Abstract
Community‐based management of acute malnutrition (CMAM) is effective in treating acute malnutrition. However, post‐discharge follow‐up often lacks. We aimed at assessing the relapse rate and the associated factors in a CMAM programme in Burkina Faso. Discharged children from the community nutrition centre were requested to return at least every 3 months for follow‐up. The data of recovered children (weight‐for‐height z‐score ≥−2) who were discharged between July 2010 and June 2011 were collected in 45 villages, randomly selected out of 210 in January 2012. Sociodemographic data, economic variables, information on household food availability and the child's food consumption in the last 24 h were collected from the parents. A multivariate Cox proportional hazards regression was used to identify the factors associated to relapse. Of the 637 children, 14 (2.2%) died and 218 (34.2%) were lost to follow‐up. The relapse rate [95% confidence interval] among the children who returned for follow‐up was 15.4 [11.8–19.0] per 100 children‐years. The associated factors to relapses in multivariate Cox regression model were mid‐upper arm circumference (MUAC) at discharge below 125 mm, no oil/fat consumption during the last 24 h and incomplete vaccination. To limit relapses, CMAM programmes should avoid premature discharge before a MUAC of at least 125 mm. Nutrition education should emphasize fat/oil as inexpensive energy source for children. Promoting immunization is essential to promote child growth. Periodic monitoring of discharged children should be organized to detect earlier those who are at risk of relapse. The relapse rate should be a CMAM effectiveness indicator.
Keywords: acute malnutrition, community‐based management, relapse, associated factors
Introduction
Undernutrition remains a major health concern in developing countries. In 2011, it accounted for 2.3 million deaths in children under 5 years (UN Inter‐Agency Group for Child Mortality Estimation 2012). Undernutrition includes micronutrient deficiencies, wasting and stunting. Wasting and acute malnutrition are synonymous and are defined by a weight‐for‐height z‐score (WHZ) below −2 using the World Health Organization (WHO) growth standards 2006. Moderate acute malnutrition (MAM) is currently defined by a WHZ <−2 but >−3 and/or a mid‐upper arm circumference (MUAC) <125 mm but >115 mm while severe acute malnutrition (SAM) is defined by a WHZ <−3 and/or a MUAC <115 mm and/or nutritional oedema (World Health Organization & United Nations Children's Fund 2009; The SPHERE Project 2011).
In 2007, WHO, the World Food Programme (WFP), the Standing Committee on Nutrition and the United Nations Children's Fund (UNICEF) recommended the community‐based management (CMAM) strategy for treating SAM (World Health Organization, World Food Programme, United Nations Standing Committee on Nutrition & The United Nations Children's Fund 2007). The CMAM approach enables to take care of children suffering from SAM before complications arise in the living place of these children so access to care increases and deaths by malnutrition decrease (Collins et al. 2006a).
The CMAM approach is widely used by humanitarian agencies both for SAM and MAM (The SPHERE Project 2011; Maleta & Amadi 2014) with successful short‐term issues (Ashworth 2006). Unfortunately, there is little information about what happens after recovery over longer period (Ashraf et al. 2012). Relapses defined as new episode of acute malnutrition after discharge, help programme managers to understand the situation outside the programme (Research and Development Programme (Valid International and Concern Worldwide) 2006). Indeed, relapses may reflect partly the impact of the nutritional education about child feeding practices provided to the mothers or caregivers during the treatment or the effectiveness of a nutritional support beyond the recovery period.
Using data collected from a CMAM programme run in Burkina Faso, we aimed at assessing the relapse rates and the associated factors among children who have been discharged from the programme from July 2010 to June 2011.
Key messages.
Children discharged from community‐based management of acute malnutrition (CMAM) programmes with a mid‐upper arm circumference <125 mm are more likely to relapse.
Consumption of oil/fat is a cheap means to provide high energy to children and therefore to avoid wasting.
Promoting immunization is essential to promote child health and growth. It is important to integrate it as a part of the treatment package for malnourished children.
Periodic monitoring of discharged children is necessary to detect early those who are at risk of relapses.
Relapse rate should be an indicator for assessing the effectiveness of the CMAM programme.
Materials and methods
Programme description
This CMAM programme is run by the Red Cross in Burkina Faso. Children (from 6 months to 5 years) suffering from uncomplicated malnutrition and whose appetite were conserved were treated in their village (in a community nutrition centre) by trained community volunteers using a ready‐to‐use‐therapeutic food (RUTF; for SAM) or corn soya flour (CSB; for MAM). The RUTF is a specially formulated food designed to be nutritionally equivalent to the WHO‐recommended therapeutic feeding milk for SAM, F100, and has some advantages: it can be stored at room temperature with no risk of bacterial growth, it does not require cooking and it can be eaten by children directly from the package. RUTF is currently recommended by WHO and UNICEF for the community‐based management of SAM (World Health Organization, World Food Programme, United Nations Standing Committee on Nutrition & The United Nations Children's Fund 2007). The CSB, flour supplied by WFP, is a mix of corn (75% to 80%), soybeans (20% to 25%) and micronutrient premix (de Pee & Bloem 2009). In this CMAM programme, mothers received a weekly home ration to feed the child until recovery. Children with MAM received weekly 1400 g of CSB, 140 g of oil and 105 g of sugar, providing 1000 kcal of energy per day. The oil was mixed with the CSB flour before distribution. Children with SAM received a weekly ration of RUTF, providing 175 kcal of energy per kilogram per day.
Routine medications consisting of one dose of vitamin A (100 000 IU for children between 6 months and 1 year of age and 200 000 IU for children above 1 year of age), mebendazole 100 mg (one tablet twice a day for 3 days for children under 23 months of age) or albendazole (one dose of 400 mg for children above 23 months of age), iron tablet (200 mg per week until recovery) and folic acid tablet (400 μg per week) were also administered. In case of SAM, the routine medications were completed by an antibiotic (amoxicillin 50 mg kg−1 day−1 or cotrimoxazole 20 mg kg−1 day−1 for 7 days).
The admission and discharge criteria followed the guidelines of the Ministry of Health of Burkina Faso in 2010 (Ministère de la santé (Burkina Faso) 2007). A child was diagnosed as SAM when the MUAC was less than 110 mm or if bilateral pitting oedema were found. MAM was diagnosed if MUAC was 110 mm or more but less than 125 mm with no oedema. Children were discharged if the WHZ index was greater than −2 using the WHO growth standards 2006 (WHO Multicentre Growth Reference Study Group 2006). In each village, a group of six trained community volunteers admitted children by checking admission criteria but they were discharged by a programme nurse who made a supervisory visit fortnightly.
Children were treated in their villages by the trained community volunteers if there were no complications (good appetite, no fever, no diarrhoea, no vomiting and no other symptom of severe sickness) and if the child was above 6 months old. In case of complications, the patients were transferred to the health centre and from the health centre to a therapeutic feeding centre in case of life‐threatening complications (Marasmus or Kwashiorkor with oedema, severe anaemia, respiratory distress, severe dehydration). A child who did not achieve the discharge criteria after 12 weeks was also transferred to the health centre.
The programme offered to mothers on a weekly basis a nutritional counselling including a cooking demonstration of enriched porridge that used local ingredients. The nutritional counselling was delivered weekly by the community volunteers.
The programme began in 2007 and was implemented in nine provinces out of 45 in Burkina Faso and covered 210 villages.
Patients
We conducted a retrospective cohort study. Children aged 6 to 59 months who were discharged from the CMAM programme 6 to 20 months prior to our study (from July 2010 to June 2011) were the target population. The parents of these children were asked to return their children for follow‐up in the nutrition centre of the village at least every 3 months even if the child was not ill. The average length of an untreated episode of SAM is 7.5 months (Garenne et al. 2009). So, a period of 3 months is suitable to observe a new case of acute malnutrition. After discharge, most of the women continued to come weekly to the nutrition centre to participate to educational activities and children were measured monthly.
We used a one‐stage stratified and clustered sampling design. As the programme was running in nine provinces and 210 villages, we randomly selected in each province five villages out of 20 to 23 (according to the province) and in each village, all the children who met the inclusion criteria were included. The inclusion criteria were: to have been discharged from the programme between July 2010 and June 2011 with a WHZ >−2 using the WHO growth standards of 2006 (WHO Multicentre Growth Reference Study Group 2006) and no oedema. Children older than 5 years on our data collection day were excluded.
We recorded from individual growth monitoring card the anthropometric measures at admission in the programme and at discharge and also during subsequent visits following discharge for each child. On that data collection day, each child was measured for anthropometric data and parents were interviewed to collect socioeconomic data.
Anthropometric measures
At each visit, the community volunteers measured the weight, the height, the MUAC of the children and checked nutritional oedema. The weight was taken with a precision of 100 g (using a Spring Scale when the child was under 2 years old or an electronic scale manufactured by SECA, France). The height was measured with a precision of 0.1 cm using local‐made boards: standing board for children above 2 years old and lying‐down board for children between 6 months and 23 months. MUAC was evaluated using a standard colour‐coded tape. The measured values were recorded on a growth monitoring card.
Anthropometric indexes were calculated using the nutrition survey software ‘emergency nutrition assessment’ for standardized monitoring and assessment of relief and transitions (SMART) 2011 that used the WHO Child Growth Standards 2006 (Golden 2011).
Food diversity assessment
The child's diet during the previous day was also checked to estimate his dietary diversity score according to WHO recommendations (World Health Organization 2008) based on consumption of the following seven food groups:
grains, roots and tubers;
legumes and nuts;
dairy products (milk, yogurt, cheese);
flesh foods (meat, fish, poultry and liver/organ meats);
eggs;
vitamin A‐rich fruits and vegetables;
other fruits and vegetables.
We checked in detail what the child ate during the past 24 h before grouping into food groups. So, oil or fat is not listed in the foods groups but we checked for its consumption. According to WHO recommendations, a child under 2 years old should have a minimal dietary diversity score of 4, which means he ate during the past 24 h foods from at least four foods groups listed earlier.
Assessment of other variables
The food security level of the household was also assessed using the validated household hunger scale (HHS) with a score which ranges from 0 to 6 (no hunger to severe hunger) (Ballard et al. 2011). With a HHS score under 3, the household is considered to have little or no hunger problem. If the HHS score ranges from 3 to 4, the household has a moderate hunger situation and when the HHS score is higher than 4 the household has a severe hunger situation.
Mothers were also asked whether they used to assist the demonstration of enriched porridge cooking and whether they had cooked it for their child during the previous week.
Definition of variables
The outcome was relapse into malnutrition defined as a new episode of acute malnutrition with a WHZ <−2 using the WHO standard growth charts 2006 (WHO Multicentre Growth Reference Study Group 2006) during the follow‐up or on the data point day. A bipedal oedema was also a relapse criterion.
The weight gain during recovery was calculated in percentage as follows: (weight at discharge time minus weight on admission) × 100/(weight on admission) (World Health Organization & United Nations Children's Fund 2009) and also in g kg−1 day−1 as (weight at discharge time minus weight on admission) × 1000/(weight on admission × length of stay in days) (SPHERE Project Team 2004). We categorized weight gain using a threshold of 5 g kg−1 day−1 (SPHERE Project Team 2004) or 15% of initial weight (without oedema) (World Health Organization, World Food Programme, United Nations Standing Committee on Nutrition & The United Nations Children's Fund 2007).
The socioeconomic level (SEL) of the child's family was estimated with a score based on ownership of some durable goods. If a good is owned, 1 point was attributed and 0 if not. The goods checked were radio, television, a house roof in metal sheet, a house with a cemented floor, a motorcycle and a car. The SEL score was categorized as follows: 0 or 1 for low SEL, 2 or 3 for medium and 4 or more for high.
The period of food scarcity in Burkina Faso run from July to September each year. So, children who were followed in that period were defined to have faced food scarcity period (Ministère de l'Agriculture 2008; United States Agency for International Development & Famine Early Warming Systems Network 2010).
Statistical analysis
WHZ index and MUAC on admission and discharged were compared with a paired Student's t‐test.
We compared the anthropometric data on admission and discharge between the children who completed the follow‐up and those who died or were lost to follow‐up using chi‐square test for categorical variable and one‐way analysis of variance or Kruskal–Wallis test for quantitative variables. The P‐values were then corrected using Bonferroni method.
For each child, the time to relapse was defined as the time from his discharge from the programme to the date of a new episode of acute malnutrition (either SAM or MAM).
The relapse‐free survival curves were estimated using the Kaplan–Meier method. Survival curves were compared using the log‐rank test and we used Cox proportional model for multivariate analysis that included as variables those which were significant in the univariate analysis (log‐rank test) at P‐value <0.05. The assumption of proportionality of hazards was checked with the verification of parallelism of the curves ln(‐ln (S (t)), where S (t) is the survival curve derived from the Cox model. In the final model, we considered a variable as significantly associated to relapses when the P‐value was <0.05.
All tests were two‐tailed and performed with STATA 12.1 (StataCorp LP, College Station, TX, USA).
Oral informed consent was obtained from the mothers of all participating children prior to the survey. The study was approved by the Ministry of Health of Burkina Faso. All the children who relapsed were treated until recovery.
Results
A total of 637 children were discharged as cured (WHZ >−2) and were looked for during the follow‐up. Of those children, 14 (2.2%) died and 218 (34.2%) were lost to follow‐up (Fig. 1). Socioeconomic, dietary and household food security data were collected only for the 405 children who returned for follow‐up. The median time for follow‐up was 395 days (range: 185–598 days).
Figure 1.
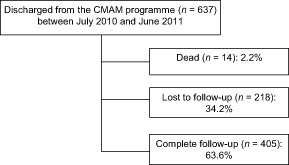
Chart showing the monitoring of children after discharge.
The median number of measurement for these children was 6 (range: 1–10).
Of 405 children who completed the follow‐up, 63 relapsed. The relapse rate [95% confidence interval] among those children was 15.4 [11.8–19.0] per 100 children‐years.
Table 1 shows the sociodemographic, economic and nutritional habits of children and their families. The median age of the children at the admission to the programme was 14.2 months (range 6–60). Girls represented 53.9% of the sample. One child out of five did not fully receive the childhood vaccines required for their age. Eight children out of 10 were not breastfed on our data collection day. Less than a third of the children (32.0%) had the minimal dietary diversity score of 4. Slightly more than half had consumed oil or fat during the last 24 h (54.9%) and eaten an enriched porridge made of local ingredients during the last week before the data point day (69.3%).
Table 1.
Sociodemographic data of the children who completed the follow‐up
| Variables | n | % |
|---|---|---|
| Age at admission (months): median (range) | 346 | 14.2 (6–60) |
| <24 months | 29.2 | |
| Sex | 405 | |
| Girls | 53.9 | |
| Vaccinal status | 394 | |
| Incompleted for the age | 19.3 | |
| Dietary diversity score: mean (SD) | 369 | 4.3 (1.6) |
| <4 | 32.0 | |
| Oil/fat consumption the last 24 h | 399 | |
| Yes | 54.9 | |
| Ate enriched porridge during last week | 398 | |
| Yes | 69.3 | |
| Family household hunger scale: mean (SD) | 390 | 1.5 (1.8) |
| Little‐No hunger | 59.2 | |
| Moderate hunger | 28.2 | |
| Severe hunger | 12.6 | |
| Socioeconomic score: mean (SD) | 289 | 2.7 (1.4) |
| High | 54.0 | |
| Intermediate | 28.4 | |
| Low | 17.7 | |
| Mother's education | 401 | |
| No school attending | 81.6 | |
| Primary school | 12.5 | |
| Secondary school | 6.0 |
SD, standard deviation.
Table 2 shows the anthropometric indexes on admission and discharge according to the final status of children.
Table 2.
Nutritional status of children on admission and discharge
| All (n = 637) | Followed (n = 405) | Lost to follow‐up (n = 218) | Died (n = 14) | P‐value* | |
|---|---|---|---|---|---|
| % | % | % | % | ||
| Age on admission (months) median (p25–p75) | 14 (11–24) | 14 (11–24) | 14 (11–24) | 19.5 (15–24) | 0.25 |
| Sex | |||||
| Girls | 54 | 53.8 | 53.2 | 71.4 | 0.41 |
| Type of acute malnutrition on admission | <0.001 | ||||
| MAM | 87.1 | 90.4 | 83.0 | 53.9 | |
| SAM | 12.9 | 09.6 | 17.0 | 46.1 | |
| WHZ on admission: mean (SD) | −2.30 (1.16) | −2.22 (1.12) | −2.45 (1.17) | −2.28 (1.17) | 0.06 |
| WHZ at discharge: mean (SD) | −0.67 (0.95) | −0.67 (0.97) | −0.67 (0.85) | −0.66 (0.80) | 0.99 |
| MUAC at admission (mm): mean (SD) | 119.6 (8.1) | 120.7 (7.6) ‡§ | 118.1 (8.3) ‡ | 113.9 (11.8) § | <0.001 |
| MUAC at discharge (mm): mean (SD) | 130.4 (6.1) | 130.9 (6.4) | 130.0 (6.0) | 128.7 (4.5) | 0.15 |
| Weight gain (g kg−1 day−1):mean (SD) | 3.2 (2.5) | 3.1 (2.5) | 3.5 (2.5) | 3.1 (2.5) | 0.33 |
| Weight gain (% of admission weight): mean (SD) | 19.6 (13.6) | 18.4 (11.3) † | 21.8 (16.4) † | 21.2 (20.3) | 0.01 |
| Length of stay (day): mean (SD) | 65.0 (25.0) | 64.6 (24.9) | 65.5 (25.6) | 71.0 (21.0) | 0.61 |
In this table, MAM and SAM were the diagnoses made on admission based on a MUAC under 110 mm (SAM) or under 125 mm (MAM). MAM, moderate acute malnutrition; MUAC, mid‐upper arm circumference; SAM, severe acute malnutrition, SD, standard deviation; WHZ, weight‐for‐height z‐score. *Chi‐square and ANOVA one‐way test or Kruskal–Wallis test. †‡§Groups significantly different with Bonferroni test.
The average WHZ (standard deviation) on admission was −2.30 (1.16) and has increased significantly at discharge to −0.67 (0.95) with P < 0.001. Similarly, the average MUAC (standard deviation) has increased significantly from 119.6 mm (8.1) on admission to 130.4 mm (6.1) at discharge (P < 0.001). One child out of 10 (12.9%) on admission was suffering from SAM defined by a MUAC <110 mm. No case of oedema was recorded. We noticed that children who died or were lost to follow‐up were more likely to have SAM (on admission) with a smaller MUAC than those who completed the follow‐up. The mean weight gain was 3.2 g kg−1 day−1 and no difference was found between children who completed the follow‐up and not or died. But the weight gain in percentage of the admission weight (mean of 19.6% of the admission weight) was higher for the children who missed the follow‐up compared with those who completed the follow‐up. No difference was found between the three groups for the length of the treatment which was slightly 2 months.
In bivariate analysis, using the log‐rank test, the associated variables with relapses (at P = 0.05) were MUAC at discharge (P = 0.003), complete vaccination (P = 0.0065) and oil or fat consumption during the last 24 h (P = 0.007). So, children with a MUAC <125 mm at discharge time, or who were not fully vaccinated for their age or who did not consume oil the previous day to the dietary recall were more likely to relapse. None of the following variables were associated to relapses (P > 0.05): age, sex, dietary diversity score, eating enriched porridge during last week, HHS, socioeconomic level, education level of the mother, facing food scarcity period, weight gain, weight gain categorized (percentage of weight gain under or at least 15%) (World Health Organization, World Food Programme, United Nations Standing Committee on Nutrition & The United Nations Children's Fund 2007), length of stay in the programme for recovery and home visits during treatment.
All the significant variables in bivariate analysis remained significant in multivariate analysis at P = 0.05 (Table 3) (Cox regression).
Table 3.
Cox regression analysis of factors associated to relapses (WHZ <−2): n = 387
| Adjusted HR | CI 95% | P | |
|---|---|---|---|
| MUAC at discharge | 0.002 | ||
| <125 mm | 3.89 | 1.66–9.2 | |
| >=125 mm | 1 | ||
| Complete vaccination | 0.02 | ||
| Yes | 1 | ||
| No | 1.89 | 1.08–3.12 | |
| Oil/fat consumption the last 24 h | 0.02 | ||
| Yes | 1 | ||
| No | 1.92 | 1.11–3.33 |
CI, confidence interval; HR, Hazard Ratio; MUAC, mid‐upper arm circumference; WHZ, weight‐for‐height z‐score.
Discussion
The objectives of this study were to determine the relapse rates and the associated factors among children who were discharged from the CMAM programme. During 1‐year follow‐up period in this CMAM programme, 15 children out of 100 relapsed.
There is no standard in the literature to assess whether this rate is high or acceptable. This is a serious lack that needs to be fulfilled, considering that beyond reducing mortality, CMAM programme should promote a good nutrition for children. According to our opinion, this could be a critical way to fight malnutrition with sustainable results. To assess the effectiveness of a CMAM programme, relapse rate among discharged children should be a critical indicator.
The relapse rate in this study using WHZ index was less than the rate reported by Chang et al. (27% in 1‐year follow‐up) in their recent study conducted in Malawi (Chang et al. 2013). Relapses in that study were defined as a WHZ <−2 or a MUAC <125 mm. Moreover, Chang et al. included as participants only the cases of MAM and the study was a trial instead of a programme follow‐up. Ashraf et al. reported a relapse rate of 17.8% in 6 months follow‐up (Ashraf et al. 2012) for a CMAM programme in Bangladesh. This rate is higher than what we reported in this study. But, participating children were cases of SAM (with WHZ <−3) and relapse was considered only when the child experienced a new episode of SAM. Similar to what Chang et al. have reported (Chang et al. 2013), in our study, among SAM cases, 10.5% relapsed into SAM (WHZ <−3) in 1‐year follow‐up.
To address malnutrition issues in a sustainable way, we suggest considering the factors associated to relapse in our study. Children who were discharged with a MUAC less than 125 mm were more likely to relapse. This may indicate an insufficient recovery which promotes a new episode of malnutrition. MUAC correlates with lean tissue before and after rehabilitation of malnutrition (Matarese et al. 2013). To promote lean tissue synthesis, nutrient requirement is high (Golden 2009). Therefore, we could advise that CMAM programme provides sufficient nutrients in diets of malnourished children in addition to calories.
To summarize, a MUAC of at least 125 mm should be required before discharge, both for SAM and MAM cases, as a MUAC threshold of 125 mm defined a MAM case. Using MUAC as discharge criterion enables to avoid shorter duration effect of weight gain of 15% that was previously recommended by WHO (Dale et al. 2013). WHO has updated that recommendation and the new discharge criterion for SAM cases is a MUAC of at least 125 mm (WHO 2013).
This study also shows that incomplete vaccination appears to be a risk factor for relapse which suggests that complete vaccination may reinforce immunization that prevents some current infections. However, immunization may also reflect SEL. Using the education level and the socioeconomic score (built based on ownership of some durable goods) as indicators, we were unable to identify SEL as a risk factor for relapse.
Nutritional education is often vague in many programmes (Asworth & Ferguson 2009). The nutrition education should focus on which foods the mothers should use to prevent malnutrition. Oil or fat is one of the cheapest ingredients which provide high energy intake. Oil or fat is recommended in a child's diet to increase energy density (Michaelsen et al. 2009); however, most of the children in developing countries are fed with porridge made only of cereals with low energy density. Shea butter is widely available in Burkina Faso, the setting of our study, and can be used to increase the energy content of porridge, while being careful to provide also micronutrients.
Surprisingly, we found that dietary diversity score and HHS were not associated with relapses. Dietary diversity is in fact a good indicator for micronutrients intake (Moursi et al. 2008) but not for energy intake which is more important to avoid wasting. The dietary diversity score used the seven food groups for children aged 6–23 months excluding oil/fat which appears in this study as an important energy source that should be checked in developing countries as a cheap means to improve food energy density of children.
The HHS scale does not capture data on food availability or food utilization, which are other components of food security typically measured at the national level (availability) and individual level (consumption/utilization) (Ballard et al. 2011). Thus, it seems that assessing HHS was not the best indicator in this case, especially given the fact that it was measured at family level instead of the child level. Moreover, food aids frequently given to families in Burkina Faso have probably improved the household food availability. Nevertheless, measuring food consumption appeared to be a relevant tool for this study and for programme implementation.
The socioeconomic factors assessed by education level and socioeconomic score did not capture any difference between children regarding the relapse. This could be explained by the fact that these factors are slightly similar among the rural people and research is still needed to prospect for most reliable indicators in this setting.
Chang et al. (2013) have found a seasonal effect on relapses during their study. However, this is not found in our case, as there was no difference in relapses between children who faced a food scarcity period and those who did not (results not shown). This might be due also to food aids in variant forms given to the population during the food scarcity period in this programme setting, especially cereals distributed at subsidized prices (discount from 40% up to 45%) that contributed to stabilize the market price of cereals (United States Agency for International Development & Famine Early Warming Systems Network 2010).
We found that most of the children who died had experienced a SAM. This observation shows that SAM cases should be followed with a special attention to limit or avoid deaths.
It would have been interesting to present relapses for different types of malnutrition (SAM or MAM) at admission, but given the low number of SAM cases, this was not carried out. Nevertheless, SAM cases seemed to relapse more frequently than MAM cases. SAM cases were treated with a RUTF, which is known as the most effective outpatient treatment of SAM cases with low rate of relapses (Ciliberto et al. 2005; Collins et al. 2006b). Our findings suggest that children did not eat the amount of RUTF they had to consume for their recovery. A close supervision by home visits to mothers may be necessary to strengthen the adherence to the treatment and to avoid possible sharing with other children. Both cases of SAM and MAM need follow‐up to avoid relapses and to promote good nutritional habits.
Our study might have some limitations. One‐third of the discharged children did not return for the follow‐up. The relapse rate in these children might be higher. Some of the children, who are lost to follow‐up after discharge, had moved with their parents to the neighbouring countries (such as Côte d'Ivoire) due to economic reasons. Others had moved with their parents for several months inside gold panning sites.
Anthropometric measurements were mostly taken monthly. However, some children have been measured only every 3 months, a period in which we could not know when the relapse first occurred. This may constitute a bias for the Cox modelling. Therefore, the results need to be carefully interpreted.
As conclusion, relapses should be limited in the CMAM programme by means of avoiding premature discharge (before a MUAC of at least 125 mm). Education programme on nutrition for mothers should emphasize on the role of nutrients especially fat as important energy component of diet for children. In addition, it should include the promotion of local food which could provide micronutrients. Distribution of micronutrients powders could be an alternative. Promoting immunization is also essential to promote child health and growth. We recommend a periodic monitoring of discharged children for early detection of those who are at risk of relapses. Home visits during and after treatment are necessary to encourage good adherence to treatment and to promote good nutritional habits.
Source of funding
We thank the Red Cross of Belgium for financial support to the study.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
YES participated in fieldwork, data collection and analysis, study design and writing of the manuscript. MD and PB participated in data analysis and writing of the manuscript. PD participated in study design, data collection and analysis and writing of the manuscript.
Acknowledgements
We deeply appreciate the dedication and commitment of the staff of the Red Cross (in Belgium and Burkina Faso) and thank them for their help and support during the study. We thank Ildephonse Ngabonziza for editing this paper.
Somassè, Y. E. , Dramaix, M. , Bahwere, P. , and Donnen, P. (2016) Relapses from acute malnutrition and related factors in a community‐based management programme in Burkina Faso. Maternal & Child Nutrition, 12: 908–917. doi: 10.1111/mcn.12197.
References
- Ashraf H., Alam N.H., Chisti M.J., Mahmud M.R., Hossain M.I., Ahmed T. et al (2012) A follow‐up experience of 6 months after treatment of children with severe acute malnutrition in Dhaka, Bangladesh. Journal of Tropical Pediatrics 58 (4), 253–257. [DOI] [PubMed] [Google Scholar]
- Ashworth A. (2006) Efficacy and effectiveness of community‐based treatment of severe malnutrition. Food and Nutrition Bulletin 27 (3), S24–S48. [DOI] [PubMed] [Google Scholar]
- Asworth A. & Ferguson E. (2009) Dietary counselling in the management of moderate malnourishment in children. Food and Nutrition Bulletin 30 (3), S405–S433. [DOI] [PubMed] [Google Scholar]
- Ballard T., Coates J., Swindale A. & Deitchler M. (2011) Household Hunger Scale: Indicator Definition and Measurement Guide, 3rd edn Food and Nutrition Technical Assistance II Project, FHI 360: Washington, DC. [Google Scholar]
- Chang C.Y., Trehan I., Wang R.J., Thakwalakwa C., Maleta K., Deitchler M. et al (2013) Children successfully treated for moderate acute malnutrition remain at risk for malnutrition and death in the subsequent year after recovery. The Journal of Nutrition 143, 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciliberto M.A., Sandige H., Ndekha M.J., Ashorn P., Briend A., Ciliberto H.M. et al (2005) Comparison of home‐based therapy with ready‐to‐use therapeutic food with standard therapy in the treatment of malnourished Malawian children: a controlled, clinical effectiveness trial. The American Journal of Clinical Nutrition 81, 864–870. [DOI] [PubMed] [Google Scholar]
- Collins S., Dent N., Binns P., Bahwere P., Sadler K. & Hallam A. (2006a) Management of severe acute malnutrition in children. The Lancet 368, 1992–2000. [DOI] [PubMed] [Google Scholar]
- Collins S., Sadler K., Dent N., Khara T., Guerrero S., Myatt M. et al (2006b) Key issues in the success of community‐based management of severe malnutrition. Food and Nutrition Bulletin 27 (3), S49–S81. [DOI] [PubMed] [Google Scholar]
- Dale N.M., Myatt M., Prudhon C. & Briend A. (2013) Using Mid‐Upper Arm Circumference to end treatment of severe acute malnutrition leads to higher weight gain in the most malnourished children. PLoS ONE 8 (2), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garenne M., Willie D., Maire B., Fontaine O., Eeckels R. & Van den Broeck J. (2009) Incidence and duration of severe wasting in two African populations. Public Health Nutrition 12 (11), 1974–1982. [DOI] [PubMed] [Google Scholar]
- Golden M. (2009) Proposed recommended nutrient densities for moderately malnourished children. Food and Nutrition Bulletin 30 (3), s267–s342. [DOI] [PubMed] [Google Scholar]
- Golden M. (2011) ENA for SMART. Available at: http://www.nutrisurvey.de/enabeta/index.htm (Accessed 5 June 2014).
- Maleta K. & Amadi B. (2014) Community‐based management of acute malnutrition (CMAM) in sub‐Saharan Africa: case studies from Ghana, Malawi, and Zambia. Food and Nutrition Bulletin 35 (2 Suppl.), S34–S38. [DOI] [PubMed] [Google Scholar]
- Matarese L.E., Steiger E., Seidner D.L. & Richmond B. (2013) Body composition changes in cachectic patients Receiving home parenteral nutrition. JPEN. Journal of Parenteral and Enteral Nutrition 26, 366–371. [DOI] [PubMed] [Google Scholar]
- Michaelsen K.F., Hoppe C., Roos N., Kaestel P., Stougaard M., Lauritzen L. et al (2009) Choice of foods and ingredients for moderately malnourished children 6 months to 5 years of age. Food and Nutrition Bulletin 30 (3), s 343–s 404. [DOI] [PubMed] [Google Scholar]
- Ministère de la santé (Burkina Faso) (2007) Protocole national de prise en charge de la malnutrition aiguë au Burkina Faso.
- Ministère de l'Agriculture (2008) Evolution du secteur agricole et des conditions de vie des ménages au Burkina‐faso Ouagadougou. DGPSA.
- Moursi M.M., Arimond M., Dewey K.G., Treche S., Ruel M.T. & Delpeuch F. (2008) Dietary diversity is a good predictor of the micronutrient density of the diet of 6‐ to 23‐month‐old children in Madagascar. The Journal of Nutrition 138, 2448–2453. [DOI] [PubMed] [Google Scholar]
- de Pee S. & Bloem M.W. (2009) Current and potential role of specially formulated foods supplements for preventing malnutrition among 6‐ to 23‐month‐old children and for treating moderate malnutrition among 6‐ to 59‐month‐old children. Food and Nutrition Bulletin 30 (3), S434–S463. [DOI] [PubMed] [Google Scholar]
- Research and Development Programme (Valid International and Concern Worldwide) (2006) Community‐Based Therapeutic Care (CTC): A Field Manual, 1st edn Valid International: Oxford. [Google Scholar]
- SPHERE Project Team (2004) The SPHERE Humanitarian Charter and Minimum Standards in Disaster Response. SPHERE: Geneva. [DOI] [PubMed] [Google Scholar]
- The SPHERE Project (2011) Humanitarian Charter and Minimum Standards in Humanitarian Response, 3rd edn SPHERE: Southampton. [Google Scholar]
- United States Agency for International Development & Famine Early Warming Systems Network (2010) Burkina‐Faso, perspectives sur la sécurité alimentaire: juillet à décembre 2010. Famine Early Warning System Network Juillet–Decembre 2010, 1–10. [Google Scholar]
- UN Inter‐Agency Group for Child Mortality Estimation (2012) Levels and Trends in Child Mortality: Report 2012.
- WHO (2013) Updates on the Management of Severe Acute Malnutrition in Infants and Children. World Health Organization: Geneva. [PubMed] [Google Scholar]
- WHO Multicentre Growth Reference Study Group (2006) WHO Child Growth Standards based on length/height, weight and age. Acta Pædiatrica 450, 76–85. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2008) Indicators for Assessing Infant and Young Child Feeding Practices‐ Part I: Definition. World Health Organization: Geneva: Available at: http://www.who.int/nutrition/publications/infantfeeding/9789241596664/en/ (Accessed 17 February 2015). [Google Scholar]
- World Health Organization & United Nations Children's Fund (2009) WHO Child Growth Standards and the Identification of Severe Acute Malnutrition in Infants and Children: A Joint Statement by the World Health Organization and the United Nations Children's Fund. World Health Organization: Geneva: Available at: http://www.who.int/nutrition/publications/severemalnutrition/9789241598163/en/ (Accessed 17 February 2015). [PubMed] [Google Scholar]
- World Health Organization, World Food Programme, United Nations Standing Committee on Nutrition & The United Nations Children's Fund (2007) Community‐Based Management of Severe Acute Malnutrition. A Joint Statement by the World Health Organization, the World Food Programme, the United Nations System Standing Committee on Nutrition and the United Nations Children's Fund. World Health Organization: Geneva: Available at: http://www.who.int/nutrition/topics/statement_commbased_malnutrition/en/ (Accessed 17 February 2015). [Google Scholar]


