Abstract
Glucocorticoid (GC) therapy induces deleterious effects on the skeleton in kidney transplantation but studies of GC discontinuation in this population are limited. This study evaluated changes in areal bone mineral density (BMD) with GC withdrawal. Subjects were enrolled one yr after renal transplantation and randomized to continue or stop prednisone; all subjects continued cyclosporine and mycophenolate mofetil. BMD measured by dual-energy X-ray absorptiometry was performed at enrollment and repeated at one yr and values were standardized. Mean ± standard deviation of annualized change in standardized BMD between GC withdrawal vs. continuation group at the lumbar spine was +4.7% ± 5.5 vs. + 0.9% ± 5.3 (p = 0.0014); total hip +2.4% ± 4.2 vs. −0.4% ± 4.2 (p = 0.013), and femoral neck +2.1% ± 4.6 vs. +1.0% ± 6.0 (p = 0.37). There was no confounding by prednisone dose prior to enrollment, change in creatinine clearance, weight, or use of bone-active medications following study entry. Multivariate analysis determined that the change in BMD was positively associated with baseline alkaline phosphatase and creatinine clearance and negatively associated with baseline BMD. BMD improves with GC withdrawal after renal transplantation, and this gain in BMD is dependent on the baseline bone turnover, renal function, and BMD.
Keywords: bone mineral density, dual-energy X-ray absorptiometry, glucocorticoid withdrawal, kidney transplant, renal transplant, steroid withdrawal
Glucocorticoid (GC) therapy results in deleterious effects on the skeleton (1, 2), leading to decline in bone mineral density (BMD) (3), deterioration in trabecular bone microarchitecture (4), and increased fracture risk (5, 6). GC-treated patients may fracture at a higher bone density relative to those without GC therapy (7–10). GCs affect bone metabolism through multiple mechanisms, the predominant of which acts to decrease bone formation via decreasing osteoblast proliferation, function, and survival (11, 12). GCs also reduce osteocyte viability (13), increase bone resorption via osteoclast activation (14), diminish gastrointestinal calcium absorption (15), and decrease sex steroids (16, 17). Additional mechanisms of increased urinary calcium and phosphorous excretion and hyperparathyroidism have also been described (18, 19). Even at low doses, GCs are associated with an increased risk for fracture (20).
Relative to the general population, fracture risk is estimated to be more than fourfold greater in end-stage renal disease (21) and in renal transplantation. (22, 23) Hip fracture risk is one-third greater during the first year following renal transplantation vs. continuing dialysis (24). Risks for hip fracture include older age, female gender, Caucasian race, longer dialysis duration, lower body mass index, and peripheral vascular disease (25) and type 1 diabetes mellitus (26). The reader is referred to excellent reviews of post-renal transplantation osteodystrophy (27–33).
GC therapy historically has been a main component of maintenance immunosuppression in renal transplantation. After renal transplantation, patients taking GCs experience BMD decreases by 4–9% at the lumbar spine and 5–8% at the hip (34–40). As immunosuppressive regimens have evolved, high doses of GCs are given during the immediate post-transplant period and weaned off within a few weeks (41). Although current immunosuppressive regimens are GC-sparing, patients receiving a kidney transplant when GCs were typically prescribed for chronic immunosuppression may continue on this treatment. There are limited studies examining the effect of GC discontinuation on BMD in kidney transplant recipients (42). We sought to evaluate this effect using data from a randomized clinical trial of GC withdrawal in renal transplant recipients (43).
Patients and methods
This study is a retrospective medical records review in a completed, non-blinded, randomized clinical trial of GC withdrawal vs. continuation (43). Subjects were adult renal transplant recipients 6–36 months post-transplantation followed at Ohio State University recruited from November 1997 to April 2002. For trial entry, subjects’ serum creatinine was <2.5 mg/dL with no increase in serum creatinine >30% within the past three months prior to enrollment, had no prior acute rejection episodes, and their urinary protein excretion was <600 mg/24 h.
Immunosuppression
All subjects were treated with prednisone (initially 2 mg/kg/d, decreased to 0.2 mg/kg/d at one month, and lowered to 0.15 mg/kg/d at one yr after transplantation), microemulsion cyclosporine (initially 5–6 mg/kg/d, starting 2–5 d after renal function was established, then adjusted to obtain whole blood trough levels of >250 mg/dL during the first year and >150 ng/mL thereafter), and mycophenolate mofetil (MMF, 2 g daily). Mean cyclosporine and MMF doses and cyclosporine trough levels were not different between groups at any time point from study entry to 60 months (data not shown). The study was initially designed to recruit 40 subjects from each of three groups (6–12, 12–18, and 18–36 months after transplantation). However, there was difficulty with recruitment from the last group, and five additional subjects were recruited from the 6–12 month post-transplantation. Sixty subjects were randomized to continue and 60 to discontinue GC therapy. Soon after obtaining consent, one subject was withdrawn because of proteinuria >600 mg/24 h and another because of non-adherence to study procedures, leaving 118 subjects eligible for the current analysis. All subjects continued cyclosporine and MMF. Subjects randomized to GC withdrawal were instructed to lower prednisone by 2.5 mg and continue reducing by 2.5 mg every two wk until discontinuation. One subject required a slower taper owing to symptoms suggesting adrenal insufficiency. Informed consent was obtained, and the study was conducted with institutional review board approval.
Data on bone-active medications such as bisphosphonate, calcitriol, estrogen, selective estrogen receptor modulator, calcitonin, and cinacalcet at the time of enrollment and in relation to dual-energy X-ray absorptiometry (DXA) scanning were captured. Cumulative doses of prednisone therapy after transplantation were calculated. Serum and urine tests were performed at clinical laboratories. Serum calcium concentration was corrected for the prevailing albumin concentration using calcium + 0.8 × (4.0-albumin) (44). Creatinine clearance was calculated with 24-h urinary collection of creatinine using the formula (urinary creatinine [mg/dL]/serum creatinine [mg/dL]) × (urinary volume [mL]/1440) (45).
Areal BMD was measured using DXA scanning of anteroposterior lumbar spine (L1–L4), total hip, and femoral neck. Clinical DXA scans were performed using various manufacturers at various sites within and outside the medical center. As study participants used different DXA manufacturers and BMD measurement by manufacturer are systematically different, BMD was standardized as shown later. All BMD units are in g/cm2, and all subsequent references to BMD denote standardized BMD.
| Spine (46) | Hologic | 1.055 (BMD − 0.972) + 1.0436 |
| Lunar | 0.9683 (BMD − 1.100) + 1.0436 | |
| Norland | 0.9743 (BMD − 0.969) + 1.0436 | |
| Total Hip (46) | Hologic | 0.006 + (1.008 × BMD) |
| Lunar | −0.031 + (0.979 × BMD) | |
| Norland | 0.026 + (1.012 × BMD) | |
| Femoral Neck (47) | Hologic | 0.019 + (1.087 × BMD) |
| Lunar | −0.023 + (0.939 × BMD) | |
| Norland | 0.006 + (0.985 × BMD) |
Baseline BMD (DXA1) was measured at the time of study enrollment (6–36 months after transplantation) and repeated after the first DXA approximately one yr later (DXA2). The time interval from DXA1 to DXA2 was slightly longer in the GC withdrawal vs. GC continuation, 0.13 yr at the lumbar spine (p = 0.0001), 0.11 yr at the total hip (p = 0.0001), and 0.14 yr at the femoral neck (p < 0.0001). To control for these intergroup difference (40–50 d), change in BMD for each subject was annualized. Annualized percent change in BMD (%DBMD) was defined as:
Annualized %ΔBMD = % change/years between DXA1 and DXA2
% Change =100 × (BMD at DXA2 – BMD at DXA1)/BMD at DXA1.
Statistical methods
All analyses were performed using SAS 9.2 (Cary, NC, USA). Descriptive statistics of the baseline subject characteristics at the time of trial enrollment were determined with two-tailed unpaired Student’s t-tests and Fisher’s exact tests to compare means and proportions between groups. For each skeletal location (lumbar spine, total hip, and femoral neck), differences in annualized % change in BMD between treatment groups were assessed using t-tests. We adjusted for other possible predictors of change in BMD using repeated-measures multiple regression analysis that included skeletal site as a predictor. Eighteen predictors were screened for model inclusion taken from the variables listed in Table 1.
Table 1.
Baseline characteristics (mean and standard deviation) in the overall, GC withdrawal, and GC continuation groups
| Variable | N | Overall (SD) N = 87 | GC withdrawal (SD) N = 45 | GC continuation (SD) N = 42 | p-Value |
|---|---|---|---|---|---|
| Age (yr) | 87 | 46.7 (13.7) | 48.3 (14.2) | 45.0 (13.0) | 0.260 |
| Sex (% male) | 87 | 56.3% | 57.8% | 54.8% | 0.831 |
| Race (% white) | 87 | 81.6% | 82.2% | 81.0% | 1.0000 |
| BMI (kg/m2) | 87 | 29.9 (5.6) | 30.0 (5.2) | 29.7 (6.1) | 0.797 |
| Transplant donor type | 87 | 0.416 | |||
| Cadaveric (%) | 56 | 64.4% | 62.2% | 66.7% | |
| Living related (%) | 27 | 31.0% | 35.6% | 26.2% | |
| Living unrelated (%) | 4 | 4.6% | 2.2% | 7.1% | |
| Diabetes (%) | 87 | 36.8% | 28.9% | 45.2% | 0.126 |
| Hemodialysis (months) | 86 | 10.6 (23.2) | 9.2 (14.2) | 12.1 (30.0) | 0.567 |
| Peritoneal dialysis (months) | 87 | 9.6 (18.9) | 6.9 (8.6) | 12.4 (25.6) | 0.198 |
| Dialysis (months) | 87 | 20.0 (28.2) | 15.9 (14.0) | 24.5 (37.7) | 0.172 |
| Time since transplant (d) | 87 | 417.6 (180.1) | 415.9 (172.4) | 419.4 (190.1) | 0.930 |
| Calcium (mg/dL) | 87 | 9.7 (0.7) | 9.7 (0.9) | 9.6 (0.5) | 0.646 |
| Phosphorous (mg/dL) | 87 | 2.9 (0.6) | 3.0 (0.7) | 2.9 (0.6) | 0.258 |
| Creatinine (mg/dL) | 87 | 1.50 (0.4) | 1.55 (0.4) | 1.44 (0.4) | 0.143 |
| Creatinine clearance (mL/min) | 86 | 64.8 (21.8) | 65.2 (19.6) | 64.4 (24.3) | 0.862 |
| Intact PTH (pg/mL) | 87 | 95.8 (72.7) | 84.8 (48.7) | 107.5 (91.0) | 0.148 |
| Alkaline phosphatase (U/L) | 87 | 90.4 (35.3) | 95.2 (38.5) | 85.3 (31.3) | 0.193 |
| Cumulative prednisone (mg) | 86 | 7862 (2536) | 8003 (2777) | 7707 (2266) | 0.592 |
| Bone-active medication (%) | 87 | 17.2 | 13.3 | 21.4 | 0.399 |
| Lumbar spine BMD (g/cm2) | 86 | 1.041 (0.145) | 1.027 (0.132) | 1.054 (0.159) | 0.390 |
| Lumbar spine Z-score | 84 | −0.7 (1.3) | −0.8 (1.2) | −0.6 (1.4) | 0.470 |
| Femoral neck BMD (g/cm2) | 80 | 0.824 (0.132) | 0.831 (0.128) | 0.816 (0.138) | 0.601 |
| Femoral neck Z-score | 77 | −0.7 (0.9) | −0.6 (0.8) | −0.8 (1.0) | 0.478 |
| Total hip BMD (g/cm2) | 76 | 0.902 (0.139) | 0.892 (0.131) | 0.913 (0.149) | 0.506 |
| Total hip Z-score | 74 | −0.6 (0.9) | −0.6 (0.8) | −0.5 (1.0) | 0.864 |
BMD, bone mineral density; GC, glucocorticoid.
In model building, we first entered each predictor independently into a regression model in which the other predictors of change were GC use, skeletal site, and two interactions (i) predictor and skeletal site and (i) GC use and skeletal site. Interactions with a p-value ≤ 0.25 put the predictor and its interaction with skeletal site into the list of predictors for consideration in a multiple regression model. Second, predictors not included after initial screening were then placed independently into a regression model in which the only other predictors of change were GC use, skeletal site, and the interaction of GC use and skeletal site. Those with parameter estimates with a p-value ≤ 0.25 were also added to the list of predictors for consideration in the multiple regression model. To arrive at a parsimonious multiple regression model, we began with all the selected predictors as well as skeletal site, GC use, and their interaction of skeletal site and GC use and sequentially removed effects with p-values >0.05, choosing for deletion at each step the effect with the largest p-value. We stopped when the statistical significance of each of the remaining effects did not exceed 0.05. All modeling was performed using SAS’s MIXED procedure, which supports modeling with repeated measures within subject. The repeated measures were the changes at up to three skeletal sites. The correlation between repeated measures was modeled through the use of a random effect at the subject level.
Results
Among eligible subjects, 87 had DXA scans within a year of study consent date and subsequent scans within two yr of their initial scans. These 87 subjects comprise the current study population whose results are described here. Each of the 87 had the potential to provide six DXA measurements, two time points from each of three measurement site, but not all subjects had data for each site. On average, each subject provided data from 5.6 scans for a total of 484 scans distributed across the DXA manufacturer as follows: 401 Hologic, 75 Lunar, and eight Norland.
Subject characteristics and biochemical data are shown in Table 1. Age at entry was in the mid-fifth decade. Gender was equally divided and more than 80% were Caucasian. Subject’s BMI was at the borderline of overweight and obese. Sixty-four percent of kidneys came from cadavers. Baseline characteristics did not differ in terms of age, gender, race, BMI, transplant donor type, or prevalence of diabetes mellitus. Among the 54 subjects who had any prior hemodialysis, duration was not different between withdrawal and continuation groups (16.2 ± 15.6 vs. 17.6 ± 34.9, respectively, p = 0.8). Among the 44 subjects with any prior peritoneal dialysis, duration in GC withdrawal(12.5 ± 8.0) vs. continuation (27.3 ± 32.6) was not different (p = 0.07). Hemodialysis and peritoneal dialysis were combined into a single variable, “dialysis,” and no difference was found between treatment groups. Subject enrollment and baseline DXA scan were on average 1¼ yr following transplantation, and there were no differences in time between transplantation to enrollment, baseline serum calcium, phosphorous, creatinine, creatinine clearance, intact parathyroid hormone (PTH), alkaline phosphatase, cumulative prednisone dose, and use of bone-active medications between the two groups. The baseline DXA scan was obtained 15 ± 56 d prior to consent and did not differ between treatment groups (data not shown). Baseline standardized BMD at the lumbar spine, total hip, and femoral neck did not differ between the two groups. BMD was within 1 standard deviation compared to an age, gender, and race-matched population (Z-score range, −0.5 to −0.8).
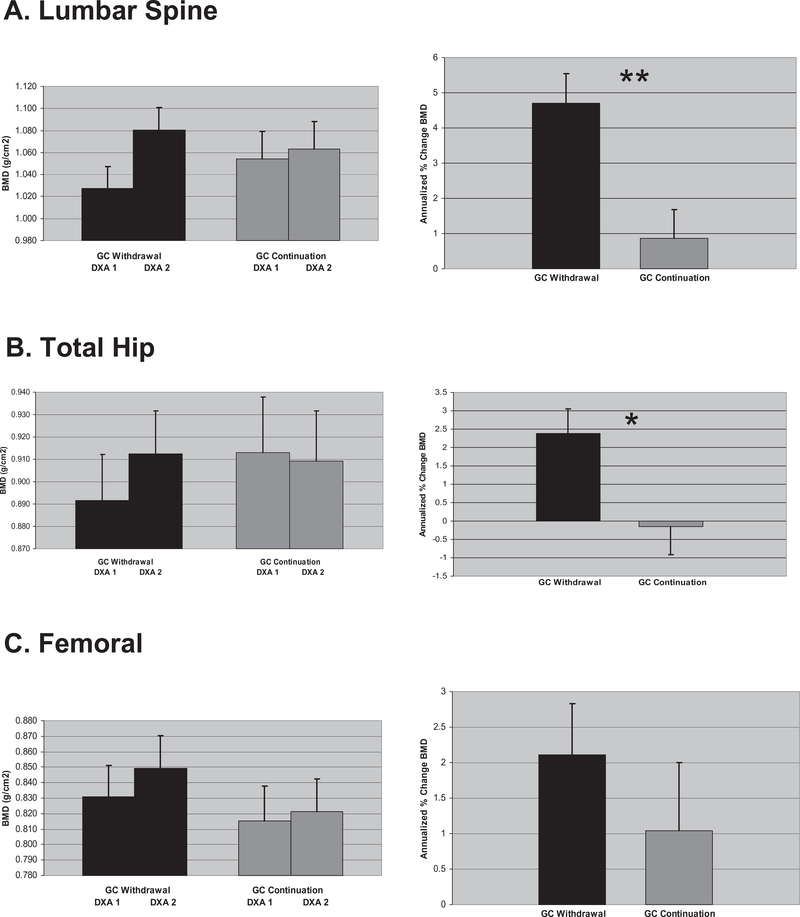
Baseline (DXA1) and follow-up (DXA2) BMD values for each anatomic site are shown in Fig. 1, left panel. Two BMD measurements at the lumbar spine, total hip, and femoral neck were retrieved in 86, 76, and 80 subjects, respectively. Mean (±SD) annualized percent change in standardized BMD (%ΔsBMD) (Fig. 1, right panel) at the lumbar spine, total hip, and femoral neck was 4.7% (5.5), 2.4% (4.2), and 2.1% (4.6), respectively, in GC withdrawal and 0.9% (5.3), −0.2% (4.5), and 1.0% (6.0), respectively, in GC continuation. Intergroup differences were statistically significant at the lumbar spine and total hip, 3.8% (p = 0.001) and 2.5% (p = 0.01), respectively, but non-significant at the femoral neck, 1.1% (p = 0.37).
Fig. 1.
Summary of bone mineral density (BMD) and annualized change in BMD by anatomic site. (A) Lumbar spine, (B) total hip, (C) femoral neck. Left panel: Observed mean standardized BMD (g/cm2) in glucocorticoid (GC) withdrawal and continuation at baseline (DXA1) and follow up (DXA2). Right panel: Mean annualized percent change in sBMD in GC withdrawal and continuation. Error bars denote standard error. Asterisks summarize results of t-tests of group differences. *p = 0.01, **p = 0.001. DXA, dual-energy X-ray absorptiometry.
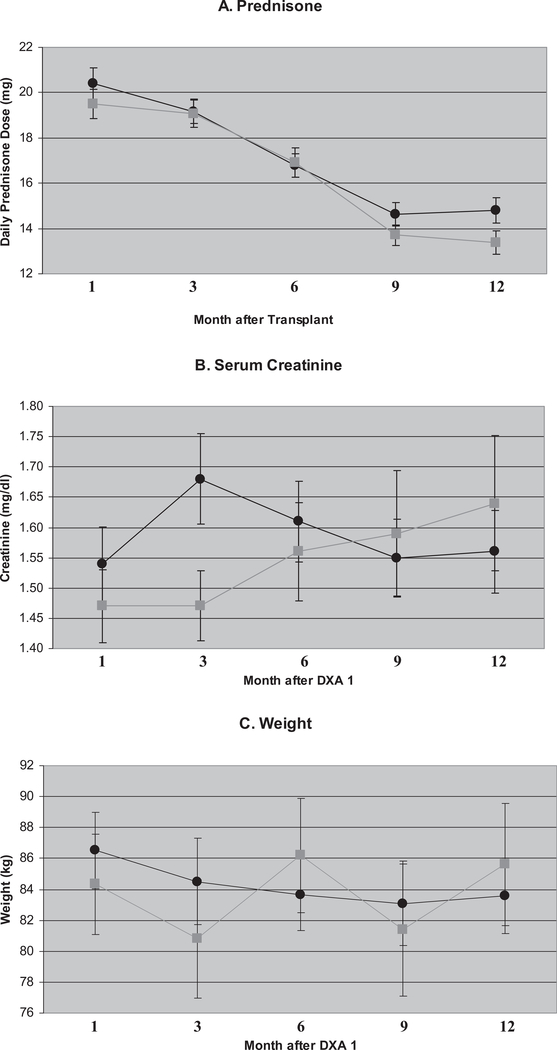
To determine whether specific factors were potentially biasing our findings, we tested intergroup differences in prednisone use, changes in serum creatinine, weight, and bone-active medications. Cumulative prednisone dose at the time of consent was not statistically different between the two groups (Table 1); however, there may have been subtle intergroup differences in prednisone dosing. Daily prednisone doses (mg) between one and 12 months post-transplant are shown in Fig. 2A. To test for group differences, we fit a repeated-measures model of prednisone dose with predictors: month since transplant, group membership, and the interaction of the two predictors. The interaction was not statistically significant (p = 0.36) and was removed from the model. In the main effects model, effect of treatment group was statistically non-significant (p = 0.56), but month since transplant was statistically significant (p < 0.0001). Over time, the average prednisone dose decreased.
Fig. 2.
Selected parameters across time by treatment group. (A) Mean daily prednisone dose (mg/d) by month since transplant. (B) Serum creatinine (mg/dL) by month after baseline dual-energy X-ray absorptiometry (DXA) scan. (C) Weight (kg) by month after baseline DXA scan. Error bars denote standard error. Black circles (•), glucocorticoid (GC) withdrawal group. Gray squares ( ), GC continuation group.
), GC continuation group.
Serum creatinine and weight after the baseline DXA scan are shown in Fig. 2B, C. Creatinine values at DXA1 are similar but not identical to those at enrollment (Table 1). To test for group differences, we fit a repeated-measures model as described earlier. The interaction between month since DXA1 and group assignment was statistically non-significant (p = 0.06) and was removed from the model. In the main effects model, neither month (p = 0.08) nor group membership (p = 0.64) was statistically significant. In modeling weight with month after baseline DXA, group membership, and the interaction of the two predictors, the interaction was statistically non-significant (p = 0.08) and was removed from the model. The main effects model also showed no effect by group assignment (p = 0.92). Time was statistically significant (p = 0.01) with a decrease by months 6–9, followed by a rise to baseline.
We examined the use of bone-active medications at the baseline and follow-up DXA scans (Table 2). There were no group differences in bisphosphonate, calcitriol, or estrogen use at either DXA scan. Likewise, there was no group difference after aggregating any bone-active medication use at either DXA scan.
Table 2.
Use of bone-active medications at baseline and follow-up DXA scan by group assignment
| GC withdrawal |
GC continuation |
||||
|---|---|---|---|---|---|
| Bone-active medication | % | N | % | N | p-Value |
| Bisphosphonate at DXA1 | 2.2 | 45 | 7.1 | 42 | 0.349 |
| Calcitriol at DXA1 | 4.4 | 45 | 11.9 | 42 | 0.255 |
| Estrogen at DXA1 | 36.8 | 19 | 15.8 | 19 | 0.269 |
| Any bone-active medication at DXA1 | 15.6 | 45 | 19.0 | 42 | 0.667 |
| Bisphosphonate at DXA2 | 6.7 | 45 | 11.9 | 42 | 0.475 |
| Calcitriol at DXA2 | 11.1 | 45 | 28.6 | 42 | 0.058 |
| Estrogen at DXA2 | 36.8 | 19 | 15.8 | 19 | 0.269 |
| Any bone-active medication at DXA2 | 26.7 | 45 | 40.5 | 42 | 0.172 |
For estrogen use, only women are included.
DXA, dual-energy X-ray absorptiometry; GC, glucocorticoid.
The multiple regression model of annualized % change in BMD estimates that for the GC withdrawal group, BMD changes at the lumbar spine, total hip, and femoral neck are +4.6%, + 2.2%, and +1.6%, respectively (Table 3). The effect of GC is estimated to decrease % BMD changes by 3.6% and 2.4% at the lumbar spine and total hip, respectively. The effect of GC at the femoral neck is not statistically significant. Change in BMD was positively associated with baseline alkaline phosphatase and creatinine clearance and negatively associated with baseline BMD. Annualized %ΔsBMD increased 0.033% per U/L of alkaline phosphatase (p = 0.003); increased 0.036% per mL/min increase in creatinine clearance (p = 0.047); and decreased 0.69% per 0.100 g/cm2 of baseline BMD (p = 0.04).
Table 3.
Multivariate analysis of annualized percent change in standardized bone mineral density
| Parameter | Estimate | CI | p-Value |
|---|---|---|---|
| GC withdrawal | |||
| Lumbar spine | 4.55 | 3.11, 5.99 | <0.0001 |
| Total hip | 2.22 | 0.70, 3.74 | 0.004 |
| Femoral neck | 1.63 | 0.14, 3.12 | 0.032 |
| GC continuation | |||
| Lumbar spine | 0.91 | −0.57, 2.40 | 0.227 |
| Total hip | −0.22 | −1.81, 1.38 | 0.791 |
| Femoral neck | 1.45 | −0.087, 2.98 | 0.064 |
| Effect of GC | |||
| Lumbar spine | −3.63 | 5.72, −1.55 | 0.001 |
| Total hip | −2.44 | −4.65, −0.22 | 0.031 |
| Femoral neck | −0.18 | −2.33, 1.97 | 0.868 |
| Per U/L change in alkaline phosphatase | 0.033 | 0.012, 0.055 | 0.003 |
| Per 0.100 g/cm2 change in baseline BMD | −0.69 | −1.33, −0.046 | 0.036 |
| Per 1 mL/min change in creatinine clearance | 0.036 | 0.00, 0.071 | 0.047 |
BMD, bone mineral density; GC, glucocorticoid.
Discussion
In this randomized clinical trial of renal transplant recipients, GC withdrawal vs. continuation was followed by an increase in BMD at the lumbar spine and total hip. Although a number of longitudinal studies have shown loss of BMD with greater cumulative prednisone doses (40), there are few longitudinal studies of GC withdrawal in renal transplantation. In a prospective trial of patients seven yr post-kidney transplant on prednisolone (6 mg daily), cyclosporine, and azathioprine, one yr changes in BMD were +2.5% at the lumbar spine and +1.0% at the total hip in GC withdrawal group (N = 32) vs. −0.5% at the lumbar spine and total hip in the GC continuation group (N = 32) (42, 48). The current study cohort had comparable bone mass prior to randomization compared to the study of Farmer et al. as subject’s Z-scores (−0.35 to −0.65) are similar to those in the current study (−0.6 to −0.7). In the current study, GC withdrawal showed increases of +4.7% at the lumbar spine, +2.4% at the total hip, and +2.1% at the femoral neck, suggesting the magnitude of BMD increase may be greater if GCs are discontinued sooner after transplantation (on average 1¼ yr in this study). Daily prednisone doses in the GC continuation group decreased at two and three yr post-transplantation and at most recent follow-up, 10.8, 10.1, and 7.9 mg, respectively.
We observed that BMD changes differed at different anatomic sites. At the lumbar spine, BMD increase was on average greater in magnitude compared to the femoral neck or total hip (Fig. 1). The multiple regression model predicts that after GC withdrawal, relative to change in BMD at the lumbar spine, is 2.3% less at the total hip and 2.9% less at the femoral neck (Table 3). This is consistent with the greater metabolic activity, increased surface area, and concomitant response to bone-active agents by the trabecular bone-enriched vertebral spine compared to the less active and responsive cortical bone-enriched femoral neck. Cortical and trabecular bone composition at the total hip is roughly equal, and its BMD recovery after GC withdrawal was intermediate between the femoral neck and the lumbar spine.
Serum alkaline phosphatase concentration was positively associated with the change in BMD. Specific bone turnover biomarkers were not measured. However, total alkaline phosphatase correlates with bone formation biomarker, bone-specific alkaline phosphatase, with a correlation coefficient as high as 0.94 in Paget’s disease (49, 50). Higher baseline alkaline phosphatase, reflecting greater bone turnover, predicted a greater improvement in BMD. For example, after doubling baseline alkaline phosphatase from its mean of 90–180 U/L, the model predicts a further 3.0% increase in BMD. A greater creatinine clearance also predicts greater BMD increase. For example, relative to the mean creatinine clearance of 64.8 mL/min (Table 1), a subject with a creatinine clearance of 74.8 mL/min (Table 1) is expected to have a 0.4% greater increase in BMD. This is also an expected finding – healing of renal osteodystrophy occurring after underlying renal function improves following kidney transplantation. BMD change was negatively associated with starting BMD values. For example, compared to the average baseline lumbar spine BMD of 1.041 g/cm2 (Table 1), an individual with an initial BMD of 0.941 g/cm2 would experience a 0.7% greater increase in BMD. In contrast to prior data reporting an inverse relationship between PTH and BMD (51), baseline intact PTH did not predict change in BMD in this study. Modest elevation and fairly narrow range in PTH in the current study (96.8 ± 73 pg/mL) might account for the lack of association.
There were three fractures in each group. In GC withdrawal, one subject each experienced fracture at L1, rib, and fibula at five yr, six yr, and two months, respectively, after study entry and in GC continuation, one subject each suffered fracture at T12, wrist, and humerus at two, one, and five yr following entry. As previously reported, there were no group differences at one yr and last follow-up at 3.7 yr in rates of patient survival, graft survival, acute and chronic rejection, or graft function (43).
The strength of this study is the randomized allocation of treatment which, as indicated by the lack of statistically significant differences in Table 1, equalized measured baseline patient characteristics. Although lack of blinding may have introduced bias, compared to the original cohort of 118 subjects, the current study population of 87 subjects had similar baseline characteristics, suggesting that this population remained balanced in terms of unmeasured baseline characteristics and potential confounders by treatment assignment.
A limitation of this study was the use of different DXA manufacturers. Restricting analysis subjects who had baseline and follow-up DXA scanner (Hologic QDR 4500) at our medical center (short-term root mean square coefficient of variation 1.1% at lumbar spine and 1.5% at total hip) did not alter the results of intergroup comparisons at the lumbar spine and total hip, showing benefit to GC withdrawal, 4.3% (p = 0.0012, n = 68) and 3.2% (p = 0.002, n = 64), respectively. Another limitation was the use of different DXA manufacturers by the same subject. There were three subjects who switched from Hologic to Lunar and three different subjects who switched from Lunar to Hologic. Most subjects stayed on the same machine throughout. The use of different DXA manufacturers in this study introduces a bias, but it is likely non-systematic, leading to a wider confidence intervals and a conservative estimate of the effect of GC withdrawal. Serum 25-hydroxy vitamin D concentration was not measured in this study nor was vitamin D supplemented; however, randomized group allocation likely distributed this factor equally between groups. Although the study cohort was a subset of the entire enrolled population, baseline data (Table 1) showed equality in the measured baseline data, suggesting that unmeasured relevant covariates remain equal between groups. The retrospective nature of this analysis may also introduce bias. However, we diligently searched for possible confounding factors such as prednisone therapy after transplantation, differences in renal function, and weight and especially use of bone-active medications between DXA scans, but found no between- group differences.
BMD by DXA in the assessment of osteoporosis in the general population has its limitations, for example, measuring areal vs. volumetric BMD, inability to distinguish cortical vs. trabecular bone, and inability to distinguish calcium content from bone vs. extra-skeletal origin. The assessment of post-renal transplantation bone disease is considerably more complex than data derived from DXA alone. For example, DXA, in contrast to quantitative bone histomorphometry, provides no information on bone turnover and mineralization (52). Hence, an important limitation of this study is the lack of bone biopsy data. Although BMD by DXA is a strong predictor for fracture risk in the general population, its predictive ability in the chronic kidney disease (CKD) population is uncertain. For this reason, the recently published clinical practice guidelines on Chronic Kidney Disease-Metabolic Bone Disease by Kidney Disease: Improving Global Outcomes (KDIGO) do not suggest routine BMD testing in CKD 3–5D. These guidelines do however suggest measuring BMD following renal transplantation in patients receiving GC therapy or who have risk factors for osteoporosis as in the general population (53). Data from this study are in keeping with these recommendations as monitoring BMD by DXA would document improvement in patients whose GC therapy is withdrawn.
The magnitude of increase in BMD between treatment groups (+ 4.7% at lumbar spine, +2.4% at the total hip, and +2.1% at the femoral neck) is comparable to the 12-month increases in BMD seen in pivotal trials of commonly prescribed oral bisphosphonates for treatment of post-menopausal osteoporosis. For alendronate 5 and 10 mg daily vs. placebo, the one yr BMD increase at the lumbar spine was +4–5%, total hip and femoral neck +2–2.5% (54–56). Similarly, risedronate 5 mg daily vs. placebo increased BMD at the lumbar spine by 3–4% and femoral neck 2% at one yr of treatment (57, 58). Although these bisphosphonate-induced gains in BMD are modest, they are associated with relatively greater reductions in fracture risk of about 50%. Nevertheless, extrapolating these data to kidney transplant recipients is difficult as DXA has not been validated as a surrogate marker for fracture risk nor is an improvement in BMD shown to decrease fracture risk in this population.
Important clinical concerns regarding GC withdrawal include risk for acute rejection and chronic allograph nephropathy. Acute rejection has been demonstrated in mycophenolate-treated patients undergoing early GC withdrawal (59). However, this study differs in that subjects were 6–24 months post-transplantation and were stable at the time of GC withdrawal. As the majority of acute rejections occur in the first six months following transplantation, this study selected those who would be at low risk for acute rejection. Meta-analyses noting an increased risk of acute rejection following late GC have included various immunosuppressants (60, 61). A subsequent study in cyclosporine-based immunosuppression reported no increased risk of acute rejection and improvement in seven-yr patient and renal graft survival in GC withdrawal group (62). Long-term kidney dysfunction and allograft fibrosis have been reported in GC withdrawal (63, 64) but not shown in the randomized of Pelletier et al. (43). Although the presence of osteopenia by DXA scanning is unlikely to be the main determinant influencing the decision to withdraw GC therapy, it may constitute one factor favoring this choice, as individuals with a lower baseline BMD experienced a greater percent gain in BMD.
In summary, we found that discontinuation vs. continuation of GC therapy in renal transplant patients one yr after transplantation was followed by an increase in BMD at the lumbar spine and total hip, but not at the femoral neck in the following year. This pattern reflects the proportion of trabecular bone in each skeletal site. Serum alkaline phosphatase levels may serve as a biomarker that predicts change in BMD after GC withdrawal.
Acknowledgements
This research was partially funded by grants, R01 AR043052, K24 AR-04884, and the Endowed Chair for Aging at U.C. Davis (To N.E. Lane). We are grateful to Dr. Todd Pesavento for his helpful review of this manuscript.
Footnotes
Conflict of interest: There were no conflicts of interest with any author in this work.
References
- 1.Canalis E Clinical review 83: mechanisms of glucocorticoid action in bone: implications to glucocorticoid-induced osteoporosis. J Clin Endocrinol Metab 1996: 81: 3441. [DOI] [PubMed] [Google Scholar]
- 2.Kream BE, Lukert BP. Clinical and basic aspects of glucocorticoid action on bone In: Bilezikian JP, Raisz LG, Rodan GA eds. Principles of Bone Biology, Vol. 1 San Diego: Academic Press, 2002: 723. [Google Scholar]
- 3.Sambrook P, Birmingham J, Kempler S et al. Corticosteroid effects on proximal femur bone loss. J Bone Miner Res 1990: 5: 1211. [DOI] [PubMed] [Google Scholar]
- 4.Chappard D, Legrand E, Basle MF et al. Altered trabecular architecture induced by corticosteroids: a bone histomorphometric study. J Bone Miner Res 1996: 11: 676. [DOI] [PubMed] [Google Scholar]
- 5.Van Staa TP, Leufkens HG, Abenhaim L, Zhang B, Cooper C. Use of oral corticosteroids and risk of fractures. J Bone Miner Res 2000: 15: 993. [DOI] [PubMed] [Google Scholar]
- 6.Naganathan V, Jones G, Nash P, Nicholson G, Eisman J, Sambrook PN. Vertebral fracture risk with long-term corticosteroid therapy: prevalence and relation to age, bone density, and corticosteroid use. Arch Intern Med 2000: 160: 2917. [DOI] [PubMed] [Google Scholar]
- 7.Luengo M, Picado C, Del Rio L, Guanabens N, Montserrat JM, Setoain J. Vertebral fractures in steroid dependent asthma and involutional osteoporosis: a comparative study. Thorax 1991: 46: 803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peel NF, Moore DJ, Barrington NA, Bax DE, Eastell R. Risk of vertebral fracture and relationship to bone mineral density in steroid treated rheumatoid arthritis. Ann Rheum Dis 1995: 54: 801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Staa TP, Laan RF, Barton IP, Cohen S, Reid DM, Cooper C. Bone density threshold and other predictors of vertebral fracture in patients receiving oral glucocorticoid therapy. Arthritis Rheum 2003: 48: 3224. [DOI] [PubMed] [Google Scholar]
- 10.Kumagai S, Kawano S, Atsumi T et al. Vertebral fracture and bone mineral density in women receiving high dose glucocorticoids for treatment of autoimmune diseases. J Rheumatol 2005: 32: 863. [PubMed] [Google Scholar]
- 11.Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest 1998: 102: 274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Canalis E, Bilezikian JP, Angelí A, Giustina A. Perspectives on glucocorticoid-induced osteoporosis. Bone 2004: 34: 593. [DOI] [PubMed] [Google Scholar]
- 13.Sambrook PN, Hughes DR, Nelson AE, Robinson BG, Mason RS. Osteocyte viability with glucocorticoid treatment: relation to histomorphometry. Ann Rheum Dis 2003: 62: 1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofbauer LC, Gori F, Riggs BL et al. Stimulation of osteoprotegerin ligand and inhibition of osteoprotegerin production by glucocorticoids in human osteoblastic lineage cells: potential paracrine mechanisms of glucocorticoid-induced osteoporosis. Endocrinology 1999: 140: 4382. [DOI] [PubMed] [Google Scholar]
- 15.Morris HA, Need AG, O’Loughlin PD, Horowitz M, Bridges A, Nordin BE. Malabsorption of calcium in corticosteroid-induced osteoporosis. Calcif Tissue Int 1990: 46: 305. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook PN, Eisman JA, Champion GD, Pocock NA. Sex hormone status and osteoporosis in postmenopausal women with rheumatoid arthritis. Arthritis Rheum 1988: 31: 973. [DOI] [PubMed] [Google Scholar]
- 17.Reid IR, Ibbertson HK, France JT, Pybus J. Plasma testosterone concentrations in asthmatic men treated with glucocorticoids. Br Med J (Clin Res Ed) 1985: 291: 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reid IR, Ibbertson HK. Evidence for decreased tubular reabsorption of calcium in glucocorticoid-treated asthmatics. Horm Res 1987: 27: 200. [DOI] [PubMed] [Google Scholar]
- 19.Cosman F, Nieves J, Herbert J, Shen V, Lindsay R. High-dose glucocorticoids in multiple sclerosis patients exert direct effects on the kidney and skeleton. J Bone Miner Res 1994: 9: 1097. [DOI] [PubMed] [Google Scholar]
- 20.van Staa TP. The pathogenesis, epidemiology and management of glucocorticoid-induced osteoporosis. Calcif Tissue Int 2006: 79: 129. [DOI] [PubMed] [Google Scholar]
- 21.Alem AM, Sherrard DJ, Gillen DL et al. Increased risk of hip fracture among patients with end-stage renal disease. Kidney Int 2000: 58: 396. [DOI] [PubMed] [Google Scholar]
- 22.Abbott KC, Oglesby RJ, Hypolite IO et al. Hospitalizations for fractures after renal transplantation in the United States. Ann Epidemiol 2001: 11: 450. [DOI] [PubMed] [Google Scholar]
- 23.Vautour LM, Melton LJ III, Clarke BL, Achenbach SJ, Oberg AL, McCarthy JT. Long-term fracture risk following renal transplantation: a population-based study. Osteoporos Int 2004: 15: 160. [DOI] [PubMed] [Google Scholar]
- 24.Ball AM, Gillen DL, Sherrard D et al. Risk of hip fracture among dialysis and renal transplant recipients. JAMA 2002: 288: 3014. [DOI] [PubMed] [Google Scholar]
- 25.Stehman-Breen CO, Sherrard DJ, Alem AM et al. Risk factors for hip fracture among patients with end-stage renal disease. Kidney Int 2000: 58: 2200. [DOI] [PubMed] [Google Scholar]
- 26.Nisbeth U, Lindh E, Ljunghall S, Backman U, Fell-strom B. Increased fracture rate in diabetes mellitus and females after renal transplantation. Transplantation 1999: 67: 1218. [DOI] [PubMed] [Google Scholar]
- 27.Torres A, Lorenzo V, Salido E. Calcium metabolism and skeletal problems after transplantation. J Am Soc Nephrol 2002: 13: 551. [DOI] [PubMed] [Google Scholar]
- 28.Heaf JG. Bone disease after renal transplantation. Transplantation 2003: 75: 315. [DOI] [PubMed] [Google Scholar]
- 29.Cohen A, Sambrook P, Shane E. Management of bone loss after organ transplantation. J Bone Miner Res 2004: 19: 1919. [DOI] [PubMed] [Google Scholar]
- 30.Cunningham J Posttransplantation bone disease. Transplantation 2005: 79: 629. [DOI] [PubMed] [Google Scholar]
- 31.Maalouf NM, Shane E. Osteoporosis after solid organ transplantation. J Clin Endocrinol Metab 2005: 90: 2456. [DOI] [PubMed] [Google Scholar]
- 32.Hamdy NA. Calcium and bone metabolism pre- and post-kidney transplantation. Endocrinol Metab Clin North Am 2007: 36: 923. [DOI] [PubMed] [Google Scholar]
- 33.Stein E, Ebeling P, Shane E. Post-transplantation osteoporosis. Endocrinol Metab Clin North Am 2007: 36: 937. [DOI] [PubMed] [Google Scholar]
- 34.Julian BA, Laskow DA, Dubovsky J, Dubovsky EV, Curtis JJ, Quarles LD. Rapid loss of vertebral mineral density after renal transplantation. N Engl J Med 1991: 325: 544. [DOI] [PubMed] [Google Scholar]
- 35.Kwan JT, Almond MK, Evans K, Cunningham J. Changes in total body bone mineral content and regional bone mineral density in renal patients following renal transplantation. Miner Electrolyte Metab 1992: 18: 166. [PubMed] [Google Scholar]
- 36.Epstein S, Inzerillo AM, Caminis J, Zaidi M. Disorders associated with acute rapid and severe bone loss. J Bone Miner Res 2003: 18: 2083. [DOI] [PubMed] [Google Scholar]
- 37.Almond MK, Kwan JT, Evans K, Cunningham J. Loss of regional bone mineral density in the first 12 months following renal transplantation. Nephron 1994: 66: 52. [DOI] [PubMed] [Google Scholar]
- 38.Grotz WH, Mundinger FA, Gugel B, Exner VM, Kirste G, Schollmeyer PJ. Bone mineral density after kidney transplantation. A cross-sectional study in 190 graft recipients up to 20 years after transplantation. Transplantation 1995: 59: 982. [PubMed] [Google Scholar]
- 39.Grotz WH, Mundinger FA, Rasenack J et al. Bone loss after kidney transplantation: a longitudinal study in 115 graft recipients. Nephrol Dial Transplant 1995: 10: 2096. [PubMed] [Google Scholar]
- 40.Pichette V, Bonnardeaux A, Prudhomme L, Gagne M, Cardinal J, Ouimet D. Long-term bone loss in kidney transplant recipients: a cross-sectional and longitudinal study. Am J Kidney Dis 1996: 28: 105. [DOI] [PubMed] [Google Scholar]
- 41.Ferguson RM, Henry ML, Elkhammas EA et al. Twenty years of renal transplantation at The Ohio State University: the results of five eras of immunosuppression. Am J Surg 2003: 186: 306. [DOI] [PubMed] [Google Scholar]
- 42.Farmer CK, Hampson G, Abbs IC et al. Late low-dose steroid withdrawal in renal transplant recipients increases bone formation and bone mineral density. Am J Transplant 2006: 6: 2929. [DOI] [PubMed] [Google Scholar]
- 43.Pelletier RP, Akin B, Ferguson RM. Prospective, randomized trial of steroid withdrawal in kidney recipients treated with mycophenolate mofetil and cyclosporine. Clin Transplant 2006: 20: 10. [DOI] [PubMed] [Google Scholar]
- 44.Vokes TJ. Blood calcium, phosphate, and magnesium In: Favus MJ ed. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. Washington, DC: American Society for Bone and Mineral Research, 2006: 123. [Google Scholar]
- 45.Stevens L PRD. Calculation of the creatinine clearance. UpToDate, 2008. [Google Scholar]
- 46.HuI SL, Gao S, Zhou XH et al. Universal standardization of bone density measurements: a method with optimal properties for calibration among several instruments. J Bone Miner Res 1997: 12: 1463. [DOI] [PubMed] [Google Scholar]
- 47.Lu Y, Fuerst T, Huí S, Genant HK. Standardization of bone mineral density at femoral neck, trochanter and Ward’s triangle. Osteoporos Int 2001: 12: 438. [DOI] [PubMed] [Google Scholar]
- 48.Farmer CK, Hampson G, Vaja S et al. Late low dose steroid withdrawal in renal transplant recipients increase bone formation and bone mineral density without altering renal function: a randomized controlled trial. J Bone Miner Res 2002: 17(S1): S158. [Google Scholar]
- 49.Gomez B Jr, Ardakani S, Ju J et al. Monoclonal antibody assay for measuring bone-specific alkaline phosphatase activity in serum. Clin Chem 1995: 41: 1560. [PubMed] [Google Scholar]
- 50.Garnero P, Delmas PD. Assessment of the serum levels of bone alkaline phosphatase with a new immunoradiometric assay in patients with metabolic bone disease. J Clin Endocrinol Metab 1993: 77: 1046. [DOI] [PubMed] [Google Scholar]
- 51.Roe SD, Porter CJ, Godber IM, Hosking DJ, Cassidy MJ. Reduced bone mineral density in male renal transplant recipients: evidence for persisting hyperparathyroidism. Osteoporos Int 2005: 16: 142. [DOI] [PubMed] [Google Scholar]
- 52.Moe S, Drueke T, Cunningham J et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2006: 69: 1945. [DOI] [PubMed] [Google Scholar]
- 53.Moe S, Drueke T, Cunningham J et al. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl 2009: 113: S1. [DOI] [PubMed] [Google Scholar]
- 54.Liberman UA, Weiss SR, Broll J et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. The Alendronate Phase III Osteoporosis Treatment Study Group. N Engl J Med 1995: 333: 1437. [DOI] [PubMed] [Google Scholar]
- 55.Black DM, Cummings SR, Karpf DB et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996: 348: 1535. [DOI] [PubMed] [Google Scholar]
- 56.Cummings SR, Black DM, Thompson DE et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA 1998: 280: 2077. [DOI] [PubMed] [Google Scholar]
- 57.Heaney RP, Zizic TM, Fogelman I et al. Risedronate reduces the risk of first vertebral fracture in osteoporotic women. Osteoporos Int 2002: 13: 501. [DOI] [PubMed] [Google Scholar]
- 58.Harris ST, Watts NB, Genant HK et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 1999: 282: 1344. [DOI] [PubMed] [Google Scholar]
- 59.Ahsan N, Hricik D, Matas A et al. Prednisone withdrawal in kidney transplant recipients on cyclosporine and mycophenolate mofetil-a prospective randomized study. Steroid Withdrawal Study Group. Transplantation 1999: 68: 1865. [DOI] [PubMed] [Google Scholar]
- 60.Pascual J, Quereda C, Zamora J, Hernandez D. Steroid withdrawal in renal transplant patients on triple therapy with a calcineurin inhibitor and mycophenolate mofetil: a meta-analysis of randomized, controlled trials. Transplantation 2004: 78: 1548. [DOI] [PubMed] [Google Scholar]
- 61.Pascual J, Quereda C, Zamora J, Hernandez D. Updated metaanalysis of steroid withdrawal in renal transplant patients on calcineurin inhibitor and myco- phenolate mofetil. Transplant Proc 2005: 37: 3746. [DOI] [PubMed] [Google Scholar]
- 62.Opelz G, Dohler B, Laux G. Long-term prospective study of steroid withdrawal in kidney and heart transplant recipients. Am J Transplant 2005: 5(4 Pt 1): 720. [DOI] [PubMed] [Google Scholar]
- 63.Sivaraman P, Nussbaumer G, Landsberg D. Lack of long-term benefits of steroid withdrawal in renal transplant recipients. Am J Kidney Dis 2001: 37: 1162. [DOI] [PubMed] [Google Scholar]
- 64.Laftavi MR, Stephan R, Stefanick B et al. Randomized prospective trial of early steroid withdrawal compared with low-dose steroids in renal transplant recipients using serial protocol biopsies to assess efficacy and safety. Surgery 2005: 137: 364. [DOI] [PubMed] [Google Scholar]