Abstract
Acute malnutrition affects millions of children each year, yet global coverage of life‐saving treatment through the community‐based management of acute malnutrition (CMAM) is estimated to be below 15%. We investigated the potential role of stigma as a barrier to accessing CMAM. We surveyed caregivers bringing children to rural health facilities in Marsabit County, Kenya, divided into three strata based on the mid‐upper arm circumference of the child: normal status (n = 327), moderate acute malnutrition (MAM, n = 241) and severe acute malnutrition (SAM, n = 143). We used multilevel mixed effects logistic regression to estimate the odds of reporting shame as a barrier to accessing health care. We found that the most common barriers to accessing child health care were those known to be universally problematic: women's time and labour constraints. These constituted the top five most frequently reported barriers regardless of child acute malnutrition status. In contrast, the odds of reporting shame as a barrier were 3.64 (confidence interval: 1.66–8.03, P < 0.05) times higher in caregivers of MAM and SAM children relative to those of normal children. We conclude that stigma is an under‐recognized barrier to accessing CMAM and may constrain programme coverage. In light of the large gap in coverage of CMAM, there is an urgent need to understand the sources of acute malnutrition‐associated stigma and adopt effective means of de‐stigmatization.
Keywords: stigma, acute malnutrition, access, community‐based management of acute malnutrition
Introduction
Acute malnutrition affects approximately 11% of children under the age of 5 each year or approximately 52 million individuals (Black et al. 2013). The condition occurs as a result of infection, disease and/or restricted dietary intake and is associated with increased risk of morbidity and mortality. Cases of acute malnutrition may be classified as moderate (MAM) or severe (SAM) and are determined by the use of mid‐upper arm circumference (MUAC), weight‐for‐height z‐scores (WHZ) or the presence of bilateral pitting oedema.1 Community‐based management of acute malnutrition (CMAM), a treatment protocol that began in 2001 and was adopted globally by 2006, is viewed as a highly efficacious approach to treating acute malnutrition (Bhutta et al. 2005; Black et al. 2013). However, of the total number of children eligible for CMAM each year globally, less than 15% are estimated to receive treatment (Guerrero & Rogers 2013a). The discrepancy in coverage is thought largely to reflect poor access to existing programmes rather than the absence of treatment options at country level (Brown et al. 2009; Guerrero & Rogers 2013b).
Studies of barriers to CMAM include one review of SAM treatment programmes across six African countries (the Democratic Republic of the Congo, Ethiopia, Malawi, Niger and North and South Sudan), which identified fear of rejection, not recognizing the child's condition as malnutrition, lack of confidence in the programme, relapse and distance to the site of programme as the most common factors (Guerrero et al. 2010). A recent evaluation of SAM treatment access across 21 countries found the primary barriers to be a lack of knowledge of malnutrition, lack of knowledge about CMAM, high opportunity costs and distance to site – all of which are consistent with pre‐existing knowledge about access barriers for other child health services (Guerrero & Rogers 2013b).
An association between stigma and acute malnutrition has been reported in two earlier contexts: in a study from Pakistan from the early 1990s, mothers of children with severe wasting reported hiding their children due to shame and fear of judgment associated with the condition, which was thought to be caused by ‘malevolent’ influences from the spirit world (Mull 1991). In Tanzania at around the same time, stigma associated with poverty was nearly inextricable from that linked to child malnutrition and was perpetuated by a discriminatory health care system (Howard & Millard 1997). Apart from these early studies, the role of stigma as a potential barrier to accessing care has not been studied. Field observations by the first author during Peace Corps service in Niger, West Africa, in 2008 and 2009 suggested that the shame of having an acutely malnourished child discouraged some caregivers from seeking timely treatment, even when caregivers were aware of CMAM services and such services were available.
Conceptualizing stigma and its consequences for health
Contemporary conceptualizations of stigma consider the dynamic social, economic and political contexts and processes that produce and intensify discrimination, expanding on Erving Goffman's pivotal early work defining stigma as the ‘deeply discrediting’ association between an attribute and a stereotype (Parker & Aggleton 2003; Goffman 2009). Discrimination may be individual, in which a person takes overt discriminatory action against another; structural, in which social or institutional norms perpetuate inequalities; and/or self‐imposed, in which members of stigmatized groups experience ‘situationally based fear that one will be judged on the basis of [negative] stereotypes’ (Steele 1997; Link & Phelan 2001; Major & O'Brien 2005). Self‐imposed stigma has also been referred to as ‘stereotype threat’ (Steele 1997), ‘identity threat’ (Major & O'Brien 2005) and ‘stigma consciousness’ (Pinel 1999).
Stigma is well documented for many health conditions, with mental illness being the focus of work prior to the 1990s and human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) stigma dominating the research through the present day (Rüsch et al. 2005; Mahajan et al. 2008). In 2006, the National Institute of Health (NIH) Fogarty International Center's Stigma and Global Health Research Program requested further research about the burden of stigma, its causes and the development of effective interventions to curb it (Keusch et al. 2006). Recent work has documented stigma in connection to tuberculosis, epilepsy, obesity and obstetric fistula (Baskind & Birbeck 2005; Mak et al. 2006; Coreil et al. 2010; Khisa & Nyamongo 2012). Although the stereotypes associated with different health conditions vary, many of the consequences of having a stigmatized health condition are foreseeable in regard to decision making about prevention, care and treatment. Common consequences for health and care include anxiety, stress, non‐disclosure about one's health status, care avoidance and potential permanent disability or even death (Keusch et al. 2006; Nyblade et al. 2009; Hatzenbuehler & Link 2014).
The relationship between stigma and acute malnutrition has not been systematically investigated since CMAM became a prominent intervention in child nutrition and health services. We proposed to bridge this gap by collecting empirical evidence on the presence of acute malnutrition‐associated stigma in the context of a rural health facility‐based survey that identified access barriers for multiple childhood conditions in Kenya. We hypothesized that caregivers of children with acute malnutrition experience feelings of shame, embarrassment or discomfort around accessing CMAM, in addition to the universal challenges known to limit access to child health services. We also hypothesized that stigma is a contributing factor in the poor coverage of CMAM programmes.
Key messages
Access to community‐based management of acute malnutrition (CMAM) may be constrained by stigma associated with acute malnutrition, in addition to the many universal barriers to child health care such as time and workload constraints of caregivers.
In order to achieve maximum coverage of potentially life‐saving CMAM programmes, thoughtful consideration of barriers unique to CMAM must be made among researchers and programmers alike.
Efforts to integrate de‐stigmatization activities into existing health service platforms would benefit from a qualitative understanding of the source of stigma associated with acute malnutrition.
Materials and methods
Study setting
Marsabit County, Kenya, covers approximately 26 000 square miles and is divided into six administrative divisions. It is a geographically remote and arid region in North Kenya, home to approximately 290 000 people and subject to drought and food insecurity. Nomadic pastoralism is the dominant livelihood and is practiced by the dominant ethnic groups in the region: the Borana, the Gabra, the Rendille, and the Burji. The Borana and Gabra share a common language, the Borana language, which is widely spoken across the region. Political and ethnic tension, primarily between the Borana and Gabra groups, dates back for decades, originating in disputes over land and livestock ownership and water use. Violent clashes over grazing land and civil disputes in the town of Moyale, on the border of Marsabit County and Ethiopia, were ongoing throughout 2013 and during the time of this study (Kenya Humanitarian Response 2013).
Rates of wasting (>12%) and stunting (>27%) among children under the age of 5 in Marsabit County are among the highest in the country (Wambua 2013).
Selection of study areas
This study was conducted in three administrative districts of Marsabit County. Districts were selected if Concern Worldwide, an international non‐profit humanitarian organization based in Dublin, Ireland, supported their child health and nutrition programming and if they had active CMAM participants in June of 2013.
Selection of health facilities
Eighteen health facilities were randomly selected for participation in this study as sites for data collection, six from each study district. We randomly selected three from above and below the 50th percentile of CMAM admissions2 according to June 2013 records for each district. We used this stratification approach to ensure even representation of population densities and to include facilities with a range of potential accessibility issues.
Selection of study participants
Eligible participants were adult women (≥18 years) who accompanied a child aged 6–59 months at a study facility between August and September 2013 and gave informed consent to participate in the study. Women accompanying children <6 months were not included, as CMAM protocol for children of this age was not established at the time of the study.
From the pool of eligible women, study participants were systematically and purposively selected into one of three study subgroups based on their child's MUAC. Women whose children had SAM (MUAC <115 mm or oedema) were eligible for the SAM group. Those whose children had MAM (MUAC <125 mm, ≥115 mm) were eligible for the MAM group and those with children with a normal MUAC (MUAC ≥125 mm) were eligible for the Normal group. Group selection was based on MUAC and oedema only and did not discriminate between other indicators of child health or nutrition status or the purpose of the clinic visit (e.g. women in any of the three groups could have been present for a well child visit or treatment for illness).
Target sample size
The minimum target sample size for this study was 144 individuals per group or 432 individuals in total. This target was calculated to detect a 20% difference in the frequency of a single access barrier between any two subgroups with 80% power, alpha 0.05 and 95% confidence. The sample size was adjusted to account for correlation among individuals from a single facility on a single day using a design effect factor of 0.05. We did not have prior data with which to estimate the design effect; our chosen value of 0.05 value reflects our assumption that participants at the same facility were only 5% more likely to have correlating values than participants selected at random.
Sampling strategy
We designed our sampling strategy knowing that it might exceed the target sample size. Due to uncertainty around CMAM admissions during the study time period, we needed to design a strategy that would reach the target sample size but not over‐sample the early weeks of the study. We also expected admissions at some facilities to be lower than in others, in which case low‐admissions facilities would need a longer period of time to reach their target sample size. To attain sampling breadth across time as well as ensure that the target sample would be reached at each clinic, we chose to operate the study for a pre‐specified period of time (5 weeks) regardless of whether the overall target sample size had already been reached.
Four women were recruited per group (SAM, MAM, Normal) per facility per day for four ‘study days’ spread across 6 weeks (August–September 2013). Study days were pre‐selected by health facility staff to coincide with CMAM activities. On each of the four study days at each study facility, every second eligible woman was systematically selected for the MAM and the Normal groups, beginning with the first eligible woman for each group and continuing with every other woman until four women in each group had been selected. This approach was used to limit oversampling of participants early in the day and to reduce the burden on enumerators. As a result of the limited number of women eligible for the SAM group, every eligible woman for that group was selected on each study day.
Data collection procedures
Enumerators were community health workers working under the supervision of one designated study nurse at each study clinic. Enumerators and study nurses were selected based on the their prior experience with data collection, a positive work record, their designation as the primary individuals working at the health facilities selected for the study and written and spoken proficiency in English and Borana. The survey took approximately 40 min and was administered at study facilities after participants had completed their clinic visits.
The survey consisted of 40 closed or open‐ended questions, presented in the English or Borana languages, as preferred by the mother. All questions were originally written in English, translated to Borana by a professional translator and then back translated to English during enumerator training before the survey was finalized. The survey was pilot tested by enumerators among patients and families at the Marsabit County Hospital, allowing for corrections and adjustments to question wording and order prior to finalization.
Child MUAC, weight and height were obtained from the child's health card using the measurements recorded by the study nurse during their clinic visit that day. MUAC was assessed using a standard MUAC tape on the left arm. Length of children less than 87 cm long was measured using a baby mat. Height of children greater than 87 cm was measured using a wooden or plastic stadiometer. Child age was recorded from the child's health card (n = 595, 84%) or estimated by the 116 mothers (16%) whose child did not have a health card. Mothers reported their own age and marital status (married monogamous, married polygamous, single, separated, divorced, widowed), their formal education level (none, primary, secondary, higher), ethnicity, the residential status of their household (resident, refugee, visitor), the number of people living in their household and the number under the age of 5 years and the primary source of income in their household. Food security was assessed using a condensed version of the Household Food Insecurity Access Scale (Swindale & Bilinsky 2006) that queried whether any of the following five events occurred within the previous 4 weeks: inability to eat healthy and nutritious foods, worry about running out of food, not having any food to eat in the household, going to sleep at night hungry due to lack of food and going a day and night without eating anything due to lack of food. We asked participants about the presence or absence of 17 locally relevant household items to assess non‐agricultural assets (lantern, cart, hoe or axe, clock or watch, radio, television, car battery, electricity, generator, tape player, bicycle, cell phone, tin roof, latrine, running water and mosquito net) in addition to recording the number and type of livestock owned by the household. Participants reported their primary reason for being at the clinic on that day, whether their child was ill, and any symptoms they noticed in their child. They also answered questions about care‐seeking behavior including choice of provider and typical time to care.
Barriers to care were assessed through open‐ended questions. Participants were asked to freely list any barriers, challenges or obstacles that they had faced, either in the past or on the day of the study, in accessing health care for a child. They were then asked to pick what they considered the three most important issues, which were recorded by enumerators. A pre‐specified list of responses was available to enumerators to aid in data collection, but this list was not made available or indicated to participants. It included a pre‐coded response for shame using the Borana term fokifade amale cherfad; this phrasing also means shyness or embarrassment and was the preferred term for identifying the concept of stigma. Responses that were not pre‐specified were written separately and coded by JRB later.
All participants were also asked directly about the concepts of stigma, regardless of whether they had freely listed shame as a barrier to accessing care. Consensus among survey staff was that using the terms ‘stigma’ or ‘shame’ in direct questions could alienate or insult participants and should be changed to ‘discomfort’. Thus all participants were asked about their level of comfort with their purpose at the clinic that day. This question was worded to elicit emotional discomfort rather than physical discomfort; enumerators were given additional prompts such as ‘worried’ and ‘unhappy’ to assist if the question was not received as intended. The Borana term used for discomfort was dansa indagau.
To get a sense of social norms and perceptions of acute malnutrition, we also asked whether participants would expect women whose children were wasted to feel uncomfortable coming to the clinic and reasons why this might be the case. Answers were pre‐coded; unique responses were noted by enumerators and coded post hoc by JRB later.
Ethical approval
The Cornell University Institutional Review Board for Human Participants granted ethical approval for this study (protocol #1306003948). In Kenya, approval was granted by the Nairobi Nutrition Information Working Group and the Marsabit County Ministry of Health. All participants gave verbal informed consent and were free to exit the survey at any point.
Analytic methods
Data were assembled and analysed using STATA 12 (StataCorp 2013). Descriptive statistics (means, medians, frequencies) were calculated for the caregiver, child and household characteristics as appropriate, and for responses regarding perceptions of acute malnutrition. We used Fisher's exact tests to identify differences in frequencies across the three study groups. We created a continuous count index of household food insecurity, ranging from zero (no measures of food insecurity indicated) to five (all five measures of food insecurity indicated). We also created a continuous count index of household assets. Of the 17 asset variables measured, eight were reported by at least 10% of the sample. We used these eight variables to create a scale ranging from zero (none of the assets indicated) to eight (all eight assets indicated) to assess household wealth. Access barriers were categorized using an adapted version of a framework proposed by Barton (Streatfield et al. 2008; Barton 2010).
We used multilevel mixed effects logistic regression to estimate the odds of reporting a barrier given the acute malnutrition status of the child using the responses to our open‐ended questions about access barriers. The 10 most commonly reported barriers were used as binary outcome variables in 10 separate models, each adjusted for the same set of covariates. We chose 11 covariates that we expected to be potentially confounding because of their association with exposure (acute malnutrition) and outcome (shame), as well as covariates that we expected to be significant predictors of at least one of the 10 outcome barriers. Covariates comprised child age (months) and sex (male = 0, female = 1), maternal age (years), marital status (single, separated, divorced or widowed = 0, married = 1), education (0 = no formal education, 1 = any formal education), household distance from the clinic (minutes), household size, food insecurity (0 to 5), physical household assets (0 to 8), district, and facility caseload (low = 0, high = 1). Health facility was treated as a random effect. We used a dummy variable to test for the association between barriers and study group. The threshold for statistical significance for all variables was P < 0.05.
Results
Descriptive statistics
Of the 711 total participants, 327 (n = 46%) had a child without acute malnutrition and comprised the Normal group, 241 (n = 34%) had a child with MAM and comprised the MAM group and 143 (n = 20%) had a child with SAM and comprised the SAM group. Oedema qualified four of the participants for the SAM group. Participants were evenly distributed across the 18 study health facilities.
Descriptive statistics of the study participants are presented by group in Table 1. Most participants were married Borana residents aged 22–31 with no formal education, living in households with 4–7 individuals, including 1–2 children under the age of 5.
Table 1.
Characteristics of participants in a survey of access barriers to rural health clinics in Marsabit County, Kenya (n = 711)*
| Normal group (n = 327) | MAM group (n = 241) | SAM group (n = 143) | |
|---|---|---|---|
| Women's age (years) | 27, 23–31 | 26, 22–31 | 26, 22–30 |
| Household status (resident) | 310 (95) | 221 (92) | 125 (88) |
| Household size | 6, 4–7 | 6, 4–7 | 6, 4–7 |
| Number of children under the age of 5 | 2, 1–2 | 2, 1–2 | 2, 1–2 |
| Food insecurity index † | 1, 0–3 | 2, 0–4 | 2, 0–4 |
| Household assets index ‡ | 3, 2–5 | 3, 1–4 | 3, 1–4 |
| Marital status | |||
| Married, monogamous | 274 (84) | 180 (75) | 111 (78) |
| Married, polygamous | 20 (6) | 16 (7) | 7 (5) |
| Divorced or separated | 20 (6) | 24 (10) | 20 (14) |
| Widowed | 9 (3) | 10 (4) | 2 (1) |
| Women's education | |||
| None | 253 (77) | 180 (75) | 110 (77) |
| Any primary | 40 (12) | 41 (17) | 19 (13) |
| Any secondary or above | 19 (6) | 8 (3) | 6 (4) |
| Ethnicity | |||
| Borana | 187 (58) | 136 (56) | 73 (51) |
| Garreh | 42 (13) | 33 (14) | 18 (13) |
| Rendille | 29 (9) | 26 (11) | 23 (16) |
| Gabra | 16 (5) | 19 (7) | 14 (10) |
| Primary source of household income | |||
| Sale of milk | 53 (16) | 43 (18) | 27 (19) |
| Sale of firewood | 34 (10) | 41 (17) | 32 (22) |
| Sale of miraa § | 53 (16) | 38 (16) | 14 (10) |
| Sale of livestock | 47 (14) | 31 (13) | 8 (6) |
| Small business | 35 (11) | 23 (10) | 12 (8) |
MAM, moderate acute malnutrition; SAM, severe acute malnutrition. *Values are n (%) or median, interquartile range as appropriate. †The number of indicators of household food insecurity reported (0–5). ‡The number of physical household assets reported (0–8). §Miraa (also known as khat or qat) is a plant chewed for its properties as a stimulant.
Table 2 presents descriptive data on the children of study participants. The sample was 45% female (n = 315) with a median age of 21 months and a median MUAC of 124 mm.
Table 2.
Characteristics of children of the participants in a survey of access barriers to rural health facilities in Marsabit County, Kenya (n = 711)*
| Normal group (n = 327) | MAM group (n = 241) | SAM group (n = 143) | |
|---|---|---|---|
| Child age (months) | 25, 14–36 | 20, 12–29 | 15, 11–24 |
| Child MUAC (mm) | 135, 129–141 | 122, 120–124 | 113, 111–114 |
| Child sex (female) | 143 (45) | 103 (43) | 68 (49) |
| Primary reasons why child was brought to the health facility | |||
| Treatment for illness | 141 (43) | 54 (23) | 23 (16) |
| Supplementary feeding | 23 (7) | 95 (40) | 7 (5) |
| Therapeutic feeding | 2 (1) | 29 (12) | 73 (51) |
| Referral from health worker | 58 (18) | 25 (11) | 18 (13) |
| Growth monitoring | 61 (19) | 26 (11) | 13 (9) |
| Other | 40 (12) | 10 (4) | 9 (6) |
| Child seem unwell to mother? | 192 (59) | 121 (50) | 71 (50) |
| If child seemed unwell, symptoms observed by mother (multiple answers allowed) | |||
| Fever | 117 (61) | 82 (68) | 39 (55) |
| Acute respiratory illness | 116 (60) | 84 (70) | 31 (44) |
| Diarrhea or vomiting | 80 (42) | 62 (51) | 45 (63) |
| Lack of appetite | 54 (28) | 41 (34) | 29 (41) |
| Crying | 27 (14) | 29 (24) | 23 (32) |
| Injury | 22 (11) | 12 (10) | 1 (1) |
| Thin | 18 (9) | 25 (21) | 28 (39) |
MAM, moderate acute malnutrition; MUAC, mid‐upper arm circumference; SAM, severe acute malnutrition. *Values are n (%) or median, interquartile range as appropriate.
When asked to free‐list barriers experienced personally, participants listed 30 different barriers to accessing child health and nutrition services; the frequency of each barrier is depicted in Fig. 1. Figure 1 also categorizes each barrier into one of five types. Temporal and geographic barriers were heavily cited: they comprised four of the top five most frequently reported responses to the question about personal barriers and accounted for 49% (n = 958) of all responses. Social and cultural barriers, although reported less frequently than temporal barriers, were the second most common type of response accounting for 23% (n = 443) of all responses. Barriers related to personal experience, knowledge or beliefs were reported 271 times accounting for 14% of the total, while infrastructural and financial barriers constituted the remainder.
Figure 1.
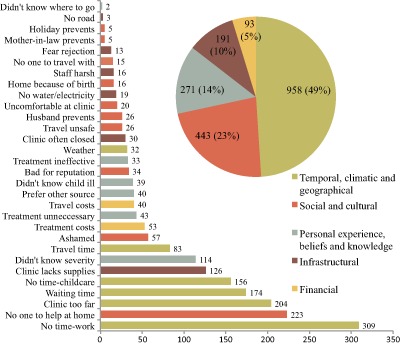
Barriers to accessing health services for children in Marsabit County, Kenya.
The 30 unique responses are named on the Y‐axis; the number of times that each response was given is shown to the right of the bars. Responses were categorized according to an adapted framework for access by Barton (2010). The total number of responses in each category is depicted in the pie chart.
Freely listed responses mentioning shame were reported as barriers 57 times, frequently enough to be the ninth most common barrier cited overall, but still only comprising 3% of all responses. When we stratified barrier results by study group, the frequency of shame was highest within the SAM group (n = 21, 15%), followed by the MAM group (n = 24, 10%). It was not one of the top 10 most frequently reported barriers within the Normal group (n = 12, 4%). The frequency of the 10 most commonly listed barriers in each study group is depicted in Fig. 2.
Figure 2.
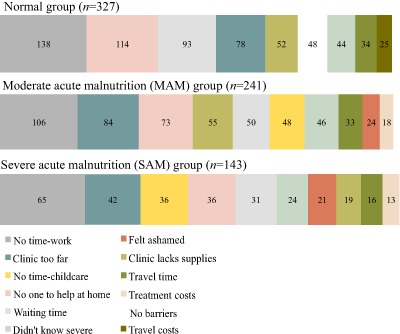
The frequency of the most commonly reported barriers to accessing child health in Marsabit County, Kenya, by study group.
The 10 most frequent barriers for each study group (SAM, MAM and Normal) are tabulated in order of decreasing frequency from left to right. The barrier of interest, shame, is depicted in red.
When asked directly, 41% (n = 58) of women in the SAM group responded that they were uncomfortable with their purpose attending the clinic on that day; the frequency of this response was lower in the MAM group (n = 71, 30%) and lowest in the Normal group (n = 61, 19%) (P < 0.001) (Table 3). Three quarters of the total sample (n = 526) reported that they would expect women with acutely malnourished children to experience more discomfort attending a clinic with their child when compared with other women. This expectation was distributed evenly across the study subgroups (P = 0.65).
Table 3.
The frequency of discomfort regarding the reason for their clinic visit, as reported by participants in a survey of access barriers to health services in rural facilities in Marsabit County, Kenya (n = 711)*
| Normal group (n = 327) | MAM group (n = 241) | SAM group (n = 143) | P‐value* | |
|---|---|---|---|---|
| Felt uncomfortable about their purpose at the clinic today (n = 190, 27%) | ||||
| 61 (19%) | 71 (30%) | 58 (41) | <0.001 | |
| Thought women whose children have acute malnutrition would be more uncomfortable coming to the clinic than other women (n = 526, 74%) | ||||
| 237 (73) | 180 (76) | 109 (76) | 0.65 | |
| Reasons they would feel more uncomfortable (n = 526, multiple answers allowed) | ||||
| Mother's ability to care for her child is questioned | 150 (64) | 105 (59) | 73 (66) | 0.40 |
| Household's ability to feed the child is questioned | 139 (60) | 114 (69) | 64 (58) | 0.54 |
| Mother's ability to provide necessities for her child is questioned | 120 (52) | 114 (64) | 63 (57) | 0.04 |
| Child is perceived to have a serious health condition | 69 (30) | 60 (33) | 33 (30) | 0.66 |
| Household is perceived to be poor | 56 (24) | 36 (20) | 28 (25) | 0.52 |
MAM, moderate acute malnutrition; SAM, severe acute malnutrition. *Values are n (%). Significance refers to Fisher's exact tests comparing the distribution of positive responses across study groups.
Participants offered a number of reasons why discomfort or shame might occur (or be expected to occur) among women attending a clinic with acutely malnourished children. Having a child with moderate or severe acute malnutrition was perceived to indicate negative characteristics about the child's mother and household. Approximately 60% of respondents mentioned that having an acutely malnourished child would invite the perception that the mother did not take proper care of her child, either in terms of food or other basic provisions (Table 3).
Multivariate regression results
Shame as a barrier, freely listed
Our first mixed effects regression model estimated the odds of reporting shame given the acute malnutrition status of the child in the SAM and MAM groups (consolidated), adjusting for child age and sex, maternal age, marital status, education, household size, distance from the clinic, food security status, household assets, district, and clinic caseload. Of the top 10 most frequently reported barriers in this study, shame was the only barrier that varied by study group. Women with acutely malnourished children had a 3.64 times higher odds of reporting shame relative to the Normal group [confidence interval (CI): 1.65–8.03, P < 0.05] (Fig. 3).
Figure 3.
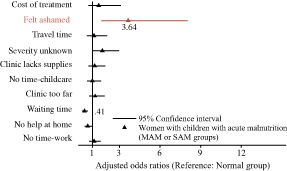
The odds of reporting one of the 10 most common health service barriers in Marsabit County, Kenya (moderate and severe groups consolidated and compared with the normal group).
Odds ratios of significant findings (P < 0.05) are noted on the figure and were estimated using a multilevel mixed effects logistic regression model that adjusted for child age and sex, maternal age, marital status, education, household size, distance from the clinic, food security status and wealth, district and facility caseload. Health facility was treated as a random effect.
When separated by study group strata, the odds of reporting shame as a barrier were highest in the SAM group [odds ratio (OR): 6.54, CI: 2.46–17.41, P < 0.001) followed by the MAM group (OR: 2.87, CI: 1.23–6.69 P < 0.05) (Fig. 4).
Figure 4.
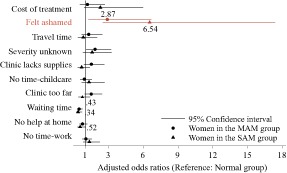
The odds of reporting one of the 10 most common barriers in a survey of access to health services at rural health facilities in Marsabit County, Kenya (moderate and severe groups stratified and compared with the normal group).
Odds ratios of significant findings (P < 0.05) are noted on the figure. Estimates were produced by our multilevel mixed effects logistic regression model, which adjusted for child age and sex, maternal age, marital status, education, household size, distance from the clinic, food security status and wealth, district and facility caseload. Health facility was treated as a random effect.
Discussion
This study systematically investigated whether stigma associated with acute malnutrition constrains access to CMAM. By asking participants with and without acutely malnourished children about their experiences in accessing care, we were able to identify those barriers that are universal across child health conditions and those that are unique to a single condition or service. Our findings support the two hypotheses posed earlier, that acute malnutrition is a stigmatized condition and that stigma is one factor limiting utilization of CMAM.
We used three approaches to triangulate the association between stigma and acute malnutrition: we asked open‐ended questions about participants' past and current experience accessing a clinic, direct questions about their own comfort level on the day of the survey and direct questions about general expectations and perceptions surrounding the condition. Each approach yielded consistent findings: many access barriers were universal, regardless of the child's nutritional status or reason for being at the clinic.
Most frequently among women whose children had acute malnutrition shame was reported as a barrier, and the majority of participants perceived acute malnutrition as a condition that would elicit emotional discomfort when seeking care. Our findings support the hypothesis that caregivers of children with acute malnutrition experience stigmatization, as revealed in the feelings of shame, embarrassment and discomfort reported in the process of accessing treatment.
Sources of stigma among stigmatized health conditions
The existing literature on health‐related stigma yields insight into sources of negative stereotyping that may be relevant to understanding the stigmatization of acute malnutrition.
Shame associated with child malnutrition in Tanzania was inseparable from that of hunger and food insecurity; this was largely a result of structural discrimination perpetuated by decades of political and economic policies that prevented households from escaping poverty (Howard & Millard 1997). Similarly, poverty and its constraints were believed to fuel HIV/AIDS stigma in Zambia (Bond 2006). Recent qualitative work from Zimbabwe showed something different: HIV/AIDS‐related stigma was distinct from poverty‐related stigma among both HIV‐affected and HIV‐unaffected children (Campbell et al. 2011). In Pakistan, the belief that child wasting was caused by spirits punishing the mother's immoral behaviour was found as the predominant source of stigma (Mull 1991). Punitive witchcraft has been documented as a perceived cause of poor health, namely epilepsy, and its consequential stigmatization in Kenya and across sub‐Saharan Africa (Baskind & Birbeck 2005; Sharkawy et al. 2006). A more recent ethnographic study from southern Malawi found that among the Yao people, child malnutrition can be seen as an indication of inadequate parental care, such as poor food provisioning or failure to abide by rules governing sexual abstinence (Flax 2013).
Wasting is highly visible and was tightly associated with HIV/AIDS during the early years of the HIV/AIDS epidemic prior to the availability of antiretroviral therapies and continues to prompt the labelling of individuals as infected with the virus (Duffy 2005). A recent study of HIV/AIDS‐related stigma in Swaziland, Namibia, Kenya, Nigeria, Burkina Faso and Senegal found Kenyan narratives about HIV/AIDS to be among the most polarized and moralistic (Winskell et al. 2011). This is consistent with recent findings from Kenya showing that HIV/AIDS‐related stigma contributes to women's refusal of HIV testing (Turan et al. 2010).
The above examples are useful for generating hypotheses about the sources of stigma associated with acute malnutrition that we found in our study. One hypothesis is that acute malnutrition‐associated stigma is driven by the stigma of poverty and food insecurity, as shown in Tanzania and Zambia (Howard & Millard 1997; Bond 2006). Alternately, it may be that household food security and acute malnutrition are independent predictors of stigma, as shown by the recent findings in Zimbabwe that HIV/AIDS stigma is distinct from poverty stigma (Campbell et al. 2011). A third hypothesis is that ‘stigma by association’, or stigma associated with HIV/AIDS infection, occurs among caregivers of visibly wasted children; this phenomenon has been documented for tuberculosis (Coreil et al. 2010). A final hypothesis, as seen among mothers of wasted children in Pakistan, some communities that stigmatize epilepsy, and the recent ethnography from Malawi, involves the presence of a belief system that views the condition as a form of supernatural or spiritual punishment (Mull 1991; Baskind & Birbeck 2005; Flax 2013).
De‐stigmatization efforts
Most stigma reduction interventions are primarily informed by sociocognitive conceptualizations of stigma and are thus limited to advocacy‐based strategies to increase empathy and tolerance among the general public (e.g. via media or political campaigns, health worker training) or to reduce fear among high‐risk groups (i.e. via support groups) (Parker & Aggleton 2003; Mahajan et al. 2008; Carr et al. 2010). Although contemporary conceptualizations of stigma recognize the broader structural and cultural processes that perpetuate stigma, such dynamics have not been targeted by most interventions (Mahajan et al. 2008). A recent review of structural stigma is likely to generate interest in creating effective multilevel de‐stigmatization strategies (Hatzenbuehler & Link 2014). The high incidence and severe consequences of untreated SAM add urgency to the need for greater attention to acute malnutrition‐related stigma.
Limitations
This study likely underestimated stigma as a barrier for several reasons. First, shame, stigma or discomfort may be difficult topics for a person to discuss or admit, which may have prevented some participants from listing it or responding positively when asked directly. Second, health workers are known to be sources of discrimination or judgment in many settings, which may have informed participants' choice of responses in this study (Reis et al. 2005; Nyblade et al. 2009). We emphasized the necessity of sensitive and respectful data collection during enumerator training to minimize this limitation, but our efforts may not have been adequate to put participants at ease or to offset caution learned from past negative experiences. Third, women for whom stigma was an especially strong barrier would be underrepresented in this facility‐based study because they are unlikely to seek care. The facility‐based aspect of this study means that it is not representative of the community at‐large and is only generalizable to women who accessed care. Fourth, our age threshold criteria (>18 years) excluded the perspectives and experience of young mothers. Fifth, our tool for measuring stigma did not attempt to separate the constructs of shame and guilt, two distinct psychological concepts that our research did not fully distinguish (Tangney & Dearing 2002).
Finally, by using MUAC to categorize our MAM and SAM groups, we applied a relatively stringent definition for acute malnutrition. There are known discrepancies between the use of MUAC and WHZ as indicators, with MUAC being more predictive of mortality risk and less sensitive to weight or body mass index (Briend et al. 2011). The potential effect of classification differences on this study's results is unknown.
Logistic and budgetary constraints led to several of the study limitations noted earlier, namely the decision to hire health care workers for data collection rather than professional enumerators and the restriction to a facility‐based study. Regarding the former, we found the expense and coordination of housing and transporting professional enumerators at the 18 rural health facilities in this study to be prohibitive. Similarly, the logistics of conducting a wide‐scale community‐based study were beyond our capacity. Future research that is able to overcome these limitations would be in a strong position to clarify the relationship between acute malnutrition and stigma.
Conclusions
Access to CMAM is constrained by stigma associated with acute malnutrition, in addition to the many universal barriers to child health care such as time and workload constraints of caregivers. Shame and discomfort at health clinics were reported more frequently among mothers of children with MAM or SAM compared with those with children of normal status, and a majority of all participants perceived acute malnutrition as an indication of poor maternal or household provisioning.
While efforts to improve access to CMAM necessarily focus on addressing the most common or pressing barriers, the extent and strength of stigmatization is potentially a much larger issue than we were able to discern and should not be overlooked. Yet if the experience of decades of HIV/AIDS research is indicative, we might expect efforts to address the stigmatization of acute malnutrition to have low priority (Mahajan et al. 2008). In order to achieve maximum coverage of potentially life‐saving CMAM programmes, thoughtful consideration of barriers unique to CMAM must be made among researchers and programmers alike (Guerrero & Rogers 2013b). Given the groundwork laid for conceptualizing stigma and its consequences for health outcomes (Earnshaw & Chaudoir 2009), the existing precedents for effective de‐stigmatization programmes (Carr et al. 2010) and the capacity of child health and nutrition programmes to influence caregiver behaviour and improve coverage (Ruel & Alderman 2013; Guerrero & Rogers 2014), many of the key requirements exist for successfully integrating de‐stigmatizing interventions into existing platforms. Such efforts could be strengthened by community‐level qualitative and ethnographic research to identify the sources of stigma associated with acute malnutrition, along with formative work to determine which approaches and targets for de‐stigmatization efforts may be most appropriate and effective.
Source of funding
This study was funded by a National Science Foundation Integrated Graduate Education and Research Traineeship and by a Fulbright‐Hays Doctoral Dissertation Research Award.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
JRB is primarily responsible for conceptualizing and designing the study, data analysis and writing the manuscript. MN contributed to study design and data collection. RJS provided feedback on data analysis and reviewed the manuscript. DLP provided feedback and supervision of the study design and data analysis and reviewed multiple drafts of the manuscript.
Acknowledgements
Many individuals from Concern Worldwide and Cornell University provided logistic and technical support to the implementation of the study. Special thanks to Rob Dabasso, Benjamin Dalziel, Kate Golden, Bob Kaugi, William Macharia, Francis Malwe, Peter Karanja, Samuel Kirichu, Regine Koppolow, Martin Kumbe, Bertha Ojany, Claire Orengo, Dan Otieno, Francoise Vermeylen, Rita Wakanyi and Yacob Yishak. Thank you to the Marsabit County District Health Management Teams in Marsabit Central, Moyale, and Sololo, the health workers and community health workers who contributed to data collection. Above all, thank you to the women who gave their time and attention to participate in this study.
Bliss, J. R. , Njenga, M. , Stoltzfus, R. J. , and Pelletier, D. L. (2016) Stigma as a barrier to treatment for child acute malnutrition in Marsabit County, Kenya. Matern Child Nutr, 12: 125–138. doi: 10.1111/mcn.12198.
Footnotes
MAM is defined as a WHZ <−2 and ≥−3 or a MUAC <125 and ≥115 mm. SAM is the more severe condition, defined by a WHZ <−3, MUAC <115 mm or the presence of bilateral pitting oedema.
CMAM admission rates are recorded by clinic staff on a weekly basis and uploaded monthly by district health management teams to Kenya's open‐access Health Information System (Kenya Ministry of Health 2013).
References
- Barton P.L. (2010) Understanding the US Health Service System, 4th edn Health Administration Press: Chicago. [Google Scholar]
- Baskind R. & Birbeck G.L. (2005) Epilepsy‐associated stigma in sub‐Saharan Africa: the social landscape of a disease. Epilepsy & Behavior 7 (1), 68–73. [DOI] [PubMed] [Google Scholar]
- Bhutta Z.A., Ahmed T., Black R.E., Cousens S., Dewey K., Guigliani E. et al (2005) Effective international action against undernutrition: why has it proven so difficult and what can be done to accelerate progress? The Lancet 368 (9551), 1992–2000. [DOI] [PubMed] [Google Scholar]
- Black R.E., Victora C.G., Walker S.P., Bhutta Z.A., Christian P., de Onis M. et al (2013) Maternal and child undernutrition and overweight in low‐income and middle‐income countries. The Lancet 382, 427–451. [DOI] [PubMed] [Google Scholar]
- Bond V. (2006) Stigma when there is no other option: understanding how poverty fuels discrimination toward people living with HIV in Zambia In: AIDS, Poverty, and Hunger: Challenges and Responses (ed. Gillespie S.), pp. 181–197. International Food Policy Research Institute: Washington, DC. AIDS [Google Scholar]
- Briend A., Maire E., Fontaine O. & Garenne M. (2011) Mid‐upper arm circumference and weight‐for‐height to identify high‐risk malnourished under‐five children. Maternal & Child Nutrition 8 (1), 130–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K.H., Nyirandutyle D.H. & Jungichann S. (2009) Management of acute malnutrition in resource‐poor settings. Nature Reviews. Endocrinology 5, 597–603. [DOI] [PubMed] [Google Scholar]
- Campbell C., Skordal M., Mupambireyi Z., Madanhire C., Robertson L., Nyamukapa C. et al (2011) Can AIDS stigma be reduced to poverty stigma? Exploring Zimbabwean children's representations of poverty and AIDS. Child: Care, Health and Development 38 (5), 732–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr D., Eckhaus T., Brady L., Watts C., Zimmerman C. & Nyblade C. (2010) Scaling up the response to HIV stigma and discrimination. Washington D.C. International Center for Research on Women [ICRW] 2010.
- Coreil J., Mayard G., Simpson K.M., Lauzardo M., Zhu Y. & Weiss M. (2010) Structural forces and the production of TB‐related stigma among Haitians in two contexts. Social Science & Medicine 71 (8), 1409–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy L. (2005) Suffering, shame, and silence: the stigma of HIV/AIDS. The Journal of the Association of Nurses in AIDS Care: JANAC 16 (1), 13–20. [DOI] [PubMed] [Google Scholar]
- Earnshaw V.A. & Chaudoir S.R. (2009) From conceptualizing to measuring HIV stigma: a review of HIV stigma mechanism measures. AIDS Behavior 13 (6), 1160–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flax V.L. (2013) ‘It was caused by the carelessness of the parents’: cultural models of child malnutrition in southern Malawi. Maternal & Child Nutrition 11 (1), 104–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffman E. (2009) Stigma: Notes on the Management of Spoiled Identity. Simon and Schuster, Inc: New York City. [Google Scholar]
- Guerrero S. & Rogers E. (2013a) Access for all: is community‐based treatment of severe acute malnutrition (SAM) at scale capable of meeting global needs? Coverage Monitoring Network 1, 1–13. [Google Scholar]
- Guerrero S. & Rogers E. (2013b) Access for all: what factors influence access to community‐based treatment of severe acute malnutrition? Coverage Monitoring Network 2, 1–13. [Google Scholar]
- Guerrero S. & Rogers E. (2014) Access for all: what can community‐based SAM treatment learn from other public health interventions to improve access and coverage? Coverage Monitoring Network 3, 1–11. [Google Scholar]
- Guerrero S., Myatt M. & Collins S. (2010) Determinants of coverage in community‐based therapeutic care programmes: towards a joint quantitative and qualitative analysis. Disasters 34 (2), 571–585. [DOI] [PubMed] [Google Scholar]
- Hatzenbuehler M.L. & Link B.G. (2014) Introduction to the special issue on structural stigma and health. Social Science & Medicine 103 (c), 1–6. [DOI] [PubMed] [Google Scholar]
- Howard M. & Millard A. (1997) Hunger and Shame: Child Malnutrition and Poverty on Mount Kilimanjaro. Routledge: London. [Google Scholar]
- Kenya Humanitarian Response (2013) Conflict Assessment Report: Moyale. Humanitarian Response. Available at: http://www.humanitarianresponse.info/operations/kenya/assessment/moyale-conflict-joint-assessment-report (Accessed 3 June 2014).
- Kenya Ministry of Health (2013) Kenya Health Information System. Available at: http://hiskenya.org/dhis-web-commons/security/login.action (Accessed 3 June 2014).
- Keusch G., Wilentz J. & Kleinman A. (2006) Stigma and global health: developing a research agenda. The Lancet 367, 525–527. [DOI] [PubMed] [Google Scholar]
- Khisa A.M. & Nyamongo I.K. (2012) Still living with fistula: an exploratory study of the experience of women with obstetric fistula following corrective surgery in West Pokot, Kenya. Reproductive Health Matters 20 (40), 59–66. [DOI] [PubMed] [Google Scholar]
- Link B.G. & Phelan J.C. (2001) Conceptualizing stigma. Annual Review of Sociology 27, 363–385. [Google Scholar]
- Mahajan A.P., Sayles J.N., Patel V.A., Remien R.H., Sawires S.R., Ortiz D.J. et al (2008) Stigma in the HIV/AIDS epidemic: a review of the literature and recommendations for the way forward. AIDS (London, England) 22 (Suppl. 2), S57–S65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Major B. & O'Brien L.T. (2005) The social psychology of stigma. Annual Review of Psychology 56 (1), 393–421. [DOI] [PubMed] [Google Scholar]
- Mak W.W.S., Mo P.K.H., Cheung R.Y.M., Woo J., Cheung F.M. & Lee D. (2006) Comparative stigma of HIV/AIDS, SARS, and Tuberculosis in Hong Kong. Social Science & Medicine 63 (7), 1912–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mull D. (1991) Traditional perceptions of marasmus in Pakistan. Social Science & Medicine 32 (2), 175–191. [DOI] [PubMed] [Google Scholar]
- Nyblade L., Stangl A., Weiss E. & Ashburn K. (2009) Combating HIV stigma in health care settings: what works? Journal of the International AIDS Society 12 (1), 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker R. & Aggleton P. (2003) HIV and AIDS‐related stigma and discrimination: a conceptual framework and implications for action. Social Science & Medicine 57, 13–24. [DOI] [PubMed] [Google Scholar]
- Pinel E.C. (1999) Stigma consciousness: the psychological legacy of social stereotypes. Journal of Personality and Social Psychology 76 (1), 114–128. [DOI] [PubMed] [Google Scholar]
- Reis C., Heisler M., Amowitz C.C., Moreland R.S., Maleni J.O., Anyamele C. et al (2005) Discriminatory attitudes and practices by health workers toward patients with HIV/AIDS in Nigeria. PLoS Medicine 2 (8), e246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel M.T. & Alderman H. (2013) Nutrition‐sensitive interventions and programmes: how can they help to accelerate progress in improving maternal and child nutrition? The Lancet 382, 1–16. [DOI] [PubMed] [Google Scholar]
- Rüsch N., Angermeyer M.C. & Corrigan P.W. (2005) Mental illness stigma: concepts, consequences, and initiatives to reduce stigma. European Psychiatry 20 (8), 529–539. [DOI] [PubMed] [Google Scholar]
- Sharkawy G.E., Newton C. & Hartley S. (2006) Attitudes and practices of families and health care personnel toward children with epilepsy in Kilifi, Kenya. Epilepsy & Behavior 8 (1), 201–212. [DOI] [PubMed] [Google Scholar]
- StataCorp (2013) Stata Statistical Software: Release 13.
- Steele C.M. (1997) A threat in the air: how stereotypes shape intellectual identity and performance. American Psychologist 52 (6), 613–629. [DOI] [PubMed] [Google Scholar]
- Streatfield P.K., Koehlmous T.P., Alan N. & Mridha M.K. (2008) Mainstreaming nutrition in maternal, newborn and child health: barriers to seeking services from existing maternal, newborn, child health programmes. Maternal & Child Nutrition 4 (s1), 237–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindale A. & Bilinsky P. (2006) Household Dietary Diversity Score (HDDS) for Measurement of Household Food Access: Indicator Guide, 2nd edn Food and Nutrition Technical Assistance (FANTA): Washington, DC. [Google Scholar]
- Tangney J.P. & Dearing R.L. (2002) Shame and Guilt. The Guilford Press: New York City. [Google Scholar]
- Turan J.M., Bakus E.A., Onono M., Holzener W.L., Miller S. & Cohen C.R. (2010) HIV/AIDS stigma and refusal of HIV testing among pregnant women in rural Kenya: results from the MAMAS study. AIDS and Behavior 15 (6), 1111–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wambua F. (2013) Marsabit County SMART Nutrition Survey. Food for the Hungry, Concern Worldwide, World Vision, and the Kenyan Ministry of Health.
- Winskell K., Hill E. & Obyerodhyambo O. (2011) Comparing HIV‐related symbolic stigma in six African countries: social representations in young people's narratives. Social Science & Medicine 73, 1257–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]


