Abstract
Background:
Tenosynovial giant cell tumor (TGCT), a rare, locally aggressive neoplasm, overexpresses colony-stimulating factor 1 (CSF1). Surgery is standard with no approved systemic therapy. This phase 3 randomized, double-blind study evaluated pexidartinib, a CSF1 receptor inhibitor, in patients with TGCT.
Methods:
In part 1 of this two-part study, 120 patients with symptomatic, advanced TGCT for whom surgery was not recommended were randomized (1:1) to pexidartinib (n=61) or placebo (n=59). Part 2 was open-label pexidartinib for all patients. Primary endpoint was centrally reviewed overall response rate (week 25) by RECIST, version 1.1. Secondary endpoints included range of motion, response rate by tumor volume score (TVS), patient-reported outcomes (PROs), and response duration. Emergence of mixed and cholestatic hepatotoxicity caused the Data Monitoring Committee to stop enrollment six patients short of target.
Findings:
The overall response rate was higher for pexidartinib versus placebo at week 25 by RECIST (39 vs 0%; p<0·0001). At 22 months median follow-up, the best overall response with pexidartinib had increased to 53% by RECIST. Hair color changes, liver enzyme increase, fatigue, and dysgeusia were the most frequent pexidartinib-associated adverse events. Three patients had transaminases ≥3×ULN with total bilirubin and alkaline phosphatase ≥2×ULN indicative of mixed and cholestatic hepatotoxicity, one lasting 7 months and confirmed by biopsy.
Interpretation:
Pexidartinib is the first systemic therapy to demonstrate a robust tumor response in TGCT with improved patient symptoms and functional outcomes; mixed and cholestatic hepatotoxicity is an identified risk.
RESEARCH IN CONTEXT
Evidence before this study
There are currently no approved systemic therapies available for patients with tenosynovial giant cell tumor (TGCT). A PubMed search was conducted using the term “tenosynovial giant cell tumor” and the commonly used alternatives “giant cell tumor of the tendon sheath” and “pigmented villonodular synovitis,” without date limits, to identify clinical studies of investigational systemic agents in this disease. All potentially relevant articles were assessed for quality and relevance. A limited number of clinical studies, case studies, and retrospective analyses were identified, and none were randomized phase 3 studies.
Added value of this study
To the best of our knowledge, ENLIVEN is the first randomized, placebo-controlled phase 3 study in patients with TGCT. The study was designed not only to evaluate the risk and benefit profile for pexidartinib as the first systemic therapy in TGCT, but also to increase knowledge of the disease and how to evaluate the effectiveness of new therapies in this rare, non-malignant tumor. In addition to evaluating tumor response rate, ENLIVEN assessed patient symptoms and physical functional outcomes of key importance to patients by using a novel TGCT-specific magnetic resonance imaging−based tumor response evaluation method and a unique TGCT-specific patient-reported outcomes score. The results of the study were overwhelmingly positive in favor of pexidartinib; mixed and cholestatic hepatotoxicity was an identified risk.
Implications of all available evidence
As the first randomized, placebo-controlled phase 3 study in TGCT, ENLIVEN is a landmark study that sets the standard for drug development in this rare disease. The results are potentially practice-changing for TGCT. The use of TGCT-specific patient-reported outcomes allows the results to readily translate into clinical practice. Pexidartinib is the first systemic therapy to demonstrate a robust tumor response in TGCT while improving patient symptoms and functional outcomes; mixed and cholestatic hepatotoxicity was an identified risk. Pexidartinib may be a relevant treatment option for TGCT, which is associated with severe morbidity or functional limitations, and which is not amenable to improvement with surgery.
INTRODUCTION
Tenosynovial giant cell tumor (TGCT), also known as giant cell tumor of the tendon sheath (GCT-TS) or pigmented villonodular synovitis (PVNS), is a rare, locally aggressive, mesenchymal neoplasm that most often arises in the synovium of joints, bursae, or tendon sheaths.1, 2 A minority of cells within TGCT are neoplastic, aberrantly expressing colony-stimulating factor 1 (CSF1) as a result of genomic alterations at the CSF1 gene locus on chromosome 1p13.3, 4 Dysregulated CSF1 attracts histiocytoid and inflammatory cells that compose the bulk of the tumor.3, 4 Annual TGCT incidence rates are estimated to be 43 cases per million, of which approximately 10% are of the diffuse subtype.5
Surgical resection, when feasible, is standard treatment for TGCT; however, recurrence of the diffuse subtype is particularly common.1, 6, 7 Repeated surgeries often result in increasing morbidity and reduced function of affected joints.1 Persistent disease can cause cartilage destruction and bone erosion, in addition to functional limitations from tumor mass and associated effusions, resulting in long-term pain and/or joint dysfunction. Joint replacement or even amputation may be necessary.1 No approved systemic therapies currently exist.6
Pexidartinib is a novel, orally administered small molecule tyrosine kinase inhibitor (TKI) with strong selective activity against CSF1 receptor (CSF1R); KIT and FLT3-ITD are also inhibited.8 In a phase 1 study of patients with recurrent or inoperable TGCT (n=23), pexidartinib treatment resulted in 52% overall response rate by Response Evaluation Criteria in Solid Tumors, version 1.1 (RECIST).8 To further evaluate pexidartinib in TGCT, a phase 3, randomized, double-blind, multinational study (ENLIVEN) was conducted to compare efficacy and safety of pexidartinib versus placebo in patients with symptomatic, advanced TGCT for whom surgery was not recommended.
METHODS
Patients
Eligible patients were aged 18 years or older, had a histologically confirmed TGCT diagnosis, and had advanced disease for which surgical resection would be associated with potentially worsening functional limitation or severe morbidity, confirmed by two surgeons or a multidisciplinary tumor board. Patients had symptomatic disease, with a score by worst pain or worst stiffness numeric rating scale (NRS) of at least 4, and measurable disease per RECIST, with a minimal tumor size of 2 cm, as assessed by a central radiologist.
Patients were excluded for prior pexidartinib or any biologic targeting CSF1 or CSF1R (previous oral TKIs were allowed), metastatic TGCT, or active cancer requiring therapy. Patients provided written informed consent. Complete eligibility criteria are in the appendix.
Study design and treatment
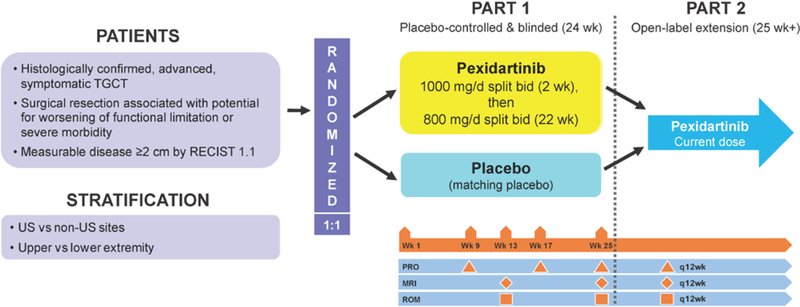
The study was conducted in two parts (figure 1; for additional details see “Methods” in the appendix). In part 1 (double-blind phase), eligible patients received pexidartinib or placebo for 24 weeks. Patients received a loading dose of 1000 mg/d orally (400 mg morning; 600 mg evening) of pexidartinib or matching placebo for the first 2 weeks, followed by 800 mg/d (400 mg twice a day) thereafter.
Figure 1: ENLIVEN study design.
bid=twice daily. RECIST 1.1=Response Evaluation Criteria in Solid Tumors, version 1.1. TGCT=tenosynovial giant cell tumor.
Patients completing part 1 were eligible to enter part 2, open-label pexidartinib, at the dose of pexidartinib or placebo they were receiving at the end of part 1. Patients with centrally confirmed disease progression before the end of part 1 were eligible for early entry into part 2 if found to have been on placebo after unblinding. Treatment continued until disease progression, unacceptable toxicity, consent withdrawal, or investigator’s decision to discontinue.
The Institutional Review Board at each participating center approved the study; ethics were in accordance with Declaration of Helsinki and Good Clinical Practice guidelines of the International Conference on Harmonisation.
Randomization and masking
Patients were centrally randomized to part 1 of the study via an integrated web response system (IWRS) in a 1:1 ratio to receive either pexidartinib or matching placebo twice daily for 24 weeks. Randomization was stratified by United States (US) versus non-US sites and by upper-extremity versus lower-extremity involvement; any location at or superior to thoracic vertebra 12 was considered upper extremity, whereas any location below or inferior to lumbar vertebra 1 was considered lower extremity. The randomization schedule was developed by an independent third-party vendor to ensure that the patients, investigators, study site personnel, safety laboratory personnel, central imaging readers, and representatives of the sponsor involved in the conduct and/or management of the study remained blinded to treatment assignment. The randomization schedule was kept strictly confidential until the time of unblinding. Clinical site staff obtained investigational medicinal product dispensing information by accessing an interactive web/voice response system. Pexidartinib and placebo capsules were identical in appearance. Part 2 of the study was open-label.
Study endpoints and assessments
The primary endpoint was overall response rate (complete response [CR] or partial response [PR]) at the end of part 1 (week 25) based on blinded, centrally read magnetic resonance imaging (MRI) and RECIST, version 1.1. MRI was performed at baseline, week 13, and week 25 during part 1, and every 12 weeks in part 2. Any disease progression before week 25 was verified by central MRI reading before unblinding and potential early entry into part 2.
Secondary endpoints included week 25 determinations of (1) mean change from baseline in range of motion (ROM) of the affected joint, relative to a reference standard for the same joint, as assessed by an independent and blinded third party; (2) centrally evaluated overall response rate based on tumor volume score (TVS), a TGCT-specific method that calculates tumor volume as a percentage of the estimated maximally distended synovial cavity8; (3) mean change from baseline in the Patient-Reported Outcomes Measurement Information System−Physical Function scale (PROMIS)9, 10; (4) mean change from baseline in worst stiffness NRS (stiffness); (5) proportion of responders based on Brief Pain Inventory (BPI) worst pain NRS and analgesic use by BPI-30 definition (Pain-30); and (6) duration of response based on RECIST and TVS. Safety was assessed using National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0.
Selection of patient-reported outcome (PRO) instruments for the study (PROMIS, worst stiffness, and worst pain) was based on a separate qualitative study identifying PRO instruments relevant to patients with TGCT.11 A detailed description of study endpoints is in the appendix.
Statistical analysis
A sample size of 126 patients was planned to provide 90% power to detect a 25% difference in response rate, assuming 10% for placebo and 35% for pexidartinib, using a two-sided, two-sample comparison at α=0·05 significance level by Fisher’s exact test. These response rates were selected to identify a clinically meaningful difference after 24 weeks of treatment. In response to two cases of mixed and cholestatic hepatotoxicity during the study, the Data Monitoring Committee (DMC) reviewed unblinded safety data of these and similar cases in the pexidartinib non-TGCT development program. Based on DMC recommendations, the protocol was amended effective September 30, 2016, to halt enrollment six patients short of target; patients who had not started treatment were discontinued. Patients were informed of new safety information and re-consented to continue the study. Patients found to have been on placebo at the end of part 1 were no longer allowed to enter part 2 to receive open-label pexidartinib. Patients treated with pexidartinib in part 1 could continue into part 2. Study results are reported here from the March 27, 2017, data cutoff, which was the timepoint when all patients had either completed the final part 1 assessment (week 25) or discontinued treatment before completing part 1. Best overall response and duration of response are based on a later data cutoff of January 31, 2018, to provide more mature data for the prespecified duration of response analysis.
Primary and secondary efficacy endpoints were analyzed using a hierarchical procedure; details are in the appendix. The primary endpoint and other binary endpoints were analyzed using one-sided α=0·025 significance level by Fisher’s exact test. The two-sided 95% confidence interval (CI) for the difference between responder proportions in the treatment groups was calculated using the Wilson method. Stiffness rating was based on an NRS ranging from 0 (“no stiffness”) to 10 (“stiffness as bad as you can imagine”). Pain-30 responders were defined as patients who experienced a ≥30% decrease in mean BPI worst pain NRS item and did not experience a ≥30% increase in narcotic analgesic use over a 7-day period at the end of part 1 compared with baseline. Duration of response was defined from date of the first recorded response to date of the first documented disease progression. For patients with no radiologic progression, duration was censored from date of the last MRI scan. The Kaplan-Meier method was used to compute the median. Continuous endpoints, including ROM and PROs, were analyzed using mixed models for repeated measurements, where change from baseline was the dependent variable. The analyses included the randomization stratification factor of geographic region, whenever appropriate.
Efficacy analyses were performed using the intention-to-treat population; safety analyses were performed using the safety population, ie, all patients who received at least one dose of study treatment. Intention-to-treat and safety analysis sets were identical. The crossover pexidartinib group included patients who received placebo in part 1 and open-label pexidartinib in part 2.
Role of funding source
Employees of the sponsor participated in the study design and conduct and were involved in collection, analysis, and interpretation of the data. An independent DMC was responsible for safeguarding patients, assessing study drug safety during the study, monitoring overall study conduct, and making recommendations about continuing, modifying, or stopping the study. Manuscript writing support was provided to the authors and funded by the sponsor. All authors contributed to writing and reviewing the manuscript, contributed to the decision for publication submission, and assume responsibility for the completeness and integrity of the data and adherence to the protocol. The corresponding author had full access to all study data and had final responsibility for the decision to submit the manuscript for publication.
RESULTS
Part 1: randomized, double-blind, placebo-controlled
Patients
From May 2015 through September 2016, 120 patients from 12 countries were randomized and received at least one dose of pexidartinib (n=61) or placebo (n=59) (see CONSORT diagram in appendix figure 1). Baseline characteristics were balanced between treatment groups (table 1). The most common disease site was the knee, present in 73 of 120 (61%) patients; 63 (53%) had at least one prior surgery for TGCT, and 11 (9%) had prior TKI treatment (imatinib or nilotinib).
Table 1:
Patient demographics and baseline disease characteristics
| Characteristic* | Pexidartinib n=61 |
Placebo n=59 |
Total N=120 |
|---|---|---|---|
| Median age (range), year | 44·0 (22–75) | 45·0 (18–79) | 44·5 (18–79) |
| Sex, n (%) | |||
| Male | 26 (43) | 23 (39) | 49 (41) |
| Female | 35 (57) | 36 (61) | 71 (59) |
| Race, n (%) | |||
| White | 52 (85) | 54 (92) | 106 (88) |
| Black | 3 (5) | 1 (2) | 4 (3) |
| Asian | 1 (2) | 2 (3) | 3 (3) |
| Native American | 2 (3) | 0 | 2 (2) |
| Hawaiian/Pacific Islander | 2 (3) | 2 (3) | 4 (3) |
| Multiracial | 1 (2) | 0 | 1 (1) |
| Geographic region, n (%) | |||
| US region | 23 (38) | 22 (37) | 45 (38) |
| Non-US region | 38 (62) | 37 (63) | 75 (63) |
| Disease location, n (%) | |||
| Knee | 34 (56) | 39 (66) | 73 (61) |
| Ankle | 14 (23) | 7 (12) | 21 (18) |
| Hip | 6 (10) | 7 (12) | 13 (11) |
| Wrist | 2 (3) | 2 (3) | 4 (3) |
| Foot | 2 (3) | 1 (2) | 3 (3) |
| Shoulder | 1 (2) | 1 (2) | 2 (2) |
| Spine | 1 (2) | 1 (2) | 2 (2) |
| Elbow | 1 (2) | 0 | 1 (1) |
| Finger | 0 | 1 (2) | 1 (1) |
| Prior surgeries for TGCT, n (%) | |||
| 0 | 29 (48) | 28 (48) | 57 (48) |
| 1 | 13 (21) | 12 (20) | 25 (21) |
| 2 | 7 (12) | 12 (20) | 19 (16) |
| ≥3 | 12 (20) | 7 (12) | 19 (16) |
| Prior systemic therapy, n (%) | |||
| No prior systemic therapy | 53 (87) | 56 (95) | 109 (91) |
| Nilotinib | 1 (2) | 0 | 1 (1) |
| Imatinib | 7 (12) | 3 (5) | 10 (8) |
| Tumor sum of longest diameters | |||
| Mean (SD), mm† | 101·3 (63·1) | 105·5 (73·5) | 103·4 (68·2) |
| Tumor volume score | |||
| Mean (SD)† | 14·8 (21·2) | 12·1 (16·2) | 13·5 (18·9) |
| Range of motion in affected joint relative to a reference standard | |||
| Mean (SD), %‡ | 62·5 (24·8) | 62·9 (21·8) | 62·7 (23·3) |
| Concomitant analgesic use, n (%) | 38 (62) | 36 (61) | 74 (62) |
Percentages may not sum to 100% due to rounding.
1 patient in the pexidartinib group and 1 patient in the placebo group were missing baseline values.
1 patient in the placebo group was missing a baseline range of motion assessment.
SD=standard deviation. TGCT=tenosynovial giant cell tumor.
Overall, 9 of 61 (15%) patients in the pexidartinib group discontinued part 1 early due to adverse events (AEs) (n=8) and consent withdrawal (n=1, after DMC recommendation), versus 11 of 59 (19%) in the placebo group discontinuing early due to disease progression (n=1), consent withdrawal (n=6; 5 after DMC recommendation), investigator decision (n=3; all after DMC recommendation), and noncompliance (n=1).
Efficacy
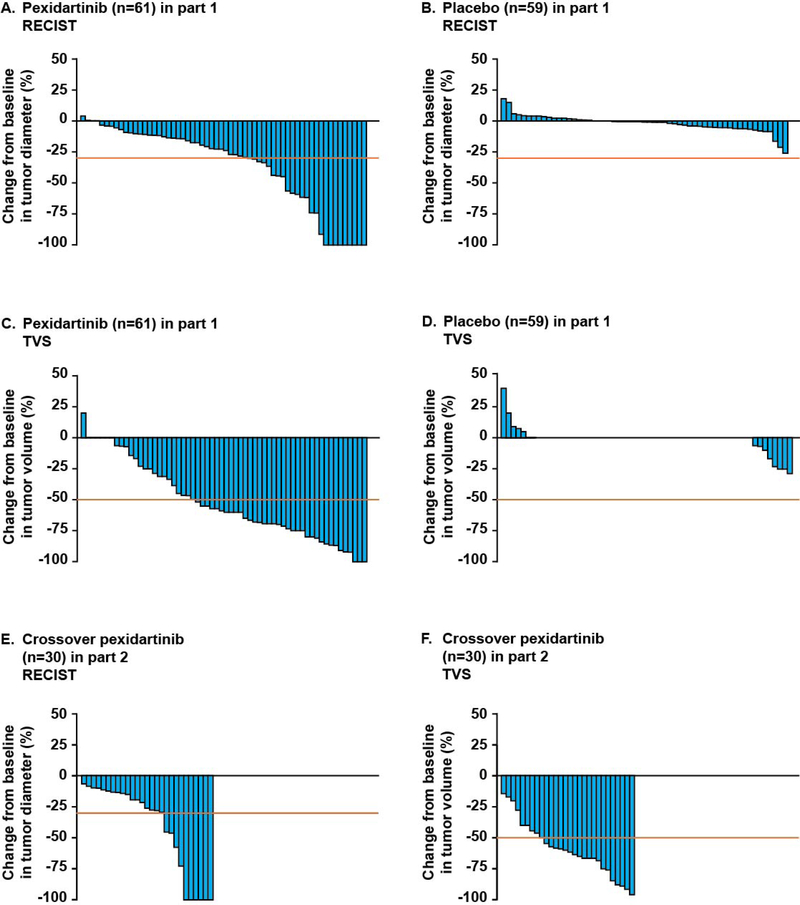
Overall response rate (CR or PR) by RECIST at week 25 was 39% in the pexidartinib group versus 0% in the placebo group (95% CI for difference, 27–52%; p<0·0001) (figure 2 and table 2). The best overall response for patients randomized to pexidartinib was 53% (95% CI 40–64%) at the January 31, 2018 data cutoff; this increase in response rate reflects additional tumor size decrease that occurred with longer pexidartinib treatment. The majority of patients who achieved a CR or PR maintained their response for all time points measured (appendix figure 2). Overall response rate by TVS at week 25 was 56% with pexidartinib versus 0% with placebo (95% CI for difference, 42–68%; p<0·0001) (figure 2 and table 2); TVS response rate increased to 64% (95% CI 51–75%) at the January 31, 2018 data cutoff when tumor assessments after week 25 were included. At the 6-month median follow-up, no patient who responded to pexidartinib (by RECIST) at week 25 had progressed. At the January 31, 2018 data cutoff, with 22 months median follow-up, median duration of response by RECIST (range: 0+ to 25+ months) or TVS (range: 0+ to 28+ months) was not reached, as few patients had experienced disease progression. An example of a patient with extensive disease at baseline and an objective response is shown in appendix figure 3.
Figure 2: Maximum change in tumor size according to RECIST and TVS.
RECIST=Response Evaluation Criteria for Adverse Events, version 1.1. TVS=tumor volume score.
Table 2:
Tumor response by RECIST and TVS at week 25
| Pexidartinib n=61 |
Placebo n=59 |
Difference in % (pexidartinib – placebo)* |
|
|---|---|---|---|
| Response rate based on RECIST for part 1 (primary endpoint) | |||
| Complete response | |||
| no. (%) | 9 (15) | 0 | |
| 95% CI | 8 to 26 | 0 to 6 | |
| Partial response | |||
| no. (%) | 15 (25) | 0 | |
| 95% CI | 16 to 37 | 0 to 6 | |
| Stable disease | |||
| no. (%) | 24 (39) | 46 (78) | |
| 95% CI | 28 to 52 | 66 to 87 | |
| Progressive disease | |||
| no. (%) | 1 (2) | 1 (2) | |
| 95% CI | 0 to 9 | 0 to 9 | |
| Not evaluable† | |||
| no. (%) | 12 (20) | 12 (20) | |
| 95% CI | 12 to 31 | 12 to 32 | |
| Overall response (complete or partial) | |||
| no. (%) | 24 (39)‡ | 0 | 39 |
| 95% CI | 28 to 52 | 0 to 6 | 27 to 52 |
| Fisher’s exact test p value (one-sided) | <0·0001 | ||
| Response rate based on TVS for part 1 | |||
| Complete response | |||
| no. (%) | 3 (5) | 0 (0) | |
| 95% CI | 2 to 14 | 0 to 6 | |
| Partial response | |||
| no. (%) | 31 (51) | 0 (0) | |
| 95% CI | 39 to 63 | 0 to 6 | |
| Stable disease | |||
| no. (%) | 14 (23) | 45 (76) | |
| 95% CI | 14 to 35 | 64 to 85 | |
| Progressive disease | |||
| no. (%) | 1 (2) | 2 (3) | |
| 95% CI | 0 to 9 | 0 to 12 | |
| Not evaluable† | |||
| no. (%) | 12 (20) | 12 (20) | |
| 95% CI | 12 to 31 | 12 to 32 | |
| Overall response (complete or partial) | |||
| no. (%) | 34 (56)‡ | 0 (0) | 56 |
| 95% CI | 43 to 68 | 0 to 6 | 42 to 68 |
| Fisher’s exact test p value (one-sided) | <0·0001 | ||
95% confidence interval was calculated using the Newcombe method.
19 patients discontinued the study prior to their end of part 1 assessment, 3 patients had their MRI completed outside of the defined week 25 window (days 155–183), and 2 patients started part 2 treatment prior to their end of part 1 assessment.
Response rate increased to 53% by RECIST and 64% by TVS at the January 31, 2018 data cutoff, when tumor assessments after week 25 were included.
CI=confidence interval. MRI=magnetic resonance imaging, RECIST=Response Evaluation Criteria for Adverse Events, version 1.1. TVS=tumor volume score.
Secondary endpoints demonstrated benefit of pexidartinib at week 25 (tables 2 and 3). Pexidartinib, versus placebo, significantly increased relative ROM (+15% [95% CI 11–19%] vs +6% [95% CI 2–11%] from baseline; p=0·0043) and significantly improved physical functioning per PROMIS (p=0.0019), with patients on pexidartinib reporting improved physical functioning compared with baseline (+4·1; 95% CI 1·8–6·3), while placebo-group patients reported no improvement (−0·9; 95% CI −3·0 to 1·2). Pexidartinib-group patients also reported significantly greater improvement in stiffness compared with baseline than placebo-group patients (−2·5 [95% CI −3·0 to −1·9] vs −0·3 [95% CI −0·9 to 0·3]; p<0·0001). The proportion of Pain-30 responders was higher with pexidartinib (31%; 95% CI 21–44%) than with placebo (15%; 95% CI 8–27%); however, the result did not reach statistical significance (one-sided p=0·032) (table 3). An exploratory analysis of pain using a mixed-model, repeat-measures analysis of mean change from baseline (same methodology as that used for other secondary endpoints) showed improved pain with pexidartinib versus placebo (−2·5 [95% CI −3·1 to −1·8] vs −0·6 [95% CI −1·2 to 0·1]; p<0·0001). Improvements in secondary endpoints correlated with tumor response (appendix figure 4).
Table 3:
Range of motion and patient-reported outcomes
| Study endpoint | Pexidartinib n=61 |
Placebo n=59 |
Difference (pexidartinib – placebo) |
|---|---|---|---|
| Range of motion assessment* | |||
| Baseline assessment | |||
| no. assessed | 61 | 58 | |
| Mean (SE) | 62·5 (3·2) | 62·9 (2·9) | |
| Change from baseline to week 25 | |||
| Least-squares mean (SE)† | +15·1 (2·1) | +6·2 (2·4) | +8·9 (3·0) |
| 95% CI | 10·9 to 19·2 | 1·5 to 10·9 | 2·9 to 14·9 |
| p value for week 25 comparison | 0·0043 | ||
| PROMIS−Physical Function scale‡ | |||
| Baseline assessment | |||
| no. assessed | 60 | 57 | |
| Mean (SE) | 37·5 (0·6) | 38·9 (0·8) | |
| Change from baseline to week 25 | |||
| Least-squares mean (SE) | +4·1 (1·1) | −0·9 (1·0) | +5·0 (1·6) |
| 95% CI | 1·8 to 6·3 | −3·0 to 1·2 | 1·9 to 8·0 |
| p value for week 25 comparison | 0·0019 | ||
| Worst stiffness NRS score§ | |||
| Baseline assessment | |||
| no. assessed | 59 | 58 | |
| Mean (SE) | 5·6 (0·2) | 5·9 (0·3) | |
| Change from baseline to week 25 | |||
| Least-squares mean (SE) | −2·5 (0·3) | −0·3 (0·3) | −2·2 (0·41) |
| 95% CI | −3·0 to −1·9 | −0·9 to 0·3 | −3·0 to −1·4 |
| p value for week 25 comparison |
<0·0001 |
||
| Response based on Pain-30‖ | |||
| Patients with valid mean worst pain NRS at baseline and week 25, n (%) | 33 (54) | 35 (59) | |
| Patients with decrease of at least 30% in the mean worst pain NRS item, n (%) | 19 (31) | 9 (15) | |
| Patients without a 30% or greater increase in narcotic analgesic data, n (%)¶ | 35 (57) | 35 (59) | |
| Patients with both valid mean worst pain NRS at baseline and week 25 and sufficient narcotic analgesic data for assessment, n (%) | 33 (54) | 35 (59) | |
| Pain-30 response** | |||
| no. (%) | 19 (31) | 9 (15) | 15·9 |
| 95% CI | 21 to 44 | 8 to 27 | 1 to 30†† |
| Fisher’s exact test p value (one-sided) | 0·032 | ||
Mean change from baseline in range of motion of the affected joint, relative to a reference standard for the same joint, at the week 25 visit.
Least-squares mean change from baseline in percent normal reference for corresponding joint and plane of motion.
The PROMIS−Physical Function scale addresses symptoms of immobility. A score of 50 represents the average level of physical functioning in the US general population with a standard deviation of 10. A three-point change in the PROMIS−Physical Function scale has been reported to represent a clinically meaningful difference large enough to have implications for a patient’s physical functioning in this population.10
The worst stiffness NRS item is a one-item self-administered questionnaire assessing the “worst” stiffness in the last 24 hours. The NRS for this item ranges from 0 (“no stiffness”) to 10 (“stiffness as bad as you can imagine”).
The worst pain NRS item is a one-item self-administered questionnaire assessing the “worst” pain in the last 24 hours. The NRS for this item ranges from 0 (“no pain”) to 10 (“pain as bad as you can imagine”).
Sufficient narcotic analgesic data was defined as a minimum of 4 of 7 days of valid recorded data, which includes recording of no narcotic analgesic use for a day. Patients with 0 narcotic analgesic usage at both baseline and week 25 were counted toward without 30% or greater increase.
Pain-30 responders were defined as patients who experienced a ≥30% decrease in mean BPI worst pain NRS item and did not experience a ≥30% increase in narcotic analgesic use over a 7-day period compared with baseline.
95% confidence interval was calculated using the Newcombe method.
BPI=Brief Pain Inventory. CI=confidence interval. NRS=numeric rating scale. PROMIS=Patient-Reported Outcomes Measurement Information System. SE=standard error.
Safety
Treatment-emergent AEs of any grade occurred in 60 of 61 (98%) patients who received pexidartinib and 55 of 59 (93%) patients who received placebo; grade 3 or 4 AEs occurred in 27 (44%) and 7 (12%) patients receiving pexidartinib or placebo, respectively. AEs occurring at ≥10% frequency are reported in table 4. The most common grade 3 or 4 AEs occurring at a higher incidence in the pexidartinib group were increases in aspartate aminotransferase (AST) (10% vs 0%), alanine aminotransferase (ALT) (10% vs 0%), alkaline phosphatase (7% vs 0%), and hypertension (5% vs 0%). Hair color changes (de-pigmentation) of any grade were also more common with pexidartinib (67% vs 3%). Eight (13%) patients discontinued pexidartinib due to AEs, of which seven were liver-related. Treatment interruption or dose reduction due to AEs occurred in 23 of 61 (38%) patients in the pexidartinib group and 6 of 59 (10%) in the placebo group; these were most commonly due to hepatic AEs including AST and ALT increase, or cholestatic hepatotoxicity in the pexidartinib group.
Table 4:
Adverse events occurring in ≥10% of patients in part 1 or part 2
| Adverse event, n (%) | Part 1 | Part 2 | ||||
|---|---|---|---|---|---|---|
| Pexidartinib n=61 |
Placebo n=59 |
Crossover pexidartinib n=30 |
||||
| Any grade | Grade 3/4 | Any grade | Grade 3/4 | Any grade | Grade 3/4 | |
| Skin disorders | ||||||
| Hair color changes | 41 (67) | 0 | 2 (3) | 0 | 25 (83) | 0 |
| Pruritus | 10 (16) | 0 | 2 (3) | 0 | 8 (27) | 0 |
| Rash | 8 (13) | 1 (2) | 2 (3) | 0 | 6 (20) | 0 |
| Rash maculopapular | 6 (10) | 0 | 1 (2) | 0 | 2 (7) | 0 |
| Erythema | 1 (2) | 0 | 0 | 0 | 5 (17) | 0 |
| Gastrointestinal disorders | ||||||
| Nausea | 23 (38) | 0 | 24 (41) | 0 | 6 (20) | 0 |
| Diarrhea | 12 (20) | 0 | 15 (25) | 0 | 8 (27) | 0 |
| Abdominal pain | 10 (16) | 0 | 6 (10) | 0 | 1 (3) | 0 |
| Vomiting | 12 (20) | 1 (2) | 3 (5) | 0 | 1 (3) | 0 |
| Dry mouth | 6 (10) | 0 | 2 (3) | 0 | 4 (13) | 0 |
| Constipation | 7 (12) | 0 | 3 (5) | 0 | 3 (10) | 0 |
| Stomatitis | 4 (7) | 0 | 1 (2) | 0 | 3 (10) | 0 |
| General disorders | ||||||
| Fatigue | 33 (54) | 0 | 21 (36) | 0 | 5 (17) | 0 |
| Asthenia | 6 (10) | 0 | 3 (5) | 0 | 5 (17) | 0 |
| Facial edema | 8 (13) | 0 | 1 (2) | 0 | 6 (20) | 1 (3) |
| Peripheral edema | 8 (13) | 0 | 2 (3) | 0 | 5 (17) | 0 |
| Investigations | ||||||
| AST increase | 24 (39) | 6 (10) | 0 | 0 | 5 (17) | 1 (3) |
| ALT increase | 17 (28) | 6 (10) | 1 (2) | 0 | 7 (23) | 2 (7) |
| Alkaline phosphatase increase | 9 (15) | 4 (7) | 0 | 0 | 1 (3) | 1 (3) |
| Lactate dehydrogenase increase | 7 (12) | 1 (2) | 0 | 0 | 3 (10) | 0 |
| Nervous disorders | ||||||
| Dysgeusia | 15 (25) | 0 | 1 (2) | 0 | 7 (23) | 0 |
| Headache | 11 (18) | 0 | 11 (19) | 0 | 5 (17) | 0 |
| Dizziness | 6 (10) | 1 (2) | 9 (15) | 0 | 4 (13) | 0 |
| Musculoskeletal disorders | ||||||
| Arthralgia | 14 (23) | 2 (3) | 15 (25) | 1 (2) | 6 (20) | 0 |
| Pain in extremity | 4 (7) | 0 | 4 (7) | 1 (2) | 3 (10) | 0 |
| Eye disorders | ||||||
| Periorbital edema | 11 (18) | 1 (2) | 1 (2) | 0 | 3 (10) | 0 |
| Eyelid edema | 1 (2) | 0 | 0 | 0 | 3 (10) | 0 |
| Metabolic/nutritional disorders | ||||||
| Decreased appetite | 10 (16) | 0 | 6 (10) | 0 | 3 (10) | 0 |
| Vascular disorders | 2 (7) | |||||
| Hypertension | 9 (15) | 3 (5) | 6 (10) | 0 | 6 (20) | 2 (7) |
ALT=alanine aminotransferase. AST=aspartate aminotransferase.
Serious AEs occurred in 8 of 61 (13%) patients in the pexidartinib group and 1 of 59 (2%) in the placebo group. Three of the patients in the pexidartinib group experienced ALT and AST ≥3 × upper limit of normal (ULN) with total bilirubin and alkaline phosphatase ≥2 × ULN. In the first case (a 75-year-old woman), hyperbilirubinemia lasted approximately 7 months and required two liver dialysis procedures; a liver biopsy revealed significant ductopenia and severe cholestasis. In the other two cases, hyperbilirubinemia recovered within 1–2 months of pexidartinib discontinuation. With longer pexidartinib treatment through the January 31, 2018 data cutoff, no additional cases of mixed and cholestatic hepatotoxicity were reported.
Part 2: open-label extension with placebo crossover to pexidartinib
Patients
A total of 30 patients who were randomized to placebo in part 1, entered part 2 and received open-label pexidartinib (crossover pexidartinib group), all starting at 800 mg/d, before the DMC-recommended stop of crossover (see CONSORT diagram in appendix figure 1).
Efficacy
Of the crossover pexidartinib patients (n=30), 9 (30%; 95% CI 17–48%) had a RECIST response at week 25 of pexidartinib treatment, and 17 (57%; 95% CI 39–73%) had a TVS response at week 25 (figure 2). At the January 31, 2018 data cutoff, response rates increased to 53% (95% CI 36–70%) by RECIST and 67% (95% CI 49–81%) by TVS in the crossover pexidartinib group. Median response duration was not reached at the January 31, 2018 data cutoff and ranged from 3+ to 23+ months by RECIST to 6+ to 23+ months by TVS. Mean change in ROM from beginning of part 2 to week 25 was +13% (95% CI 8–19%), PROMIS was +4·9 (95% CI 1·5–8·3), stiffness was -3·0 (95% CI −4·5 to −1·5), and pain was −2·6 (95% CI −4·1 to −1·1).
Safety
Crossover pexidartinib patients had fewer liver enzyme elevations than in part 1, and no bilirubin increases or signs of drug-induced cholestatic hepatotoxicity (table 4 and appendix table 1). One patient with cardiovascular disease history died of type A aortic dissection reported as unrelated to study treatment.
DISCUSSION
In this first randomized, placebo-controlled phase 3 study in TGCT (ENLIVEN), pexidartinib significantly improved overall tumor response rate when compared with placebo in patients with symptomatic, advanced TGCT for whom surgery would be associated with potentially worsening functional limitation or severe morbidity. In addition, secondary endpoints favored pexidartinib over placebo at week 25, including TVS, a TGCT-specific imaging method to better assess tumor responses, and a TGCT-adapted version of PROMIS.
Analysis of the primary endpoint demonstrated a significantly greater response rate by RECIST at week 25 in patients receiving pexidartinib versus placebo (39 vs 0%; p<0·0001); response rates increased further with longer pexidartinib treatment. Response rate at week 25 with pexidartinib was also significantly greater by TVS assessment (56 vs 0%; p<0·0001), which considers the overall size of this irregularly shaped tumor relative to the normal synovial cavity, rather than one-dimensional measurements used by RECIST.
TGCT has a highly variable clinical presentation and is associated with pain, swelling, limitation of motion, hemorrhagic joint effusions, and progressive cartilage destruction,1 which may dramatically impact activities of daily living (eg, walking, working, sports). In ENLIVEN, objectively measured ROM showed significant improvement with pexidartinib versus placebo. Patient-reported outcome measures of physical function and stiffness improved significantly with pexidartinib. Changes of ≥3 for physical functioning and ≥1 for stiffness have been demonstrated as changes that are meaningful to patients from the TGCT population (H. L. Gelhorn, PhD, personal communication, 2018). In ENLIVEN, mean changes from baseline to week 25 in the PROMIS–Physical Function scale (+4·1 vs −0·9; p=0·0019) and worst stiffness scale (−2·5 vs −0·3; p<0·0001) suggest that patients receiving pexidartinib versus placebo experienced clinically meaningful changes. The proportion of Pain-30 responders at week 25 was higher with pexidartinib versus placebo; differences missed statistical significance. A prespecified exploratory analysis of mean change in pain showed reduced pain with pexidartinib versus placebo.
The safety profile of pexidartinib warrants special attention, particularly regarding the potential for hepatic AEs. Overall, treatment-emergent AEs of any grade occurred at a high incidence in both the pexidartinib and placebo groups (98% vs 93%, respectively). The high incidence of AEs in the placebo group reflects the severe and debilitating nature of the disease. Hair color changes (de-pigmentation) were the most common AEs with pexidartinib (67% vs 3%); these AEs and other skin disorders were attributed to the KIT inhibitory activity of pexidartinib. Grade 3 or 4 AEs occurred at a higher rate in the pexidartinib versus placebo group (44% vs 12%, respectively), and the most common grade 3 or 4 AEs occurring at a higher incidence in pexidartinib-treated patients were increases in liver enzymes. Hepatic AEs were also the most common cause of treatment interruption, dose reduction, or treatment discontinuation in the pexidartinib group.
Hepatic AEs were comprised of two clinically distinct types: (1) liver enzyme abnormalities, and (2) mixed and cholestatic hepatotoxicity. Liver enzyme abnormalities consisted of asymptomatic, reversible, mostly grade 1–2 transaminase increase, which has been attributed to the known class effect of CSF1R inhibition on Kupffer cells.12, 13 Pexidartinib also caused unexpected mixed and cholestatic hepatotoxicity aside from what may be occurring with Kupffer cell inhibition. Three patients in the pexidartinib group experienced ALT and AST ≥3 × ULN with total bilirubin and alkaline phosphatase ≥2 × ULN; one case had biopsy-confirmed ductopenia and prolonged bilirubin increase lasting ~7 months. Mixed and cholestatic hepatotoxicity was also observed in non-TGCT studies of pexidartinib (n=637; Daiichi Sankyo, unpublished data, 2018); the two most concerning cases were a case needing liver transplant (pexidartinib 1200 mg/d combined with paclitaxel) and a case associated with death (pexidartinib 1000 mg/d monotherapy in a patient with advanced, loco-regionally progressing mucosal melanoma). In these cases, serious hepatotoxicity emerged within the first 2 months of treatment and was associated with increased alkaline phosphatase, consistent with mixed and cholestatic hepatotoxicity and thus not fulfilling Hy’s law criteria. The pattern of liver test abnormalities in patients with mixed and cholestatic hepatotoxicity mandates frequent monitoring for elevations in bilirubin and alkaline phosphatase in addition to transaminases during the first 8 weeks of pexidartinib.
Proof-of-principle for the clinical utility of TKIs in TGCT was demonstrated with imatinib and nilotinib; however, response rates with these agents were inferior to that observed with pexidartinib. In a retrospective study of advanced TGCT patients treated with imatinib (n=29), overall response rate was 19% (5 of 27 evaluable patients), while in a single-arm study of nilotinib (n=56), overall response at 1 year was 6% (3 of 51 evaluable patients).14, 15 Pexidartinib is a potent and selective inhibitor of the CSF1R,16 which may explain the superior efficacy observed in the ENLIVEN study.
ENLIVEN was designed not only to evaluate the risk and benefit profile of pexidartinib in TGCT, but also to characterize the highly variable clinical presentation of the disease and prospectively assess the natural history of TGCT, providing a benchmark to evaluate the effectiveness of new therapies in this non-malignant, locally aggressive, potentially morbid tumor. Study strengths included the use of a panel of endpoints evaluating tumor response, symptoms, and function, particularly the TGCT-specific endpoints (ie, TVS and PROMIS), to better understand the clinical impact of TGCT as well as the extent of improvement with systemic therapy in a disease where none is available. TVS was included as a secondary (rather than primary) endpoint to validate the methodology as providing an accurate measurement of disease response with less error and variability than RECIST.
ENLIVEN included patients with symptomatic, advanced TGCT for whom surgical resection would be associated with potentially worsening functional limitation or severe morbidity. For some of these patients, as well as others with severe, debilitating disease that is amenable to surgery, pexidartinib would provide an important treatment option despite risk of mixed and cholestatic hepatotoxicity. In these cases, we recommend that informed decisions regarding pexidartinib should be made between the patient and an experienced healthcare team, particularly regarding the potential for hepatic AEs as weighed against the severity of their disease.
Patients who crossed over to pexidartinib in part 2 of the ENLIVEN study started pexidartinib at a dose of 800 mg/d and had fewer liver enzyme and no bilirubin increases or signs of drug-induced cholestatic hepatotoxicity elevations. The comparable response rate in this crossover cohort and the lower hepatic AEs among patients who crossed over to pexidartinib in part 2 suggest that a starting dose of 800 mg/d (400 mg twice daily) is preferable to the 1000 mg/d starting dose. The role and timing of pexidartinib in multimodality TGCT treatment require further study. Optimal treatment duration and discontinuation strategies remain to be determined.
Limitations of the ENLIVEN study included early termination of patient enrollment and increased patient withdrawal from the study following the emergence of mixed and cholestatic hepatotoxicity and subsequent revision of the study design. This occurred in response to two cases of mixed and cholestatic hepatotoxicity that occurred in this study and similar cases that occurred in the pexidartinib non-TGCT development program, prompting a review of unblinded safety data by the DMC. Although enrollment was terminated early, 120 (95%) patients of the 126 target were enrolled. Patients were informed of the new safety information and the majority re-consented to continue the study, which proceeded to completion preserving the integrity of the primary and secondary efficacy endpoints.
In conclusion, pexidartinib is the first systemic therapy to display a robust tumor response in TGCT with improved patient symptoms and functional outcomes. Mixed and cholestatic hepatotoxicity is an identified risk associated with pexidartinib and should be highlighted in informed discussions between patients and their healthcare team. Pexidartinib offers a relevant treatment option for carefully selected TGCT patients who are suffering from severe morbidity or functional limitations and for whom no proven treatment options exist.
Supplementary Material
ACKNOWLEDGMENTS
We thank the patients who volunteered to participate in this study; their family members and caregivers; the study center staff members who cared for the patients; the sponsor staff involved in data collection and analyses; Stefano Ferrari, MD, (IRCCS Istituto Ortopedico Rizzoli, Bologna, Italy) for his contributions to the study; and Heather Nyce, PhD (SciStrategy Communications) for medical writing assistance in the development of the manuscript. Research and manuscript support was provided by Daiichi Sankyo, Co., Ltd., Tokyo, Japan. All research at Memorial Sloan Kettering is supported in part by a grant from the National Institutes of Health/National Cancer Institute (#P30 CA008748).
Funding: Sponsored by Daiichi Sankyo, Co., Ltd.; ClinicalTrials.gov number, NCT02371369.
Disclaimers/Research support: supported by Daiichi Sankyo, Co., Ltd., Tokyo, Japan. All research at Memorial Sloan Kettering is supported in part by a grant from the National Institutes of Health/National Cancer Institute (#P30 CA008748).
Footnotes
DECLARATION OF INTERESTS
WDT reports a standard budget for site participation in the clinical trial from Plexxikon and Daiichi Sankyo, and personal fees from Eli Lilly, EMD Serono, Novartis, Eisai, Janssen, Immune Design, Adaptimmune, Daiichi Sankyo, Blueprint, Loxo, GlaxoSmithKline, Agios Pharmaceuticals, Plexxikon, and Nanocell, outside the submitted work. In addition, WDT has a patent Companion Diagnostic for CDK4 inhibitors—14/854,329 pending to MSKCC/SKI, and a patent Methods of Treating Metastatic Sarcoma Using Talimogene Laherparepvec (T-Vec) and Pembrolizumab Combination Therapy—62/671,625 pending to MSKCC/SKI, and is on the Scientific Advisory Boards for Certis Oncology Solutions and Atropos Therapeutics. HG reports grants to his institution from Daiichi Sankyo, Novartis, and Five Prime Therapeutics, during the conduct of the study. EP reports personal fees from Amgen, Daiichi Sankyo, Lilly, PharmaMar, and Takeda, and grants to her institution from Bristol-Myers Squibb, Pfizer, Daiichi Sankyo, and PharmaMar, outside the submitted work. JD reports grants from Roche/Genentech, GSK, and Lilly, and personal fees from GSK, Lilly, Novartis, and Ignyta, outside the submitted work. SB reports personal fees from Novartis and Lilly and grants from Novartis, during the conduct of the study; personal fees from Deciphera, Blueprint Medicines, PharmaMar, ADC Therapeutics, and Nanobiotix; and grants from Incyte and Blueprint Medicines, outside the submitted work. J-YB reports grants and personal fees from Plexxikon, during the conduct of the study; grants and personal fees from Novartis and Roche, and grants and other from Five Prime, outside the submitted work. TA reports grants to his institution from Plexxikon, during the conduct of the study; grants and personal fees from EMD Serono, and personal fees from Adaptimmune, Lilly, Esai, Novartis, Shire, and Taiho, outside the submitted work. JM-B reports grants from Eisai, PharmaMar, and Novartis, and personal fees from PharmaMar and Lilly, outside the submitted work. CWR reports grants to his institution from Daiichi Sankyo, during the conduct of the study; grants to his institution from Argos Therapeutics, Bristol-Myers Squibb, CytRx Corporation, Eisai, Exelixis, Genentech, Novartis, Janssen, Karyopharm Therapeutics, MabVax, Merck, Morphotek, Threshold Pharmaceuticals, and TRACON Pharmaceuticals, and personal fees from Eisai, Exelixis, Genentech, Novartis, and Pfizer, outside the submitted work. CP reports being the owner of Spire Sciences, outside the submitted work. JHH reports personal fees from Daiichi Sankyo, during the conduct of the study; personal fees from Daiichi Sankyo, outside the submitted work. MVDS reports grants to his institution from Daiichi Sankyo, outside the submitted work. HLG reports employment with Evidera, during the conduct of the study. DES, QW, and AY report employment with Daiichi Sankyo. HHH reports personal medical consulting fees from Plexxikon, during the conduct of the study. PSL reports employment with Plexxikon, and a patent US 9358235 B2 issued. ST-S reports employment with Plexxikon, during the conduct of the study. SS reports research funding to her institution from Daiichi Sankyo and Novartis, outside the submitted work. AJW reports grants to his institution from Daiichi Sankyo, during the conduct of the study; grants to his institution from Eli Lilly, Five Prime Therapeutics, Plexxikon, Karyopharm Therapeutics, AADi Inc, and Celldex, and personal fees from Eli Lilly, Novartis, Loxo, Five Prime Therapeutics, and Daiichi Sankyo outside the submitted work. No other conflicts of interest were reported.
DATA SHARING
De-identified individual participant data (IPD) and applicable supporting clinical study documents may be available upon request at https://vivli.org. In cases where clinical study data and supporting documents are provided pursuant to the sponsor’s policies and procedures, the sponsor will continue to protect the privacy of the clinical study participants. Details on data sharing criteria and the procedure for requesting access can be found at this web address: https://vivli.org/ourmember/daiichi-sankyo/.
Prior presentation: presented in part at the 2018 American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago, Illinois, 1–5 June 2018
A complete list of investigators is provided in the appendix
APPENDIX
The authors have provided an appendix to give readers additional information about their work
REFERENCES
- 1.Staals EL, Ferrari S, Donati DM, Palmerini E. Diffuse-type tenosynovial giant cell tumour: current treatment concepts and future perspectives. Eur J Cancer 2016; 63: 34–40. [DOI] [PubMed] [Google Scholar]
- 2.de Saint Aubain Somerhausen N, van de Rijn M. Tenosynovial giant cell tumor: localized type, diffuse type In: Fletcher CB J; Hogendoorn P; Martens F, ed. World Health Organization classification of tumours of soft tissue and bone. 4 ed. Lyon, France: IARC Press; 2013: 100–3. [Google Scholar]
- 3.West RB, Rubin BP, Miller MA, et al. A landscape effect in tenosynovial giant-cell tumor from activation of CSF1 expression by a translocation in a minority of tumor cells. Proc Natl Acad Sci U S A 2006; 103(3): 690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cupp JS, Miller MA, Montgomery KD, et al. Translocation and expression of CSF1 in pigmented villonodular synovitis, tenosynovial giant cell tumor, rheumatoid arthritis and other reactive synovitides. Am J Surg Pathol 2007; 31(6): 970–6. [DOI] [PubMed] [Google Scholar]
- 5.Mastboom MJL, Verspoor FGM, Verschoor AJ, et al. Higher incidence rates than previously known in tenosynovial giant cell tumors. Acta Orthop 2017; 88(6): 688–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brahmi M, Vinceneux A, Cassier PA. Current systemic treatment options for tenosynovial giant cell tumor/pigmented villonodular synovitis: targeting the CSF1/CSF1R AXis. Curr Treat Options Oncol 2016; 17(2): 10. [DOI] [PubMed] [Google Scholar]
- 7.Palmerini E, Staals EL, Maki RG, et al. Tenosynovial giant cell tumour/pigmented villonodular synovitis: outcome of 294 patients before the era of kinase inhibitors. Eur J Cancer 2015; 51(2): 210–7. [DOI] [PubMed] [Google Scholar]
- 8.Tap WD, Wainberg ZA, Anthony SP, et al. Structure-guided blockade of CSF1R kinase in tenosynovial giant-cell tumor. N Engl J Med 2015; 373(5): 428–37. [DOI] [PubMed] [Google Scholar]
- 9.Rose M, Bjorner JB, Gandek B, Bruce B, Fries JF, Ware JE Jr. The PROMIS Physical Function item bank was calibrated to a standardized metric and shown to improve measurement efficiency. J Clin Epidemiol 2014; 67(5): 516–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.PROMIS®. HealthMeasures Web site. http://www.healthmeasures.net/score-and-interpret/interpret-scores/promis. 2018.
- 11.Gelhorn HL, Tong S, McQuarrie K, et al. Patient-reported symptoms of tenosynovial giant cell tumors. Clin Ther 2016; 38(4): 778–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, Ruttinger D. Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. J Immunother Cancer 2017; 5(1): 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radi ZA, Koza-Taylor PH, Bell RR, et al. Increased serum enzyme levels associated with Kupffer cell reduction with no signs of hepatic or skeletal muscle injury. Am J Pathol 2011; 179(1): 240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cassier PA, Gelderblom H, Stacchiotti S, et al. Efficacy of imatinib mesylate for the treatment of locally advanced and/or metastatic tenosynovial giant cell tumor/pigmented villonodular synovitis. Cancer 2012; 118(6): 1649–55. [DOI] [PubMed] [Google Scholar]
- 15.Gelderblom H, Cropet C, Chevreau C, et al. Nilotinib in locally advanced pigmented villonodular synovitis: a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol 2018; 19(5): 639–48. [DOI] [PubMed] [Google Scholar]
- 16.Zhang C, Ibrahim PN, Zhang J, et al. Design and pharmacology of a highly specific dual FMS and KIT kinase inhibitor. Proc Natl Acad Sci U S A 2013; 110(14): 5689–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.