Abstract
In this multi-center study, voxel-based relaxometry (VBR), a novel technique to automatically quantify localized cartilage change, was used to investigate T1ρ and T2 relaxation times of patients with anterior cruciate ligament (ACL) tears at the time of injury and 6 months after reconstructive surgery. Sixty-four ACL-injured patients from three sites underwent bilateral 3T MR T1ρ and T2 mapping; 56 patients returned 6 months after surgery. Cross-sectional and longitudinal VBR comparisons of relaxation times were calculated. Noyes Score (NS) clinical grades of cartilage lesions were noted at both times and correlated with relaxation times. Lastly, patients were divided into two groups based on baseline NS grades in the injured knee. T1ρ times of each group were assessed with VBR and compared. Results illustrate the feasibility of VBR for efficiently analyzing data from patients at different sites. Significant relaxation time elevations at baseline were observed in the injured knee compared to the uninjured, particularly in the posterolateral tibia (pLT). Longitudinally, a decrease was observed in the pLT and patella, while an increase was noted in the trochlea. Stratifying patients by baseline lesion presence revealed T1ρ increased more 6 months after surgery in patients with lesions. Such findings propose that the presence of cartilage lesions at baseline are associated with the longitudinal progression of T1ρ and T2 after ACL injury, and may contribute to early cartilage degeneration. Furthermore, the speed and localized specificity of automatic VBR analysis may translate well for clinical application, as seen in this multicenter study.
Keywords: T1ρ, T2, voxel-based relaxometry, ACL injury, cartilage degeneration
The anterior cruciate ligament (ACL), a principal stabilizer of the knee joint that prevents anterior tibial translation and internal tibial rotation, is susceptible to damage from rotational and hyperextension injury, resulting in functional instability, abnormal loading patterns, and osteoarthritis (OA).1–3 Although the association between abnormal joint biomechanics following ACL injury and subsequent cartilage degeneration is not fully understood, it has been suggested that ACL injuries predispose patients to posttraumatic OA, despite ACL reconstructive surgery (ACLR).1–14 Following ACL injury, characteristic subchondral bone impaction sites on the lateral femoral condyle (LF) and posterior tibial plateau (pLT) can be seen on MR images; however, the location of LF impaction may be dependent on knee flexion during the injury.7,15 Despite this femoral variability, it is clear that the bone marrow edema pattern sustained from the pivot shift-induced transchondral fracture most frequently occurs over the anterior aspect of the LF and posterior margin of the inner plateau, secondary to tibial translation and abnormal rotation.7,9 Research has suggested that bone contusions associated with ACL injury could develop into degenerative lesions.7,9,10,15
Conventional radiography and MRI are currently employed to image damaged and diseased joints, and have been deemed clinically accurate in grading cartilage lesions based on an arthroscopic standard.16–20 A widely accepted articular cartilage grading method is the Noyes Score (NS), which offers a semi-quantitative scale to score the appearance and quantity of knee cartilage.21 Many studies have correlated this grading method with established metrics of cartilage health, positioning NS as an accurate and accepted method of identifying lesions.16,18,19 Furthermore, baseline cartilage damage has been associated with longitudinal symptoms of OA.22 However, this relationship between clinical and radiographic characteristics of OA is most present in patients with severely worsening lesions, and may not detect early degenerative changes.4,11,23,24 In fact, it has been suggested that 10% of knee cartilage is lost by the time radiographs detect chondral change.23
Noninvasive MR methods, such as T1ρ and T2 relaxation mapping, have provided complementary indications of early and longitudinal cartilage matrix depletion.25 T1ρ, the spin-lattice relaxation in the rotating frame, has shown to be sensitive to proteoglycan (PG) content, while T2 is associated with collagen fibril orientation of cartilage.12,13,24,26,27 Due to PG loss, water content fluctuations, and molecular changes associated with early cartilage matrix degeneration, elevations in both values have been observed in patients with OA.2,11,17,24,27–29 Traditionally, Region Of Interest (ROI)-based approaches are used to quantify changes in relaxation times. As cartilage composition changes occur before radiographic evidence is observed, voxel-based relaxometry (VBR), a highly sensitive and novel technique, could capture early localized differences in relaxation times compared to standard ROI-based methods.11
Additionally, the fully automatic nature of VBR makes the translation of this compositional imaging technique to clinical application more feasible; previous manual segmentations would no longer be needed, drastically reducing the time between scans and results, as well as effort from the clinician. This fully automatic method would also allow for the analysis of other large data sets from multiple center trials in an efficient and timely manner, a feat too laborious and prone to errors with traditional ROI-based manual methods. Moreover, despite promising spatial assessments provided by texture and laminar ROI-based analyses, the local spatial distribution of relaxation times in different patient populations remains a challenge.30 Thus, a detailed study employing VBR to analyze the effects of ACL tears on early degeneration could quickly identify localized regions first displaying cartilage changes and direct disease-modifying strategies following the injury.
The goal of this study is to investigate the relationship between ACL injury and subsequent cartilage degeneration, as seen by relaxation time changes, with data acquired from three sites, and correlate these findings with a previously established clinical cartilage grading method. Using VBR, this study analyzes T1ρ and T2 in patients with ACL injuries at baseline and 6 months following ACLR, correlates relaxation times with Noyes Score grades, and assesses the progression of T1ρ in two cohorts defined by the presence of baseline cartilage lesions.
We first hypothesize that VBR will accurately analyze the data collected from the different sites, indicating VBR as a step towards clinical translation. Next, that there will be clear relaxation time differences between injured and uninjured knees at both time points, particularly in the lateral injured knee. Furthermore, we postulate that VBR will be able to detect longitudinal increases in relaxation times due to early cartilage degeneration, as previously detailed in literature,12,13 revealing additional localized patterns. Regarding NS correlations, we expect an association between the NS of the cartilage compartment with lesions and elevated relaxation times in that compartment. Finally, we hypothesize that patients with baseline cartilage lesions will demonstrate a greater longitudinal degenerative progression than those without.
METHODS
Subjects
In this Institutional Review Board (IRB)-approved analytic case-control study (Level of Evidence: III), all patients provided informed consent by the Committee on Human Research of the home institution prior to scanning. Sixty-four patients (28 Female; Age = 28.3 ± 12.5 years; BMI = 24.5 ± 3.1 kg/m2) were scanned at three sites: University of California, San Francisco (San Francisco, CA), Mayo Clinic (Rochester, MN), and Hospital for Special Surgery (New York City, NY). Sixty patients sustained acute, unilateral ACL tears with no previous history of knee trauma or disease, two patients had previous contralateral ACLR, and two patients did not undergo ACLR (n = 64 patients). To date, 56 patients (24 Female; Age = 29.3 ± 12.7 years; BMI = 24.7 ± 3.1kg/m2) returned 6 months after ACLR, or 6 months following injury in the two cases with no ACLR (Table 1). All patients underwent standard postoperative rehabilitation protocol.
Table 1.
Patient Demographic Characteristics
| Characteristic | Baseline | 6 Months |
|---|---|---|
| Total | n = 64 | n = 56 |
| Malea | 36 (56%) | 32 (57%) |
| Femalea | 28 (44%) | 24 (43%) |
| Age (years)b | 28.3±12.5 | 29.3±12.7 |
| BMI (kg/m2)b | 24.5±3.1 | 29.3±3.1 |
| Time from injury to baseline scan (days)b | 18.5±7.9 | – |
| Time from injury to surgery (days)b | 49.1±31.2 | – |
| ACL graft (baseline n = 63; 6-month n = 56)a | ||
| Hamstring-semitendinosus + gracilis | 17 (27%) | 16 (29%) |
| Hamstring-semitendinosus | 4 (6%) | 4 (7%) |
| Posterior tibialis | 6 (10%) | 6 (11%) |
| Bone-patella tendon-bone (B-PT-B) | 28 (44%) | 22 (39%) |
| Achilles tendon | 6 (10%) | 6 (11%) |
| No ACLR | 2 (3%) | 2 (4%) |
| Type of graft (baseline n = 60, 6-month n = 53)a | ||
| Allograft | 13 (22%) | 13 (25%) |
| Autograft | 47 (78%) | 40 (75%) |
| Meniscal tear (baseline n = 63; 6-month n = 56)a | ||
| Yes meniscal tear | 40 (63%) | 35 (63%) |
| Repair | 18 (45%) | 16 (46%) |
| Excision | 12 (30%) | 8 (23%) |
| Repair + Excision | 4 (10%) | 5 (14%) |
| Other/None | 6 (15%) | 6 (17%) |
| No meniscal tear | 23 (37%) | 21 (37%) |
| MCL Lesion (baseline n = 61; 6-month n = 54)a | ||
| Presence of lesion (WORMS ≥2) | 17 (28%) | 13 (24%) |
| No presence of lesion (WORMS <2) | 44 (72%) | 41 (76%) |
Data expressed as count (%).
Data expressed as mean ± standard deviation.
MRI Protocol
All bilateral knee scans were acquired on a 3T MR (General Electric Healthcare, Milwaukee, WI) using an eight-channel phased array knee coil (Invivo, Inc., Gainesville, FL) following ACL injury (baseline) and 6 months post-ACLR (Table 1). MRI sequence protocol included: (i) sagittal intermediate-weighted, fluid sensitive, fat-saturated three-dimensional (3D) fast spin-echo (CUBE) images (TR/TE = 1,500/25 ms, FOV = 16 cm, 384 × 384 matrix, slice thickness = 1 mm, echo train length = 50, BW = 50 kHz, NEX = 0.5); and (ii) sagittal combined 3D Tlρ/T2 (Tlρ TSL = 0/10/40/80 ms, FSL = 500 Hz, FOV = 14 cm, 256 × 128 matrix, slice thickness = 4 mm, T2 preparation TE = 0/12.87/25.69/51.39 ms).31
Image Quality Control and Site Cross-Calibration
All images underwent an automatic quality control procedure to check the stability of the MRI protocol settings. Identical agarose phantoms were scanned monthly at each site to ensure longitudinal cross-calibration. Initial calibration was established by analyzing two traveling volunteers at all three sites, scanned at the beginning of patient enrollment and 12 months later.32 Phantom longitudinal RMS-CVs ranged from 1.3% to 2.6% for T1ρ and 1.2% to 2.7% for T2. No significant differences were observed in T1ρ and T2 of the traveling volunteers between the sites (RMS-CV T1ρ and T2: 4.9% and 4.4%, respectively).32
Image Processing
All image post-processing was conducted at a single site with in-house programs written in MatLab (MathWorks, Natick, MA), integrated with the elastix toolbox for non-rigid image registration.11,33,34 Analysis of the Jacobian determinant (J) determined the minimum deformation template reference, ensuring absence of local volume vanishing in the cartilage region (J < 0), and minimizing local volume contraction (J > 1) and expansion (J > 0). Using the VTK CISG registration toolkit, reference sagittal high-resolution CUBE images were rigidly registered with the first TSL = 0, T1ρ-weighted image, and used for segmentation. Cartilage compartments were defined as the medial femoral condyle (MF), medial tibia (MT), lateral femoral condyle (LF), lateral tibia (LT), femoral trochlea (TrF), and patella (P), and were semiautomatically segmented using a Bezier spline and edge detection-based method.35 The non-rigid registration technique, developed using elastix, was applied between the reference and each first TSL = 0, T1ρ-weighted image in the dataset. Five-level recursive pyramidal multi-resolutions with a random sampler approach estimated the non-rigid transformation between the fixed and moving image. The transformation field was applied to all later TSL images. ROIs established by the previously described cartilage compartments were used to constrain a second iteration of the non-rigid registration procedure between the outputs of the first non-rigid registration phase. Employing a Levenberg-Marquardt mono-exponential (S[TSL]∝exp[–TSL/T1ρ] and S-[TE]∝exp[–TE/T2]) applied to each voxel, T1ρ maps were acquired by fitting the morphed T1ρ-weighted images from different TSLs.36 Finally, the reference-ROIs were applied to the morphed maps, establishing a fully automatic atlas-based segmentation procedure.
Automatic Segmentation Reproducibility
Cartilage compartments of an ACL-tear cohort demonstrated an average CV of 3.81% when comparing atlas-based automatic results and semiautomatic, classical ROI-based technique. No significant differences were observed in algorithm performances between baseline and 1-year ACL-tear subjects (1-year average CV = 3.78%).11 Scan/rescan repeatability on six healthy volunteers resulted in an average CV of 2.38% for fully automatic segmentation, slightly overcoming the performance obtained by the semiautomatic technique when applied to the same cohort (average CV = 2.76%).11
Quality Control (QC) and Semi-Automatic Adjustment of Mis-Registered Images
To avoid bias from applying an automatic segmentation technique, every registered image was put through a graded Quality Control (QC) before statistical analyses were conducted. ROIs were overlaid onto Echo 1 and 3, using the clearly defined edges of the articular cartilage surface to gauge the accuracy of segmentation. Images were visually rated on a 1–5 scale (1: ROI correctly overlaid; 2: <5% ROI incorrectly overlaid; 3: <20% ROI incorrectly overlaid; 4: ROI shifted off correct position; 5: unreadable image). For compartments with QC scores 3–5, an ad hoc procedure was applied using a manually cropped region of Echo 1 in the reference and the case images, targeting the specific compartment of interest. The coordinates of these cropped regions were used to initialize the non-rigid registration with a roto-translation, aligning cropped regions and constraining the sequential non-rigid registration. Following this reregistration procedure, images were rechecked for accuracy. After this second phase, images with scores below two were not included in statistical analyses.
Cartilage Grading and Group Determination
A single board-certified musculoskeletal radiologist (HP) with 25 years of experience performed blinded Noyes Score grading of cartilage lesions at baseline and 6 months on MR images.16,21 In the NS system, Grade I lesions indicate a closed chondromalacia lesion. Grade II lesions are characterized by clear cartilage surface damage, such as fissures and fibrillations, and grade III lesions signify bone surface exposure. Further sub-classifications specify the extent of degradation and lesion size.
A summation of the baseline medial and patellofemoral (PFJ) NS for the injured knees of patients who underwent ACLR with 6-month scans (n = 54) determined two cohorts: 37 with no lesions (Σ(NS) = 0) at baseline (16 Female; Age = 22.8 ± 9.4 years; BMI = 23.9 ± 2.5 kg/m2), and 17 with lesions or abnormalities (Σ(NS)>1) at baseline (7 Female; Age = 40.8 ± 10.4 years; BMI = 26.1 ± 3.5kg/m2). Table 2 details the distribution of NS grading by compartment. Lateral NS of the injured knee were not included, as observed lesions may be due to femoral-tibial impact during injury and pivot shift, thus designating medial and PFJ compartments as indicators of baseline cartilage quality. To further support this, using PFJ and medial NS from the uninjured knee yielded almost identical groups and cartilage defect distributions were highly correlated between the injured and uninjured sides (R = 0.70).
Table 2.
Baseline Clinical Characteristics of the Injured Knee for Patients With 6 Month Scans as Assessed by Noyes Score (NS)a
| Characteristic (n = 54) | |||
|---|---|---|---|
| LF cartilage lesion | LT cartilage lesion | ||
| NS = 0 | 7 (13%) | NS = 0 | 11 (20%) |
| NS = 1 | 0 (0%) | NS = 1 | 0 (0%) |
| NS = 2 | 39 (72%) | NS = 2 | 20 (37%) |
| NS = 3 | 6 (11%) | NS = 3 | 12 (22%) |
| NS = 4 | 0 (0%) | NS = 4 | 0 (0%) |
| NS ≥ 5 | 2 (4%) | NS ≥ 5 | 11 (20%) |
| MF cartilage lesion | MT cartilage lesion | ||
| NS = 0 | 44 (81%) | NS = 0 | 48 (89%) |
| NS = 1 | 0 (0%) | NS = 1 | 0 (0%) |
| NS = 2 | 8 (15%) | NS = 2 | 4 (7%) |
| NS = 3 | 1 (2%) | NS = 3 | 2 (4%) |
| NS = 4 | 0 (0%) | NS = 4 | 0 (0%) |
| NS ≥ 5 | 1 (2%) | NS ≥ 5 | 0 (0%) |
| Trochlea cartilage lesion | Patella cartilage lesion | ||
| NS = 0 | 48 (89%) | NS = 0 | 42 (78%) |
| NS = 1 | 1 (2%) | NS = 1 | 0 (0%) |
| NS = 2 | 5 (9%) | NS = 2 | 8 (15%) |
| NS = 3 | 0 (0%) | NS = 3 | 1 (2%) |
| NS = 4 | 0 (0%) | NS = 4 | 3 (6%) |
| NS ≥ 5 | 0 (0%) | NS ≥ 5 | 0 (0%) |
NS, Noyes score; LF, lateral femoral condyle; LT, lateral tibia; MF, medial femoral condyle; MT, medial tibia.
Data expressed as count (%).
Statistical Analyses
Statistical Parametric Mapping (SPM) was performed to study the local cross-sectional differences between injured and uninjured knees. Voxel-based summary statistics, such as mean and standard deviation, were computed for the different groups. Group comparisons were performed through paired student t-tests, obtaining p-value SPMs. Average percent differences were analyzed in the areas of the SPMs that showed significance (p < 0.05). The same procedure was adopted to analyze T1ρ and T2 longitudinal changes. Percentages of voxels showing significance (PSV), average p-values in the overall compartment (p-value) and average percentage differences (APD) for each compartment were summarized by SPMs. T1ρ and T2 Pearson Partial correlations with NS were computed, obtaining R-value SPMs, color-coded to distinguish weak, moderate and strong positive and negative correlations (R: −0.8 to +0.8). Significant averaged R and p-value clusters were summarized by SPMs. Longitudinal and cross-sectional analyses of T1ρ were performed on the two groups defined by baseline NS in the injured knee. Age, gender, BMI, meniscal tears, MCL lesions, surgery type, and graft source were considered as adjusting factors in statistical analyses (Table 1).
RESULTS
QC Grading
After QC grading, 100% of baseline MF, 99% MT, 100% LF, 99% LT, 100% TrF, and 98% P cartilage compartments were used for statistical analyses. For the 6-month scans, 99% of MF, 99% MT, 94% LF, 98% LT, 98% TrF, and 91% P compartments were used for statistical analyses.
T1ρ and T2 VBR Comparisons
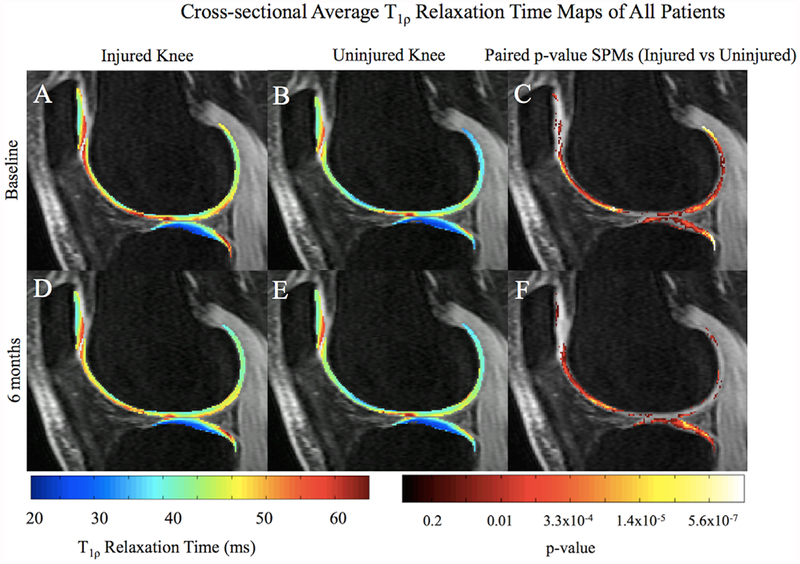
Considering two patients had previous contralateral ACLR and two patients did not undergo ACLR, we compared data with and without these four patients. No significant changes were observed, so all patients were included in analyses. Significant T1ρ and T2 elevations were observed in the injured knee compared to the uninjured at baseline, particularly in the pLT (Fig. 1A–C; T1ρ PSV = 42.4%; p-value = 0.01). After 6 months, T1ρ in the injured LT remained elevated compared to the uninjured (Fig. 1D–F; T1ρ PSV = 53.0%, p-value = 0.01). SPMs reveal a more diffuse and anteriorly shifted region of elevated T1ρ in the injured knee after 6 months, no longer concentrated in the most posterior region of the pLT, as at baseline (Fig. 1D–F). Elevated baseline relaxation times were also observed in the LF (Fig. 1A–C; T1ρ PSV = 40.2%; p-value = 0.01) and patella (T1ρ PSV = 31.6%; p-value = 0.009). A similar trend of elevation in the injured knee compared to uninjured was observed in the MT (baseline: T1ρ PSV = 61.6%; p-value = 0.009; 6 months: T1ρ PSV = 45.0%; p-value = 0.01), MF (baseline: T1ρ PSV = 47.5%; p-value = 0.01; 6 months: T1ρ PSV = 45.9%; p-value = 0.01), and TrF (baseline: T1ρ PSV = 34.5%; p-value = 0.008; 6 months: T1ρ PSV = 55.3%; p-value = 0.009). T2 followed similar trends observed in T1ρ findings.
Figure 1.
Average cross-sectional T1ρ SPMs of all patients overlaid onto registered image. Paired p-value SPMs (C and F) show significant differences between injured and uninjured knees at both time points, with a threshold set at p < 0.05. The most posterior aspect of the pLT exhibits a highly focused region with elevated T1ρ values (A–C), becoming more diffuse and anterior after 6 months (D–F).
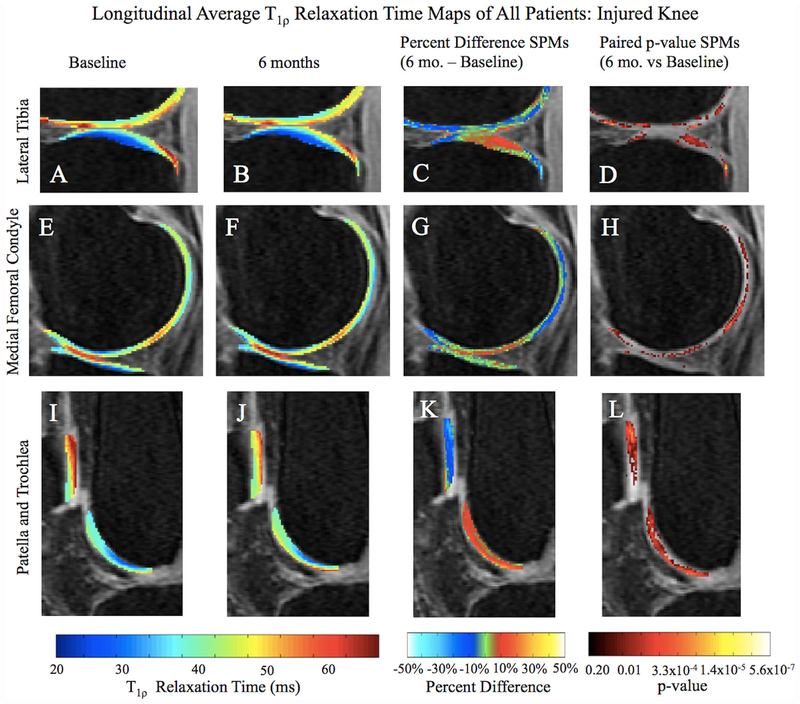
A longitudinal decrease of T1ρ in the injured knee over 6 months was observed in the most posterior aspect of the pLT (PSV = 6.6%; APD = −22.7%; p-value 0.02), while an increase was seen in the more central part of the compartment (PSV = 15.9%; APD = 14.3%; p-value = 0.02; Fig. 2A–D). The medial compartments, particularly the posterior aspect of MF (PSV = 9.3%; APD = −13.8%; p-value = 0.02) also indicated a longitudinal decrease as well as a slight increase in the weight-bearing region (Fig. 2E–H; PSV = 7.5%; APD = 9.9%; p-value = 0.03). A significant decrease in relaxation times was noted in the upper region of the patella (Fig. 2I–L; PSV = 18.5%; APD = −15.6%; p-value = 0.02). The trochlea did not follow the same general decreasing relaxation time pattern and, in fact, demonstrated higher T1ρ values after 6 months (Fig. 2I–L; PSV = 37.3%; APD = 14.3%; p-value = 0.01). T2 followed similar trends observed in T1ρ findings. Due to the particular results seen in the trochlea, the corresponding NS of this compartment was correlated with T1ρ and T2 relaxation times.
Figure 2.
Average longitudinal T1ρ SPM of all patients overlaid onto registered image. Paired p-value with a threshold set at p < 0.05 and Percent Difference SPMs (C, D, G, H, K, and L) show regions of significant differences between baseline and 6-month T1ρ relaxation times, particularly in the pLT, posterior MF, patella, and superficial trochlea. In the Percent Difference SPMs, red regions indicate higher T1ρ times at 6 months, while blue regions indicate lower T1ρ times at 6 months.
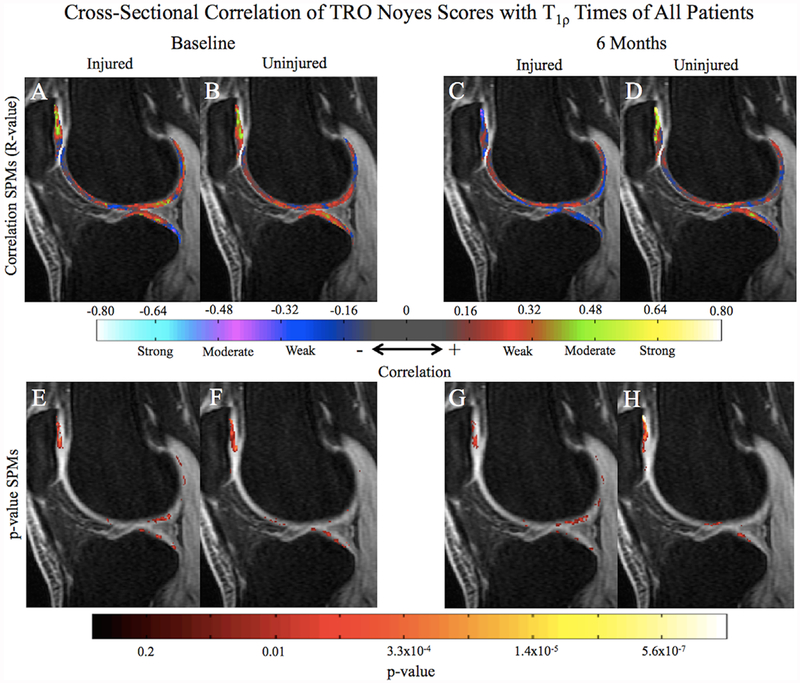
Relaxation Time Correlations With Trochlea Noyes Score
In the injured knee at baseline, trochlea NS showed the greatest correlation with T1ρ of the LT (Fig. 3A and E; PSV correlated = 20.2%; Average R-value = 0.33; p-value = 0.02), patella (PSV correlated = 20.0%; Average R-value = 0.34; p-value = 0.02), and LF (PSV correlated = 7.2%; Average R-value = 0.32; p-value = 0.02). Few voxels in the trochlea correlated with the trochlea NS (Fig. 3A; PSV correlated = 3.6%; Average R-value = 0.32; p-value = 0.02). The uninjured knee at baseline similarly displayed a high correlation between the uninjured trochlea NS and T1ρ of the LT (Fig. 3B and F; PSV correlated = 7.7%; Average R-value = 0.31; p-value = 0.02), and patella (PSV correlated = 31.1%; Average R-value = 0.35; p-value = 0.01), as well as showing little correlation with trochlea T1ρ (PSV correlated = 4.3%; Average R-value = 0.29; p-value = 0.03). At 6 months, trochlea NS of the injured knee did not significantly correlate with more than 3% of voxels (PSV) in any region (Fig. 3C and G). The uninjured trochlea NS remained correlated with T1ρ in the patella (Fig. 3D and H; PSV correlated = 19.2%; Average R-value = 0.42; p-value = 0.01). T2 relaxation time correlations with the corresponding injured and uninjured trochlea NS for both knees were similar to the correlations observed in the T1ρ results.
Figure 3.
Average cross-sectional SPMs of all patients correlating T1ρ values of both knees with the corresponding trochlea Noyes Scores (NS). At baseline, both sides (A and B) are correlated with T1ρ values in the patella, LT, and LF. At 6 months, the trochlea NS of the injured knee (C) are no longer correlated with any T1ρ values, while the uninjured trochlea NS remains correlated with the patella (D). p-value SPMs (E-F) identify regions of significance within the correlation SPMs (A–D) with a threshold set at p < 0.05.
Cross-Sectional Differences Between Cohorts
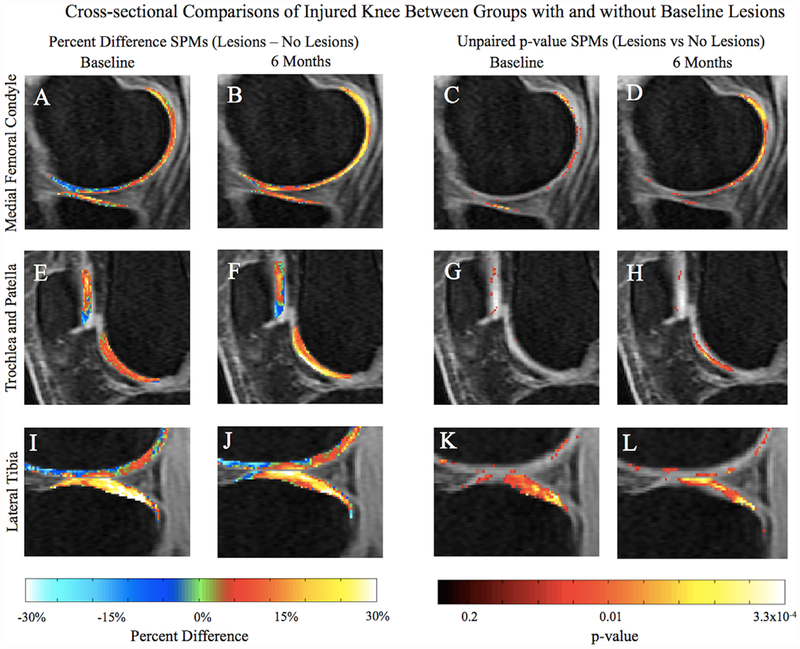
NS defined cohorts based on the lesion presence in the medial side and PFJ of the injured knee at baseline. There were no significant differences between the cohorts regarding MCL lesion presence (p-value = 0.32), meniscal tears (p-value = 0.52), or gender (p-value = 0.89). The cohort with lesions had a significantly higher BMI (p-value = 0.01), was older (p-value = 6.9 × 10−8), and sustained more allografts than autographs (p-value = 2.3 × 10−5). Table 3 displays cross-sectional comparisons of T1ρ between patients with (n = 17) and without (n = 37) cartilage lesions at baseline for both time points, with PSV, the APD within this volume, and associated p-values. At baseline, significant T1ρ cross-sectional differences between the cohorts in the injured knee were most observed in the MF, MT, and LT (Fig. 4A, C, E, G, I, and K), and in the MF, MT, LT, and TrF at 6 months (Fig. 4B, D, F, H, J, and L; Table 3).
Table 3.
VBR Cross-Sectional Comparison of T1ρ Between Patients With and Without Baseline Cartilage Lesions or Abnormalities
| Baseline |
6 Months |
|||||
|---|---|---|---|---|---|---|
| % volume of significant correlated area (PSV) | % difference of significant correlated area (APD) | Average p-value of significant correlated area | % volume of significant correlated area (PSV) | % difference of significant correlated area (APD) | Average p-value of significant correlated area | |
| MF | 15.4 | 18.2 | 0.02 | 33.8 | 19.5 | 0.02 |
| MT | 13.5 | 21.5 | 0.02 | 11.7 | 22.4 | 0.03 |
| LF | 8.5 | 20.4 | 0.02 | 8.5 | 20.3 | 0.03 |
| LT | 27.4 | 27.8 | 0.02 | 22.4 | 25.2 | 0.02 |
| TrF | 0.8 | 16.8 | 0.03 | 10.3 | 24.2 | 0.02 |
| P | 2.1 | 24.9 | 0.03 | 0.3 | 17.6 | 0.03 |
MF, medial femoral condyle; MT, medial tibia; LF, lateral femoral condyle; LT, lateral tibia; TrF, trochlea; P, patella. A positive APD indicates that the T1ρ relaxation times are higher in the group with baseline lesions than thegroup without lesions.
Figure 4.
Average cross-sectional percent difference (A, B, E, F, I, and J) and unpaired p-value (C, D, G, H, K, and L) SpMs with a threshold set at p < 0.05 of all patients in each cohort in the injured knee. There is greater T1ρ deviation in the MF at 6 months (B and D) between the two groups than at baseline (A and C). Significant differences between groups can also be seen in the superficial trochlea, while the patella at both time points remain similar (F and H). Significantly higher T1ρ relaxation times in the group with lesions can be seen in the LT at both time points (I–L).
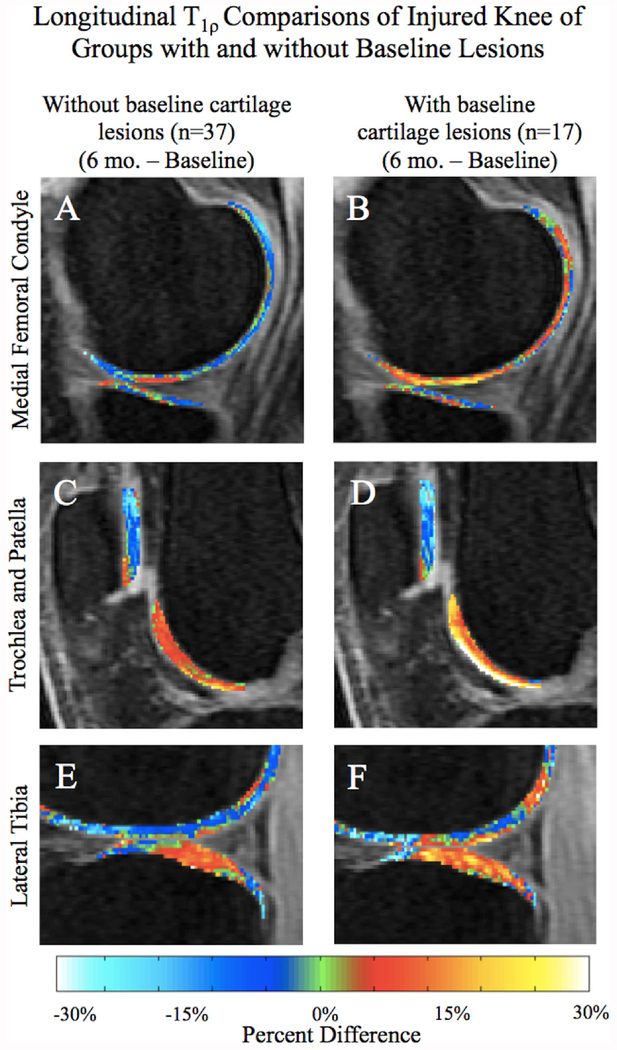
Longitudinal Differences Within Cohorts
Within the two cohorts, longitudinally assessing the regions previously identified from the cross-sectional analysis (MF, MT, LT, and TrF) revealed a differing longitudinal T1ρ progression between the cohorts. Patients with baseline cartilage lesions showed greater average T1ρ longitudinal increases in MF and the superficial TrF of the injured knee compared to those without abnormalities (Fig. 5A–D). Significant longitudinal changes were further observed in the patella and LT of both groups, though the trend appeared similar between the groups (Fig. 5C–F).
Figure 5.
Average longitudinal percent difference SPMs (A–F) of all patients in the injured knee. The group with lesions at baseline shows a greater longitudinal increase in T1ρ than the group without initial lesions, especially in the MF (A–B) and superficial trochlea (C–D). There is almost no longitudinal difference observed in the patella between the cohorts (C–D), and the LT remains fairly similar (E–F).
DISCUSSION
This is the first study, to the best of our knowledge, to extensively apply VBR on ACL-reconstructed patients, and analyze cross-sectional and longitudinal differences in both knees from patients at three different sites. Furthermore, we studied the local correlation between cartilage morphological defects identified by Noyes Score and relaxation times to assess the contribution of medial and PFJ cartilage lesion presence on chondral degeneration. Our results illustrate the feasibility of VBR for efficiently analyzing images acquired from different sites and effectively assessing localized cartilage changes on an atlas-model.
In the initial T1ρ and T2 analyses, VBR was employed to isolate localized relaxation differences between injured and uninjured knees at baseline and 6 months after ACLR. Many previous ACL studies have similarly used the contralateral knee of the patient as a control.2,5,6 As hypothesized, significant relaxation time differences were noted on the lateral side of baseline injured knees compared to the uninjured knees, most likely due to the contusion during the injury.7,9,10 An “MRI triad” has been described in literature, encapsulating the ACL tear, subsequent bone bruise to the terminal sulcus of the LF, and regions of abnormal signal in the pLT.10 Elevated pLT relaxation times remained 6 months following ACLR, appearing to diffuse and shift anteriorly (Fig. 2A and B). While a longitudinal decrease in T1ρ and T2 was observed in the most posterior aspect of the injured pLT and MF, an increase was also seen in the more central weight-bearing regions (Fig. 2A–H). Additionally, the injured patella demonstrated a significant decrease between time points, while the trochlea had greater relaxation times 6 months after ACLR than baseline (Fig. 2I–L). Further studies would need to determine if these regions, particularly the pLT and patella, are in fact healing over this window of time.
With the ability to evaluate each individual voxel, we were able to localize changes from this sizeable dataset that may have otherwise been overlooked by traditional ROI-based or subcompartmental analyses. For example, subcompartmental analysis of the LT divides the compartment into three subcompartments based on the position of the meniscus.12 In this study, such a division would encompass the decreasing region of the most posterior aspect of the pLT as well as the increasing region of the more central pLT (Fig. 2C), subsequently washing out these highly localized changes.
Next, we sought to correlate a previously established lesion grading method with T1ρ and T2 to ultimately assess the link between lesions in one compartment with relaxation times in the entire knee. An association between the trochlea compartment NS and elevated T1ρ and T2 in the trochlea was expected, evident of the present lesions. However, other compartments were significantly correlated with the trochlea NS, indicating intricate connections between compartments. In particular, the patella of both knees at baseline was correlated with the corresponding trochlea NS (Fig. 3A–B). Yet at 6 months, only the uninjured patella remained correlated, suggesting a change in the injured patella (Fig. 3C–D). This detected change may be due to the observed longitudinal decrease of relaxation times in the patella (Fig. 2I–L). Further research analyzing the effect of a cartilage lesion on nonadjacent cartilage degeneration could reveal more about these intricately connected knee regions.
In the latter part of this study, two groups classified by baseline medial and PFJ cartilage lesions were analyzed. Ralles et al. demonstrated that prolonged time between injury and ACLR on the scale of months increased incidence of chondral injury,37 while the patients in this study were scanned 18.5 ± 7.9 days following injury. Thus, it is reasonable to assume the lesions in the medial and PFJ compartments were unrelated to the injury, and ultimately highlighting cartilage abnormalities already present at the time of injury. In other words, we employed NS to demonstrate that presence of cartilage lesions in compartments not directly involved in the injury may contribute to later cartilage degeneration, as seen by elevated T1ρ and T2. A variety of factors were adjusted for in the statistical comparison to minimize group differences, such as graft source (Table 1), which is known to affect degree of cartilage degeneration.1 Nevertheless, there were significant demographic differences between the cohorts, such as age and BMI. Kumar et al. similarly separated patients with ACL tears based on a baseline characteristic.2 This study identified two groups and found a trend associated with elevated relaxation times.
Assessing cross-sectional differences between the cohorts first identified the MF, TrF, and LT as dynamic regions with significant differences between baseline lesion cohorts. The large, positive cross-sectional differences in these compartments seen at both times (Fig. 4) indicated that the group with baseline lesions sustained more elevated T1ρ times compared to the cohort without lesions. Following with a longitudinal analysis, from the intense change seen in the group with baseline lesions, it is clear that this cohort had larger T1ρ elevations in the MF and superficial TrF than the cohort without baseline lesions (Fig. 5B and D). The LT elevations appeared somewhat similar across both time points.
Combining results from T1ρ to T2 VBR analyses with the NS-separated cohorts, it is interesting to note that the injured patella also remained fairly similar between the cohorts despite lesion presence (Table 3; Figs. 4E–F and 5C–D). From the initial VBR analysis, injured patella cartilage revealed decreasing relaxation times over 6 months. Considering the homogeneity between cohorts, the observed relaxation time decrease may also involve biomechanical changes from ACLR, such as loading pattern changes. Nevertheless, the trochlea of the injured knee, which demonstrated a significant relaxation time increase over 6 months, also displayed a large difference between cohorts, pointing towards the significance of cartilage quality at the time of injury. Results ultimately suggest that cartilage of patients with baseline lesions had more elevated relaxation times in the injured knee, and consequently deteriorated at a faster rate compared to those without baseline lesions, particularly in the MF and superficial TrF. Such findings emphasize the identification of existing chondral abnormalities at the time of injury, and their contribution to posttraumatic cartilage degeneration.
Identifying patients at risk for accelerated cartilage degeneration is a crucial step to treat OA. Luyten et al. propose three criteria to distinguish patients with early OA38; however, they do not discuss pending degeneration following ACL injury. More research on early degenerative factors, such as the suggested presence of cartilage lesions unrelated to the injury, would help identify patients who are at risk for more severe chondral degeneration following ligament injuries. The data and techniques presented here also document the utility of VBR analysis with ACL-injured patients, and could assist with medical intervention at the time of injury and postponement of OA progression.
Ultimately, many of the findings from this study confirmed our initial hypotheses. Relaxation times were clearly different in the injured and uninjured knees at both time points, particularly in the lateral side, as anticipated (Fig. 1). There was a longitudinal increase in the trochlea T1ρ and T2, possibly illustrating the early stages of degeneration (Fig. 2I–L). Dividing the population into two cohorts revealed that the cartilage of those with baseline lesions had more elevated relaxation times, suggesting more rapid cartilage degeneration. Contrary to the hypothesis of longitudinally increasing relaxation times, the local nature of VBR allowed us to observe the pLT and patella both demonstrated decreasing relaxation times 6 months following ACLR. However, future research will have to analyze whether these regions are truly recovering from cartilage degeneration.
Despite encouraging results obtained from this study, there are limitations. Many studies show cartilage change over the period of years, while this study is limited to several months14; a larger population and extended analysis over several years would bolster our findings. Confounding factors, such as meniscal tears, despite being adjusted for in statistical analyses, were not incorporated into the primary focus of the study. In future, larger studies, incorporating the severity of meniscal lesions, rather than a binary yes/no adjustment as in this study, as well as the choice of treatment (repair, excision, etc.) could greatly benefit the findings, as meniscal lesions and their method of treatment are correlated with longitudinal clinical outcomes.39 Additionally, using the VBR method, while providing highly sensitive measurements of cartilage relaxation times, may also come with some limitations. For example, diffuse changes occurring in different morphological locations in different subjects may not be detected, due to the localized nature of VBR; this is one aspect where a ROI-based methodology might be used in tandem with VBR. Lastly, although T1ρ and T2 have been shown to accurately reflect PG and collagen changes, there are MR parameter-dependencies and different field strength effects on relaxation times that were not considered.40
ACKNOWLEDGMENTS
This study was possible thanks to the combined efforts of the AF-ACL Consortium. HSS and UCSF received funding from AF and General Electric Healthcare. Mayo received funding from AF.
Conflicts of interest: The consortium is listed in the Acknowledgments, as well as the research grant funding statement.
Grant sponsor: AF-ACLConsortium; Grant sponsor: Arthritis Foundation (AF); Grant sponsor: General Electric Healthcare.
REFERENCES
- 1.Shirazi AN, Chrzanowski W, Khademhosseini A, et al. 2015. Anterior cruciate ligament: structure, injuries and regenerative treatments. Adv Exp Med Bio 881:161–186. [DOI] [PubMed] [Google Scholar]
- 2.Kumar D, Kothari A, Souza RB, et al. 2014. Frontal plane knee mechanics and medial cartilage MR relaxation times in individuals with ACL reconstruction: a pilot study. Knee 21:881–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu-Ambrose T 2003. The anterior cruciate ligament and functional stability of the knee joint. BCMJ 45:495–499. [Google Scholar]
- 4.Culvenor AG, Collins NJ, Guermazi A, et al. 2015. Early patellofemoral osteoarthritis features 1 year after anterior cruciate ligament reconstruction predict symptoms and quality of life at 3 years. Arthritis Care Res [Epub ahead of print]. DOI: 10.1002/acr.22761 [DOI] [PubMed] [Google Scholar]
- 5.Theologis AA, Haughom B, Liang F, et al. 2014. Comparison of T1rho relaxation times between ACL-reconstructed knees and contralateral uninjured knees. Knee Surg Sports Traumatol Arthrosc 22:298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Fontenay BP, Argaud S, Blache Y, et al. 2014. Motion alterations after anterior cruciate ligament reconstruction: comparison of the injured and uninjured lower limbs during a single-legged jump. J Athl Train 49:311–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy BJ, Smith RL, Uribe JW, et al. 1992. Bone signal abnormalities in the posterolateral tibia and lateral femoral condyle in complete tears of the anterior cruciate ligament: a specific sign? Radiology 182:221–224. [DOI] [PubMed] [Google Scholar]
- 8.Wellsandt E, Gardinier ES, Manal K, et al. 2015. Decreased knee joint loading associated with early knee osteoarthritis after anterior cruciate ligament injury. Am J Sports Med 44:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bisson LJ, Kluczynski MA, Hagstrom LS, et al. 2013. A prospective study of the association between bone contusion and intra-articular injuries associated with acute anterior cruciate ligament tear. Am J Sports Med 41: 1801–1807. [DOI] [PubMed] [Google Scholar]
- 10.Maffulli N, Binfield PM, King JB. 2003. Articular cartilage lesions in the symptomatic anterior cruciate ligament-deficient knee. Arthroscopy 19:685–690. [DOI] [PubMed] [Google Scholar]
- 11.Pedoia V, Li X, Su F, et al. 2016. Fully automatic analysis of the knee articular cartilage T1ρ relaxation time using voxel-based relaxometry. J Magn Reson Imaging 43:970–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li X, Kuo D, Theologis A, et al. 2011. Cartilage in anterior cruciate ligament-reconstructed knees: MR imaging T1{rho} and T2-initial experience with 1-year follow-up. Radiology 258:505–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Su F, Hilton JF, Nardo L, et al. 2013. Cartilage morphology and T1ρ and T2 quantification in ACL-reconstructed knees: a 2-year follow-up. Osteoarthritis Cartilage 21:1058–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potter HG, Jain SK, Ma Y, et al. 2012. Cartilage injury after accute, isolated anterior cruciate ligament tear: immediate and longitudinal effect with clinical/MRI follow-up. Am J Sports Med 40:276–285. [DOI] [PubMed] [Google Scholar]
- 15.Zamber RW, Teitz CC, McGuire DA, et al. 1989. Articular cartilage lesions of the knee. Arthroscopy 5:258–268. [DOI] [PubMed] [Google Scholar]
- 16.McGibbon CA, Trahan CA. 2003. Measurement accuracy of focal cartilage defects from MRI and correlation of MRI graded lesions with histology: a preliminary study. Osteoarthritis Cartilage 11:483–493. [DOI] [PubMed] [Google Scholar]
- 17.Pedersen DR, Martin JA, Thedens DR, et al. 2013. Imaging biopsy composition at ACL reconstruction. Orthop Res Rev 5:35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alizai H, Roemer FW, Hayashi D, et al. 2015. An update on risk factors for cartilage loss in knee osteoarthritis assessed using MRI-based semiquantitative grading methods. Eur Radiol 25:883–893. [DOI] [PubMed] [Google Scholar]
- 19.Li XM, Peng WJ, Wu H, et al. 2009. MRI findings in injured articular cartilage of the knee correlated with surgical findings. Chin Med J (Engl) 122:2624–2630. [PubMed] [Google Scholar]
- 20.Potter HG, Linklater JM, Allen AA, et al. 1998. Magnetic resonance imaging of articular cartilage in the knee: an evaluation with use of fast-spin-echo imaging. J Bone Joint Surg 80:1276–1284. [DOI] [PubMed] [Google Scholar]
- 21.Noyes FR, Stabler CL. 1989. A system for grading articular cartilage lesions at arthroscopy. Am J Sports Med 17: 505–513. [DOI] [PubMed] [Google Scholar]
- 22.Sharma L, Nevitt M, Hochberg M, et al. 2015. Clinical significance of worsening versus stable preradiographic MRI lesions in a cohort study of persons at higher risk for knee osteoarthritis. Ann Rheum Dis 0:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ding C, Jones G, Wluka AE, et al. 2010. What can we learn about osteoarthritis by studing a healthy person against a person with early onset of disease? Curr Opin Rheumatol 22:520–527. [DOI] [PubMed] [Google Scholar]
- 24.Li X, Ma CB, Link TM, et al. 2007. In vivo T1ρ and T2 mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage 15:789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prasad AP, Nardo L, Schooler J, et al. 2013. T1rho and T2 relaxation times predict progression of knee osteoarthritis. Osteoarthritis Cartilage 21:69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xia Y, Moody JB, Burton-Wurster N, et al. 2001. Quantitative in situ correlation between microscopic MRI and polarized light microscopy studies of articular cartilage. Osteoarthritis Cartilage 9:393–406. [DOI] [PubMed] [Google Scholar]
- 27.Wheaton AJ, Casey FL, Gougoutas AJ, et al. 2004. Correlation of T1rho with fixed charge density in cartilage. JMRI 20:519–525. [DOI] [PubMed] [Google Scholar]
- 28.Nieminen MT, Töoyröas J, Rieppo J, et al. 2000. Quantitative MR microscopy of enzymatically degraded articular cartilage. Magn Reson Med 43:675–681. [DOI] [PubMed] [Google Scholar]
- 29.Duvvuri U, Reddy R, Patel SD, et al. 1997. T1rho-relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med 38:863–867. [DOI] [PubMed] [Google Scholar]
- 30.Carballido-Gamio J, Stahl R, Blumenkrantz G, et al. 2009. Spatial analysis of magnetic resonance T1rho and T2 relaxation times improves classification between subjects with and without osteoarthritis. Med Phys 36: 4059–4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Wyatt C, Rivoire J, et al. 2014. Simultaneous acquisition of T1rho and T2 quantification in knee cartilage—reproducibility and diurnal variation. JMRI 39:1287–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Pedoia V, Kumar D, et al. 2015. Cartilage T1rho and T2 relaxation times: longitudinal reproducibility and variations using different coils, MR systems and sites. Osteoarthritis Cartilage 3:2214–2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shamonin DP, Bron EE, Lelieveldt BPF, et al. 2014. Fast parallel image registration on CPU and GPU for diagnostic classification of Alzheimer’s disease. Front Neuroinform 7:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klein S, Staring M, Murphy K, et al. 2010. Elastix: a toolbox for intensity based medical image registration. IEEE Trans Med Imaging 29:196–205. [DOI] [PubMed] [Google Scholar]
- 35.Carballido-Gamio J, Bauer JS, Stahl R, et al. 2008. Inter-subject comparison of MRI knee cartilage thickness. Med Image Anal 12:120–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marquardt D 1963. An algorithm for least-squares estimation of nonlinear parameters. J Soc Indust Appl Math 11:431–441. [Google Scholar]
- 37.Ralles S, Agel J, Obermeier M, et al. 2015. Incidence of secondary intra-articular injuries with time to anterior cruciate ligament reconstruction. Am J Sports Med 43:1373–1379. [DOI] [PubMed] [Google Scholar]
- 38.Luyten FP, Denti M, Filardo G, et al. 2012. Definition and classification of early osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc 20:401–406. [DOI] [PubMed] [Google Scholar]
- 39.Bonasia DE, Pellegrino P, D’Amelio A, et al. 2015. Meniscal root tear repair: why, when and how? Orthop Rev (Pavia) 7: 5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang P, Block J, Gore JC. Chemical exchange in knee cartilage assessed by R1rho (1/T1rho) dispersion at 3T. JMRI 2014;33:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]