Abstract
Observational studies suggest that high iron intake during pregnancy is associated with the risk of gestational diabetes. As such studies are prone to bias, we re‐analysed data from a randomised controlled trial of iron supplementation to see whether it supports the risk found in observational studies. The trial was conducted in primary health care setting in five municipalities in Finland in 1985–1986. The participants were 2944 women (95% of pregnant women in the area) who were randomly allocated either to (1) the selective iron group (elemental iron 50 mg twice a day only if diagnosed as anaemic, continuing until their haemoglobin increased to 110 g L−1) or (2) the routine iron group (elemental iron 100 mg day−1 throughout the pregnancy regardless of haemoglobin level). The numbers of women in the analyses were 1358 and 1336, respectively. The main outcome measure was a composite variable including any glucose intolerance‐related outcome (e.g. glucosuria, gestational diabetes, large‐for‐gestational‐age child) in mothers' or children's patient records during pregnancy and post‐partum. There were no statistically significant differences in the incidence of the primary outcome between the selective iron and the routine iron groups (13.0 vs. 11.0%, P = 0.12). The most common outcome was large‐for‐gestational‐age calculated from children's hospital data (8.3 vs. 8.2%, P = 0.95). The results were mainly similar when stratified by the mothers' baseline haemoglobin level, body mass index or gestational weight gain. Routine iron supplementation throughout pregnancy did not increase the risk of glucose intolerance during pregnancy. The results need to be confirmed in future trials.
Keywords: iron supplementation, randomised controlled trial, pregnancy, glucose intolerance, gestational diabetes
Introduction
Gestational diabetes mellitus (GDM) is defined as glucose intolerance that is recognised during pregnancy for the first time (American Diabetes Association 2013). GDM and milder glucose intolerance increase the risk of macrosomia and other adverse pregnancy outcomes (Stotland et al. 2004; HAPO Study Cooperative Research Group et al. 2008; Ju et al. 2008). In the long term, GDM predicts the risk of overweight, type 2 diabetes and metabolic syndrome in the child (Dabelea 2007; Clausen et al. 2008, 2009; Pirkola et al. 2010) and type 2 diabetes in the mother (Kim et al. 2002; Lee et al. 2007). The main risk factors for GDM are being overweight in pre‐pregnancy, advanced maternal age and family history of diabetes (American Diabetes Association 2003). As the incidence of GDM has been increasing over the last 20 years (Ferrara 2007; Galtier 2010) and it has been estimated that only about half of the cases are explained by people being overweight (Kim et al. 2010), it is important to search for other modifiable risk factors.
It has been hypothesised that iron may have a role in the development of GDM. High haemoglobin (Hb) level in early pregnancy has been positively (Lao et al. 2002) and low Hb level negatively (Lao & Ho 2004) associated with the risk of GDM, although not in all studies (Chen et al. 2006). Higher levels of serum ferritin, transferrin and some other markers of iron stores have been found among women with GDM compared with women without GDM (Lao et al. 2001; Chen et al. 2006; Afkhami‐Ardekani & Rashidi 2009; Soubasi et al. 2010; Derbent et al. 2013). Serum ferritin level is also elevated in inflammatory states and therefore may not necessarily indicate increased body iron stores (Williams et al. 2002; Mainous et al. 2004). Higher levels of hepcidin, a hormone‐regulating iron metabolism, have also been associated with GDM (Derbent et al. 2013).
Regarding iron intake from food and/or supplements, the results of the previous observational studies are partly contradictory (Bo et al. 2009; Bowers et al. 2011; Qiu et al. 2011; Helin et al. 2012). As observational studies are prone to confounding, the effect of iron supplementation on the risk of GDM should be examined in randomised controlled trials. One such study from Hong Kong has been reported to date (Chan et al. 2009). It found no difference in the incidence of GDM between women randomised to take 60 mg elemental iron or a placebo daily, but the overall compliance to the intervention as well as participation in the follow‐up were relatively low. More evidence is needed on this topic because iron supplementation and GDM are common in pregnancy (e.g. 78% and 13% of pregnant women in Finland, respectively; Arkkola et al. 2006; National Institute for Health and Welfare 2012). We examined the effects of a trial comparing routine and selective iron supplementation on the risk of glucose intolerance in pregnant women.
Key messages
Previous observational studies have suggested that high iron intake during pregnancy might increase the risk of gestational diabetes, but more evidence is needed from randomised controlled trials.
We used the opportunity to analyse data from a well‐implemented randomised controlled trial of iron supplementation from the 1980s.
There were no differences in the composite outcome variable (including any glucose intolerance‐related outcome) between women in the routine iron supplementation group and women who received iron supplementation only if they were diagnosed as anaemic.
The results suggest that routine iron supplementation may not increase the risk of glucose intolerance in pregnancy.
Material and methods
Study design and participants
We used the opportunity to analyse data from an old trial conducted in Finland in the mid‐1980s (Hemminki et al. 1989). The original trial was a population based multi‐centre randomised controlled trial aiming to study the health effects of routine iron supplementation on mothers' and infants' health. The methods and the main results have been described previously (Hemminki et al. 1989, 1991; Hemminki & Rimpela 1991a, 1991b; Hemminki & Merilainen 1995). Pregnant women were recruited in municipal maternity clinics in Tampere and five neighbouring municipalities in Southern Finland between April 1985 and May 1986. Briefly, all 51 municipal midwives (or public health nurses) working at 27 different clinics recruited the participants and carried out the study. All women attending the maternity clinics for the first time during their pregnancy (n = 3098) were screened for eligibility (Fig. 1). The exclusion criteria for participation were a serious chronic disease, Hb ≤ 110 g L−1, high probability of moving from the area before the birth of the child or enrolling later than at 16 weeks gestation. A total of 154 (5.0%) women were excluded before randomisation, nine of them would have been eligible.
Figure 1.
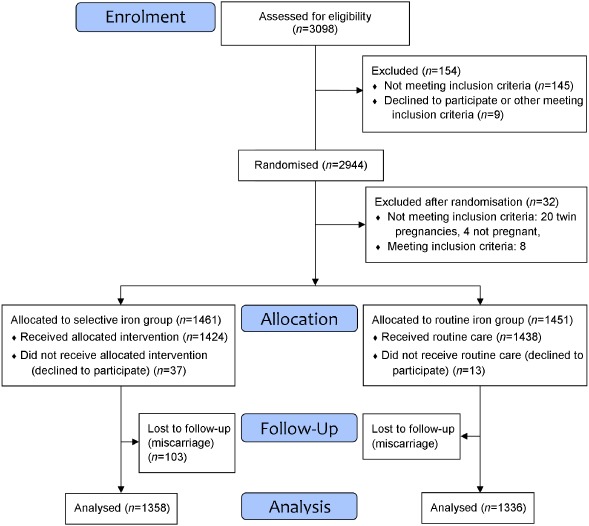
Flow diagram of the randomised controlled trial.
In total, 2944 women were randomised either to the selective iron group or the routine iron group by using random codes created by computer in blocks of 10 per clinic. The codes were kept in sealed envelopes stored in containers and the midwives were told to take them in order. The midwives gave the participants oral and written information on the study only after the randomisation and asked them to participate and to sign the informed consent form. Thirty‐two women were excluded after randomisation (Fig. 1). In addition, 103 (7.0%) participants in the selective iron group and 115 (7.9%) participants in the routine iron group had a miscarriage and were not included in the analyses. The final study population was therefore 1358 (93.0%) and 1336 (92.1%) participants in the two groups, respectively. This included also 37 and 13 women, respectively, who declined participation in the intervention, but were willing for data collection. They were asked to take iron as they wished. The study was conducted in accordance with Helsinki Declaration and the study protocol was accepted in seven ethics committees in the area.
Intervention
The participants in the routine iron group were advised to take elemental iron 100 mg day−1 throughout the pregnancy regardless of their Hb level (Hemminki et al. 1989). This advice was very similar to that in usual care practices in the 1980s. The participants in the selective iron group were advised to take elemental iron (50 mg twice a day) only if their Hb fell below 100 g L−1 after 14 weeks gestation on two consecutive visits and anaemia was confirmed in the laboratory. They were asked to continue supplementation for 2 months or until their Hb increased to 110 g L−1. As the midwives requested, the protocol was slightly changed in the middle of the study so that the participants in the selective iron group were also allowed to start iron supplementation if their Hb was <105 g L−1 after 33 weeks gestation.
The participants' compliance to their assigned treatment was assessed by questionnaires filled in during the routine prenatal visits at 28 and 36 weeks gestation. The participants were asked if they had used any iron containing supplements during the preceding 2 weeks and what the dosage had been. In the routine iron group of those answering this question (86–92% of all), 82% at 28 weeks and 73% at 36 weeks gestation had used iron containing supplements daily or almost daily (Hemminki et al. 1989). Among participants using iron supplements, the reported mean dosage of elemental iron was 124 mg day−1 at both time points. In the selective iron group of those answering this question (84–89% of all), 92% at 28 weeks and 84% at 36 weeks gestation had not used any iron containing supplements. Among participants using iron supplements, the reported mean dosage of elemental iron was 107 mg day−1 at 28 weeks and 121 mg day−1 at 36 weeks gestation.
Outcomes and data collection
GDM was not originally an outcome in the trial and it was not assessed systematically among all participants, but was abstracted from patient records as recognised in the usual care. In the 1980s, GDM was usually screened among women with risk factors (e.g. diabetes in close relatives, overweight, glucosuria, birthweight ≥4500 g in any previous pregnancy; Hyvönen 1991). All outcome data were based on children's hospital records or on routinely and prospectively recorded midwives' notes during prenatal and post‐natal visits (Hemminki et al. 1989). In total, the percentage of missing forms was 0.5% for children's records and 0.4% for the midwives forms. All births occurred in a hospital. Midwives themselves abstracted the data into study forms. The hospital data were abstracted by a trained research assistant who was not aware of the intervention assignment.
The primary outcome of the present study was a composite variable including any glucose intolerance‐related outcome described in detail in Table 1. Large for gestational age (LGA) was considered one of the indicators of glucose intolerance as women with GDM are more likely to have a LGA child as compared with other pregnant women (Wendland et al. 2012).
Table 1.
Definitions of the study outcomes
| Outcome | Description of the outcome* | The primary data source |
|---|---|---|
| 1. The primary reason for referring to a specialist in maternity hospital | Abnormal blood glucose values in daily follow‐up measurements, elevated blood sugar level or glucosuria | Midwives' notes |
| 2. The primary reason for inpatient care before delivery | Abnormal blood glucose values in daily follow‐up measurements, elevated blood sugar level or glucosuria | Midwives' notes |
| 3. Discharge diagnosis from birth hospital | Abnormal glucose tolerance or gestational diabetes | Midwives' notes |
| 4. Discharge diagnosis from birth hospital | Grouped using ICD 8 codes, including glucosuria, abnormal result in the oral glucose tolerance test, pre‐diabetes (impaired glucose tolerance) or diabetes mellitus during pregnancy | Children's hospital records |
| 5. Discharge diagnosis from birth hospital | ICD 8 code: A large for date child | Children's hospital records |
| 6. Large for gestational age | Birthweight above the 90th percentile of birthweight, defined separately for both sexes and each week of gestational age † | Children's hospital records |
| 7. Composite outcome (the primary outcome) | Any of the above outcomes | Midwives' notes, children's hospital records |
*Most common terms used in the primary data source or in the eighth revision of the International Classification of Diseases (ICD 8). †The researchers calculated the variable based on birthweight, gestational age at birth and sex. The reference data for the 90th percentile of birthweight were obtained from the Finnish Medical Birth Register (year 1987).
The following data were obtained from the midwives' forms: mothers' age, parity, height (all at the first visit), and gestation weeks, body weight and Hb at the four data collection visits (around 12, 20, 28 and 36 weeks gestation). Data on pre‐pregnancy weight (self‐reported at 12 weeks gestation) and socio‐economic status and smoking during pregnancy (at 28 weeks gestation) were obtained from the mothers' questionnaires. The definition of socio‐economic status was based on the classification of Statistics Finland for the year 1980 (Central Statistical Office of Finland 1981). The eight occupational categories were recoded into five by combining categories ‘employers’ and ‘entrepreneurs’ into ‘self‐employed persons’ and categories ‘retired’, ‘students’ and ‘others, e.g. unemployed’ into ‘others’ (Table 2). Gestation weeks at delivery were categorised into four categories (<37, 37.0–38.9, 39.0–41.9 and ≥42.0 weeks).
Table 2.
Background characteristics and pregnancy data, by the iron supplementation groups, means (SD) or numbers (%)
| Missing values (%)* | Selective iron (n = 1358) | Routine iron (n = 1336) | t/χ 2 | df | P‐value † | |
|---|---|---|---|---|---|---|
| Background characteristics | ||||||
| Age, years, mean (SD) | 0, 0 | 27.8 (5.6) | 27.7 (4.9) | |||
| Pre‐pregnancy body mass index (BMI), kg m−2, mean (SD) | 5.2, 3.6 | 22.4 (3.4) | 22.3 (3.5) | |||
| Categorised pre‐pregnancy BMI, n (%) | 5.2, 3.6 | |||||
| Underweight, BMI < 18.5 kg m−2 | 80 (6.2) | 108 (8.4) | ||||
| Normal weight, BMI 18.5–24.9 kg m−2 | 1004 (78.0) | 961 (74.6) | ||||
| Overweight, BMI 25.0–29.9 kg m−2 | 153 (11.9) | 174 (13.5) | ||||
| Obese, BMI ≥ 30 kg m−2 | 50 (3.9) | 45 (3.5) | ||||
| Previous births, n (%) | 4.5, 4.6 | |||||
| 0 | 559 (43.1) | 557 (43.7) | ||||
| 1 | 506 (39.0) | 499 (39.1) | ||||
| ≥2 | 232 (17.9) | 219 (17.2) | ||||
| Socio‐economic status, n (%) | 4.2, 2.4 | |||||
| Upper level employees | 179 (13.8) | 186 (14.3) | ||||
| Lower level employees | 659 (50.7) | 635 (48.7) | ||||
| Workers | 355 (27.3) | 363 (27.8) | ||||
| Self‐employed persons | 27 (2.1) | 29 (2.2) | ||||
| Others (e.g. unemployed, pensioners, students, unknown status) | 81 (6.2) | 91 (7.0) | ||||
| Smokers, n (%) ‡ | 8.7, 7.5 | |||||
| Non‐smoker | 966 (77.9) | 939 (76.0) | ||||
| Quitted smoking during pregnancy | 79 (6.4) | 90 (7.3) | ||||
| Occasional smoker | 73 (5.9) | 71 (5.7) | ||||
| Smoker | 122 (9.8) | 136 (11.0) | ||||
| Pregnancy data | ||||||
| Weeks gestation at the first prenatal visit, weeks, mean (SD) | 8.0, 8.0 | 11.5 (2.0) | 11.6 (2.1) | −0.322 | 2476 | 0.75 |
| Haemoglobin, g L−1, mean (SD) | ||||||
| At 12 weeks gestation (baseline) | 2.3, 2.1 | 136.5 (9.4) | 135.7 (9.3) | 2.162 | 2633 | 0.031 |
| At 20 weeks gestation | 2.1, 2.4 | 126.4 (8.9) | 127.0 (8.6) | −1.622 | 2632 | 0.11 |
| At 28 weeks gestation | 2.9, 2.9 | 123.9 (9.5) | 127.0 (8.6) | −8.718 | 2614 | <0.001 |
| At 36 weeks gestation | 7.9, 7.0 | 124.8 (9.6) | 131.2 (9.3) | −17.117 | 2491 | <0.001 |
| Body weight, kg, mean (SD) | ||||||
| Pre‐pregnancy | 4.8, 3.1 | 60.9 (10.0) | 60.8 (10.8) | 0.315 | 2586 | 0.75 |
| At 12 weeks gestation | 1.9, 2.0 | 63.1 (10.3) | 62.9 (10.2) | 0.443 | 2639 | 0.66 |
| At 20 weeks gestation | 1.5, 1.6 | 66.1 (10.2) | 66.0 (10.2) | 0.336 | 2650 | 0.74 |
| At 28 weeks gestation | 2.1, 1.9 | 70.0 (10.3) | 69.8 (10.5) | 0.329 | 2639 | 0.74 |
| At 36 weeks gestation | 5.5, 5.6 | 73.6 (10.6) | 73.5 (10.6) | 0.420 | 2542 | 0.68 |
| Gestational weight gain § , kg, mean (SD) | 9.6, 8.2 | 12.6 (4.1) | 12.6 (4.1) | −0.131 | 2453 | 0.90 |
| Categorised gestational weight gain, n (%) ¶ | 10.0, 8.5 | 1.408 | 2 | 0.50 | ||
| Below the recommendations | 404 (33.1) | 431 (35.3) | ||||
| Within the recommendations | 569 (46.6) | 545 (44.6) | ||||
| Above the recommendations | 249 (20.4) | 246 (20.1) | ||||
| Weeks gestation at delivery, weeks, mean (SD) | 0.7, 1.0 | 39.8 (1.9) | 39.9 (1.8) | −1.766 | 2669 | 0.08 |
| Categorised weeks gestation at delivery, n (%) | 0.7, 1.0 | 4.285 | 3 | 0.23 | ||
| <37 weeks | 52 (3.8) | 36 (2.7) | ||||
| 37.0–38.9 weeks | 139 (10.2) | 150 (11.2) | ||||
| 39.0–41.9 weeks | 995 (73.3) | 964 (72.2) | ||||
| ≥42.0 weeks | 171 (12.6) | 186 (13.9) |
*Percentage of women with missing data in the selective iron and the routine iron groups, respectively. †Two‐sided independent samples t‐test for continuous variables, two‐sided χ 2‐test for categorised variables. Differences in the baseline values were not tested statistically. ‡At 28 weeks gestation on average in both groups. §From pre‐pregnancy to 36 weeks gestation. ¶Compared with the recommendations of the Institute of Medicine 2009.
In sub‐analyses, the participants were categorised by their pre‐pregnancy body mass index (BMI) to underweight (BMI < 18.5 kg m−2), normal weight (BMI 18.5–24.9 kg m−2), overweight (BMI 25.0–29.9 kg m−2) and obese (BMI ≥ 30 kg m−2) women. They were also categorised based on whether their gestational weight gain was below, within or above the recommended weight gain, using the recommendations of the Institute of Medicine (IOM): 12.5–18.0 kg for underweight, 11.5–16.0 kg for normal weight, 7.0–11.5 kg for overweight and 5.0–9.0 kg for obese women (IOM 2009).
Statistical methods
The data were analysed by the intention to treat. Additionally, per‐protocol analyses were performed for the outcomes after excluding the 50 women who refused participation in the intervention but agreed to data collection. Post‐hoc power calculations showed that our sample size was adequate to detect a statistically significant between‐group difference in the composite outcome if the incidence of the outcome was 11% among the routine iron group and <7.8% or >14.7% in the selective iron group (assuming 80% power and two‐tailed P‐value 0.05).
Descriptive data are given as numbers (%) and means (SD). A chi‐square test was used to compare percentages and a t‐test to compare means between the groups. When comparing the incidence of the composite outcome between the groups, the analyses were also stratified by baseline Hb (<130, 130–139.9, ≥140 g L−1), BMI and gestational weight gain groups. All tests were two‐sided and P‐values < 0.05 were regarded as statistically significant. The data were analysed using the SPSS statistical software package version 20 (SPSS Inc., Chicago, IL, USA).
Results
Participants' background characteristics and pregnancy data
The participants' background characteristics were very similar in the two groups (Table 2). The participants were 28 years old and had a BMI of 22 kg m−2, on average, in both groups. The percentages of overweight or obese participants were relatively low in both groups. The groups were also very similar regarding the number of previous births, socio‐economic status and smoking.
The timing of the first visit was similar in both groups (Table 2). Regardless of randomisation the mean Hb level was slightly higher in the selective iron group than in the routine iron group at the first visit (137 vs. 136 g L−1, P = 0.031). As expected, from 28 weeks gestation onwards, the routine iron group had higher mean Hb level than the selective iron group (131 vs. 125 g L−1 at 36 weeks gestation, P < 0.001). There were no differences between the groups in body weight measurement at any of the visits, in the average gestational weight gain from pre‐pregnancy to 36 weeks gestation or in the percentages of participants gaining weight below, within or above the IOM's recommendations. The average duration of the pregnancy and the percentages of participants giving birth pre‐ or post‐term were also similar between the groups.
The incidence of glucose intolerance‐related outcomes
The incidence of the composite outcome was 13.0% in the selective iron group and 11.0% in the routine iron group (P = 0.12; Table 3). When the six outcome variables were compared separately, some of the outcomes were slightly, but non‐significantly, more common in the selective iron group. ‘Large for gestational age’ (outcome 6) was the most common outcome (8% in both groups). The length and birthweight of the child and the proportion of children with birthweight ≥4000 g or ≥4500 g were similar in the two groups (Table 3). The results of the per‐protocol analyses were very similar.
Table 3.
Incidence of glucose intolerance‐related outcomes*, and child birthweight and length by the iron supplementation groups, numbers (%) or means (SD)
| Selective iron (n = 1358) | Routine iron (n = 1336) | t/χ 2 | df | P‐value † | |
|---|---|---|---|---|---|
| Glucose intolerance‐related outcomes, n (%) | |||||
| 1. The primary reason for referring to a specialist in maternity hospital | 11 (0.8) | 6 (0.4) | 1.399 | 1 | 0.24 |
| 2. The primary reason for inpatient care before delivery | 11 (0.8) | 11 (0.8) | 0.001 | 1 | 0.97 |
| 3. Discharge diagnosis from birth hospital | 3 (0.2) | 3 (0.2) | 0.000 | 1 | 0.98 |
| 4. Discharge diagnosis from birth hospital | 62 (4.6) | 47 (3.5) | 1.904 | 1 | 0.17 |
| 5. Discharge diagnosis from birth hospital | 12 (0.9) | 8 (0.6) | 0.742 | 1 | 0.39 |
| 6. Large for gestational age | 112 (8.3) | 109 (8.2) | 0.004 | 1 | 0.95 |
| 7. Composite outcome | 176 (13.0) | 147 (11.0) | 2.445 | 1 | 0.12 |
| Child birthweight and length ‡ | |||||
| Length of the child, cm, mean (SD) | 50.1 (2.5) | 50.2 (2.8) | −1.585 | 2687 | 0.11 |
| Birthweight, g, mean (SD) | 3566 (565) | 3580 (570) | −0.646 | 2688 | 0.52 |
| Birthweight ≥4000 g, n (%) | 288 (21.3) | 295 (22.1) | 0.281 | 1 | 0.60 |
| Birthweight ≥4500 g, n (%) | 44 (3.2) | 53 (4.0) | 1.011 | 1 | 0.32 |
*The outcomes are defined in Table 1. †Two‐sided χ 2‐test for categorised variables and two‐sided independent samples t‐test for continuous variables. ‡Missing values were 0.2% in the selective iron and 0.1% in the routine iron groups.
When stratifying the composite outcome by Hb level at the first study visit, the results were fairly similar in the two higher strata (Table 4). Among women with Hb < 130 g L−1, the selective iron group had a higher incidence of the outcome than the routine iron group. Among participants with Hb < 120 g L−1, the incidence of the outcome was 29.4% (n = 15) in the selective iron group and 21.8% (n = 12) in the routine iron group (P = 0.37). Among participants with Hb ≥ 150 g L−1, the respective numbers were 12.9% (n = 9) and 9.4% (n = 6; P = 0.52). Regardless of the iron group, the incidence of the outcome was not associated with baseline Hb level.
Table 4.
Incidence of the composite outcome* by the iron supplementation groups, stratified by haemoglobin level, pre‐pregnancy body mass index (BMI) and gestational weight gain, n (%)
| n † | Selective iron (n = 1358) | Routine iron (n = 1336) | χ 2 | df | P‐value ‡ | |
|---|---|---|---|---|---|---|
| Haemoglobin level at the first visit | ||||||
| <130 g L−1 | 337, 375 | 53 (15.7) | 39 (10.4) | 4.476 | 1 | 0.034 |
| 130–139.9 g L−1 | 598, 595 | 71 (11.9) | 69 (11.6) | 0.022 | 1 | 0.88 |
| ≥140 g L−1 | 392, 338 | 48 (12.2) | 37 (10.9) | 0.297 | 1 | 0.59 |
| 0.18 § | 0.80 § | |||||
| Pre‐pregnancy BMI | ||||||
| Underweight, <18.5 kg m−2 | 80, 108 | 4 (5.0) | 8 (7.4) | 0.446 | 1 | 0.50 |
| Normal weight, 18.5–24.9 kg m−2 | 1004, 961 | 116 (11.6) | 96 (10.0) | 1.248 | 1 | 0.26 |
| Overweight, 25.0–29.9 kg m−2 | 153, 174 | 34 (22.2) | 28 (16.1) | 1.991 | 1 | 0.16 |
| Obese, ≥30 kg m−2 | 50, 45 | 13 (26.0) | 12 (26.7) | 0.005 | 1 | 0.94 |
| <0.001 § | <0.001 § | |||||
| Gestational weight gain, n (%) ¶ | ||||||
| Below the recommendations | 404, 431 | 31 (7.7) | 36 (8.4) | 0.130 | 1 | 0.72 |
| Within the recommendations | 569, 545 | 93 (16.3) | 60 (11.0) | 6.688 | 1 | 0.010 |
| Above the recommendations | 249, 246 | 39 (15.7) | 45 (18.3) | 0.607 | 1 | 0.44 |
| 0.001 § | <0.001 § | |||||
*The composite outcome is defined in Table 1. †Numbers in the selective iron and the routine iron groups, respectively. ‡Two‐sided χ 2‐test. § P‐value for trend between the strata within the selective iron and the routine iron groups. ¶Compared with the recommendations of the Institute of Medicine 2009.
When stratifying the composite outcome by the pre‐pregnancy BMI, no significant differences were observed between the groups within any of the strata (Table 4). Regardless of the iron group, the incidence of the outcome increased remarkably with increasing BMI, being 4‐ to 5‐fold in obese women compared with underweight women.
When stratifying the composite outcome by gestational weight gain, the selective iron group had a higher incidence of the outcome than the routine iron group among women who gained weight within the recommendations (Table 4). No differences were observed between the groups among women gaining weight either below or above the recommendations. Regardless of the iron group, women with lower than recommended weight gain had lower incidence of the outcome than those with recommended or above recommended weight gain.
Discussion
As opposite to the hypothesis, this randomised controlled trial found no statistically significant differences in the incidence of the glucose intolerance‐related outcomes during pregnancy between women who were prescribed iron supplements only if they were diagnosed as anaemic and women who were prescribed elemental iron (100 mg day−1) routinely regardless their Hb level. The results were mainly the same when stratified by the baseline BMI. Thus, our trial does not support the hypothesis that iron supplements during pregnancy increase the risk of GDM.
The present study has several strengths. A randomised controlled trial is the best design to explore the effects of iron supplementation. Compliance with the selective or the routine iron group was good based on both self‐reported supplement use and the differences observed in Hb measurements at different stages of pregnancy. The follow‐up was almost complete (Fig. 1), the proportion of missing data was low and the data were analysed according to the intention‐to‐treat principle. The study population was large and representative. The results are applicable to healthy European pregnant women who are mostly normal weight.
The main limitation of the present study is that the oral glucose tolerance test had not been performed systematically to all participants. Consequently, the composite outcome covers also milder forms of glucose intolerance and may include some women who did not have glucose intolerance. However, the finding of a good correlation between pre‐pregnancy BMI and GDM supports the validity of our outcome. Furthermore, the risk of information bias regarding the outcome should be low because the same criteria were applied to both groups and the risk of GDM was not generally suspected to be related to iron intake in the 1980s. However, the results may be biased towards a null finding. Another important limitation is that data on iron stores or dietary intake of iron was not available. The association between high iron intake and glucose intolerance may be related to iron metabolism and iron stores rather than iron intake per se. We did not have data on some potential confounders such as family history of diabetes mellitus or GDM in any previous pregnancy. The large number of women randomised suggests that these factors were equally distributed between the groups, but we could not confirm it. Although our data were collected nearly 30 years ago, the biological mechanism of the potential association between iron supplementation and glucose intolerance should have remained the same.
Our findings are in concordance with those of the only previous randomised controlled trial conducted in Hong Kong (n = 1164; Chan et al. 2009). In that study, the incidence of GDM was similar (11%) in the iron supplementation group (60 mg day−1 elemental iron) and the placebo group. A strength of the study was that GDM was defined by oral glucose tolerance test and data on serum ferritin and transferrin were also available. However, 40% of the women were lost to follow‐up, compliance with the intervention was low (53–56%) and 43–46% of the women had used other dietary supplements (possibly including iron), which may explain why the Hb levels were similar between the groups at 28 weeks gestation.
Previous observational studies have generally supported the hypothesis that excessive iron intake in pregnancy could be associated with increased risk of GDM. A prospective cohort study (n = 3158) in the United States found that the intake of dietary heme iron during pregnancy was directly associated but the intake of dietary non‐heme iron tended to be inversely associated with the risk of GDM (Qiu et al. 2011). An Italian case–control study (n = 1000) reported that the risk of GDM was elevated among women using iron supplements during pregnancy compared with women not using them (Bo et al. 2009). In our previous cohort study (n = 399; Helin et al. 2012), high total iron intake (from both diet and supplements) increased the risk for GDM, but the result was statistically significant only after excluding women with baseline Hb < 120 g L−1. In addition, the results from the US Nurses' Health Study (n = 13 475) suggested that higher pre‐pregnancy intake of dietary heme iron increased linearly the risk for GDM, but the intake of total iron, non‐heme iron or iron supplements were not related to the risk for GDM (Bowers et al. 2011). The measurement methods of iron intake and GDM were heterogeneous in these observational studies.
In the present study, the risk of glucose intolerance‐related outcomes was unexpectedly higher in the selective iron group than in the routine iron group when including women with Hb < 130 g L−1 or women with weight gain within the recommendations only. These observations are difficult to explain, especially because two of the major risk factors of GDM – BMI and age – were similar between the groups within these two subgroups of women (results not shown). Moreover, iron supplement use was not higher than expected in the selective iron group (results not shown). We cannot exclude the possibility that low Hb may have led women in the selective iron group to eat iron‐rich food. The observations might also be explained by effect modification or they could be chance findings resulting from multiple testing.
There are some potential explanations for why the results of the observational studies and the randomised controlled trials are different. Firstly, the original hypothesis may be wrong and the findings in observational studies may be due to residual confounding (e.g. iron stores or metabolism as a confounder). Secondly, the phenomenon may be different depending on the main source, type (supplements and diet vs. diet only) and the dosage of iron intake. Thirdly, the findings of the present study may be wrong for example due to limitations of our outcome measure.
There are still many open questions regarding the potential association between dietary or supplementary iron intake and the risk of GDM. Absorption of different forms of iron varies and is affected for example by body iron stores and other dietary factors (Cao & O'Brien 2013). The roles of heme and non‐heme iron need to be better understood (Cao & O'Brien 2013). In addition, better understanding of iron metabolism during pregnancy, the markers of body iron stores and the possible effects of iron on glucose regulation at the cell level is warranted.
In conclusion, the study found no effect of iron supplementation on the risk of glucose intolerance in Finnish women pregnant in the 1980s. The findings of the previous observational studies may be confounded or biased. To confirm our results, future trials should include the glucose tolerance test to define GDM, measures of iron intake from diet and additional markers of iron status.
Source of funding
None.
Conflict of interest
The authors declare that they have no conflicts of interest.
Contributions
EH was the principal investigator of the original trial conducted in the 1980s; TIK, EH and RL designed the research; TIK performed statistical analyses and wrote the paper. All authors interpreted the findings, commented the drafts and approved the final draft of the manuscript.
Acknowledgements
None.
Kinnunen, T. I. , Luoto, R. , Helin, A. , and Hemminki, E. (2016) Supplemental iron intake and the risk of glucose intolerance in pregnancy: re‐analysis of a randomised controlled trial in Finland. Matern Child Nutr, 12: 74–84. doi: 10.1111/mcn.12139.
Trial registration: Not done (recruitment in 1985–1986)
References
- Afkhami‐Ardekani M. & Rashidi M. (2009) Iron status in women with and without gestational diabetes mellitus. Journal of Diabetes and Its Complications 23 (3), 194–198. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association (2003) Gestational diabetes mellitus. Diabetes Care 26 (Suppl. 1), S103–S105. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association (2013) Diagnosis and classification of diabetes mellitus. Diabetes Care 36 (Suppl. 1), S67–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arkkola T., Uusitalo U., Pietikainen M., Metsala J., Kronberg‐Kippila C., Erkkola M. et al (2006) Dietary intake and use of dietary supplements in relation to demographic variables among pregnant Finnish women. The British Journal of Nutrition 96 (5), 913–920. [DOI] [PubMed] [Google Scholar]
- Bo S., Menato G., Villois P., Gambino R., Cassader M., Cotrino I. et al (2009) Iron supplementation and gestational diabetes in midpregnancy. American Journal of Obstetrics and Gynecology 201 (2), 158.e1–158.e6. [DOI] [PubMed] [Google Scholar]
- Bowers K., Yeung E., Williams M.A., Qi L., Tobias D.K., Hu F.B. et al (2011) A prospective study of prepregnancy dietary iron intake and risk for gestational diabetes mellitus. Diabetes Care 34 (7), 1557–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C. & O'Brien K.O. (2013) Pregnancy and iron homeostasis: an update. Nutrition Reviews 71 (1), 35–51. [DOI] [PubMed] [Google Scholar]
- Central Statistical Office of Finland (1981) Classification of Occupations 1980. Handbooks, No 14 Central Statistical Office of Finland: Helsinki. [Google Scholar]
- Chan K.K., Chan B.C., Lam K.F., Tam S. & Lao T.T. (2009) Iron supplement in pregnancy and development of gestational diabetes – a randomised placebo‐controlled trial. BJOG: An International Journal of Obstetrics and Gynaecology 116 (6), 789–797; discussion 797–798. [DOI] [PubMed] [Google Scholar]
- Chen X., Scholl T.O. & Stein T.P. (2006) Association of elevated serum ferritin levels and the risk of gestational diabetes mellitus in pregnant women: the Camden study. Diabetes Care 29 (5), 1077–1082. [DOI] [PubMed] [Google Scholar]
- Clausen T.D., Mathiesen E.R., Hansen T., Pedersen O., Jensen D.M., Lauenborg J. et al (2008) High prevalence of type 2 diabetes and pre‐diabetes in adult offspring of women with gestational diabetes mellitus or type 1 diabetes: the role of intrauterine hyperglycemia. Diabetes Care 31 (2), 340–346. [DOI] [PubMed] [Google Scholar]
- Clausen T.D., Mathiesen E.R., Hansen T., Pedersen O., Jensen D.M., Lauenborg J. et al (2009) Overweight and the metabolic syndrome in adult offspring of women with diet‐treated gestational diabetes mellitus or type 1 diabetes. The Journal of Clinical Endocrinology and Metabolism 94 (7), 2464–2470. [DOI] [PubMed] [Google Scholar]
- Dabelea D. (2007) The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care 30 (Suppl. 2), S169–S174. [DOI] [PubMed] [Google Scholar]
- Derbent A.U., Simavli S.A., Kaygusuz I., Gumus I.I., Yilmaz S., Yildirim M. et al (2013) Serum hepcidin is associated with parameters of glucose metabolism in women with gestational diabetes mellitus. The Journal of Maternal‐Fetal & Neonatal Medicine 26(11), 1112–1115. [DOI] [PubMed] [Google Scholar]
- Ferrara A. (2007) Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 30 (Suppl. 2), S141–S146. [DOI] [PubMed] [Google Scholar]
- Galtier F. (2010) Definition, epidemiology, risk factors. Diabetes & Metabolism 36 (6 Pt 2), 628–651. [DOI] [PubMed] [Google Scholar]
- HAPO Study Cooperative Research Group , Metzger B.E., Lowe L.P., Dyer A.R., Trimble E.R., Chaovarindr U. et al (2008) Hyperglycemia and adverse pregnancy outcomes. The New England Journal of Medicine 358 (19), 1991–2002. [DOI] [PubMed] [Google Scholar]
- Helin A., Kinnunen T.I., Raitanen J., Ahonen S., Virtanen S.M. & Luoto R. (2012) Iron intake, haemoglobin and risk of gestational diabetes: a prospective cohort study. BMJ Open 2 (5), 1–8. doi: 10.1136/bmjopen-2012-001730. Print 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki E. & Merilainen J. (1995) Long‐term follow‐up of mothers and their infants in a randomized trial on iron prophylaxis during pregnancy. American Journal of Obstetrics and Gynecology 173 (1), 205–209. [DOI] [PubMed] [Google Scholar]
- Hemminki E. & Rimpela U. (1991a) Iron supplementation, maternal packed cell volume, and fetal growth. Archives of Disease in Childhood 66 (4 Spec No), 422–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki E. & Rimpela U. (1991b) A randomized comparison of routine versus selective iron supplementation during pregnancy. Journal of the American College of Nutrition 10 (1), 3–10. [DOI] [PubMed] [Google Scholar]
- Hemminki E., Uski A., Koponen P. & Rimpela U. (1989) Iron supplementation during pregnancy – experiences of a randomized trial relying on health service personnel. Controlled Clinical Trials 10 (3), 290–298. [DOI] [PubMed] [Google Scholar]
- Hemminki E., Rimpela U. & Yla‐Outinen A. (1991) Iron prophylaxis during pregnancy and infections. International Journal for Vitamin and Nutrition Research. Internationale Zeitschrift fur Vitamin‐ und Ernahrungsforschung. Journal international de vitaminologie et de nutrition 61 (4), 370–371. [PubMed] [Google Scholar]
- Hyvönen K. (1991) Gestaatiodiabeteksen esiintyvyys ja seulonta. University of Kuopio.
- IOM (Institute of Medicine) (2009) Weight Gain During Pregnancy: Reexamining the Guidelines. The National Academies Press: Washington, DC. [PubMed] [Google Scholar]
- Ju H., Rumbold A.R., Willson K.J. & Crowther C.A. (2008) Borderline gestational diabetes mellitus and pregnancy outcomes. BMC Pregnancy and Childbirth 8, 31‐2393‐8‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Newton K.M. & Knopp R.H. (2002) Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 25 (10), 1862–1868. [DOI] [PubMed] [Google Scholar]
- Kim S.Y., England L., Wilson H.G., Bish C., Satten G.A. & Dietz P. (2010) Percentage of gestational diabetes mellitus attributable to overweight and obesity. American Journal of Public Health 100 (6), 1047–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lao T.T. & Ho L.F. (2004) Impact of iron deficiency anemia on prevalence of gestational diabetes mellitus. Diabetes Care 27 (3), 650–656. [DOI] [PubMed] [Google Scholar]
- Lao T.T., Chan P.L. & Tam K.F. (2001) Gestational diabetes mellitus in the last trimester – a feature of maternal iron excess? Diabetic Medicine: A Journal of the British Diabetic Association 18 (3), 218–223. [DOI] [PubMed] [Google Scholar]
- Lao T.T., Chan L.Y., Tam K.F. & Ho L.F. (2002) Maternal hemoglobin and risk of gestational diabetes mellitus in Chinese women. Obstetrics and Gynecology 99 (5 Pt 1), 807–812. [DOI] [PubMed] [Google Scholar]
- Lee A.J., Hiscock R.J., Wein P., Walker S.P. & Permezel M. (2007) Gestational diabetes mellitus: clinical predictors and long‐term risk of developing type 2 diabetes: a retrospective cohort study using survival analysis. Diabetes Care 30 (4), 878–883. [DOI] [PubMed] [Google Scholar]
- Mainous A.G. 3rd, Wells B.J., Everett C.J., Gill J.M. & King D.E. (2004) Association of ferritin and lipids with C‐reactive protein. The American Journal of Cardiology 93 (5), 559–562. [DOI] [PubMed] [Google Scholar]
- National Institute for Health and Welfare (2012) Perinatal statistics: parturients, deliveries and newborns 2011. Official Statistics of Finland, Health 2012. Statistical report 20/2012. National Institute for Health and Welfare, Helsinki.
- Pirkola J., Pouta A., Bloigu A., Hartikainen A.L., Laitinen J., Jarvelin M.R. et al (2010) Risks of overweight and abdominal obesity at age 16 years associated with prenatal exposures to maternal prepregnancy overweight and gestational diabetes mellitus. Diabetes Care 33 (5), 1115–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu C., Zhang C., Gelaye B., Enquobahrie D.A., Frederick I.O. & Williams M.A. (2011) Gestational diabetes mellitus in relation to maternal dietary heme iron and nonheme iron intake. Diabetes Care 34 (7), 1564–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soubasi V., Petridou S., Sarafidis K., Tsantali C., Diamanti E., Buonocore G. et al (2010) Association of increased maternal ferritin levels with gestational diabetes and intra‐uterine growth retardation. Diabetes & Metabolism 36 (1), 58–63. [DOI] [PubMed] [Google Scholar]
- Stotland N.E., Caughey A.B., Breed E.M. & Escobar G.J. (2004) Risk factors and obstetric complications associated with macrosomia. International Journal of Gynaecology and Obstetrics: The Official Organ of the International Federation of Gynaecology and Obstetrics 87 (3), 220–226. [DOI] [PubMed] [Google Scholar]
- Wendland E.M., Torloni M.R., Falavigna M., Trujillo J., Dode M.A., Campos M.A. et al (2012) Gestational diabetes and pregnancy outcomes – a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy and Childbirth 12, 23‐2393‐12‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.J., Poulton R. & Williams S. (2002) Relationship of serum ferritin with cardiovascular risk factors and inflammation in young men and women. Atherosclerosis 165 (1), 179–184. [DOI] [PubMed] [Google Scholar]


