Abstract
Vitamin D insufficiency during pregnancy is associated with disturbed skeletal homeostasis during infancy. Our aim was to investigate the influence of adherence to recommendations for vitamin D supplement intake of 10 μg per day (400 IU) during pregnancy (mother) and in the first months of life (child) on the occurrence of positional skull deformation of the child at the age of 2 to 4 months. In an observational case–control study, two hundred seventy‐five 2‐ to 4‐month‐old cases with positional skull deformation were compared with 548 matched controls. A questionnaire was used to gather information on background characteristics and vitamin D intake (food, time spent outdoors and supplements). In a multiple variable logistic regression analysis, insufficient vitamin D supplement intake of women during the last trimester of pregnancy [adjusted odds ratio (aOR) 1.86, 95% (CI) 1.27–2.70] and of children during early infancy (aOR 7.15, 95% CI 3.77–13.54) were independently associated with an increased risk of skull deformation during infancy. These associations were evident after adjustment for the associations with skull deformation that were present with younger maternal age and lower maternal education, shorter pregnancy duration, assisted vaginal delivery, male gender and milk formula consumption after birth. Our findings suggest that non‐adherence to recommendations for vitamin D supplement use by pregnant women and infants are associated with a higher risk of positional skull deformation in infants at 2 to 4 months of age. Our study provides an early infant life example of the importance of adequate vitamin D intake during pregnancy and infancy.
Keywords: case–control study, infant, plagiocephaly, non‐synostotic, skull, vitamin D
Introduction
Background
Positional skull deformation refers to a condition in young infants in which the shape of the head and often also the face are deformed as a result of positional preference or external moulding forces. The deformation, which occurs because the cranium is malleable and growing rapidly, may result in flattening of part of the cranium, ear misalignment and facial asymmetry (Littlefield et al. 2002, 2004; Hutchison et al. 2004). When the occipital skull is deformed symmetrically, this is defined as deformational brachycephaly; when the deformation is asymmetrically, this is defined as deformational plagiocephaly (DP). Figure 1 shows both components of positional skull deformation, however, mixed forms are frequently observed. The term skull deformation is generally used as a collective name for both types of deformation (Litva et al. 2002).
Figure 1.
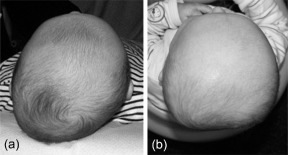
Components of skull deformation: (a) plagiocephaly, (b) brachycephaly.
Prevalence of skull deformation
The prevalence of positional skull deformation varies with age and measurement method used. In infants, van Vlimmeren et al. (2007) reported a DP prevalence of 6% at birth, 22% at age 7 weeks and 17% at age 6 months. DP was measured using plagiocephalometry, in which the two oblique skull diameters are compared as a ratio (van Vlimmeren et al. 2006). In another cohort of healthy infants, Hutchison et al. (2004), using the HeadsUp method, digital photography and head shape quantification using custom written software, found a prevalence of skull deformation at age 6 weeks and 4 months of 16% and 20%, respectively (Hutchison et al. 2004, 2005). After the age of 6 months, the prevalence of skull deformation in both studies gradually declined.
Risk factors for skull deformation
Several risk factors for positional skull deformation have been identified (Bialocerkowski et al. 2008), primarily in studies of the plagiocephaly rather than the brachycephaly component of skull deformation. Obstetric risk factors for skull deformation include unusual shape of the uterus, primiparity, multiple birth pregnancy, premature gestation, prolonged labour, breech presentation and assisted delivery (vacuum/forceps) (Littlefield et al. 2002; Peitsch et al. 2002; Hutchison et al. 2003; van Vlimmeren et al. 2007). Infant risk factors for skull deformation that have been identified include male sex, torticollis and delayed motor development (Hutchison et al. 2003, 2004; van Vlimmeren et al. 2007). The known infant care risk factors are cumulative exposure to the supine position, positional preference, lack of variation in head positioning when sleeping in the first 6 weeks of life and position during bottle‐feeding. Tummy time when awake more than three times a day protects against skull deformation (Hutchison et al. 2003; van Vlimmeren et al. 2007; McKinney et al. 2008; Lennartsson 2011). In one study, excessive daily intake of folic acid during pregnancy was associated with skull deformation (Michels et al. 2011).
Consequences of skull deformation
Although skull deformation is considered to be a minor and purely cosmetic condition (Collett et al. 2005), associations in the longer term have been described with auditory processing disorders (Balan et al. 2002), mandibular asymmetry (St John et al. 2002) and strabismus (Rekate 1998). Skull deformation has the potential for negative physical and psychosocial effects, e.g. teasing and poor self‐perception (Collett et al. 2005).
Vitamin D status as a potential risk factor for skull deformation
Vitamin D is known to be important for the intestinal absorption of calcium and for bone mineralization. The vitamin D status of the newborn and the breastfed infant is determined by maternal vitamin D status during pregnancy and lactation (Dawodu & Wagner 2007). The higher concentrations of 1,25‐dihydroxy‐vitamin D that occur during pregnancy are believed to be important for adequate calcium levels to be available for fetal bone mineralization (Coulter 2012). Congenital rickets is a recognized complication of maternal vitamin D deficiency during pregnancy (Innes et al. 2002). Vitamin D deficiency is associated with an increased risk of craniotabes, the term used to describe a newborn's skull that is softer and thinner than normal and that deforms reversibly when localized pressure is applied (Yorifuji et al. 2008). If vitamin D deficiency persists during infancy then this has the potential to cause skull bones to remain relatively soft and more malleable for a longer period and thus predispose the infant to skull deformation.
Vitamin D can be obtained from sun exposure, food and supplements. The major source of vitamin D for most humans is exposure of the skin to sunlight. However, the ability to make vitamin D from sunlight exposure is dependent on several factors including seasonal variation, time of day and geographical location. When insufficient exposure to sunlight occurs, dietary and supplement sources of vitamin D become important determinants of vitamin D status (Holick 2007). As few natural food products contain significant quantities of vitamin D, diet is an inadequate source of vitamin D in countries which do not have mandatory vitamin D fortification. Even in those countries which have mandatory food fortification dietary vitamin D intake is not always sufficient (Calvo et al. 2004, 2005). The most rational approach therefore to reduce the risk of vitamin D deficiency in pregnant women (and infants) is through supplementation (Compston 1998).
Vitamin D supplements
According to a Cochrane review in 2012, the use of supplementation during pregnancy improves vitamin D concentrations at birth. However, the review concluded that there is currently insufficient high quality evidence for any clinical benefit from such supplementation (De‐Regil et al. 2012). The uncertainty created by this lack of evidence is reflected in the variability in recommendations for vitamin D supplementation during pregnancy in different countries and continents. Dosing recommendations for supplementation vary fourfold in Australasia: 5 μg per day (200 IU) National Health and Medical Research Council (2006), the United Kingdom: 10 μg per day (400 IU) (Scientific Advisory Committee on Nutrition 2007), the Netherlands: 10 μg per day (400 IU) (Health Council of the Netherlands 2008), Scandinavia: 10 μg per day (400 IU) (Nordic Council of Ministers 2004), the United States: 15 μg per day (600 IU) (Ross et al. 2011) and Germany, Switzerland, Austria and Belgium: 20 μg per day (800 IU) (Hoge Gezondheidsraad 2009; Deutsche Gesellschaft für Ernährung, Österreichische Gesellschaft für Ernährung, Schweizerische Gesellschaft für Ernährungsforschung & Schweizerische Vereinigung für Ernährung 2013).
Study objectives
In order to determine if positional skull deformation is associated with vitamin D status, we report findings from our case–control study which investigated the influence of adherence to recommendations for vitamin D supplement intake in the third trimester of pregnancy (woman) and in the first months of life (child) on the development of positional skull deformation in 2‐ to 4‐month‐old children. We hypothesized that lower vitamin D supplement intake during later pregnancy and early infancy would be associated with an increased risk of positional skull deformation. We also investigated the contribution of other vitamin D‐related factors (dietary sources and sun exposure) and the known sociodemographic, obstetric and infant care risk factors for positional skull deformation.
Key messages
Vitamin D insufficiency during pregnancy has been described to be associated with disturbed skeletal homeostasis in the infant.
Insufficient vitamin D supplement intake of woman during the last trimester of pregnancy is associated with a higher risk of positional skull deformation in her 2‐ to 4‐month‐old child.
Insufficient vitamin D intake of the child in the first months of life is associated with an increased risk of positional skull deformation (independent of the maternal vitamin D intake).
Our study shows the importance of promoting a lifestyle with sufficient vitamin D intake in pregnant women and provides evidence for the potential that these recommendations prevent skull deformation.
Material and methods
Study design
We conducted a population‐based case–control study among Dutch children 2 to 4 months old and born between 22 November 2009 and 9 June 2010. A total of two hundred seventy‐five 2‐ to 4‐month‐old cases with mild to severe skull deformation from the Helmet Therapy Assessment in Deformed Skulls (HEADS) study (van Wijk et al. 2012) were compared with 548 controls from a survey on infant milk feeding in 2010 (Lanting & Rijpstra 2011).
Cases and controls
Cases that were referred to a paediatric physical therapist because of positional preference or skull deformation were approached to participate in the HEADS study. They were eligible as cases for this case–control study if they had a diagnosis of clinically relevant skull deformation and were aged between 2 and 4 months. Premature children (<36 weeks of pregnancy time), children with a diagnosis of craniosynostosis, congenital muscular torticollis or craniofacial dysmorphism were excluded (van Wijk et al. 2012). The diagnosis ‘skull deformation’ was based on plagiocephalometry performed by the paediatric physical therapist at the first visit in their practice.
Plagiocephalometry is an easy‐to‐apply, non‐invasive and reliable measurement to assess skull shape with good clinical accuracy (van Vlimmeren et al. 2006; van Adrichem et al. 2008). The largest transverse circumference of the head with perpendicular marks of both ears and nose represents the different skull flattening aspects. An oblique diameter difference index (ratio of the longest and the smallest oblique diameter × 100%, representing severity of plagiocephaly) of >104% is defined to be clinically relevant for an asymmetrically deformed skull; a cranio proportional index (the ratio of the width and length diameter × 100%, representing severity of brachycephaly) of >90% for symmetrical flattening. The deformation is not dichotomous; combinations of both aspects of skull deformation often occur simultaneously (Litva et al. 2002; van Vlimmeren et al. 2006). Controls were recruited from a survey on type of infant milk feeding in 2010 (Lanting & Rijpstra 2011). A questionnaire was handed out to parents of children aged 2 to 6 months at participating well‐baby clinics (250) across the Netherlands. Children were eligible as controls in our study when they were between 2 and 4 months of age and when parents responded negatively to the question whether their child consulted a paediatric physical therapist for skull deformation or positional preference.
Questionnaire
Using a structured questionnaire, data were collected on sociodemographic, obstetric and infant risk factors and vitamin D intake. The sociodemographic factors measured included maternal age, education and ethnicity (as defined by country of birth) of the woman. Obstetric factors included pregnancy duration and delivery method (caesarean, assisted delivery, normal delivery). Infant factors included gender, birthweight and type of milk feeding.
Vitamin D intake was measured with questions about the various factors known to influence vitamin D status. The intake assessment comprised of the use of vitamin D supplements, consumption of dietary sources of vitamin D and exposure to sunlight. The reference period was the last trimester of pregnancy for mothers and the first months of life for the child. Adequacy of vitamin D intake was defined using Dutch Health Council recommendations (Health Council of the Netherlands 2008). Adequate vitamin D intake during pregnancy was defined as taking a vitamin D supplement containing the recommended amount of vitamin D [10 μg (400 IU)] at least five times a week. Certain brands of vitamin D supplements or multivitamins for adults/pregnant woman do not contain the recommended amount of vitamin D. Therefore, the brand of the vitamin D supplements taken was retrieved to verify the vitamin D supplement intake. Adequate vitamin D intake in the first months of life was defined as 10 μg per day (400 IU) of vitamin D supplements (if breastfed) or daily consumption of at least 0.5 L of fortified formula milk. To our knowledge, all specific child vitamin D supplements available in the Netherlands contain the recommended amount of vitamin D (10 μg per 400 IU).
Exposure to sunlight was operationalized as the time spent outdoors per day from April to September. In the Netherlands, situated at latitude 52°N, sunlight exposure only generates vitamin D during these months of the year. Adequate sunlight exposure was defined as more than 15 min outside on more than five occasions per week from April to September for both pregnant women and children. Due to our enrollment period (birth date from November until June), the pregnant women included in this study could not meet the ‘time spent outdoors’ conditions for their entire pregnancy. Therefore, this variable was not included in the analysis.
In addition to supplement intake and sunlight exposure, dietary sources of vitamin D for the pregnant women were determined. We determined maternal intake, during the last trimester of pregnancy, of foods that are either natural (fish consumption per week) or fortified (milk, yoghurt, cheese and margarine consumption per day) sources of vitamin D.
Data analysis
Logistic regression was used to estimate associations between each risk factor and the presence of a skull deformation (univariate analyses). Because the importance of certain risk factors may not be found when their effects are confounded by other risk factors, we performed a multiple logistic regression analysis. In the multivariate model of factors associated with skull deformation, vitamin D variables specific to the mother during late pregnancy and specific to the child during early infancy were both entered. Other factors with a P‐value < 0.10 at the univariate level were included in the multivariate model. Covariates that did not contribute to the full model based on examination of the Wald statistic and comparison between its unadjusted odds ratio and adjusted odds ratio (aOR) were removed from the final model (Hosmer & Lemeshow 2000). In addition, the Chi‐square test was used to determine whether covariates that were independently associated with skull deformation were also associated with each other.
Results
Demographic data, obstetric and infant characteristics of the 275 cases and 548 controls are presented in Table 1. Mothers of children included in the cases were younger (P < 0.001) and had a lower level of education (P < 0.001). Cases were more likely to be born at an earlier gestation (P = 0.012) or following an assisted delivery (P = 0.018). Cases were more likely to be male (P < 0.001) and to have commenced formula rather than breast milk feeding (P = 0.021). No significant differences were found for birthweight and country of birth. There was also no significant difference between the birth seasons of cases and controls.
Table 1.
Univariate associations of maternal sociodemographic, obstetric and infant factors with infant skull deformation
| Variable | Skull deformation | |||
|---|---|---|---|---|
| Cases (n 1 = 275) | Controls (n 2 = 548) | OR (95% CI) | P | |
| Maternal sociodemographic factors | ||||
| Mother's age in years (n 1 = 272, n 2 = 546), mean ± SD | 30.13 ± 4.24 | 31.40 ± 4.49 | 0.94 (0.91–0.97) | <0.001 |
| Mother's education level*, (n 1 = 272, n 2 = 543), n (%) | <0.001 | |||
| Low | 64 (23) | 85 (16) | 2.15 (1.43–3.22) | <0.001 |
| Medium | 121 (45) | 210 (39) | 1.64 (1.18–2.29) | 0.003 |
| High | 87 (32) | 248 (45) | 1.00 | |
| Mother's country of birth, (n 1 = 272, n 2 = 543), n (%) | ||||
| Netherlands | 261 (96) | 520 (95) | 1.00 | |
| Other country | 12 (4) | 28 (5) | 0.85 (0.43–1.71) | 0.655 |
| Obstetric factors | ||||
| Birth season, (n 1 = 275, n 2 = 548), n (%) | 0.113 | |||
| January–March (Winter) | 156 (57) | 349 (64) | 1.24 (0.72–2.13) | 0.138 |
| April–June (Spring) | 90 (33) | 158 (29) | 1.00 | |
| July–September (Summer) | 0 (0) | 0 (0) | – | |
| October–December (Autumn) | 29 (10) | 41 (7) | 0.79 (0.57–1.1) | 0.433 |
| Pregnancy duration in weeks, (n 1 = 274, n 2 = 548), mean ± SD | 39.4 ± 1.5 | 39.6 ± 1.4 | 0.88 (0.79–0.97) | 0.012 |
| Assisted delivery, (n 1 = 270, n 2 = 513), n (%) | ||||
| Yes | 30 (11) | 32 (6) | 1.88 (1.12–3.17) | 0.018 |
| Normal delivery or cesarean section | 240 (89) | 481 (94) | 1.00 | |
| Infant factors | ||||
| Gender, (n 1 = 275, n 2 = 547), n (%) | ||||
| Boy | 186 (68) | 255 (47) | 2.39 (1.77–3.24) | <0.001 |
| Girl | 89 (32) | 292 (53) | 1.00 | |
| Birthweight in grams, (n 1 = 273, n 2 = 545), mean ± SD | 3495 ± 575 | 3524 ± 512 | 1.00 (1.00–1.00) | 0.476 |
| Type of milk feeding started after birth, (n 1 = 275, n 2 = 548), n (%) | ||||
| Breastfeeding | 188 (68) | 416 (76) | 1.00 | |
| Formula milk | 87 (32) | 132 (24) | 1.46 (1.06–2.01) | 0.021 |
CI, confidence interval; OR, odds ratio. *Low educational level: lower technical and vocational training and lower general secondary education; Medium educational level: intermediate vocational training and advanced secondary education; High educational level: higher vocational education and university.
Table 2 shows the vitamin D intake of mothers and infants and associations between vitamin intake and skull deformation. Insufficient vitamin D supplement intake during the last trimester of pregnancy was associated with an increased risk of skull deformation (P < 0.001). The risk of skull deformation was decreased in infants of mothers who consumed fish one to two times per week during the last trimester of pregnancy (P = 0.018). Insufficient vitamin D supplement intake during early infancy was associated with an increased risk of skull deformation (P < 0.001).
Table 2.
Univariate associations of vitamin D related risk factors with infant skull deformation
| Variable | Skull deformation | |||
|---|---|---|---|---|
| Cases (n 1 = 275) | Controls (n 2 = 548) | OR (95% CI) | P | |
| Pregnant woman | ||||
| Supplement use*, (n 1 = 247, n 2 = 489), n (%) | ||||
| Insufficient | 173 (70) | 273 (56) | 1.85 (1.34–2.56) | <0.001 |
| Sufficient | 74 (30) | 216 (44) | 1.00 | |
| Dietary vitamin D, (n 1 = 275, n 2 = 548), n (%) | ||||
| Daily milk/yoghurt | 248 (90) | 485 (89) | 0.83 (0.52–1.30) | 0.467 |
| Daily cheese | 168 (61) | 358 (65) | 1.20 (0.89–1.60) | 0.233 |
| Daily (diet) margarine | 217 (79) | 443 (81) | 1.10 (0.79–1.60) | 0.512 |
| Fish 1–2 times a week | 104 (38) | 255 (47) | 1.43 (1.06–1.92) | 0.018 |
| Child | ||||
| Time spent outdoors † (n 1 = 273, n 2 = 543), n (%) | ||||
| Insufficient | 203 (74) | 427 (79) | 0.79 (0.56–1.11) | 0.170 |
| Sufficient | 70 (26) | 116 (21) | 1.00 | |
| Vitamin D supplement intake ‡ (n 1 = 269, n 2 = 548), n (%) | ||||
| Insufficient | 17 (6) | 19 (4) | 5.74 (3.29–10.02) | <0.001 |
| Sufficient | 252 (94) | 529 (96) | 1.00 | |
CI, confidence interval; OR, odds ratio. *Sufficient defined as 10 μg per day of vitamin D at least five times per week. †Time spent outdoors after the age of 4 weeks. Sufficient defined as being outside for more than 15 min on more than five occasions per week, from April until September. ‡Sufficient defined as 10 μg per day vitamin D supplement intake if breastfeeding or >0.5 L per day of formula milk.
The multiple variable logistic regression analysis (Table 3) showed an independent association with skull deformation of both inadequate maternal vitamin D supplement use (aOR = 1.86) during pregnancy and insufficient vitamin D supplement intake during infancy (aOR = 7.15) either as a vitamin D supplement or vitamin D fortified milk. Lower maternal dietary intake of vitamin D as defined by fish consumption less than once weekly was also associated with an increased risk of skull deformation (aOR = 1.50). No significant association was found between the time the child spent outdoors and the risk of skull deformation. Other factors independently associated with an increased risk of skull deformation were younger maternal age (aOR = 0.94), lower maternal education (aOR = 1.97), shorter pregnancy duration (aOR = 0.84), assisted vaginal delivery (aOR = 2.55), infant male gender (aOR = 2.34) and formula milk consumption after birth (aOR = 1.51).
Table 3.
Multiple logistic regression analysis of risk factors for infant skull deformation
| Variable | aOR (95% CI) | P |
|---|---|---|
| Pregnant woman vitamin D intake | ||
| Insufficient vitamin D supplement use* | 1.86 (1.27–2.70) | 0.001 |
| Not consuming fish 1–2 times a week | 1.50 (1.04–2.16) | 0.031 |
| Child vitamin D intake | ||
| Insufficient time spent outdoors † | 0.68 (0.45–1.03) | 0.071 |
| Insufficient vitamin D supplement intake ‡ | 7.15 (3.77–13.54) | 0.000 |
| Maternal sociodemographic, obstetric and infant factors | ||
| Maternal age in years | 0.94 (0.90–0.98) | 0.002 |
| Educational level of mother | 0.023 | |
| Low | 1.97 (1.19–3.26) | 0.009 |
| Medium | 1.51 (1.00–2.27) | 0.049 |
| Pregnancy duration in weeks | 0.84 (0.74–0.95) | 0.006 |
| Assisted vaginal delivery | 2.55 (1.33–4.86) | 0.005 |
| Male gender | 2.34 (1.63–3.35) | <0.001 |
| Milk formula consumption after birth | 1.51 (1.00–2.27) | 0.049 |
aOR, adjusted odds ratio; CI, confidence interval. Pseudo (Nagelkerke) R 2 = 0.216. *Not taking 10μg per day of vitamin D at least five times per week. †Time spent outdoors after the age of 4 weeks. Sufficient defined as being outside for more than 15 min on more than five occasions per week, from April until September. ‡Not taking 10 μg per day if vitamin D supplement intake if breastfeeding or ≤0.5 L of formula milk per day.
In view of the association observed between formula milk consumption and skull deformation, we determined whether formula milk consumption was also associated with socioeconomic status and with maternal vitamin D intake. In comparison with breastfed infants, a larger proportion of the mothers of formula milk‐fed infants had a low education level (28% vs. 15%, P < 0.0001) and an insufficient vitamin D intake (74% vs. 62%, P = 0.002).
Discussion
Summary of results
Our case–control study showed that the risk of skull deformation at 2 to 4 months of age is increased in infants if vitamin D intake by the mother during late pregnancy or by the infant during early infancy is insufficient as defined by the current recommendations in the Netherlands. These associations were evident after adjustment for maternal sociodemographic, obstetric and infant factors that are already acknowledged as risk factors for skull deformation and for which independent associations with skull deformation were also evident in this study.
The association between skull deformation and adequacy of vitamin D intake was stronger for infant [aOR = 7.15, 95% confidence interval (CI) 3.77–13.54] rather than maternal adequacy of vitamin D supplement intake (aOR = 1.86, 95% CI 1.27–2.70). However, a sufficient vitamin D supplement intake by the children themselves in the first months of life did not reverse the consequences of the insufficient vitamin D intake during pregnancy. These findings are consistent with observations made on the effects of maternal vitamin intake during pregnancy on bone mineral density during early childhood (Viljakainen et al. 2011). While post‐natal vitamin D supplementation improves infant vitamin D status and bone mineral content, it only partly eliminates the differences in bone mineral content, measured at age 14 months, that were associated with poorer maternal vitamin D status during pregnancy (Viljakainen et al. 2011).
Milk formula consumption after birth was associated with an increased risk of skull deformation, despite milk formula being a source of vitamin D. This association was not as strong as that seen with adequate vitamin D intake by the mother and infant. Possibly, the usually unilateral position during bottle‐feeding, in the context of a child born with poor vitamin D status secondary to inadequate maternal vitamin D intake, outweighed the protective effect of post‐natal vitamin D supplements in formula milk. This potentially supports our observation that sufficient vitamin D supplement intake by the children themselves in the first months of life cannot reverse the consequences of the insufficient maternal vitamin D intake during pregnancy. The finding of more skull deformities among the formula‐fed infants reflected their socioeconomic status as well. The mothers who chose formula‐feeding were less well educated and a larger portion of them had an insufficient vitamin D supplement intake during pregnancy. Thus, these infants had a lower starting point with respect to their vitamin D status, and it would take them longer to reach vitamin D sufficiency, even with formula milk that contains 10 μg per 0.5 L (400 IU per 0.5 L).
Food fortification is a population‐prevention approach to reducing the population risk of vitamin D deficiency. Some specific target groups, such as children and pregnant women, [especially women with more pigmented skin (McAree et al. 2013)] will need extra vitamin D. Supplementation use is then the best (individual) treatment approach to enable these higher risk groups to achieve adequate vitamin D status. Pregnant women should be strongly recommended to take vitamin D supplements, particularly during seasons when the body is unable to create vitamin D without the exposure to sunlight.
In this study, insufficient or no vitamin D supplement intake in the third trimester of pregnancy was observed in the majority of women (70% of the mothers of infants with skull deformation and 56% of the mothers of infants without skull deformation). This frequency of inadequate vitamin intake during pregnancy is comparable with the study of Belderbos et al. of 1000 healthy term neonates born in the Netherlands from 2006 to 2009, in which only 46% of the mothers of these newborns had used the recommended dose of vitamin supplements during pregnancy (Belderbos et al. 2011). Of note, almost 14% of all vitamin D supplements taken in this study by pregnant women (six of the 25 brands) neither contained 10 μg (400 IU) of vitamin D nor advised the right amount for pregnant women.
Vitamin D dietary references and folic acid recommendations have common target populations: women who are pregnant or trying to get pregnant. In a recent study in the Netherlands, 52% of pregnant women reported using folic acid during the entire recommended period (Zetstra‐van der Woude et al. 2012). Hence, the uptake of folic acid is somewhat higher compared with the vitamin D supplement uptake in this study. Dietary intake advice specific to pregnancy can be combined into a single ‘healthy lifestyle’ advice which could potentially enable more effective promotion. Special attention should be paid to less educated women, as in our study pregnant women who were less educated had two times greater risk of having a child with skull deformation compared with highly educated woman.
The vitamin D supplement intake advice for infants had a better uptake: 94% of the cases and 97% of controls met the daily required intake of 10 μg (400 IU) of vitamin D. This is due either to the use of formula milk or, in case of breastfeeding, to starting with the recommended vitamin D supplementation within 3 weeks. However, if vitamin D intake is insufficient during pregnancy then infant supplementation will not be sufficient to prevent all cases of skull deformation for which vitamin D deficiency is a contributory factor.
Recently, van Wijk et al. showed that skull deformation does not completely resolve in all infants by natural course, but also that helmet therapy does not increase the likelihood of resolution. This emphasizes the importance of prevention, early detection and early treatment with pediatric physiotherapy (van Wijk et al. 2014). Our study shows the importance of promoting a lifestyle with sufficient vitamin D intake in pregnant women and provides evidence for the potential that these recommendations prevent skull deformation, which is a prevalent contemporary infant health issue.
Furthermore, vitamin D insufficiency during pregnancy and early childhood has been associated with an increased risk of a number of serious diseases, including type 1 diabetes (Zipitis & Akobeng 2008), multiple sclerosis (Munger et al. 2004), schizophrenia (McGrath et al. 2004), early childhood wheeze (Camargo et al. 2007) and respiratory infections (Wayse et al. 2004; Karatekin et al. 2009; Belderbos et al. 2011). As a number of these health outcomes do not become apparent until later life, they are more difficult concepts to use in the promotion to pregnant women of the importance of adequate vitamin D intake. Our study provides an early infant life example of the importance of adequate vitamin D intake during pregnancy and infancy.
Strengths and limitations
Access to a large and well‐defined case group in which skull deformation was based upon a robust definition was the strength of our study. That these cases were not from a highly referred and specialized population make the comparison with population controls from a survey on child milk feeding feasible. A few children with skull deformation may have been included in the control group because exclusion was only based on one question. If this was the case, this would mean that the findings of our study are an underestimation of the true effect of vitamin D supplement use on skull deformation. Furthermore, cases as well as controls were born in the same period of the year and because of our inclusion period (November–June), not enough data were gathered to study the association between birth season and skull deformation for all seasons.
Important limitations of this study were the precision of measurement of the intake of vitamin D and the lack of measurement of vitamin D status. Vitamin D intake is a multidimensional concept, made up out of several components. Precisely measuring all these aspects with a questionnaire is difficult because the contribution of each factor to the persons' total vitamin D intake cannot be estimated. For example, the concentration of vitamin D in breast milk was considered negligible in this study because quite large doses of vitamin D [65–90 μg per day (1600–3600 IU per day)] are required to achieve significant increases in breast milk vitamin D content (Hollis & Wagner 2004). However, when a pregnant woman's vitamin D intake is optimal, the vitamin D content in her breast milk content would be increased and her infant would be at lower risk of developing skull deformation. Because we did not collect the data on which brand of formula milk was given to the child, we were unable to consider variability in vitamin D content of different commercial milk products.
Serum 25‐hydroxy‐vitamin D (25(OH)D) concentration reflects the adequacy of vitamin D intake and production and is generally used to assess vitamin D status. However, obtaining blood samples and measuring serum 25(OH)D concentration was beyond the scope of this study. To explore our findings further, a randomized controlled trial of vitamin D supplementation during pregnancy and infancy with measurement of maternal and infant serum 25(OH)D concentrations should be conducted to establish whether there is a causal link between vitamin D supplement use and skull deformation.
Our study highlights the importance of asking not only the frequency of vitamin D supplement use during pregnancy but also the brand of the supplement used. This enabled us to more precisely estimate the vitamin D supplement intake of the pregnant woman. The information on brand is required to determine vitamin D intake and also implies that it is likely that some mothers will commence supplementation during pregnancy without realizing that the supplement they are taking is an inadequate source of vitamin D. Reducing variance in the vitamin and mineral content of pregnancy supplements would be a potentially important way of improving the status of pregnant women with respect to other key micronutrients including folate and iodine (Gallego et al. 2010).
Selection bias in the control group is another potential limitation of our study. In the control group, in comparison with the average Dutch population, there was an overrepresentation of highly educated women. This could potentially lead to an overestimation of the vitamin D intake in the control group leading to an overestimation of the odds ratio for risk of vitamin D‐related skull deformation in the general population.
The case–control design of this study might have led to sampling and information bias, especially recall bias. Cases compared with controls may have given more detailed information on their vitamin D intake; it is, however, questionable if the recall of this beforehand unknown risk factor differs substantially for cases and controls. The occurrence of socially desirable answers will probably be equal in both groups. This study identified well‐known risk factors for skull deformation such as shorter pregnancy duration, assisted vaginal delivery and male gender. However, identified risk factors such as younger maternal age and lower maternal education are less well defined in the literature (Bialocerkowski et al. 2008). Further research of those risk factors and the mechanism by which they lead to skull deformation is required.
Conclusion
Our findings suggest that insufficient vitamin D supplement intake by the pregnant woman is associated with a higher risk of positional skull deformation in her offspring at 2 to 4 months of age. We also showed that only 40% of all pregnant women had a sufficient vitamin D supplement intake during the last trimester of pregnancy. Our study shows the importance of promoting a lifestyle with sufficient vitamin D intake in pregnant women and provides evidence for the potential that these recommendations prevent skull deformation.
Source of funding
The HEADS study was funded by ZonMw, the Netherlands Organization for Health Research and Development (grant number 170.992.501). Besides the initial review process before funding and amendments, ZonMw did not have any involvement in the study design, management of the study, data analysis, writing and publications. All researcher activities were independent of the funding source.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
C.I. Lanting and R.M. van Wijk provided data on the control population and the cases from their research on type of infant milk feeding (2010) and the HEADS study (2009–2013), respectively. M.G.M. Weernink analysed and interpreted the data and wrote the main article. C.G.M. Groothuis‐Oudshoorn supervised the data analysis. C.C. Grant and R.M. van Wijk advised on the measurement of vitamin D intake and interpreted the data. C.C. Grant edited the main article. L.A. van Vlimmeren designed the measurement instrument (plagiocephalometry) to assess skull asymmetry. All authors discussed the results and implications and commented on the manuscript at all stages. M.M. Boere‐Boonekamp had the final responsibility for the design of the study and the manuscript.
Acknowledgements
We would like to thank the reviewers for their helpful and constructive comments; the HEADS project group and TNO Child Health for distribution of the questionnaire; the participating well‐baby clinics and paediatric physiotherapists for recruiting participants and acquiring the data; and all participating mothers for taking the time and effort to complete the questionnaire.
Weernink, M. G. M. , van Wijk, R. M. , Groothuis‐Oudshoorn, C. G. M. , Lanting, C. I. , Grant, C. C. , van Vlimmeren, L. A. , and Boere‐Boonekamp, M. M. (2016) Insufficient vitamin D supplement use during pregnancy and early childhood: a risk factor for positional skull deformation. Matern Child Nutr, 12: 177–188. doi: 10.1111/mcn.12153.
References
- van Adrichem L.N., van Vlimmeren L.A., Cadanová D., Helders P.J., Engelbert R.H., van Neck H.J. et al (2008) Validation of a simple method for measuring cranial deformities (plagiocephalometry). The Journal of Craniofacial Surgery 19, 15–21. [DOI] [PubMed] [Google Scholar]
- Balan P., Kushnerenko E., Sahlin P., Huotilainen M., Naatanen R. & Hukki J. (2002) Auditory ERPs reveal brain dysfunction in infants with plagiocephaly. The Journal of Craniofacial Surgery 13, 520–525, discussion 526. [DOI] [PubMed] [Google Scholar]
- Belderbos M.E., Houben M.L., Wilbrink B., Lentjes E., Bloemen E.M., Kimpen J.L. et al (2011) Cord blood vitamin D deficiency is associated with respiratory syncytial virus bronchiolitis. Pediatrics 127, e1513–e1520. [DOI] [PubMed] [Google Scholar]
- Bialocerkowski A.E., Vladusic S.L. & Wei Ng C. (2008) Prevalence, risk factors, and natural history of positional plagiocephaly: a systematic review. Developmental Medicine and Child Neurology 50, 577–586. [DOI] [PubMed] [Google Scholar]
- Calvo M.S., Whiting S.J. & Barton C.N. (2004) Vitamin D fortification in the United States and Canada: current status and data needs. The American Journal of Clinical Nutrition 80, 1710S–1716S. [DOI] [PubMed] [Google Scholar]
- Calvo M.S., Whiting S.J. & Barton C.N. (2005) Vitamin D intake: a global perspective of current status. Journal of Nutrition 135, 310–316. [DOI] [PubMed] [Google Scholar]
- Camargo C.A. Jr, Rifas‐Shiman S.L., Litonjua A.A., Rich‐Edwards J.W. et al (2007) Maternal intake of vitamin D during pregnancy and risk of recurrent wheeze in children at 3 y of age. The American Journal of Clinical Nutrition 85, 788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collett B., Breiger D., King D., Cunningham M. & Speltz M. (2005) Neurodevelopmental implications of ‘deformational’ plagiocephaly. Journal of Developmental and Behavioral Pediatrics 26, 379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compston J.E. (1998) Vitamin D deficiency: time for action. British Medical Journal 317, 1466–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter A. (2012) Patient engagement – what works? The Journal of Ambulatory Care Management 35, 80–89. [DOI] [PubMed] [Google Scholar]
- Dawodu A. & Wagner C.L. (2007) Mother‐child vitamin D deficiency: an international perspective. Archives of Disease in Childhood 92, 737–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De‐Regil L.M., Palacios C., Ansary A., Kulier R. & Peña‐Rosas J.P. (2012) Vitamin D supplementation for women during pregnancy. Cochrane Database of Systematic Reviews (Online) 2, CD008873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutsche Gesellschaft für Ernährung, Österreichische Gesellschaft für Ernährung, Schweizerische Gesellschaft für Ernährungsforschung & Schweizerische Vereinigung für Ernährung (2013) Referenzwerte für die Nährstoffzufuhr [Reference values for nutrient provision]. Vitamin D. 1.Auflage, 5., korrigierte Nachdruck. Neustadt a.d.Weinstrasse: Neuer Umschau Buchverlag; 2013. [Google Scholar]
- Gallego G., Goodall S. & Eastman C.J. (2010) Iodine deficiency in Australia: is iodine supplementation for pregnant and lactating women warranted? The Medical Journal of Australia 193, 310–311. [DOI] [PubMed] [Google Scholar]
- Health Council of the Netherlands (2008) Towards an Adequate Intake of Vitamin D. Health Council of the Netherlands: The Hague. [Google Scholar]
- Hoge Gezondheidsraad (2009) Voedingsaanbevelingen voor België. herziening 2009. Brussel: Hoge Gezondheidsraad; 2009: HGR nr. 8309.
- Holick M.F. (2007) Vitamin D deficiency. New England Journal of Medicine 357, 266–281. [DOI] [PubMed] [Google Scholar]
- Hollis B.W. & Wagner C.L. (2004) Vitamin D requirements during lactation: high‐dose maternal supplementation as therapy to prevent hypovitaminosis D for both the mother and the nursing infant. The American Journal of Clinical Nutrition 80, 1752S–1758. [DOI] [PubMed] [Google Scholar]
- Hosmer D.W. & Lemeshow S. (2000) Applied Logistic Regression. John Wiley & Sons: New York. [Google Scholar]
- Hutchison B.L., Thompson J.M. & Mitchell E.A. (2003) Determinants of nonsynostotic plagiocephaly: a case‐control study. Pediatrics 112, e316. [DOI] [PubMed] [Google Scholar]
- Hutchison B.L., Hutchison L.A., Thompson J.M. & Mitchell E.A. (2004) Plagiocephaly and brachycephaly in the first two years of life: a prospective cohort study. Pediatrics 114, 970–980. [DOI] [PubMed] [Google Scholar]
- Hutchison B.L., Hutchison L.A., Thompson J.M. & Mitchell E.A. (2005) Quantification of plagiocephaly and brachycephaly in infants using a digital photographic technique. The Cleft Palate‐Craniofacial Journal 42, 539–547. [DOI] [PubMed] [Google Scholar]
- Innes A.M., Seshia M.M., Prasad C., Al Saif S., Friesen F.R., Chudley A.E. et al (2002) Congenital rickets caused by maternal vitamin D deficiency. Paediatrics and Child Health 7, 455–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatekin G., Kaya A., Salihoglu O., Balci H. & Nuhoglu A. (2009) Association of subclinical vitamin D deficiency in newborns with acute lower respiratory infection and their mothers. European Journal of Clinical Nutrition 63, 473–477. [DOI] [PubMed] [Google Scholar]
- Lanting C.I. & Rijpstra A. (2011) Survey on Type of Infant Milk Feeding in 2010. Breastfeeding in the Province Zeeland. [Peiling Melkvoeding van Zuigelingen 2010. Borstvoeding in de Provincie Zeeland.]. Netherlands Organisation for Applied Scientific Research: Leiden. [Google Scholar]
- Lennartsson F. (2011) Developing guidelines for child health care nurses to prevent nonsynostotic plagiocephaly: searching for the evidence. Journal of Pediatric Nursing 26, 348–358. [DOI] [PubMed] [Google Scholar]
- Littlefield T.R., Kelly K.M., Pomatto J.K. & Beals S.P. (2002) Multiple‐birth infants at higher risk for development of deformational plagiocephaly: II. is one twin at greater risk? Pediatrics 109, 19–25. [DOI] [PubMed] [Google Scholar]
- Littlefield T.R., Saba N.M. & Kelly K.M. (2004) On the current incidence of deformational plagiocephaly: an estimation based on prospective registration at a single center. Seminars in Pediatric Neurology 11, 301–304. [DOI] [PubMed] [Google Scholar]
- Litva A., Coast J., Donovan J., Eyles J., Shepard M., Tacchi J. et al (2002) ‘The public is too subjective’: public involvement at different levels of health‐care decision making. Social Science and Medicine 54, 1825–1837. [DOI] [PubMed] [Google Scholar]
- McAree T., Jacobs B., Manickavasagar T., Sivalokanathan S., Brennan L., Bassett P. et al (2013) Vitamin D deficiency in pregnancy – still a public health issue. Maternal and Child Nutrition 9, 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J., Saari K., Hakko H., Jokelainen J., Jones P., Järvelin M.R. et al (2004) Vitamin D supplementation during the first year of life and risk of schizophrenia: a Finnish birth cohort study. Schizophrenia Research 67, 237–245. [DOI] [PubMed] [Google Scholar]
- McKinney C.M., Cunningham M.L., Holt V.L., Leroux B. & Starr J.R. (2008) Characteristics of 2733 cases diagnosed with deformational plagiocephaly and changes in risk factors over time. The Cleft Palate‐Craniofacial Journal 45, 208–216. [DOI] [PubMed] [Google Scholar]
- Michels A.C., Van den Elzen M.E., Vles J.S. & Van der Hulst R.R. (2011) Prevalence of positional plagiocephaly and excessive folic acid intake during pregnancy. The Cleft Palate‐Craniofacial Journal 49, 1–4. [DOI] [PubMed] [Google Scholar]
- Munger K.L., Zhang S.M., O'Reilly E., Hernan M.A., Olek M.J., Willett W.C. et al (2004) Vitamin D intake and incidence of multiple sclerosis. Neurology 62, 60–65. [DOI] [PubMed] [Google Scholar]
- National Health and Medical Research Council (2006) Nutrient reference values for Australia and New Zealand including Recommended Dietary Intakes. Canberra, Australia: Commonwealth of Australia; 2006. [Google Scholar]
- Nordic Council of Ministers (2004) Nordic Nutrition Recommendations 2004, 4th edn, Integrating Nutrition and Physical Activity Nordic Council of Ministers: Copenhagen. [Google Scholar]
- Peitsch W.K., Keefer C.H., Labrie R.A. & Mulliken J.B. (2002) Incidence of cranial asymmetry in healthy newborns. Pediatrics 110, e72. [DOI] [PubMed] [Google Scholar]
- Rekate H.L. (1998) Occipital plagiocephaly: a critical review of the literature. Journal of Neurosurgery 89, 24–30. [DOI] [PubMed] [Google Scholar]
- Ross A.C., Manson J.E., Abrams S.A., Aloia J.F., Brannon P.M., Clinton S.K. et al (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. Journal of Clinical Endocrinology and Metabolism 96, 53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scientific Advisory Committee on Nutrition (2007) Update on Vitamin D. Position Statement by the Scientific Advisory Committee on Nutrition. The Stationery Office: London. [Google Scholar]
- St John D., Mulliken J.B., Kaban L.B. & Padwa B.L. (2002) Anthropometric analysis of mandibular asymmetry in infants with deformational posterior plagiocephaly. Journal of Oral and Maxillofacial Surgery 60, 873–877. [DOI] [PubMed] [Google Scholar]
- Viljakainen H.T., Korhonen T., Hytinantti T., Laitinen E.K., Andersson S., Mäkitie O. et al (2011) Maternal vitamin D status affects bone growth in early childhood – a prospective cohort study. Osteoporosis International 22, 883–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vlimmeren L.A., Takken T., van Adrichem L.N., van der Graaf Y., Helders P.J. & Engelbert R.H. (2006) Plagiocephalometry: a non‐invasive method to quantify asymmetry of the skull; a reliability study. European Journal of Pediatrics 165, 149–157. [DOI] [PubMed] [Google Scholar]
- van Vlimmeren L.A., van der Graaf Y., Boere‐Boonekamp M.M., L'Hoir M.P., Helders P.J. & Engelbert R.H. (2007) Risk factors for deformational plagiocephaly at birth and at 7 weeks of age: a prospective cohort study. Pediatrics 119, e408–e418. [DOI] [PubMed] [Google Scholar]
- Wayse V., Yousafzai A., Mogale K. & Filteau S. (2004) Association of subclinical vitamin D deficiency with severe acute lower respiratory infection in Indian children under 5 y. European Journal of Clinical Nutrition 58, 563–567. [DOI] [PubMed] [Google Scholar]
- van Wijk R.M., Boere‐Boonekamp M.M., Groothuis‐Oudshoorn C.G., van Vlimmeren L.A. & IJzerman M.J. (2012) HElmet therapy Assessment in infants with Deformed Skulls (HEADS): protocol for a randomised controlled trial. Trials 13, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wijk R.M., van Vlimmeren L.A., Groothuis‐Oudshoorn C.G., van der Ploeg C.P., IJzerman M.J. & Boere‐Boonekamp M.M. (2014) Helmet therapy in infants with positional skull deformation: randomised controlled trial. BMJ (Clinical Research Ed.) 348, g2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorifuji J., Yorifuji T., Tachibana K., Nagai S., Kawai M., Momoi T. et al (2008) Craniotabes in normal newborns: the earliest sign of subclinical vitamin D deficiency. The Journal of Clinical Endocrinology and Metabolism 93, 1784–1788. [DOI] [PubMed] [Google Scholar]
- Zetstra‐van der Woude P.A., de Walle H.E.K. & de Jong‐van den Berg L.T.W. (2012) Periconceptional folic acid use: still room to improve. Birth Defects Research. Part A, Clinical and Molecular Teratology 94, 96–101. [DOI] [PubMed] [Google Scholar]
- Zipitis C.S. & Akobeng A.K. (2008) Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta‐analysis. Archives of Disease in Childhood 93, 512–517. [DOI] [PubMed] [Google Scholar]


