Abstract
Dietary intake during pregnancy influences maternal health. Poor dietary practices during pregnancy have been linked to maternal complications. The objective was to determine the effect of dietary intervention before or during pregnancy on pregnancy outcomes. A systematic review was conducted without date restrictions. Randomised controlled trials (RCTs) evaluating whole diet or dietary components and pregnancy outcomes were included. Two authors independently identified papers for inclusion and assessed methodological quality. Meta‐analysis was conducted separately for each outcome using random effects models. Results were reported by type of dietary intervention: (1) counselling; (2) food and fortified food products; or (3) combination (counselling + food); and collectively for all dietary interventions. Results were further grouped by trimester when the intervention commenced, nutrient of interest, country income and body mass index. Of 2326 screened abstracts, a total of 28 RCTs were included in this review. Dietary counselling during pregnancy was effective in reducing systolic [standardised mean difference (SMD) −0.26, 95% confidence interval (CI) −0.45 to −0.07; P < 0.001] and diastolic blood pressure (SMD −0.57, 95% CI −0.75 to −0.38; P < 0.001). Macronutrient dietary interventions were effective in reducing the incidence of preterm delivery (SMD −0.19, 95% CI −0.34 to −0.04; P = 0.01). No effects were seen for other outcomes. Dietary interventions showed some small, but significant differences in pregnancy outcomes including a reduction in the incidence of preterm birth. Further high‐quality RCTs, investigating micronutrient provision from food, and combination dietary intervention, are required to identify maternal diet intakes that optimise pregnancy outcomes.
Keywords: diet, pregnancy, randomised controlled trial, systematic review, meta‐analysis
Introduction
The association between early life nutrition and long‐term health has been of interest for decades (Bhutta 2013). There is an abundance of evidence to suggest that when a woman has a good state of health and nutrition prior to and during pregnancy, there is a greater chance of a successful pregnancy and birth outcome (Goldberg 2002; Erick 2008; Ritchie & King 2008; Nichols‐Richardson 2011). Inadequate nutrition during this time, particularly the first trimester, impairs fetal growth (Antal et al. 1997; Derbyshire et al. 2006; Northstone et al. 2008) and has long‐term negative consequences for the mother and the developing fetus (Godfrey & Robinson 1998; Moore et al. 2004; Northstone et al. 2008; Derbyshire et al. 2009).
Gestational diabetes mellitus (GDM) and pregnancy hypertensive disorders are among the most common complications of pregnancy (Sibai 2003; Zhang et al. 2006) affecting 2–5% (Gilmartin et al. 2008), and 10% (Roberts et al. 2011) of all pregnancies, respectively. Pregnancies complicated by these metabolic conditions are associated with adverse maternal, fetal and neonatal outcomes (Brown et al. 2000; Östlund et al. 2003; Roberts et al. 2005, 2003). GDM is associated with an increased risk of pregnancy hypertensive disorders and caesarean section (American Diabetes Association 2003), while increasing the risk of neonatal outcomes: intrauterine fetal death, preterm delivery and fetal macrosomia (American Diabetes Association 2003). In the United States, pregnancy hypertensive disorders, are one of the leading causes of maternal death (Peters & Flack 2004), associated with an increased risk of placental abruption, ante‐ and post‐partum haemorrhage, and acute renal or hepatic failure for the mother (National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy 2000). Pregnancy hypertensive disorders are also associated with preterm birth, intrauterine growth restriction and perinatal death for the neonate (Brown et al. 2000, Roberts et al. 2003, National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy 2000, Lowe et al. 2009). Maternal diet is one of the key risk factors for developing pregnancy hypertensive disorders and GDM and therefore may be a key preventative strategy on these and other pregnancy outcomes (King 2006). However, little is known about the effectiveness of dietary intervention during pregnancy (Roseboom et al. 2011).
The objective of this study was to synthesise the best of the available evidence by conducting a systematic review and meta‐analysis, to determine whether dietary interventions have any effect on pregnancy outcome. Dietary interventions could consist of dietary counselling, food and/or fortified food products, or a combination of both, administered either before or during pregnancy.
Key messages
Dietary counselling during pregnancy reduces maternal blood pressure, but not hypertensive disorders.
Dietary interventions focused on modifying macronutrient intakes during pregnancy reduces the incidence of preterm delivery.
Further research is needed to elucidate the role of maternal diet, particularly micronutrient provision and combination dietary interventions to optimise pregnancy outcomes.
Materials and methods
The review protocol was developed using the Cochrane Handbook for Systematic Reviews of Interventions (The Cochrane Collaboration 2011) and Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) for reporting the methods and outcomes (Liberati et al. 2009; Moher et al. 2009).
Eligibility criteria
Table 1 highlights the inclusion and exclusion criteria for the selection of publications. Any publication identified in the search reporting one or more pregnancy, neonatal or infant outcomes was considered. At least three randomised controlled trials (RCTs) needed to be identified for each outcome for inclusion in the results to allow for meta‐analysis. The outcome definitions were those used by the authors; however, where multiple definitions existed between publications, the term was used broadly. For example, preterm delivery included those classified as <36 weeks gestation (de Groot et al. 2004), <37 weeks (Mora et al. 1978; Rush et al. 1980; McDonald et al. 1981; Kafatos et al. 1989; Van Buul et al. 1997; Smuts et al. 2003b; Khoury et al. 2005; Bech et al. 2007; Aaltonen et al. 2008; Thornton et al. 2009; Vinter et al. 2011) and unspecified (Mardones‐Santander et al. 1988; Briley et al. 2002; Smuts et al. 2003a; Quinlivan et al. 2011). Nutrient deficiencies were excluded from this review as they were considered risk factors rather than clinical outcomes. This review focuses on dietary intake from food, rather than nutritional supplement use. Publications that targeted the whole diet, single food groups, individual food and fortified foods were included. Fortified foods included the provision of one or more foods that has essential vitamins and/or minerals added to enhance the foods nutritional content; for example docosahexaenoic acid (DHA) added to a cereal‐based bar to increase the omega 3 fatty acid content. Fortified foods modify both the macro‐ (carbohydrate, protein, fat) and micronutrient (vitamins and minerals) content of the maternal diet. Supplement‐only trials including the provision of a pill, capsule or tablet containing vitamins, minerals or amino acids were excluded. The types of dietary interventions included dietary counselling, modifying or providing food and fortified food products, or a combination of both.
Table 1.
Criteria for the selection of studies
| Inclusion | Exclusion |
|---|---|
| Studies that reported any pregnancy, neonatal or infant outcomes in preconception or pregnant women, any age, weight or body mass index, without date limits. | Health conditions that may influence dietary intake (i.e. unrepresentative sample such as gestational diabetes). |
| At least three randomised controlled trials per outcome. | Studies published in languages other than English. |
| Any healthy, human population. | Studies in animals. |
| Randomised or pseudo‐randomised controlled trials on dietary interventions, including counselling, food and/or both provided by any health professional. | Case study, editorial, conference proceeding. |
| Any intensity, frequency or timing of intervention. | Studies on gestational weight gain. |
| Positive or neutral methodological quality.* | Trials solely on nutrient supplementation (i.e. tablet form; no macronutrient change). |
| Multiple births. | |
| Duplicate populations (the data reporting the smaller number of participants for the same pregnancy outcome was excluded). | |
| Negative methodological quality.* |
*American Dietetic Association Quality Criteria Checklist for Primary Research (see Academy of Nutrition and Dietetics 2012).
Search strategy
A research librarian (DB) guided the systematic search for publications in December 2011. The search was conducted without date limits, using 10 electronic databases: EMBASE; Pre‐Medline; MEDLINE; Proquest; Web of Science; CINAHL; Scopus; The Cochrane Library; Mosby Index; and Maternity and Infant Care. The following MeSH terms, words and combinations of words were searched: pregnancy; diet OR food OR beverage OR nutrient OR macro‐nutrient OR micro‐nutrient; and randomised controlled trial OR randomized controlled trial. Keywords were searched as free text in the title, abstract, or topic and combined using the Boolean operator ‘AND’. Our MeSH terms were intentionally broad so as to capture as many dietary interventions and outcomes as possible. Preconception was defined as up to 3 months before pregnancy; however, we did not explicitly include ‘preconception’ as a search term in isolation from the term ‘pregnancy’. Limits included English language and humans. Additional publications were identified from the reference lists of included papers. All primary trials included in systematic reviews and meta‐analyses were independently assessed for eligibility. Outcomes were divided into two groups: pregnancy (this paper), and neonatal and infant (presented elsewhere).
Selection process
All records identified were first assessed for eligibility based on the information contained in the title and abstract, by two independent reviewers (EG and AH), as recommended by the PRISMA guidelines for systematic reviews of randomised trials (Liberati et al. 2009). When multiple publications were available for the same trial, the publication reporting the greatest number of participants for each outcome was selected. The full text of all publications that appeared to meet the eligibility screening (Table 1) was retrieved and subjected to a second assessment for relevance (EG and AH). Any discrepancy in assessment between reviewers was resolved through discussion. Selected full texts were then assessed for methodological quality by two independent reviewers (EG and SD) using the Quality Criteria Checklist for Primary Research in the American Dietetic Association Evidence Analysis Manual (Academy of Nutrition and Dietetics 2012). Any discrepancies were discussed. The Quality Criteria Checklist includes 10 structured validity questions that address scientific quality and soundness, including bias, confounding and the appropriateness of the interventions and measures. The Quality Criteria Checklist enabled a systematic and objective rating to be given to each publication. The highest methodological quality papers were designated ‘Positive’, meeting most of the validity criteria including all priority criteria (Academy of Nutrition and Dietetics 2012). ‘Neutral’ quality publications met most of the validity criteria, but failed one or more of the four priority criteria, indicating the study was not exceptionally strong (Academy of Nutrition and Dietetics 2012). ‘Negative’ publications failed to meet six or more of the validity criteria (Academy of Nutrition and Dietetics 2012) and were excluded from the results.
Data extraction
One reviewer (EG) extracted relevant data from all included publications, with a second independent reviewer (AH/JL) extracting data from approximately half to ensure accuracy. The following variables were data extracted: study design, aim, quality, participant characteristics, intervention type, dietary modification, assessment, compliance and outcome. For the meta‐analysis, one reviewer (EG) extracted the data from each publication into an Excel spread sheet, verified by a second reviewer (AB) for the following variables: author, year, outcome, type of intervention, trimester when the intervention commenced, nutrient of interest (macro‐ or micronutrient), body mass index (BMI; underweight/nutritional risk, overweight/obese, or no restriction), country income (high or low), quality rating (positive or neutral), participant numbers per group and by outcome, mean, standard deviation, 95% confidence interval (CI), median, range and odds ratio where possible for each outcome. Trials were divided into either macro‐ or micronutrient, depending on their focus. For example, an intervention using a high‐energy and high‐protein beverage would be classified ‘macronutrient’, while an intervention targeting calcium using either dairy or fortified orange juice would be classified ‘micronutrient’. BMI was defined according to the baseline nutritional status of the mother. The Organisation for Economic Co‐operation and Development (OECD) criteria (The World Bank Group 2012) was used to classify ‘high‐’ and ‘low‐’ income countries. Corresponding authors were emailed for additional data or clarification when needed. In trials reporting a range of gestational ages at study commencement, the most advanced week was used to calculate length of intervention.
Statistical analysis
The main measure of effect was the standardised mean difference (SMD). The SMD is determined by taking the difference in the mean outcome between the intervention and control group in one publication, and dividing it by the pooled standard deviation for the outcome across the whole trial. The SMD accounts for differences in variance between studies by allowing the size of the intervention effect in each publication to be expressed relative to the variability observed within that study. A secondary measure of effect, the raw mean difference (RMD), presented in the common units for each outcome [e.g. weeks gestation, mmHg for blood pressure (BP) ] was also included. Trials containing more than one intervention (Rush et al. 1980; Ross et al. 1985; Chan et al. 2006), or control group (Smuts et al. 2003a) had their effects averaged, as there were no significant differences on pregnancy outcomes (Lipsey & Wilson 2001). Dichotomised outcomes had the log‐odds and standard errors converted to SMD via the method created by Hasselblad and Hedges (1995; Chinn 2000). Means and variance were approximated for trials reporting medians using the method reported by Hozo et al. (2005). Trials reporting outcomes with zero counts for both intervention and control groups were excluded from the analysis. For outcomes reporting zero counts for one intervention group only, the Firth penalized likelihood method was used to approximate the odds ratio (Firth 1993; Heinze & Schemper 2002) and 95% CIs.
Meta‐analyses were performed for each outcome and dietary intervention type using a random effects model with random weights applied for each study. Subgroup analyses were performed for each outcome by dietary intervention, trimester when the intervention commenced, nutrient of interest, BMI and country income. The I 2 statistic was applied to describe the total variation in study estimates because of heterogeneity. Funnel plots were used as a visual tool for investigating the presence of potential bias and Egger's test used to test for funnel plot asymmetry (Egger et al. 1997). Statistical analyses were performed using the metan command in the statistical software package Intercooled Stata, version 12 (Stata, College Station, Texas, USA) (StataCorp 2011). P‐values ≤0.01 were considered statistically significant to adjust for the multiple comparisons that were made.
Results
Description of studies
The trial selection process is summarised in Fig. 1. Of the 2326 papers screened, data were extracted from 28 publications, which included 8322 participants. The earliest published study was in 1978 (Mora et al. 1978) and the latest in 2011 (Luoto et al. 2011; Courville et al. 2011; Quinlivan et al. 2011; Vinter et al. 2011). Twenty‐four publications were performed in high‐income OECD countries: 10 in the United States, 3 each in Finland, Denmark and the Netherlands, and 1 each in Australia, Norway, Italy, Greece and Chile (Supporting Information http://onlinelibrary.wiley.com/doi/10.1111/mcn.12142/suppinfo).
Figure 1.
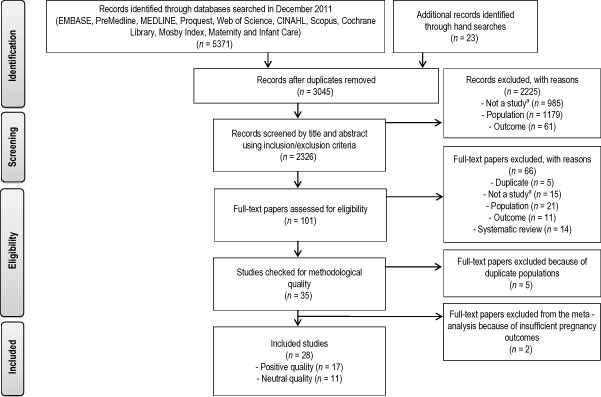
Flow diagram for the selection of included studies.
aNot a study means not a study design of interest. It includes observational trials, editorials and conference papers.
The methodological quality and characteristics of included publications are shown in Supporting Information http://onlinelibrary.wiley.com/doi/10.1111/mcn.12142/suppinfo. All studies were of positive or neutral quality according to the American Dietetic Association Quality Criteria Checklist (Academy of Nutrition and Dietetics 2012). Fourteen publications compared dietary counselling with standard antenatal care (no intervention) (Kafatos et al. 1989; Van Buul et al. 1997; Knuist et al. 1998; Briley et al. 2002; Bonomo et al. 2005; Khoury et al. 2005; Chan et al. 2006; O'Connor & Whaley 2007; Wolff et al. 2008; Asbee et al. 2009; Thornton et al. 2009; Luoto et al. 2010; Quinlivan et al. 2011; Vinter et al. 2011). Twelve publications evaluated the effect of specific food and fortified food products (Mora et al. 1978, 1979; Rush et al. 1980; McDonald et al. 1981; Metcoff et al. 1985; Ross et al. 1985; Mardones‐Santander et al. 1988; Smuts et al. 2003a, 2003b; de Groot et al. 2004; Bech et al. 2007; Courville et al. 2011), two of which came from the Columbian Longitudinal Study of Malnutrition and Intellectual Development (Mora et al. 1978, 1979). Two publications from the Finnish Mother–Infant Nutrition and Probiotic Intervention assessed the effect of combined dietary counselling, and food and fortified food products (Aaltonen et al. 2008; Luoto et al. 2010).
All but one publication (McDonald et al. 1981) studied the effect of dietary intervention during pregnancy on pregnancy outcomes. McDonald et al. (1981) included pre‐pregnancy (periconception) dietary intervention. Twenty (of 28) publications, included two groups: dietary intake (intervention) vs. usual care or dietary intake (control). Dietary intervention during the second and third trimester was the most frequently reported period of intervention (22 publications). Dietary intervention ranged in duration from 10 (Rush et al. 1980) to greater than 40 weeks (pre‐pregnancy intervention) (McDonald et al. 1981). Dietitians or nutritionists were the most frequent dietary intervention providers (12 publications) and macronutrients (24 publications) were more commonly targeted in the intervention than micronutrients (4 publications). Fourteen publications did not report any dietary data (Metcoff et al. 1985; Ross et al. 1985; Van Buul et al. 1997; Knuist et al. 1998; Smuts et al. 2003a, 2003b; Bonomo et al. 2005; Bech et al. 2007; O'Connor & Whaley 2007; Asbee et al. 2009; Thornton et al. 2009; Courville et al. 2011; Quinlivan et al. 2011; Vinter et al. 2011), with 12 of these not conducting nutritional assessments during the intervention period (Metcoff et al. 1985; Ross et al. 1985; Van Buul et al. 1997; Knuist et al. 1998; Smuts et al. 2003a, 2003b; Bonomo et al. 2005; Bech et al. 2007; O'Connor & Whaley 2007; Asbee et al. 2009; Thornton et al. 2009; Vinter et al. 2011). Six trials (7 publications) recruited women who were underweight or nutritionally at risk (Mora et al. 1978, 1979; Rush et al. 1980; McDonald et al. 1981; Metcoff et al. 1985; Ross et al. 1985; Mardones‐Santander et al. 1988), while four trials recruited overweight or obese women as their target population (Wolff et al. 2008; Thornton et al. 2009; Quinlivan et al. 2011; Vinter et al. 2011). The pregnancy outcomes included: hypertensive disorders (pregnancy‐induced hypertension and preeclampsia), BP, GDM, caesarean section, length of gestation, preterm and post‐term delivery.
Effects of dietary intervention
Table 2 shows the effect of dietary intervention components and all dietary intervention trials on meta‐analysed pregnancy outcomes. There was one trial (2 publications) in our review on the combination (counselling + food) dietary intervention (Aaltonen et al. 2008; Luoto et al. 2010). Aaltonen et al. (2008) analysed the effect of combination dietary intervention on the pregnancy outcomes preeclampsia, GDM, BP, length of gestation, preterm and post‐term (Aaltonen et al. 2008), while Luoto et al. (2010) analysed combination dietary intervention and caesarean section only (Luoto et al. 2010). However, because of insufficient data for pooling, this trial was meta‐analysed as part of all dietary interventions only.
Table 2.
Effect of dietary interventions during pregnancy on pregnancy outcomes*
| Number of trials (references) | Number of participants | Intervention Number or Mean ± SD | Control Number or Mean ± SD | SMD † (95% CI) | RMD ‡ | P‐value | I 2 (%) | ||
|---|---|---|---|---|---|---|---|---|---|
| Int | Con | ||||||||
| Dietary counselling | |||||||||
| Hypertensive disorders | 7 (Van Buul et al. 1997; Khoury et al. 2005; Wolff et al. 2008; Asbee et al. 2009; Thornton et al. 2009; Luoto et al. 2011; Vinter et al. 2011) | 811 | 791 | 77 | 96 | −0.12 (−0.30 to 0.06) | 0.85 | 0.20 | 0 |
| Systolic BP (mmHg) | 3 (Chan et al. 2006; Wolff et al. 2008; Vinter et al. 2011) | 225 | 207 | 115.65 ± 5.80 | 117.81 ± 7.61 | −0.26 (−0.45 to −0.07) | −0.66 | <0.001 | 0 |
| Diastolic BP (mmHg) | 3 (Chan et al. 2006; Wolff et al. 2008; Vinter et al. 2011) | 225 | 207 | 71.81 ± 4.31 | 75.02 ± 6.50 | −0.57 (−0.75 to −0.38) | −2.76 | <0.001 | 0 |
| Gestational diabetes | 6 (Wolff et al. 2008; Asbee et al. 2009; Thornton et al. 2009; Luoto et al. 2011; Quinlivan et al. 2011; Vinter et al. 2011) | 624 | 582 | 191 | 172 | −0.27 (−0.72 to 0.17) | 0.70 | 0.23 | 69 |
| Length of gestation (weeks) | 12 (Kafatos et al. 1989; Van Buul et al. 1997; Knuist et al. 1998; Bonomo et al. 2005; Khoury et al. 2005; Chan et al. 2006; O'Connor & Whaley 2007; Wolff et al. 2008; Asbee et al. 2009; Thornton et al. 2009; Luoto et al. 2011; Vinter et al. 2011) | 1577 | 1526 | 39.60 ± 1.40 | 39.49 ± 1.59 | 0.06 (−0.05 to 0.16) | 0.08 | 0.29 | 49 |
| Preterm delivery | 7 (Kafatos et al. 1989; Van Buul et al. 1997; Briley et al. 2002; Khoury et al. 2005; Thornton et al. 2009; Quinlivan et al. 2011; Vinter et al. 2011) | 884 | 875 | 33 | 56 | −0.25 (−0.56 to 0.05) | 0.67 | 0.10 | 19 |
| Post‐term delivery | 1 (Thornton et al. 2009) | 116 | 116 | 15 | 16 | −0.04 (−0.46 to 0.38) | 0.95 | 0.85 | NA |
| Caesarean section | 6 (Knuist et al. 1998; Bonomo et al. 2005; Wolff et al. 2008; Asbee et al. 2009; Thornton et al. 2009; Vinter et al. 2011) | 680 | 667 | 206 | 206 | −0.02 (−0.17 to 0.14) | 0.99 | 0.85 | 12 |
| Food and food products | |||||||||
| Hypertensive disorders | 2 (Smuts et al. 2003a, 2003b) | 160 | 184 | 6 | 10 | −0.29 (−0.86 to 0.29) | 1.04 | 0.33 | 0 |
| Gestational diabetes | 3 (Smuts et al. 2003a, 2003b; de Groot et al. 2004) | 200 | 223 | 4 | 7 | 0.20 (−0.50 to 0.89) | 0.86 | 0.58 | 0 |
| Length of gestation (weeks) | 7 (Ross et al. 1985; Mardones‐Santander et al. 1988; Smuts et al. 2003a, 2003b; de Groot et al. 2004; Bech et al. 2007; Courville et al. 2011) | 1399 § | 895 § | 39.68 ± 1.09 | 39.65 ± 1.37 | 0.09 (−0.13 to 0.25) | 0.25 | 0.27 | 48 |
| Preterm delivery | 7 (Mora et al. 1978; Rush et al. 1980; McDonald et al. 1981; Smuts et al. 2003a, 2003b; de Groot et al. 2004; Bech et al. 2007) | 1620 | 1349 | 170 | 145 | −0.10 (−0.23 to 0.04) | 0.88 | 0.16 | 0 |
| Post‐term delivery | 2 (Mora et al. 1978; McDonald et al. 1981) | 315 | 310 | 18 | 26 | −0.16 (−0.68 to 0.36) | 0.76 | 0.54 | 43 |
| Caesarean section | 3 (Smuts et al. 2003a, 2003b; de Groot et al. 2004) | 189 | 213 | 23 | 35 | −0.15 (−0.48 to 0.17) | 0.82 | 0.35 | 0 |
| Combination | |||||||||
| Hypertensive disorders | 1 (Aaltonen et al. 2008) | 86 | 85 | 5 | 6 | −0.11 (−0.79 to 0.56) | 0.83 | 0.74 | NA |
| Systolic BP (mmHg) | 1 (Aaltonen et al. 2008) | 86 | 85 | 116 ± 14.10 | 112 ± 11 | 0.32 (0.01 to 0.62) | 4.00 | 0.04 | NA |
| Diastolic BP (mmHg) | 1 (Aaltonen et al. 2008) | 86 | 85 | 70 ± 9.60 | 69.00 ± 9.20 | 0.11 (−0.20 to 0.41) | 1.00 | 0.49 | NA |
| Gestational diabetes | 1 (Aaltonen et al. 2008) | 86 | 85 | 8 | 6 | 0.17 (−0.44 to 0.77) | 1.29 | 0.59 | NA |
| Length of gestation (weeks) | 1 (Aaltonen et al. 2008) | 86 | 85 | 38.25 ± 1.98 | 39.69 ± 1.4 | −0.55 (−0.84 to −0.26) | −1.43 | <0.001 | NA |
| Preterm delivery | 1 (Aaltonen et al. 2008) | 86 | 85 | 4 | 1 | 0.78 (−0.44 to 2.00) | 3.82 | 0.21 | NA |
| Post‐term delivery | 1 (Aaltonen et al. 2008) | 86 | 85 | 0 | 1 | −0.62 (−2.40 to 1.16) | 0.33 | 0.50 | NA |
| Caesarean section | 1 (Luoto et al. 2010) | 77 | 76 | 12 | 11 | 0.05 (−0.44 to 0.54) | 1.07 | 0.85 | NA |
| All dietary interventions | |||||||||
| Hypertensive disorders | 10 | 1057 | 1060 | 88 | 112 | −0.13 (−0.30 to 0.04) | 0.84 | 0.12 | 0 |
| Systolic BP (mmHg) | 4 | 311 | 292 | 115.74 ± 7.87 | 116.35 ± 8.46 | −0.12 (−0.45 to 0.21) | −0.35 | 0.48 | 71 |
| Diastolic BP (mmHg) | 4 | 311 | 292 | 71.36 ± 5.63 | 73.51 ± 7.18 | −0.40 (−0.79 to −0.01) | −0.45 | 0.04 | 80 |
| Gestational diabetes | 10 | 910 | 890 | 203 | 185 | −0.12 (−0.44 to 0.20) | 0.82 | 0.47 | 52 |
| Length of gestation (weeks) | 20 | 3062 | 2506 | 39.60 ± 1.31 | 39.55 ± 1.51 | 0.04 (−0.06 to 0.14) | 0.10 | 0.42 | 61 |
| Preterm delivery | 15 | 2590 | 2309 | 207 | 202 | −0.13 (−0.27 to 0.02) | 0.84 | 0.09 | 15 |
| Post‐term delivery | 4 | 517 | 511 | 33 | 43 | −0.16 (−0.42 to 0.11) | 0.79 | 0.24 | 0 |
| Caesarean section | 10 | 946 | 956 | 241 | 252 | −0.03 (−0.16 to 0.10) | 0.98 | 0.65 | 0 |
BP, blood pressure; CI, confidence interval; Con, control; Int, intervention; NA, not applicable; RMD, raw mean difference; SD, standard deviation; SMD, standardised mean difference.
*The main measure of effect was SMD. The SMD was determined by taking the difference in the mean of an outcome between the intervention and control group in one publication, and dividing it by the pooled standard deviation for the outcome across the whole trial. †The trials by Metcoff et al. (1985) and Mora et al. (1979) were not included in the meta‐analysed outcome: length of gestation, as authors did not present results. ‡The secondary measure of effect was RMD. Categorical outcomes are reported as odds ratios. §The total number of participants (Int + Con) was 3307. The trial by Metcoff et al. (1985) provided withdrawals for the trial (n = 61); however, it did not specify participant withdrawals per Int and Con group. Because authors did not provide this data, all enrolled participants were included in the number of participants (n = 3368).
Pregnancy hypertensive disorders
Seven RCTs studied the effect of dietary counselling on pregnancy hypertensive disorders (n = 1602 women) (Van Buul et al. 1997; Khoury et al. 2005; Wolff et al. 2008; Asbee et al. 2009; Thornton et al. 2009; Luoto et al. 2010; Vinter et al. 2011), with two trials reporting more than one hypertensive outcome (Wolff et al. 2008; Thornton et al. 2009). Trials examining the effect of dietary counselling on pregnancy hypertensive disorders included: three trials on pregnancy‐induced hypertension (Van Buul et al. 1997; Wolff et al. 2008; Thornton et al. 2009), four trials on preeclampsia/eclampsia (Wolff et al. 2008; Asbee et al. 2009; Thornton et al. 2009; Luoto et al. 2010), and two trials combining pregnancy‐induced hypertension and preeclampsia (Khoury et al. 2005; Vinter et al. 2011). Two RCTs studied the effect of food and fortified food products on pregnancy hypertensive disorders (n = 344 women) (Smuts et al. 2003a, 2003b). Meta‐analysis demonstrated no effect of dietary intervention components or all dietary interventions combined on pregnancy hypertensive disorders, with no evidence of heterogeneity or bias.
Maternal BP
Three RCTs studied the effect of dietary counselling on maternal BP (systolic and diastolic) (n = 432 women) (Chan et al. 2006; Wolff et al. 2008; Vinter et al. 2011). Meta‐analysis demonstrated significant effects for a reduction in both systolic (SMD −0.26, 95% CI −0.45 to −0.07; P < 0.001; I 2 = 0%) (Fig. 2) and diastolic BP (SMD −0.57, 95% CI −0.75 to −0.38; P < 0.001; I 2 = 0%) for dietary counselling (Fig. 3). Using RMD, this roughly translates to a mean change in systolic and diastolic BP of −0.66 mmHg and −2.76 mmHg, respectively, although, this could vary by population. Meta‐analysis demonstrated an effect for all dietary interventions combined on diastolic BP, with very high heterogeneity (I 2 = 80%), and no evidence of bias. The four trials contributing to this analysis all commenced in the second trimester. Therefore, any effect from earlier or later intervention during pregnancy could not be determined.
Figure 2.
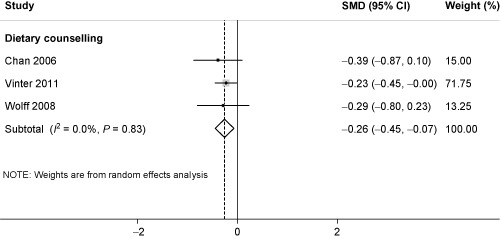
Standardised mean difference (SMD) for systolic blood pressure and dietary counselling in pregnancy. The overall effect size was estimated by SMD. The black dot represents the point estimate, and square size the weight of each study in the meta‐analysis and the horizontal lines represent the 95% confidence interval (CI). The vertical solid line represents the line of no effect. The diamond represents the overall pooled estimate effect of dietary counselling on systolic blood pressure.
Figure 3.
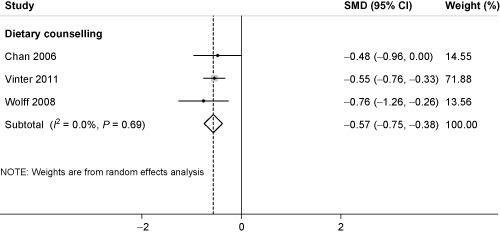
Standardised mean difference (SMD) for diastolic blood pressure and dietary counselling in pregnancy. The overall effect size was estimated by SMD. The black dot represents the point estimate, and square size the weight of each study in the meta‐analysis and the horizontal lines represent the 95% confidence interval (CI). The vertical solid line represents the line of no effect. The diamond represents the overall pooled estimate effect of dietary counselling on diastolic blood pressure.
GDM
Six RCTs studied the effect of dietary counselling on GDM (n = 1206 women) (Wolff et al. 2008; Asbee et al. 2009; Thornton et al. 2009; Luoto et al. 2010; Quinlivan et al. 2011; Vinter et al. 2011). Three RCTs studied the effect of food and fortified food products on GDM (n = 423 women) (Smuts et al. 2003a, 2003b; de Groot et al. 2004). Meta‐analysis demonstrated no effect of dietary intervention components or all dietary interventions combined on GDM. The data showed heterogeneity (I 2 = 52%), with no presence of bias.
Caesarean section
Six RCTs studied the effect of dietary counselling on caesarean section (n = 1347 women) (Knuist et al. 1998; Bonomo et al. 2005; Wolff et al. 2008; Asbee et al. 2009; Thornton et al. 2009; Vinter et al. 2011). Three RCTs studied the effect of food and fortified food products on caesarean section (n = 402 women) (Smuts et al. 2003a, 2003b; de Groot et al. 2004). Meta‐analysis demonstrated no effect of dietary intervention components or all dietary interventions combined on caesarean section, with no evidence of heterogeneity or bias.
Length of gestation
Twelve RCTs studied the effect of dietary counselling on length of gestation (n = 3103 women) (Kafatos et al. 1989; Van Buul et al. 1997; Knuist et al. 1998; Bonomo et al. 2005; Khoury et al. 2005; Chan et al. 2006; O'Connor & Whaley 2007; Wolff et al. 2008; Asbee et al. 2009; Thornton et al. 2009; Luoto et al. 2010; Vinter et al. 2011). Nine RCTs studied the effect of food and fortified food products on length of gestation (n = 3307 women) (Mora et al. 1979; Metcoff et al. 1985; Ross et al. 1985; Mardones‐Santander et al. 1988; Smuts et al. 2003a, 2003b; de Groot et al. 2004; Bech et al. 2007; Courville et al. 2011), with seven of these trials meta‐analysed (n = 2294). There were no effects of dietary intervention components or all dietary interventions combined on length of gestation. The data showed heterogeneity (I 2 = 61%), with no evidence of bias.
Fourteen RCTs (n = 4728) studied the effect of dietary intervention on preterm delivery (Mora et al. 1978; Rush et al. 1980; McDonald et al. 1981; Kafatos et al. 1989; Van Buul et al. 1997; Briley et al. 2002; Smuts et al. 2003a, 2003b; de Groot et al. 2004; Khoury et al. 2005; Bech et al. 2007; Thornton et al. 2009; Quinlivan et al. 2011; Vinter et al. 2011). Seven RCTs studied the effect of dietary counselling on preterm delivery (n = 1759 women) (Kafatos et al. 1989; Van Buul et al. 1997; Briley et al. 2002; Khoury et al. 2005; Thornton et al. 2009; Quinlivan et al. 2011; Vinter et al. 2011): the other seven trials provided food and fortified food products (n = 2969) (Mora et al. 1978; Rush et al. 1980; McDonald et al. 1981; Smuts et al. 2003a, 2003b; de Groot et al. 2004; Bech et al. 2007). Meta‐analysis demonstrated no effect of dietary intervention components or all dietary interventions combined, with no evidence of heterogeneity or bias.
One RCT studied the effect of dietary counselling on post‐term delivery (n = 232 women) (Thornton et al. 2009). Two RCTs studied the effect of food and fortified food products on post‐term delivery (n = 625 women) (Mora et al. 1978; McDonald et al. 1981). Meta‐analysis found no effect of dietary intervention components or all dietary interventions combined on post‐term delivery, with no evidence of heterogeneity or bias.
Sub‐analysis: trimester when the intervention commenced, nutrient of interest, BMI and country income
There were very few effects of diet on pregnancy outcome by trimester when the intervention commenced, nutrient of interest, BMI and country income subgroupings (Table 3). This was largely due to the small number of outcomes where three or more trials contributed data for each group. The trials for trimester when the intervention commenced on diastolic BP, and high‐income country on hypertensive disorders, systolic and diastolic BP, GDM and caesarean section did not differ from the results presented in Table 2, therefore have not been repeated.
Table 3.
Subgroup analyses for pregnancy outcomes in evaluation of dietary intervention during pregnancy*
| Number of trials | SMD (95% CI) | RMD † | P‐value | |
|---|---|---|---|---|
| Hypertensive disorders | ||||
| Trimester when the intervention commenced | ||||
| One | 1 | −0.16 (−1.70 to 1.39) | 0.76 | 0.84 |
| Two | 9 | −0.13 (−0.30 to 0.04) | 0.84 | 0.13 |
| Nutrient of interest | ||||
| Macronutrients | 9 | −0.16 (−0.34 to 0.02) | 0.80 | 0.08 |
| Micronutrient ‡ | 1 | 0.04 (−0.37 to 0.45) | 1.05 | 0.86 |
| BMI | ||||
| Overweight and obese | 3 | −0.30 (−0.63 to 0.02) | 0.69 | 0.07 |
| All weight categories § | 7 | −0.03 (−0.25 to 0.18) | 0.96 | 0.76 |
| Systolic BP (mmHg) | ||||
| Nutrient of interest | ||||
| Macronutrients | 3 | −0.05 (−0.45 to 0.35) | −0.28 | 0.81 |
| Micronutrient § | 1 | −0.39 (−0.87 to 0.10) | −3.51 | 0.12 |
| BMI | ||||
| Overweight and obese | 2 | −0.24 (−0.44 to −0.03) | −0.51 | 0.02 |
| All weight categories | 2 | −0.01 (−0.07 to 0.68) | −0.43 | 0.98 |
| Diastolic BP (mmHg) | ||||
| Nutrient of interest | ||||
| Macronutrients | 3 | −0.38 (−0.88 to 0.12) | −1.43 | 0.13 |
| Micronutrient ¶ | 1 | −0.48 (−0.96 to 0.00) | −3.50 | 0.05 |
| BMI | ||||
| Overweight and obese | 2 | −0.58 (−0.78 to −0.38) | −2.69 | <0.001 |
| All weight categories | 2 | −0.15 (−0.72 to 0.41) | −0.92 | 0.60 |
| Gestational diabetes | ||||
| Trimester when the intervention commenced | ||||
| One | 1 | −0.77 (−2.57 to 1.02) | 0.26 | 0.40 |
| Two | 9 | −0.10 (−0.43 to 0.23) | 0.83 | 0.55 |
| Nutrient of interest | ||||
| Macronutrients | 10 | −0.12 (−0.44 to 0.20) | 0.82 | 0.47 |
| BMI | ||||
| Overweight and obese | 4 | −0.42 (−0.91 to 0.06) | 0.57 | 0.09 |
| All weight categories | 6 | 0.22 (−0.00 to 0.43) | 1.11 | 0.05 |
| Length of gestation (weeks)** | ||||
| Trimester when the intervention commenced | ||||
| One | 2 | 0.11 (−1.31 to 1.53) | −0.06 | 0.88 |
| Two | 17 | 0.06 (−0.05 to 0.17) | 0.14 | 0.30 |
| Three | 1 | −1.44 (−1.64 to −1.23) | −0.20 | <0.001 |
| Nutrient of interest | ||||
| Macronutrients | 15 | 0.01 (−0.10 to 0.13) | 0.03 | 0.83 |
| Micronutrients | 5 | 0.11 (−0.08 to 0.31) | 0.31 | 0.27 |
| BMI | ||||
| Underweight/nutritional risk | 2 | 0.00 (‒0.21 to 0.21) | 0.00 | 1.00 |
| Overweight and obese | 3 | 0.02 (−0.14 to 0.18) | 0.00 | 0.83 |
| All weight categories | 15 | 0.05 (−0.07 to 0.18) | 0.13 | 0.41 |
| Country income | ||||
| Low | 2 | 0.00 (−0.21 to 0.21) | 0.00 | 1.00 |
| High | 18 | 0.05 (−0.06 to 0.15) | 0.11 | 0.40 |
| Preterm delivery | ||||
| Trimester when the intervention commenced | ||||
| One | 1 | −0.44 (−1.22 to 0.34) | 0.49 | 0.27 |
| Two | 13 | −0.13 (−0.30 to 0.05) | 0.84 | 0.17 |
| Three | 1 | −0.08 (−0.41 to 0.26) | 0.89 | 0.66 |
| Nutrient of interest | ||||
| Macronutrients | 13 | −0.19 (−0.34 to −0.04) | 0.79 | 0.01 |
| Micronutrients | 2 | 0.10 (−0.16 to 0.37) | 1.18 | 0.44 |
| BMI | ||||
| Underweight/nutritional risk | 3 | −0.15 (−0.32 to 0.01) | 0.83 | 0.07 |
| Overweight and obese | 3 | −0.04 (−0.48 to 0.41) | 0.94 | 0.88 |
| All weight categories | 9 | −0.16 (−0.46 to 0.14) | 0.80 | 0.32 |
| Country income | ||||
| Low | 2 | −0.13 (−0.44 to 0.17) | 0.83 | 0.40 |
| High | 13 | −0.13 (−0.30 to 0.05) | 0.82 | 0.17 |
| Post‐term delivery | ||||
| Trimester when the intervention commenced | ||||
| One | 1 | 0.20 (−0.52 to 0.91) | 1.38 | 0.59 |
| Two | 2 | −0.07 (−0.48 to 0.34) | 0.91 | 0.73 |
| Three | 1 | −0.36 (−0.76 to 0.04) | 0.57 | 0.08 |
| Nutrient of interest | ||||
| Macronutrients | 4 | −0.16 (−0.42 to 0.11) | 0.79 | 0.24 |
| BMI | ||||
| Underweight/nutritional risk | 2 | −0.16 (−0.68 to 0.36) | 0.76 | 0.54 |
| Overweight and obese | 1 | −0.04 (−0.46 to 0.38) | 0.95 | 0.85 |
| All weight categories | 1 | −0.62 (−2.40 to 1.16) | 0.33 | 0.50 |
| Country income | ||||
| Low | 2 | −0.16 (−0.68 to 0.36) | 0.76 | 0.54 |
| High | 2 | −0.07 (−0.48 to 0.34) | 0.91 | 0.73 |
| Caesarean section | ||||
| Trimester when the intervention commenced | ||||
| One | 1 | −0.48 (−1.03 to 0.08) | 0.56 | 0.09 |
| Two | 8 | −0.02 (−0.16 to 0.13) | 0.99 | 0.84 |
| Three | 1 | 0.04 (−0.24 to 0.31) | 1.04 | 0.80 |
| Nutrient of interest | ||||
| Macronutrients | 9 | −0.00 (−0.14 to 0.13) | 1.00 | 0.96 |
| Micronutrients | 1 | −0.18 (−0.52 to 0.15) | 0.77 | 0.28 |
| BMI | ||||
| Overweight and obese | 3 | 0.10 (−0.11 to 0.31) | 1.05 | 0.36 |
| All weight categories | 7 | −0.10 (−0.26 to 0.06) | 0.89 | 0.41 |
BMI, body mass index; BP, blood pressure; CI, confidence interval; RMD, raw mean difference; SMD, standardised mean difference. *The main measure of effect was SMD. Meta‐analysis focused on outcomes with three or more trials contributing data to pooled results. There were no studies for subgroup analyses for trimester commencement: one on systolic BP and diastolic BP, and three on hypertensive disorders, systolic BP, diastolic BP and gestational diabetes; or nutrient of interest: micronutrients on gestational diabetes and post‐term delivery; BMI: underweight/nutritional risk on hypertensive disorders, systolic BP, diastolic BP and caesarean section; or country income: low on hypertensive disorders, systolic BP, diastolic BP, gestational diabetes and caesarean section. †The secondary measure of effect was RMD. Categorical outcomes are reported as odds ratios. ‡Micronutrient of interest is sodium. §All weight categories included those trials not restricting BMI within the target population. ¶Micronutrient of interest is calcium. **The trials by Metcoff et al. (1985) and Mora et al. (1979) were not included in the meta‐analysed outcome: length of gestation, as authors did not present results.
Trials altering the macronutrient composition of dietary interventions demonstrated a reduction in the incidence of preterm delivery (P = 0.01). Using RMD, this translates to a 21% reduction (0.79 odds ratio) in the incidence of preterm birth.
Discussion
Summary of main findings
To develop appropriate dietary guidelines for pregnancy we need to understand the effects of diet and dietary modification on a range of pregnancy outcomes. This systematic review summarises the best available evidence of dietary intervention during pregnancy on pregnancy outcomes. Results indicate that dietary interventions during pregnancy, particularly dietary counselling, slightly reduce BP (0.66 mmHg systolic and 2.76 mmHg diastolic), but not hypertensive disorders. Dietary interventions focusing on macronutrient intake reduce the incidence of preterm delivery (21% decrease in the odds), while interventions commencing in the second trimester reduce diastolic BP (0.45 mmHg). No other significant effects were observed for the other pregnancy outcomes.
Interpretation
The effect on BP was not consistent across trial populations. There were variations in the types of dietary intervention and the effect of specific nutrient components. There was no heterogeneity for dietary intervention components, but considerate heterogeneity for all dietary interventions combined. Dietary counselling interventions shown to lower BP varied in frequency from one to 10 sessions. Target populations ranged from adolescent girls (Chan et al. 2006) to obese women (Wolff et al. 2008; Vinter et al. 2011), limiting the generalisability of the findings to a broader group of women of child‐bearing age. Those interventions shown to be effective in reducing BP included a balanced diet complying with National recommendations (Vinter et al. 2011), energy intake individualised for the needs of the mother (Wolff et al. 2008) and modifying calcium intake (Chan et al. 2006). Identifying specific components of successful interventions can assist in understanding how the intervention exerts its effect (Michie et al. 2011). Calcium has been studied in large supplemental trials (Palacios & Pena‐Rosas 2011), demonstrating a reduction in the risk of hypertensive disorders during pregnancy, particularly for women at high‐risk or with low calcium intakes (Hofmeyr et al. 2010; Palacios & Pena‐Rosas 2011; Imdad & Bhutta 2012). Our review identified no effect of dietary intervention on hypertensive disorders. However, the pooled trials involved whole diet recommendations, or the modification of fat intake, and there were no trials on hypertensive disorders specifically targeting calcium intake.
Like the results in our review, calcium intake has been shown to lower BP among pregnant women (Carroli et al. 1994; Van Mierlo et al. 2006; Hofmeyr et al. 2010; Palacios & Pena‐Rosas 2011), and the effects appear stronger in women with low calcium intakes prior to intervention (Carroli et al. 1994; Van Mierlo et al. 2006; Hofmeyr et al. 2010; Palacios & Pena‐Rosas 2011). Calcium intakes were not analysed for each included study as part of this review. The effect of low calcium during pregnancy is thought to exert its effect via an increase in parathyroid hormone secretion, which increases intracellular calcium, smooth muscle contractibility and/or releases renin from the kidney, leading to vasoconstriction and retention of sodium (Hacker et al. 2012). These physiological changes (from low calcium intakes during pregnancy) increase BP and potentially contribute to the development of hypertensive disorders (Hacker et al. 2012). Women beginning pregnancy with adequate intakes of at least 1000 mg calcium per day may not need additional amounts, while those with suboptimal intakes (<500 mg per day) may benefit from intervention (Hacker et al. 2012).
Our review also found that women consuming a balanced diet, including enough energy based on their individual requirements, had lower BP, paralleling current lifestyle recommendations for individuals with high BP (National Institutes of Health 2003). The effect size of 1–3 mmHg was very small compared with using antihypertensive agents (Patel et al. 2012). However, the effect was evident in normotensive rather than hypertensive women. Furthermore, treatment with antihypertensive drugs during pregnancy carries known and unknown risks to the fetus, because these drugs cross the placenta (e.g. nifedapine is Category C) (Department of Health, 2014). Therefore, any reductions in BP gained from dietary intervention offers clear advantages when BP is of clinical concern.
Macronutrient interventions demonstrating a reduction in the incidence of preterm delivery varied in frequency and included supplemental beverages (Rush et al. 1980; McDonald et al. 1981), dietary counselling on the nutritional needs during pregnancy (Kafatos et al. 1989; Briley et al. 2002; Quinlivan et al. 2011), limiting cholesterol and reducing saturated fat (Khoury et al. 2005), GDM‐specific dietary recommendations (Thornton et al. 2009), providing DHA‐fortified eggs (Smuts et al. 2003a, 2003b), and a range of energy‐ and protein‐based foods (Mora et al. 1978). Observational studies (Kramer 1987; Institute of Medicine 1990; Rush 2001) have reported that energy intake may be strongly and positively associated with a reduced risk of preterm birth. Our review confirms these findings, demonstrating a reduction in the incidence of preterm birth with macronutrient or whole diet intervention.
The pooled studies on preterm delivery showed little evidence of heterogeneity, with a narrow spread of data and overlapping CIs. Six of the macronutrient dietary interventions were conducted in low‐income populations (Mora et al. 1978; Rush et al. 1980; McDonald et al. 1981; Kafatos et al. 1989; Briley et al. 2002; Quinlivan et al. 2011). Four interventions contained small sample sizes of less than 125 pregnant women (Briley et al. 2002; Smuts et al. 2003a; de Groot et al. 2004; Quinlivan et al. 2011) meaning these trials were individually underpowered. Of the 13 RCTs contributing data, only two trials (Kafatos et al. 1989; Khoury et al. 2005) demonstrated statistically significant effects in their respective publications. Based on the pooled dietary interventions, there were particular nutrients impacting on the result rather than the type of intervention. Modifying fat intake, particularly long‐chain polyunsaturated fatty acids (LC‐PUFA) were shown to reduce the incidence of preterm delivery (Smuts et al. 2003a, 2003b; Khoury et al. 2005). Paralleling these findings, Horvath et al. (2007), Szajewska et al. (2006) and a recent Cochrane Review (Makrides et al. 2012) concluded that women allocated to LC‐PUFA supplementation had longer gestation than women receiving placebo or no treatment, which remained true for both low‐ and high‐risk pregnancies. Observational studies, mainly in populations with high consumption of seafood, have also suggested that marine LC‐PUFA intake during pregnancy promotes longer gestation (Olsen et al. 1986, 1990, 1993). DHA and arachidonic acid are essential nutrients that are supplied during pregnancy to the fetus by preferential placental transfer (Al et al. 1995; Otto et al. 1997; Berghaus et al. 2000; Larquè et al. 2003). The mechanism behind their role in increasing gestational length may be an imbalance between DHA and arachidonic acid, which is associated with disturbances in the production of prostacyclin and thromboxane involved in the initiation of labour (Herrera 2002). Therefore, adequate dietary intake or supplementation of LC‐PUFA may prolong gestational length, and in turn, decrease the risk of preterm delivery (Horvath et al. 2007).
The conversion of each publication's results to SMD for statistical inference effectively creates a common scale that would otherwise not be possible because of the differences in variance between publications. The SMD express differences as units of standard deviations, and thus cannot be used to interpret absolute or relative differences in clinically meaningful outcomes (e.g. for BP, the absolute reduction in mmHg). Therefore, we have also provided the RMD to give some indication of effect size in the standard units for each outcome, including odds ratios for outcomes like pre‐ and post‐term delivery.
Implications for practice and research
Dietary intervention during pregnancy slightly reduces maternal BP and the incidence of preterm delivery. No strong evidence was found for any effect of dietary intervention during pregnancy on the other outcomes. To develop appropriate dietary guidelines for pregnancy, we need to understand the role of everyday diet on pregnancy. This review advances our understanding of the role of nutrition for a healthy pregnancy by observing small reductions in BP and slight increases in the length of gestation. Further research, with larger sample sizes and robust methodology is required to better understand. Quantifying dietary intakes before, during and after an intervention would provide an important measure of compliance with the dietary intervention regime, which was lacking from most of the included trials (n = 14).
Limitations
This systematic review is broader in scope when compared with other published systematic reviews and meta‐analyses (Dodd et al. 2010; Streuling et al. 2010; Tanentsapf et al. 2011; Oteng‐Ntim et al. 2012; Thangaratinam et al. 2012), and the quality of the included studies was mostly positive. Gestational weight gain was not included as an outcome in this review, as others have focused on this (Dodd et al. 2010; Streuling et al. 2010; Tanentsapf et al. 2011; Oteng‐Ntim et al. 2012; Thangaratinam et al. 2012). Despite the broad scope of this review, very few trials contributed data to each pregnancy outcome, with the exception of length of gestation (n = 12). For this reason, some of the outcomes were not reported and others are underpowered, particularly with the subgroup analysis. Dietary intervention trials should measure and report on a range of pregnancy outcomes so the effects of diet on pregnancy outcomes can be determined. There was heterogeneity for BP, GDM and length of gestation only, which was not explained by subgroup analyses, but may be due to the varying intensity and duration of included trials.
Conclusion
There is evidence that dietary intervention during pregnancy can reduce maternal BP and the incidence of preterm delivery. Interventions focusing on national recommendations and modifying calcium, saturated fat and cholesterol are the most promising dietary interventions to reduce BP and the incidence of preterm delivery. Further large high‐quality RCTs investigating combination dietary intervention and micronutrient provision from food are needed. Future trials beginning in preconception and spanning for the duration of pregnancy, as well as between pregnancies are needed to advance our understanding of optimal nutrition for maternal–child health.
Source of funding
EG is funded by an Australian Postgraduate Award (PhD scholarship) provided by the University of Newcastle, New South Wales.
Conflict of interest
The authors declare that they have no conflicts of interest.
Contributions
EG, AB, JEB, AJH designed research; EG and AJH conducted research; EG and AB analysed data; EG wrote the paper and had primary responsibility for final content. All authors read and approved the final paper.
Supporting information
Table S1. Characteristics of included studies in a systematic review of dietary interventions during pregnancy on pregnancy outcomes
Acknowledgements
The authors thank Research Librarian Debbie Booth for her assistance with the database searches; Accredited Practising Dietitians Samantha Diamond and Jun Lai for their assistance with assessing methodological quality and data extraction.
Gresham, E. , Bisquera, A. , Byles, J. E. , and Hure, A. J. (2016) Effects of dietary interventions on pregnancy outcomes: a systematic review and meta‐analysis. Matern Child Nutr, 12: 5–23. doi: 10.1111/mcn.12142.
References
- Aaltonen J., Ojala T., Laitinen K., Piirainen T.J., Poussa T.A. & Isolauri E. (2008) Evidence of infant blood pressure programming by maternal nutrition during pregnancy: a prospective randomized controlled intervention study. Journal of Pediatrics 152, 79–84. [DOI] [PubMed] [Google Scholar]
- Academy of Nutrition and Dietetics (2012) Evidence Analysis Manual: Steps in the Academy Evidence Analysis Process, Research and Strategic Business Development: Chicago. [Google Scholar]
- Al M.D., Van Houwelingen A.C., Kester A.D., Hasaart T.H., de Jong A.E. & Hornstra G. (1995) Maternal essential fatty acid patterns during normal pregnancy and their relationship to the neonatal essential fatty acid status. British Journal of Nutrition 74, 55–68. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association (2003) Gestational diabetes mellitus. Diabetes Care 26, s103–s105. [DOI] [PubMed] [Google Scholar]
- Antal M., Regoly‐Merei A., Varsanyi H., Biro L., Sagi K., Molnar D.V. et al (1997) Nutritional survey of pregnant women in Hungary. International Journal for Vitamin and Nutrition Research 67, 115–122. [PubMed] [Google Scholar]
- Asbee S.M., Jenkins T.R., Butler J.R., White J., Elliot M. & Rutledge A. (2009) Preventing excessive weight gain during pregnancy through dietary and lifestyle counseling: a randomized controlled trial. Obstetrics and Gynecology 113, 305–312. [DOI] [PubMed] [Google Scholar]
- Bech B.H., Obel C., Henriksen T.B. & Olsen J. (2007) Effect of reducing caffeine intake on birth weight and length of gestation: randomised controlled trial. BMJ (Clinical Research Ed.) 334, 409–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghaus T., Demmelmair H. & Koletzko B. (2000) Essential fatty acids and their long‐chain polyunsaturated metabolites in maternal and cord plasma triglycerides during late gestation. Biology of the Neonate 77, 96–100. [DOI] [PubMed] [Google Scholar]
- Bhutta Z.A. (2013) Early nutrition and adult outcomes: pieces of the puzzle. Lancet 382, 486–487. [DOI] [PubMed] [Google Scholar]
- Bonomo M., Corica D., Mion E., Goncalves D., Motta G., Merati R. et al (2005) Evaluating the therapeutic approach in pregnancies complicated by borderline glucose intolerance: a randomized clinical trial. Diabetic Medicine 22, 1536–1541. [DOI] [PubMed] [Google Scholar]
- Briley C., Flanagan N.L. & Lewis N. (2002) In‐home prenatal nutrition intervention increased dietary iron intakes and reduced low birthweight in low‐income African‐American women. Journal of the American Dietetic Association 102 (7), 984–987. [DOI] [PubMed] [Google Scholar]
- Brown M.A., Hague W.M., Higgins J., Lowe S., McCowan L., Oats J. et al (2000) The detection, investigation and management of hypertension in pregnancy: full consensus statement. Australian and New Zealand Journal of Obstetrics and Gynaecology 40, 139–155. [DOI] [PubMed] [Google Scholar]
- Carroli G., Duley L., Belizan J.M. & Villar J. (1994) Calcium supplementation during pregnancy: a systematic review of randomised controlled trials. British Journal of Obstetrics & Gynaecology 101, 753–758. [DOI] [PubMed] [Google Scholar]
- Chan G.M., McElligott K., McNaught T. & Gill G. (2006) Effects of dietary calcium intervention on adolescent mothers and newborns: a randomized controlled trial. Obstetrics and Gynecology 108, 565–571. [DOI] [PubMed] [Google Scholar]
- Chinn S. (2000) A simple method for converting an odds ratio to effect size for use in meta‐analysis. Statistics in Medicine 19, 3127–3131. [DOI] [PubMed] [Google Scholar]
- Courville A., Harel O. & Lammi‐Keefe C. (2011) Consumption of a DHA‐containing functional food during pregnancy is associated with lower infant ponderal index and cord plasma insulin concentration. The British Journal of Nutrition 106, 208–212. [DOI] [PubMed] [Google Scholar]
- Department of Health (2014) Therapeutic Goods Administration. Australian Government.
- Derbyshire E., Davies J., Costarelli V. & Dettmar P. (2006) Prepregnancy body mass index and dietary intake in the first trimester of pregnancy. Journal of Human Nutrition and Dietetics 19, 267–273. [DOI] [PubMed] [Google Scholar]
- Derbyshire E., Davies G., Costarelli V. & Dettmar P. (2009) Habitual micronutrient intake during and after pregnancy in Caucasian Londoners. Maternal & Child Nutrition 5, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd J.M., Grivell R.M., Crowther C.A. & Robinson J.S. (2010) Antenatal interventions for overweight or obese pregnant women: a systematic review of randomised trials. BJOG: An International Journal of Obstetrics & Gynaecology 117, 1316–1326. [DOI] [PubMed] [Google Scholar]
- Egger M., Smith G.D., Schneider M. & Minder C. (1997) Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed.) 315, 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erick M. (2008) Nutrition during pregnancy and lactation In: Krause's Food & Nutrition Therapy (ed. Alexopoulos Y.), pp. 160–198. Saunder's Elsevier Inc.: Toronto, Ontario. [Google Scholar]
- Firth D. (1993) Bias reduction of maximum likelihood estimates. Biometrika 80, 27–38. [Google Scholar]
- Gilmartin A.B., Ural S.H. & Repke J.T. (2008) Gestational diabetes mellitus. Reviews in Obstetrics and Gynecology 1, 129–134. [PMC free article] [PubMed] [Google Scholar]
- Godfrey K. & Robinson S. (1998) Maternal nutrition, placental growth and fetal programming. Proceedings of the Nutrition Society 57, 105–111. [DOI] [PubMed] [Google Scholar]
- Goldberg G. (2002) Nutrition in pregnancy and lactation In: Nutrition through the Lifecycle (ed. Shetty P.), pp. 63–90. Leatherhead Publishing: Surrey, UK. [Google Scholar]
- de Groot R.H.M., Hornstra G., van Houwelingen A.C. & Roumen F. (2004) Effect of alpha‐linolenic acid supplementation during pregnancy on maternal and neonatal polyunsaturated fatty acid status and pregnancy outcome. American Journal of Clinical Nutrition 79, 251–260. [DOI] [PubMed] [Google Scholar]
- Hacker A.N., Fung E.B. & King J.C. (2012) Role of calcium during pregnancy: maternal and fetal needs. Nutrition Reviews 70, 397–409. [DOI] [PubMed] [Google Scholar]
- Hasselblad V. & Hedges L.V. (1995) Meta‐analysis of screening and diagnostic tests. Psychological Bulletin 117, 167–178. [DOI] [PubMed] [Google Scholar]
- Heinze G. & Schemper M. (2002) A solution to the problem of separation in logistic regression. Statistics in Medicine 21, 2409–2419. [DOI] [PubMed] [Google Scholar]
- Herrera E. (2002) Implications of dietary fatty acids during pregnancy on placental, fetal and postnatal development – a review. Placenta 23, S9–S19. [DOI] [PubMed] [Google Scholar]
- Hofmeyr G.J., Lawrie T.A., Atallah A.N. & Duley L. (2010) Calcium supplementation during pregnancy for preventing hypertensive disorders and related problems. Cochrane Database of Systematic Reviews 4, CD001059. [DOI] [PubMed] [Google Scholar]
- Horvath A., Koletzko B. & Szajewska H. (2007) Effect of supplementation of women in high‐risk pregnancies with long‐chain polyunsaturated fatty acids on pregnancy outcomes and growth measures at birth: a meta‐analysis of randomized controlled trials. British Journal of Nutrition 98, 253–259. [DOI] [PubMed] [Google Scholar]
- Hozo S., Djulbegovic B. & Hozo I. (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 5, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imdad A. & Bhutta Z.A. (2012) Effects of calcium supplementation during pregnancy on maternal, fetal and birth outcomes. Paediatric and Perinatal Epidemiology 26, 138–152. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (1990) Nutrition in pregnancy, National Academy Press: Washington, DC. [Google Scholar]
- Kafatos A., Vlachonikolis I. & Codrington C. (1989) Nutrition during pregnancy: the effects of an educational intervention program in Greece. The American Journal of Clinical Nutrition 50, 970–979. [DOI] [PubMed] [Google Scholar]
- Khoury J., Henriksen T., Christophersen B. & Tonstad S. (2005) Effect of a cholesterol‐lowering diet on maternal, cord, and neonatal lipids, and pregnancy outcome: a randomized clinical trial. American Journal of Obstetrics and Gynecology 193, 1292–1301. [DOI] [PubMed] [Google Scholar]
- King J.C. (2006) Maternal obesity, metabolism, and pregnancy outcomes. Annual Review of Nutrition 26, 271–291. [DOI] [PubMed] [Google Scholar]
- Knuist M., Bonsel G.J., Zondervan H.A. & Treffers P.E. (1998) Low sodium diet and pregnancy‐induced hypertension: a multi‐centre randomised controlled trial. British Journal of Obstetrics and Gynaecology 105, 430–434. [DOI] [PubMed] [Google Scholar]
- Kramer M. (1987) Determinants of low birth weight: methodological assessment and meta‐analysis. Bulletin of the World Health Organization 65, 663–737. [PMC free article] [PubMed] [Google Scholar]
- Larquè E.D., Demmelmair H., Berger B., Hasbargen U. & Koletzko B. (2003) In vivo investigation of the placental transfer of C‐labeled fatty acids in humans. The Journal of Lipid Research 44, 49–55. [DOI] [PubMed] [Google Scholar]
- Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P.A. et al (2009) The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 6, e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsey M.W. & Wilson D.B. (2001) Practical Meta‐Analyisis, Sage Publications Inc.: Thousand Oaks, CA. [Google Scholar]
- Lowe S.A., Brown M.A., Dekker G.A., Gatt S., McLintock C.K., McMahon L.P. et al (2009) Guidelines for the management of hypertensive disorders of pregnancy 2008. Australian & New Zealand Journal of Obstetrics & Gynaecology 49, 242–246. [DOI] [PubMed] [Google Scholar]
- Luoto R., Laitinen K., Nermes M. & Isolauri E. (2010) Impact of maternal probiotic‐supplemented dietary counseling on pregnancy outcome and prenatal and postnatal growth: a double‐blind, placebo‐controlled study. The British Journal of Nutrition 103, 1792–1799. [DOI] [PubMed] [Google Scholar]
- Luoto R., Kinnunen T.I., Aittasalo M., Kolu P., Raitanen J., Ojala K. et al (2011) Primary prevention of gestational diabetes mellitus and large‐for‐gestational‐age newborns by lifestyle counseling: a cluster‐randomized controlled trial. PLoS (Public Library of Science) Medicine 8, e1001036̀. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makrides M., Duley L. & Olsen S.F. (2012) Marine oil, and other prostaglandin precursor, supplementation for pregnancy uncomplicated by pre‐eclampsia or intrauterine growth restriction. Cochrane Database of Systematic Reviews 19, CD003402. [DOI] [PubMed] [Google Scholar]
- Mardones‐Santander F., Rosso P., Stekel A., Ahumada E., Llaguno S., Pizarro F. et al (1988) Effect of a milk‐based food supplement on maternal nutritional status and fetal growth in underweight Chilean women. The American Journal of Clinical Nutrition 47, 413–419. [DOI] [PubMed] [Google Scholar]
- McDonald E.C., Pollitt E. & Mueller W. (1981) The bacon chow study: maternal nutritional supplementation and birth weight of offspring. American Journal of Clinical Nutrition 34, 2133–2144. [DOI] [PubMed] [Google Scholar]
- Metcoff J., Costiloe P., Crosby W.M., Dutta S., Sandstead H.H., Milne D. et al (1985) Effect of food supplementation (WIC) during pregnancy on birth weight. American Journal of Clinical Nutrition 41, 933–947. [DOI] [PubMed] [Google Scholar]
- Michie S., Abraham C., Eccles M.P., Francis J.J., Hardeman W. & Johnston M. (2011) Strengthening evaluation and implementation by specifying components of behaviour change interventions: a study protocol. Implementation Science 6, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. & The P.G. (2009) Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine 6, e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore V., Davies M., Willson K., Worsley A. & Robinson J. (2004) Dietary composition of pregnant women is related to size of the baby at birth. Journal of Nutrition 134, 1820–1826. [DOI] [PubMed] [Google Scholar]
- Mora J.O., Clement J., Christiansen N., Suescun J., Wagner M. & Herrera M.G. (1978) Nutritional supplementation and the outcome of pregnancy. III. Perinatal and neonatal mortality. Nutrition Reports International 18, 167–175. [Google Scholar]
- Mora J.O., de Paredes B., Wagner M., de Navarro L., Suescun J., Christiansen N. et al (1979) Nutritional supplementation and the outcome of pregnancy. I. Birth weight. American Journal of Clinical Nutrition 32, 455–462. [DOI] [PubMed] [Google Scholar]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy (2000) Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. American Journal of Obstetrics and Gynecology 183, S1–S22. [PubMed] [Google Scholar]
- National Institutes of Health (2003) The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC 7). Hypertension 42, 1206–1252. [DOI] [PubMed] [Google Scholar]
- Nichols‐Richardson S.M. (2011) Nutrition during pregnancy and lactation In: Williams' Essentials of Nutrition and Diet Therapy (ed. Alexopoulos A.), pp. 236–261. Mosby Inc.: St Louis. [Google Scholar]
- Northstone K., Emmett P. & Rogers I. (2008) Dietary patterns in pregnancy and associations with socio‐demographic and lifestyle factors. European Journal of Clinical Nutrition 62, 471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor M.J. & Whaley S.E. (2007) Brief intervention for alcohol use by pregnant women. American Journal of Public Health 97, 252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen S.F., Hansen H.S., Sorensen T.I., Jensen B., Secher N.J., Sommer S. et al (1986) Intake of marine fat, rich in (n‐3)‐polyunsaturated fatty acids, may increase birthweight by prolonging gestation. Lancet 2, 367–369. [DOI] [PubMed] [Google Scholar]
- Olsen S.F., Olsen J. & Frische G. (1990) Does fish consumption during pregnancy increase fetal growth? A study of the size of the newborn, placental weight and gestational age in relation to fish consumption during pregnancy. International Journal of Epidemiology 19, 971–977. [DOI] [PubMed] [Google Scholar]
- Olsen S.F., Grandjean P., Weihe P. & Videro T. (1993) Frequency of seafood intake in pregnancy as a determinant of birth weight: evidence for a dose‐dependent relationship. Journal of Epidemiology & Community Health 47, 436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oteng‐Ntim E., Varma R., Croker H., Poston L. & Doyle P. (2012) Lifestyle interventions for overweight and obese pregnant women to improve pregnancy outcome: systematic review and meta‐analysis. BMC Medicine 10, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto S.J., Houwelingen A.C., Antal M., Manninen A., Godfrey K., Lopez‐Jaramillo P. et al (1997) Maternal and neonatal essential fatty acid status in phospholipids: an international comparative study. European Journal of Clinical Nutrition 51, 232–242. [DOI] [PubMed] [Google Scholar]
- Östlund I., Hanson U., Björklund A., Hjertberg R., Eva N., Nordlander E. et al (2003) Maternal and fetal outcomes if gestational impaired glucose tolerance is not treated. Diabetes Care 26, 2107–2111. [DOI] [PubMed] [Google Scholar]
- Palacios C. & Pena‐Rosas J.P. (2011) Calcium Supplementation during Pregnancy for Preventing Hypertensive Disorders and Related Problems, The World Health Organisation: Geneva. [Google Scholar]
- Patel N.K., Gadhavi M., Gorasia D., & Pandya M.R. (2012) Comparative evaluation of antihypertensive drugs in the management of pregnancy‐induced hypertension. Int J Basic Clin Pharmacol 1, 174–177. [Google Scholar]
- Peters R.M. & Flack J.M. (2004) Hypertensive disorders of pregnancy. Journal of Obstetric, Gynecologic, & Neonatal Nursing 33, 209–220. [DOI] [PubMed] [Google Scholar]
- Quinlivan J.A., Lam L.T. & Fisher J. (2011) A randomised trial of a four‐step multidisciplinary approach to the antenatal care of obese pregnant women. Australian & New Zealand Journal of Obstetrics & Gynaecology 51, 141–146. [DOI] [PubMed] [Google Scholar]
- Ritchie L.D. & King J.C. (2008) Nutrient recommendations and dietary guidelines for pregnant women In: Handbook of Nutrition and Pregnancy (eds Lammi‐Keefe C.J., Couch S.C. & Philipson E.H.), pp. 3–25. Humana Press: Clifton USA. [Google Scholar]
- Roberts C.L., Algert C.S., Morris J.M., Ford J.B. & Henderson‐Smart D.J. (2005) Hypertensive disorders in pregnancy: a population‐based study. The Medical Journal of Australia 182, 332–335. [DOI] [PubMed] [Google Scholar]
- Roberts C.L., Ford J.B., Algert C.S., Antonsen S., Chalmers J., Cnattingius S. et al (2011) Population‐based trends in pregnancy hypertension and pre‐eclampsia: an international comparative study. British Medical Journal Open 1, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J.M., Pearson G., Cutler J. & Lindheimer M. (2003) Summary of the NHLBI Working Group on Research on Hypertension During Pregnancy. Hypertension 41, 437–445. [DOI] [PubMed] [Google Scholar]
- Roseboom T.J., Painter R.C., van Abeelen A.F., Veenendaal M.V. & de Rooij S.R. (2011) Hungry in the womb: what are the consequences? Lessons from the Dutch famine. Maturitas 70, 141–145. [DOI] [PubMed] [Google Scholar]
- Ross S.M., Nel E. & Naeye R.L. (1985) Differing effects of low and high bulk maternal dietary supplements during pregnancy. Early Human Development 10, 295–302. [DOI] [PubMed] [Google Scholar]
- Rush D. (2001) Maternal nutrition and perinatal survival. Journal of Health, Population, and Nutrition 19, S217–S264. [PubMed] [Google Scholar]
- Rush D., Stein Z. & Susser M. (1980) A randomized controlled trial of prenatal nutritional supplementation in New York City. Pediatrics 65, 683–697. [PubMed] [Google Scholar]
- Sibai B.M. (2003) Diagnosis and management of gestational hypertension and preeclampsia. Obstetrics & Gynecology 102, 181–192. [DOI] [PubMed] [Google Scholar]
- Smuts C.M., Borod E., Peeples J.M. & Carlson S.E. (2003a) High‐DHA eggs: feasibility as a means to enhance circulating DHA in mother and infant. Lipids 38, 407–414. [DOI] [PubMed] [Google Scholar]
- Smuts C.M., Huang M., Mundy D., Plasse T., Major S. & Carlson S.E. (2003b) A randomized trial of docosahexaenoic acid supplementation during the third trimester of pregnancy. Obstetrics and Gynecology 101, 469–479. [DOI] [PubMed] [Google Scholar]
- StataCorp (2011) Stata Release 12, Stata Press, College Station, TX. [Google Scholar]
- Streuling I., Beyerlein A. & von Kries R. (2010) Can gestational weight gain be modified by increasing physical activity and diet counseling? A meta‐analysis of interventional trials. The American Journal of Clinical Nutrition 92, 678–687. [DOI] [PubMed] [Google Scholar]
- Szajewska H., Horvath A. & Koletzko B. (2006) Effect of n–3 long‐chain polyunsaturated fatty acid supplementation of women with low‐risk pregnancies on pregnancy outcomes and growth measures at birth: a meta‐analysis of randomized controlled trials. The American Journal of Clinical Nutrition 83, 1337–1344. [DOI] [PubMed] [Google Scholar]
- Tanentsapf I., Heitmann B. & Adegboye A. (2011) Systematic review of clinical trials on dietary interventions to prevent excessive weight gain during pregnancy among normal weight, overweight and obese women. BMC Pregnancy and Childbirth 11, 81‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thangaratinam S., Rogozińska E., Jolly K., Glinkowski S., Roseboom T., Tomlinson J.W. et al (2012) Effects of interventions in pregnancy on maternal weight and obstetric outcomes: meta‐analysis of randomised evidence. BMJ (Clinical Research Ed.) 344, e2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cochrane Collaboration (2011). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] (eds Higgins J.P.T. & Green S.). Available from http://www.cochrane-handbook.org. [Google Scholar]
- The World Bank Group (2012) Country and Lending Groups: High‐Income OECD Members.
- Thornton Y.S., Smarkola C., Kopacz S.M. & Ishoof S.B. (2009) Perinatal outcomes in nutritionally monitored obese pregnant women: a randomized clinical trial. Journal of the National Medical Association 101, 569–577. [DOI] [PubMed] [Google Scholar]
- Van Buul B.J.A., Steegers E.A.P., Van Der Maten G.D., Delemarre F.M.C., Jongsma H.W., Oosterbaan H.P. et al (1997) Dietary sodium restriction does not prevent gestational hypertension: a Dutch two‐center randomized trial. Hypertension in Pregnancy 16, 335–346. [Google Scholar]
- Van Mierlo L.A., Arends L.R., Streppel M.T., Zeegers M.P., Kok F.J., Grobbee D.E. et al (2006) Blood pressure response to calcium supplementation: a meta‐analysis of randomized controlled trials. Journal of Human Hypertension 20, 571–580. [DOI] [PubMed] [Google Scholar]
- Vinter C.A., Jensen D.M., Ovesen P., Beck‐Nielsen H. & Jørgensen J.S. (2011) The LiP (Lifestyle in Pregnancy) study: a randomized controlled trial of lifestyle intervention in 360 obese pregnant women. Diabetes Care 34, 2502–2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S., Legarth J., Vangsgaard K., Toubro S. & Astrup A. (2008) A randomized trial of the effects of dietary counseling on gestational weight gain and glucose metabolism in obese pregnant women. International Journal of Obesity 32, 495–501. [DOI] [PubMed] [Google Scholar]
- Zhang C., Liu S., Solomon C.G. & Hu F.B. (2006) Dietary fiber intake, dietary glycemic load, and the risk for gestational diabetes mellitus. Diabetes Care 29, 2223–2230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of included studies in a systematic review of dietary interventions during pregnancy on pregnancy outcomes


