Abstract
In Papua New Guinea, intermittent preventive treatment with sulphadoxine‐pyrimethamine and azithromycin (SPAZ‐IPTp) increased birthweight despite limited impact on malaria and sexually transmitted infections. To explore possible nutrition‐related mechanisms, we evaluated associations between gestational weight gain (GWG), enrolment body mass index (BMI) and mid‐upper arm circumference (MUAC), and birthweight. We investigated whether the increase in birthweight associated with SPAZ‐IPTp may partly be driven by a treatment effect on GWG. The mean GWG rate was 393 g/week (SD 250; n = 948). A 100 g/week increase in GWG was associated with a 14 g (95% CI 2.6, 25.4) increase in birthweight (P = 0.016). Enrolment BMI and MUAC also positively correlated with birthweight. SPAZ‐IPTp was associated with increased GWG [58 g/week (26, 900), P < 0.001, n = 948] and with increased birthweight [48 g, 95% CI (8, 880), P = 0.019] when all eligible women were considered (n = 1947). Inclusion of GWG reduced the birthweight coefficient associated with SPAZ‐IPTp by 18% from 44 to 36 g (n = 948), although SPAZ‐IPTp was not significantly associated with birthweight among women for whom GWG data were available (P = 0.13, n = 948). One month post‐partum, fewer women who had received SPAZ‐IPTp had a low post‐partum BMI (<18.5 kg m−2) [adjusted risk ratio: 0.55 (95% CI 0.36, 0.82), P = 0.004] and their babies had a reduced risk of wasting [risk ratio 0.39 (95% CI 0.21, 0.72), P = 0.003]. SPAZ‐IPTp increased GWG, which could explain its impact on birthweight and maternal post‐partum BMI. Future trials of SPAZ‐IPTp must incorporate detailed anthropometric evaluations to investigate mechanisms of effects on maternal and child health.
Keywords: mid‐upper arm circumference; body mass index, intermittent preventive treatment in pregnancy; low birthweight; antimalarials; antibiotics
Introduction
Adequate nutrition and growth during pregnancy, early childhood and adolescence translate into immediate and long‐term health benefits (Barker et al. 1993; Black et al. 2013; Prentice et al. 2013). Macronutrient undernutrition in pregnancy has been associated with adverse health outcomes and is particularly common in resource‐limited settings (Black et al. 2008, 2013). Undernutrition may increase the risk of infectious disease, while infection itself increases maternal energy requirements and drives nutritional depletion (Schaible & Kaufmann 2007; Prentice et al. 2008). Interventions that improve nutritional status of pregnant women by preventing and treating infection and inflammation, or through improving nutrient absorption, may significantly reduce the high burden of low birthweight (LBW, <2500 g) in developing countries.
Intermittent preventive treatment in pregnancy (IPTp) with sulphadoxine‐pyrimethamine (SP) aims to protect pregnant women and their fetuses from the deleterious effects of gestational malaria, including LBW (WHO 2014). Pregnant women are often also at risk of bacterial infections which predispose to LBW (Chico et al. 2012; Romero et al. 2014). This, together with the threat of antimalarial drug resistance, has led to clinical trials evaluating the role of adding azithromycin (AZ, a macrolide antibiotic with antimalarial properties) to SP‐IPTp. SPAZ‐IPTp was associated with a reduced risk of LBW in one of two clinical trials in sub‐Saharan Africa (van den Broek et al. 2009; Luntamo et al. 2010). In Papua New Guinea (PNG), SPAZ‐IPTp increased mean birthweight (∼50 g) and reduced LBW by 26%. These benefits occurred in the context of a relatively low prevalence of malaria, and apparently limited effects on carriage of sexually transmitted infections (STIs) (Unger et al. 2015). This suggests there may be additional unknown mechanisms by which SPAZ‐IPTp improves birthweight. One important predictor of LBW in the trial was low mid‐upper arm circumference (MUAC) at first antenatal visit, suggesting maternal undernutrition contributes to the burden of LBW in PNG (Unger et al. 2015).
Gestational weight gain (GWG) has been previously shown to be an important predictor of birthweight (IOM 2009). In low‐resource settings, GWG is strongly dependent on pre‐pregnancy nutritional status and nutrient supply during gestation. When the nutrient requirements of the mother, placenta and fetus are not met, fetal growth may be reduced and adverse pregnancy outcomes, such as LBW, may ensue (Abrams & Selvin 1995; Carmichael et al. 1997; Butte & King 2005; Mola et al. 2011). The impact of sulphadoxine‐pyrimethamine/azithromycin‐based intermittent preventive treatment (SPAZ‐IPTp) on GWG is unknown, but it is conceivable that SPAZ‐IPTp partly achieves its effect on birthweight by improving GWG. By preventing and clearing maternal infections, SPAZ‐IPTp might increase the energy available to mother and fetus.
In this exploratory secondary analysis of the SPAZ‐IPTp trial data from PNG, we evaluated the association of anthropometric indicators of maternal macronutrient nutritional status, in particular GWG, with birthweight. We subsequently evaluated the impact of SPAZ‐IPTp on GWG and maternal post‐partum nutritional status at 1 month, and examined whether the previously observed effect of SPAZ‐IPTp on birthweight is in part mediated by improved GWG.
Key messages.
Gestational weight gain (GWG), body mass index and mid‐upper arm circumference are important predictors of birthweight in Papua New Guinea.
Sulphadoxine‐pyrimethamine/azithromycin‐based intermittent preventive treatment (SPAZ‐IPTp) increases GWG, which appears to be one of the mechanisms by which the intervention improves birthweight.
SPAZ‐IPTp may reduce the risk of maternal underweight and infant wasting at 4–6 weeks post‐partum.
Future trials of SPAZ‐IPTp must incorporate detailed anthropometric evaluations.
Methods
Study setting and participants
The original trial was conducted in malaria‐endemic areas of Madang Province on the North Coast of PNG (Unger et al. 2015). LBW is common in the study area (18–20%), and infant mortality rates remain unacceptably high (47 per 1000 live births in 2013) (Unger et al. 2015; UN 2014). A small number of studies have linked undernutrition with adverse pregnancy outcomes, and pregnancy with maternal nutritional depletion, in PNG (Primhak & MacGregor 1991; Garner et al. 1994a, 1994b; Allen et al. 1998).
We used data collected as part of a participant‐blinded parallel group randomised controlled clinical trial evaluating the effect on LBW of monthly IPTp with SPAZ‐IPTp (SP: Micro Labs Ltd., Bangalore, India; AZ: Pfizer, New York, USA) (mean number of treatment courses: 2.8, median 3, range 0–4) given from second trimester onwards, compared with the current malaria in pregnancy regimen in PNG, i.e. a single treatment course of SP and chloroquine (CQ, Medopharm, Chennai, India) at first antenatal visit (NCT01136850) (Unger et al. 2015). Enrolment took place between November 2009 and August 2012, and follow‐up was completed in February 2013. Pregnant women presenting for their first antenatal visit at one of nine participating antenatal clinics were invited to group information sessions. If interested in participating, women were screened and counselled individually and written informed consent was obtained. Study exclusion criteria included pregnancy >26 gestational weeks by fundal height, symptomatic anaemia, previous serious adverse reaction to trial medications, permanent disability or chronic medical conditions, known multiple pregnancy, not available for follow‐up, and <16 years of age (legal age of consent in PNG) (Unger et al. 2015). Women were randomised (participant blinded) to monthly treatments with a single dose of SP (1500/75 mg) plus 4 g of AZ (1 g twice daily for 2 days), or a single treatment with SP plus 450–600 mg of CQ daily over 3 days, followed by monthly placebo courses. Administration of SP and the first dose of AZ or CQ were supervised and accompanied by a dry biscuit.
To be eligible for inclusion in the present study, women were additionally required to have had both MUAC and body mass index (BMI) measured at first antenatal visit.
Study design
We conducted a secondary prospective cohort analysis using data collected as part of the SPAZ‐IPTp clinical trial. The analysis was conducted in two parts. First, we examined the relationship between maternal nutritional status (GWG, enrolment BMI, enrolment MUAC) and birth outcomes. We subsequently investigated the impact of SPAZ‐IPTp on GWG; on maternal BMI and MUAC 4–6 weeks post‐partum; and on birthweight and infant nutritional status 4–6 weeks post‐partum.
Data collection
At enrolment, socio‐demographic and clinical data were collected; MUAC, height and weight were measured; and venous blood was drawn. When available, a transabdominal ultrasound scan was performed within a week of enrolment to determine gestational age (Hadlock et al. 1984; Loughna et al. 2009). Participants were scheduled for additional antenatal study visits during which a subset of women had further body weight measurements taken. Women were subsequently followed up to delivery when a detailed newborn examination was performed, and newborn anthropometric measurements were taken. Participants and their infants were scheduled for postnatal follow‐up visit (4–6 weeks after delivery) for repeat maternal and infant anthropometric evaluation.
Haemoglobin concentrations were measured (HemoCue Ltd, Ängelholm, Sweden), blood smears were evaluated for malaria infection (Laman et al. 2014), and Plasmodium species‐specific qPCR was performed (Rosanas‐Urgell et al. 2010). Malaria positivity at enrolment was defined as detection of malaria infection by light microscopy and/or qPCR. Women found to be moderate‐to‐severely anaemic (haemoglobin <90 g L−1) were provided with iron/folate supplements and treated with albendazole.
Women's weight and height were evaluated with the participants in bare feet, wearing light clothing and without headwear. Height was measured using a stadiometer and rounded to the nearest full centimetre, and weight was determined using mechanical scales (capacity 150 kg, sensitivity of 250 g). The mean (± SD) height‐for‐age z‐score of the cohort was −1.37 (± 0.90) (WHO 2006). MUAC (circumference halfway between the acromion and the olecranon process) was evaluated to the nearest centimetre using a non‐stretchable flexible measuring tape.
GWG was evaluated for 948 women with ultrasound‐dated pregnancies and sequential weight measurements. Weight measurements were taken at least 30 days apart. Because of late antenatal presentation, only weights measured during the second and third trimesters were available for GWG analyses. For women with ≥3 measurements, GWG was evaluated using the largest weight gain time interval available. Lack of information on pre‐pregnancy weight precluded an evaluation of total GWG.
Infant weights were measured using digital infant scales (Cupid 1, Charder Electronic Co., Taichung City, Taiwan, precision 10 g). A non‐stretchable measuring tape was used to assess specific neonatal anthropometric measurements: head circumference (HC; across occipital fontanelle and above both ears), abdominal circumference (AC; at umbilical level) and crown‐heel length (CHL; superior aspect of the head to heel, with legs fully extended and infant in supine position).
Definitions
GWG was expressed in g/week when evaluated as the dependent variable (outcome), and in 100 g/week when assessed as an explanatory variable. Average weekly GWG rates were calculated using the formula:
| (1) |
Low weight gain was defined as a weekly GWG rate in the first quartile of the overall GWG distribution (<226 g/week). We used this cohort‐derived cut‐off as pre‐pregnancy BMI was unavailable, not all women had gestational age estimates or had gestational age estimated by a late pregnancy scan, and neither charts to estimate pre‐pregnancy BMI nor published GWG standards have been validated in Melanesian populations.
An MUAC <23 cm at first antenatal visit or postnatal follow‐up visit was defined as low. This corresponded with the first quartile of the overall distribution of enrolment MUAC in the cohort, and is similar to cut‐off points previously suggested to identify undernourished non‐pregnant and pregnant women in both developed and developing country settings (Krasovec & Anderson 1991; Liljestrand & Bergstrom 1991; James et al. 1994; Ververs et al. 2013).
A BMI <19.8 kg m−2 at first antenatal clinic was defined as low. This cut‐off was chosen for the following reasons: (1) few women [3.8% (102/2671)] had a BMI less than 18.5 kg m−2 at first antenatal visit probably as a result of GWG; (2) it corresponded with the 10th centile for BMI at the end of the first trimester in other similar cohorts (Calvo et al. 2009); and (3) this cut‐off has been previously identified as a potentially useful cut‐off to identify women at risk of adverse pregnancy outcome due to undernutrition when pre‐pregnancy BMI was unavailable (WHO 1995). At the post‐natal visit, underweight was defined as a BMI <18.5 kg m−2 (non‐pregnant adult).
Outcomes
All outcome analyses were restricted to pregnancies that resulted in live singleton births with no congenital abnormalities (Rijken et al. 2011).
Maternal outcome measures included rate of GWG (g/week), and BMI and MUAC at the postnatal visit.
Infant outcome measures at birth included birthweight, HC, AC, CHL, LBW, measuring small‐for‐gestational age (SGA, defined as a birthweight <10th centile of a population‐specific fetal weight standard) (Unger et al. 2014) and preterm birth (PTB, <37 gestational weeks). Given home birth is common in our setting, anthropometric measurements undertaken after 24 yet within 7 days of delivery were included in analyses, but adjusted for post‐partum weight loss (Turner et al. 2013; Unger et al. 2015). Infant outcome measures at the postnatal visit included underweight [weight‐for‐age z‐score (WAZ) < −2 standard deviations (SD)], stunting [length‐for‐age z‐score (LAZ) < −2 SD] and wasting [weight‐for‐length z‐score (WLZ) < −2 SD], according to the 2006 WHO (World Health Organization) sex‐specific child growth standards (WHO 2006).
Statistical analysis
Data were doubled‐entered in the study database (FoxPro 9.0, Microsoft, Redmond, USA) and analysed with Stata 12.0 (StataCorp, College Station, USA). Univariable comparisons of variables were undertaken using the chi‐square test for binary data, and the Student's t‐test and the Mann–Whitney U‐test for parametric data and nonparametric data, respectively.
Linear regression was used to calculate mean differences or regression coefficients for continuous parametric outcome variables. A multivariable Poisson regression model with robust error variance was applied to calculate adjusted risk ratios for binary outcome measures (Zou 2004). Multivariable models assessing outcome measures at birth were adjusted for factors previously found to be associated with, for example, birthweight in this and other similar cohorts (a priori selection) (Unger et al. 2015). These included maternal age, gravidity, newborn sex, and time difference between delivery and weight measurement, in addition to explanatory variables of particular interest in this analysis, namely enrolment MUAC and BMI, GWG, and treatment arm (SPCQ vs. SPAZ). Factors associated with GWG and enrolment BMI and MUAC on univariable analysis (P < 0.1) were included in multivariable models evaluating the association of SPAZ‐IPTp with GWG and post‐ natal maternal anthropometrics (BMI, MUAC), respectively. Interactions terms were fitted, and presence of effect measure modification was defined as P (interaction term) < 0.15. Analyses of the association between SPAZ‐IPTp and infant outcomes at post‐natal visit were adjusted a priori for maternal age, parental income status and maternal literacy status. A sample size calculation was performed for the original trial, powered to examine the impact of SPAZ‐IPTp on birthweight and malaria at delivery, but not for the present, exploratory, secondary analysis. All analyses used α < 0.05 as the cut‐off for statistical significance.
Ethical considerations
Ethical approval was obtained from the PNG Institute of Medical Research Institutional Review Board (0815), the PNG Medical Research Advisory Council (08.01) and the Melbourne Health Human Research Ethics Committee (2008.162). The clinical trial was registered with the U.S. National Institutes of Health Clinical Trials Registry (http://Clinicaltrials.gov, registration NCT01136850). All women gave written informed consent for participation in the original trial and the present study.
Results
Of 2793 women enrolled in the trial, 2671 were eligible for inclusion in the present study at baseline (Fig. 1). The median symphysis pubis fundal height at enrolment was 22 cm (interquartile range: 19, 24). A total of 2094 women (78.4%) had a documented congenitally normal singleton live birth (Fig. 1) and outcome analyses were performed in this cohort. Women excluded from outcome analyses due to formal withdrawal, lost contact, stillbirth, twin pregnancy or congenital abnormality (n = 577) were more likely to be primigravid (χ2 = 5.48, P = 0.018), and more likely to be illiterate (χ2 = 7.05, P = 0.008), but they were similar to women included in the analyses in terms of other baseline characteristics, including enrolment anthropometrics (Supporting Information http://onlinelibrary.wiley.com/doi/10.1111/mcn.12215/suppinfo).
Figure 1.
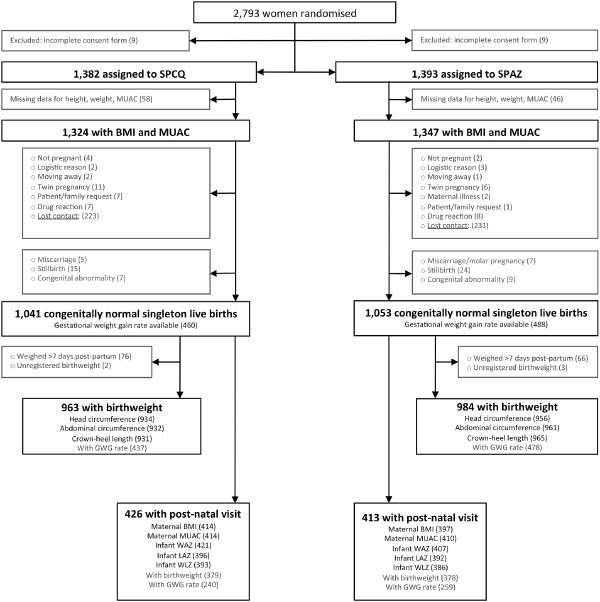
Participants' flow diagram chart. Up to 96.3% (2671/2775) of women assigned to treatment were included in the present study at baseline. Of those, 2094 women, 78.4% had a known congenitally normal singleton live birth and outcome analyses were performed on these pregnancies. The proportion of participants with GWG (χ2 2 = 0.64, P = 0.42), birthweight (χ2 2 = 0.03, P = 0.85), preterm birth (χ2 2 = 0.64, P = 0.51) and post‐natal visit data (χ2 2 = 0.71, P = 0.40) was similar between trial treatment groups.
The mean (± SD) BMI and MUAC at enrolment were 22.5 kg m−2 (± 2.9) and 24.0 cm (± 2.6), respectively. A total of 12.1% (321/2671) of women had a BMI below 19.8 kg m−2, 27.6% (737/2671) had an MUAC below 23 cm. Factors associated with BMI and MUAC at enrolment included living in a rural area, age, ethnicity and height, among others (Supporting Information http://onlinelibrary.wiley.com/doi/10.1111/mcn.12215/suppinfo).
Factors associated with GWG
The mean (± SD) weekly GWG rate in the second and third trimesters of pregnancy was 393 g/week (± 250) (n = 948, corresponding to 45.3% of all women with a live birth). An evaluation of the association between maternal characteristics and anthropometry at enrolment and GWG is presented in Table 1. Predictors of GWG in our cohort were SPAZ‐IPTp (P < 0.001) and maternal height (P = 0.006). Figure 2 depicts the GWG distribution by treatment arm. There were no interactions between covariates and GWG. The proportion of participants with GWG data (χ2 2 = 0.64, P = 0.42) was similar between treatment arms.
Table 1.
Association of SPAZ‐IPTp and maternal characteristics with second and third trimester gestational weight gain (g/week) (n = 948)
| Characteristics | Univariable regression | Multivariable regression | ||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | (95% CI) | R2 | P | Coefficient | (95% CI) | R2 | P | |
| Assigned to SPAZ‐IPTp | 52.8 | (21.7, 84.0) | 0.012 | 0.001 | 62.1* | (29.9, 94.4) | 0.033 | <0.001 |
| BMI (kg m−2) | 0.2 | (−5.3, 5.6) | 0.000 | 0.95 | −1.2 | (−6.8, 4.3) | 0.024 | 0.66 |
| MUAC (cm) | 3.5 | (−2.7, 9.7) | 0.001 | 0.27 | 1.1* | (−6.9, 9.0) | 0.033 | 0.79 |
| Weight at enrolment | 1.7 | (−0.3, 3.6) | 0.003 | 0.10 | −0.6* | (−3.5, 2.3) | 0.033 | 0.69 |
| Height (cm) | 4.4 | (1.7, 7.1) | 0.011 | <0.001 | 4.4* | (1.3, 7.5) | 0.033 | 0.006 |
| Maternal age (years) | 1.7 | (−1,1, 4.6) | 0.001 | 0.23 | 1.8* | (−1.1, 4.7) | 0.033 | 0.23 |
| Rural residence | −36.6 | (−69.0, −4.2) | 0.004 | 0.027 | −26.0* | (−61.9, 9.8) | 0.033 | 0.16 |
| Madang ethnic group | −27.4 | (−59.1, 4.3) | 0.003 | 0.09 | −13.1* | (−48.9, 22.7) | 0.033 | 0.47 |
| Haemoglobin (g dL−1) (n = 913) | 12.1 | (1.5, 22.7) | 0.006 | 0.025 | 10.2 † | (−0.2, 22.0) | 0.035 | 0.07 |
Note: Gravidity, literacy, parental income status and malaria infection at enrolment were not associated with gestational weight gain at P < 0.1. Multivariable regression included treatment group, gestational age at first measurement, time interval between measurements, maternal age, residence and ethnicity as covariates. *Model additionally included MUAC, height, weight. †Inclusion of haemoglobin as a covariate in the model (n = 913) did no alter the association between treatment arm and height with gestational weight gain (treatment arm: 62.1, 95% CI 29.9, 94.4; P < 0.001; height: 4.5, 95% CI 1.4, 7.7; P = 0.005).
Figure 2.
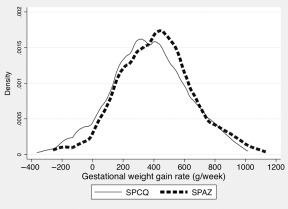
Distribution of second/third trimester gestational weight gain rate (g/week) by treatment group (n = 948). Women assigned to sulphadoxine‐pyrimethamine (SP) plus chloroquine (CQ), and recipients of SP plus azithromycin (AZ) were more likely to have a mean gestational weight gain rate in the lower half and upper half of the overall weight gain distribution curve (χ2 2 = 9.8, P = 0.002), respectively.
Association between GWG, BMI and MUAC, and birthweight
Birthweight was available for analysis for 1947 infants (93.0%) (Fig. 1). Overall, the mean birthweight (± SD) was 2941 g (± 479), and prevalence of LBW was 15.1% (293/1947). Among women with ultrasound‐dated pregnancies, the overall prevalence of PTB and SGA was 8.6% (110/1276) and 13.6% (173/1276), respectively. The proportion of participants with birthweight (χ2 2 = 0.03, P = 0.85) and SGA/PTD (χ2 2 = 0.64, P = 0.51) data was similar between treatment groups.
GWG was a predictor of birthweight (Table 2) . Women in the lowest quartile of GWG (<226 g/week) compared with women with a GWG of ≥226 g/week had infants with a decreased birthweight (2874 vs. 2994 g, adjusted mean difference 88 g, 95% CI −173, −3; P = 0.043) and an increased risk of LBW (adjusted risk ratio 1.63, 95% CI 1.19, 2.23; P = 0.002), independent of maternal height and MUAC (Table 3). The effect of GWG on birthweight appeared to be largely driven by mechanisms affecting fetal soft tissue accumulation (AC) (Table 3). Enrolment MUAC and BMI were also predictors of birthweight: a low MUAC (<23 cm) and BMI (<19.8 kg m−2) at enrolment were associated with reduced mean birthweight and an increased risk of LBW (Tables 2,3). Among women with ultrasound‐dated pregnancies, all three indicators of maternal nutrition were associated with measuring SGA, but only BMI and MUAC were associated with PTB. Enrolment haemoglobin was not significantly associated with birthweight [10.0 g per 10 g L−1 (−4.6, 24.6), P = 0.18, n = 1864].
Table 2.
Association of gestational weight gain rate (100 g/week) (n = 915), and BMI/MUAC at enrolment (n = 1947), with birthweight
| Univariable regression | Multivariable regression | |||||
|---|---|---|---|---|---|---|
| Coefficient | (95% CI) | P | Coefficient | (95% CI) | P | |
| Gestational weight gain (100 g/week) | 21.0 | (9.0, 33.0) | 0.001 | 14.0* | (2.6, 25.4) | 0.016 |
| Enrolment MUAC (cm) | 37.8 | (29.7, 45.9) | <0.001 | 29.1 † | (21.1, 37.0) | <0.001 |
| Enrolment BMI (kg m−2) | 31.3 | (24.0, 38.6) | <0.001 | 30.2 | (23.1, 37.3) | <0.001 |
| Height (cm) | 16.5 | (12.9, 20.1) | <0.001 | 13.2 † | (9.7, 16.7) | <0.001 |
Note: Coefficient denotes change in birthweight in grams associated with a 1 U increment of the predictor variable. All adjusted analyses include age, gravidity, treatment arm, timing of birthweight measurement and newborn sex as covariates. *Additionally adjusted for height and MUAC. †Additionally adjusted for height or MUAC.
Table 3.
Impact of low gestational weight gain, and low MUAC and BMI at enrolment, on newborn size and adverse birth outcomes
| GWG rate (g/week) | <226 | ≥226 | aRR or MD (95% CI)* | P | |
|---|---|---|---|---|---|
| Birthweight (g) | 915 | 2874 (2795, 2952) | 2994 (2953, 3034) | −88 (−173, −3) | 0.043 |
| Head circumference (cm) | 894 | 31.9 (31.7, 32.5) | 32.2 (31.7, 32.6) | −0.2 (−0.4, 0.1) | 0.29 |
| Abdominal circumference (cm) | 896 | 27.2 (24.2, 30.3) | 30.1 (29.0, 31.2) | −0.4 (−0.8, −0.0) | 0.043 |
| Crown‐heel length (cm) | 999 | 45.4 (44.3, 46.6) | 46.0 (44.7, 47.2) | −0.5 (−1.1, −0.0) | 0.06 |
| Low birthweight | 915 | 21.2 (48/226) | 11.8 (81/689) | 1.63 (1.19, 2.23) | 0.002 |
| Preterm birth | 915 | 9.3 (21/226) | 6.1 (42/689) | 1.38 (0.83, 2.28) | 0.22 |
| Small‐for‐gestational age | 915 | 20.4 (46/226) | 13.2 (91/689) | 1.41 (1.04, 1.92) | 0.030 |
| MUAC (cm) | <23 | ≥23 | aRR or MD (95% CI) † | P | |
|---|---|---|---|---|---|
| Birthweight (g) | 1947 | 2833 (2794, 2873) | 2983 (2958, 3008) | −111 (−157, −65) | <0.001 |
| Head circumference (cm) | 1890 | 32.7 (32.5, 32.9) | 33.1 (33.0, 33.2) | −0.3 (−0.5, −0.1) | 0.001 |
| Abdominal circumference (cm) | 1893 | 31.3 (31.0, 31.5) | 31.9 (31.8, 32.0) | −0.6 (−0.8, −0.3) | <0.001 |
| Crown‐heel length (cm) | 1896 | 47.5 (47.2, 47.7) | 47.7 (47.5, 47.9) | −0.5 (−0.9, −0.1) | 0.008 |
| Low birthweight | 1947 | 20.5 (111/541) | 12.9 (182/1406) | 1.46 (1.18, 1.80) | <0.001 |
| Preterm birth | 1276 | 12.6 (45/357) | 7.1 (65/919) | 1.75 (1.21, 2.52) | 0.003 |
| Small‐for‐gestational age | 1276 | 18.0 (64/356) | 11.9 (109/917) | 1.43 (1.08, 1.88) | 0.011 |
| BMI (kg m−2) | <19.8 | ≥19.8 | aRR or MD (95% CI) ‡ | P | |
|---|---|---|---|---|---|
| Birthweight (g) | 1947 | 2819 (2760, 2879) | 2957 (2934, 2980) | −139 (−204, −74) | <0.001 |
| Head circumference (cm) | 1890 | 32.7 (32.4, 32.9) | 33.0 (32.9, 33.1) | −0.4 (−0.7, −0.1) | 0.009 |
| Abdominal circumference (cm) | 1893 | 31.2 (30.9, 31.6) | 31.8 (31.7, 31.9) | −0.6 (−1.0, −0.3) | 0.002 |
| Crown‐heel length (cm) | 1896 | 47.2 (47.6, 47.8) | 47.6 (47.4, 47.8) | −0.5 (−1.0, 0.1) | 0.90 |
| Low birthweight | 1947 | 20.5 (45/220) | 14.4 (248/1,727) | 1.41 (1.07, 1.86) | 0.014 |
| Preterm birth | 1276 | 12.9 (20/155) | 8.0 (90/1121) | 1.59 (1.01, 2.50) | 0.043 |
| Small‐for‐gestational age | 1276 | 19.4 (30/156) | 12.8 (143/1121) | 1.49 (1.05, 2.11) | 0.027 |
Note: Data are % (n) or mean (95% CI). RR, risk ratio; CI, confidence interval; MD, mean difference. *Adjusted for height, MUAC, age, gravidity, treatment arm, timing of birthweight measurement (except preterm birth) and newborn sex. †Adjusted for height, age, gravidity, treatment arm, timing of birthweight measurement (except preterm birth) and newborn sex. ‡Adjusted for age, gravidity, treatment arm, timing of birthweight measurement (except preterm birth) and newborn sex.
Association of SPAZ‐IPTp with birthweight and adverse infant growth outcomes
Akin to observations made in the original trial, SPAZ‐IPTp was associated with an increase in mean birthweight (P = 0.019) and a reduction in the risk of LBW (P = 0.002) (Table 4). Similar trends were observed among a subset of women who had both GWG and birthweight data available, although the association between SPAZ‐IPTp and birthweight was not statistically significant in this subset of women (P = 0.13) (Table 4). Inclusion of GWG as a covariate in the model evaluating the impact of SPAZ‐IPTp on birthweight reduced the adjusted birthweight coefficient associated with the intervention by 18% from 44 g (95% CI −12, 100; P = 0.13, Table 4) to 36 g (95% CI −21, 92; P = 0.21). Inclusion of GWG in the adjusted model examining the effect of SPAZ‐IPTp on LBW resulted in a change in the risk ratio from 0.69 (0.50, 0.95; P = 0.018, Table 4) to 0.72 (0.52, 0.99; P = 0.042). GWG was associated with increased birthweight (P = 0.014) and a reduction of LBW risk (P = 0.022) in both multivariable models. These observations suggest that the positive effect of SPAZ‐IPTp on birthweight may be partly mediated by its effect on GWG.
Table 4.
Association of SPAZ‐IPTp with maternal nutritional status and infant growth outcomes (adjusted analysis)
| Total | SPCQ | SPAZ |
aRR or MD (95% CI) |
P | |
|---|---|---|---|---|---|
| Mother | |||||
| Gestational weight gain (g/week) * | |||||
| All | 948 | 364 (341, 387) | 419 (398, 441) | 58 (26, 90) | <0.001 |
| With post‐natal BMI data | 480 | 382 (349, 415) | 415 (383, 446) | 33 (−13, 79) | 0.15 |
| Postnatal visit | |||||
| BMI (kg m−2) † | 811 | 21.9 (21.6, 22.2) | 22.3 (21.9, 22.6) | 0.4 (0.0, 0.9) | 0.043 |
| With gestational weight gain data | 480 | 21.8 (21.3, 22.3) | 22.5 (22.1, 22.9) | 0.7 (0.2, 1.3) | 0.012 |
| BMI categories † | |||||
| Underweight (<18.5) | 55 (13.1) | 28 (7.0) | 0.55 (0.36, 0.82) | 0.004 | |
| Normal (≥18.5, <25) | 299 (72.2) | 309 (77.8) | Reference | ||
| Overweight (≥25, <30) | 53 (12.8) | 51 (12.9) | 1.01 (0.72, 1.42) | 0.95 | |
| Obese (≥30) | 7 (1.7) | 10 (2.5) | 1.29 (0.51, 3.27) | 0.59 | |
| MUAC (cm) ‡ | 824 | 24.3 (24.0, 24.5) | 24.6 (24.3, 24.9) | 0.4 (0.0, 0.8) | 0.049 |
| With gestational weight gain data | 491 | 24.4 (24.1, 24.7) | 24.7 (24.4, 25.0) | 0.4 (−0.1, 0.9) | 0.11 |
| MUAC <23 cm | 824 | 101 (24.4) | 86 (21.0) | 0.84 (0.65, 1.08) | 0.17 |
| Newborn/Infant | |||||
| Birth § | |||||
| Birthweight | 1,947 | 2922 (2889, 2954) | 2961 (2933, 2989) | 48 (8, 88) | 0.019 |
| With gestational weight gain data | 915 | 2947 (2900, 2993) | 2976 (2937, 3015) | 44 (−12, 100) | 0.13 |
| Low birthweight | 1947 | 168 (17.5) | 125 (12.7) | 0.72 (0.58, 0.88) | 0.002 |
| With gestational weight gain data | 915 | 72 (16.5) | 57 (11.9) | 0.69 (0.50, 0.94) | 0.018 |
| Postnatal visit ¶ | |||||
| Underweight (WAZ < −2) | 828 | 49 (11.6) | 29 (7.1) | 0.61 (0.39, 0.94) | 0.025 |
| Stunted (LAZ < −2) | 788 | 167 (42.2) | 166 (42.4) | 1.00 (0.84, 1.19) | 0.99 |
| Wasted (WLZ < −2) | 779 | 33 (8.4) | 13 (3.4) | 0.39 (0.21, 0.72) | 0.003 |
Note: Numbers are n (%) or mean (95% CI). BMI, body mass index; MUAC, mid‐upper arm circumference; WAZ, weight‐for‐age z‐score; LAZ, length‐for‐age z‐score; WLZ, weight‐for‐length z‐score. *Adjusted for maternal height, GA at first measurement, and length of time interval between weight measurements. †Adjusted for age, gravidity, rural residence, ethnicity, literacy and income status. ‡Adjusted for age, gravidity, rural residence, ethnicity, literacy, income status and height. §Adjusted for age, gravidity, height, MUAC, timing of weight measurement, newborn sex. ¶Adjusted for age, parent income status and literacy.
At the postnatal visit, SPAZ‐IPTp was associated with a reduced risk of the infant being underweight or wasted, but not stunted (Table 4). When gestational age at delivery was included in the model for the subset of 578 infants with ultrasound‐estimated gestation, SPAZ‐IPTp remained associated with a reduced risk for wasting [0.40 (0.20, 0.81), P = 0.011], but not underweight [0.87 (0.53, 1.45), P = 0.60].
Association of SPAZ‐IPTp with maternal postnatal nutritional status
Because SPAZ‐IPTp was associated with higher GWG, we evaluated whether this increase in GWG may be reflected in changes in maternal nutritional status following delivery. A total of 839 (40.1%) mother–infant pairs had a follow‐up postnatal visit (Fig. 1). The mean [median] time between delivery and the follow‐up visit was 56 days [34], and was similar in each treatment arm (P = 0.69). Over 95% of women were exclusively breastfeeding at the time of their postnatal visit.
Receipt of SPAZ‐IPTp was associated with an increase in maternal postnatal BMI and in MUAC (Table 4). Specifically, SPAZ‐IPTp was associated with a reduced risk of women being underweight at postnatal visit (Table 4), but did not affect the prevalence of overweight or obese women. Similar observations were made when only women who had complete data for both GWG and postnatal BMI/MUAC were analysed (Table 4). Background characteristics of women with complete data for GWG and post‐natal anthropometric assessment and those who had data for one or the other were similar (Supporting Information http://onlinelibrary.wiley.com/doi/10.1111/mcn.12215/suppinfo).
Discussion
We observed that macronutrient undernutrition in pregnancy is not uncommon in rural PNG. We found that GWG, enrolment BMI and enrolment MUAC are important predictors of birthweight. Low GWG, BMI and MUAC were all associated with increased risk of LBW delivery. SPAZ‐IPTp was associated with increased GWG. This apparent effect may in part explain why women who received SPAZ‐IPTp are at a reduced risk of being underweight 4–6 weeks post‐partum. Furthermore, our analysis suggests that increased GWG may be one of the mechanisms by which SPAZ‐IPTp improves birthweight. The effect of SPAZ‐IPTp on birthweight, irrespective of mechanism, may translate into downstream effects for infant growth: SPAZ‐IPTp was associated with a reduced risk of wasting 4–6 weeks post‐partum. Limitations of this exploratory analysis include the high level of (non‐differential) loss to follow‐up for the postpartum visit and partial availability of GWG data.
A low MUAC was associated with LBW, decreased birthweight and PTB, as previously reported in Indonesia (Sebayang et al. 2011). Because MUAC fluctuates less with gestation than BMI, it is a useful tool to identify women with macronutrient undernutrition in antenatal clinics in developing countries, where presentation is often late (Ververs et al. 2013). Other measures also appear to be of value. A pregnancy BMI <19.8 kg m−2 has been recommended by WHO as identifying women who require intensified antenatal follow‐up (WHO 1995). In the Democratic Republic of Congo, babies of women with malaria and BMI <19.8 kg m−2 were especially likely to be SGA (Landis et al. 2009), and in our cohort, a low BMI at first antenatal visit was associated with an increased risk of both LBW and SGA.
Our findings suggest that macronutrient undernutrition represents a significant risk factor for decreased birthweight in North Coast women. In addition to interventions reducing the burden of infection and inflammation in pregnancy, nutritional interventions may improve pregnancy outcomes (Imdad & Bhutta 2012). Provision of culturally appropriate nutritional advice by health workers, and facilitating access to nutritious and affordable foods may be useful in the PNG context (Muller et al. 2002). Balanced energy and protein supplements to undernourished pregnant women may improve fetal growth, and could reduce the risk of stillbirth and SGA (Ceesay et al. 1997; Imdad & Bhutta 2012; Ota et al. 2012), although most supplementation trials report moderate‐to‐small effects on birthweight.
The SPAZ‐IPTp trial in PNG, which formed the basis for the present analysis, was the second trial that convincingly demonstrated an association between SPAZ‐IPTp and increased mean birthweight (Luntamo et al. 2010; Unger et al. 2015). We now show that SPAZ‐IPTp also increased GWG, an important predictor of birth size in our and other similar cohorts (Hanieh et al. 2014). More importantly, increasing GWG may be one of the mechanisms by which SPAZ‐IPTp improved birthweight: our analysis suggests that the association between SPAZ‐IPTp and birthweight is in part driven by GWG. There are several possible explanations for the SPAZ‐IPTp effect on GWG. In addition to reducing malaria, SPAZ‐IPTp may reduce the burden of other infectious diseases (Porco et al. 2009; Chico et al. 2013; Keenan et al. 2013). This reduction in infection and inflammation may reduce maternal energy requirements (Prentice et al. 2008), thus potentially making more energy and nutrients available to mother and fetus. Another explanation for improved GWG may be the effect of antibiotics on the gut microbiome (Angelakis et al. 2013). In rural PNG, low‐protein high‐fibre diets are common (Muller et al. 2002). AZ (and SP) therapy may temporarily alter bacterial species composition in favour of organisms that are better at breaking down, for example, complex carbohydrates, thereby increasing ‘active’ nutrient availability in the absence of dietary changes (Larsbrink et al. 2014), potentially translating into increased GWG and fat storage (Backhed et al. 2004; Cox & Blaser 2013).
Improved GWG among women receiving SPAZ‐IPTp may explain the reduced risk for women to be underweight at their postnatal visit. This may be of importance in settings where nutritional depletion as a result of pregnancy continues to be a problem, including PNG (Garner et al. 1994b). SPAZ‐IPTp also appeared to reduce fetal and infant wasting, but we did not evaluate its effects on body composition in our participants. In a previous study from Malawi, SPAZ‐IPTp reduced the risk of being stunted and underweight at 1 month (Luntamo et al. 2013), findings that are broadly similar to the reduced risk of wasting and underweight observed in the present study. Further study is required to determine whether the effects of SPAZ‐IPTp on maternal and child anthropometry are primarily mediated through positive benefits for increased lean tissue mass or reflect fat accumulation, which could increase the risk of later obesity and insulin resistance (Yajnik et al. 2003).
Our study has limitations. First, there was significant loss to follow‐up for mother/infant outcomes assessed at delivery and during the postnatal visit, only a subset of women had weight gain data collected, and stillbirths were excluded from analyses. This demands for cautious interpretation of our findings as the risk of selection bias is high. Reassuringly, there was no differential loss to follow‐up by treatment arm for any outcome measure, and sub‐analyses among women with complete data yielded similar results (Table 4); background characteristics were also similar between women with complete and partial data for GWG and postnatal BMI (Supporting Information http://onlinelibrary.wiley.com/doi/10.1111/mcn.12215/suppinfo). Second, we were unable to obtain pre‐pregnancy weight data, precluding an evaluation of the associations between total pregnancy weight gain or pre‐pregnancy BMI with outcomes. Third, exclusion criteria of the original trial may have resulted in selection bias and could affect generalisability of our findings: most women presented at >26 weeks gestation, and nutritional and socio‐economic features among these women may have been different. Women included at baseline, but without pregnancy outcome follow‐up resulting in a live birth (n = 577), were excluded from analyses. These women had similar anthropometric characteristics but were more likely to be in their first pregnancy and illiterate (Supporting Information http://onlinelibrary.wiley.com/doi/10.1111/mcn.12215/suppinfo). Fourth, only a subset of women had ultrasound‐dated pregnancies and most pregnancies were dated based on mid‐pregnancy fetal biometry: this may result in selection bias and could affect accuracy of gestational age estimation, respectively. Furthermore, maternal height and weight measurements were made with relatively crude instruments, which may reduce the precision of GWG, BMI and MUAC measurements. Women in the intervention arm received three courses of active antimalarial treatment, and AZ was given together with SP: as such we are unable to distinguish between the effects of these components. Lastly, we did not measure dietary intake, micronutrient levels or other possible confounders, such as iron/folate supplements and albendazole treatment.
In conclusion, undernutrition is common in pregnant women in coastal PNG, and is associated with adverse pregnancy outcomes, including LBW. Interventions that improve maternal nutritional status and promote adequate pregnancy weight gain are urgently required. Further research evaluating SPAZ‐IPTp may be warranted, given that, in addition to its previously shown impact on malaria and sexually transmitted infections, it appears to increase GWG, and it may prevent maternal nutritional depletion and lead to improved infant growth. Greater understanding of the mechanisms underlying these possible positive benefits is needed. Given that a large number of pregnant women in developing countries are at risk of infection and undernutrition, a strategy such as SPAZ‐IPTp, which aims to address a number of risk factors underlying the ‘syndrome’ of LBW, may be worth exploring further.
Sources of funding
This research was supported by the Malaria in Pregnancy Consortium, through a grant from the Bill & Melinda Gates Foundation (46099); the Pregvax Consortium, through a grant from the European Union's Seventh Framework Programme FP7‐2007‐HEALTH (PREGVAX 201588); and Pfizer Inc (investigator‐initiated research grant WS394663). SK is supported through an NHMRC Early Career Fellowship (GNT1052960), and IM received an NHMRC Senior Research Fellowship (#1043345). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributor statement
Conceptualised and designed the study: SJR, HWU. Collected data: MO, RW, HWU. Analysed the data: HWU, SJR. Interpreted the data: HWU, SJR, IM, SK, PB. Wrote the first draft of the manuscript: HWU, SJR. Read, edited and approved the final version of the manuscript: HWU, RW, MO, PB, SK, IM, SJR.
Supporting information
Table S1. Comparisons of background characteristics of women included in, and excluded from, all outcome analyses; overall, and by treatment arm.
Table S2. Characteristics of pregnant women at first antenatal visit by body mass index and mid‐upper arm circumference groupings.
Table S3. Background characteristics of pregnant women with complete data for gestational weight gain and postnatal visit compared to women with incomplete data; overall, and by treatment arm.
Supporting info item
Acknowledgements
The authors would like to thank all participating women for their help with this research. We are also grateful for the support received by the staff at participating health centres. Further thanks go to the IPTp study clinical, administrative, and laboratory teams, in particular Dr Sarah Hanieh, Tiamarie Badem, Lynette Bureng, Anna Samuel, Elvin Lufele, Samantha Mal, Dr Leanne Robinson, Dr Valerie Briand, and Professor Peter Siba.
Unger, H. W. , Wangnapi, R. A. , Ome‐Kaius, M. , Boeuf, P. , Karl, S. , Mueller, I. , and Rogerson, S. J. (2016) Azithromycin‐containing intermittent preventive treatment in pregnancy affects gestational weight gain, an important predictor of birthweight in Papua New Guinea – an exploratory analysis. Maternal & Child Nutrition, 12: 699–712. doi: 10.1111/mcn.12215.
References
- Abrams B. & Selvin S. (1995) Maternal weight gain pattern and birth weight. Obstetrics and Gynecology 86, 163–169. [DOI] [PubMed] [Google Scholar]
- Allen S.J., Raiko A., O'Donnell A., Alexander N.D. & Clegg J.B. (1998) Causes of preterm delivery and intrauterine growth retardation in a malaria endemic region of Papua New Guinea. Archives of Disease in Childhood. Fetal and Neonatal Edition 79, F135–F140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelakis E., Merhej V. & Raoult D. (2013) Related actions of probiotics and antibiotics on gut microbiota and weight modification. The Lancet Infectious Diseases 13, 889–899. [DOI] [PubMed] [Google Scholar]
- Backhed F., Ding H., Wang T., Hooper L.V., Koh G.Y., Nagy A. et al (2004) The gut microbiota as an environmental factor that regulates fat storage. Proceedings of the National Academy of Sciences of the United States of America 101, 15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D.J., Gluckman P.D., Godfrey K.M., Harding J.E., Owens J.A. & Robinson J.S. (1993) Fetal nutrition and cardiovascular disease in adult life. Lancet 341, 938–941. [DOI] [PubMed] [Google Scholar]
- Black R.E., Allen L.H., Bhutta Z.A., Caulfield L.E., de Onis M., Ezzati M. et al (2008) Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371, 243–260. [DOI] [PubMed] [Google Scholar]
- Black R.E., Victora C.G., Walker S.P., Bhutta Z.A., Christian P., de Onis M. et al (2013) Maternal and child undernutrition and overweight in low‐income and middle‐income countries. Lancet 382, 427–451. [DOI] [PubMed] [Google Scholar]
- van den Broek N.R., White S.A., Goodall M., Ntonya C., Kayira E., Kafulafula G. et al (2009) The APPLe study: a randomized, community‐based, placebo‐controlled trial of azithromycin for the prevention of preterm birth, with meta‐analysis. PLoS Medicine 6, e1000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butte N.F. & King J.C. (2005) Energy requirements during pregnancy and lactation. Public Health Nutrition 8, 1010–1027. [DOI] [PubMed] [Google Scholar]
- Calvo E.B., Lopez L.B., Balmaceda Ydel V., Poy M.S., Gonzalez C., Quintana L. et al (2009) Reference charts for weight gain and body mass index during pregnancy obtained from a healthy cohort. The Journal of Maternal‐fetal and Neonatal Medicine 22, 36–42. [DOI] [PubMed] [Google Scholar]
- Carmichael S., Abrams B. & Selvin S. (1997) The association of pattern of maternal weight gain with length of gestation and risk of spontaneous preterm delivery. Paediatric and Perinatal Epidemiology 11, 392–406. [DOI] [PubMed] [Google Scholar]
- Ceesay S.M., Prentice A.M., Cole T.J., Foord F., Weaver L.T., Poskitt E.M. et al (1997) Effects on birth weight and perinatal mortality of maternal dietary supplements in rural Gambia: 5 year randomised controlled trial. BMJ (Clinical Research Ed.) 315, 786–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chico R.M., Mayaud P., Ariti C., Mabey D., Ronsmans C. & Chandramohan D. (2012) Prevalence of malaria and sexually transmitted and reproductive tract infections in pregnancy in sub‐Saharan Africa: a systematic review. JAMA: The Journal of the American Medical Association 307, 2079–2086. [DOI] [PubMed] [Google Scholar]
- Chico R.M., Hack B.B., Newport M.J., Ngulube E. & Chandramohan D. (2013) On the pathway to better birth outcomes? A systematic review of azithromycin and curable sexually transmitted infections. Expert Review of Anti‐infective Therapy 11, 1303–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox L.M. & Blaser M.J. (2013) Pathways in microbe‐induced obesity. Cell Metabolism 17, 883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner P., Dubowitz L., Baea M., Lai D., Dubowitz M. & Heywood P. (1994a) Birthweight and gestation of village deliveries in Papua New Guinea. Journal of Tropical Pediatrics 40, 37–40. [DOI] [PubMed] [Google Scholar]
- Garner P., Smith T., Baea M., Lai D. & Heywood P. (1994b) Maternal nutritional depletion in a rural area of Papua New Guinea. Tropical and Geographical Medicine 46, 169–171. [PubMed] [Google Scholar]
- Hadlock F.P., Deter R.L., Harrist R.B. & Park S.K. (1984) Estimating fetal age: computer‐assisted analysis of multiple fetal growth parameters. Radiology 152, 497–501. [DOI] [PubMed] [Google Scholar]
- Hanieh S., Ha T.T., Simpson J.A., Thuy T.T., Khuong N.C., Thoang D.D. et al (2014) Postnatal growth outcomes and influence of maternal gestational weight gain: a prospective cohort study in rural Vietnam. BMC Pregnancy and Childbirth 14, 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imdad A. & Bhutta Z.A. (2012) Maternal nutrition and birth outcomes: effect of balanced protein‐energy supplementation. Paediatric and Perinatal Epidemiology 26 (Suppl. 1), 178–190. [DOI] [PubMed] [Google Scholar]
- IOM (Institute of Medicine) (2009) Weight Gain during Pregnancy, The National Academies Press: Washington, DC. [Google Scholar]
- James W.P., Mascie‐Taylor G.C., Norgan N.G., Bistrian B.R., Shetty P.S. & Ferro‐Luzzi A. (1994) The value of arm circumference measurements in assessing chronic energy deficiency in Third World adults. European Journal of Clinical Nutrition 48, 883–894. [PubMed] [Google Scholar]
- Keenan J.D., Emerson P.M., Gaynor B.D., Porco T.C. & Lietman T.M. (2013) Adult mortality in a randomized trial of mass azithromycin for trachoma. JAMA Internal Medicine 173, 821–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krasovec K. & Anderson M. (1991) Maternal Nutrition and Pregnancy Outcomes: Anthropometric Assessment, Pan American Health Organization and World Health Organisation: Washington, DC. [Google Scholar]
- Laman M., Moore B.R., Benjamin J., Padapu N., Tarongka N., Siba P. et al (2014) Comparison of an assumed versus measured leucocyte count in parasite density calculations in Papua New Guinean children with uncomplicated malaria. Malaria Journal 13, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis S.H., Lokomba V., Ananth C.V., Atibu J., Ryder R.W., Hartmann K.E. et al (2009) Impact of maternal malaria and under‐nutrition on intrauterine growth restriction: a prospective ultrasound study in Democratic Republic of Congo. Epidemiology and Infection 137, 294–304. [DOI] [PubMed] [Google Scholar]
- Larsbrink J., Rogers T.E., Hemsworth G.R., McKee L.S., Tauzin A.S., Spadiut O. et al (2014) A discrete genetic locus confers xyloglucan metabolism in select human gut Bacteroidetes. Nature 506, 498–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liljestrand J. & Bergstrom S. (1991) Antenatal nutritional assessment: the value of upper arm circumference. Gynecologic and Obstetric Investigation 32, 81–83. [DOI] [PubMed] [Google Scholar]
- Loughna P., Chitty L., Evans T. & Chudleigh T. (2009) Fetal size and dating: charts recommended for clinical obstetric practice. Ultrasound 17, 161–167. [Google Scholar]
- Luntamo M., Kulmala T., Mbewe B., Cheung Y.B., Maleta K. & Ashorn P. (2010) Effect of repeated treatment of pregnant women with sulfadoxine‐pyrimethamine and azithromycin on preterm delivery in Malawi: a randomized controlled trial. The American Journal of Tropical Medicine and Hygiene 83, 1212–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luntamo M., Kulmala T., Cheung Y.B., Maleta K. & Ashorn P. (2013) The effect of antenatal monthly sulphadoxine‐pyrimethamine, alone or with azithromycin, on foetal and neonatal growth faltering in Malawi: a randomised controlled trial. Tropical Medicine and International Health 18, 386–397. [DOI] [PubMed] [Google Scholar]
- Mola G.D., Kombuk B. & Amoa A.B. (2011) Poor weight gain in late third trimester: a predictor of poor perinatal outcome for term deliveries? Papua and New Guinea Medical Journal 54, 164–173. [PubMed] [Google Scholar]
- Muller I., Betuela I. & Hide R. (2002) Regional patterns of birthweights in Papua New Guinea in relation to diet, environment and socio‐economic factors. Annals of Human Biology 29, 74–88. [DOI] [PubMed] [Google Scholar]
- Ota E., Tobe‐Gai R., Mori R. & Farrar D. (2012) Antenatal dietary advice and supplementation to increase energy and protein intake. Cochrane Database of Systematic Reviews (9), CD000032. [DOI] [PubMed] [Google Scholar]
- Porco T.C., Gebre T., Ayele B., House J., Keenan J., Zhou Z. et al (2009) Effect of mass distribution of azithromycin for trachoma control on overall mortality in Ethiopian children: a randomized trial. JAMA: The Journal of the American Medical Association 302, 962–968. [DOI] [PubMed] [Google Scholar]
- Prentice A.M., Gershwin M.E., Schaible U.E., Keusch G.T., Victora C.G. & Gordon J.I. (2008) New challenges in studying nutrition‐disease interactions in the developing world. The Journal of Clinical Investigation 118, 1322–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice A.M., Ward K.A., Goldberg G.R., Jarjou L.M., Moore S.E., Fulford A.J. et al (2013) Critical windows for nutritional interventions against stunting. The American Journal of Clinical Nutrition 97, 911–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primhak R.A. & MacGregor D.F. (1991) Ethnic and environmental factors affecting fetal growth in Papua New Guinea. Annals of Human Biology 18, 235–243. [DOI] [PubMed] [Google Scholar]
- Rijken M.J., Rijken J.A., Papageorghiou A.T., Kennedy S.H., Visser G.H., Nosten F. et al (2011) Malaria in pregnancy: the difficulties in measuring birthweight. BJOG: An International Journal of Obstetrics and Gynaecology 118, 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R., Dey S.K. & Fisher S.J. (2014) Preterm labor: one syndrome, many causes. Science 345, 760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosanas‐Urgell A., Mueller D., Betuela I., Barnadas C., Iga J., Zimmerman P.A. et al (2010) Comparison of diagnostic methods for the detection and quantification of the four sympatric Plasmodium species in field samples from Papua New Guinea. Malaria Journal 9, 361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaible U.E. & Kaufmann S.H. (2007) Malnutrition and infection: complex mechanisms and global impacts. PLoS Medicine 4, e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sebayang S.K., Dibley M.J., Kelly P., Shankar A.V. & Shankar A.H. (2011) Modifying effect of maternal nutritional status on the impact of maternal multiple micronutrient supplementation on birthweight in Indonesia. European Journal of Clinical Nutrition 65, 1110–1117. [DOI] [PubMed] [Google Scholar]
- Turner C., Carrara V., Thien N.A., Paw N.M., Rijken M., McGready R. et al (2013) Changes in the body weight of term infants, born in the tropics, during the first seven days of life. BMC Pediatrics 13, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger H.W., Karl S., Wangnapi R.A., Siba P., Mola G., Walker J. et al (2014) Fetal size in a rural Melanesian population with minimal risk factors for Growth restriction: an observational ultrasound study from Papua New Guinea. The American Journal of Tropical Medicine and Hygiene 92, 178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger H.W., Ome‐Kaius M., Wangnapi R.A., Umbers A.J., Hanieh S., Suen C.S. et al (2015) Sulphadoxine‐pyrimethamine plus azithromycin for the prevention of low birthweight in Papua New Guinea: a randomised controlled trial. BMC Medicine 13, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UN (2014) Child mortality estimates. United Nations, Geneva.
- Ververs M.T., Antierens A., Sackl A., Staderini N. & Captier V. (2013) Which anthropometric indicators identify a pregnant woman as acutely malnourished and predict adverse birth outcomes in the humanitarian context? PLoS Currents 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (1995) Maternal anthropometry and pregnancy outcomes. a WHO Collaborative Study. Bulletin of the World Health Organization 73 (Suppl. ), 1–98. [PMC free article] [PubMed] [Google Scholar]
- WHO (2006) WHO Child Growth Standards: Length/Height‐for‐Age, Weight‐for‐Age, Weight‐for‐Length, Weight‐for‐Height, and Body Mass Index‐for‐Age: Methods and Development. World Health Organisation (WHO): Geneva. [Google Scholar]
- WHO (2014) WHO Policy Brief for the Implementation of Intermittent Preventive Treatment in Malaria in Pregnancy using Sulfadoxine‐Pyrimethamine (IPTp‐SP), World Health Organisation (WHO): Geneva. January 2014. [Google Scholar]
- Yajnik C.S., Fall C.H., Coyaji K.J., Hirve S.S., Rao S., Barker D.J. et al (2003) Neonatal anthropometry: the thin‐fat Indian baby. The Pune Maternal Nutrition Study. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity 27, 173–180. [DOI] [PubMed] [Google Scholar]
- Zou G. (2004) A modified poisson regression approach to prospective studies with binary data. American Journal of Epidemiology 159, 702–706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparisons of background characteristics of women included in, and excluded from, all outcome analyses; overall, and by treatment arm.
Table S2. Characteristics of pregnant women at first antenatal visit by body mass index and mid‐upper arm circumference groupings.
Table S3. Background characteristics of pregnant women with complete data for gestational weight gain and postnatal visit compared to women with incomplete data; overall, and by treatment arm.
Supporting info item


