SUMMARY
Many randomized controlled trials (RCTs) have investigated drug treatment for women at high risk of fracture, with a reduction in fracture risk as their end point. There has also been progress in identifying women at the highest risk of fractures. The most important clinical determinant contributing to the clinical decision of initiating and choosing drug therapy for fracture prevention is a woman’s fracture risk, which, in RCTs, was determined by menopausal state, age, bone mineral density, fracture history, fall risks and glucocorticoid use. Women with secondary osteoporosis were excluded, except in studies of glucocorticoid use. A second determinant of drug therapy is the evidence for fracture prevention in terms of spectrum (vertebral, nonvertebral and/or hip fractures), size and speed of effect. In the absence of head-to-head RCTs with fracture risk as the end point, however, the efficacy of antifracture drugs cannot be directly compared. Other determinants include the potential extraskeletal benefits and safety concerns of the drug, patient preferences and reimbursement issues.
Keywords: drug safety, drug treatment, fracture risk, glucocorticoid osteoporosis, postmenopausal osteoporosis
INTRODUCTION
During the past two decades, many randomized controlled trials (RCTs) have investigated the use of drug treatment to reduce fracture risk in women with postmenopausal osteoporosis, who are at high risk of fractures. The results of these RCTs guide therapy for postmenopausal osteoporosis and, to a lesser degree of evidence, for glucocorticoid-induced osteoporosis (GIOP).1,2 Meanwhile, the process of identifying those women with the highest risk of fractures has progressed, initially from the measurement of bone mineral density (BMD) alone to the development of algorithms that are based on an integrated approach combining BMD, BMD-independent clinical risk factors (e.g. age, personal and family fracture history, low body weight, smoking, excessive alcohol intake, rheumatoid arthritis and glucocorticoid use)3,4 and BMD-independent, fall-related risk factors.5
The aim of this Review is to identify patient-and drug-related determinants that contribute to the clinical decision about choosing and initiating drug therapy for the prevention of fractures.6 Cost-effectiveness is an issue of increasing interest, but is not the focus of this article.7 References to RCTs published before 2006 are available elsewhere in general reviews on postmenopausal osteoporosis and GIOP.1,8
MECHANISMS OF ACTION OF FRACTURE PREVENTION DRUGS
Fractures can be prevented by drugs that have different, and often opposite, effects on bone remodeling.1,9–12 Antiresorptive drugs (e.g. bisphosphonates [etidronate, alendronate, risedronate, ibandronate, zoledronate], raloxifene, calcitonin and estrogens) decrease bone turnover. The recombinant human parathyroid hormone N-terminal fragment 1–34 (rhPTH [1–34], known as teriparatide) and the full-length form (rhPTH [1–84]), by contrast, increase bone turnover, but preferentially affect bone formation over bone resorption. In between these two extremes is strontium ranelate, which stimulates bone formation and inhibits bone resorption in animal models. These drugs have a wide spectrum of effects in bone: from preserving to rebuilding bone architecture and from preserving to profoundly affecting its material, such as its mineralization (Table 1).
Table 1.
Mechanisms of action of drugs that prevent fractures.
| Drug class | Changes to relevant markers |
|||
|---|---|---|---|---|
| Bone resorption | Bone formation | Architecture | Mineralization | |
| Antiresorptive drugs | ↓ | ↓ | U | ↑ |
| Strontium ranelate | ↓a | ↑a | ↑a | U |
| rhPTH (teriparatide or rhPTH [1–84]) | ↑ | ↑↑ | ↑ | ↓ |
Demonstrated in animal studies.
Key: U, unchanged; ↑, increased; ↑↑, strongly increased; ↓, decreased.
Abbreviation: rhPTH, recombinant human parathyroid hormone.
PATIENT CHARACTERISTICS
The most important clinical determinant regarding the initiation of osteoporosis treatment is a woman’s fracture risk profile.13 All of the aforementioned drugs have been studied in postmenopausal women with a high risk of fractures on the basis of menopausal state, age, low BMD and/or presence of a morphometric vertebral fracture and, in some studies, other clinical risks. Algorithms for predicting vertebral and nonvertebral fractures are available.2,3,14 Women with a fracture after menopause need immediate attention for counseling about subsequent fracture risk, which is higher in the short-term than the long-term.15,16 Zoledronate is the only agent that has been studied in patients selected on the basis of a recent hip fracture, irrespective of BMD.11 Some of the drugs mentioned above have been studied in women with GIOP.8,17–19
Women with postmenopausal osteoporosis are advised to adopt nonpharmacological life-style interventions, such as correcting calcium and vitamin D deficiencies (which was part of all RCTs),20 optimizing protein intake, exercising, preventing falls, stopping smoking and moderating alcohol intake.2 Women with postmenopausal osteoporosis need differential diagnosis to identify factors that frequently contribute to secondary osteoporosis (which was an exclusion criterion in postmenopausal osteoporosis studies), many of which are treatable.21 These approaches are considered necessary but insufficient to offer maximum protection against fractures in women with postmenopausal osteoporosis or GIOP who are at high risk of fractures (Figure 1).1,2,22
Figure 1.
Pyramidal approach to prevention of fractures in patients at high risk of fracture. All women with postmenopausal osteoporosis and glucocorticoid-induced osteoporosis need lifestyle advice (bottom level), which relates to diet, exercise, fall prevention, cessation of smoking and moderation of alcohol intake. The next step is the differential diagnosis of contributors to secondary osteoporosis, such as diseases and drugs (middle level), followed by treatment with antiresorptive or anabolic drugs in those at high risk of fractures (top level). Modified from US Department of Health and Human Services (2004) Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, MD: US Department of Health and Human Services, made available by the National Library of Medicine.
ANTIFRACTURE EFFECTS OF DRUGS
Patients with postmenopausal osteoporosis
The antifracture effects in patients with postmenopausal osteoporosis vary between drugs (Table 2). In the absence of head-to-head studies, however, differences in fracture reduction compared to placebo should not be compared directly and no inferences should be made regarding superiority of one efficacious treatment over another, as the antifracture studies differed in patient and study characteristics.6
Table 2.
Fracture reduction by drugs in postmenopausal women with osteoporosis.a
| Drug | Type of fracture prevented |
||||
|---|---|---|---|---|---|
| Vertebral fractures |
Nonvertebral fractures |
Hip fractures |
|||
| Primary analysis | Primary analysis | Post hoc subgroup analysis | Primary analysis | Post hoc subgroup analysis | |
| Alendronate | + | + | NA | + | NA |
| Risedronate | + | + | NA | + | NA |
| Ibandronate | + | ND | +b | ND | ND |
| Zoledronate | + | + | NA | + | NA |
| Raloxifene | + | ND | +c | ND | ND |
| Calcitonin | + | ND | ND | NA | NA |
| Strontium ranelate | + | + | NA | ND | +d |
| Teriparatide (rhPTH [1–34]) | + | + | NA | ND | ND |
| rhPTH (1–84) | + | ND | ND | ND | ND |
The data are derived from women with a low T-score and/or previous vertebral fracture from primary analyses, which give the highest level of evidence in randomized controlled trials, and from post hoc analyses in high-risk subgroups.
In women with T-score <−3.0, or women with T-score <−2.5 and a fracture that has occurred during previous 5 years; possibly dose-related.
In the subgroup with severe previous vertebral fractures (>40% of height loss).
In women with T-score <−2.4 and age >74 years. Abbreviations: NA, not applicable; ND, no data or not significant; rhPTH, recombinant human parathyroid hormone.
In primary analyses, which give the highest level of evidence of fracture prevention in RCTs,6 all agents tested significantly decreased the risk of morphometric vertebral fractures. Alendronate, risedronate, zoledronate, strontium ranelate and teriparatide also reduced the risk of nonvertebral fractures, and alendronate, risedronate and zoledronate also reduced the risk of hip fractures. Post hoc analyses in high-risk subgroups indicated that the selective estrogen receptor modulator (SERM) raloxifene and the bisphosphonate ibandronate prevented nonvertebral fractures23,24 and that strontium ranelate prevented hip fractures.1
The reported reduction in fracture risk varied between drugs, and was typically around 40–60% for vertebral fractures with all drugs, 15–30% for those that reduced the risk of nonvertebral fractures and 15–60% for those that reduced hip fractures (Figure 2).1
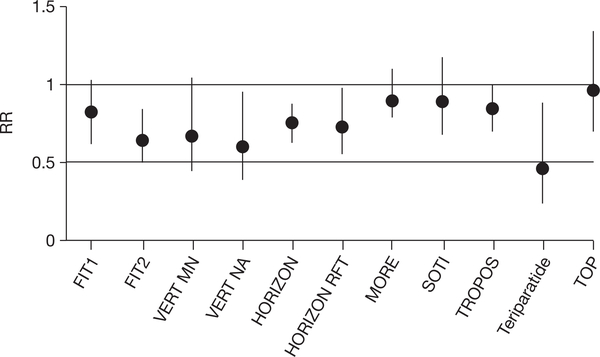
Figure 2.
Reduction in nonvertebral fractures versus placebo as reported in analyses at the end of primary antifracture studies. The reduction in fracture risk is expressed as a relative risk (RR) with 95% confidence intervals.1 The risk reduction varied between studies, but the size of effect cannot be compared in the absence of head-to-head trials between drugs.6 Abbreviations: BMD, bone mineral density; FIT, Fracture Intervention Trial with alendronate in patients with an existing vertebral fracture (FIT1) and in patients with low BMD but without vertebral fracture (FIT2); HORIZON, Health Outcomes and Reduced Incidence with Zoledronic acid ONce yearly pivotal fracture trial; HORIZON RFT, HORIZON recurrent fracture trial; MORE, Multiple Outcomes of Raloxifene Evaluation study; SOTI, Spinal Osteoporosis Therapeutic Intervention; teriparatide, study using recombinant human parathyroid hormone 1–34; TOP, Treatment of Osteoporosis with Parathyroid hormone study (with recombinant human parathyroid hormone 1–84); TROPOS, TReatment Of Peripheral OSteoporosis studies with strontium ranelate; VERT MN and VERT NA, Vertebral Efficacy with Risedronate Therapy studies (Multinational and North America). The following studies were not included: BONE (oral iBandronate Osteoporosis vertebral fracture trial in North America and Europe), as no RRs were indicated for nonvertebral fractures (no effect in total group); and PROOF (the Prevent Recurrence Of Osteoporotic Fractures study), as the RR for nonvertebral fractures was only significant at the low dose (100 IU/day; RR = 0.64 (95% CI 0.41–0.99) but not at 200 and 400 IU/day.
The speed of onset of antifracture effects also varied between drugs. Early effects on nonvertebral fractures have been reported within 6 months for risedronate,25 12 months for alendronate and strontium ranelate (in patients aged over 80 years)26 and 18 months for teriparatide.
Postmenopausal osteoporosis in the elderly
Beyond postmenopausal osteoporosis, secondary analyses of data can indicate antifracture effects in the elderly. In patients aged over 80 years, strontium ranelate had an antifracture effect for vertebral, nonvertebral and clinical fractures, and for hip fractures in patients with low T-scores in the spine and hip.26 The antifracture effect of alendronate did not wane at the age of 80–85 years.27 Risedronate reduced the risk of morphometric vertebral fractures in women aged 80 years and over.28 Teriparatide decreased the risk of morphometric vertebral fractures in those older than 75 years.29 Antifracture data are available from elderly patients receiving zoledronate in a study of those with a recent hip fracture, in which half of the cohort was older than 74 years.11 In women aged 80–89 years with fall-related risk factors for hip fracture, but without documented BMD-evidence of osteoporosis, risedronate did not significantly reduce the risk of hip fracture.1 In postmenopausal women with a T-score >–2.0 and no previous radiographic vertebral fracture, alendronate was not effective at reducing nonvertebral fracture risk.1
Comparing the antifracture effects of different drugs in patients with postmenopausal osteoporosis
Head-to-head RCTs that directly compare different drugs and use antifracture effects as the primary end point could resolve some of the uncertainty regarding the differences between drugs, but are unlikely to be conducted for postmenopausal osteoporosis as such studies need enormous numbers of patients and would prove costly.1
One way to overcome this sample size restriction is to estimate antifracture effects in systematic reviews or meta-analyses, which are considered the highest level of evidence-based medicine;6,30 however, the results of meta- analyses vary greatly as a function of the treatment dose, duration of follow-up, selection of studied fractures and time of analysis.30 Meta-analyses that include only patient groups with documented fracture risk31 give more precise point estimates for use in daily practice than meta-analyses that include patients without osteoporosis or without baseline BMD data.32
Head-to-head randomized studies comparing the effects of alendronate and risedronate on surrogate markers of fracture risk (which favored the effects of alendronate on BMD and markers of bone turnover)33,34 and nonrandomized postmarketing antifracture studies using a large-scale pharmacy insurance database and a rigorous methodological approach (which favored the effects of risedronate on hip fracture risk)35 have shown differences between drugs, but at a low level of evidence-based medicine (level 2B and level 3, respectively).6 Clearly, there are differences between classes of drugs in terms of their mechanisms of action, but also between drugs of the same class, as shown for bisphosphonates, which differ in bone and enzyme affinity.36 How strongly these pharmacological variations are related to clinical differences in antifracture effects, and whether they can therefore be used in clinical decision-making, remains unclear.
Glucocorticoid-induced osteoporosis
All patients being started on glucocorticoid treatment should, at the very least, receive prophylactic calcium and vitamin D supplementation.37 In patients with GIOP (in starters or chronic users of variable doses of glucocorticoid), bisphosphonates increase BMD and reduce the risk of morphometric vertebral fractures on the basis of secondary end points, safety analyses, extension studies, and combinations of study populations.8,17,18 Specifically, there is evidence that alendronate, etidronate and risedronate can prevent vertebral fractures, although the evidence for etidronate is of a lower grade than for alendronate and risedronate. Teriparatide was associated with increased BMD in women who were also taking estrogens.18
Head-to-head trials with antifracture data in post hoc or secondary end point analyses indicate that alendronate has greater effects than calcitriol8,17 and calcitonin8 on BMD and bone markers in patients with GIOP, without altering the incidence of vertebral fractures. In an interim analysis at 18 months, a significantly greater increase in BMD (at the hip and spine) and a significant reduction in semiquantitatively measured morphometric vertebral fractures were seen with teriparatide compared with alendronate.19
Combination and sequential treatment regimens
Combinations of antiresorptive drugs can induce a greater increase in BMD than each drug separately, but the relationship between this change in BMD and fracture risk is unknown.48 Zoledronate reduced the risk of fractures in patients receiving SERMs, estrogens or calcitonin in a prespecified subgroup analysis.10 The increases in bone formation, levels of resorption markers and BMD that are usually observed during treatment with either teriparatide or rhPTH (1–84) are blunted when rhPTH is combined with alendronate.48,49 It is recommended, therefore, that antiresorptive drugs other than raloxifene are avoided during treatment with either teriparatide or rhPTH (1–84).
In patients who have previously been treated with antiresorptive agents, teriparatide and rhPTH (1–84) can increase BMD and the levels of bone turnover markers in the serum, which varied according to the previously used antiresorptive drug.51,52 Teriparatide and rhPTH (1–84) are now recognized for the treatment of postmenopausal osteoporosis when incident fractures occur during adequate treatment with antiresorptive drugs, and for the treatment of patients with postmenopausal osteoporosis who have intolerance or contraindications to bisphosphonates and SERMs. Teriparatide and rhPTH (1–84) treatment is continued for 18–24 months, and the antifracture effect persists after withdrawal of teriparatide.53 The increase in BMD that is achieved by teriparatide therapy can be preserved by subsequent treatment with antiresorptive drugs.53 In this setting, a 50-month, open-label, follow-up study indicated ongoing nonvertebral fracture reduction.53
EXTRASKELETAL BENEFITS OF DRUG TREATMENT
Extraskeletal benefits and risks also influence clinical decisions. For example, raloxifene reduced the risk of invasive breast cancer by 66% over 8 years.38 This agent has recently been approved by the FDA for the prevention of estrogen-receptor-positive breast cancer in women at high risk, as well as in women with postmenopausal osteoporosis.
Estrogens attenuate severe climacteric symptoms, such as hot flushes;39 however, estrogen treatment alone is generally not considered as a first-line therapy for osteoporosis, as the potential adverse effects (breast cancer, venous thromboembolisms and cardiovascular thrombotic events, including stroke) are greater than the antifracture benefits, particularly in older women.39
Zoledronate, administered within 90 days of a hip fracture and then yearly for up to 3 years, is the first antiresorptive drug to show an association with a decrease in all-cause mortality, reducing the levels by 28% over 2 years.11 The reason for the reduced mortality in this study is not clear.
SAFETY AND TOLERANCE OF DRUGS
Oral bisphosphonates are poorly absorbed and can cause esophageal irritation; therefore, they are taken with water, on an empty stomach, without lying down and at least 30 min before eating (60 min for ibandronate). Patients with mild gastrointestinal intolerance to one type of bisphosphonate can add an H2 blocker or proton pump inhibitor to ameliorate gastro intestinal tolerance, or switch to other oral or intravenous bisphosphonates or to drugs of other classes. Osteonecrosis of the jaw has been associated with the use of bisphosphonates.40 This unusual disorder is most commonly reported in cancer patients who have been treated with high-dose regimens of intravenous pamidronate or zoledronate. The incidence is considered very low (<1/10,000 to <1/100,000) in patients taking oral bisphosphonates at the doses used to treat osteoporosis, and most of these cases can be related to the length of administration of these bisphosphonates. Atypical fractures (such as subtrochanteric fractures) have been reported during long-term treatment with oral bisphosphonates administered for osteoporosis. In one study, all reported patients had low, but still ongoing, bone formation (measured by tetracycline labeling) on bone histology.41 In cases of osteonecrosis of the jaw or atypical fractures, the consensus is that bisphosphonates should be withdrawn.40 In dogs treated with bisphosphonates, an increased frequency of microcracks was found, but among postmenopausal osteoporotic women undergoing long-term bisphosphonate treatment, microcrack frequency in the iliac bone was low, despite a marked reduction in bone turnover.42 Bisphosphonates should only be used when creatinine clearance is >30 ml/min, as they can be nephrotoxic, especially when used at high doses for an extended duration. Renal problems have been reported in patients treated with zoledronate, but almost exclusively in those patients treated for malignancies.43 Adequate hydration of the patient at the time of drug infusion, together with calcium and vitamin D supplementation, is recommended to avoid hypocalcemia. In one study, atrial fibrillation was associated with the use of zoledronate10 (although this did not apply to patients with Paget’s disease of the bone or after hip fractures);11 this association was not seen with alendronate44 or risedronate.45
Raloxifene is contraindicated in patients who have had a venous thrombosis, and the treatment must be stopped in situations of prolonged decubitus. Raloxifene increases the risk of hot flushes and should not be given too soon after menopause has started.
In clinical trials, strontium ranelate caused a higher incidence of diarrhea during the first 3 months of treatment than did placebo. Postmarketing data indicate that drug rash with eosinophilia systemic symptoms and Stevens–Johnson syndrome can occur, very rarely, as a result of treatment with strontium ranelate.46 This treatment is also associated with an increased risk of thromboembolic events.1 Strontium ranelate should be used with caution in patients who have had a thrombosis, and the treatment must be stopped in prolonged decubitus situations and in patients with skin reactions.
Teriparatide is associated with dizziness and leg cramps in 9% and 3% of patients, respectively. A post-treatment increase in serum calcium levels was found in 11% of patients, and in 3% when later retested. Routine calcium monitoring during therapy is not required for teriparatide, but is advocated for rhPTH (1–84) treatment because of the higher frequency of hypercalcemia (28%) associated with this agent. Teriparatide is associated with an increased risk of developing osteosarcoma during lifelong treatment in rats;47 however, no increased risk of cancer has been reported in clinical studies or in primate models with teriparatide. Teriparatide is contraindicated for treatment of Paget’s disease of the bone and in situations where there are, or have been, unexplained elevations of serum alkaline phosphatase levels, prior external beam or implant radiation involving the skeleton, bone metastases or a history of skeletal malignancies, metabolic bone diseases other than osteoporosis or pre-existing hypercalcemia. It should only be used in adults with fully fused epiphyses.
ROUTE AND FREQUENCY OF ADMINISTRATION
In many chronic diseases, including osteoporosis, low adherence to drug regimens is a common problem.54 One reason for this is that anti osteoporosis drugs are prescribed for fracture prevention, and not for symptomatic pain relief. Adherence and persistence for bisphosphonates is low (40–60%), even when it is given weekly or monthly.54 Below a persistence threshold of 50% there is no antifracture effect;54 it is unclear to what extent low compliance and persistence are related to the physician or to the patient.55 In patients who do not comply with a weekly intake, monthly treatment with ibandronate (or intra venous infusion every 3 months) or yearly infusions of zoledronate should be considered.
AVAILABILITY AND REIMBURSEMENT ISSUES
In spite of the indications put forward by the European and US governmental authorities, the availability of drugs and reimbursement for their cost differ between countries and continents. For example, reimbursement for teriparatide varies between countries in the EU according to patient characteristics and incident fractures. Strontium ranelate is reimbursed in EU countries for the treatment of postmenopausal osteoporosis in women older than 50 years in some countries, or only in women older than 80 years in other countries (e.g. Belgium), but is not currently available in the US.
GENERIC DRUGS
Owing to economic considerations, a number of generic forms of bisphosphonates are now available in the EU, based on the efficacy of these formulations in small, single-dose, bioavailability studies, and will also be available in the US in early 2008. Differences in the disintegration/dissolution profiles of generic forms of alendronate suggest that bioavailability studies might not be adequate for the meaningful assessment of the safety and efficacy of generic drugs.56
UNRESOLVED QUESTIONS AND FUTURE RESEARCH
Many patients who experience a clinical fracture would not be classed as having osteoporosis on the basis of BMD measurements alone.4 The upcoming WHO case-finding strategy includes clinical risk factors that predict the individual 5-year and 10-year absolute fracture risk independently of BMD.3 Further clinical research is needed to translate the results of RCTs into clinical practice, and thereby to match drug treatment with individual absolute fracture risk and its pathophysiology.
Vertebral fractures are, independently of BMD, a risk for new fractures.3 Their presence is an indication to initiate drug therapy and, when occurring during treatment with antiresorptive agents, for considering a switch to teriparatide or rhPTH (1–84). The diagnosis of vertebral fractures, however, is often overlooked as a sign of bone fragility, as many such fractures occur subclinically. Studies are needed to specify the indications for spinal X-rays or vertebral fracture assessment using dual X-ray absorptiometry to allow timely identification of patients with vertebral fractures.57
Studies are needed to refine fracture prediction by measuring other components of bone that contribute to its resistance to fracture (such as its microarchitecture, material composition and level of remodeling),58 by increasing our knowledge about genetic backgrounds59 and by integrating approaches to extraskeletal factors, such as fall risks, that are frequently present in patients with a recent fracture.4,60
Could mechanisms of action be helpful in making a clinical choice between drugs? The limited vertebral antifracture data in the aforementioned head-to-head RCTs suggest that—at least for GIOP—stimulation of formerly suppressed bone formation by teriparatide is a more effective approach than suppression of bone turnover by alendronate.19 Such observations should stimulate further research into fine-tuning drug therapy according to the pathophysiology that underlies fracture risk and the presence of clinical risk factors proposed by the WHO.5 The effects of teriparatide and other upcoming bone-forming agents61 open new horizons for bone repair in patients in whom bone microarchitecture is severely disturbed. The use of sequential treatment regimens that switch between currently available and upcoming antiresorptive62 and anabolic61 agents during lifetime treatment is, therefore, an attractive idea, but more data will be needed to substantiate such clinical pathways.
CONCLUSIONS
There is strong evidence in support of the initiation of effective antifracture medications in women that have either postmenopausal osteoporosis or GIOP and a high risk of fracture. The selection of the drugs can be based on answers to questions about patient characteristics (menopausal state, age, BMD, fracture history, risk of vertebral, nonvertebral and hip fracture, time of fracture [recent or old], GIOP, need for an extraskeletal benefit, previous nonpersistence or noncompliance, and preferences of the patient) and drug characteristics (mechanisms of action, antifracture effects, safety, and route and frequency of administration); however, more work is needed to link drug characteristics with the underlying causes of fracture risk in individual patients and to find new medications with both antifracture and extraskeletal effects so as to increase patient acceptance and efficacy of therapy. The perspective of fracture prevention by sequential treatment, which first restores the lost bone and then maintains the newly formed bone, combined with fall prevention strategies, provides a window of opportunity for the prevention of further fractures.
KEY POINTS.
There is strong evidence that supports the initiation of effective antifracture medications in women with postmenopausal osteoporosis or glucocorticoid-induced osteoporosis who have a high risk of fracture
The selection of a specific drug treatment can be based on characteristics of the patient and of the drug, and should include adequate calcium and vitamin D supplementation
More work is needed to match drug treatment with the underlying causes of fracture risk in an individual patient
Sequential treatment with anabolic and antiresorptive agents, combined with fall prevention strategies, provides a window of opportunity for the prevention of further fractures
Learning objectives.
Upon completion of this activity, participants should be able to:
Identify differences in action between different classes of fracture prevention drugs.
List the most likely factors determining fracture risk profile in women.
Describe risk reduction for vertebral, nonvertebral, and hip fractures for different fracture prevention drugs.
Describe risk reduction in all-cause mortality associated with the use of fracture prevention drugs.
Identify adverse effects of fracture prevention drugs.
Acknowledgments
Désirée Lie, University of California, Irvine, CA, is the author of and is solely responsible for the content of the learning objectives, questions and answers of the Medscape-accredited continuing medical education activity associated with this article.
Footnotes
Competing interests
PP Geusens, CH Roux, WF Lems, JD Adachi, KG Saag, DM Reid and MC Hochberg have declared associations with the following companies/organizations: Actelion, the Alliance for Better Bone Health (Procter and Gamble Pharmaceuticals and Sanofi-Aventis), Amgen, Eli Lily, GlaxoSmithKline, Merck, Novartis Pharmaceuticals, Nycomed, Pfizer, Procter and Gamble Pharmaceuticals, Roche, Sanofi-Aventis and Servier. See the article online for full details of the relationship(s). The other authors, the managing editor R Ashton and the CME questions author D Lie declared no competing interests.
Medscape Continuing Medical Education online
Medscape, LLC is pleased to provide online continuing medical education (CME) for this journal article, allowing clinicians the opportunity to earn CME credit. Medscape, LLC is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide CME for physicians. Medscape, LLC designates this educational activity for a maximum of 1.0 AMA PRA Category 1 Credits™. Physicians should only claim credit commensurate with the extent of their participation in the activity. All other clinicians completing this activity will be issued a certificate of participation. To receive credit, please go to http://www.medscape.com/cme/ncp and complete the post-test.
Contributor Information
Piet P Geusens, University Hospital, Maastricht, The Netherlands, and allied with Biomedical Research Institute, University of Hasselt, Belgium..
Christian H Roux, Paris University, France..
David M Reid, University of Aberdeen, UK..
Willem F Lems, VU University Medical Centre in Amsterdam, The Netherlands..
Silvano Adami, University of Verona, Italy..
Jonathan D Adachi, McMaster University in Hamilton, Canada..
Philip N Sambrook, University of Sydney, Australia..
Kenneth G Saag, University of Alabama at Birmingham, AL, USA..
Nancy E Lane, University of California at Davis Medical Center in Sacramento, CA, USA..
Marc C Hochberg, University of Maryland School of Medicine, MD, USA..
References
- 1.Sambrook P and Cooper C (2006) Osteoporosis. Lancet 367: 2010–2018 [DOI] [PubMed] [Google Scholar]
- 2.Geusens PP (2003) Review of guidelines for testing and treatment of osteoporosis. Curr Osteoporos Rep 1: 59–65 [DOI] [PubMed] [Google Scholar]
- 3.Kanis JA et al. (2005) Assessment of fracture risk. Osteoporos Int 16: 581–589 [DOI] [PubMed] [Google Scholar]
- 4.Khosla S and Melton LJ III (2007) Clinical practice. Osteopenia. N Engl J Med 22: 2293–2300 [DOI] [PubMed] [Google Scholar]
- 5.Kelsey JL et al. (1992) Risk factors for fractures of the distal forearm and proximal humerus. The Study of Osteoporotic Fractures Research Group. Am J Epidemiol 135: 477–489 [DOI] [PubMed] [Google Scholar]
- 6.Guyatt GH et al. (1994) Users’ guides to the medical literature. II. How to use an article about therapy or prevention. B. What were the results and will they help me in caring for my patients? JAMA 271: 59–63 [DOI] [PubMed] [Google Scholar]
- 7.Stevenson M et al. (2005) A systematic review and economic evaluation of alendronate, etidronate, risedronate, raloxifene and teriparatide for the prevention and treatment of postmenopausal osteoporosis. Health Technol Assess 9: 1–160 [DOI] [PubMed] [Google Scholar]
- 8.van Staa TP (2006) The pathogenesis, epidemiology and management of glucocorticoid-induced osteoporosis. Calcif Tissue Int 79: 129–137 [DOI] [PubMed] [Google Scholar]
- 9.Riggs BL and Parfitt AM (2005) Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action on bone remodeling. J Bone Miner Res 20: 177–184 [DOI] [PubMed] [Google Scholar]
- 10.Black DM et al. (2007) Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 356: 1809–1822 [DOI] [PubMed] [Google Scholar]
- 11.Lyles KW et al. (2007) Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 357: 1799–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenspan SL et al. (2007) Effect of recombinant human parathyroid hormone (1–84) on vertebral fracture and bone mineral density in postmenopausal women with osteoporosis: a randomized trial. Ann Intern Med 146: 326–339 [DOI] [PubMed] [Google Scholar]
- 13.Seeman E and Eisman JA (2004) 7: Treatment of osteoporosis: why, whom, when and how to treat. The single most important consideration is the individual’s absolute risk of fracture. Med J Aust 180: 298–303 [PubMed] [Google Scholar]
- 14.Roux C et al. (2007) Assessment of non-vertebral fracture risk in post-menopausal women. Ann Rheum Dis 66: 931–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Helden S et al. (2006) Risk of new clinical fractures within 2 years following a fracture. Osteoporos Int 17: 348–354 [DOI] [PubMed] [Google Scholar]
- 16.Center JR et al. (2007) Risk of subsequent fracture after low-trauma fracture in men and women. JAMA 297: 387–394 [DOI] [PubMed] [Google Scholar]
- 17.de Nijs RN et al. (2006) Alendronate or alfacalcidol in glucocorticoid-induced osteoporosis. N Engl J Med 355: 675–684 [DOI] [PubMed] [Google Scholar]
- 18.Lane NE et al. (1998) Parathyroid hormone treatment can reverse corticosteroid-induced osteoporosis. Results of a randomized controlled clinical trial. J Clin Invest 102: 1627–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saag KG et al. (2007) Teriparatide or alendronate in glucocorticoid-induced osteoporosis. N Engl J Med 357: 2028–2039 [DOI] [PubMed] [Google Scholar]
- 20.Bischoff-Ferrari HA (2007) How to select the doses of vitamin D in the management of osteoporosis. Osteoporos Int 18: 401–407 [DOI] [PubMed] [Google Scholar]
- 21.Tannenbaum C et al. (2002) Yield of laboratory testing to identify secondary contributors to osteoporosis in otherwise healthy women. J Clin Endocrinol Metab 87: 4431–4437 [DOI] [PubMed] [Google Scholar]
- 22.US Department of Health and Human Services (2004) Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville, M: D: US Department of Health and Human Services [Google Scholar]
- 23.Delmas PD et al. (2003) Severity of prevalent vertebral fractures and the risk of subsequent vertebral and nonvertebral fractures: results from the MORE trial. Bone 33: 522–532 [DOI] [PubMed] [Google Scholar]
- 24.Delmas PD et al. (2004) Daily and intermittent oral ibandronate normalize bone turnover and provide significant reduction in vertebral fracture risk: results from the BONE study. Osteoporos Int 15: 792–798 [DOI] [PubMed] [Google Scholar]
- 25.Roux C et al. (2004) Efficacy of risedronate on clinical vertebral fractures within six months. Curr Med Res Opin 20: 433–439 [DOI] [PubMed] [Google Scholar]
- 26.Seeman E et al. (2006) Strontium ranelate reduces the risk of vertebral and nonvertebral fractures in women eighty years of age and older. J Bone Miner Res 21: 1113–1120 [DOI] [PubMed] [Google Scholar]
- 27.Hochberg MC et al. (2005) Effect of alendronate on the age-specific incidence of symptomatic osteoporotic fractures. J Bone Miner Res 20: 971–976 [DOI] [PubMed] [Google Scholar]
- 28.Boonen S et al. (2004) Safety and efficacy of risedronate in reducing fracture risk in osteoporotic women aged 80 and older: implications for the use of antiresorptive agents in the old and oldest old. J Am Geriatr Soc 52: 1832–1839 [DOI] [PubMed] [Google Scholar]
- 29.Marcus R et al. (2003) The skeletal response to teriparatide is largely independent of age, initial bone mineral density, and prevalent vertebral fractures in postmenopausal women with osteoporosis. J Bone Miner Res 18: 18–23 [DOI] [PubMed] [Google Scholar]
- 30.Gøtzsche PC et al. (2007) Data extraction errors in meta-analyses that use standardized mean differences. JAMA 298: 430–437 [DOI] [PubMed] [Google Scholar]
- 31.Liberman UA et al. (2006) Hip and non-spine fracture risk reductions differ among antiresorptive agents: Evidence from randomised controlled trials. Int J Clin Pract 60: 1394–1400 [DOI] [PubMed] [Google Scholar]
- 32.Boonen S et al. (2005) Effect of osteoporosis treatments on risk of non-vertebral fractures: review and meta-analysis of intention-to-treat studies. Osteoporos Int 16: 1291–1298 [DOI] [PubMed] [Google Scholar]
- 33.Rosen CJ et al. (2005) Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res 20: 141–151 [DOI] [PubMed] [Google Scholar]
- 34.Ferrari S and Rizzoli R (2007) Risedronate or alendronate for the prevention of osteoporotic fractures: is there a real difference? Bonekey Osteovision 4: 141–143 [Google Scholar]
- 35.Silverman SL et al. (2007) Effectiveness of bisphosphonates on nonvertebral and hip fractures in the first year of therapy: the risedronate and alendronate (REAL) cohort study. Osteoporos Int 18: 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Russell RG (2006) Bisphosphonates: from bench to bedside. Ann N Y Acad Sci 1068: 367–401 [DOI] [PubMed] [Google Scholar]
- 37.Homik J et al. Calcium and vitamin D for corticosteroid-induced osteoporosis. Cochrane Database of Systematic Reviews 1998, Issue 1 Art. No.: CD000952. doi: 10.1002/14651858.CD000952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Martino S et al. (2004) Continuing outcomes relevant to Evista: breast cancer incidence in postmenopausal osteoporotic women in a randomized trial of raloxifene. J Natl Cancer Inst 96: 1751–1761 [DOI] [PubMed] [Google Scholar]
- 39.Rossouw JE et al. (2002) Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principle results from the Women’s Health Initiative randomized controlled trial. JAMA 288: 321–333 [DOI] [PubMed] [Google Scholar]
- 40.Shane E et al. (2006) Osteonecrosis of the jaw: more research needed. J Bone Miner Res 21: 1503–1505 [DOI] [PubMed] [Google Scholar]
- 41.Odvina CV et al. (2005) Severely suppressed bone turnover: a potential complication of alendronate therapy. J Clin Endocrinol Metab 90: 1294–1301 [DOI] [PubMed] [Google Scholar]
- 42.Chapurlat RD et al. (2007) Microcrack frequency and bone remodeling in postmenopausal osteoporotic women on long-term bisphosphonates: a bone biopsy study. J Bone Miner Res 22: 1502–1509 [DOI] [PubMed] [Google Scholar]
- 43.Berenson JR (2005) Recommendations for zoledronic acid treatment of patients with bone metastases. Oncologist 10: 52–62 [DOI] [PubMed] [Google Scholar]
- 44.Cummings SR et al. (2007) Alendronate and atrial fibrillation. N Engl J Med 356: 1895–1896 [DOI] [PubMed] [Google Scholar]
- 45.Karam R et al. (2007) Yearly zoledronic acid in postmenopausal osteoporosis. N Engl J Med 357: 712–713 [PubMed] [Google Scholar]
- 46.European Medicines Agency (2007) Press Release: EMEA recommends changes in the product information for Protelos/Osseor due to the risk of severe hypersensitivity reactions [www.emea.europa.eu./humandocs/PDFs/EPAR/protelos/PressRelease_Protelos_41745807en.pdf] (accessed 13 February 2008)
- 47.Vahle JL et al. (2004) Bone neoplasms in F344 rats given teriparatide [rhPTH(1–34)] are dependent on duration of treatment and dose. Toxicol Pathol 32: 426–438 [DOI] [PubMed] [Google Scholar]
- 48.Greenspan SL et al. (2003) Combination therapy with hormone replacement and alendronate for prevention of bone loss in elderly women: a randomized controlled trial. JAMA 289: 2525–2533 [DOI] [PubMed] [Google Scholar]
- 49.Black DM et al. (2003) The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med 349: 1207–1215 [DOI] [PubMed] [Google Scholar]
- 50.Finkelstein JS et al. (2003) The effects of parathyroid hormone, alendronate, or both in men with osteoporosis. N Engl J Med 349: 1216–1226 [DOI] [PubMed] [Google Scholar]
- 51.Cosman F et al. (2005) Daily and cyclic parathyroid hormone in women receiving alendronate. N Engl J Med 353: 566–575 [DOI] [PubMed] [Google Scholar]
- 52.Black DM et al. (2005) One year of alendronate after one year of parathyroid hormone (1–84) for osteoporosis. N Engl J Med 353: 555–565 [DOI] [PubMed] [Google Scholar]
- 53.Rubin MR and Bilezikian JP (2005) Parathyroid hormone as an anabolic skeletal therapy. Drugs 65: 2481–2498 [DOI] [PubMed] [Google Scholar]
- 54.Siris ES et al. (2006) Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc 81: 1013–1022 [DOI] [PubMed] [Google Scholar]
- 55.Bliuc D et al. (2005) Barriers to effective management of osteoporosis in moderate and minimal trauma fractures: a prospective study. Osteoporos Int 16: 977–982 [DOI] [PubMed] [Google Scholar]
- 56.Epstein S et al. (2005) Disintegration and esophageal irritation profiles of alendronate formulations: implications for clinical safety and efficacy. J Applied Res 5: 253–265 [Google Scholar]
- 57.Damiano J et al. (2006) Diagnosis of vertebral fractures by vertebral fracture assessment. J Clin Densitom 9: 66–71 [DOI] [PubMed] [Google Scholar]
- 58.Bouxsein ML (2005) Determinants of skeletal fragility. Best Pract Res Clin Rheumatol 19: 897–911 [DOI] [PubMed] [Google Scholar]
- 59.Ralston SH and de Crombrugghe B (2006) Genetic regulation of bone mass and susceptibility to osteoporosis. Genes Dev 20: 2492–2506 [DOI] [PubMed] [Google Scholar]
- 60.van Helden S et al. (2008) Bone and fall-related fracture risks in women and men with a recent clinical fracture. J Bone Joint Surg Am 90: 241–248 [DOI] [PubMed] [Google Scholar]
- 61.Canalis E et al. (2007) Mechanisms of anabolic therapies for osteoporosis. N Engl J Med 357: 905–916 [DOI] [PubMed] [Google Scholar]
- 62.Margolis RN and Wimalawansa SJ (2006) Novel targets and therapeutics for bone loss. Ann N Y Acad Sci 1068: 402–409 [DOI] [PubMed] [Google Scholar]