids, showing clear association between the species and plasmids (158). K. pneumoniae ST14 was the most common clone in this Canadian study. K. pneumoniae ST11 with blaOXA-181 situated in Tn2013 on pKP3-A-like plasmids was described in the United Kingdom (134). K. pneumoniae ST147, an international AMR high-risk clone (11, 184) with chromosomal Tn2013, was responsible for interhospital outbreaks and was obtained from North America (157, 158) and the Middle East (153, 179). In the United States, K. pneumoniae ST34 and ST43 were the most common clones associated with blaOXA-181 (155). A large-scale country-wide outbreak occurred in South Africa from 2014 to 2016 due to the high-risk clone K. pneumoniae ST307 with OXA-181, involving 350 patients across 42 hospitals from 23 cities (185). blaOXA-181 was part of Tn2013 that was housed on a 51-kb IncX3 plasmid identical to pOXA181_EC14828.
Among E. coli isolates, ST410 is the most common high-risk global clone associated with blaOXA-181, usually situated within pOXA181_EC14828-like IncX3 types of plasmids. ST410 has been described in Canada (158), the Czech Republic (121), China (151), France (108), and Poland (95).
In summary, Tn2013, which contains ISEcp1 upstream of blaOXA-181 and harbored on different plasmids belonging to the ColE2, IncX3, IncN1, and IncT replicon types, has been mainly responsible for the global dispersion of OXA-181. Clonal outbreaks (e.g., K. pneumoniae ST147 and ST307 and E. coli ST410) have also contributed to the international spread of blaOXA-48 into various hospitals, regions, and countries.
OXA-232
OXA-232 is currently the 3rd most common global OXA-48-like derivative and was described in 2013 from E. coli (1 isolate) and K. pneumoniae (2 isolates) from rectal swabs obtained from three French patients who had recently visited India (26). One of the patients was hospitalized during the visit to India. The E. coli isolate tested nonsusceptible to all β-lactams (excluding imipenem, ertapenem, and meropenem), fluoroquinolones, aminoglycosides, sulfonamides, and tetracycline, while the K. pneumoniae isolates had similar susceptibility patterns but were also resistant to the carbapenems. The blaOXA-232 gene was situated downstream of ISEcp1 harbored within Tn2013 located on a 6.1-kb ColE-type plasmid (a scenario previously described with blaOXA-181) (26).
This was followed by a publication from a Singapore hospital that reported OXA-232 from eight K. pneumoniae isolates obtained from several types of clinical specimens (189). Interestingly, the blaOXA-232 was also harbored on a 6.1-kb ColE-type plasmid.
Geographical Distribution of OXA-232
In 2014 to 2015, OXA-232-producing K. pneumoniae and E. coli were reported in the United States (190), Malaysia (191), South Korea (192), and Mexico (91). The majority of these reports were associated with the coproduction of NDM-1 and previous travel to the Indian subcontinent.
Since 2015, OXA-232 has been reported from Europe (e.g., Germany [193], the Czech Republic [121], Italy [194], Switzerland [195], Poland [95], and the United Kingdom [30]), Asia (e.g., Brunei [196], China [197], and Thailand [198]), and Africa (e.g., Tunisia [199]).
Published evidence suggests that Enterobacterales with blaOXA-232 are endemic in India, and nosocomial outbreaks have been described in South Korea (192, 200), Mexico (91), the United States (201), Brunei (196), and China (197).
Bacteria Producing OXA-232
OXA-232 was first described for K. pneumoniae and E. coli from humans, and these organisms remain the most common bacteria associated with blaOXA-232 (193, 194, 196, 197).
Molecular Epidemiology of Enterobacterales with blaOXA-232
The blaOXA-232 gene was first identified in 2012 from E. coli ST2968 and two K. pneumoniae ST14 isolates from French patients who had returned from India (26). OXA-232 was associated with ISEcp1 within a truncated Tn2013 that was situated within a ColKP3-type plasmid named pOXA-232.
Genetic environments surrounding blaOXA-232.
The genetic environment surrounding blaOXA-232 is very similar to the environment surrounding blaOXA-181 except that Tn2013 contains a large deletion on the 5′ end of ISEcp1 (26). The deletion which disrupted the ISEcp1 transposase activity most likely stabilized blaOXA-232 onto pOXA-232-like plasmids. Tn2013 is the only reported genetic environment associated with blaOXA-232 to date.
Plasmids harboring blaOXA-232.
The ColE2-type pOXA-232 plasmid is 6,141 bp in length, and its backbone is identical to pKP3-A with blaOXA-181, except for the 5′-end deletion of ISEcp1 within Tn2013 (26). blaOXA-232 differs from blaOXA-181 by one nucleotide substitution (A642T) that leads to one amino acid alteration (Arg214Ser). The pOXA-232 plasmid was nonconjugative but transformative. Almost all isolates with blaOXA-232 harbored pOXA-232-like plasmids (i.e., with plasmids highly similar or identical to pOXA-232) and have been described globally, including in Brunei (196), Canada (158), China (197, 202), France (26), Germany (193), South Korea (192), Italy (203), Singapore (189), Switzerland (195), and the United States (201). A K. pneumoniae ST15 isolate from the Czech Republic harbored a pOXA-232-like plasmid named pOXA-232_30929, which contained a 5,981-bp segment insertion consisting of Tn1000 situated between Tn2013 and repA (121). The similarities of the genes, transposons, and plasmids between blaOXA-181 and blaOXA-232 suggest a common origin and transposition followed by subsequent evolution of OXA-232 from OXA-181 (Fig. 2).
A large (110-kb) nontypeable plasmid containing a truncated Tn2013 that included blaOXA-232 was reported from Poland (95). Detailed analysis and sequencing of this plasmid have not yet been performed.
Chromosomal insertion of blaOXA-232.
A single K. pneumoniae ST14 isolate from Canada that lacked a ColKP3 plasmid showed chromosomal integration of blaOXA-232 (158).
Clonal dissemination of Enterobacterales with blaOXA-232.
Hospital outbreaks due to K. pneumoniae strains with blaOXA-232 on pOXA-232-like plasmids have been reported worldwide and include the following: ST14 in Western Canada (158) and in a burn intensive care unit in South Korea (192), ST15 in an intensive care unit (202) and in a neonatal intensive care unit in China (197), ST16 from three institutions in Italy (203), ST17 in a U.S. hospital (201), and ST231 in two hospitals in Switzerland (195), two hospitals in Brunei (196), and two hospitals in Singapore (189). K. pneumoniae ST14 and ST16 were the most common U.S. STs associated with blaOXA-232 obtained from the United States (155). The majority of K. pneumoniae isolates with blaOXA-232 coproduced CTX-M-15 (26, 121, 155, 189, 192, 195–197, 201–203), and NDM-1 (26, 121, 155, 190, 193, 203) or contained the rmtF 16S rRNA methyltransferase (195, 201, 202) situated on different plasmids than those that contained the blaOXA-232. K. pneumoniae ST15 responsible for a NICU outbreak in China also contained a hypervirulent plasmid (i.e., pLVPK-like IncHI1B/IncFIB plasmid) containing different virulence-associated genes, including rmpA2 (202).
Sporadic global appearances of E. coli with blaOXA-232 on pOXA-232-like plasmids have been reported, and the isolates belonged to the following clones: ST131, ST167, ST448, ST457, ST2003, and ST2968 (26, 112, 158, 190, 193).
OXA-204
OXA-204 is a rarely reported OXA-48-like carbapenemase and was first described in 2013 when a K. pneumoniae strain with reduced susceptibility to ertapenem was isolated from a urine of a Tunisian patient (25). The isolate, K. pneumoniae 204, also tested nonsusceptible to the penicillins, the β-lactamase inhibitor combinations, broad-spectrum cephalosporins, aminoglycosides, fluoroquinolones, sulfonamides, and tetracycline but remained susceptible to meropenem and imipenem. K. pneumoniae 204 coproduced SHV-1, CTX-M-14, OXA-1, and CMY-4. The blaOXA-232 gene was situated downstream of ISEcp1 harbored within Tn2016 located on a 150-kb IncA/C-type plasmid that also contained blaCMY-4 (25). Subsequently OXA-204 has also been found in E. coli obtained in Tunisia (204).
Geographical Distribution of OXA-204
Enterobacterales with OXA-204 outside Tunisia have been described only in France, where it is often associated with the coproduction of CMY-4, Tn2016, and previous travel to Tunisia (87, 108, 115).
Bacteria Producing OXA-204
OXA-204 was first described for K. pneumoniae and E. coli from humans, and these organisms remain the most common bacteria associated with blaOXA-204 (25, 204). This carbapenemase has also been found in P. mirabilis, C. freundii, and S. marcescens (87).
Molecular Epidemiology of Enterobacterales with blaOXA-204
The blaOXA-204 gene was first reported in 2012 from a K. pneumoniae ST383 isolate from a Tunisian patient (25). It was associated with Tn2016 that was located on a 150-kb A/C-type plasmid named p204-B.
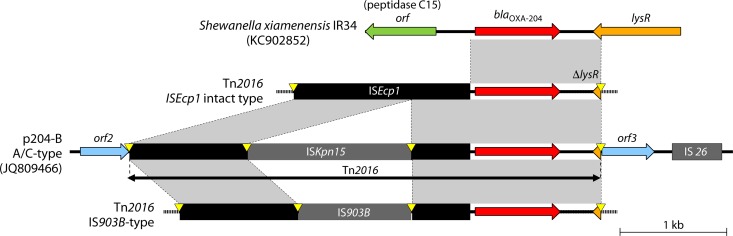
Genetic environments surrounding blaOXA-204.
The genetic environment of the 4,420-bp transposon Tn2016 is similar to that of Tn2013 associated with blaOXA-181, but it differs in that ISKpn15 was inserted within ISEcp1 upstream of blaOXA-204 and Tn2013 and does not contain the Δere downstream of blaOXA-204 (Fig. 10) (25). Interestingly, the segment separating blaOXA-204 from the ISEcp1 element was 46 bp in length (as opposed to 128 bp for blaOXA-181), suggesting that blaOXA-204 and blaOXA-181 had been mobilized independently through two distinct transposition processes from their natural progenitor (Fig. 2). In addition to the original IRL and IRR of ISEcp1, Tn2016 contains IRR2, which is responsible for one-sided transposition. Tn2016 was inserted into the IncA/C-type p204-B plasmid within a target site duplication of 5 bp (AAATA) (Fig. 10). ISKpn15 did not disrupt promoter sequences of ISEcp1, and it is likely involved in the expression of blaOXA-204 (25). However, the ISEcp1 transposase was disrupted by ISKpn15, and this may have stabilized blaOXA-204 onto p204-B-like A/C plasmids (25). ISKpn15 is flanked by the 8-bp target site duplication, which indicates a transposition event that occurred after mobilization of Tn2013-like ISEcp1–blaOXA-204–ΔlysR element onto the p204-B plasmid. This functional genetic structure has also been described for OXA-204-producing E. coli, K. pneumoniae, and P. mirabilis from France (87) and Tunisia (204). A Tn2016 variant that contained IS903B instead of ISKpn15 was reported from an E. coli ST617 isolate in Tunisia (Fig. 10). IS903B also did not disrupt promoter sequences of ISEcp1.
FIG 10.
Genetic environments of blaOXA-204 in the S. xiamenensis chromosome, Tn2016 (p204-B plasmid), and Tn2016 variants. Only partial sequences of Tn2016 ISEcp1 intact type (pKP49) and Tn2016 IS903B type (pEC25) were available (GenBank accession numbers KP027886 and KP027885, respectively), but the whole structures of the transposons are drawn. Mobile elements are indicated with black (ISEcp1) or gray (others). Genes from background plasmids are indicated with cyan, and those from the chromosome are indicated with green. Yellow triangles indicate target site duplications.
The origin of blaOXA-204 is most likely the chromosome of S. xiamenensis (Fig. 2) (45). The blaOXA-204 gene on the chromosome of S. xiamenensis strain IR34 was flanked by a gene encoding peptidase C15 and lysR, a transcriptional regulator (45) (Fig. 10).
Plasmids harboring blaOXA-204.
K. pneumoniae ST383 strain 204 contained a 150-kb A/C-type plasmid named p204-B, which contained Tn2016 (40). A/C-type plasmids (formerly called IncA/C) can be divided into IncA (A/C1), IncA (A/C2), and IncC plasmids (205). IncC plasmids have two antimicrobial resistance islands (ARI-A and ARI-B) which harbor various antimicrobial resistance genes (205). IncC type 1 plasmids frequently have the ISEcp1-blaCMY element inserted downstream of traA (205). The exact plasmid type of p204-B is unknown because the complete sequence has not yet been reported. The p204-B plasmid also contains blaCMY-4 (40). A/C-type plasmids are broad-range plasmids (123), and this was confirmed with p204-B by electrotransformation using A. baumannii and transconjugation using E. coli and P. aeruginosa (40). K. pneumoniae 204 also contained an approximately 70-kb IncL/M-type conjugative plasmid, named p204-A, that harbors blaCTX-M-14 (40).
Studies from France and Tunisia have shown the predominance of p204-B-like A/C-type plasmids with Tn2016 or variants that contain blaCMY-4 (87, 206) among OXA-204-producing Enterobacterales, including K. pneumoniae, E. coli, C. freundii, P. mirabilis, and S. marcescens (87). The Tn2016 variant containing the intact ISEcp1 is associated with the functional transposon, suggesting that blaOXA-204 can disperse among various bacterial species.
A K. pneumoniae ST11 isolate and an E. coli ST617 isolate from Tunisia harbored 2 nontypeable plasmids containing blaOXA-204; one plasmid was approximately 180 kb, while the other plasmid was approximately 150 kb (204).
Clonal dissemination of Enterobacterales with blaOXA-204.
An endoscopy-related outbreak involving 10 health institutions occurred in France between 2012 and 2014 (87) that was due to E. coli ST90 and K. pneumoniae ST147 harboring p205-B-like A/C plasmids with blaCMY-4 and blaCTX-M-15 (87). K. pneumoniae ST147 and ST11 with blaOXA-204 on A/C plasmids have also been detected in Tunisia (204, 206).
OXA-162
OXA-162 is a rarely reported OXA-48-like carbapenemase and was first described for a K. pneumoniae isolate from Turkey in 2008 (23). This was followed by a German report in 2012 when carbapenem-nonsusceptible Enterobacterales with OXA-162 (including E. coli, Raoultella ornithinolytica, and C. freundii) were recovered in 2008 to 2009 from different patients without recent travel history (207). The blaOXA-162 gene was situated downstream of IS1999 located on a 60-kb IncL/M-type plasmid (similar to blaOXA-48). R. ornithinolytica and C. freundii coproduced SHV-5.
Geographical Distribution of OXA-162
Since 2012, OXA-162 has been reported in K. pneumoniae from Turkey (23), Hungary (208), and Greece (209). The Hungarian isolates coproduced CTX-M-15, and the Greek isolates coproduced DHA-1.
Bacteria Producing OXA-162
OXA-162 has been described in different Enterobacterales, including K. pneumoniae, E. coli, R. ornithinolytica, and C. freundii (207, 209).
Molecular Epidemiology of Enterobacterales with blaOXA-162
The blaOXA-162 gene was first identified in a K. pneumoniae isolate in Turkey in 2008 (23). Compared with blaOXA-48, blaOXA-162 has only one nucleotide substitution (A637G), resulting in one amino acid alteration (Thr213Ala). The blaOXA-162 was situated in Tn1999.2 (Tn1999 variant with disruption of IS1999 by IS1R) (23, 207) and was harbored on a 60-kb pOXA-48a-like IncL conjugative plasmid (207–209). Tn1999.2 with blaOXA-162 was identical to Tn1999.2 with blaOXA-48, except one nucleotide difference in blaOXA-162, suggesting that OXA-162 originated from OXA-48 through a point mutation (Fig. 2) (23, 207).
The following Enterobacterales with blaOXA-162 have been reported: K. pneumoniae ST15 isolates from two patients in a pediatric intensive care unit in Hungary (208), K. pneumoniae ST11 from a patient in Greece (209), K. pneumoniae (ST unknown) from a patient in Turkey (23), E. coli, R. ornithinolytica, and C. freundii from a patient in Germany (207). Identical pOXA-48a-like plasmids with blaOXA-162 were identified among three German Enterobacterales species obtained from the same patient, indicating that horizontal transfer had occurred in vivo (207).
OXA-244
OXA-244 is a rarely reported OXA-48-like carbapenemase and was first described in 2011 from a K. pneumoniae isolate from ascitic fluid from a patient in Malaga, Spain (27). The blaOXA-244 gene was located on a 60-kb IncL type plasmid, and the K. pneumoniae isolate coproduced CTX-M-15.
Geographical Distribution of OXA-244
Subsequently, OXA-244 has been described in Germany (210), Russia (41), France (211), the Netherlands (212), and the United Kingdom (30). A French report described OXA-244-producing E. coli from a patient with epidemiological links to Egypt (42), while Dutch travelers returning from Indonesia were colonized with OXA-244-producing E. coli (212).
Bacteria Producing OXA-244
OXA-244 has been described for different Enterobacterales species, including K. pneumoniae, E. coli, and K. aerogenes isolates (27, 30, 41).
Molecular Epidemiology of Enterobacterales with blaOXA-244
The blaOXA-244 gene was first identified in a K. pneumoniae ST392 isolate from Spain (27). Compared with blaOXA-48, blaOXA-244 contains a single nucleotide substitution (A640G) that leads to one amino acid alteration (Arg214Gly) (27). The blaOXA-244 gene is also situated within Tn1999 on a ca. 60-kb IncL plasmid, suggesting that OXA-244 originated from OXA-48 through a point mutation (Fig. 2). A Russian study described P. mirabilis and K. aerogenes isolates with blaOXA-244 on identical 60-kb IncL plasmids, suggesting that horizontal transfer of the conjugative plasmids had occurred (41).
Tn51098 is a 21.9-kb IS1R-based composite transposon that includes an inverted ΔTn1999.2 with blaOXA-244 and a plasmid-derived fragment of pOXA-48a (stretching from Δtir-pemI to korC-orf25), which is 99.9% identical to Tn6237 with blaOXA-48 (Fig. 11). Tn51098 was described in 4 different E. coli clones (namely, ST38, ST361, ST1722, and ST3541) obtained from French patients with epidemiological links to Egypt. Tn51098 was integrated into the same chromosomal position among the different STs (42, 211). Genetic analysis revealed that the genes upstream of Tn51098 was identical to those of Tn6237 containing blaOXA-48 previously described in the United Kingdom among epidemic E. coli ST38 cluster 2 isolates (130) (Fig. 11). E. coli ST38 with blaOXA-244 was also found in fecal samples of two Dutch travelers that had returned from Indonesia (212) as well as ST38 obtained in Canada (158). The association of Tn51098 and Tn6237 with E. coli ST38 suggests that the evolution of blaOXA-48 to blaOXA-244 through a point mutation most likely occurred after the mobilization of Tn6237 onto the E. coli ST38 chromosome.
FIG 11.
Genetic environments of blaOXA-244 in Tn51098 (E. coli chromosome) and blaOXA-48 in Tn6237 (E. coli chromosome). Mobile elements are indicated with black (IS1999) or gray (others). The upstream regions of Tn51098 and Tn6237 in isolate 2 are identical, whereas the downstream regions are different. The Tn6237 flanking regions in isolate 2 and EC15 are different. Genes from background plasmids are indicated with cyan, and those from the chromosome are indicated with green. Yellow triangles indicate target site duplications.
A community fecal surveillance study for ESBL-producing E. coli in Germany detected an isolate with blaOXA-244 (210). The UK national AMR surveillance program detected blaOXA-244 among 10 out of 114 E. coli isolates with OXA-48-like enzymes (30).
OTHER OXA-48-LIKE CARBAPENEMASES (e.g., OXA-245, OXA-436, OXA-484, AND OXA-519)
OXA-245 was first reported in 2011 from a K. pneumoniae isolate obtained from a surveillance culture in Malaga, Spain (27). OXA-245 differs from OXA-48 by a single amino acid substitution: Glu125Tyr. Similar to the OXA-244 gene, blaOXA-245 was located upstream of IS1999 situated on a 60-kb IncL/M-type plasmid (27). In Spain, OXA-245 has been associated with K. pneumoniae ST11 (213) and the coproduction of CTX-M-15 (84). OXA-245 has also been reported from the United Kingdom (30).
OXA-436 was first reported in 2017 from Enterobacter asburiae, C. freundii, and K. pneumoniae obtained from 4 Danish patients without recent travel history (214). The blaOXA-436 gene was harbored on a 314-kb IncHI2 plasmid. Shewanella bicestrii sp. nov. has been identified as the most likely the progenitor of OXA-436 (29).
K. pneumoniae isolates with OXA-484 and OXA-519 were, respectively, obtained from the United Kingdom (30) and Belgium (31). OXA-519 differed from OXA-48 by a Val120Leu substitution and was located within Tn1999 harbored on a 60.7-kb IncL plasmid.
LABORATORY DETECTION OF ENTEROBACTERALES WITH OXA-48-LIKE CARBAPENEMASES
The detection of carbapenemase-producing Enterobacterales (CPE) normally consists of a two-step approach, namely, a screening process using the appropriate screening agents followed by a phenotypic or genotypic confirmation test to detect the presence of a carbapenemase. Enterobacterales with OXA-48-like enzymes can test susceptible to the carbapenems and the extended-spectrum cephalosporins (e.g., 3rd- and 4th-generation cephalosporins). Enterobacterales with OXA-48-like enzymes often coproduce ESBLs of the CTX-M types that cause resistance to the extended-spectrum cephalosporins and increase the MICs to carbapenems (20). Clinical laboratories are more likely to identify and report Enterobacterales coproducing OXA-48-like and CTX-M enzymes, while the detection of those isolates with only blaOXA-48-like genes remains challenging. It is important to remember that the majority of diagnostic tests are evaluated against bacteria producing OXA-48 and OXA-181 (to a lesser extent) and often do not include other OXA-48-like enzymes. This might be one of the reasons why OXA-48 enzymes are more commonly reported than other OXA-48-like enzymes in the published literature. The laboratory detection of bacteria with different OXA-48-like enzymes is problematic for some clinical laboratories, especially those in regions of nonendemicity.
Screening Agents
CPE in general test nonsusceptible to carbapenems, and this approach still remains a simple initial screen for the presence of carbapenemases among Enterobacterales. However, the OXA-48-like producers often show only slight increases in the carbapenem MICs (215, 216), which makes the strategy of considering nonsusceptibility to carbapenems more problematic. The lowering of the carbapenem breakpoints did increase the sensitivity for the detection CPE but decreased the specificity because the production of ESBLs or AmpC enzymes combined with decreased permeability is also responsible for nonsusceptibility to the carbapenems. Thus, screening criteria using other antimicrobial agents (such as temocillin) have been studied (see below for details). Temocillin, as a treatment option, is not currently available in the United States.
The Clinical and Laboratory Standards Institute (CLSI) in 2010 revised the carbapenem breakpoints. For epidemiological or infection control purposes, isolates that test nonsusceptible to one or more carbapenems should be tested for the presence of carbapenemases (217). The European Committee on Antimicrobial Susceptibility Testing (EUCAST) uses different carbapenem screening breakpoints for carbapenemases than for clinical purposes. The latest EUCAST guidelines (breakpoint tables version 9.0, 2019 [218], and guidelines on detection of resistance mechanisms version 2.0, 2017 [219]) state the following: Enterobacterales with meropenem MICs of >0.125 μg/ml or disk diameter <28 mm should be tested for carbapenemase production. Isolates with meropenem zones sizes between 25 and 27 mm should be tested for carbapenemase production if they are coresistant to piperacillin-tazobactam and/or temocillin (218). The disk diameter of <25 mm was stated in the previous version of the EUCAST guidelines (220), but this criterion was not able to detect 25% of OXA-48 producers in Europe (216). The combination of the disk zone diameter cutoffs for piperacillin-tazobactam (≤16 mm) and temocillin (≤12 mm) with meropenem zones of 25 to 27 mm showed excellent negative predictive value (99.2%) for the detection of OXA-48-like carbapenemases (216). A Canadian study investigated 189 CPE, including 30 OXA-48-like producers, to determine the best MIC screening criteria (221). That study, using meropenem (i.e., >1 μg/ml) as a screening agent, was able to capture 86% of carbapenemase-producing isolates, including only 40% of OXA-48-like producers, whereas application of EUCAST recommendations detected 98.4% of CPE isolates, including 93% of OXA-48 producers (221).
Meropenem, as a single screening agent, provides the best balance between sensitivity and specificity for the detection of OXA-48-like carbapenemases (215, 219, 222, 223). Ertapenem has a high sensitivity but lacks specificity (215, 217, 219, 222–224), while imipenem cannot reliably distinguish between wild-type isolates and carbapenemase producers in species such as Proteus spp., Providencia spp., and M. morganii (215). The EUCAST guidelines no longer support imipenem as a sole screening agent (219). Faropenem is an oral penem antibiotic, and a UK study evaluated its use as a potential screening agent for CPE (224). Growth up to the edge of a 10-μg faropenem disk showed 99% sensitivity and 94% specificity for the detection of CPE, including 90% of OXA-48-like producers (n = 21) that were flagged as possible CPE (224). French investigators published algorithms with faropenem and temocillin disks that showed mixed results for detecting OXA-48-like CPE (225, 226). Further evaluation of faropenem as a screening agent that includes a large number of OXA-48-like carbapenemases is needed.
The most challenging aspect for the successful screening of CPE with OXA-48-like enzymes is the difference in results obtained with automated susceptibility platforms. A multicenter Nordic study found significant variations in carbapenem MICs among CPE that included OXA-48 and OXA-181 producers (227), while a UK study showed that susceptibility profiles generated with BD Phoenix, MicroScan, and Vitek 2 were poor at detecting CPE with OXA-48-like enzymes (228). Currently, BD Phoenix CPO Detect panels are available for clinical use, including integrated phenotypic tests for the detection of carbapenemase (229).
Phenotypic Confirmation Methods
Phenotypic methods for the specific detection of carbapenemase activity will be able (in theory at least) to identify all types of OXA-48-like derivatives. The characteristics of these tests are summarized in Table 3. For the detection of carbapenemase activity specifically for infection and prevention or surveillance purposes, the Carba NP and modified carbapenem inactivation method (mCIM) tests are endorsed by the CLSI for use in clinical laboratories (for details, please see “Carba NP test” and “Carbapenem inactivation method” below). Other methods, including improved Carba NP or CIM-based methods, spectrophotometry, electrochemical assays, and matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS)-based methods, are also available. For the rapid, easy, and specific detection of CPE with OXA-48-like enzymes, the lateral flow immunoassay methodologies are promising. The specific details of these methods are described below.
TABLE 3.
Characteristics of phenotypic tests for carbapenemase detectiona
| Test method | Specific detection of OXA-48 | Sensitivity/specificity (%) | Sensitivity for OXA-48 (%) | Com | Special equipment | Interpretation | Exper | TAT | Cost (U.S. dollars) | Ref |
|---|---|---|---|---|---|---|---|---|---|---|
| Inhibitor-based, MASTDISCS Combi Carba plus | Indirectly | 100/91.1 | 100 | Yes | No | Disk zone diam | Low | 18–24 h | 234 | |
| Carba NP test, RAPIDEC CARBA NP | No | 97.8–99/98.5–100 | 93–96.3 | Yes | No | Color change | Low | 2.5 h | $10 | 244 |
| Colorimetric, β-CARBA test | No | 85.1/92.7 | 80.5b | Yes | No | Color change | Low | 35 minb | 258 | |
| mCIM, CLSI | No | 97/99 | 100 | No | No | Disk zone diam | Low | 22 h | $1 | 244 |
| rCIM | No | 99/100 | 97.4 | No | No | Disk zone diam | Low | 2.5 h | $0.25 | 260 |
| Lateral flow immunoassay, O.O.K. K-SeT | Yesc | OXA-48 and KPC only | 100 | Yes | No | Visible lines | Low | 20 min | $11 | |
| Lateral flow immunoassay, NG-Test CARBA5 | Yes | 97.3/99.8 | 100 | Yes | No | Visible lines | Low | 20 min | 268 | |
| Electrochemical assay, BYG Carba v2.0 | No | 96.3/99.7 | 97.2 | No | Homemade potentiostat | Conductance signal with cutoff value | High | 30 min | $2.5 | 270 |
| Spectrophotometry | No | 100/98.5 | 100 | No | UV spectrophotometer | Absorbance slope with cutoff value | High | 12–24 h | $2–3 | 271 |
| MALDI-TOF MS, MBT STAR-Carba IVD | No | 100/98.2 | 100 | Yes | MALDI-TOF MS (Bruker) | Automatic interpretation by software | Low | 55 min | ? | 280 |
Com, commercially available; Exper, expertise needed; TAT, turnaround time; Ref, reference.
A higher sensitivity for OXA-48 producers was reported when an extended incubation time (60 min) was used.
OXA-48 groups and OXA-163 groups can be separately detected.
Inhibitor-based synergy tests.
Inhibitor-based synergy tests are based on the ability of certain substrates to inhibit the action of carbapenemases (230). This process involves the testing of a carbapenem with and without the addition of an inhibitor with the ability to inhibit different types of carbapenemase. The inhibitor-based methods often use disk susceptibility testing principles (e.g., the double-disk synergy test [DDST] and combined disk test [CDT]). The interpretation is subjective with the DDST but is standardized with the CDT (e.g., zone diameter difference of x mm is indicative of a positive test) (12). The main disadvantage with these methods is the long turnaround time, between 18 and 24 h. The inhibitors most often used include boronic acid derivatives for inhibition of KPCs and metal chelators such as EDTA and dipicolinic acid for inhibition of metallo-β-lactamases (MBLs) (230). They are often used in combination with AmpC inhibitors, such as cloxacillin, to distinguish carbapenemases from isolates with porin mutations that produce high levels of AmpC enzymes.
Chemical compounds with specific inhibition properties for OXA-48-like enzymes are not yet available for clinical use. OXA-48-like enzymes confer high-level resistance to temocillin (216). Temocillin resistance is not specific for OXA-48-type carbapenemases, as other resistance mechanisms, including certain non-OXA-48-like carbapenemases, ESBLs, and AmpCs combined with porin loss, can also cause resistance to temocillin (231, 232). When combined with negative results obtained with inhibitor-based tests for carbapenemases, temocillin resistance has the ability to predict the presence of OXA-48-like enzymes (219, 232). The EUCAST guidelines advocate that in the absence of synergy for KPC, AmpC, and MBLs, high-level temocillin resistance (>128 μg/ml or disk zone diameter of <11 mm) is suggestive for the presence of OXA-48-like enzymes (219). However, EUCAST recommended that the presence of OXA-48-like enzymes be confirmed with other methods (219). More complicated algorithms for routine workflow that aimed for a higher sensitivity and specificity for detection different types of carbapenemases (including OXA-48-like enzymes) have been published (223, 233). Maurer et al. recently proposed an extensive algorithm that includes synergy tests using disks containing cloxacillin supplemented agar with boronic acid, EDTA, and temocillin, which resulted in 100% sensitivity and specificity for detecting CPE, including OXA-48 (other OXA-48 variants were not included for evaluation) (223). The authors do recommend that molecular testing be performed for confirmation of OXA-48-like enzymes.
Commercial disk kits that use inhibitors combined with temocillin are available from 3 companies: the MASTDISCS Combi Carba plus (Mast Group Ltd., Merseyside, UK), the KPC/MBL and OXA-48 Confirm kit (Rosco Diagnostica, Taastrup, Denmark), and the KPC&MBL&OXA-48 disk kit (Liofilchem, Roseto degli Abruzzi, Italy). All of them were based on the EUCAST recommendation (220) and contained 5 disks with carbapenem, carbapenem with MBL inhibitor, carbapenem with AmpC inhibitor, carbapenem with KPC inhibitor, and temocillin. The combinations of carbapenems and inhibitors differ among the different methodologies. MASTDISCS Combi Carba plus was reported to have 100% sensitivity and 91.1% specificity for CPE, including 5 OXA-48 and 1 OXA-181/NDM-1 producers (234). The other kits have not been extensively evaluated, but promising results have been published (235).
Avibactam is a recently developed non β-lactam β-lactamase inhibitor that inhibits class A, C, and selected D β-lactamases (including OXA-48-like enzymes) (236, 237). Huang et al. evaluated an avibactam-supplemented combination disk test approach with a zone diameter difference between temocillin and temocillin-avibactam disks of ≥5 mm for the detection of OXA-48 producers. When this approach was combined with inhibitors specifically for KPCs and MBLs, it yielded a 97% sensitivity and an 84% specificity for the detection of OXA-48 producers (238).
Greek investigators designed an OXA-48 disk verification method that incorporated imipenem disks saturated with EDTA and EDTA with phenylboronic acid (PBA) (239). The evaluation of the test is based on the contortion of zone sizes, and the test is very problematic to read.
Carba NP test.
The Carba NP (Nordmann and Poirel) method was initially reported by French investigators in 2012 (240). Since 2015, the CLSI has advocated the use of a modified version of this methodology for the routine verification of carbapenemases among Enterobacterales (217). The procedure incorporated an incubation process of a microbe within a solution containing imipenem, zinc sulfate, and phenol red, and carbapenemase activity is shown by a color change from red to orange or yellow due to imipenem breakdown products. The original publication by Nordmann and Poirel documented 100% sensitivity and specificity for identifying different carbapenemases, including Enterobacterales with OXA-48-like enzymes. The authors advocated incubating the lysate for at least 2 h to ensure ample time for the detection of OXA-48-like β-lactamases (241). The Carba NP test has been ratified with bacteria obtained from blood agar, Mueller-Hinton agar, and Trypticase soy agar plates (241).
Enterobacterales with OXA-48-like β-lactamases can test false negative with the Carba-NP test, and a sensitivity of 21% has been documented by investigators from Canada (242). The sensitivity of the methodology can be enhanced by enlarging the inoculum of mucoid K. pneumoniae (242). Different shades of orange obtained with OXA-48-like microbes make the Carba NP test hard to interpret. In 2015, the CLSI published a modified version and recommended specific bacterial controls for routine clinical use (217). The CLSI approach showed high sensitivities and specificities, and the majority of results were available within 15 min, including for microbes with OXA-48-like enzymes (243). Commercial applications such as RAPIDEC CARBA NP (bioMérieux) and Neo-Rapid CARB (Rosco Diagnostica) have been available and evaluated by several different investigators (243–245). Overall, the commercial versions show results similar to those with the original and CLSI-modified versions. The detection of OXA-48-like bacteria using the Carba NP or Rapidec Carba NP can be significantly improved by using 10-μl bacterial loops as opposed to 1-μl loops.
Further modifications to improve interpretation of color change, accuracy, or procedures have been reported. The Blue-Carba test is a modification of the Carba NP test developed in Portugal by Peixe and colleagues (246). This method uses bromothymol blue as the indicator and imipenem and cilastatin as the antibiotic and substrate, and it can be performed directly on bacterial colonies (246). The performance of the Blue-Carba test is similar to that of the Carba NP test. Sensitivities for CPE with KPCs and MBLs were close to 100%, but this modification has the same issues as the Carba NP test regarding mucoid K. pneumoniae and false negatives for OXA-48-like CPE (the sensitivities range from 89 to 100%) (247–249). A commercial version has been recently launched as the Rapid Carb Blue kit (Rosco Diagnostica) (250). The Carba NP test direct (251) can be performed directly on bacterial cultures, as opposed to bacterial extracts, with an enhanced sensitivity for the detection of OXA-48-like enzymes from 83% to 94% (251). The modified paper strip Carba NP method uses filter paper strips with the Carba NP solutions and bacterial cultures (252). The results can be obtained within 5 min, and the strip method had higher overall sensitivity than the CLSI method (86% versus 75%) for the detection of CPEs (252).
Colorimetric test.
The β-CARBA test (Bio-Rad, Marne la Coquette, France) is a commercially available, easy-to-use, and rapid chromogenic test (253). This test is based on the change of color of an undisclosed chromogenic substrate in the presence of carbapenem-hydrolyzing enzymes (254). A single colony is suspended in a reaction mixture and incubated at room temperature for 30 min. A color change from yellow to orange-red or purple is indicative of the presence of carbapenem-hydrolyzing activity. A validation study found that overall sensitivity and specificity of the β-CARBA test for the detection of CPEs (e.g., 85.1% and 92.7%, respectively) were lower than with the Carba NP test (254). OXA-48-like enzymes were detected in only 80.5% of isolates (254). The test gave false-positive results for OXA-163 and OXA-405 producers (254). The extended incubation of 1 h allows the additional detection of some OXA-48 variants (253).
Carbapenem inactivation method.
The carbapenem inactivation method (CIM) was described in 2015 and consists of the initial incubation for 2 h of the isolate in water that contains a meropenem disk (255). The meropenem disk is then plated on an agar plate inoculated with a susceptible E. coli indicator bacterium and incubated for at least 8 h. If a carbapenemase is present, the activity of the meropenem disk will be inactivated, allowing uninhibited growth of the susceptible E. coli. The advantages of CIM included cost-effectiveness, ease of performance, and the availability of reagents in most laboratories. Tijet et al. evaluated the CIM and reported a sensitivity of 98.8% and specificity of 100% for the detection of CPE that also included OXA-48-like-, NDM-, and KPC-producing isolates that previously tested negative with the Carba NP test (256). However, some groups reported lower detection rates for OXA-48-like enzymes (50 to 91%) (257).
The mCIM, which uses tryptic soy broth for incubation and a longer incubation time (4 h), was published by the CLSI (257). The overall sensitivity increased from 82% to 93%, and a validation study involving 9 laboratories confirmed high reproducibility, with a mean sensitivity and specificity of 97% and 99%, respectively (257). A recent evaluation by Tamma et al. of 11 phenotypic assays, including the mCIM, found 100% sensitivity of the mCIM for the detection of OXA-48-like-producing CPE, with 98% sensitivity and 99% specificity for all CPE (258). The interpretation of mCIM results is less subjective than that of the Carba NP test. The mCIM was added to the CLSI M100 document in the 2018 edition (217) as a suitable carbapenemase detection method. However, this test is time-consuming, since the best results are obtained when agar plates are incubated for 12 to 18 h (256). Rapid versions of the CIM have been published recently and include the CIMplus test, which allows for the detection of carbapenemases within 8 h, with an overall sensitivity of 95.7% and specificity of 94.4% for the detection of different carbapenemases, including 38 isolates producing OXA-48-like enzymes (259). Muntean et al. published the rapid carbapenem inactivation method (rCIM), which is rapid (i.e., results are available within 3 h), cost-effective, and accurate for the detection of CPE (i.e., overall sensitivity of 99% and specificity of 100%) (260). Among OXA-48 variants, the rCIM was able to detect isolates with OXA-162, OXA-181, OXA-204, OXA-232, and OXA-372; however, isolates with OXA-244 tested negative (260).
Indirect carbapenemase test.
The MAST indirect carbapenemase test is a paper test device that has been recently developed based on the principles of the indirect carbapenemase test (ICT) (261). The ICT method utilizes a cell permeabilizing agent to release carbapenemase enzymes for hydrolyzing a carbapenem. This will allow a carbapenem-susceptible reporter organism to grow, producing a distorted zone of inhibition. The MAST ICT had 100% sensitivity for the detection of the most common carbapemases but was more subjective to interpret than the mCIM test, showing a specificity of 70.3% (262).
Lateral flow immunoassay.
The immunochromatographic lateral flow assay is based on an antigen-antibody reaction performed on chromatographic paper and is easy to perform with rapid results, usually within 15 min after an extract solution is applied to a test cartridge. The OXA-48 K-SeT assay (Coris Bioconcept, Gembloux, Belgium) was designed to capture two epitopes specific to the OXA-48-like enzyme, including OXA-48, -181, -204, -232, and -244 (263). This assay has been upgraded to a multiplex form (RESIST-3 O.K.N. K-SeT assay; Coris BioConcept) that can simultaneously detect the presence of KPC and NDM enzymes. The assay showed 100% sensitivity and 100% specificity for these carbapenemases (264). The RESIST-4 O.K.N.V. assay, which contains two cassettes (OXA-48-like/KPC and NDM/VIM), is also available (265). Recently, Coris BioConcept developed the RESIST-3 O.O.K. K-SeT assay, which detects OXA-48-like and KPC enzymes. This product was developed to distinguish between OXA-48-like enzymes with high (i.e., OXA-48, -162, -181, and -232) and weak (i.e., OXA-163, -108, -247, -405, and -438) carbapenemase activities. The manufacturer claimed 100% sensitivity and 100% specificity, but the performance has not been validated by other researchers.
The Carba5 assay is another immunochromatographic assay that can detect the most common carbapenemases (including OXA-48, -162, -181, -204, -232, -244, -517, -519, and -535) in one cassette with 100% sensitivity and 95.3% specificity (266). Three isolates with OXA-163 and OXA-405 gave false-positive results. These OXA-48-like enzymes have weak carbapenemase activity (267). A commercial version is available as the NG-Test CARBA5 assay (NG Biotech, Guipry, France) (268). All isolates with KPC, NDM, VIM, and OXA-48-like enzymes could be detected, but Enterobacterales with IMP-13 and IMP-14 were not detected in the validation study (268).
Electrochemical assay.
The BYG Carba test recognizes carbapenem hydrolysis via an electrochemical reaction to show a pH change (269). Results are available within 30 min, and evaluation by different investigators showed sensitivities and specificities of up to 97% and 99%, respectively, for Enterobacterales with OXA-48-like β-lactamases (270).
Spectrophotometry.
UV spectrophotometry with excellent sensitivities and specificities for showing carbapenem hydrolysis among OXA-48-like bacteria was described in 2012 (271). This methodology is not available for clinical diagnostics (44, 272).
Mass spectrometry.
The Bruker MALDI-TOF MS system that incorporates specific software for the detection of carbapenemases within 2 to 3 h has been available in certain microbiology laboratories worldwide (273, 274). Enterobacterales with OXA-48-like enzymes can test negative using this approach (275). Imipenem is the best carbapenem for the detection of bacteria with OXA-48-like β-lactamases (276), and adding ammonium bicarbonate to the test lysate will increase the ability of MALDI-TOF to detect Enterobacterales with OXA-48-like enzymes (277).
Recently, Bruker Daltonik launched a software module, named MBT STAR-BL, for the automatic analysis of drug hydrolysis mass spectra that has been integrated into the MBT Compass software for species identification (278, 279). The software is accompanied by detailed protocols for the manual preparation of reagents. More recently, the MBT STAR-Carba IVD kit (Bruker Daltonik), the first CE-IVD (in vitro diagnostic)-certified mass spectrometric resistance detection assay, was launched and in combination with the MBT STAR-BL showed 100% sensitivity and 98.2% specificity for the detection of CPE, including those with OXA-48 variants of OXA-162, -181, -204, -232, and -244, within a turnaround time of 55 min (280).
Genotypic Methods
Molecular methods are highly sensitive and specific for the detection of OXA-48-like genes among Enterobacterales and are considered to be the best confirmatory methods. The basic strategy for the identification of OXA-48-like enzymes is first to amplify the blaOXA-48-like genes, followed by sequencing of the gene for the identification of the specific subtypes (e.g., OXA-48, OXA-181, etc.) (12). For diagnostic purposes, screening for OXA-48-like genes is sufficient, while subtype identification is more relevant for genomic surveillance programs.
Molecular assays are mainly performed on cultured bacterial isolates, and some assays have also been validated for the identification of carbapenemase genes directly on clinical samples. Several in-house PCR, commercial real-time PCR, and DNA microarray methods are available for routine clinical use and usually aimed to identify different carbapenemases simultaneously. It is important to keep in mind that some blaOXA-48-like genes have significant variation in nucleotide sequences (e.g., OXA-181 variants), leading to false-negative results (281), while certain OXA-48 subtypes without carbapenemase activity (i.e., OXA-163, OXA-247, and OXA-405) can produce false-positive results (282). Disadvantages of molecular methods include the relatively high costs (compared to most phenotypic tests), the ability to only detect carbapenemase genes targeted in the assay (compared to hydrolysis assays), and relatively longer turnaround times (compared to rapid phenotypic tests such as the lateral flow technologies, which have turnaround times of minutes versus hours).
In-house assays.
In-house multiplex PCR assays that can simultaneously detect the most common carbapenemase genes (e.g., blaOXA-48, blaKPC, blaNDM, blaVIM, and blaIMP) are useful and sometimes implemented in clinical laboratories (283). Table 4 shows an up-to-date list of published in-house PCR assays for different carbapenemases. We performed in silico PCR (284) to assess the detection performances of these assays for 32 blaOXA-48-like genes as defined in the BLDB website (37), and the results are shown in Table 4. The most extensive multiplex assay available has the ability to identify 11 different carbapenemase genes in three separate reactions (285).
TABLE 4.
In-house molecular methods for the detection of blaOXA-48-like and other genes
| Test method | blaOXA-48-like genes detecteda | Other genes detected | Reference |
|---|---|---|---|
| Conventional multiplex PCR | All except for blaOXA-54, blaOXA-436, and blaOXA-535 | blaKPC, blaGES, blaNDM, blaVIM, blaIMP, blaSPM, blaBIC, blaAIM, blaGIM, blaSIM, and blaDIM | 285 |
| All except for blaOXA-54, blaOXA-436, and blaOXA-535 | blaKPC, blaNDM, blaVIM, and blaIMP | 283 | |
| Real-time multiplex PCR, melting-curve analysis | All except for blaOXA-54, blaOXA-436, and blaOXA-535 | blaKPC, blaGES, blaNDM, blaVIM, and blaIMP | 286 |
| All except for blaOXA-54, blaOXA-436, and blaOXA-535 | blaKPC, blaGES, blaNDM, blaVIM, blaIMP, and blaOXA-23 | 300 | |
| Real-time multiplex PCR, hydrolysis probe | All except for blaOXA-54, blaOXA-436, blaOXA-535, and blaOXA-538 | blaKPC and blaNDM | 287 |
| All except for blaOXA-54, blaOXA-416, blaOXA-436, and blaOXA-535 | blaKPC, blaNDM, blaVIM, and blaIMP | 288 | |
| All except for blaOXA-54, blaOXA-436, and blaOXA-535 | 16S rRNA (internal control) | 155 | |
| Real-time multiplex PCR, melting-curve analysis, and LunaProbe | All except for blaOXA-416, blaOXA-436, and blaOXA-535b | 289 | |
| LAMP,c visible color change | blaOXA-48, blaOXA-162, blaOXA-163, blaOXA-181, blaOXA-199, blaOXA-204, blaOXA-232, blaOXA-244, blaOXA -245, blaOXA-247, blaOXA-370, blaOXA-405, and blaOXA-438 | blaKPC, blaNDM, blaVIM, and blaIMP-14 | 290 |
| Modular multiplex oligonucleotide ligation-PCR, xTAG probe | blaOXA-48, blaOXA-162, blaOXA-163, blaOXA-181, blaOXA-199, blaOXA-204, blaOXA-232, blaOXA-244, blaOXA-245, and blaOXA-370 | blaKPC, blaGES, blaIMI, blaSEM, blaNDM, blaVIM, blaIMP, ESBLs, AmpCs | 291 |
In silico PCR analysis was performed for the detection of blaOXA-48, blaOXA-48b, blaOXA-54, blaOXA-162, blaOXA-163, blaOXA-181, blaOXA-199, blaOXA-204, blaOXA-232, blaOXA-244, blaOXA-245, blaOXA-247, blaOXA-252, blaOXA-370, blaOXA-405, blaOXA-416, blaOXA-436, blaOXA-438, blaOXA-439, blaOXA-484, blaOXA-505, blaOXA-514, blaOXA-515, blaOXA-517, blaOXA-519, blaOXA-535, blaOXA-538, blaOXA-546, blaOXA-547, and blaOXA-566.
Melting-curve analysis followed by data analysis may detect specific blaOXA-48 variant groups.
LAMP, loop-mediated isothermal amplification.
A conventional PCR method includes the following steps: DNA extraction, PCR amplification, and gel electrophoresis, and it usually take 3 to 6 h to get results. Real-time PCR is superior in terms of specificity and speed, is less labor-intensive, but is more expensive than conventional PCR. Monteiro et al. developed a one-reaction assay for blaOXA-48, blaKPC, blaNDM, blaVIM, blaIMP, and blaGES using melting-curve analysis (286). Probe-based assays for the simultaneous detection of blaOXA-48, blaKPC, and blaNDM (287) as well as for blaOXA-48, blaKPC, blaNDM, blaVIM, and blaIMP (288) have also been reported. A probe-based assay specifically for blaOXA-48 was developed at the U.S. Centers for Disease Control and Prevention (155) and included 2 sets of blaOXA-48 primers/probes combined with a bacterial 16S rRNA gene as an internal control. Peirano et al. recently developed a simple and cost-effective PCR method for the rapid identification of Enterobacterales with OXA-181 (306).
Hemarajata et al. developed a real-time PCR assay with LunaProbe-enhanced high-resolution melt analysis using the LightCycler 480 instrument and software (Roche Molecular Systems, Pleasanton, CA) (289). The assay detected the presence of blaOXA-48-like genes, and subsequent data analysis enabled distinction between blaOXA-48/245, blaOXA-162, blaOXA-244, blaOXA-181/204, and blaOXA-232. The assay is unique for its capability to detect common subtypes without sequencing. However, this approach cannot identify the exact subtypes due to the limited length of probe-binding sequence. For example, the blaOXA-48/245 subgroup also includes blaOXA-199, blaOXA-505, blaOXA-519, blaOXA-546, blaOXA-547, and blaOXA-566, and the blaOXA-181 subgroup also includes blaOXA-484.
Loop-mediated isothermal amplification (LAMP) is a rapid, cost-effective, easy-to-perform alternative nucleic acid amplification methodology that requires only a water bath or heating block to obtain a constant temperature. Results can be interpreted with the naked eye or UV light. Srisrattakarn et al. published a LAMP assay for the detection of blaOXA-48, blaKPC, blaNDM, blaVIM, and blaIMP-14 with a reaction time of 60 min (290).
A novel low-cost molecular method based on the Luminex xTAG assay (Luminex Corp., Austin, TX) that can detect 46 β-lactamases, including blaOXA-48 subtypes, within 5 h has been reported (291). The assay utilizes modular multiplex oligonucleotide ligation-PCR and xTAG-compatible probe-based detection using the MAGPIX Dx system (Luminex) to interpret results.
Commercial assays.
The commercial multiplex PCR assays for the detection of different carbapenemase genes (including OXA-like genes) that are available for clinical laboratories are listed in Table 5. Advantages of commercial assays include federal/national approval as clinical diagnostic tests, quality controlled materials, and ready-to-use formats. These assays are mainly validated for direct detection of carbapenemase genes on clinical samples. Disadvantages include high cost and uncertain detection performance for blaOXA-48 subtypes due to unavailability of targeted oligonucleotide sequences.
TABLE 5.
Commercial molecular assays for the detection of blaOXA-48-like and other genes
| Test method (name) | blaOXA-48-like genes detected | Other genes detected | Reference |
|---|---|---|---|
| Real-time multiplex PCR, molecular beacon probe (Check-Direct CPE; Checkpoints, Wageningen, the Netherlands) | blaOXA-48, blaOXA-162, blaOXA-163, blaOXA-181, blaOXA-204, blaOXA-232, blaOXA-244, blaOXA-245, and blaOXA -370 | blaKPC, blaNDM, and blaVIM (blaKPC/blaNDM signal cannot be differentiated) | 292 |
| Real-time multiplex PCR (EntericBio CPE; Serosep Limited, Limerick, Ireland) | Yes | blaKPC, blaGES, blaNDM, blaVIM, and blaIMP | 293 |
| Real-time multiplex PCR, hydrolysis probe (Amplidiag CarbaR+VRE; Mobidiag Limited, Espoo, Finland) | blaOXA-48, blaOXA-162, blaOXA-181, blaOXA-204, blaOXA-232, and blaOXA-244 | blaKPC, blaNDM, blaVIM, blaIMP, ISAba1-blaOXA-51, blaOXA-23, 24/40, -58, vanA, and vanB | 294 |
| Real-time multiplex PCR, hydrolysis probe (Amplidiag CarbaR+MCR; Mobidiag Limited, Espoo, Finland) | blaOXA-48, blaOXA-162, blaOXA-181, blaOXA-204, blaOXA-232, and blaOXA-244 | blaKPC, blaGES, blaNDM, blaVIM, blaIMP, ISAba1-blaOXA-51, blaOXA-23, blaOXA24/40, blaOXA-58, and mcr | 295 |
|
Conventional PCR, DNA hybridization (AID carbapenemase line probe assay; Autoimmun Diagnostika GmbH, Strassberg, Germany) |
Yes | blaKPC, blaIMI, blaNCM-A, blaNDM, blaVIM, blaIMP, blaSPM, blaBIC, blaAIM, blaGIM, blaSIM, and blaDIM | 223 |
| LAMP, Genie II platform (eazyplex SuperBug CRE; Amplex, Giessen, Germany) | blaOXA-48, blaOXA-162, blaOXA-204, and blaOXA-244; blaOXA-181 and blaOXA-232a | blaKPC, blaNDM, blaVIM, blaCTX-M-1 group, and blaKCTX-M-9 group | 297 |
| Microarray (Check-MDR CT103 XL; Checkpoints, Wageningen, the Netherlands) | blaOXA-48, blaOXA-162, blaOXA-163, blaOXA-181, blaOXA-204, blaOXA-232, blaOXA-244, blaOXA-245, and blaOXA-370 | blaKPC, blaGES, blaNDM, blaVIM, blaIMP, blaSPM, blaGIM, blaOXA-23-like, blaOXA-24-like, blaOXA-58-like genes, ESBLs, and AmpCs | 236 |
These groups were separately detected in different tubes according to the manufacturer. The initial assay could not detect blaOXA-181.
Methodologies such as Check-Direct CPE (Checkpoints, Wageningen, the Netherlands) (292), EntericBio CPE (Serosep Limited, Limerick Ireland) (293), Amplidiag CarbaR+VRE assay (Mobidiag Limited, Espoo, Finland) (294), and Amplidiag CarbaR+MCR (Mobidiag) (295) include PCR reagents and use real-time multiplex PCR instruments (e.g., LightCycler 480 instrument and Applied Biosystems 7500 real-time system [Thermo Fisher Scientific, Waltham, MA]). The AID carbapenemase line probe assay (Autoimmun Diagnostika GmbH, Strassberg, Germany) can detect 13 carbapenemase genes, including blaOXA-48, in a single reaction (296). Line probe assays are tests that use conventional PCR followed by hybridization of labeled PCR products with specific oligonucleotide probes immobilized on a strip. A rapid molecular assay using LAMP has been available as Eazyplex superbug CRE (Amplex Diagnostics, Bahnhof, Germany (297). This assay provides results within 15 min and consists of 8‑microtube test strips containing freeze-dried, ready-to-use reagents for the amplification of 7 resistance genes as well as an internal control. The company claims that blaOXA-48, blaOXA-162, blaOXA-204, blaOXA-244, blaOXA-181, and blaOXA-232 can be detected, but external validation studies have not yet been reported.
The advantage of microarray-based assays is the ability to simultaneously detect a large number of genes. The major limitations are the high cost, turnaround time, and labor associated with the procedure. The Check-MDR CT103 XL (Checkpoints, Wageningen, the Netherlands) kit can simultaneously detect 11 different carbapenemase genes, including blaOXA-48, in approximately 8 h on 16 isolates (298).
Fully automated systems.
The use of fully automated commercial PCR systems is easier to incorporate into clinical laboratories and will reduce workload and turnaround time. Unfortunately, they are often more expensive than in-house methods (Table 6). These include the GeneXpert system (Cepheid, CA), which is an easy-to-use cartridge-based system requiring only sample loading. The initial Xpert Carba-R (or MDRO) kit failed to detect blaOXA-181 (281). Later the Xpert Carba-R kit, version 2, which can detect blaOXA-181 and blaOXA-232, was developed (282). The BD MAX system (Becton, Dickinson) has the commercial ready-to-use BD MAX CPE kit (299). The BD MAX system allows user-developed protocols, and several in-house (300) and commercial (301) assays have been reported.
TABLE 6.
Fully automated systems for the detection of blaOXA-48-like and other genes
| Test method (name) | blaOXA-48-like genes detected | Other genes detected | Clinical sample | Turnaround time | Reference |
|---|---|---|---|---|---|
| Real-time PCR (Xpert Carba-R version 2; Cepheid, CA) | blaOXA-48, blaOXA-162, blaOXA-163, and blaOXA-181, blaOXA-204, blaOXA-232, blaOXA-244, and blaOXA-405 | blaKPC, blaGES, blaNDM, blaVIM, and blaIMP-1 | Rectal swabs | 50 min | 282 |
| Real-time PCR (BD MAX CPEa ; Becton, Dickinson, NJ) | blaOXA-48, blaOXA-162, blaOXA-181, blaOXA-199, and blaOXA-204 | blaKPC and blaNDM | Swabs | 2.5 h | 299 |
| Microarray (Verigene BC-GN; Luminex, IL) | blaOXA-48, blaOXA-23, blaOXA-24, and blaOXA-58 were reported as blaOXA | blaKPC, blaNDM, blaVIM, blaIMP, and genes for identification of 9 Gram-negative bacteria | Blood culture | 2 h | 302 |
Research use only.
The Verigene system (Luminex, IL) is an automated microarray-based gene detection system. The Verigene BC-GN test has the ability to identify Gram-negative bacteria and detects carbapenemase genes, including blaOXA genes, from positive blood culture bottles within 2 h (302). However, the test cannot distinguish blaOXA-48 from other blaOXA genes (e.g., blaOXA-23, blaOXA-24, and blaOXA-58).
Some recent additions on which published data are presently lacking include the following: GenMark’s (Steinhausen, Switzerland) ePlex blood culture identification (BCID) test also includes blaOXA genes but cannot distinguish blaOXA-48 from blaOXA-23. The BioFire FilmArray pneumonia panel (BioFire Diagnostics, Salt Lake City, UT) contains a blaOXA-48-like target, and the Unyvero LRT panel (Curetis USA, Inc., San Diego, CA) contained several blaOXA targets, including blaOXA-48-like. The GenePOC Carba assay was recently released as a point-of-care test for detecting blaKPC, blaNDM, blaVIM, blaOXA-48-like genes, and blaIMP.
Whole-genome sequencing.
Whole-genome sequencing (WGS) based on next-generation sequencing technology can reconstruct bacterial genomes, enabling the detection of all known carbapenemases and their variants. Recent advances in sequencing technologies and data analysis methods have made WGS more cost-effective and less time-consuming than standard sequencing techniques. WGS is becoming the mainstay for molecular epidemiological studies due to its accuracy and ability for comprehensive analysis, including clonal relatedness, plasmid, virulence genes, mobile genetic elements, and phage types (42, 155, 157).
Resistance gene databases, such as ResFinder (303), ARG-ANNOT (304), and CARD (305), are frequently used for the comprehensive detection of AMR genes. ResFinder has been commonly used by researchers and clinical laboratories due to the availability of an easy-to-use web-based interface (https://cge.cbs.dtu.dk/services/ResFinder/). However, for the definitive identification of the blaOXA-48 variants, the reference NCBI database (Bacterial Antimicrobial Resistance Reference Gene Database [https://www.ncbi.nlm.nih.gov/bioproject/PRJNA313047]) should be consulted.
CONCLUSIONS
Antimicrobial resistance has become one of the major global health and development challenges of the 21st century. One of the most pressing current AMR concerns is the international spread of carbapenem-resistant Gram-negative bacteria, especially those that produce carbapenemases. Carbapenemases belong to the Ambler class A (i.e., KPC types), class B (i.e., VIM, IMP, and NDM types), and class D OXA β-lactamases.
The OXA β-lactamases with activity against the carbapenems can be divided into those that are mainly present in A. baumannii (referred to as OXA-23-like, OXA-24-like, OXA-51-like, and OXA-58-like enzymes) and those that have found a niche among the Enterobacterales (referred to as OXA-48-like enzymes). The OXA-48-like enzymes efficiently hydrolyze the amino-, carboxy-, and ureidopenicillins and narrow-spectrum cephalosporins (e.g., cephalothin) and weakly hydrolyze the carbapenems but not the extended-spectrum cephalosporins (e.g., ceftriaxone, ceftazidime, and cefepime).
Global genomic surveillance and clinical studies, as well as several case reports, suggest that Enterobacterales with OXA-48-like carbapenemases are endemic in certain parts of the world and are being introduced on a regular basis into regions of nonendemicity, where they are responsible for nosocomial outbreaks.
OXA-48, OXA-181, OXA-232, OXA-204, OXA-162, and OXA-244, in that order, are the most common enzymes identified among the OXA-48-like carbapenemase group. The characteristics (i.e., geographical distribution, bacteria responsible for their production, and molecular epidemiology) of Enterobacterales with these β-lactamases are summarized in Table 7. OXA-48 is associated with Tn1999 on IncL plasmids and is endemic in North Africa and the Middle East. OXA-181 and OXA-232 are associated with ISEcp1, Tn2013 on IncX3, and ColE2 plasmids and are endemic on the Indian subcontinent (e.g., India, Bangladesh, Pakistan, and Sri Lanka) and certain Sub-Saharan African countries. Overall clonal dissemination plays a minor role, but certain high risk clones (e.g., K. pneumoniae ST147, ST307, ST15, and ST14 and E. coli ST38 and ST410) have been associated with the global distribution of OXA-48, OXA-181, OXA-232, and OXA-204. Other OXA48-like carbapenemases, such as OXA-436, OXA-245, OXA-484, and OXA-519, are less often reported.
TABLE 7.
Summary of the characteristics of Enterobacterales with OXA-48-like carbapenemases
| Enzyme | Country and yr of isolation | OXA-48 cluster | Geographical distribution | Bacteria | Genetic environment | Plasmids | Clonal dissemination |
|---|---|---|---|---|---|---|---|
| OXA-48 | Turkey, 2004 | 48 | Regions of endemicity: Middle East (Turkey, Lebanon, Jordan, Oman, Iran, and Saudi Arabia), North Africa (Morocco, Algeria, Tunisia, and Egypt). Hospital outbreaks: Greece, Australia, Israel, UK, Netherlands, Ireland, Spain, France, Germany, Mexico, China, Belgium, Norway, Poland, Taiwan, South Africa, Croatia, and Slovenia. Case reports: global. | K. pneumoniae, E. coli, E. cloacae complex, C. freundii, S. marcescens, P. mirabilis, K. oxytoca, Kluyvera spp., Salmonella spp., P. aeruginosa | Tn1999 variants (1–5, with 2 the most common) Tn2016-like (rare) | IncL (common); others (A/C, IncF) very rare | K. pneumoniae: global, ST11, ST15, and ST147; UK, ST101; Poland, ST395; Spain, ST405. E. coli: global, ST38 with Tn6237 chromosomal integration, ST410. |
| OXA-181 | India, 2006 | 181 | Region of endemicity: Indian subcontinent (India, Bangladesh, Pakistan, and Sri Lanka). Hospital outbreaks: Nigeria, Angola, South Africa, São Tomé and Príncipe, Romania, and United Arab Emirates. Case reports: global. | K. pneumoniae, E. coli, E. cloacae complex, C. freundii, M. morganii, P. mirabilis, Aeromonas caviae | ISEcp1 within Tn2013 | ColE2, IncX3, IncN1, and IncT | K. pneumoniae: global, ST147; Canada, ST14; South Africa, ST307; USA, ST34 and ST43. E. coli: global, ST410. |
| OXA-232 | France, 2013 | 181 | Region of endemicity: India. Hospital outbreaks: South Korea, Mexico, USA, Brunei, and China. Case reports: global. |
K. pneumoniae, E. coli | ISEcp1 within truncated Tn2013 | ColE2-type | K. pneumoniae: Canada, ST14; South Korea, ST14 and ST15. |
| OXA-204 | Tunisia, 2013 | 48 | Tunisia and France | K. pneumoniae, E. coli, P. mirabilis, C. freundii, S. marcescens | ISEcp1 within Tn2016 | A/C type | K. pneumoniae ST147 and E. coli ST90 |
| OXA-162 | Turkey, 2008 | 48 | Turkey, Germany, Hungary, and Greece | K. pneumoniae, E. coli, C. freundii, R. ornithinolytica | Tn1999.2 | IncL | |
| OXA-244 | Spain, 2011 | 48 | Spain, Germany, Russia, France, Netherlands, UK | K. pneumoniae, E. coli, K. aerogenes | Tn1999.2 within Tn51098 | IncL | |
| OXA-436 | Denmark, 2017 | 436 | Denmark | E. asburiae, C. freundii, K. pneumoniae | IncHI2 | ||
| OXA-245 | Spain, 2011 | 48 | Spain, UK | K. pneumoniae | Tn1999 | IncL | |
| OXA-484 | UK, 2017 | 181 | UK | ||||
| OXA-519 | Belgium, 2018 | 48 | Belgium | K. pneumoniae | Tn1999 | IncL |
The laboratory detection of Enterobacterales with OXA-48-like carbapenemases is challenging for some clinical laboratories, especially for those that are situated in regions of nonendemicity. The detection of OXA-48-like carbapenemases is an essential initial step required for appropriate management of patients during infection prevention and control efforts. Identification is also appropriate for surveillance and epidemiological studies. The detection of OXA-48-like Enterobacterales with phenotypic methods has improved recently but remains challenging due to their weak carbapenemase activities. The susceptibility profiles created with automated susceptibility platforms, in general, performed poorly in screening for bacteria with OXA-48-like carbapenemases. Meropenem provides the best balance between sensitivity and specificity, but the ideal single screening agent for screening purposes is still wanting. PCR-based molecular confirmation methods have excellent sensitivities and specificities but are unfortunately rather expensive and time-consuming (compared to some phenotypic tests). Lateral flow immunoassays show promise for the rapid and accurate detection of Enterobacterales with OXA-48-like carbapenemases.
In summary, Enterobacterales with OXA-48-like carbapenemases are important causes of carbapenem resistance in certain parts of the globe; OXA-48 is associated with Tn1999 on IncL plasmids and is endemic in North Africa and the Middle East. OXA-181 is associated with ISEcp1, Tn2013 on IncX3, and ColE2 plasmids and is endemic on the Indian subcontinent (e.g., India, Bangladesh, Pakistan, and Sri Lanka) and certain Sub-Saharan African countries. Bacteria with blaOXA-48 and blaOXA-181 are emerging in different parts of the world and are most likely underreported due to problems with the laboratory detection of these enzymes. The medical community at large, especially those interested in antimicrobial resistance, should be aware of the looming threat that is posed by bacteria with OXA-48-like carbapenemases.
ACKNOWLEDGMENTS
All the authors have no conflicts of interest pertaining to writing the manuscript.
No direct financial support was received for writing the manuscript. Funding was acquired through Calgary Laboratory Services research grants 10015169 (J.D.D.P.) and 10017905 (G.P.).
Biographies

Johann D. D. Pitout, M.D., is a professor at the Cummings Medical School, University of Calgary, and medical microbiologist at Calgary Laboratory Services, Alberta, Canada. His main research interests are resistance to antimicrobial agents among Gram-negative bacteria, especially the laboratory detection, characterization, molecular epidemiology, and evolution of bacteria with newer β-lactamases such as AmpC, extended-spectrum β-lactamases, and carbapenemases. He has also been involved in population-based surveillance studies and the role of high-risk clones and mobile genetic elements (such as plasmids) among bacteria producing these newer types of β-lactamases. He serves on the editorial boards of several journals and has published over 170 peer-reviewed manuscripts in this field.

Gisele Peirano, Ph.D., is a research associate in the Microbiology section of Calgary Laboratory Services and at the Department of Pathology & Laboratory Medicine, University of Calgary in Calgary, Alberta, Canada. Her main research interests include the mechanisms and molecular epidemiology of extended-spectrum β-lactamases and carbapenemase-producing Enterobacteriaceae and other Gram-negative organisms. She has published over 60 papers in this field.

Marleen M. Kock received her Ph.D. degree in Microbiology in 2004 from the University of Pretoria (UP), South Africa (SA). Her Ph.D. project focused on identifying and characterizing nitrogen-fixing root-nodulating bacteria of honeybush tea (Cyclopia spp.). She worked as a postdoctoral fellow at the Sappi Technology Centre, SA, describing the bacteria responsible for paper breaks in the paper mills. In 2006, she was appointed as a postdoctoral fellow in the Department of Medical Microbiology, UP. She was appointed as a lecturer/medical scientist in the Department of Medical Microbiology, UP/National Health Laboratory Service, in 2007. Kock was promoted to the position of associate professor in 2018. She has a special interest in bacterial taxonomy (identification, characterization, and classification), which formed the basis of her research, which focuses on antimicrobial resistance mechanisms and epidemiology of important nosocomial and community-acquired pathogens, specifically Acinetobacter baumannii, Escherichia coli, Klebsiella pneumoniae, and Pseudomonas aeruginosa.

Kathy-Anne Strydom, M.D., is a pathologist specializing in medical microbiology, currently working in the Molecular Biology department of the Ampath National Reference Laboratory, Centurion, South Africa. She graduated in 2008 from the University of Pretoria’s medical school, where she also completed her postgraduate training in 2015. She maintains her affiliations with the University of Pretoria, where she is appointed as extraordinary lecturer in the Department of Medical Microbiology.

Yasufumi Matsumura, M.D., Ph.D., is an associate professor in clinical laboratory medicine at the Kyoto University Graduate School of Medicine in Kyoto, Japan, and is Deputy Director of Clinical Laboratory and the Department of Infection Control and Prevention at the Kyoto University Hospital. He completed his medical degree at Kyoto Prefectural University of Medicine and his residency and fellowship at Kyoto City Hospital. In 2008, he started his Ph.D. studies at the Kyoto University and has been working on clinical and microbiological research on antimicrobial resistance in Enterobacterales. His main research focuses on resistance mechanisms and molecular epidemiology. He has been involved in identification and characterization of epidemic clones producing extended-spectrum β-lactamases or carbapenemases using whole-genome sequencing and bioinformatics analysis.
REFERENCES
- 1.Seale AC, Gordon NC, Islam J, Peacock SJ, Scott J. 2017. AMR surveillance in low and middle-income settings—a roadmap for participation in the Global Antimicrobial Surveillance System (GLASS). Wellcome Open Res 2:92. doi: 10.12688/wellcomeopenres.12527.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. 2003. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis 36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 3.Marchaim D, Gottesman T, Schwartz O, Korem M, Maor Y, Rahav G, Karplus R, Lazarovitch T, Braun E, Sprecher H, Lachish T, Wiener-Well Y, Alon D, Chowers M, Ciobotaro P, Bardenstein R, Paz A, Potasman I, Giladi M, Schechner V, Schwaber MJ, Klarfeld-Lidji S, Carmeli Y. 2010. National multicenter study of predictors and outcomes of bacteremia upon hospital admission caused by Enterobacteriaceae producing extended-spectrum beta-lactamases. Antimicrob Agents Chemother 54:5099–5104. doi: 10.1128/AAC.00565-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush K, Bradford PA. 2016. β-Lactams and β-lactamase inhibitors: an overview. Cold Spring Harb Perspect Med 6:a025247. doi: 10.1101/cshperspect.a025247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonomo RA. 2017. β-Lactamases: a focus on current challenges. Cold Spring Harb Perspect Med 7:a025239. doi: 10.1101/cshperspect.a025239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drawz SM, Bonomo RA. 2010. Three decades of beta-lactamase inhibitors. Clin Microbiol Rev 23:160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bush K. 2013. Proliferation and significance of clinically relevant beta-lactamases. Ann N Y Acad Sci 1277:84–90. doi: 10.1111/nyas.12023. [DOI] [PubMed] [Google Scholar]
- 8.Ambler RP, Coulson AF, Frere JM, Ghuysen JM, Joris B, Forsman M, Levesque RC, Tiraby G, Waley SG. 1991. A standard numbering scheme for the class A beta-lactamases. Biochem J 276:269–270. doi: 10.1042/bj2760269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bush K, Jacoby GA. 2010. Updated functional classification of beta-lactamases. Antimicrob Agents Chemother 54:969–976. doi: 10.1128/AAC.01009-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bush K. 1998. Metallo-beta-lactamases: a class apart. Clin Infect Dis 27(Suppl 1):S48–S53. doi: 10.1086/514922. [DOI] [PubMed] [Google Scholar]
- 11.Pitout JD, Nordmann P, Poirel L. 2015. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother 59:5873–5884. doi: 10.1128/AAC.01019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumura Y, Pitout JD. 2016. Recent advances in the laboratory detection of carbapenemase-producing Enterobacteriaceae. Expert Rev Mol Diagn 16:783–794. doi: 10.1586/14737159.2016.1172964. [DOI] [PubMed] [Google Scholar]
- 13.Mathers AJ, Peirano G, Pitout JD. 2015. The role of epidemic resistance plasmids and international high-risk clones in the spread of multidrug-resistant Enterobacteriaceae. Clin Microbiol Rev 28:565–591. doi: 10.1128/CMR.00116-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walther-Rasmussen J, Høiby N. 2006. OXA-type carbapenemases. J Antimicrob Chemother 57:373–383. doi: 10.1093/jac/dki482. [DOI] [PubMed] [Google Scholar]
- 15.Datta N, Kontomichalou P. 1965. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature 208:239–241. doi: 10.1038/208239a0. [DOI] [PubMed] [Google Scholar]
- 16.Hedges RW, Datta N, Kontomichalou P, Smith JT. 1974. Molecular specificities of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol 117:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evans BA, Amyes SG. 2014. OXA beta-lactamases. Clin Microbiol Rev 27:241–263. doi: 10.1128/CMR.00117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Antunes NT, Fisher JF. 2014. Acquired class D beta-lactamases. Antibiotics (Basel) 3:398–434. doi: 10.3390/antibiotics3030398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poirel L, Naas T, Nordmann P. 2010. Diversity, epidemiology, and genetics of class D beta-lactamases. Antimicrob Agents Chemother 54:24–38. doi: 10.1128/AAC.01512-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poirel L, Potron A, Nordmann P. 2012. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother 67:1597–1606. doi: 10.1093/jac/dks121. [DOI] [PubMed] [Google Scholar]
- 21.Docquier JD, Mangani S. 2016. Structure-function relationships of class D carbapenemases. CDT 17:1061–1071. doi: 10.2174/1389450116666150825115824. [DOI] [PubMed] [Google Scholar]
- 22.Poirel L, Héritier C, Tolün V, Nordmann P. 2004. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob Agents Chemother 48:15–22. doi: 10.1128/aac.48.1.15-22.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kasap M, Torol S, Kolayli F, Dundar D, Vahaboglu H. 2013. OXA-162, a novel variant of OXA-48 displays extended hydrolytic activity towards imipenem, meropenem and doripenem. J Enzyme Inhib Med Chem 28:990–996. doi: 10.3109/14756366.2012.702343. [DOI] [PubMed] [Google Scholar]
- 24.Potron A, Nordmann P, Lafeuille E, Al Maskari Z, Al Rashdi F, Poirel L. 2011. Characterization of OXA-181, a carbapenem-hydrolyzing class D beta-lactamase from Klebsiella pneumoniae. Antimicrob Agents Chemother 55:4896–4899. doi: 10.1128/AAC.00481-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potron A, Nordmann P, Poirel L. 2013. Characterization of OXA-204, a carbapenem-hydrolyzing class D beta-lactamase from Klebsiella pneumoniae. Antimicrob Agents Chemother 57:633–636. doi: 10.1128/AAC.01034-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Potron A, Rondinaud E, Poirel L, Belmonte O, Boyer S, Camiade S, Nordmann P. 2013. Genetic and biochemical characterisation of OXA-232, a carbapenem-hydrolysing class D beta-lactamase from Enterobacteriaceae. Int J Antimicrob Agents 41:325–329. doi: 10.1016/j.ijantimicag.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 27.Oteo J, Hernandez JM, Espasa M, Fleites A, Saez D, Bautista V, Perez-Vazquez M, Fernandez-Garcia MD, Delgado-Iribarren A, Sanchez-Romero I, Garcia-Picazo L, Miguel MD, Solis S, Aznar E, Trujillo G, Mediavilla C, Fontanals D, Rojo S, Vindel A, Campos J. 2013. Emergence of OXA-48-producing Klebsiella pneumoniae and the novel carbapenemases OXA-244 and OXA-245 in Spain. J Antimicrob Chemother 68:317–321. doi: 10.1093/jac/dks383. [DOI] [PubMed] [Google Scholar]
- 28.Gomez S, Pasteran F, Faccone D, Bettiol M, Veliz O, De Belder D, Rapoport M, Gatti B, Petroni A, Corso A. 2013. Intrapatient emergence of OXA-247: a novel carbapenemase found in a patient previously infected with OXA-163-producing Klebsiella pneumoniae. Clin Microbiol Infect 19:E233–E235. doi: 10.1111/1469-0691.12142. [DOI] [PubMed] [Google Scholar]
- 29.Jousset AB, Dabos L, Bonnin RA, Girlich D, Potron A, Cabanel N, Dortet L, Glaser P, Naas T. 2018. CTX-M-15-producing Shewanella species clinical isolate expressing OXA-535, a chromosome-encoded OXA-48 variant, putative progenitor of the plasmid-encoded OXA-436. Antimicrob Agents Chemother 62:e01879-17. doi: 10.1128/AAC.01879-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Findlay J, Hopkins KL, Loy R, Doumith M, Meunier D, Hill R, Pike R, Mustafa N, Livermore DM, Woodford N. 2017. OXA-48-like carbapenemases in the UK: an analysis of isolates and cases from 2007 to 2014. J Antimicrob Chemother 72:1340–1349. doi: 10.1093/jac/dkx012. [DOI] [PubMed] [Google Scholar]
- 31.Dabos L, Bogaerts P, Bonnin RA, Zavala A, Sacre P, Iorga BI, Huang DT, Glupczynski Y, Naas T. 2018. Genetic and biochemical characterization of OXA-519, a novel OXA-48-like beta-lactamase. Antimicrob Agents Chemother 62:e00469-18. doi: 10.1128/AAC.00469-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Poirel L, Castanheira M, Carrer A, Rodriguez CP, Jones RN, Smayevsky J, Nordmann P. 2011. OXA-163, an OXA-48-related class D β-lactamase with extended activity toward expanded-spectrum cephalosporins. Antimicrob Agents Chemother 55:2546–2551. doi: 10.1128/AAC.00022-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dortet L, Oueslati S, Jeannot K, Tande D, Naas T, Nordmann P. 2015. Genetic and biochemical characterization of OXA-405, an OXA-48-type extended-spectrum beta-lactamase without significant carbapenemase activity. Antimicrob Agents Chemother 59:3823–3828. doi: 10.1128/AAC.05058-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dortet L, Naas T. 2017. Noncarbapenemase OXA-48 variants (OXA-163 and OXA-405) falsely detected as carbapenemases by the β Carba test. J Clin Microbiol 55:654–655. doi: 10.1128/JCM.02086-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sampaio JL, Ribeiro VB, Campos JC, Rozales FP, Magagnin CM, Falci DR, da Silva RC, Dalarosa MG, Luz DI, Vieira FJ, Antochevis LC, Barth AL, Zavascki AP. 2014. Detection of OXA-370, an OXA-48-related class D beta-lactamase, in Enterobacter hormaechei from Brazil. Antimicrob Agents Chemother 58:3566–3567. doi: 10.1128/AAC.02510-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Magagnin CM, Rozales FP, Antochevis L, Nunes LS, Martins AS, Barth AL, Sampaio JM, Zavascki AP. 2017. Dissemination of bla OXA-370 gene among several Enterobacteriaceae species in Brazil. Eur J Clin Microbiol Infect Dis 36:1907–1910. doi: 10.1007/s10096-017-3012-x. [DOI] [PubMed] [Google Scholar]
- 37.Naas T, Oueslati S, Bonnin RA, Dabos ML, Zavala A, Dortet L, Retailleau P, Iorga BI. 2017. Beta-lactamase database (BLDB)—structure and function. J Enzyme Inhib Med Chem 32:917–919. doi: 10.1080/14756366.2017.1344235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antonelli A, Di Palo DM, Galano A, Becciani S, Montagnani C, Pecile P, Galli L, Rossolini GM. 2015. Intestinal carriage of Shewanella xiamenensis simulating carriage of OXA-48-producing Enterobacteriaceae. Diagn Microbiol Infect Dis 82:1–3. doi: 10.1016/j.diagmicrobio.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 39.Poirel L, Heritier C, Nordmann P. 2004. Chromosome-encoded ambler class D beta-lactamase of Shewanella oneidensis as a progenitor of carbapenem-hydrolyzing oxacillinase. Antimicrob Agents Chemother 48:348–351. doi: 10.1128/AAC.48.1.348-351.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zong Z. 2012. Discovery of bla(OXA-199), a chromosome-based bla(OXA-48)-like variant, in Shewanella xiamenensis. PLoS One 7:e48280. doi: 10.1371/journal.pone.0048280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fursova NK, Astashkin EI, Knyazeva AI, Kartsev NN, Leonova ES, Ershova ON, Alexandrova IA, Kurdyumova NV, Sazikina SY, Volozhantsev NV, Svetoch EA, Dyatlov IA. 2015. The spread of bla OXA-48 and bla OXA-244 carbapenemase genes among Klebsiella pneumoniae, Proteus mirabilis and Enterobacter spp. isolated in Moscow, Russia. Ann Clin Microbiol Antimicrob 14:46. doi: 10.1186/s12941-015-0108-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hoyos-Mallecot Y, Naas T, Bonnin RA, Patino R, Glaser P, Fortineau N, Dortet L. 2017. OXA-244-producing Escherichia coli isolates, a challenge for clinical microbiology laboratories. Antimicrob Agents Chemother 61:e00818-17. doi: 10.1128/AAC.00818-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Docquier JD, Calderone V, De Luca F, Benvenuti M, Giuliani F, Bellucci L, Tafi A, Nordmann P, Botta M, Rossolini GM, Mangani S. 2009. Crystal structure of the OXA-48 beta-lactamase reveals mechanistic diversity among class D carbapenemases. Chem Biol 16:540–547. doi: 10.1016/j.chembiol.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 44.Oueslati S, Nordmann P, Poirel L. 2015. Heterogeneous hydrolytic features for OXA-48-like β-lactamases. J Antimicrob Chemother 70:1059–1063. doi: 10.1093/jac/dku524. [DOI] [PubMed] [Google Scholar]
- 45.Tacão M, Correia A, Henriques I. 2013. Environmental Shewanella xiamenensis strains that carry blaOXA-48 or blaOXA-204 genes: additional proof for blaOXA-48-like gene origin. Antimicrob Agents Chemother 57:6399–6400. doi: 10.1128/AAC.00771-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Potron A, Poirel L, Nordmann P. 2011. Origin of OXA-181, an emerging carbapenem-hydrolyzing oxacillinase, as a chromosomal gene in Shewanella xiamenensis. Antimicrob Agents Chemother 55:4405–4407. doi: 10.1128/AAC.00681-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.de Jonge BL, Karlowsky JA, Kazmierczak KM, Biedenbach DJ, Sahm DF, Nichols WW. 2016. In vitro susceptibility to ceftazidime-avibactam of carbapenem-nonsusceptible Enterobacteriaceae isolates collected during the INFORM global surveillance study (2012 to 2014). Antimicrob Agents Chemother 60:3163–3169. doi: 10.1128/AAC.03042-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karlowsky JA, Lob SH, Kazmierczak KM, Badal RE, Young K, Motyl MR, Sahm DF. 2017. In vitro activity of imipenem against carbapenemase-positive Enterobacteriaceae isolates collected by the SMART global surveillance program from 2008 to 2014. J Clin Microbiol 55:1638–1649. doi: 10.1128/JCM.02316-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carrer A, Poirel L, Eraksoy H, Cagatay AA, Badur S, Nordmann P. 2008. Spread of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in Istanbul, Turkey. Antimicrob Agents Chemother 52:2950–2954. doi: 10.1128/AAC.01672-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gulmez D, Woodford N, Palepou MF, Mushtaq S, Metan G, Yakupogullari Y, Kocagoz S, Uzun O, Hascelik G, Livermore DM. 2008. Carbapenem-resistant Escherichia coli and Klebsiella pneumoniae isolates from Turkey with OXA-48-like carbapenemases and outer membrane protein loss. Int J Antimicrob Agents 31:523–526. doi: 10.1016/j.ijantimicag.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 51.Cuzon G, Naas T, Bogaerts P, Glupczynski Y, Huang TD, Nordmann P. 2008. Plasmid-encoded carbapenem-hydrolyzing beta-lactamase OXA-48 in an imipenem-susceptible Klebsiella pneumoniae strain from Belgium. Antimicrob Agents Chemother 52:3463–3464. doi: 10.1128/AAC.00543-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Matar GM, Dandache I, Carrer A, Khairallah MT, Nordmann P, Sabra A, Araj GF. 2010. Spread of OXA-48-mediated resistance to carbapenems in Lebanese Klebsiella pneumoniae and Escherichia coli that produce extended spectrum beta-lactamase. Ann Trop Med Parasitol 104:271–274. doi: 10.1179/136485910X12647085215651. [DOI] [PubMed] [Google Scholar]
- 53.Carrer A, Poirel L, Yilmaz M, Akan OA, Feriha C, Cuzon G, Matar G, Honderlick P, Nordmann P. 2010. Spread of OXA-48-encoding plasmid in Turkey and beyond. Antimicrob Agents Chemother 54:1369–1373. doi: 10.1128/AAC.01312-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Decre D, Birgand G, Geneste D, Maury E, Petit JC, Barbut F, Arlet G. 2010. Possible importation and subsequent cross-transmission of OXA-48-producing Klebsiella pneumoniae, France, 2010. Euro Surveill 15:19718 10.2807/ese.15.46.19718-en. [DOI] [PubMed] [Google Scholar]
- 55.Cuzon G, Naas T, Lesenne A, Benhamou M, Nordmann P. 2010. Plasmid-mediated carbapenem-hydrolysing OXA-48 beta-lactamase in Klebsiella pneumoniae from Tunisia. Int J Antimicrob Agents 36:91–93. doi: 10.1016/j.ijantimicag.2010.02.014. [DOI] [PubMed] [Google Scholar]
- 56.Ktari S, Mnif B, Louati F, Rekik S, Mezghani S, Mahjoubi F, Hammami A. 2011. Spread of Klebsiella pneumoniae isolates producing OXA-48 beta-lactamase in a Tunisian university hospital. J Antimicrob Chemother 66:1644–1646. doi: 10.1093/jac/dkr181. [DOI] [PubMed] [Google Scholar]
- 57.Benouda A, Touzani O, Khairallah MT, Araj GF, Matar GM. 2010. First detection of oxacillinase-mediated resistance to carbapenems in Klebsiella pneumoniae from Morocco. Ann Trop Med Parasitol 104:327–330. doi: 10.1179/136485910X12743554760108. [DOI] [PubMed] [Google Scholar]
- 58.Hays C, Benouda A, Poirel L, Elouennass M, Nordmann P. 2012. Nosocomial occurrence of OXA-48-producing enterobacterial isolates in a Moroccan hospital. Int J Antimicrob Agents 39:545–547. doi: 10.1016/j.ijantimicag.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 59.Goren MG, Chmelnitsky I, Carmeli Y, Navon-Venezia S. 2011. Plasmid-encoded OXA-48 carbapenemase in Escherichia coli from Israel. J Antimicrob Chemother 66:672–673. doi: 10.1093/jac/dkq467. [DOI] [PubMed] [Google Scholar]
- 60.Moquet O, Bouchiat C, Kinana A, Seck A, Arouna O, Bercion R, Breurec S, Garin B. 2011. Class D OXA-48 carbapenemase in multidrug-resistant enterobacteria, Senegal. Emerg Infect Dis 17:143–144. doi: 10.3201/eid1701.100244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalpoe JS, Al Naiemi N, Poirel L, Nordmann P. 2011. Detection of an Ambler class D OXA-48-type beta-lactamase in a Klebsiella pneumoniae strain in The Netherlands. J Med Microbiol 60:677–678. doi: 10.1099/jmm.0.028308-0. [DOI] [PubMed] [Google Scholar]
- 62.Pitart C, Sole M, Roca I, Fabrega A, Vila J, Marco F. 2011. First outbreak of a plasmid-mediated carbapenem-hydrolyzing OXA-48 beta-lactamase in Klebsiella pneumoniae in Spain. Antimicrob Agents Chemother 55:4398–4401. doi: 10.1128/AAC.00329-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.O’Brien DJ, Wrenn C, Roche C, Rose L, Fenelon C, Flynn A, Murphy V, FitzGerald SF, Fenelon LE, Crowley B, Schaffer K. 2011. First isolation and outbreak of OXA-48-producing Klebsiella pneumoniae in an Irish hospital, March to June 2011. Euro Surveill 16:19921 https://www.eurosurveillance.org/content/10.2807/ese.16.29.19921-en. [PubMed] [Google Scholar]
- 64.Pirs M, Andlovic A, Cerar T, Zohar-Cretnik T, Kobola L, Kolman J, Frelih T, Presern-Strukelj M, Ruzic-Sabljic E, Seme K. 2011. A case of OXA-48 carbapenemase-producing Klebsiella pneumoniae in a patient transferred to Slovenia from Libya, November 2011. Euro Surveill 16:20042 https://www.eurosurveillance.org/content/10.2807/ese.16.50.20042-en. [PubMed] [Google Scholar]
- 65.Levast M, Poirel L, Carrer A, Deiber M, Decroisette E, Mallaval FO, Lecomte C, Nordmann P. 2011. Transfer of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae from Turkey to France. J Antimicrob Chemother 66:944–945. doi: 10.1093/jac/dkq504. [DOI] [PubMed] [Google Scholar]
- 66.Poirel L, Ros A, Carrer A, Fortineau N, Carricajo A, Berthelot P, Nordmann P. 2011. Cross-border transmission of OXA-48-producing Enterobacter cloacae from Morocco to France. J Antimicrob Chemother 66:1181–1182. doi: 10.1093/jac/dkr023. [DOI] [PubMed] [Google Scholar]
- 67.Cuzon G, Ouanich J, Gondret R, Naas T, Nordmann P. 2011. Outbreak of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in France. Antimicrob Agents Chemother 55:2420–2423. doi: 10.1128/AAC.01452-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Glupczynski Y, Huang TD, Bouchahrouf W, Rezende de Castro R, Bauraing C, Gerard M, Verbruggen AM, Deplano A, Denis O, Bogaerts P. 2012. Rapid emergence and spread of OXA-48-producing carbapenem-resistant Enterobacteriaceae isolates in Belgian hospitals. Int J Antimicrob Agents 39:168–172. doi: 10.1016/j.ijantimicag.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 69.Mairi A, Pantel A, Sotto A, Lavigne JP, Touati A. 2018. OXA-48-like carbapenemases producing Enterobacteriaceae in different niches. Eur J Clin Microbiol Infect Dis 37:587–604. doi: 10.1007/s10096-017-3112-7. [DOI] [PubMed] [Google Scholar]
- 70.Avgoulea K, Di Pilato V, Zarkotou O, Sennati S, Politi L, Cannatelli A, Themeli-Digalaki K, Giani T, Tsakris A, Rossolini GM, Pournaras S. 2018. Characterization of extensively drug-resistant or pandrug-resistant sequence type 147 and 101 OXA-48-producing Klebsiella pneumoniae causing bloodstream infections in patients in an intensive care unit. Antimicrob Agents Chemother 62:e02457-17. doi: 10.1128/AAC.02457-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Voulgari E, Zarkotou O, Ranellou K, Karageorgopoulos DE, Vrioni G, Mamali V, Themeli-Digalaki K, Tsakris A. 2013. Outbreak of OXA-48 carbapenemase-producing Klebsiella pneumoniae in Greece involving an ST11 clone. J Antimicrob Chemother 68:84–88. doi: 10.1093/jac/dks356. [DOI] [PubMed] [Google Scholar]
- 72.Espedido BA, Steen JA, Ziochos H, Grimmond SM, Cooper MA, Gosbell IB, van Hal SJ, Jensen SO. 2013. Whole genome sequence analysis of the first Australian OXA-48-producing outbreak-associated Klebsiella pneumoniae isolates: the resistome and in vivo evolution. PLoS One 8:e59920. doi: 10.1371/journal.pone.0059920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adler A, Solter E, Masarwa S, Miller-Roll T, Abu-Libdeh B, Khammash H, Najem K, Dekadek S, Stein-Zamir C, Nubani N, Kunbar A, Assous MV, Carmeli Y, Schwaber MJ. 2013. Epidemiological and microbiological characteristics of an outbreak caused by OXA-48-producing Enterobacteriaceae in a neonatal intensive care unit in Jerusalem, Israel. J Clin Microbiol 51:2926–2930. doi: 10.1128/JCM.01049-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Regev-Yochay G, Smollan G, Tal I, Pinas Zade N, Haviv Y, Nudelman V, Gal-Mor O, Jaber H, Zimlichman E, Keller N, Rahav G. 2018. Sink traps as the source of transmission of OXA-48-producing Serratia marcescens in an intensive care unit. Infect Control Hosp Epidemiol 39:1307–1315. doi: 10.1017/ice.2018.235. [DOI] [PubMed] [Google Scholar]
- 75.Gharbi M, Moore LS, Gilchrist M, Thomas CP, Bamford K, Brannigan ET, Holmes AH. 2015. Forecasting carbapenem resistance from antimicrobial consumption surveillance: lessons learnt from an OXA-48-producing Klebsiella pneumoniae outbreak in a West London renal unit. Int J Antimicrob Agents 46:150–156. doi: 10.1016/j.ijantimicag.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thomas CP, Moore LS, Elamin N, Doumith M, Zhang J, Maharjan S, Warner M, Perry C, Turton JF, Johnstone C, Jepson A, Duncan ND, Holmes AH, Livermore DM, Woodford N. 2013. Early (2008–2010) hospital outbreak of Klebsiella pneumoniae producing OXA-48 carbapenemase in the UK. Int J Antimicrob Agents 42:531–536. doi: 10.1016/j.ijantimicag.2013.08.020. [DOI] [PubMed] [Google Scholar]
- 77.Dautzenberg MJ, Ossewaarde JM, de Greeff SC, Troelstra A, Bonten MJ. 2016. Risk factors for the acquisition of OXA-48-producing Enterobacteriaceae in a hospital outbreak setting: a matched case-control study. J Antimicrob Chemother 71:2273–2279. doi: 10.1093/jac/dkw119. [DOI] [PubMed] [Google Scholar]
- 78.Dautzenberg MJ, Ossewaarde JM, de Kraker ME, van der Zee A, van Burgh S, de Greeff SC, Bijlmer HA, Grundmann H, Cohen Stuart JW, Fluit AC, Troelstra A, Bonten MJ. 2014. Successful control of a hospital-wide outbreak of OXA-48 producing Enterobacteriaceae in the Netherlands, 2009 to 2011. Euro Surveill 19:20723 10.2807/1560-7917.ES2014.19.9.20723. [DOI] [PubMed] [Google Scholar]
- 79.Morris D, O’Connor M, Izdebski R, Corcoran M, Ludden CE, McGrath E, Buckley V, Cryan B, Gniadkowski M, Cormican M. 2016. Dissemination of clonally related multidrug-resistant Klebsiella pneumoniae in Ireland. Epidemiol Infect 144:443–448. doi: 10.1017/S0950268815001041. [DOI] [PubMed] [Google Scholar]
- 80.Wrenn C, O’Brien D, Keating D, Roche C, Rose L, Ronayne A, Fenelon L, Fitzgerald S, Crowley B, Schaffer K. 2014. Investigation of the first outbreak of OXA-48-producing Klebsiella pneumoniae in Ireland. J Hosp Infect 87:41–46. doi: 10.1016/j.jhin.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 81.Arana DM, Saez D, Garcia-Hierro P, Bautista V, Fernandez-Romero S, Angel de la Cal M, Alos JI, Oteo J. 2015. Concurrent interspecies and clonal dissemination of OXA-48 carbapenemase. Clin Microbiol Infect 21:148.e1–148.e4. doi: 10.1016/j.cmi.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 82.Fernandez J, Montero I, Martinez O, Fleites A, Poirel L, Nordmann P, Rodicio MR. 2015. Dissemination of multiresistant Enterobacter cloacae isolates producing OXA-48 and CTX-M-15 in a Spanish hospital. Int J Antimicrob Agents 46:469–474. doi: 10.1016/j.ijantimicag.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 83.López-Camacho E, Paño-Pardo JR, Ruiz-Carrascoso G, Wesselink J-J, Lusa-Bernal S, Ramos-Ruiz R, Ovalle S, Gómez-Gil R, Pérez-Blanco V, Pérez-Vázquez M, Gómez-Puertas P, Mingorance J. 2018. Population structure of OXA-48-producing Klebsiella pneumoniae ST405 isolates during a hospital outbreak characterised by genomic typing. J Glob Antimicrob Resist 15:48–54. doi: 10.1016/j.jgar.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 84.Perez-Vazquez M, Oteo J, Garcia-Cobos S, Aracil B, Harris SR, Ortega A, Fontanals D, Hernandez JM, Solis S, Campos J, Dougan G, Kingsley RA. 2016. Phylogeny, resistome and mobile genetic elements of emergent OXA-48 and OXA-245 Klebsiella pneumoniae clones circulating in Spain. J Antimicrob Chemother 71:887–896. doi: 10.1093/jac/dkv458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Robustillo-Rodela A, Pérez-Blanco V, Espinel Ruiz MA, Ruiz Carrascoso G, Figueira Iglesias JC, Abad Martín D. 2017. Successful control of 2 simultaneous outbreaks of OXA-48 carbapenemase-producing Enterobacteriaceae and multidrug-resistant Acinetobacter baumannii in an intensive care unit. Am J Infect Control 45:1356–1362. doi: 10.1016/j.ajic.2017.07.018. [DOI] [PubMed] [Google Scholar]
- 86.Pantel A, Richaud-Morel B, Cazaban M, Bouziges N, Sotto A, Lavigne JP. 2016. Environmental persistence of OXA-48-producing Klebsiella pneumoniae in a French intensive care unit. Am J Infect Control 44:366–368. doi: 10.1016/j.ajic.2015.09.021. [DOI] [PubMed] [Google Scholar]
- 87.Potron A, Bernabeu S, Cuzon G, Ponties V, Blanchard H, Seringe E, Naas T, Nordmann P, Dortet L. 2017. Analysis of OXA-204 carbapenemase-producing Enterobacteriaceae reveals possible endoscopy-associated transmission, France, 2012 to 2014. Euro Surveill 22:17-00048 10.2807/1560-7917.ES.2017.22.49.17-00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Semin-Pelletier B, Cazet L, Bourigault C, Juvin ME, Boutoille D, Raffi F, Hourmant M, Blancho G, Agard C, Connault J, Corvec S, Caillon J, Batard E, Lepelletier D. 2015. Challenges of controlling a large outbreak of OXA-48 carbapenemase-producing Klebsiella pneumoniae in a French university hospital. J Hosp Infect 89:248–253. doi: 10.1016/j.jhin.2014.11.018. [DOI] [PubMed] [Google Scholar]
- 89.Borgmann S, Pfeifer Y, Becker L, Rieß B, Siegmund R, Sagel U. 2018. Findings from an outbreak of carbapenem-resistant Klebsiella pneumoniae emphasize the role of antibiotic treatment for cross transmission. Infection 46:103–112. doi: 10.1007/s15010-017-1103-3. [DOI] [PubMed] [Google Scholar]
- 90.Gottig S, Gruber TM, Stecher B, Wichelhaus TA, Kempf VA. 2015. In vivo horizontal gene transfer of the carbapenemase OXA-48 during a nosocomial outbreak. Clin Infect Dis 60:1808–1815. doi: 10.1093/cid/civ191. [DOI] [PubMed] [Google Scholar]
- 91.Torres-Gonzalez P, Cervera-Hernandez ME, Niembro-Ortega MD, Leal-Vega F, Cruz-Hervert LP, García-García L, Galindo-Fraga A, Martinez-Gamboa A, Bobadilla-del Valle M, Sifuentes-Osornio J, Ponce-de-Leon A. 2015. Factors associated to prevalence and incidence of carbapenem-resistant Enterobacteriaceae fecal carriage: a cohort study in a Mexican tertiary care hospital. PLoS One 10:e0139883. doi: 10.1371/journal.pone.0139883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guo L, An J, Ma Y, Ye L, Luo Y, Tao C, Yang J. 2016. Nosocomial outbreak of OXA-48-producing Klebsiella pneumoniae in a Chinese hospital: clonal transmission of ST147 and ST383. PLoS One 11:e0160754. doi: 10.1371/journal.pone.0160754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.De Geyter D, Blommaert L, Verbraeken N, Sevenois M, Huyghens L, Martini H, Covens L, Pierard D, Wybo I. 2017. The sink as a potential source of transmission of carbapenemase-producing Enterobacteriaceae in the intensive care unit. Antimicrob Resist Infect Control 6:24. doi: 10.1186/s13756-017-0182-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Samuelsen Ø, Overballe-Petersen S, Bjørnholt JV, Brisse S, Doumith M, Woodford N, Hopkins KL, Aasnæs B, Haldorsen B, Sundsfjord A, Norwegian Study Group on CPE. 2017. Molecular and epidemiological characterization of carbapenemase-producing Enterobacteriaceae in Norway, 2007 to 2014. PLoS One 12:e0187832. doi: 10.1371/journal.pone.0187832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Izdebski R, Baraniak A, Żabicka D, Machulska M, Urbanowicz P, Fiett J, Literacka E, Bojarska K, Kozińska A, Zieniuk B, Hryniewicz W, Gniadkowski M, Chrystyniuk P, Durnaś B, Kędzierska J, Mól A, Swoboda-Kopeć E, Wróblewska M, Tomanek E, Wcisło-Wach B, OXA-48-PL Study Group. 2018. Enterobacteriaceae producing OXA-48-like carbapenemases in Poland, 2013–January 2017. J Antimicrob Chemother 73:620–625. doi: 10.1093/jac/dkx457. [DOI] [PubMed] [Google Scholar]
- 96.Chen CM, Guo MK, Ke SC, Lin YP, Li CR, Vy Nguyen HT, Wu LT. 2018. Emergence and nosocomial spread of ST11 carbapenem-resistant Klebsiella pneumoniae co-producing OXA-48 and KPC-2 in a regional hospital in Taiwan. J Med Microbiol 67:957–964. doi: 10.1099/jmm.0.000771. [DOI] [PubMed] [Google Scholar]
- 97.Brink AJ, Coetzee J, Corcoran C, Clay CG, Hari-Makkan D, Jacobson RK, Richards GA, Feldman C, Nutt L, van Greune J, Deetlefs JD, Swart K, Devenish L, Poirel L, Nordmann P. 2013. Emergence of OXA-48 and OXA-181 carbapenemases among Enterobacteriaceae in South Africa and evidence of in vivo selection of colistin resistance as a consequence of selective decontamination of the gastrointestinal tract. J Clin Microbiol 51:369–372. doi: 10.1128/JCM.02234-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bedenić B, Slade M, Starčević LŽ, Sardelić S, Vranić-Ladavac M, Benčić A, Zujić Atalić V, Bogdan M, Bubonja-Šonje M, Tomić-Paradžik M, Tot T, Lukić-Grlić A, Drenjančević D, Varda-Brkić D, Bandić-Pavlović D, Mihaljević S, Zarfel G, Gužvinec M, Conzemius R, Barišić I, Tambić-Andraševic A. 2018. Epidemic spread of OXA-48 beta-lactamase in Croatia. J Med Microbiol 67:1031–1041. doi: 10.1099/jmm.0.000777. [DOI] [PubMed] [Google Scholar]
- 99.Pirš M, Cerar Kišek T, Križan Hergouth V, Seme K, Mueller Premru M, Jeverica S, Logar M, Mrvič T, Žnidaršič B, Jordan Markočič O, Lejko Zupanc T. 2019. Successful control of the first OXA-48 and/or NDM carbapenemase-producing Klebsiella pneumoniae outbreak in Slovenia 2014–2016. J Hosp Infect 101:142–149. doi: 10.1016/j.jhin.2018.10.022. [DOI] [PubMed] [Google Scholar]
- 100.Vanegas JM, Ospina WP, Felipe Higuita-Gutiérrez L, Natalia Jiménez J. 2016. First reported case of an OXA-48-producing isolate from a Colombian patient. J Glob Antimicrob Resist 6:67–68. doi: 10.1016/j.jgar.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 101.Mushi MF, Mshana SE, Imirzalioglu C, Bwanga F. 2014. Carbapenemase genes among multidrug resistant gram negative clinical isolates from a tertiary hospital in Mwanza, Tanzania. Biomed Res Int 2014:303104. doi: 10.1155/2014/303104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nagano N, Endoh Y, Nagano Y, Toyama M, Matsui M, Shibayama K, Arakawa Y. 2013. First report of OXA-48 carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in Japan from a patient returned from Southeast Asia. Jpn J Infect Dis 66:79–81. doi: 10.7883/yoken.66.79. [DOI] [PubMed] [Google Scholar]
- 103.Marimuthu K, Teo JW, Fong PB, Chin JO, Qi KJ, Boon DL, Ping AC, Krishnan P, Peng BA. 2014. First report of emergence of OXA-48 carbapenemase-producing Enterobacteriaceae in Singapore: proactive or reactive infection control strategy? Am J Infect Control 42:577–578. doi: 10.1016/j.ajic.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 104.Ma L, Wang JT, Wu TL, Siu LK, Chuang YC, Lin JC, Lu MC, Lu PL. 2015. Emergence of OXA-48-producing Klebsiella pneumoniae in Taiwan. PLoS One 10:e0139152. doi: 10.1371/journal.pone.0139152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Low YM, Yap PS, Abdul Jabar K, Ponnampalavanar S, Karunakaran R, Velayuthan R, Chong CW, Abu Bakar S, Md Yusof MY, Teh CS. 2017. The emergence of carbapenem resistant Klebsiella pneumoniae in Malaysia: correlation between microbiological trends with host characteristics and clinical factors. Antimicrob Resist Infect Control 6:5. doi: 10.1186/s13756-016-0164-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Howard JC, Anderson T, Creighton J, Freeman JT. 2018. Geographical and temporal clustering of OXA-48-producing Escherichia coli ST410 causing community-onset urinary tract infection in Christchurch, New Zealand. J Antimicrob Chemother 73:2900–2901. doi: 10.1093/jac/dky269. [DOI] [PubMed] [Google Scholar]
- 107.Breurec S, Bastian S, Cuzon G, Bernabeu S, Foucan T, Galanth S, Naas T, Dortet L. 2015. Emergence of OXA-48-producing Escherichia coli in the Caribbean islands. J Glob Antimicrob Resist 3:217–218. doi: 10.1016/j.jgar.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 108.Gauthier L, Dortet L, Cotellon G, Creton E, Cuzon G, Ponties V, Bonnin RA, Naas T. 2018. Diversity of carbapenemase-producing Escherichia coli isolates in France in 2012–2013. Antimicrob Agents Chemother 62:e00266-18. doi: 10.1128/AAC.00266-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Peirano G, Matsumura Y, Adams MD, Bradford P, Motyl M, Chen L, Kreiswirth BN, Pitout J. 2018. Genomic epidemiology of global carbapenemase-producing Enterobacter spp., 2008–2014. Emerg Infect Dis 24:1010–1019. doi: 10.3201/eid2406.171648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen L, Al Laham N, Chavda KD, Mediavilla JR, Jacobs MR, Bonomo RA, Kreiswirth BN. 2015. First report of an OXA-48-producing multidrug-resistant Proteus mirabilis strain from Gaza, Palestine. Antimicrob Agents Chemother 59:4305–4307. doi: 10.1128/AAC.00565-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hernandez-Garcia M, Leon-Sampedro R, Perez-Viso B, Morosini MI, Lopez-Fresnena N, Diaz-Agero C, Coque TM, Ruiz-Garbajosa P, Canton R. 2018. First report of an OXA-48- and CTX-M-213-producing Kluyvera species clone recovered from patients admitted in a university hospital in Madrid, Spain. Antimicrob Agents Chemother 62:e01238-18. doi: 10.1128/AAC.01238-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aquino-Andrade A, Merida-Vieyra J, Arias de la Garza E, Arzate-Barbosa P, De Colsa Ranero A. 2018. Carbapenemase-producing Enterobacteriaceae in Mexico: report of seven non-clonal cases in a pediatric hospital. BMC Microbiol 18:38. doi: 10.1186/s12866-018-1166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Seiffert SN, Perreten V, Johannes S, Droz S, Bodmer T, Endimiani A. 2014. OXA-48 carbapenemase-producing Salmonella enterica serovar Kentucky isolate of sequence type 198 in a patient transferred from Libya to Switzerland. Antimicrob Agents Chemother 58:2446–2449. doi: 10.1128/AAC.02417-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zurfluh K, Nüesch-Inderbinen MT, Poirel L, Nordmann P, Hächler H, Stephan R. 2015. Emergence of Escherichia coli producing OXA-48 beta-lactamase in the community in Switzerland. Antimicrob Resist Infect Control 4:9. doi: 10.1186/s13756-015-0051-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Woerther P-L, Jardak T, Ben Hassine I, Forget S, Chachaty E, Arlet G, Decré D. 2018. A long-term study of the diversity of OXA-48-like carbapenemase-producing bacterial strains in infected patients and carriers. Microb Drug Resist 24:181–189. doi: 10.1089/mdr.2017.0060. [DOI] [PubMed] [Google Scholar]
- 116.Poirel L, Bonnin RA, Nordmann P. 2012. Genetic features of the widespread plasmid coding for the carbapenemase OXA-48. Antimicrob Agents Chemother 56:559–562. doi: 10.1128/AAC.05289-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Poirel L, Decousser JW, Nordmann P. 2003. Insertion sequence ISEcp1B is involved in expression and mobilization of a bla(CTX-M) beta-lactamase gene. Antimicrob Agents Chemother 47:2938–2945. doi: 10.1128/aac.47.9.2938-2945.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Beyrouthy R, Robin F, Delmas J, Gibold L, Dalmasso G, Dabboussi F, Hamzé M, Bonnet R. 2014. IS1R-mediated plasticity of IncL/M plasmids leads to the insertion of blaOXA-48 into the Escherichia coli chromosome. Antimicrob Agents Chemother 58:3785–3790. doi: 10.1128/AAC.02669-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Giani T, Conte V, Di Pilato V, Aschbacher R, Weber C, Larcher C, Rossolini GM. 2012. Escherichia coli from Italy producing OXA-48 carbapenemase encoded by a novel Tn1999 transposon derivative. Antimicrob Agents Chemother 56:2211–2213. doi: 10.1128/AAC.00035-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Potron A, Nordmann P, Rondinaud E, Jaureguy F, Poirel L. 2013. A mosaic transposon encoding OXA-48 and CTX-M-15: towards pan-resistance. J Antimicrob Chemother 68:476–477. doi: 10.1093/jac/dks397. [DOI] [PubMed] [Google Scholar]
- 121.Skalova A, Chudejova K, Rotova V, Medvecky M, Studentova V, Chudackova E, Lavicka P, Bergerova T, Jakubu V, Zemlickova H, Papagiannitsis CC, Hrabak J. 2017. Molecular characterization of OXA-48-like-producing Enterobacteriaceae in the Czech Republic and evidence for horizontal transfer of pOXA-48-like plasmids. Antimicrob Agents Chemother 61:e01889-16. doi: 10.1128/AAC.01889-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Carattoli A, Seiffert SN, Schwendener S, Perreten V, Endimiani A. 2015. Differentiation of IncL and IncM plasmids associated with the spread of clinically relevant antimicrobial resistance. PLoS One 10:e0123063. doi: 10.1371/journal.pone.0123063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Carattoli A. 2009. Resistance plasmid families in Enterobacteriaceae. Antimicrob Agents Chemother 53:2227–2238. doi: 10.1128/AAC.01707-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Power K, Wang J, Karczmarczyk M, Crowley B, Cotter M, Haughton P, Lynch M, Schaffer K, Fanning S. 2014. Molecular analysis of OXA-48-carrying conjugative IncL/M-like plasmids in clinical isolates of Klebsiella pneumoniae in Ireland. Microb Drug Resist 20:270–274. doi: 10.1089/mdr.2013.0022. [DOI] [PubMed] [Google Scholar]
- 125.Mierzejewska J, Kulińska A, Jagura-Burdzy G. 2007. Functional analysis of replication and stability regions of broad-host-range conjugative plasmid CTX-M3 from the IncL/M incompatibility group. Plasmid 57:95–107. doi: 10.1016/j.plasmid.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 126.Potron A, Poirel L, Nordmann P. 2014. Derepressed transfer properties leading to the efficient spread of the plasmid encoding carbapenemase OXA-48. Antimicrob Agents Chemother 58:467–471. doi: 10.1128/AAC.01344-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carattoli A, Bertini A, Villa L, Falbo V, Hopkins KL, Threlfall EJ. 2005. Identification of plasmids by PCR-based replicon typing. J Microbiol Methods 63:219–228. doi: 10.1016/j.mimet.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 128.Sabtcheva S, Ivanov IN, Todorova B, Simeonov Y, Dobreva E, Ivanova K, Velinov T, Kantardjiev T. 2016. Detection and characterization of OXA-48-producing Klebsiella pneumoniae originated in Bulgaria. J Chemother 28:450–453. doi: 10.1179/1973947815Y.0000000047. [DOI] [PubMed] [Google Scholar]
- 129.Potron A, Poirel L, Rondinaud E, Nordmann P. 2013. Intercontinental spread of OXA-48 beta-lactamase-producing Enterobacteriaceae over a 11-year period, 2001 to 2011. Euro Surveill 18:20549. doi: 10.2807/1560-7917.ES2013.18.31.20549. [DOI] [PubMed] [Google Scholar]
- 130.Turton JF, Doumith M, Hopkins KL, Perry C, Meunier D, Woodford N. 2016. Clonal expansion of Escherichia coli ST38 carrying a chromosomally integrated OXA-48 carbapenemase gene. J Med Microbiol 65:538–546. doi: 10.1099/jmm.0.000248. [DOI] [PubMed] [Google Scholar]
- 131.Zautner AE, Bunk B, Pfeifer Y, Spröer C, Reichard U, Eiffert H, Scheithauer S, Groß U, Overmann J, Bohne W. 2017. Monitoring microevolution of OXA-48-producing Klebsiella pneumoniae ST147 in a hospital setting by SMRT sequencing. J Antimicrob Chemother 72:2737–2744. doi: 10.1093/jac/dkx216. [DOI] [PubMed] [Google Scholar]
- 132.Grundmann H, Glasner C, Albiger B, Aanensen DM, Tomlinson CT, Andrasević AT, Cantón R, Carmeli Y, Friedrich AW, Giske CG, Glupczynski Y, Gniadkowski M, Livermore DM, Nordmann P, Poirel L, Rossolini GM, Seifert H, Vatopoulos A, Walsh T, Woodford N, Monnet DL, European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) Working Group. 2017. Occurrence of carbapenemase-producing Klebsiella pneumoniae and Escherichia coli in the European survey of carbapenemase-producing Enterobacteriaceae (EuSCAPE): a prospective, multinational study. Lancet Infect Dis 17:153–163. doi: 10.1016/S1473-3099(16)30257-2. [DOI] [PubMed] [Google Scholar]
- 133.Pérez-Blanco V, Redondo-Bravo L, Ruíz-Carrascoso G, Paño-Pardo JR, Gómez-Gil R, Robustillo-Rodela A, García-Rodríguez J, Mingorance J, Herruzo R. 2018. Epidemiology and control measures of an OXA-48-producing Enterobacteriaceae hospital-wide oligoclonal outbreak. Epidemiol Infect 146:656–662. doi: 10.1017/S0950268818000249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Dimou V, Dhanji H, Pike R, Livermore DM, Woodford N. 2012. Characterization of Enterobacteriaceae producing OXA-48-like carbapenemases in the UK. J Antimicrob Chemother 67:1660–1665. doi: 10.1093/jac/dks124. [DOI] [PubMed] [Google Scholar]
- 135.Jayol A, Poirel L, Dortet L, Nordmann P. 2016. National survey of colistin resistance among carbapenemase-producing Enterobacteriaceae and outbreak caused by colistin-resistant OXA-48-producing Klebsiella pneumoniae, France, 2014. Euro Surveill 21:30339. doi: 10.2807/1560-7917.ES.2016.21.37.30339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Stoesser N, Sheppard AE, Peirano G, Sebra R, Lynch T, Anson L, Kasarskis A, Motyl MR, Crook DW, Pitout JD. 2016. Complete sequencing of plasmids containing blaOXA-163 and blaOXA-48 in Escherichia coli sequence type 131. Antimicrob Agents Chemother 60:6948–6951. doi: 10.1128/AAC.01130-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Qin S, Cheng J, Wang P, Feng X, Liu HM. 2018. Early emergence of OXA-181-producing Escherichia coli ST410 in China. J Glob Antimicrob Resist 15:215–218. doi: 10.1016/j.jgar.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 138.Al Bayssari C, Olaitan AO, Dabboussi F, Hamze M, Rolain JM. 2015. Emergence of OXA-48-producing Escherichia coli clone ST38 in fowl. Antimicrob Agents Chemother 59:745–746. doi: 10.1128/AAC.03552-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Stolle I, Prenger-Berninghoff E, Stamm I, Scheufen S, Hassdenteufel E, Guenther S, Bethe A, Pfeifer Y, Ewers C. 2013. Emergence of OXA-48 carbapenemase-producing Escherichia coli and Klebsiella pneumoniae in dogs. J Antimicrob Chemother 68:2802–2808. doi: 10.1093/jac/dkt259. [DOI] [PubMed] [Google Scholar]
- 140.Castanheira M, Deshpande LM, Mathai D, Bell JM, Jones RN, Mendes RE. 2011. Early dissemination of NDM-1- and OXA-181-producing Enterobacteriaceae in Indian hospitals: report from the SENTRY Antimicrobial Surveillance Program, 2006–2007. Antimicrob Agents Chemother 55:1274–1278. doi: 10.1128/AAC.01497-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Poirel L, Ros A, Carricajo A, Berthelot P, Pozzetto B, Bernabeu S, Nordmann P. 2011. Extremely drug-resistant Citrobacter freundii isolate producing NDM-1 and other carbapenemases identified in a patient returning from India. Antimicrob Agents Chemother 55:447–448. doi: 10.1128/AAC.01305-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Williamson DA, Heffernan H, Sidjabat H, Roberts SA, Paterson DL, Smith M, Freeman JT. 2011. Intercontinental transfer of OXA-181-producing Klebsiella pneumoniae into New Zealand. J Antimicrob Chemother 66:2888–2890. doi: 10.1093/jac/dkr396. [DOI] [PubMed] [Google Scholar]
- 143.Villa L, Carattoli A, Nordmann P, Carta C, Poirel L. 2013. Complete sequence of the IncT-type plasmid pT-OXA-181 carrying the blaOXA-181 carbapenemase gene from Citrobacter freundii. Antimicrob Agents Chemother 57:1965–1967. doi: 10.1128/AAC.01297-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Koh TH, Cao DY, Chan KS, Wijaya L, Low SB, Lam MS, Ooi EE, Hsu LY. 2012. bla(OXA-181)-positive Klebsiella pneumoniae, Singapore. Emerg Infect Dis 18:1524–1525. doi: 10.3201/eid1809.111727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Samuelsen O, Naseer U, Karah N, Lindemann PC, Kanestrom A, Leegaard TM, Sundsfjord A. 2013. Identification of Enterobacteriaceae isolates with OXA-48 and coproduction of OXA-181 and NDM-1 in Norway. J Antimicrob Chemother 68:1682–1685. doi: 10.1093/jac/dkt058. [DOI] [PubMed] [Google Scholar]
- 146.Székely E, Damjanova I, Jánvári L, Vas KE, Molnár S, Bilca DV, Lőrinczi LK, Tóth A. 2013. First description of bla(NDM-1), bla(OXA-48), bla(OXA-181) producing Enterobacteriaceae strains in Romania. Int J Med Microbiol 303:697–700. doi: 10.1016/j.ijmm.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 147.Peirano G, Ahmed-Bentley J, Fuller J, Rubin JE, Pitout JD. 2014. Travel-related carbapenemase-producing Gram-negative bacteria in Alberta, Canada: the first 3 years. J Clin Microbiol 52:1575–1581. doi: 10.1128/JCM.00162-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hall JM, Corea E, Sanjeewani HD, Inglis TJ. 2014. Molecular mechanisms of beta-lactam resistance in carbapenemase-producing Klebsiella pneumoniae from Sri Lanka. J Med Microbiol 63:1087–1092. doi: 10.1099/jmm.0.076760-0. [DOI] [PubMed] [Google Scholar]
- 149.Kayama S, Koba Y, Shigemoto N, Kuwahara R, Kakuhama T, Kimura K, Hisatsune J, Onodera M, Yokozaki M, Ohge H, Sugai M. 2015. Imipenem-susceptible, meropenem-resistant Klebsiella pneumoniae producing OXA-181 in Japan. Antimicrob Agents Chemother 59:1379–1380. doi: 10.1128/AAC.04330-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cho SY, Huh HJ, Baek JY, Chung NY, Ryu JG, Ki CS, Chung DR, Lee NY, Song JH. 2015. Klebsiella pneumoniae co-producing NDM-5 and OXA-181 carbapenemases, South Korea. Emerg Infect Dis 21:1088–1089. doi: 10.3201/eid2106.150048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Liu Y, Feng Y, Wu W, Xie Y, Wang X, Zhang X, Chen X, Zong Z. 2015. First report of OXA-181-producing Escherichia coli in China and characterization of the isolate using whole-genome sequencing. Antimicrob Agents Chemother 59:5022–5025. doi: 10.1128/AAC.00442-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lunha K, Chanawong A, Lulitanond A, Wilailuckana C, Charoensri N, Wonglakorn L, Saenjamla P, Chaimanee P, Angkititrakul S, Chetchotisakd P. 2016. High-level carbapenem-resistant OXA-48-producing Klebsiella pneumoniae with a novel OmpK36 variant and low-level, carbapenem-resistant, non-porin-deficient, OXA-181-producing Escherichia coli from Thailand. Diagn Microbiol Infect Dis 85:221–226. doi: 10.1016/j.diagmicrobio.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 153.Nahid F, Zahra R, Sandegren L. 2017. A blaOXA-181-harbouring multi-resistant ST147 Klebsiella pneumoniae isolate from Pakistan that represent an intermediate stage towards pan-drug resistance. PLoS One 12:e0189438. doi: 10.1371/journal.pone.0189438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Aung MS, San N, Maw WW, San T, Urushibara N, Kawaguchiya M, Sumi A, Kobayashi N. 2018. Prevalence of extended-spectrum beta-lactamase and carbapenemase genes in clinical isolates of Escherichia coli in Myanmar: dominance of blaNDM-5 and emergence of blaOXA-181. Microb Drug Resist 24:1333–1344. doi: 10.1089/mdr.2017.0387. [DOI] [PubMed] [Google Scholar]
- 155.Lutgring JD, Zhu W, de Man TJB, Avillan JJ, Anderson KF, Lonsway DR, Rowe LA, Batra D, Rasheed JK, Limbago BM. 2018. Phenotypic and genotypic characterization of Enterobacteriaceae producing oxacillinase-48-like carbapenemases, United States. Emerg Infect Dis 24:700–709. doi: 10.3201/eid2404.171377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.McGann P, Snesrud E, Ong AC, Appalla L, Koren M, Kwak YI, Waterman PE, Lesho EP. 2015. War wound treatment complications due to transfer of an IncN plasmid harboring bla(OXA-181) from Morganella morganii to CTX-M-27-producing sequence type 131 Escherichia coli. Antimicrob Agents Chemother 59:3556–3562. doi: 10.1128/AAC.04442-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Rojas LJ, Hujer AM, Rudin SD, Wright MS, Domitrovic TN, Marshall SH, Hujer KM, Richter SS, Cober E, Perez F, Adams MD, van Duin D, Bonomo RA. 2017. NDM-5 and OXA-181 beta-lactamases, a significant threat continues to spread in the Americas. Antimicrob Agents Chemother 61:e00454-17. doi: 10.1128/AAC.00454-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mataseje LF, Boyd DA, Fuller J, Haldane D, Hoang L, Lefebvre B, Melano RG, Poutanen S, Van Caeseele P, Mulvey MR. 2018. Characterization of OXA-48-like carbapenemase producers in Canada, 2011–14. J Antimicrob Chemother 73:626–633. doi: 10.1093/jac/dkx462. [DOI] [PubMed] [Google Scholar]
- 159.Hammerum AM, Littauer P, Hansen F. 2015. Detection of Klebsiella pneumoniae co-producing NDM-7 and OXA-181, Escherichia coli producing NDM-5 and Acinetobacter baumannii producing OXA-23 in a single patient. Int J Antimicrob Agents 46:597–598. doi: 10.1016/j.ijantimicag.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 160.Valentin T, Feierl G, Masoud-Landgraf L, Kohek P, Luxner J, Zarfel G. 2018. Proteus mirabilis harboring carbapenemase NDM-5 and ESBL VEB-6 detected in Austria. Diagn Microbiol Infect Dis 91:284–286. doi: 10.1016/j.diagmicrobio.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 161.Zurfluh K, Poirel L, Nordmann P, Klumpp J, Stephan R. 2015. First detection of Klebsiella variicola producing OXA-181 carbapenemase in fresh vegetable imported from Asia to Switzerland. Antimicrob Resist Infect Control 4:38. doi: 10.1186/s13756-015-0080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Dortet L, Cuzon G, Ponties V, Nordmann P. 2017. Trends in carbapenemase-producing Enterobacteriaceae, France, 2012 to 2014. Euro Surveill 22:30461. doi: 10.2807/1560-7917.ES.2017.22.6.30461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Piazza A, Comandatore F, Romeri F, Pagani C, Floriano AM, Ridolfo A, Antona C, Brilli M, Mattioni Marchetti V, Bandi C, Gismondo MR, Rimoldi SG. 2018. First report of an ST410 OXA-181 and CTX-M-15 coproducing Escherichia coli clone in Italy: a whole-genome sequence characterization. Microb Drug Resist 24:1207–1209. doi: 10.1089/mdr.2017.0366. [DOI] [PubMed] [Google Scholar]
- 164.Machuca J, Lopez-Cerero L, Fernandez-Cuenca F, Mora-Navas L, Mediavilla-Gradolph C, Lopez-Rodriguez I, Pascual A. 2019. OXA-48-like-producing Klebsiella pneumoniae in Southern Spain in 2014–2015. Antimicrob Agents Chemother 63:e01396-18. doi: 10.1128/AAC.01396-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Ouédraogo A-S, Compain F, Sanou M, Aberkane S, Bouzinbi N, Hide M, Sangaré L, Ouédraogo-Traoré R, Jean-Pierre H, Vendrell J, Solassol J, Decré D, Godreuil S. 2016. First description of IncX3 plasmids carrying blaOXA-181 in Escherichia coli clinical isolates in Burkina Faso. Antimicrob Agents Chemother 60:3240–3242. doi: 10.1128/AAC.00147-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gamal D, Fernández-Martínez M, El-Defrawy I, Ocampo-Sosa AA, Martínez-Martínez L. 2016. First identification of NDM-5 associated with OXA-181 in Escherichia coli from Egypt. Emerg Microbes Infect 5:e30. doi: 10.1038/emi.2016.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kieffer N, Nordmann P, Aires-de-Sousa M, Poirel L. 2016. High prevalence of carbapenemase-producing Enterobacteriaceae among hospitalized children in Luanda, Angola. Antimicrob Agents Chemother 60:6189–6192. doi: 10.1128/AAC.01201-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sangare SA, Rondinaud E, Maataoui N, Maiga AI, Guindo I, Maiga A, Camara N, Dicko OA, Dao S, Diallo S, Bougoudogo F, Andremont A, Maiga II, Armand-Lefevre L. 2017. Very high prevalence of extended-spectrum beta-lactamase-producing Enterobacteriaceae in bacteriemic patients hospitalized in teaching hospitals in Bamako, Mali. PLoS One 12:e0172652. doi: 10.1371/journal.pone.0172652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Uwaezuoke NS, Kieffer N, Iregbu KC, Nordmann P. 2017. First report of OXA-181 and NDM-1 from a clinical Klebsiella pneumoniae isolate from Nigeria. Int J Infect Dis 61:1–2. doi: 10.1016/j.ijid.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 170.Poirel L, Aires-de-Sousa M, Kudyba P, Kieffer N, Nordmann P. 2018. Screening and characterization of multidrug-resistant Gram-negative bacteria from a remote African area, Sao Tome and Principe. Antimicrob Agents Chemother 62:e01021-18. doi: 10.1128/AAC.01021-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Bitar I, Dagher C, Salloum T, Araj G, Tokajian S. 2018. First report of an Escherichia coli from Lebanon carrying an OXA-181 carbapenemase resistance determinant. J Glob Antimicrob Resist 12:113–114. doi: 10.1016/j.jgar.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 172.Abd El Ghany M, Sharaf H, Al-Agamy MH, Shibl A, Hill-Cawthorne GA, Hong PY. 2018. Genomic characterization of NDM-1 and 5, and OXA-181 carbapenemases in uropathogenic Escherichia coli isolates from Riyadh, Saudi Arabia. PLoS One 13:e0201613. doi: 10.1371/journal.pone.0201613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sidjabat HE, Townell N, Nimmo GR, George NM, Robson J, Vohra R, Davis L, Heney C, Paterson DL. 2015. Dominance of IMP-4-producing Enterobacter cloacae among carbapenemase-producing Enterobacteriaceae in Australia. Antimicrob Agents Chemother 59:4059–4066. doi: 10.1128/AAC.04378-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Walkty A, Gilmour M, Simner P, Embil JM, Boyd D, Mulvey M, Karlowsky J. 2015. Isolation of multiple carbapenemase-producing Gram-negative bacilli from a patient recently hospitalized in Nigeria. Diagn Microbiol Infect Dis 81:296–298. doi: 10.1016/j.diagmicrobio.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 175.Mahalingam N, Manivannan B, Khamari B, Siddaramappa S, Adak S, Bulagonda EP. 2018. Detection of antibiotic resistance determinants and their transmissibility among clinically isolated carbapenem-resistant Escherichia coli from South India. Med Princ Pract 27:428–435. doi: 10.1159/000489885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mohanty S, Gajanand M, Gaind R. 2017. Identification of carbapenemase-mediated resistance among Enterobacteriaceae bloodstream isolates: a molecular study from India. Indian J Med Microbiol 35:421–425. doi: 10.4103/ijmm.IJMM_16_386. [DOI] [PubMed] [Google Scholar]
- 177.Begum N, Shamsuzzaman SM. 2016. Emergence of carbapenemase-producing urinary isolates at a tertiary care hospital in Dhaka, Bangladesh. Ci Ji Yi Xue Za Zhi 28:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jacobson RK, Manesen MR, Moodley C, Smith M, Williams SG, Nicol MP, Bamford CM. 2015. Molecular characterisation and epidemiological investigation of an outbreak of blaOXA-181 carbapenemase-producing isolates of Klebsiella pneumoniae in South Africa. S Afr Med J 105:1030–1035. doi: 10.7196/SAMJ.2015.v105i12.9926. [DOI] [PubMed] [Google Scholar]
- 179.Sonnevend Á, Ghazawi A, Hashmey R, Haidermota A, Girgis S, Alfaresi M, Omar M, Paterson DL, Zowawi HM, Pal T. 2017. Multihospital occurrence of pan-resistant Klebsiella pneumoniae sequence type 147 with an ISEcp1-directed blaOXA-181 insertion in the mgrB gene in the United Arab Emirates. Antimicrob Agents Chemother 61:e00418-17. doi: 10.1128/AAC.00418-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Hayashi W, Togashi M, Taniguchi Y, Koide S, Nagano Y, Nagano N. 2018. First report of colistin resistance in OXA-181 carbapenemase-producing Klebsiella pneumoniae ST3130 in Japan. J Glob Antimicrob Resist 12:179–180. doi: 10.1016/j.jgar.2018.01.020. [DOI] [PubMed] [Google Scholar]
- 181.Anandan S, Gopi R, Devanga Ragupathi NK, Muthuirulandi Sethuvel DP, Gunasekaran P, Walia K, Veeraraghavan B. 2017. First report of blaOXA-181-mediated carbapenem resistance in Aeromonas caviae in association with pKP3-A: threat for rapid dissemination. J Glob Antimicrob Resist 10:310–314. doi: 10.1016/j.jgar.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 182.Meunier D, Doumith M, Findlay J, Mustafa N, Mallard K, Anson J, Panagea S, Pike R, Wright L, Woodford N, Hopkins KL. 2016. Carbapenem resistance mediated by blaOXA-181 in Pseudomonas aeruginosa. J Antimicrob Chemother 71:2056–2057. doi: 10.1093/jac/dkw087. [DOI] [PubMed] [Google Scholar]
- 183.Johnson TJ, Bielak EM, Fortini D, Hansen LH, Hasman H, Debroy C, Nolan LK, Carattoli A. 2012. Expansion of the IncX plasmid family for improved identification and typing of novel plasmids in drug-resistant Enterobacteriaceae. Plasmid 68:43–50. doi: 10.1016/j.plasmid.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 184.Wu W, Feng Y, Tang G, Qiao F, McNally A, Zong Z. 2019. NDM metallo-β-lactamases and their bacterial producers in health care settings. Clin Microbiol Rev 32:e00115-18. doi: 10.1128/CMR.00115-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Lowe M, Kock MM, Coetzee J, Hoosien E, Peirano G, Strydom KA, Ehlers MM, Mbelle NM, Shashkina E, Haslam DB, Dhawan P, Donnelly RJ, Chen L, Kreiswirth BN, Pitout J. 2019. Klebsiella pneumoniae ST307 with blaOXA-181, South Africa, 2014–2016. Emerg Infect Dis 25:739–747. doi: 10.3201/eid2504.181482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhao Y, Wang L, Zhang Z, Feng J, Kang H, Fang L, Jiang X, Zhang D, Zhan Z, Zhou D, Tong Y. 2017. Structural genomics of pNDM-BTR harboring In191 and Tn6360, and other bla. Future Microbiol 12:1271–1281. doi: 10.2217/fmb-2017-0067. [DOI] [PubMed] [Google Scholar]
- 187.Murata T, Ohnishi M, Ara T, Kaneko J, Han CG, Li YF, Takashima K, Nojima H, Nakayama K, Kaji A, Kamio Y, Miki T, Mori H, Ohtsubo E, Terawaki Y, Hayashi T. 2002. Complete nucleotide sequence of plasmid Rts1: implications for evolution of large plasmid genomes. J Bacteriol 184:3194–3202. doi: 10.1128/jb.184.12.3194-3202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Zowawi HM, Forde BM, Alfaresi M, Alzarouni A, Farahat Y, Chong TM, Yin WF, Chan KG, Li J, Schembri MA, Beatson SA, Paterson DL. 2015. Stepwise evolution of pandrug-resistance in Klebsiella pneumoniae. Sci Rep 5:15082. doi: 10.1038/srep15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Teo JW, Kurup A, Lin RT, Hsien KT. 2013. Emergence of clinical Klebsiella pneumoniae producing OXA-232 carbapenemase in Singapore. New Microbes New Infect 1:13–15. doi: 10.1002/2052-2975.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Doi Y, O’Hara JA, Lando JF, Querry AM, Townsend BM, Pasculle AW, Muto CA. 2014. Co-production of NDM-1 and OXA-232 by Klebsiella pneumoniae. Emerg Infect Dis 20:163–165. doi: 10.3201/eid2001.130904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Al-Marzooq F, Ngeow YF, Tay ST. 2015. Emergence of Klebsiella pneumoniae producing dual carbapenemases (NDM-1 and OXA-232) and 16S rRNA methylase (armA) isolated from a Malaysian patient returning from India. Int J Antimicrob Agents 45:445–446. doi: 10.1016/j.ijantimicag.2014.12.013. [DOI] [PubMed] [Google Scholar]
- 192.Jeong SH, Lee KM, Lee J, Bae IK, Kim JS, Kim HS, Song W. 2015. Clonal and horizontal spread of the blaOXA-232 gene among Enterobacteriaceae in a Korean hospital. Diagn Microbiol Infect Dis 82:70–72. doi: 10.1016/j.diagmicrobio.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 193.Both A, Huang J, Kaase M, Hezel J, Wertheimer D, Fenner I, Günther T, Grundhoff A, Büttner H, Aepfelbacher M, Rohde H, Hentschke M. 2016. First report of Escherichia coli co-producing NDM-1 and OXA-232. Diagn Microbiol Infect Dis 86:437–438. doi: 10.1016/j.diagmicrobio.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 194.Avolio M, Vignaroli C, Crapis M, Camporese A. 2017. Co-production of NDM-1 and OXA-232 by ST16 Klebsiella pneumoniae, Italy, 2016. Future Microbiol 12:1119–1122. doi: 10.2217/fmb-2017-0041. [DOI] [PubMed] [Google Scholar]
- 195.Mancini S, Poirel L, Tritten ML, Lienhard R, Bassi C, Nordmann P. 2018. Emergence of an MDR Klebsiella pneumoniae ST231 producing OXA-232 and RmtF in Switzerland. J Antimicrob Chemother 73:821–823. doi: 10.1093/jac/dkx428. [DOI] [PubMed] [Google Scholar]
- 196.Abdul Momin MHF, Liakopoulos A, Phee LM, Wareham DW. 2017. Emergence and nosocomial spread of carbapenem-resistant OXA-232-producing Klebsiella pneumoniae in Brunei Darussalam. J Glob Antimicrob Resist 9:96–99. doi: 10.1016/j.jgar.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 197.Yin D, Dong D, Li K, Zhang L, Liang J, Yang Y, Wu N, Bao Y, Wang C, Hu F. 2017. Clonal dissemination of OXA-232 carbapenemase-producing Klebsiella pneumoniae in neonates. Antimicrob Agents Chemother 61:e00385-17. doi: 10.1128/AAC.00385-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Laolerd W, Akeda Y, Preeyanon L, Ratthawongjirakul P, Santanirand P. 2018. Carbapenemase-producing carbapenem-resistant Enterobacteriaceae from Bangkok, Thailand, and their detection by the Carba NP and modified carbapenem inactivation method tests. Microb Drug Resist 24:1006–1011. doi: 10.1089/mdr.2018.0080. [DOI] [PubMed] [Google Scholar]
- 199.Lahlaoui H, Bonnin RA, Moussa MB, Khelifa ABH, Naas T. 2017. First report of OXA-232-producing Klebsiella pneumoniae strains in Tunisia. Diagn Microbiol Infect Dis 88:195–197. doi: 10.1016/j.diagmicrobio.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 200.Jeong SH, Kim HS, Kim JS, Shin DH, Kim HS, Park MJ, Shin S, Hong JS, Lee SS, Song W. 2016. Prevalence and molecular characteristics of carbapenemase-producing Enterobacteriaceae from five hospitals in Korea. Ann Lab Med 36:529–535. doi: 10.3343/alm.2016.36.6.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yang S, Hemarajata P, Hindler J, Li F, Adisetiyo H, Aldrovandi G, Sebra R, Kasarskis A, MacCannell D, Didelot X, Russell D, Rubin Z, Humphries R. 2017. Evolution and transmission of carbapenem-resistant Klebsiella pneumoniae expressing the blaOXA-232 gene during an institutional outbreak associated with endoscopic retrograde cholangiopancreatography. Clin Infect Dis 64:894–901. doi: 10.1093/cid/ciw876. [DOI] [PubMed] [Google Scholar]
- 202.Shu L, Dong N, Lu J, Zheng Z, Hu J, Zeng W, Sun Q, Wai-Chi Chan E, Zhou H, Hu F, Chen S, Zhang R. 2019. Emergence of OXA-232 carbapenemase-producing Klebsiella pneumoniae that carries a pLVPK-like virulence plasmid among elderly patients in China. Antimicrob Agents Chemother 63:e02246-18. doi: 10.1128/AAC.02246-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Espinal P, Nucleo E, Caltagirone M, Mattioni Marchetti V, Fernandes MR, Biscaro V, Rigoli R, Carattoli A, Migliavacca R, Villa L. 2019. Genomics of Klebsiella pneumoniae ST16 producing NDM-1, CTX-M-15, and OXA-232. Clin Microbiol Infect 25:385.e1–385.e5. doi: 10.1016/j.cmi.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 204.Charfi K, Mansour W, Khalifa AB, Mastouri M, Aouni M, Mammeri H. 2015. Emergence of OXA-204 beta-lactamase in Tunisia. Diagn Microbiol Infect Dis 82:314–317. doi: 10.1016/j.diagmicrobio.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 205.Harmer CJ, Hall RM. 2015. The A to Z of A/C plasmids. Plasmid 80:63–82. doi: 10.1016/j.plasmid.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 206.Grami R, Mansour W, Ben Haj Khalifa A, Dahmen S, Chatre P, Haenni M, Aouni M, Madec JY. 2016. Emergence of ST147 Klebsiella pneumoniae producing OXA-204 carbapenemase in a university hospital. Tunisia Microb Drug Resist 22:137–140. doi: 10.1089/mdr.2014.0278. [DOI] [PubMed] [Google Scholar]
- 207.Pfeifer Y, Schlatterer K, Engelmann E, Schiller RA, Frangenberg HR, Stiewe D, Holfelder M, Witte W, Nordmann P, Poirel L. 2012. Emergence of OXA-48-type carbapenemase-producing Enterobacteriaceae in German hospitals. Antimicrob Agents Chemother 56:2125–2128. doi: 10.1128/AAC.05315-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Janvari L, Damjanova I, Lazar A, Racz K, Kocsis B, Urban E, Toth A. 2014. Emergence of OXA-162-producing Klebsiella pneumoniae in Hungary. Scand J Infect Dis 46:320–324. doi: 10.3109/00365548.2013.879993. [DOI] [PubMed] [Google Scholar]
- 209.Voulgari E, Poulou A, Dimitroulia E, Politi L, Ranellou K, Gennimata V, Markou F, Pournaras S, Tsakris A. 2015. Emergence of OXA-162 carbapenemase- and DHA-1 AmpC cephalosporinase-producing sequence type 11 Klebsiella pneumoniae causing community-onset infection in Greece. Antimicrob Agents Chemother 60:1862–1864. doi: 10.1128/AAC.01514-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Valenza G, Nickel S, Pfeifer Y, Eller C, Krupa E, Lehner-Reindl V, Höller C. 2014. Extended-spectrum-beta-lactamase-producing Escherichia coli as intestinal colonizers in the German community. Antimicrob Agents Chemother 58:1228–1230. doi: 10.1128/AAC.01993-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Potron A, Poirel L, Dortet L, Nordmann P. 2016. Characterisation of OXA-244, a chromosomally-encoded OXA-48-like beta-lactamase from Escherichia coli. Int J Antimicrob Agents 47:102–103. doi: 10.1016/j.ijantimicag.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 212.van Hattem JM, Arcilla MS, Bootsma MC, van Genderen PJ, Goorhuis A, Grobusch MP, Molhoek N, Oude Lashof AM, Schultsz C, Stobberingh EE, Verbrugh HA, de Jong MD, Melles DC, Penders J. 2016. Prolonged carriage and potential onward transmission of carbapenemase-producing Enterobacteriaceae in Dutch travelers. Future Microbiol 11:857–864. doi: 10.2217/fmb.16.18. [DOI] [PubMed] [Google Scholar]
- 213.Oteo J, Ortega A, Bartolome R, Bou G, Conejo C, Fernandez-Martinez M, Gonzalez-Lopez JJ, Martinez-Garcia L, Martinez-Martinez L, Merino M, Miro E, Mora M, Navarro F, Oliver A, Pascual A, Rodriguez-Bano J, Ruiz-Carrascoso G, Ruiz-Garbajosa P, Zamorano L, Bautista V, Perez-Vazquez M, Campos J, GEIH-GEMARA (SEIMC) and REIPI. 2015. Prospective multicenter study of carbapenemase-producing Enterobacteriaceae from 83 hospitals in Spain reveals high in vitro susceptibility to colistin and meropenem. Antimicrob Agents Chemother 59:3406–3412. doi: 10.1128/AAC.00086-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Samuelsen O, Hansen F, Aasnaes B, Hasman H, Lund BA, Leiros HS, Lilje B, Janice J, Jakobsen L, Littauer P, Soes LM, Holzknecht BJ, Andersen LP, Stegger M, Andersen PS, Hammerum AM. 2018. Dissemination and characteristics of a novel plasmid-encoded carbapenem-hydrolyzing class D beta-lactamase, OXA-436, found in isolates from four patients at six different hospitals in Denmark. Antimicrob Agents Chemother 62:e01260-17. doi: 10.1128/AAC.01260-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Nordmann P, Gniadkowski M, Giske CG, Poirel L, Woodford N, Miriagou V, European Network on Carbapenemases. 2012. Identification and screening of carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 18:432–438. doi: 10.1111/j.1469-0691.2012.03815.x. [DOI] [PubMed] [Google Scholar]
- 216.Huang TD, Poirel L, Bogaerts P, Berhin C, Nordmann P, Glupczynski Y. 2014. Temocillin and piperacillin/tazobactam resistance by disc diffusion as antimicrobial surrogate markers for the detection of carbapenemase-producing Enterobacteriaceae in geographical areas with a high prevalence of OXA-48 producers. J Antimicrob Chemother 69:445–450. doi: 10.1093/jac/dkt367. [DOI] [PubMed] [Google Scholar]
- 217.CLSI. 2018. Performance standards for antimicrobial susceptibility testing, 28th ed CLSI supplement M100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 218.The European Committee on Antimicrobial Susceptibility Testing. 2019. Breakpoint tables for interpretation of MICs and zone diameters, version 9.0. http://www.eucast.org.
- 219.EUCAST subcommittee for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance. July 2017. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance, version 2.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_170711.pdf.
- 220.EUCAST Subcommittee for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance. December 2013. EUCAST guidelines for detection of resistance mechanisms and specific resistances of clinical and/or epidemiological importance, version 1.0. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_v1.0_20131211.pdf.
- 221.Fattouh R, Tijet N, McGeer A, Poutanen SM, Melano RG, Patel SN. 2015. What is the appropriate meropenem MIC for screening of carbapenemase-producing Enterobacteriaceae in low-prevalence settings? Antimicrob Agents Chemother 60:1556–1559. doi: 10.1128/AAC.02304-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Nordmann P, Poirel L. 2014. The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin Microbiol Infect 20:821–830. doi: 10.1111/1469-0691.12719. [DOI] [PubMed] [Google Scholar]
- 223.Maurer FP, Castelberg C, Quiblier C, Bloemberg GV, Hombach M. 2015. Evaluation of carbapenemase screening and confirmation tests with Enterobacteriaceae and development of a practical diagnostic algorithm. J Clin Microbiol 53:95–104. doi: 10.1128/JCM.01692-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Day KM, Pike R, Winstanley TG, Lanyon C, Cummings SP, Raza MW, Woodford N, Perry JD. 2013. Use of faropenem as an indicator of carbapenemase activity in the Enterobacteriaceae. J Clin Microbiol 51:1881–1886. doi: 10.1128/JCM.00720-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Dortet L, Bernabeu S, Gonzalez C, Naas T. 2017. Comparison of two phenotypic algorithms to detect carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 61:e00796-17. doi: 10.1128/AAC.00796-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Dortet L, Bernabeu S, Gonzalez C, Naas T. 2018. Evaluation of the carbapenem detection set for the detection and characterization of carbapenemase-producing Enterobacteriaceae. Diagn Microbiol Infect Dis 91:220–225. doi: 10.1016/j.diagmicrobio.2018.02.012. [DOI] [PubMed] [Google Scholar]
- 227.Haldorsen B, Giske CG, Hansen DS, Helgason KO, Kahlmeter G, Löhr IH, Matuschek E, Österblad M, Rantakokko-Jalava K, Wang M, Småbrekke L, Samuelsen Ø, Sundsfjord A, NordicAST CPE Study Group. 2018. Performance of the EUCAST disc diffusion method and two MIC methods in detection of Enterobacteriaceae with reduced susceptibility to meropenem: the NordicAST CPE study. J Antimicrob Chemother 73:2738–2747. doi: 10.1093/jac/dky276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Woodford N, Eastaway AT, Ford M, Leanord A, Keane C, Quayle RM, Steer JA, Zhang J, Livermore DM. 2010. Comparison of BD Phoenix, Vitek 2, and MicroScan automated systems for detection and inference of mechanisms responsible for carbapenem resistance in Enterobacteriaceae. J Clin Microbiol 48:2999–3002. doi: 10.1128/JCM.00341-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Ong CH, Ratnayake L, Ang MLT, Lin RTP, Chan D. 2018. Diagnostic accuracy of BD Phoenix CPO detect for carbapenemase production in 190 Enterobacteriaceae isolates. J Clin Microbiol 56:e01043-18. doi: 10.1128/JCM.01043-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Doi Y, Paterson DL. 2015. Carbapenemase-producing Enterobacteriaceae. Semin Respir Crit Care Med 36:74–84. doi: 10.1055/s-0035-1544208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Hartl R, Widhalm S, Kerschner H, Apfalter P. 2013. Temocillin and meropenem to discriminate resistance mechanisms leading to decreased carbapenem susceptibility with focus on OXA-48 in Enterobacteriaceae. Clin Microbiol Infect 19:E230–E232. doi: 10.1111/1469-0691.12146. [DOI] [PubMed] [Google Scholar]
- 232.van Dijk K, Voets GM, Scharringa J, Voskuil S, Fluit AC, Rottier WC, Leverstein-Van Hall MA, Cohen Stuart JW. 2014. A disc diffusion assay for detection of class A, B and OXA-48 carbapenemases in Enterobacteriaceae using phenyl boronic acid, dipicolinic acid and temocillin. Clin Microbiol Infect 20:345–349. doi: 10.1111/1469-0691.12322. [DOI] [PubMed] [Google Scholar]
- 233.Robert J, Pantel A, Merens A, Meiller E, Lavigne JP, Nicolas-Chanoine MH, ONERBA’s carbapenem resistance study group. 2017. Development of an algorithm for phenotypic screening of carbapenemase-producing Enterobacteriaceae in the routine laboratory. BMC Infect Dis 17:78. doi: 10.1186/s12879-016-2174-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ohsaki Y, Kubo R, Hobson J, Davies M, Osaka S, Hayashi W, Taniguchi Y, Koide S, Nagano Y, Nagano N. 2018. MASTDISCS combi Carba plus, a simple method for discriminating carbapenemase-producing Enterobacteriaceae, including OXA-48-type producers. Microbiol Immunol 62:60–65. doi: 10.1111/1348-0421.12553. [DOI] [PubMed] [Google Scholar]
- 235.Sabtcheva S, Todorova B, Ivanov I, Ivanova K, Dobrinov V, Dobreva E, Hristova R, Nedyalkov M, Kantardjiev T. 2016. Comparison of two combination disc tests for phenotypic detection of carbapenemase-producing Enterobacteriaceae. Probl Inf Parasit Dis 44:8–11. [Google Scholar]
- 236.Vasoo S, Cunningham SA, Cole NC, Kohner PC, Menon SR, Krause KM, Harris KA, De PP, Koh TH, Patel R. 2015. In vitro activities of ceftazidime-avibactam, aztreonam-avibactam, and a panel of older and contemporary antimicrobial agents against carbapenemase-producing Gram-negative bacilli. Antimicrob Agents Chemother 59:7842–7846. doi: 10.1128/AAC.02019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Livermore DM, Mushtaq S, Warner M, Zhang J, Maharjan S, Doumith M, Woodford N. 2011. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 55:390–394. doi: 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Huang TD, Berhin C, Bogaerts P, Glupczynski Y. 2014. Evaluation of avibactam-supplemented combination disk tests for the detection of OXA-48 carbapenemase-producing Enterobacteriaceae. Diagn Microbiol Infect Dis 79:252–254. doi: 10.1016/j.diagmicrobio.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 239.Tsakris A, Poulou A, Bogaerts P, Dimitroulia E, Pournaras S, Glupczynski Y. 2015. Evaluation of a new phenotypic OXA-48 disk test for differentiation of OXA-48 carbapenemase-producing Enterobacteriaceae clinical isolates. J Clin Microbiol 53:1245–1251. doi: 10.1128/JCM.03318-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Nordmann P, Poirel L, Dortet L. 2012. Rapid detection of carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis 18:1503–1507. doi: 10.3201/eid1809.120355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Dortet L, Bréchard L, Poirel L, Nordmann P. 2014. Impact of the isolation medium for detection of carbapenemase-producing Enterobacteriaceae using an updated version of the Carba NP test. J Med Microbiol 63:772–776. doi: 10.1099/jmm.0.071340-0. [DOI] [PubMed] [Google Scholar]
- 242.Tijet N, Boyd D, Patel SN, Mulvey MR, Melano RG. 2013. Evaluation of the Carba NP test for rapid detection of carbapenemase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 57:4578–4580. doi: 10.1128/AAC.00878-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.AbdelGhani S, Thomson GK, Snyder JW, Thomson KS. 2015. Comparison of the Carba NP, modified Carba NP, and updated Rosco Neo-Rapid Carb kit tests for carbapenemase detection. J Clin Microbiol 53:3539–3542. doi: 10.1128/JCM.01631-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Dortet L, Agathine A, Naas T, Cuzon G, Poirel L, Nordmann P. 2015. Evaluation of the RAPIDEC CARBA NP, the Rapid CARB Screen and the Carba NP test for biochemical detection of carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 70:3014–3022. doi: 10.1093/jac/dkv213. [DOI] [PubMed] [Google Scholar]
- 245.Kabir MH, Meunier D, Hopkins KL, Giske CG, Woodford N. 2016. A two-centre evaluation of RAPIDEC CARBA NP for carbapenemase detection in Enterobacteriaceae, Pseudomonas aeruginosa and Acinetobacter spp. J Antimicrob Chemother 71:1213–1216. doi: 10.1093/jac/dkv468. [DOI] [PubMed] [Google Scholar]
- 246.Pires J, Novais A, Peixe L. 2013. Blue-carba, an easy biochemical test for detection of diverse carbapenemase producers directly from bacterial cultures. J Clin Microbiol 51:4281–4283. doi: 10.1128/JCM.01634-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.García-Fernández S, Morosini MI, Gijón D, Beatobe L, Ruiz-Garbajosa P, Domínguez L, Cantón R, Valverde A. 2016. Detection of carbapenemase production in a collection of Enterobacteriaceae with characterized resistance mechanisms from clinical and environmental origins by use of both Carba NP and Blue-Carba tests. J Clin Microbiol 54:464–466. doi: 10.1128/JCM.02580-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Pires J, Tinguely R, Thomas B, Luzzaro F, Endimiani A. 2016. Comparison of the in-house made Carba-NP and Blue-Carba tests: considerations for better detection of carbapenemase-producing Enterobacteriaceae. J Microbiol Methods 122:33–37. doi: 10.1016/j.mimet.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 249.Pasteran F, Veliz O, Ceriana P, Lucero C, Rapoport M, Albornoz E, Gomez S, Corso A, Group RN. 2015. Evaluation of the Blue-Carba test for rapid detection of carbapenemases in gram-negative bacilli. J Clin Microbiol 53:1996–1998. doi: 10.1128/JCM.03026-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Novais Â, Brilhante M, Pires J, Peixe L. 2015. Evaluation of the recently launched rapid Carb Blue kit for detection of carbapenemase-producing Gram-negative bacteria. J Clin Microbiol 53:3105–3107. doi: 10.1128/JCM.01170-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Pasteran F, Tijet N, Melano RG, Corso A. 2015. Simplified protocol for Carba NP test for enhanced detection of carbapenemase producers directly from bacterial cultures. J Clin Microbiol 53:3908–3911. doi: 10.1128/JCM.02032-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Ho PL, Wang Y, Wing-Sze Tse C, Fung KS, Cheng VC, Lee R, To WK, Lai RW, Luk WK, Que TL, Tsang DN. 2018. Rapid detection of carbapenemase production in Enterobacteriaceae by use of a modified paper strip Carba NP method. J Clin Microbiol 56:e01110-17. doi: 10.1128/JCM.01110-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Compain F, Gallah S, Eckert C, Arlet G, Ramahefasolo A, Decré D, Lavollay M, Podglajen I. 2016. Assessment of carbapenem resistance in Enterobacteriaceae with the rapid and easy-to-use chromogenic β Carba test. J Clin Microbiol 54:3065–3068. doi: 10.1128/JCM.01912-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Bernabeu S, Dortet L, Naas T. 2017. Evaluation of the β-CARBA™ test, a colorimetric test for the rapid detection of carbapenemase activity in Gram-negative bacilli. J Antimicrob Chemother 72:1646–1658. doi: 10.1093/jac/dkx061. [DOI] [PubMed] [Google Scholar]
- 255.van der Zwaluw K, de Haan A, Pluister GN, Bootsma HJ, de Neeling AJ, Schouls LM. 2015. The carbapenem inactivation method (CIM), a simple and low-cost alternative for the Carba NP test to assess phenotypic carbapenemase activity in gram-negative rods. PLoS One 10:e0123690. doi: 10.1371/journal.pone.0123690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Tijet N, Patel SN, Melano RG. 2015. Detection of carbapenemase activity in Enterobacteriaceae: comparison of the carbapenem inactivation method versus the Carba NP test. J Antimicrob Chemother 71:274–276. doi: 10.1093/jac/dkv283. [DOI] [PubMed] [Google Scholar]
- 257.Pierce VM, Simner PJ, Lonsway DR, Roe-Carpenter DE, Johnson JK, Brasso WB, Bobenchik AM, Lockett ZC, Charnot-Katsikas A, Ferraro MJ, Thomson RB, Jenkins SG, Limbago BM, Das S. 2017. Modified carbapenem inactivation method for phenotypic detection of carbapenemase production among Enterobacteriaceae. J Clin Microbiol 55:2321–2333. doi: 10.1128/JCM.00193-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Tamma PD, Opene BN, Gluck A, Chambers KK, Carroll KC, Simner PJ. 2017. Comparison of 11 phenotypic assays for accurate detection of carbapenemase-producing Enterobacteriaceae. J Clin Microbiol 55:1046–1055. doi: 10.1128/JCM.02338-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Caméléna F, Cointe A, Mathy V, Hobson C, Doit C, Bercot B, Decré D, Podglajen I, Dortet L, Monjault A, Bidet P, Bonacorsi S, Birgy A. 2018. Within-a-day detection and rapid characterization of carbapenemase by use of a new carbapenem inactivation method-based test, CIMplus. J Clin Microbiol 56:e00137-18. doi: 10.1128/JCM.00137-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Muntean MM, Muntean AA, Gauthier L, Creton E, Cotellon G, Popa MI, Bonnin RA, Naas T. 2018. Evaluation of the rapid carbapenem inactivation method (rCIM): a phenotypic screening test for carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 73:900–908. doi: 10.1093/jac/dkx519. [DOI] [PubMed] [Google Scholar]
- 261.Mathers AJ, Carroll J, Sifri CD, Hazen KC. 2013. Modified Hodge test versus indirect carbapenemase test: prospective evaluation of a phenotypic assay for detection of Klebsiella pneumoniae carbapenemase (KPC) in Enterobacteriaceae. J Clin Microbiol 51:1291–1293. doi: 10.1128/JCM.03240-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Creighton J, Tibbs C. 2017. Evaluation of the MAST indirect carbapenemase test and comparison with a modified carbapenem inactivation method for the detection of carbapenemase enzymes in Gram-negative bacteria. N Z J Med Lab Sci 71:136–140. [Google Scholar]
- 263.Wareham DW, Shah R, Betts JW, Phee LM, Momin MH. 2016. Evaluation of an immunochromatographic lateral flow assay (OXA-48 K-SeT) for rapid detection of OXA-48-like carbapenemases in Enterobacteriaceae. J Clin Microbiol 54:471–473. doi: 10.1128/JCM.02900-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Glupczynski Y, Jousset A, Evrard S, Bonnin RA, Huang TD, Dortet L, Bogaerts P, Naas T. 2017. Prospective evaluation of the OKN K-SeT assay, a new multiplex immunochromatographic test for the rapid detection of OXA-48-like, KPC and NDM carbapenemases. J Antimicrob Chemother 72:1955–1960. doi: 10.1093/jac/dkx089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Kolenda C, Benoit R, Carricajo A, Bonnet R, Dauwalder O, Laurent F. 2018. Evaluation of the new multiplex immunochromatographic O.K.N.V. K-SeT assay for rapid detection of OXA-48-like, KPC, NDM, and VIM carbapenemases. J Clin Microbiol 56:e01247-18. doi: 10.1128/JCM.01247-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Boutal H, Vogel A, Bernabeu S, Devilliers K, Creton E, Cotellon G, Plaisance M, Oueslati S, Dortet L, Jousset A, Simon S, Naas T, Volland H. 2018. A multiplex lateral flow immunoassay for the rapid identification of NDM-, KPC-, IMP- and VIM-type and OXA-48-like carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother 73:909–915. doi: 10.1093/jac/dkx521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Arlet G, Decré D, Lavollay M, Podglajen I. 2017. Reply to “Noncarbapenemase OXA-48 variants (OXA-163 and OXA-405) falsely detected as carbapenemases by the β Carba test.” J Clin Microbiol 55:656–657. doi: 10.1128/JCM.02114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Hopkins KL, Meunier D, Naas T, Volland H, Woodford N. 2018. Evaluation of the NG-Test CARBA 5 multiplex immunochromatographic assay for the detection of KPC, OXA-48-like, NDM, VIM and IMP carbapenemases. J Antimicrob Chemother 73:3523–3526. doi: 10.1093/jac/dky342. [DOI] [PubMed] [Google Scholar]
- 269.Bogaerts P, Yunus S, Massart M, Huang TD, Glupczynski Y. 2016. Evaluation of the BYG Carba test, a new electrochemical assay for rapid laboratory detection of carbapenemase-producing Enterobacteriaceae. J Clin Microbiol 54:349–358. doi: 10.1128/JCM.02404-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Bogaerts P, Oueslati S, Meunier D, Nonhoff C, Yunus S, Massart M, Denis O, Woodford N, Hopkins KL, Naas T, Dortet L, Huang TD, Glupczynski Y. 2017. Multicentre evaluation of the BYG Carba v2.0 test, a simplified electrochemical assay for the rapid laboratory detection of carbapenemase-producing Enterobacteriaceae. Sci Rep 7:9937. doi: 10.1038/s41598-017-09820-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Bernabeu S, Poirel L, Nordmann P. 2012. Spectrophotometry-based detection of carbapenemase producers among Enterobacteriaceae. Diagn Microbiol Infect Dis 74:88–90. doi: 10.1016/j.diagmicrobio.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 272.Dortet L, Bréchard L, Cuzon G, Poirel L, Nordmann P. 2014. Strategy for rapid detection of carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 58:2441–2445. doi: 10.1128/AAC.01239-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Hrabák J, Studentová V, Walková R, Zemlicková H, Jakubu V, Chudácková E, Gniadkowski M, Pfeifer Y, Perry JD, Wilkinson K, Bergerová T. 2012. Detection of NDM-1, VIM-1, KPC, OXA-48, and OXA-162 carbapenemases by matrix-assisted laser desorption ionization–time of flight mass spectrometry. J Clin Microbiol 50:2441–2443. doi: 10.1128/JCM.01002-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Burckhardt I, Zimmermann S. 2011. Using matrix-assisted laser desorption ionization-time of flight mass spectrometry to detect carbapenem resistance within 1 to 2.5 hours. J Clin Microbiol 49:3321–3324. doi: 10.1128/JCM.00287-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Hrabák J, Chudáčková E, Papagiannitsis CC. 2014. Detection of carbapenemases in Enterobacteriaceae: a challenge for diagnostic microbiological laboratories. Clin Microbiol Infect 20:839–853. doi: 10.1111/1469-0691.12678. [DOI] [PubMed] [Google Scholar]
- 276.Sauget M, Cabrolier N, Manzoni M, Bertrand X, Hocquet D. 2014. Rapid, sensitive and specific detection of OXA-48-like-producing Enterobacteriaceae by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J Microbiol Methods 105:88–91. doi: 10.1016/j.mimet.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 277.Studentova V, Papagiannitsis CC, Izdebski R, Pfeifer Y, Chudackova E, Bergerova T, Gniadkowski M, Hrabak J. 2015. Detection of OXA-48-type carbapenemase-producing Enterobacteriaceae in diagnostic laboratories can be enhanced by addition of bicarbonates to cultivation media or reaction buffers. Folia Microbiol (Praha) 60:119–129. doi: 10.1007/s12223-014-0349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Papagiannitsis CC, Študentová V, Izdebski R, Oikonomou O, Pfeifer Y, Petinaki E, Hrabák J. 2015. Matrix-assisted laser desorption ionization–time of flight mass spectrometry meropenem hydrolysis assay with NH4HCO3, a reliable tool for direct detection of carbapenemase activity. J Clin Microbiol 53:1731–1735. doi: 10.1128/JCM.03094-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Lee AWT, Lam JKS, Lam RKW, Ng WH, Lee ENL, Lee VTY, Sze PP, Rajwani R, Fung KSC, To WK, Lee RA, Tsang DNC, Siu G. 2018. Comprehensive evaluation of the MBT STAR-BL module for simultaneous bacterial identification and β-lactamase-mediated resistance detection in Gram-negative rods from cultured isolates and positive blood cultures. Front Microbiol 9:334. doi: 10.3389/fmicb.2018.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Dortet L, Tandé D, de Briel D, Bernabeu S, Lasserre C, Gregorowicz G, Jousset AB, Naas T. 2018. MALDI-TOF for the rapid detection of carbapenemase-producing Enterobacteriaceae: comparison of the commercialized MBT STAR-Carba IVD kit with two in-house MALDI-TOF techniques and the RAPIDEC CARBA NP. J Antimicrob Chemother 73:2352–2359. doi: 10.1093/jac/dky209. [DOI] [PubMed] [Google Scholar]
- 281.Findlay J, Hopkins KL, Meunier D, Woodford N. 2015. Evaluation of three commercial assays for rapid detection of genes encoding clinically relevant carbapenemases in cultured bacteria. J Antimicrob Chemother 70:1338–1342. doi: 10.1093/jac/dku571. [DOI] [PubMed] [Google Scholar]
- 282.Dortet L, Fusaro M, Naas T. 2016. Improvement of the Xpert Carba-R kit for the detection of carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother 60:3832–3837. doi: 10.1128/AAC.00517-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Doyle D, Peirano G, Lascols C, Lloyd T, Church DL, Pitout JD. 2012. Laboratory detection of Enterobacteriaceae that produce carbapenemases. J Clin Microbiol 50:3877–3880. doi: 10.1128/JCM.02117-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Gans JD, Wolinsky M. 2008. Improved assay-dependent searching of nucleic acid sequence databases. Nucleic Acids Res 36:e74. doi: 10.1093/nar/gkn301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Poirel L, Walsh TR, Cuvillier V, Nordmann P. 2011. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis 70:119–123. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 286.Monteiro J, Widen RH, Pignatari AC, Kubasek C, Silbert S. 2012. Rapid detection of carbapenemase genes by multiplex real-time PCR. J Antimicrob Chemother 67:906–909. doi: 10.1093/jac/dkr563. [DOI] [PubMed] [Google Scholar]
- 287.Lee TD, Adie K, McNabb A, Purych D, Mannan K, Azana R, Ng C, Tang P, Hoang LM. 2015. Rapid detection of KPC, NDM, and OXA-48-like carbapenemases by real-time PCR from rectal swab surveillance samples. J Clin Microbiol 53:2731–2733. doi: 10.1128/JCM.01237-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.van der Zee A, Roorda L, Bosman G, Fluit AC, Hermans M, Smits PH, van der Zanden AG, Te Witt R, Bruijnesteijn van Coppenraet LE, Cohen Stuart J, Ossewaarde JM. 2014. Multi-centre evaluation of real-time multiplex PCR for detection of carbapenemase genes OXA-48, VIM, IMP, NDM and KPC. BMC Infect Dis 14:27. doi: 10.1186/1471-2334-14-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Hemarajata P, Yang S, Hindler JA, Humphries RM. 2015. Development of a novel real-time PCR assay with high-resolution melt analysis to detect and differentiate OXA-48-like β-lactamases in carbapenem-resistant Enterobacteriaceae. Antimicrob Agents Chemother 59:5574–5580. doi: 10.1128/AAC.00425-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Srisrattakarn A, Lulitanond A, Wilailuckana C, Charoensri N, Wonglakorn L, Saenjamla P, Chaimanee P, Daduang J, Chanawong A. 2017. Rapid and simple identification of carbapenemase genes, blaNDM, blaOXA-48, blaVIM, blaIMP-14 and blaKPC groups, in Gram-negative bacilli by in-house loop-mediated isothermal amplification with hydroxynaphthol blue dye. World J Microbiol Biotechnol 33:130. doi: 10.1007/s11274-017-2295-5. [DOI] [PubMed] [Google Scholar]
- 291.Ceyssens PJ, Garcia-Graells C, Fux F, Botteldoorn N, Mattheus W, Wuyts V, De Keersmaecker S, Dierick K, Bertrand S. 2016. Development of a Luminex xTAG assay for cost-effective multiplex detection of β-lactamases in Gram-negative bacteria. J Antimicrob Chemother 71:2479–2483. doi: 10.1093/jac/dkw201. [DOI] [PubMed] [Google Scholar]
- 292.Lau AF, Fahle GA, Kemp MA, Jassem AN, Dekker JP, Frank KM. 2015. Clinical performance of Check-Direct CPE, a multiplex PCR for direct detection of bla(KPC), bla(NDM) and/or bla(VIM), and bla(OXA)-48 from perirectal swabs. J Clin Microbiol 53:3729–3737. doi: 10.1128/JCM.01921-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Vanstone GL, Wey E, Mack D, Smith ER, Balakrishnan I. 2018. Evaluation of the EntericBio CPE assay for the detection of carbapenemase-producing organisms. J Med Microbiol 67:1728–1730. doi: 10.1099/jmm.0.000851. [DOI] [PubMed] [Google Scholar]
- 294.Oueslati S, Girlich D, Dortet L, Naas T. 2018. Evaluation of the Amplidiag CarbaR+VRE kit for accurate detection of carbapenemase-producing bacteria. J Clin Microbiol 56:e01092-17. doi: 10.1128/JCM.01092-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Girlich D, Bernabeu S, Grosperrin V, Langlois I, Begasse C, Arangia N, Creton E, Cotellon G, Sauvadet A, Dortet L, Naas T. 2019. Evaluation of the Amplidiag CarbaR+MCR kit for accurate detection of carbapenemase-producing and colistin-resistant bacteria. J Clin Microbiol 57:e01800-18. doi: 10.1128/JCM.01800-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Bloemberg GV, Braun-Kiewnick A, Tedrup J, Meijerink C, Durer E, Ritter C, Keller PM, Hombach M. 2017. Evaluation of the AID carbapenemase line probe assay for rapid detection and identification of carbapenemase genes in Gram-negative bacilli. J Antimicrob Chemother 72:1948–1954. doi: 10.1093/jac/dkx100. [DOI] [PubMed] [Google Scholar]
- 297.García-Fernández S, Morosini MI, Marco F, Gijón D, Vergara A, Vila J, Ruiz-Garbajosa P, Cantón R. 2015. Evaluation of the eazyplex SuperBug CRE system for rapid detection of carbapenemases and ESBLs in clinical Enterobacteriaceae isolates recovered at two Spanish hospitals. J Antimicrob Chemother 70:1047–1050. doi: 10.1093/jac/dku476. [DOI] [PubMed] [Google Scholar]
- 298.Cunningham SA, Vasoo S, Patel R. 2016. Evaluation of the Check-Points Check MDR CT103 and CT103 XL microarray kits by use of preparatory rapid cell lysis. J Clin Microbiol 54:1368–1371. doi: 10.1128/JCM.03302-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Roger-Dalbert C, Labourdette R, Brochu V, Lapointe S, Roy S, Galarneau H, Bouchy P, Nordmann P, Fortineau N, Girlich D. 2013. First clinical evaluation of the BD MAX™ CRE RUO assay on rectal swabs from intensive care unit patients. Abstracts of the 23rd European Congress of Clinical Microbiology and Infectious Diseases, Berlin, Germany.
- 300.Hofko M, Mischnik A, Kaase M, Zimmermann S, Dalpke AH. 2014. Detection of carbapenemases by real-time PCR and melt curve analysis on the BD Max system. J Clin Microbiol 52:1701–1704. doi: 10.1128/JCM.00373-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Antonelli A, Arena F, Giani T, Colavecchio OL, Valeva SV, Paule S, Boleij P, Rossolini GM. 2016. Performance of the BD MAX™ instrument with Check-Direct CPE real-time PCR for the detection of carbapenemase genes from rectal swabs, in a setting with endemic dissemination of carbapenemase-producing Enterobacteriaceae. Diagn Microbiol Infect Dis 86:30–34. doi: 10.1016/j.diagmicrobio.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 302.Ledeboer NA, Lopansri BK, Dhiman N, Cavagnolo R, Carroll KC, Granato P, Thomson R, Butler-Wu SM, Berger H, Samuel L, Pancholi P, Swyers L, Hansen GT, Tran NK, Polage CR, Thomson KS, Hanson ND, Winegar R, Buchan BW. 2015. Identification of Gram-negative bacteria and genetic resistance determinants from positive blood culture broths by use of the Verigene Gram-negative blood culture multiplex microarray-based molecular assay. J Clin Microbiol 53:2460–2472. doi: 10.1128/JCM.00581-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Gupta SK, Padmanabhan BR, Diene SM, Lopez-Rojas R, Kempf M, Landraud L, Rolain JM. 2014. ARG-ANNOT, a new bioinformatic tool to discover antibiotic resistance genes in bacterial genomes. Antimicrob Agents Chemother 58:212–220. doi: 10.1128/AAC.01310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Jia B, Raphenya AR, Alcock B, Waglechner N, Guo P, Tsang KK, Lago BA, Dave BM, Pereira S, Sharma AN, Doshi S, Courtot M, Lo R, Williams LE, Frye JG, Elsayegh T, Sardar D, Westman EL, Pawlowski AC, Johnson TA, Brinkman FS, Wright GD, McArthur AG. 2017. CARD 2017: expansion and model-centric curation of the comprehensive antibiotic resistance database. Nucleic Acids Res 45:D566–D573. doi: 10.1093/nar/gkw1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Peirano G, Matsumura Y, Nobrega D, Pitout JDD. 2019. A cost-effective method for identifying Enterobacterales with OXA-181. J Clin Microbiol 57:e01281-19. doi: 10.1128/JCM.01281-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Lund BA, Thomassen AM, Carlsen TJO, Hanna-Kirsti S, Leiros H-KS. 2017. Structure, activity and thermostability investigations of OXA-163, OXA-181 and OXA-245 using biochemical analysis, crystal structures and differential scanning calorimetry analysis. Acta Crystallogr F Struct Biol Commun 73:579–587. doi: 10.1107/S2053230X17013838. [DOI] [PMC free article] [PubMed] [Google Scholar]