Abstract
Background/Aims
Bicarbonate-containing alginate formulations are reported to be effective for controlling reflux symptoms. However, the efficacy of Lamina G alginate without gas production has not been reported. The aim is to evaluate the efficacy of a non-bicarbonate alginate in individuals with reflux symptoms without reflux esophagitis.
Methods
Participants who had experienced heartburn or regurgitation for 7 consecutive days were randomized to one of the following treatment groups: proton pump inhibitors (PPI) plus alginate (combination) or PPI plus placebo (PPI only). In addition, as a reference group, patients received placebo plus alginate (alginate only). The primary endpoint compared the percentage of patients with complete resolution of symptoms for the final 7 days of the treatment. Secondary endpoints compared changes in symptom score, symptom-free days during the treatment period, the Reflux Disease Questionnaire, Patient Assessment of Upper Gastrointestinal Disorders (PAGI)-Quality of Life and PAGI-Symptoms Severity Index scores, the investigator’s assessment of symptoms, and incidence of adverse events.
Results
Complete resolution of heartburn or regurgitation was not significantly different between the combination and PPI only groups (58.7% vs 57.5%, p=0.903). The secondary endpoints were not significantly different between the two groups. Complete resolution of heartburn or regurgitation, did not differ between the alginate only reference group and the PPI only group (75.0% vs 57.5%, p=0.146).
Conclusions
The addition of non-bicarbonate alginate to PPI was no more effective than PPI alone in controlling reflux symptoms.
Keywords: Gastroesophageal reflux, Proton pump inhibitors, Alginates, Bicarbonates (CRIS KCT0002297)
INTRODUCTION
Gastroesophageal reflux disease (GERD) is a representative gastrointestinal disease with a multifactorial pathophysiology.1 Proton pump inhibitors (PPIs) have previously been shown to be the most effective treatment for GERD. However, other medications based on pathophysiology have also been investigated as treatments for GERD. One of the pathophysiologies typical of GERD is the presence of an acid pocket. The acid pocket is an area containing unbuffered, highly acidic gastric secretions that are located in the proximal stomach after meals. This zone acts as a reservoir from which acid can enter the esophagus when the esophagogastric junction opens.2 Thus, the acid pocket represents an effective target for the treatment of GERD.2 Alginate-based formulations act primarily on these acid pockets by forming a physical barrier. That is, when contacting with gastric acid, alginate rapidly forms a gel “raft” of near-neutral pH as a protective mechanical barrier above the acidic gastric contents.3 Several studies have investigated the efficacy of sodium alginate, in particular the branded antacid product Gaviscon, in the alleviation of symptoms associated with GERD.1,3–6
Most alginate formulations, including Gaviscon, consist of three chemical components: sodium alginate; sodium bicarbonate, which reacts with stomach acid to yield carbon dioxide; and calcium carbonate, which reacts with stomach acid to yield free calcium ions.7 This combination of components results in the production of an alginate antacid raft with gas production.7–9 Although pure sodium alginate without gas production can act as a gel raft of matrix-forming polymers,8,9 there is no evidence that pure sodium alginate can be effective in controlling reflux symptoms on its own. Thus, the aim of study was to evaluate the efficacy of a non-bicarbonate alginate (Lamina G; Taejoon Pharm Co, Seoul, Korea) in reducing reflux symptoms in individuals with reflux symptoms without evidence of reflux esophagitis in endoscopy.
MATERIALS AND METHODS
1. Study design
A randomized, double-blind, parallel-group, comparative study was conducted over 4 weeks in the outpatient Gastroenterology Clinics of Severance and Gangnam Severance Hospitals, Seoul, Korea, between July 2014 and August 2016. The study was conducted involving patients with heartburn or regurgitation symptoms without evidence of reflux esophagitis in a screening endoscopy.
Patients received one of the following treatments for 4 weeks: 20 mg omeprazole once daily plus placebo (PPI only group) or 20 mg omeprazole plus 20 mL sodium alginate suspension (Lamina G, Taejoon Pharm Co.) three times daily (t.i.d.) (50 mg/mL) (combination group). A reference group of patients received placebo plus 20 mL sodium alginate suspension t.i.d. (50 mg/mL) (alginate only group).
The primary aim was to assess the percentage of patients with complete resolution of heartburn or regurgitation symptoms over the final 7 days of the 4-week treatment. The secondary aim was to assess symptom-free days or overnight symptom-free days over the 4-week treatment period. Any change from the baseline Reflux Disease Questionnaire (RDQ),10,11 Patient Assessment of Upper Gastrointestinal Disorders-Quality of Life (PAGI-QOL)12 and PAGI-Symptoms Severity Index (PAGI-SYM)13 scores, as well as the clinical investigator’s assessment for symptoms, were also assessed. The safety profile of subjects was investigated based on the incidences of adverse events (AEs).
Subjects were enrolled to participate in the study during 1-week screening and 4-week treatment period. After the initial screening period, subjects who met the inclusion criteria without exclusion criteria were randomized and assigned to treatment groups. Subjects received the study drug on day 1 and returned to scheduled clinic appointments at week 4 for assessment of GERD symptoms based on patient diaries; RDQ, PAGI-QOL, and PAGI-SYM scores; concomitant medication use; and the incidences of AEs. The remaining study medication was collected at week 4. All subjects had a physical examination with blood tests at both the initial appointment and the end of week 4. Female subjects had pregnancy tests performed at the initial and the week 4 appointments, and results were documented. The protocol was approved by the institutional review boards of each institution. Written informed consents were obtained. The study was registered at cris.nih.go.kr (No. KCT0002297).
2. Patients
Subjects who met the inclusion criteria were eligible to participate in the present study: males and females of Korean ethnicity aged 20 to 80 years old; outpatients who had a history of episodes of heartburn or regurgitation symptoms for at least 3 months without evidence of reflux esophagitis in a screening endoscopy; symptoms of heartburn or regurgitation during the 1-week screening period (either ≥4 days of mild symptom or ≥ 2days of moderate to severe symptoms); and agreement to sign the informed consent form. The severity of symptoms was documented on a 4-point scale: 0=none (no symptoms), 1=mild (easily tolerated symptoms; symptoms were minimal and did not disturb normal activities), 2=moderate (symptoms disturbed normal activities), and 3=severe (symptoms markedly disturbed normal activities).1
Patients were ineligible to participate in the study when one or more of the following conditions were present: erosive reflux disease, Barrett’s esophagus or esophageal stricture; active or healing gastroduodenal ulcer with the exception of evidence of a healed ulcer with scarring; history of esophageal, gastric, or duodenal surgery; any kind of malignant disease; ischemic heart disease by electrocardiogram; pregnant or nursing mothers; allergic history to the study drugs; alcohol or drug abuse; clinically significant liver problems (AST/SGOT, ALT/SGPT >2.5 upper limits of normal); clinically significant renal problems (serum creatinine >1.5 mg/dL); using a PPI within 2 weeks before screening, or use of prokinetics, H2-blocker, or antacids within 3 days before screening; participation in another clinical study within 4 weeks before screening; and any condition that an investigator considered to be inappropriate for the study (for example, reflux-like symptoms according to other organic disease).
3. Treatment allocation
Treatment allocation was done according to a computer-generated randomization code list provided by a statistician. Randomization was performed at the investigational sites. Participants were randomly assigned to receive one of the treatments for 4 weeks with a 2:2:1 ratio (PPI only group: combination group: alginate only group). The prescribed treatment schedule included a 20 mg omeprazole capsule before breakfast and 20 mL (50 mg/mL) Lamina G t.i.d. before meals. All processes were performed using a double-blind approach. The compliance with the medication was checked by counting the residual study drugs during every visit. If the final compliance rate was less than 80%, the data of the patient was excluded in the per protocol analysis.
4. Efficacy evaluation
The primary efficacy endpoint was measured as the percentage of subjects with complete resolution of reflux symptoms over the final 7 days of the treatment based on the patient diary.
The secondary efficacy endpoint was evaluated by the following: (1) changes of symptom score (patient diary) from the baseline, (2) symptom-free days during the 4-week treatment period (%, patient diary), (3) overnight symptom-free days during the 4-week treatment period (%, patient diary), changes from the baseline (4) RDQ, (5) PAGI-QOL, (6) PAGI-SYM scores, and (7) the clinical investigator’s assessment of symptom improvement.
Safety was assessed by vital signs and physical examinations, including blood tests, at both the initial appointment and the end of week 4. All AEs were recorded including the onset date, end date, severity, the relationship with study drugs, the treatment modification, and outcomes.
5. Sample size calculation
Sample size calculation was based on the assumption the combination group had a higher adequate heartburn-relief rate than the PPI only group (56.7% vs 25.7%, respectively).4 The following formula was used to calculate the sample size for each group:
More than 36 patients were required per group for a 5% significance level and a statistical power of 80%. Therefore, we decided to enroll at least 48 patients in each group (PPI only and combination group) with an estimated drop-out rate of 25% due to follow up loss and protocol violations. Given the 2:2:1 group ratio used in this study, 24 patients were recruited for the reference group.
6. Statistical analysis
Baseline characteristics were summarized according to treatment groups. Quantitative data were reported as means and standard deviation, whereas categorical data were expressed as proportions. The chi-square and Fisher exact tests were used to evaluate the associations among various categorical variables, and the t-test or Wilcoxon rank sum tests was used to compare non-categorical variables. All analyses were performed using SAS 9.2 (SAS institute Inc., Cary, NC, USA), and a p-value <0.05 was considered to indicate statistical significance.
RESULTS
1. Participant demographics and baseline characteristics
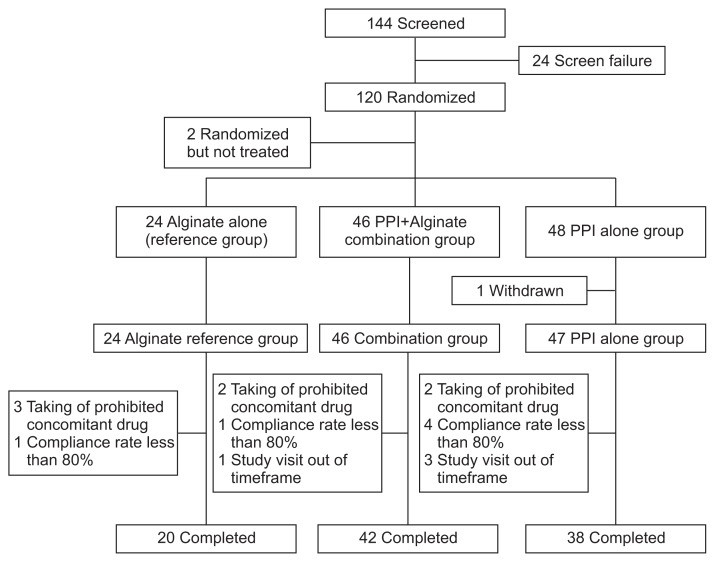
One hundred twenty patients were enrolled and randomized (Fig. 1). Two patients did not take any study drug resulting in a population of 118 patients. All subjects (46 in the combination group and 47 in the PPI only group) reported some efficacy data and the outcomes were analyzed (intention-to-treat population). The demographic and baseline characteristics among the study groups, including the reference group, are summarized in Table 1.
Fig. 1.
Patient flowchart.
PPI, proton pump inhibitor.
Table 1.
Baseline Patient Characteristics
| Characteristic | PPI+alginate | PPI | Alginate | p-value* | p-value† |
|---|---|---|---|---|---|
| Male sex | 8 (17.4) | 11 (23.4) | 4 (16.7) | 0.472 | 0.511 |
| Age, yr | 49.2±12.1 | 47.7±12.1 | 50.4±10.8 | 0.583 | 0.361 |
| Weight, kg | 57.3±8.2 | 59.1±10.4 | 56.5±8.8 | 0.410 | 0.240 |
| BMI, kg/m2 | 22.2±2.3 | 22.3±2.8 | 22.0±3.2 | 0.892 | 0.733 |
| Heartburn | |||||
| Day per week | 6.4±1.4 | 5.7±2.3 | 6.2±1.8 | 0.460 | 0.422 |
| Sum of score‡ | 8.7±4.0 | 7.8±4.7 | 8.0±3.8 | 0.341 | 0.615 |
| Acid regurgitation | |||||
| Day per week | 6.3±1.7 | 6.0±2.1 | 5.9±2.0 | 0.593 | 0.538 |
| Sum of score‡ | 8.8±4.3 | 7.7±4.3 | 7.4±3.4 | 0.199 | 0.875 |
Data are presented as number (%) or mean±SD.
PPI, proton pump inhibitor; BMI, body mass index.
p-value (PPI+alginate vs PPI);
p-value (PPI vs alginate);
Score: none (0), mild (1), moderate (2), and severe (3).
2. Primary efficacy analysis
The primary endpoint compared the percentage of patients with complete resolution of symptoms over the final 7 days of treatment (Table 2). Complete resolution of heartburn or regurgitation was not significantly different between the combination and PPI only groups (58.7% vs 57.5%, p=0.903). In addition, complete resolution of heartburn or regurgitation was also not significantly different between the PPI only and the alginate only groups (57.5% vs 75.0%, p=0.146).
Table 2.
Percentage of the Patients with Complete Resolution of Symptoms for the Last 7 Consecutive Days
| PPI+alginate | PPI | Alginate | p-value* | p-value† | |
|---|---|---|---|---|---|
| Heartburn | 21 (45.7) | 20 (47.6) | 13 (54.2) | 0.853 | 0.609 |
| Regurgitation | 20 (45.5) | 23 (52.3) | 10 (45.5) | 0.522 | 0.602 |
| Heartburn or regurgitation | 27 (58.7) | 27 (57.5) | 18 (75.0) | 0.903 | 0.146 |
Data are presented as number (%).
PPI, proton pump inhibitor.
p-value (PPI+alginate vs PPI);
p-value (PPI vs alginate).
3. Secondary efficacy analysis
The symptom scores were significantly lower than the baseline in all study groups, including the reference group (Table 3). However, the changes in symptom scores from baseline were not significantly different between the combination and PPI only groups (−10.2±8.4 vs −10.4±8.4, p=0.951). There was also no significant difference between the alginate only reference group and the PPI only group (−10.2±9.2 vs −10.4±8.4, p=0.879).
Table 3.
Changes in Symptom Score from Baseline
| PPI+alginate | PPI | Alginate | p-value* | p-value† | |
|---|---|---|---|---|---|
| Heartburn & regurgitation‡ | 7.3±8.7 | 5.2±6.6 | 5.2±7.3 | 0.218 | 0.639 |
| Changes of symptom score§ | −10.2±8.4 | −10.4±8.4 | −10.2±9.2 | 0.951 | 0.879 |
| p-value (intragroup) | <0.0001 | <0.0001 | <0.0001 |
Data are presented as mean±SD.
PPI, proton pump inhibitor.
p-value (PPI+alginate vs PPI);
p-value (PPI vs alginate);
Sum of symptom scores for the last 7 consecutive days;
From the sum of symptom scores at baseline.
Other factors, including symptom-free days during treatment period (Table 4), the change in RDQ, PAGI-QOL, and PAGI-SYM scores (Table 5), and the clinical investigator’s assessment of symptoms (data not shown), were not significantly different between the combination and PPI only groups. There was also no significant difference when comparing all secondary endpoints of the alginate only reference group with those of the PPI only group.
Table 4.
Symptom-Free Days during the 28-Day Treatment Period
| PPI+alginate | PPI | Alginate | p-value* | p-value† | |
|---|---|---|---|---|---|
| Heartburn-free days, % | 44.7±34.7 | 57.4±37.4 | 56.6±37.9 | 0.102 | 0.937 |
| Regurgitation-free days, % | 50.8±38.0 | 57.8±35.3 | 56.2±34.1 | 0.317 | 0.733 |
| Overnight symptom-free days, % | 74.8±33.1 | 83.7±21.3 | 80.7±28.1 | 0.454 | 0.915 |
Data are presented as mean±SD.
PPI, proton pump inhibitor.
p-value (PPI+alginate vs PPI);
p-value (PPI vs alginate).
Table 5.
RDQ, PAGI-QOL, and PAGI-SYM Survey Score Change
| PPI+alginate | PPI | Alginate | p-value* | p-value† | |
|---|---|---|---|---|---|
| RDQ | |||||
| Baseline | 17.5±5.4 | 15.5±4.9 | 15.8±4.7 | 0.046 | 0.562 |
| 28-day | 9.3±8.5 | 6.0±5.9 | 5.9±5.7 | 0.074 | 0.955 |
| Change from baseline | −8.2±7.6 | −9.5±5.7 | −9.8±7.2 | 0.421 | 0.747 |
| PAGI-QOL | |||||
| Baseline | 35.5±26.3 | 29.9±25.9 | 34.1±25.4 | 0.240 | 0.375 |
| 28-day | 17.0±20.3 | 12.3±17.0 | 12.6±16.6 | 0.212 | 0.750 |
| Change from baseline | −18.4±21.5 | −17.7±20.8 | −21.5±20.5 | 0.969 | 0.193 |
| PAGI-SYM | |||||
| Baseline | 32.3±21.0 | 23.2±16.3 | 27.2±17.6 | 0.036 | 0.371 |
| 28-day | 16.5±17.1 | 10.8±12.9 | 11.8±13.2 | 0.047 | 0.956 |
| Change from baseline | −15.9±17.3 | −12.4±11.9 | −15.4±14.7 | 0.562 | 0.555 |
Data are presented as mean±SD.
RDQ, Reflux Disease Questionnaire; PAGI-QOL, Patient Assessment of Upper Gastrointestinal Disorders-Quality of Life; PAGI-SYM, PAGI-Symptoms Severity Index; PPI, proton pump inhibitor.
p-value (PPI+alginate vs PPI);
p-value (PPI vs alginate).
The incidence of AEs was investigated. Seven patients (14.9%) in the combination group, 12 patients (25.5%) in the PPI only group, and six patients (25.0%) in the alginate only group reported mild to moderate AEs. However, no severe AEs were reported in any of the treatment groups, and the incidence of AEs was not significantly different among treatment groups. One patient in the PPI only group withdrew from the study treatment due to an AE of mild dyspepsia.
DISCUSSION
This study showed that the addition of a non-bicarbonate alginate (Lamina G) together with PPI was not more effective than a PPI regime on its own in the alleviation of reflux symptoms. However, when analyzing the data from the reference group, a pure alginate regimen may be as effective as a PPI regimen on its own in reducing reflux symptoms without evidence of reflux esophagitis in endoscopy.
Since a longer and more robust treatment is necessary for successful mucosal healing,14–17 initial PPI treatment is often recommended for more than 8 weeks in erosive reflux disease patients.14–17 Conversely, the treatment goal of nonerosive reflux disease (NERD) patients is only symptom relief. Previous studies have reported symptom relief in GERD patients without evidence of reflux esophagitis following 4 weeks of PPI treatment.17–20 Furthermore, patients with NERD are not treated well despite taking high-dose PPI. Considering the risks of long-term PPI use, the shortest duration or the lowest dose of PPI is an important factor when managing GERD treatment.21,22 Effective alternatives to PPI for symptom relief in GERD patients without evidence of reflux esophagitis would thus also be clinically valuable. Although further evidence is required to validate our data, the present study showed that monotherapy with an alginate alone can provide reflux symptom reduction in GERD patients without evidence of reflux esophagitis comparable to that with PPI treatment alone.
Pathophysiological mechanisms of GERD are categorized into factors that promote the occurrence of reflux and/or increase the perception of reflux.23 The latter can be of particular importance in patients without diagnosed esophagitis.23 An alginate acts on both mechanisms; the acid pocket as the factor promoting the occurrence of reflux, and mucosal integrity as the factor increasing the perception of reflux.
A floating drug-delivery system can act as a physical barrier against the acid pocket. Floating drug-delivery systems are divided into effervescent and noneffervescent systems.8,24 The effervescent system includes gas-generating treatments such as the Gaviscon formulation, whereas the noneffervescent system includes matrix-forming polymers such as sodium alginate used in the present study.8,24 To the best of our knowledge, no study has evaluated the effectiveness of noneffervescent, pure sodium alginate treatment in GERD patients. Our study is the first to investigate the efficacy of pure sodium alginate in GERD patients. According to a previous report, raft formation was observed by endoscopy after 28 seconds of infusion with Lamina G.24 Considering this rapid raft formation, sodium alginate could be an effective therapy for rapid symptom relief in GERD patients.
Microscopic impairment of the esophageal epithelium can be a pathophysiological factor of symptoms in GERD patients, explained as a relationship between impaired mucosal integrity and sensitivity to acid.23,25 Recent studies measuring mucosal integrity by baseline esophageal impedance (mean nocturnal baseline impedance, MNBI)23,26 highlight an important tool in predicting experimental sensitivity to acid. MNBI was significantly lower in NERD patients than in healthy control patients.26,27 According to the previous studies, the esophageal mucosa in NERD patients showed distinct vulnerability when exposed to acids/weak acids, and vulnerable mucosa could be protected by an alginate-containing topical solution.28,29 That is, alginates have bioadhesive potential, and can be prescribed as a topical treatment to maintain esophageal mucosal integrity against acid reflux from the stomach.28–30
Based on our findings here, pure sodium alginate may be an effective monotherapy for symptom relief in GERD patients without evidence of reflux esophagitis. However, we designed the study to compare a PPI treatment with a combination treatment (PPI and alginate), not with alginate only group. The alginate only group was designed arbitrarily as a reference group to show whether alginate monotherapy could be effective in GERD patients without evidence of reflux esophagitis. Thus, further study is required to validate the efficacy of pure sodium alginate in GERD patients without evidence of reflux esophagitis.
Our study is the first to evaluate the effect of pure sodium alginate in controlling reflux symptoms. However, it has several limitations. First, the study participants were selected to have reflux symptoms without evidence of reflux esophagitis in endoscopy. Ambulatory pH monitoring was not performed to exclude functional heartburn or hypersensitive esophagus. This may be one of the reasons not to show additional effect of pure sodium alginate to PPI in the study. Second, rescue medications for uncontrolled symptoms were not planned in the study. Thus, the possibility of self-rescue medication was not fully excluded. Third, the alginate only group was designed arbitrarily as a reference group without statistical evidence. Thus, further study is required to evaluate the efficacy of pure sodium alginate in GERD patients without evidence of reflux esophagitis. Fourth, psychiatric disorders such as depression and anxiety may play a role in GERD symptom manifestation. However, the use of psychiatric medication or a history of psychiatric disorder was not evaluated.
In conclusion, the addition of pure sodium alginate to PPI was no more effective than PPI alone in GERD patients without evidence of reflux esophagitis. Further study designed to compare pure sodium alginate with PPI only is necessary in the future.
ACKNOWLEDGEMENTS
This study was supported by Taejoon Pharm Co. Ltd., Seoul, South Korea.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTIONS
Data analysis and interpretation, drafting of the manuscript: J.H.K. Study concept and design, critical revision of the manuscript for important, obtained funding, study supervision: Y.C.L. Revising it critically for important intellectual content: E.H.K., J.C.P., S.K.S., S.K.L., D.H.J., J.J.P., Y.H.Y., H.P.
REFERENCES
- 1.Chiu CT, Hsu CM, Wang CC, et al. Randomised clinical trial: sodium alginate oral suspension is non-inferior to omeprazole in the treatment of patients with non-erosive gastroesophageal disease. Aliment Pharmacol Ther. 2013;38:1054–1064. doi: 10.1111/apt.12482. [DOI] [PubMed] [Google Scholar]
- 2.Kahrilas PJ, McColl K, Fox M, et al. The acid pocket: a target for treatment in reflux disease? Am J Gastroenterol. 2013;108:1058–1064. doi: 10.1038/ajg.2013.132. [DOI] [PubMed] [Google Scholar]
- 3.Mandel KG, Daggy BP, Brodie DA, Jacoby HI. Review article: alginate-raft formulations in the treatment of heartburn and acid reflux. Aliment Pharmacol Ther. 2000;14:669–690. doi: 10.1046/j.1365-2036.2000.00759.x. [DOI] [PubMed] [Google Scholar]
- 4.Manabe N, Haruma K, Ito M, et al. Efficacy of adding sodium alginate to omeprazole in patients with nonerosive reflux disease: a randomized clinical trial. Dis Esophagus. 2012;25:373–380. doi: 10.1111/j.1442-2050.2011.01276.x. [DOI] [PubMed] [Google Scholar]
- 5.Reimer C, Lødrup AB, Smith G, Wilkinson J, Bytzer P. Randomised clinical trial: alginate (Gaviscon Advance) vs. placebo as add-on therapy in reflux patients with inadequate response to a once daily proton pump inhibitor. Aliment Pharmacol Ther. 2016;43:899–909. doi: 10.1111/apt.13567. [DOI] [PubMed] [Google Scholar]
- 6.De Ruigh A, Roman S, Chen J, Pandolfino JE, Kahrilas PJ. Gaviscon Double Action Liquid (antacid & alginate) is more effective than antacid in controlling post-prandial oesophageal acid exposure in GERD patients: a double-blind crossover study. Aliment Pharmacol Ther. 2014;40:531–537. doi: 10.1111/apt.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dettmar PW, Gil-Gonzalez D, Fisher J, et al. A comparative study on the raft chemical properties of various alginate antacid raft-forming products. Drug Dev Ind Pharm. 2018;44:30–39. doi: 10.1080/03639045.2017.1371737. [DOI] [PubMed] [Google Scholar]
- 8.Lopes CM, Bettencourt C, Rossi A, Buttini F, Barata P. Overview on gastroretentive drug delivery systems for improving drug bioavailability. Int J Pharm. 2016;510:144–158. doi: 10.1016/j.ijpharm.2016.05.016. [DOI] [PubMed] [Google Scholar]
- 9.Singh BN, Kim KH. Floating drug delivery systems: an approach to oral controlled drug delivery via gastric retention. J Control Release. 2000;63:235–259. doi: 10.1016/S0168-3659(99)00204-7. [DOI] [PubMed] [Google Scholar]
- 10.Shaw MJ, Talley NJ, Beebe TJ, et al. Initial validation of a diagnostic questionnaire for gastroesophageal reflux disease. Am J Gastroenterol. 2001;96:52–57. doi: 10.1111/j.1572-0241.2001.03451.x. [DOI] [PubMed] [Google Scholar]
- 11.Cao Y, Yan X, Ma XQ, et al. Validation of a survey methodology for gastroesophageal reflux disease in China. BMC Gastroenterol. 2008;8:37. doi: 10.1186/1471-230X-8-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Loge C, Trudeau E, Marquis P, et al. Cross-cultural development and validation of a patient self-administered questionnaire to assess quality of life in upper gastrointestinal disorders: the PAGI-QOL. Qual Life Res. 2004;13:1751–1762. doi: 10.1007/s11136-004-8751-3. [DOI] [PubMed] [Google Scholar]
- 13.Rentz AM, Kahrilas P, Stanghellini V, et al. Development and psychometric evaluation of the Patient Assessment of Upper Gastrointestinal Symptom Severity Index (PAGI-SYM) in patients with upper gastrointestinal disorders. Qual Life Res. 2004;13:1737–1749. doi: 10.1007/s11136-004-9567-x. [DOI] [PubMed] [Google Scholar]
- 14.Richter JE, Kahrilas PJ, Johanson J, et al. Efficacy and safety of esomeprazole compared with omeprazole in GERD patients with erosive esophagitis: a randomized controlled trial. Am J Gastroenterol. 2001;96:656–665. doi: 10.1111/j.1572-0241.2001.03600.x. [DOI] [PubMed] [Google Scholar]
- 15.Bate CM, Griffin SM, Keeling PW, et al. Reflux symptom relief with omeprazole in patients without unequivocal oesophagitis. Aliment Pharmacol Ther. 1996;10:547–555. doi: 10.1046/j.1365-2036.1996.44186000.x. [DOI] [PubMed] [Google Scholar]
- 16.Chiba N, De Gara CJ, Wilkinson JM, Hunt RH. Speed of healing and symptom relief in grade II to IV gastroesophageal reflux disease: a meta-analysis. Gastroenterology. 1997;112:1798–1810. doi: 10.1053/gast.1997.v112.pm9178669. [DOI] [PubMed] [Google Scholar]
- 17.Jung HK, Hong SJ, Jo YJ, et al. Updated guidelines 2012 for gastroesophageal reflux disease. Korean J Gastroenterol. 2012;60:195–218. doi: 10.4166/kjg.2012.60.4.195. [DOI] [PubMed] [Google Scholar]
- 18.Dean BB, Gano AD, Jr, Knight K, Ofman JJ, Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clin Gastroenterol Hepatol. 2004;2:656–664. doi: 10.1016/S1542-3565(04)00288-5. [DOI] [PubMed] [Google Scholar]
- 19.Richter JE, Campbell DR, Kahrilas PJ, Huang B, Fludas C. Lansoprazole compared with ranitidine for the treatment of nonerosive gastroesophageal reflux disease. Arch Intern Med. 2000;160:1803–1809. doi: 10.1001/archinte.160.12.1803. [DOI] [PubMed] [Google Scholar]
- 20.Richter JE, Peura D, Benjamin SB, Joelsson B, Whipple J. Efficacy of omeprazole for the treatment of symptomatic acid reflux disease without esophagitis. Arch Intern Med. 2000;160:1810–1816. doi: 10.1001/archinte.160.12.1810. [DOI] [PubMed] [Google Scholar]
- 21.Vaezi MF, Yang YX, Howden CW. Complications of proton pump inhibitor therapy. Gastroenterology. 2017;153:35–48. doi: 10.1053/j.gastro.2017.04.047. [DOI] [PubMed] [Google Scholar]
- 22.Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152:706–715. doi: 10.1053/j.gastro.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 23.Herregods TV, Bredenoord AJ, Smout AJ. Pathophysiology of gastroesophageal reflux disease: new understanding in a new era. Neurogastroenterol Motil. 2015;27:1202–1213. doi: 10.1111/nmo.12611. [DOI] [PubMed] [Google Scholar]
- 24.Kim HM. Raft formation of sodium alginate in the stomach. J Neurogastroenterol Motil. 2016;22:705–706. doi: 10.5056/jnm16068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woodland P, Sifrim D. Esophageal mucosal integrity in nonerosive reflux disease. J Clin Gastroenterol. 2014;48:6–12. doi: 10.1097/MCG.0b013e318299f181. [DOI] [PubMed] [Google Scholar]
- 26.Frazzoni M, de Bortoli N, Frazzoni L, Tolone S, Savarino V, Savarino E. Impedance-pH monitoring for diagnosis of reflux disease: new perspectives. Dig Dis Sci. 2017;62:1881–1889. doi: 10.1007/s10620-017-4625-8. [DOI] [PubMed] [Google Scholar]
- 27.de Bortoli N, Martinucci I, Savarino E, et al. Association between baseline impedance values and response proton pump inhibitors in patients with heartburn. Clin Gastroenterol Hepatol. 2015;13:1082–1088. doi: 10.1016/j.cgh.2014.11.035. [DOI] [PubMed] [Google Scholar]
- 28.Woodland P, Lee C, Duraisamy Y, Farré R, Dettmar P, Sifrim D. Assessment and protection of esophageal mucosal integrity in patients with heartburn without esophagitis. Am J Gastroenterol. 2013;108:535–543. doi: 10.1038/ajg.2012.469. [DOI] [PubMed] [Google Scholar]
- 29.Woodland P, Batista-Lima F, Lee C, Preston SL, Dettmar P, Sifrim D. Topical protection of human esophageal mucosal integrity. Am J Physiol Gastrointest Liver Physiol. 2015;308:G975–G980. doi: 10.1152/ajpgi.00424.2014. [DOI] [PubMed] [Google Scholar]
- 30.Smart JD, Kellaway IW, Worthington HE. An in-vitro investigation of mucosa-adhesive materials for use in controlled drug delivery. J Pharm Pharmacol. 1984;36:295–299. doi: 10.1111/j.2042-7158.1984.tb04377.x. [DOI] [PubMed] [Google Scholar]