1. Importance of vaccine dose-response curve shape
Vaccines are a key public health discovery and are one of the most cost-efficient interventions available in medicine (Han, 2015). Finding optimal vaccine dose amounts (hereafter dose), as well as identifying appropriate regimens, are key factors in reaching maximal vaccine efficacy at the requisite safety level. However, taking a vaccine from discovery to licensure can cost in the range of US$0.8 billion (Dickson and Gagnon, 2004). With these enormous costs, there is an intense pressure to make well-informed decisions at each stage of the development process. However, the current use of antiquated methods, may lead to sub-optimal dosing decisions.
Recent conditions including increased demand, resource limitation and cost have led the World Health Organization (WHO) to conduct retrospective dose ranging studies where the immunogenicity and/or efficacy of fractional doses (of full licensed doses) were tested. For diseases such as yellow fever (Campi-Azevedo et al., 2014; Martins et al., 2013), meningitis (Guerin et al., 2008) and malaria (Regules et al., 2016), assuming the same administration route, fractional doses were found to be equivalent to, or in some cases more immunogenic than full licensed doses. Retrospective dose ranging studies have led to a policy change in yellow fever vaccines, where the WHO now recommends that a fractional 1/5th (volume) yellow fever vaccine dose may be used in outbreak situations where supply is low (WHO, 2016). This fractional dose has been administered to five million persons in Brazil to date (WHO, 2018). These studies highlight an important question; if smaller doses are optimal with respect to immunogenicity (and participant exposure to pathogen) as compared to a large licensed dose, then why were they either missed or not initially selected for licensure during development?
Another example of sub-optimal dosing decisions is evident in the development of the novel subunit TB vaccines, H-series, which are currently in phase 1/2a clinical trials. Development of the H-series vaccine has benefitted from robust dose escalation studies. The dose ranging data from small animal studies suggested that the immunogenicity of the vaccine was highest at middle doses (0.05 to 1 μg vaccine antigen) and then decreased with the higher doses (5 and 15 μg vaccine antigen) (Aagaard et al., 2011, 2009; Rhodes et al., 2016). This phenomenon creates a peaked or n-shaped dose-response curve, commonly referred to as the goldilocks effect (Baldi and Bucherelli, 2005). A suggested explanation for this, in the context of TB immunogenicity, is that after a higher vaccine dose, T cells tend more towards an exhaustive state, i.e. increased differentiation into a terminal state (Billeskov et al., 2017). Clinical testing showed a similar peaked dose-response curve; smaller doses (5, 15 μg vaccine antigen) were more immunogenic than higher doses (50, 150 μg) (Luabeya et al., 2015; Norrby et al., 2017). However, in the early phase 1/2 clinical trials, a single dose of 50 μg vaccine antigen was chosen (Lenz et al., 2015; Reither et al., 2014; van Dissel et al., 2014). Thus, despite the pre-clinical dose-response data showing lower doses were more immunogenic, the lower end of the dose-response curve in humans has not been fully explored and higher doses were selected.
Peaked dose-response curves are not unique to TB vaccines; similar dose-response curves have also been seen in adenovirus (Ad35) (Darrah et al., 2007; Ophorst et al., 2006), HIV (Evans et al., 2001), malaria (Regules et al., 2016) and influenza (Nassim et al., 2012) vaccines. This is contrary to a long-standing vaccine development assumption that the relationship between dose and host response is saturating (sigmoidal) (Semenova et al., 2012); i.e. there is a minimum vaccine dose that gives no host response, a window of vaccine doses where the response rapidly escalates and a plateau above a certain dose threshold. Following this assumption, the goal of vaccine development is to then increase dose until a response plateaus and assume that it is the highest, safe optimal dose (with some margin of error to allow for host variation). In contrast, peaked curves suggest that there is a risk that high, sub-optimal doses could be progressing to later clinical development stages. Thus, more in-depth analysis of the shape of the vaccine dose-immune response curve, which has been largely ignored to date, is essential to understanding how to select optimal dose.
2. Difficulties with current vaccine dose-finding approaches
It is likely that in many cases, sub-optimal vaccines doses have been selected. Surprisingly, the definitive text on vaccine development does not include strategies for dose finding (Plotkin et al., 2013) and there is limited regulatory guidance on dose-finding methodologies from licensing organizations such as the FDA.
Currently, estimates for effective human doses are based on responses in small animal models (such as mice and rats) in which large dose ranges are tested over short timeframes. Typically, “low” doses that are used in mice or other small animals are selected and increased by half log to one log increments until an assumed maximum plateau in response is met. The next step is then to translate vaccine responses from these animal studies to humans, known as allometric scaling. Briefly, allometric scaling is the quantifiable relationship between animal body size and characteristic, e.g. the physiological relationship between animal size and metabolism or life span. In humans, allometric scaling is applied to common PK parameters such as volume of distribution, absorption and clearance by using the host’s weight, (e.g. for the drug Isoniazid e.g. (Wilkins et al., 2011)). Challenges are faced when applying vaccine dose allometric scaling between species, as the immunological relationships are still not well characterized, and fraught with issues of not only scale, but physiological differences between species. For example, assumed vaccine dose allometric scaling factors between mice and human vary over large ranges from 5–20 for HPV vaccines (Han et al., 2010; Hassett et al., 2015) to 0.5–100 for TB vaccines (Fletcher et al., 2013; Loxton et al., 2017; Tameris et al., 2013; Tchilian et al., 2009). There is a significant gap and lack of research into how vaccine doses translate across species and current vaccine dose allometric scaling assumptions are commonly not explicitly disclosed.
Additional technical issues are present with vaccine development studies. First, an inability to dilute vaccines to small enough dose, lack of more than one dose formulation starting material, and/or assay variation could also be contributing to under-researched dose-response curves. For example, a trial of a gp120 vaccine for HIV-1 infection in humans with different adjuvants, revealed that the surrogate response (which in this case was binding and homologous virus neutralization) at a dose of 30 μg formulated with QS-21 was equivalent to that of 300 μg of the same vaccine in alum. A further study with 0.5, 3, and 30 μg of the vaccine in the adjuvant, QS-21, revealed no decrease in response. Thus, neither the lower bound nor the shape of the dose-response curve has been established, primarily due to an inability to further accurately dilute this vaccine. On the other end of the dose-response curve, accurate assessment can be limited by an inability to achieve sufficient concentrations to reach a maximal dose as defined by immunologic, clinical, or safety parameters.
Second, a common barrier to efficient vaccine development is the potential lack of biological marker of protection, or biomarker. Without such a biomarker, validation of early decisions on developmental variables, such as dose, are unattainable. However, vaccines are often progressed through to clinical trials based on a hypothetical surrogate of protection - only after efficacy has been confirmed can a chosen surrogate be properly validated (Plotkin, 2008).
In summary, it is likely that the current empirical methods used in vaccine dose finding are leading to sub-optimal vaccines dose selections. Thus, to identify an optimal combination of developmental variables with current approaches (e.g. dose, dose regimen, vaccine composition (adjuvant dose)) will require a large, expensive multi-dimensional factorial design trial. As a result, vaccine doses are moving forward without extensive evaluation, which is often due to insufficient funding, developmental time pressures and lack of a clear optimized pipeline. Can a more effective and systematic identification protocol for optimal vaccine dosing be achieved?
3. Immunostimulation (IS) /Immunodynamic (ID) modelling: Mathematical modelling for improved vaccine dose decision making
The world of drug development has faced similar drug-dosing questions, yet is far more advanced in the use of systematic methods for dose optimization. This can be attributed partly to the use of pharmacometrics (or systems pharmacology): mathematical models that describe within host drug dynamics. The most commonly models used are pharmacokinetic/pharmacodynamic (PK/PD) models that employ mechanistic mathematical models to quantify drug concentration dynamics within the host over time (PK) and track drug effect and dynamics as drug concentration varies (PD) (Upton and Mould, 2014). Model-Based Drug Development (MBDD) is recognized as an efficient tool to accelerate and streamline drug development by minimizing developmental time and resources and is regularly used to establish optimal drug doses (Sherwin et al., 2014). MBDD has been established for decades in the pharmaceutical industry to improve dose selection for small molecule drugs (Kimko and Pinheiro, 2014) and is often required by regulatory agencies in all stages of drug development. As an example, modelling was able to tease through the different doses and protocols to derive optimal values for TB drug treatments, which previously had never been formally compared (Pienaar et al., 2015).
As yet, there is no such parallel to these methods used for vaccine dosing, which may be due to the diversity and complexity of immune responses measured or a lack of appreciation of potential quantitative tools. Application of similar methods to those used in drug development could lead to better evaluation of vaccine dose-response data derived from animals and its translation to humans to potentially improve vaccine development. Vaccine development is now in a position (decades later) to borrow from the experiences, expertise and technical utilities of MBDD. Consequently, we propose the new field of vaccine Immunostimulation/Immunodynamic (IS/ID) modelling as a method to improve vaccine dose decision-making and ultimately vaccine discovery. Analogous to PK/PD modelling, IS/ID modelling applies mathematical models to describe the underlying mechanisms, the immune response stimulation (IS) that produces measured immune response dynamics following vaccination (ID).
The application of IS/ID modelling to accelerate vaccine development could provide the following benefits. Firstly, a mathematical and computational modelling framework would have the capability to more effectively evaluate in silico greater combinations of vaccine trial design variables and narrow the design space before trials ever begin. Secondly, the incorporation of mathematical modelling into pre-clinical vaccine development could eventually result in a reduction in laboratory animals by replacing empirical experimentation with in silico simulation that optimizes the selection of doses and number of animals (Tanner and McShane, 2016). Similarly, the application of IS/ID modelling to clinical vaccine trial design could also reduce trial sample size and thus the total human exposure to investigational agents. Finally, the immune response required for protection against a disease relies on complex interactions that behave nonlinearly over time and across multiple biological scales (e.g. molecular to cellular to whole systems). IS/ID models will allow us to quantify this complexity to obtain meaningful biological predictions. To ensure model identifiably, IS/ID models will be as simple as the relevant response dataset will allow, and relevant biological model parameters will be collated from prior IS/ID modelling work and literature reviews.
IS/ID modelling vaccine development is limited only by the issues already facing the vaccine development world. Decisions on vaccine dose and dose regimen are currently being made regardless of a developer’s knowledge on dose allometry, an established correlate of protection, or extensive immune response data. Our goal with IS/ID modelling is not to discuss the correct vaccine induced immune measure, but to suggest a more systematic framework to inform key vaccine dose decisions based on a chosen surrogate and applying established methods currently used in drug development. Consequently, we would like to highlight that application of IS/ID modelling to vaccine development is not limited to a particular disease, type of vaccine or induction of a specific type of immune response and is also adaptable to vaccine requirements and available data.
4. IS/ID modelling implementation
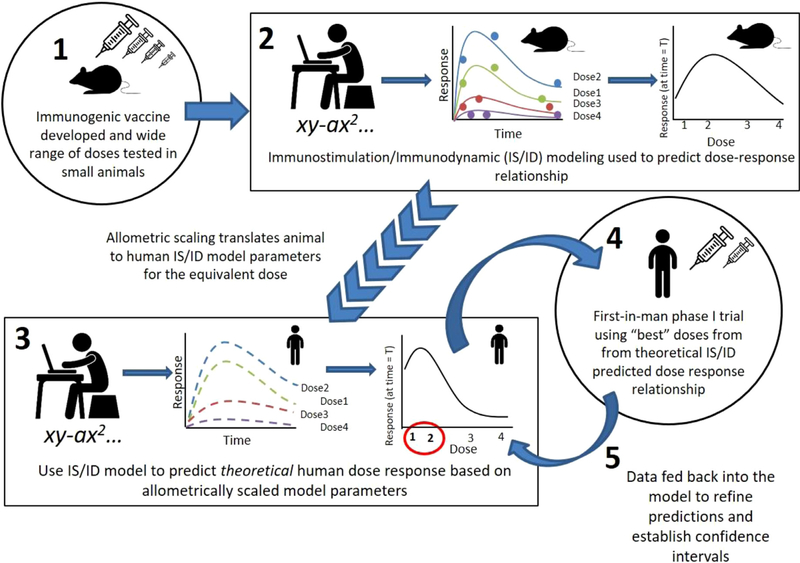
The generalized steps (for any vaccine) to integrate modelling into dose finding are outlined below, (a scheme of the steps is in Fig. 1):
Fig. 1.
Schema depicting the steps required to incorporate immunostimulation (IS) /immunodynamic (ID) modeling into vaccine development.
A wide range of doses of a new immunogenic vaccine are tested in small animal models to find minimum and maximum doses that provide the bounds of the dose-response curve (note adjuvant dose could/should also be varied). The dose range, although wide, can be based on historical work of similar vaccines.
IS/ID mathematical modelling is applied to estimate the parameters that describe the underlying dynamics of the initial animal-derived dose-response relationship. Optimal experimental design is then generated to yield the maximum information on the dose-response curve (with a pre-specified confidence interval), given limitations on animal numbers, ability to achieve desired concentrations of the product, and cost.
The IS/ID model is calibrated to human response data on limited doses and using allometric dose scaling assumptions (or tested in a human-immune response computational model (Segovia-Juarez et al., 2004)), the animal IS/ID model parameters are used to predict the theoretical human dose-response relationship and can be tested in the human-immune response computational model.
As in step 1, a selection of doses are chosen to define an approximate shape and the confidence bounds of the human dose-response curve based on the theoretical prediction in steps 1–3.
These data are then fed back into the model to gain understanding of the confidence intervals around the chosen doses. As further human data are collected, the IS/ID model is refined and used to hone in on best dose and it’s confidence interval.
5. Current IS/ID modelling
The first steps toward including IS/ID modelling into the vaccine development pipeline are under way. We conducted an intensive animal vaccine multi-dose study for a candidate TB vaccine, H56 adjuvanted with IC31 (H56 + IC31, presently in phase 2) designed by Rhodes et al. to specifically generate data for dose-response identification using our proposed translational IS/ID methods. IS/ID modelling was applied to determine the dose-response curve and showed a definitive n-shape for multiple times points after vaccination (Rhodes et al., 2016). Our modelling results suggested that the most immunogenic dose in mice was lower than empirically tested (0.25 μg for day 56 (late), Figure 3c in (Rhodes et al., 2016)). We concluded that future development of the H-series vaccines should evaluate lower doses. Using a mathematical model of key T cell dynamics following vaccination (Fig. 1 in Rhodes et al. (2018)) and well established model parameter estimation methods employed in population PK/PD modelling (i.e. nonlinear mixed effects modelling), we calibrated the model to longitudinal H56 + 1C31 multi-dose data in mice and longitudinal data H56/H1 + 1C31 data in humans for one dose. Following this, we used a vaccine dose allometric scaling assumption to ‘map’ the model parameters cross-species and predict the human dose-dependent response over time. We predicted that the most immunogenic dose in humans was between 0.8 and 8 ug H56 + 500nmolIC31 (Figures 4B–D in Rhodes et al. (2018)). This was lower than the 50 μg dose selected by the vaccine developers in clinical trials. Independently, since this prediction was made, this result has been corroborated by a phase1/2a clinical trial (Suliman et al., 2018) where preliminary findings suggest that doses 5, 15 and 50 μg H56 + 500nmolIC31 were equally immunogenic in healthy, BCG vaccinated participants. The vaccine developers have now decided to use the lower doses in future clinical trials (Suliman et al., 2018). In other work (Joslyn et al., 2018), that examined datasets from human and NHP data in anH56 immunogenicity context, we were able to make additional predictions. First, we were able to explain differences between the human and NHP study outcomes using a mathematical model that captured both species datasets. This is important for any animal model to be compared with the human scenario to understand better how results from one system translate to another. Second, in that same study, we predicted that the second booster vaccine that was given in the human study was not necessary, as it gave no additional benefit. This was also later confirmed in another human study with H56 (Suliman et al., 2018). Finally, preliminary work has also been conducted to use models to design experiments and trials. Here, like in PK/PD, models will be integrated into a statistical framework to determine optimal combinations of design variables (dose amount, subject numbers, number and occurrence of sampling times) to maximize information obtained from experiments and hence reduce costs and the number of subjects required (Aarons and Ogungbenro, 2010).
6. Future work to inform IS/ID vaccine dose modelling
In order to apply IS/ID modelling effectively to vaccine development, further datasets are required. For example, a thorough investigation into vaccine allometry, which is vital to scale vaccine dose across species, should be undertaken. Additionally, more extensive studies on immune system dynamics by dose should be introduced early on to pre-clinical investigations. In drug development, modelers use all available (relevant and standardized) data to refine understanding of PK/PD findings throughout product development. To maximize information on vaccine allometric scaling information and model parameterization across vaccines in the same way, we recommend the creation and curation of large shared dose-response data platforms through large-scale collaborative efforts with multinational pharmaceutical companies.
Second, a collaborative group of interested parties from academia, biotech, large vaccine manufacturers, regulators, governmental and non-governmental agencies must be established to aid communication, data access and development of methodology. The first meeting of such a group of individuals occurred in May 2015 at the headquarters of TB vaccine developers Aeras (Rockville, MD), where a multidisciplinary team met to discuss the current state of vaccine dose finding and the potential for mathematical modelling to assist in this arena. Creating such a network, would provide links between existing modelling consortia such as the TB, HIV, Malaria, NCD modelling consortia and International Society of Pharmacometrics to facilitate access to modelling expertise. Incentives such as open access to large historical data packages, and a commitment by vaccine developers, should be put in place to encourage modelers with experience in drug dosing and immune response modeling to move to vaccine development, which can have a potentially greater impact on human health. We believe that vaccine regulatory bodies also lack critical evaluations of vaccine product dose selection, and that agencies such as FDA or EMA should encourage modelers to move from the drug development focus into vaccine development. Thus, the motivation and investments must come in both a bottom-up and top-down fashion.
Finally, and most critically, we encourage the NIH and other vaccine funding agencies to consider head to head studies in which conventional methods for selecting vaccine do are used in parallel with the outlined modelling techniques, to better understand the impact on speed of development, number of participants exposed and cost of vaccine design. Only then can the true value of the endeavor be assessed.
Acknowledgments
Funding
SR is funded by a studentship from Aeras. RGW is funded the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement that is also part of the EDCTP2 programme supported by the European Union (MR/P002404/1), the Bill and Melinda Gates Foundation (TB Modelling and Analysis Consortium: OPP1084276/OPP1135288, CORTIS: OPP1137034, Vaccines: OPP1160830) and UNITAID (4214-LSHTM-Sept15; PO 8477–0-600).
Contributor Information
Sophie J. Rhodes, TB Modelling Group, CMMID, TB Centre, London School of Hygiene, and Tropical Medicine, UK.
Gwenan M. Knight, TB Modelling Group, CMMID, TB Centre, London School of Hygiene, and Tropical Medicine, UK
Denise E. Kirschner, University of Michigan Medical School, Ann Arbor, MI, USA
Richard G. White, TB Modelling Group, CMMID, TB Centre, London School of Hygiene, and Tropical Medicine, UK.
Thomas G. Evans, Vaccitech, Oxford, UK.
References
- Aagaard C, Hoang T, Dietrich J, Cardona PJ, Izzo A, Dolganov G, Andersen P, 2011. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nat. Med. 17 (2), 189–194. doi: 10.1038/nm.2285. [DOI] [PubMed] [Google Scholar]
- Aagaard C, Hoang TT, Izzo A, Billeskov R, Troudt J, Arnett K, Dietrich J, 2009. Protection and polyfunctional T cells induced by Ag85B-TB10.4/IC31 against Mycobacterium tuberculosis is highly dependent on the antigen dose. PLoS One 4 (6), e5930. doi: 10.1371/journal.pone.0005930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarons L, Ogungbenro K, 2010. Optimal design of pharmacokinetic studies. Basic Clin. Pharmacol. Toxicol. 106 (3), 250–255. doi: 10.1111/j.1742-7843.2009.00533.x. [DOI] [PubMed] [Google Scholar]
- Baldi E, Bucherelli C, 2005. The inverted “u-shaped” dose-effect relationships in learning and memory: modulation of arousal and consolidation. Nonlinearity Biol. Toxicol. Med. 3 (1), 9–21. doi: 10.2201/nonlin.003.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeskov R, Lindenstrom T, Woodworth J, Vilaplana C, Cardona PJ, Cassidy JP, Andersen P, 2017. High antigen dose is detrimental to post-exposure vaccine protection against tuberculosis. Front Immunol. 8, 1973. doi: 10.3389/fimmu.2017.01973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campi-Azevedo AC, de Almeida Estevam P, Coelho-Dos-Reis JG, Peruhype-Magalhaes V, Villela-Rezende G, Quaresma PF, Martins-Filho OA, 2014. Subdoses of 17DD yellow fever vaccine elicit equivalent virological/immunological kinetics timeline. BMC Infect. Dis. 14 (391), 1–12. doi: 10.1186/1471-2334-14-391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Seder RA, 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13 (7), 843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- Dickson M, Gagnon JP, 2004. The cost of new drug discovery and development. Discov. Med. 4 (22), 172–179. [PubMed] [Google Scholar]
- Evans TG. McElrath MJ. Matthews T. Montefiori D. Weinhold K. Wolff M, Group NAVE. 2001. QS-21 promotes an adjuvant effect allowing for reduced antigen dose during HIV-1 envelope subunit immunization in humans. Vaccine 19 (15–16), 2080–2091. [DOI] [PubMed] [Google Scholar]
- Fletcher HA, Tanner R, Wallis RS, Meyer J, Manjaly ZR, Harris S, McShane H, 2013. Inhibition of mycobacterial growth in vitro following primary but not secondary vaccination with Mycobacterium bovis BCG. Clin. Vaccine Immunol. 20 (11), 1683–1689. doi: 10.1128/CV1.00427-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerin PJ, Naess LM, Fogg C, Rosenqvist E, Pinoges L, Bajunirwe F, Caugant DA, 2008. Immunogenicity of fractional doses of tetravalent a/c/y/w135 meningococcal polysaccharide vaccine: results from a randomized non-inferiority controlled trial in Uganda. PLoS Negl. Trop. Dis. 2 (12), e342. doi: 10.1371/journal.pntd.0000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JE, Kim HK, Park SA, Lee SJ, Kim HJ, Son GH, Lee NG, 2010. A non-toxic derivative of lipopolysaccharide increases immune responses to Gardasil HPV vaccine in mice. Int. Immunopharmacol. 10 (2), 169–176. doi: 10.1016/j.intimp.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Han S, 2015. Clinical vaccine development. Clin. Exp. Vaccine Res. 4 (1), 46–53. doi: 10.7774/cevr.2015.4.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassett KJ, Meinerz NM, Semmelmann F, Cousins MC, Garcea RL, Randolph TW, 2015. Development of a highly thermostable, adjuvanted human papillomavirus vaccine. Eur. J. Pharm. Biopharm. 94, 220–228. doi: 10.1016/j.ejpb.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joslyn LR, Pienaar E, DiFazio RM, Suliman S, Kagina BM, Flynn JL, Kirschner DE, 2018. Integrating non-human primate, human, and mathematical studies to determine the influence of BCG timing on H56 vaccine outcomes. Front. Microbiol. 9, 1–6. ARTN 1734. doi: 10.3389/fmicb.2018.01734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimko H, Pinheiro J, 2014. Model-based clinical drug development in the past, present & future: a commentary. Br. J. Clin. Pharmacol. doi: 10.1111/bcp.12341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenz N, Schindler T, Kagina BM, Zhang JD, Lukindo T, Mpina M, Daubenberger CA, 2015. Antiviral innate immune activation in H1V-infected adults negatively affects H1/1C31-induced vaccine-specific memory CD4+ T cells. Clin. Vaccine 1mmunol. 22 (7), 688–696. doi: 10.1128/CVI.00092-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loxton AG, Knaul JK, Grode L, Gutschmidt A, Meller C, Eisele B, Group VPMS, 2017. Safety and immunogenicity of the recombinant mycobacterium bovis BCG vaccine VPM1002 in HIV-unexposed newborn infants in South Africa. Clin. Vaccine 1mmunol. 24 (2). doi: 10.1128/CVI.00439-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luabeya AK, Kagina BM, Tameris MD, Geldenhuys H, Hoff ST, Shi Z, Hussey GD, 2015. First-in-human trial of the post-exposure tuberculosis vaccine H56:IC31 in Mycobacterium tuberculosis infected and non-infected healthy adults. Vaccine 33 (33), 4130–4140. doi: 10.1016/j.vaccine.2015.06.051. [DOI] [PubMed] [Google Scholar]
- Martins RM, Mde, Maia L, Farias H,R, Camacho LA, Freire MS, Galler R, Homma A, 2013. 17DD yellow fever vaccine: a double blind, randomized clinical trial of immunogenicity and safety on a dose-response study. Hum. Vaccin Immunother. 9 (4), 879–888. doi: 10.4161/hv.22982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassim C, Christensen S, Henry D, Holmes S, Hohenboken M, Kanesa-Thasan N, 2012. Identification of antigen and adjuvant doses resulting in optimal immunogenicity and antibody persistence up to one year after immunization with a pandemic A/H1N1 influenza vaccine in children 3 to <9 years of age. Pediatr. Infect. Dis. J. doi: 10.1097/INF.0b013e3180343104. [DOI] [PubMed] [Google Scholar]
- Norrby M, Vesikari T, Lindqvist L, Maeurer M, Ahmed R, Mahdavifar S, Brighenti S, 2017. Safety and immunogenicity of the novel H4:IC31 tuberculosis vaccine candidate in BCG-vaccinated adults: two phase I dose escalation trials. Vaccine 35 (12), 1652–1661. doi: 10.1016/j.vaccine.2017.01.055. [DOI] [PubMed] [Google Scholar]
- Ophorst OJ, Radosevic K, Havenga MJ, Pau MG, Holterman L, Berkhout B, Tsuji M, 2006. Immunogenicity and protection of a recombinant human adenovirus serotype 35-based malaria vaccine against Plasmodium yoelii in mice. Infect. Immun. 74 (1), 313–320. doi: 10.1128/IAI.74.1.313-320.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienaar E, Dartois V, Linderman JJ, Kirschner DE, 2015. In silico evaluation and exploration of antibiotic tuberculosis treatment regimens. BMC Syst. Biol. 9, 79. doi: 10.1186/s12918-015-0221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotkin SA, 2008. Vaccines: correlates of vaccine-induced immunity. Clin. Infect. Dis. 47 (3), 401–409. doi: 10.1086/589862. [DOI] [PubMed] [Google Scholar]
- Plotkin SA, Orenstein WA, Offit PA, 2013. Vaccines, 6 ed. Saunders. [Google Scholar]
- Regules JA, Cicatelli SB, Bennett JW, Paolino KM, Twomey PS, Moon JE, Vekemans J, 2016. Fractional third and fourth dose of RTS,S/AS01 malaria candidate vaccine: a phase 2a controlled human malaria parasite infection and immunogenicity study. J. Infect. Dis. 214 (5), 762–771. doi: 10.1093/infdis/jiw237. [DOI] [PubMed] [Google Scholar]
- Reither K, Katsoulis L, Beattie T, Gardiner N, Lenz N, Said K, Churchyard GJ, 2014. Safety and immunogenicity of H1/IC31(R), an adjuvanted TB subunit vaccine, in HIV-infected adults with CD4+ Lymphocyte counts greater than 350 cells/mm3: a phase II, multi-centre, double-blind, randomized, placebo-controlled trial. PLoS One 9 (12), e114602. doi: 10.1371/journal.pone.0114602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SJ, Guedj J, Fletcher HA, Lindenstrom T, Scriba TJ, Evans TG, White RG, 2018. Using vaccine Immunostimulation/Immunodynamic modelling methods to inform vaccine dose decision-making. NPJ Vaccines 3, 36. doi: 10.1038/s41541-018-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes SJ, Zelmer A, Knight GM, Prabowo SA, Stockdale L, Evans TG, Fletcher H, 2016. The TB vaccine H56+IC31 dose-response curve is peaked not saturating: data generation for new mathematical modelling methods to inform vaccine dose decisions. Vaccine 34 (50), 6285–6291. doi: 10.1016/j.vaccine.2016.10.060. [DOI] [PubMed] [Google Scholar]
- Segovia-Juarez JL, Ganguli S, Kirschner D, 2004. Identifying control mechanisms of granuloma formation during M. tuberculosis infection using an agent-based model. J. Theor. Biol. 231 (3), 357–376. doi: 10.1016/j.jtbi.2004.06.031. [DOI] [PubMed] [Google Scholar]
- Semenova VA, Schiffer J, Steward-Clark E, Soroka S, Schmidt DS, Brawner MM, Quinn C, 2012. Validation and long term performance characteristics of a quantitative enzyme linked immunosorbent assay (ELISA) for human anti-PA IgG. J. Immunol. Methods 376 (1–2), 97–107. doi: 10.1016/j.jim.2011.12.002. [DOI] [PubMed] [Google Scholar]
- Sherwin CM, Zobell JT, Stockmann C, McCrory BE, Wisdom M, Young DC, Spigarelli MG, 2014. Pharmacokinetic and pharmacodynamic optimisation of intravenous tobramycin dosing among children with cystic fibrosis. J. Pharmacokinet. Pharmacodyn. 41 (1), 71–79. doi: 10.1007/s10928-013-9348-7. [DOI] [PubMed] [Google Scholar]
- Suliman S, Luabeya AKK, Geldenhuys H, Tameris M, Hoff ST, Shi Z, Group HT, 2018. Dose optimization of H56:IC31 vaccine for TB endemic populations: a double-blind, placebo-controlled, dose-selection trial. Am. J. Respir. Crit. Care Med. doi: 10.1164/rccm.201802-03660C. [DOI] [PubMed] [Google Scholar]
- Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Team MATS, 2013. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381 (9871), 1021–1028. doi: 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner R, McShane H, 2016. Replacing, reducing and refining the use of animals in tuberculosis vaccine research. ALTEX doi: 10.14573/altex.1607281. [DOI] [PubMed] [Google Scholar]
- Tchilian EZ, Desel C, Forbes EK, Bandermann S, Sander CR, Hill AV, Kaufmann SH, 2009. Immunogenicity and protective efficacy of prime-boost regimens with recombinant (delta)ureC hly+ Mycobacterium bovis BCG and modified vaccinia virus ankara expressing M. tuberculosis antigen 85A against murine tuberculosis. Infect. Immun. 77 (2), 622–631. doi: 10.1128/IAI.00685-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton RN, Mould DR, 2014. Basic concepts in population modeling, simulation, and model-based drug development: part 3-introduction to pharmacodynamic modeling methods. CPT Pharmacomet. Syst. Pharmacol. 3, e88. doi: 10.1038/psp.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dissel JT, Joosten SA, Hoff ST, Soonawala D, Prins C, Hokey DA, Andersen P, 2014. A novel liposomal adjuvant system, CAF01, promotes long-lived Mycobacterium tuberculosis-specific T-cell responses in human. Vaccine 32 (52), 7098–7107. doi: 10.1016/j.vaccine.2014.10.036. [DOI] [PubMed] [Google Scholar]
- WHO, 2016. Meeting of the Strategic Advisory Group of Experts on Immunization. October 2016 - conclusions and recommendations. https://www.who.int/wer/2016/wer9121.pdf?ua=1. Accessed May 2018.
- WHO, 2018. Yellow fever - Brazil Disease outbreak news - 9 March 2018. https://www.who.int/csr/don/09-march-2018-yellow-fever-brazil/en/. Accessed May 2018.
- Wilkins JJ, Langdon G, McIlleron H, Pillai G, Smith PJ, Simonsson US, 2011. Variability in the population pharmacokinetics of isoniazid in South African tuberculosis patients. Br. J. Clin. Pharmacol. 72 (1), 51–62. doi: 10.1111/j.1365-2125.2011.03940.x. [DOI] [PMC free article] [PubMed] [Google Scholar]