Abstract
Prior studies have reported a significant, inverse association between adiponectin in human milk and offspring growth velocity. Less is known about this association in populations characterised by a loss of weight for age z‐scores (WAZs) in early life. We investigated the association between maternal body composition and milk adiponectin in a sample of Filipino mothers. We then tested for an association between milk adiponectin and size for age in their infants. A total of 117 Filipino mothers nursing infants from 0 to 24 months were recruited from Cebu, Philippines. Anthropometrics, interviews and milk samples were collected and analysed using standard protocols. Mean milk adiponectin in this sample was 7.47 ± 5.75 ng mL−1. Mean infant WAZ and weight for length (WLZ) decreased with age. Maternal body composition was not associated with milk adiponectin content. Milk adiponectin had a significant, positive association with infant WAZ and WLZ. Prior reports have found an inverse association between milk adiponectin and infant WAZ. Here, we report that in lean populations with lower milk adiponectin, there is a positive association with infant WAZ, possibly reflecting pleiotropic biological functions of adiponectin for post‐natal growth. This study increases the understanding of normal biological variation in milk adiponectin and the consequences of low levels of milk adiponectin for offspring growth.
Keywords: human milk, adiponectin, infant growth, breast milk
Introduction
Human milk is the ideal first food for the majority of infants. In addition to balanced nutrition capable of exclusively meeting nutritional needs for the first 4–6 months of life, it has been well established that breastfeeding is associated with reduced risks of infant morbidity and mortality (Yoon et al. 1996; Bartick & Reinhold 2010; Liu & Newburg 2013). Recently, there has been increasing evidence for the long‐term benefits of breastfeeding (Kramer 2010), with studies suggesting that breastfeeding is associated with reduced risk of certain childhood cancers, paediatric overweight and type 2 diabetes when compared with infants who never received human milk (Dewey 2003; de Moura & Passos 2005; Guardamagna et al. 2012). This reduction in risk may persist into adulthood, although the evidence is somewhat mixed (Gillman & Mantzoros 2007; Robinson & Fall 2012).
This reduction in the risk of metabolic dysfunction and overweight may come from either long‐term appetite control or metabolic programming, possibly through human milk (Dewey 2003; Guilloteau et al. 2009; Koletzko et al. 2009). Human milk contains numerous metabolic hormones and cytokines, such as leptin, epidermal growth factor, tumour necrosis factor‐α, transforming growth factor‐β and adiponectin (Savino et al. 2009, 2013). Adiponectin, in particular, is thought to be important in the establishment of metabolism, appetite regulation and systemic insulin sensitivity (Robinson et al. 2011). Although its functions are best understood in adults, there is growing evidence that breast milk‐borne adiponectin survives digestion and may be bioactive in neonates (Weyermann et al. 2007). Breastfed infants have higher serum adiponectin than formula‐fed infants (de Zegher et al. 2012); it is hypothesised that higher circulating adiponectin in breastfed infants may reflect exogenous adiponectin of maternal origin.
In general, adiponectin is produced primarily by adipocytes in inverse proportion to the concentrations of fat stored in the cell; people with more body fat have decreased serum adiponectin relative to weight (Harwood 2012). In adult circulation, adiponectin promotes fatty acid oxidation and insulin sensitivity, while also down‐regulating hepatic glucose production and increasing skeletal muscle glucose uptake (Knights et al. 2014). Higher circulating levels of adiponectin are thought to be protective against the risk of overweight, obesity and type 2 diabetes. During lactation, maternal adiponectin levels are suppressed compared with levels during pregnancy or those of non‐pregnant, non‐lactating women under normal reproductive cycles (Asai‐Sato et al. 2006; Fuglsang et al. 2010). In vitro evidence suggests a functional role for prolactin in suppressing adiponectin during lactation (Asai‐Sato et al. 2006). Although suppressed compared with levels during pregnancy or reproductive cycling, maternal circulating adiponectin is typically correlated with milk adiponectin (Savino et al. 2012); however, not all studies report a significant association (Ozarda et al. 2012), and the associations between maternal body fat and milk adiponectin are less clear. It has been hypothesised that some of the variation may be explained by mammary synthesis of adiponectin, either in the mammary epithelial cells themselves or in adjacent adipocytes (Newburg et al. 2010).
For infants, the association between feeding, growth and milk‐borne adiponectin is only beginning to emerge. Several studies have found an inverse association between milk adiponectin and infant weight gain over the first few months of life (Woo et al. 2009; Cesur et al. 2012). Infant weight for age, but not length for age, shows a general pattern of significant, inverse association with milk adiponectin collected during the prior interval. Greater exposure to milk adiponectin during the first 6 months of life predicts slower growth to 6 months, but faster growth from 6 months to 2 years (Woo et al. 2012).
However, little is known about the potential function of adiponectin in lean populations, especially those characterised by reduced post‐natal growth velocity. Almost all prior work has focused on children in primarily Western, well‐fed populations with minimal post‐natal growth failure (Martin et al. 2006; Weyermann et al. 2006; Savino et al. 2008), with no consideration for how milk‐borne adiponectin may function in populations with regular post‐natal growth faltering. Here, we examine these associations in a population of lean Filipino mothers and infants. Infants in this sample have declining weight for age (WAZ) and weight for length z‐scores (WLZ) over the first 2 years of life, with the majority of loss after the first 6 months of life. In this sample, we investigated two primary questions: (i) what is the association between maternal body composition, measured using body mass index (BMI) and total body fat, and milk adiponectin; and (ii) is there an association between milk adiponectin and infant weight or BMI after adjustment for age?
Key messages.
Prior studies on milk adiponectin and infant growth have been conducted in well nourished populations.
Less is known about how such associations may persist in populations with declining postnatal growth status but high breastfeeding rates.
In lean Filipino mothers, there is no association between maternal body composition and milk adiponectin.
Milk adiponectin is positively associated with weight for age in this sample.
In populations with declining postnatal growth status, human milk adiponectin may be protective for growth, possibly illustrating the pleiotrophic effects of adiponectin on infant physiology.
Methodology
Subjects and study design
This study was part of a larger follow‐up study of female participants in the Cebu Longitudinal Health and Nutrition Survey (Cebu Study). The Cebu Study is a longitudinal birth cohort study of more than 3000 mothers and their offspring from 33 communities in the greater Cebu metropolitan area. The mothers in the present study were delivered from 1 May 1983 to 30 April 1984 (Adair et al. 2011). These now adult offspring have continued participation in the study, with approximately 55% of the original singleton participants participating in the 2009 survey (more recent data not available). Participants from the original birth cohort, now young adults, have been visited 20 times from birth to 2014, with separate surveys conducted on males and females after 2007.
In 2007–2008, during a follow‐up survey, currently lactating female participants were recruited into a study of human milk composition and early growth (Quinn et al. 2012). One hundred and thirty‐two women were eligible; all agreed to participate. Women were nursing infants from 10 days to 4 years of age; only those women nursing infants less than 2 years of age were included in analyses.
Each mother was interviewed in her home by two of the study authors (EAQ, FL). Interviews included 24‐h dietary recalls and collected information, health care behaviours, infant care practices, household assets, maternal work histories and health histories for both mothers and infants. Supplemental health data collected on all household members. Anthropometrics of weight, height, mid‐upper arm circumference and four skinfolds (triceps, biceps, subscapular, suprailiac) were collected on mothers. Recumbent length, weight, head circumference, mid‐upper arm circumference and seven skinfolds (triceps, biceps, subscapular, suprailiac, thigh, calf, abdominal) were collected on all infants. From these skinfold measurements, maternal percent body fat was calculated using the formula of Durnin Womersley, previously used on this population (Kuzawa et al. 2013). Intra‐observer error was reduced by having the same two researchers conduct all field measurements; intra‐observer error was <4%.
Milk collection
Milk samples were collected following standard milk nutrition collection protocols (Ruel et al. 1997; Miller et al. 2013). Between 6 and 10 am, mothers were visited in their homes. The night before, text messages were sent to mothers to schedule the visit and remind them not to breastfeed for 2 h prior to the study visit. When the infant was hungry, mothers began breastfeeding as they usually would. After 2 min, the infant was removed from the breast and a 10‐mL sample was collected by hand expression into a sterile polypropylene tube. The infant was returned to the breast. Samples were immediately transported on freezer packs to the laboratory facilities at the University of San Carlos, where they were frozen at −20°C for 1–3 months. Samples were then shipped on dry ice to the United States, where they were analysed at Washington University in St. Louis.
Milk analysis
Milk adiponectin was measured using a modified enzyme‐linked immunosorbent assay (R & D Systems, Minneapolis, MN, USA) modified for use with human milk (Bernstein & Dominy 2013). Samples were skimmed prior to analysis. Briefly, samples were diluted 1:5 in reagent diluent and run in duplicate using a sandwich enzyme linked immunoabsorbant assay (EIA) protocol. Any sample with a coefficient of variation (CV) greater than 10% was rerun. Inter‐assay variation was 14%, well within normal ranges for EIA protocols.
Statistical analysis
Data were analysed with Stata 12.1 (College Station, TX, USA). Descriptive characteristics (mean, standard deviation) were calculated on untransformed data. Multivariate linear regression was used to test for linear relationships between maternal body composition (BMI, percent body fat) and milk adiponectin, and between milk adiponectin content and infant WAZ. However, as there was considerable skew in the data, both adiponectin and maternal body composition data were log‐transformed prior to analyses. All models were adjusted for infant age. Regression diagnostics were performed on all regression models, with the largest change in AIC score used to determine the best fit model.
Of the 132 women who provided samples, 117 are included in the analysis. Four mothers were excluded because of non‐nutritive suckling/insufficient milk volume, one mother was excluded for concurrent pregnancy and the rest (10) were nursing infants greater than 2 years of age.
To assess hypothesis 1, that maternal body composition was significantly associated with milk adiponectin, we used multivariate linear regression to test for associations between maternal BMI, percent body fat, sum of skinfolds and milk adiponectin, before and after adjustment for infant age.
To investigate hypothesis 2, that the association between infant weight and milk adiponectin, infant weight was converted to WAZ and WLZ using the zanthro function in Stata 12.1, with the World Health Organization standards set as the reference category. Both WAZ and WLZ were normally distributed in this population. Multivariate linear regression was used to test for an association between WAZ, WLZ and milk adiponectin before and after adjustment for infant age and infant mid‐upper arm circumference. Infant age was included in the WAZ and WLZ models as both WAZ and WLZ declined with age, as did milk adiponectin, and the inclusion of age was used to statistically adjust for the age‐associated declines.
Results
Descriptive characteristics of the sample are shown in Table 1. Mean milk adiponectin was 7.47 ± 5.75 ng mL−1 (range 1.38–19.1 ng mL−1). Mean infant age was 9.3 ± 6.2 months. Using regression to test hypothesis 1, we found that milk adiponectin was inversely associated with infant age, but was not significantly correlated with any measure of maternal body composition (BMI, weight, percent body fat from skinfold thicknesses) (Table 2). For each 1‐month increase in infant age, the amount of adiponectin in milk decreased by 0.4 ± 0.09 ng mL−1 of milk (P = 0.000; r 2 = 0.165). There was no significant association between daily breastfeeding frequency or sampled milk volume (full volume measurements were not available) and the adiponectin content of milk. Daily breastfeeding frequency showed only a modest age‐associated decline, from 13.3 ± 3.2 daily feedings at 6 months to 11.7 ± 4.0 feedings at 18 months.
Table 1.
Descriptive characteristics for the sample of 117 mothers and their infants
| Variable | Mean | SD | Min | Max |
|---|---|---|---|---|
| Mother age (years) | 24.1 | 0.3 | 24 | 25 |
| Mother BMI | 19.9 | 3.1 | 13.8 | 31 |
| Mother percent body fat | 24.9 | 3.6 | 15.9 | 34.8 |
| Mother dietary energy (kcal) | 1390 | 659 | 257.7 | 3 478.1 |
| Mother education (years) | 9.7 | 2.8 | 2 | 15 |
| Adiponectin (ng mL−1) | 7.47 | 5.74 | 1.37 | 19.11 |
| Infant age (months) | 9.3 | 6.2 | 0.3 | 24 |
| % Female | 47.6 | |||
| Infant weight (g) | 7150 | 1840 | 3170 | 10 215 |
| Infant length (cm) | 66.6 | 8.9 | 39.5 | 80.5 |
| Weight for age z‐score | −1 | 1.05 | −3.5 | 1.7 |
| Breastfeeding frequency (#/24 h) | 12.1 | 3.6 | 5 | 24 |
BMI, body mass index; SD, standard deviation.
Table 2.
Regression models testing for a linear association between maternal body composition using three different measures (BMI, percent body fat, sum of skinfolds) and milk adiponectin
| β (SE) | P‐value | β (SE) | P‐value | β (SE) | P‐value | β (SE) | P‐value | |
|---|---|---|---|---|---|---|---|---|
| Maternal BMI | 0.004 (0.023) | 0.869 | ||||||
| Maternal body fat | 0.006 (0.020) | 0.760 | ||||||
| Maternal skinfolds | 0.002 (0.006) | 0.707 | ||||||
| Infant age | −0.057 (0.012) | 0.0005 | −0.057 (0.012) | 0.0005 | −0.057 (0.012) | 0.0005 | −0.057 (0.012) | 0.0005 |
| Constant | 9.059 (0.482) | 0.0005 | 8.976 (0.536) | 0.0005 | 9.025 (0.321) | 0.0005 | 9.136 (0.129) | 0.0005 |
| R 2 | 0.173 | 0.174 | 0.175 | 0.181 |
BMI, body mass index; SE, standard error. There was no significant association between any measure of maternal body composition and milk adiponectin in this sample. Infant age was a significant predictor of milk adiponectin, with infant age inversely associated with milk adiponectin as has been reported elsewhere.
For hypothesis 2, multivariate linear regression was also used to test for a linear relationship between milk adiponectin and infant size for age as z‐score, before and after adjustment for infant age, maternal BMI and nursing frequency. Milk adiponectin was a significant but positive predictor of WAZ and WLZ score in this sample both before and after adjustment for infant age (Table 3). As this is a cross‐sectional study, these are general trends within the sample, not associations within individuals over time. In this sample, weight and length for age z‐scores decline with age (Fig. 1), but higher milk adiponectin predicts higher WAZs in children over the age of 1.
Table 3.
Regression models testing for an association between milk adiponectin and infant weight for age and BMI for age z‐scores
| Infant WAZ | Infant BMI z‐score | |||
|---|---|---|---|---|
| β (SE) | P | β (SE) | P | |
| Milk adiponectin | 0.255 (0.110) | 0.022 | 0.464 (0.208) | 0.028 |
| Infant age | −0.218 (0.029) | 0.0005 | 0.039 (0.055) | 0.477 |
| Infant length | 0.119 (0.021) | 0.0005 | 0.116 (0.039) | 0.004 |
| Constant | −9.075 (1.652) | 0.0005 | −22.410 (3.137) | 0.0005 |
| R 2 | 0.398 | 0.335 | ||
BMI, body mass index; SE, standard error; WAZ, weight for age z‐scores. All models were adjusted for infant age and infant length.
Figure 1.
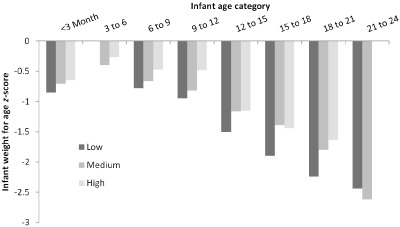
Association between infant weight for age z‐score and milk adiponectin by offspring age. Offspring were divided into trimesters of lactation to allow for more precise comparisons within age groups. Milk adiponectin was stratified into tertiles. There were no infants in the 3–6 months of age (peak lactation) receiving milk in the lowest tertile of milk adiponectin and no infants in the 21–24 months of age bracket receiving milk with high adiponectin. Within each group, infants receiving milk with the highest tertile of adiponectin had higher weight for age z‐scores than infants in the same age category receiving milk from lower adiponectin tertiles.
In a smaller subset of the sample (n = 29) with at least two longitudinal milk samples, data show modest age‐associated declines over the short study period; however, this subset was all less than 6 months old, which may have confounded the model.
Discussion
Predictors of milk adiponectin
As discussed previously, mean milk adiponectin in this sample was low compared with prior published studies but within the ranges reported by previous studies (Martin et al. 2006; Weyermann et al. 2006; Savino et al. 2008; Newburg et al. 2010; Ozarda et al. 2012). In this sample of 117 Filipino mothers nursing offspring up to 24 months of age, milk adiponectin content was not associated with any available measure of maternal body composition (BMI, percent body fat, sum of skinfolds). Additional evidence also supports an association between plasma and milk adiponectin in breastfeeding women. Circulating adiponectin levels have been reported with possible correlation to milk adiponectin (Ozarda et al. 2012; Savino et al. 2012). Similarly, the associations between maternal BMI and milk adiponectin have been reported in some, but not all, populations (Martin et al. 2006).
To our knowledge, there is only one similar report of a population with as low a sample mean for milk adiponectin (Bernstein & Dominy 2013). This study was also conducted with two Filipino populations: the Ilocanos and Aeta, two populations living in Luzon (northern Philippines). The Ilocanos are primarily small‐scale farmers and the Aeta are foragers exhibiting a pygmy phenotype (average adult male stature <150 cm). Bernstein & Dominy (2013) reported mean milk adiponectin levels of 4.2 and 4.4 ng mL−1, respectively. Presently, the milk adiponectin content of the Ilocanos and Aeta are the lowest reported in a human population. However, this study did not include comparisons with measures of maternal body composition or infant age. It is therefore impossible to know if the lack of association between maternal body composition and milk adiponectin reported in this study was also present among the Ilocanos and Aeta. Likely, as has been consistently reported elsewhere, milk adiponectin among the Ilocanos and Aeta was significantly, inversely associated with infant age, as we also demonstrate here (Martin et al. 2006; Weyermann et al. 2006; Savino et al. 2008; Newburg et al. 2010; Ozarda et al. 2012).
Associations between milk adiponectin and infant size
The positive association between human milk adiponectin and infant weight, as measured here using WAZ, is somewhat contradictory to those reported in prior populations. Most prior studies have reported that higher milk adiponectin is associated with lower early post‐natal weight gain (Woo et al. 2009; Newburg et al. 2010; Cesur et al. 2012; Brunner et al. 2015), frequently followed by rebound growth up to 2 years (Woo et al. 2012).
The mean milk adiponectin concentration for this sample was 7.47 ± 5.75 ng mL−1, well below the sample means reported for most prior studies. This sample may be situated on the low end of the global distribution for adiponectin in milk, but illustrates the considerable potential for both individual‐ and population‐level variation in milk hormones. In this sample, adiponectin content of milk was positively associated with WAZ, before and after adjustment for infant age (Fig. 1). Prior studies from Mexico and the United States have reported that milk adiponectin is inversely associated with infant weight gain during the first 4 months of life, but then positively associated with weight gain at 2 years of age (Woo et al. 2009). However, in some populations as illustrated in Woo et al. (2009), there is visible evidence that the association is considerably flattened at the lower end of the distribution of milk adiponectin, and even shows a modest positive association in samples from both the United States and Mexico (Fig. 2). At approximately 15 ng mL−1, the inverse association between milk adiponectin and infant size for age becomes visible in these graphs. However, the distribution of milk adiponectin in the Cebu sample is much lower – the mean for the Cebu sample is almost two standard deviations below the mean reported for Mexico or the United States in the aforementioned study. Ninety per cent of milk samples from Cebu are below this 15 ng mL−1 point of inflection.
Figure 2.
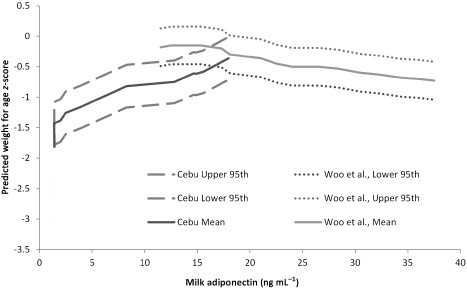
Comparison of predicted weight for age z‐scores (WAZ) for the Cebu sample (mean plus 95th percentiles) compared with published data reproduced from Woo et al. (2009) using combined data from Mexico and US mothers. Woo et al. (2009) had previously shown that in the lower end of the distribution in their sample, there was not an inverse association between milk adiponectin and infant WAZ. The data from this study provide additional information on normal biological variation in human milk, and suggest that the association between milk adiponectin and infant WAZ may be parabolic, with low levels of milk adiponectin positively associated with infant WAZ and high levels of milk adiponectin inversely associated with infant WAZ.
Tests of the association between milk adiponectin and infant growth have also been limited to well‐nourished, primarily Western populations. These populations are characterised by generally good post‐natal weight gain – and minimal to zero loss of WAZs over the first year of life. However, in Cebu, as with many other populations, especially those in low‐resource, high‐stress environments, there is instead age‐related post‐natal decline in WAZ and WLZ scores. In the Cebu Study, mean WAZ for infants <6 months was −0.41, for infants 6–12 months −1.11, for toddlers 12–18 months −1.28 and −1.98 for toddlers 18–24 months, with no differences in WAZ or WLZ by infant sex (P = 0.566). Across all age categories, the incidence of underweight (WAZ < −2) was approximately 20% (16/104; with three additional infants having WAZ < −1.98); incidence of underweight increased with age. The age‐associated decline in WAZ and WLZ has been previously reported in the prior generation (the mothers generation) of the Cebu Study (Adair & Guilkey 1997).
In lean populations such as this, with lower milk adiponectin and general declines in WAZ and WLZ scores during infancy, the positive association between milk adiponectin and infant growth may speak instead to the pleiotropic effects of adiponectin throughout the body, including increasing fatty acid oxidation, insulin sensitivity promotion and immune function.
Evolutionary context
Although some studies have reported significant positive associations between maternal BMI and milk adiponectin (Martin et al. 2006) other studies, including this one, have reported no association between maternal BMI and milk adiponectin (Weyermann et al. 2006; Ozarda et al. 2012). Martin et al. (2006) have suggested that the lack of a consistent association across samples may reflect reduced inhibition of adiponectin synthesis by prolactin in heavier women. Changes to prolactin regulation and downstream effects on local and systemic adiponectin production may contribute to population variation in milk adiponectin (Ozarda et al. 2012). Increased nursing frequency, resulting in increased circulating prolactin, may act to down‐regulate mammary synthesis of adiponectin. In Cebu, infants were nursed an average of 12.1 ± 3.6 times per 24 h (range 5–24); however, there was no significant association between nursing frequency and milk adiponectin, nor an interaction between nursing frequency and maternal BMI. This high frequency of nursing may maintain elevated prolactin levels in mothers which, in turn, suppress adiponectin synthesis (Nilsson et al. 2005) and lead to the reduction in milk adiponectin observed here.
The findings reported here for this sample do not detract from prior discussions of the long‐term effects of adiponectin on infant programming and growth. Rather, we suggest that these data provide important insights into the full range of normal human variation in milk adiponectin and its potential function in nurslings. Although the overall mean and individual values reported for this sample are within the ranges reported in prior studies, the overall distribution is shifted much lower. Given the available evidence, it seems feasible, rather than unusual, that the positive association we report here between milk adiponectin and infant growth is part of normal biological variation. For example, Woo et al. (2009) graphically represented the association between WAZs and adiponectin as an inverted parabola; however, the left side of the graph was truncated at 10 ng mL−1 (Fig. 1). Most of the Cebu sample falls within this lower range (0.5–10 ng mL−1), and further illustrates these associations between milk adiponectin and WAZ at the lower end of the milk adiponectin distribution. For comparison between the Cebu sample and the previously described sample from the United States, we calculated median, fifth and 95th confidence intervals for WAZs by milk adiponectin for the Cebu sample and plotted them with the US data from Woo et al. (2009). The comparison is shown in Fig. 2. The confidence intervals for WAZ shown in this interval are specific to the samples, with 95% of children in the Cebu growing at or below the fifth centile for the US sample.
It has previously been hypothesised that milk adiponectin may play an important role in maternal regulation of infant growth (Weyermann et al. 2007; Woo et al. 2009; Cesur et al. 2012; Brunner et al. 2015). One leading hypothesis is that increased milk adiponectin may be a maternal mechanism for decreasing infant weight gain during the early post‐natal period when infant growth is entirely dependent on maternal metabolism (Hinde 2012). By reducing growth during this time period, the energetic costs of infant growth are displaced to later infancy, when others, such as fathers, grandmothers or older siblings, can contribute to the energy costs of reproduction, freeing the mother up earlier for a subsequent pregnancy. In milk with low levels of adiponectin, such as milk found in the Cebu Study, low milk adiponectin is growth promoting, with maternal physiology bearing an increased share of the energetic burden of fuelling offspring growth. This, too, may be an adaptive mechanism for maximising child survivorship: if milk adiponectin is associated with either maternal BMI or circulating adiponectin, then low milk adiponectin may signal a limited nutritional environment with reduced capacity for supporting offspring growth. Increasing growth during the periods of total or partial breastfeeding, when maternal metabolism can support growth, may be a mechanism for promoting increased child survival, especially if adiponectin is important in the regulation of lean mass vs. fat mass. Murine models have shown that low‐energy diets increase circulating adiponectin (Berg et al. 2001), and it is hypothesised, but not demonstrated, that this may result in lower milk adiponectin (Savino et al. 2008). However, Savino et al. (2008) also speculate that ‘dynamic changes in adiponectin levels may be an adaptive response of adipose [cells] to environmental energy supply’ (p. 704).
Although the exact maternal‐ or population‐specific characteristics that predispose populations to low milk adiponectin (perhaps low maternal BMI, low‐energy intakes or high physical activity levels) are uncertain, there is a general trend towards associations between milk adiponectin and maternal BMI among populations with higher BMIs. One possible interpretation of these findings may be that the majority of milk adiponectin is synthesised in the mammary gland under local control. Additional adiponectin, should circulating adiponectin levels be high or in cases of higher maternal BMI, may be recruited from extra‐mammary sources. This may explain why in well‐nourished populations with a combination of both high body fat and high dietary fat intake, milk adiponectin is significantly associated with maternal BMI, but is not associated with maternal BMI in lean populations with relatively low‐fat diets. In such high‐resource environments, high milk adiponectin signals to infants that they are likely in environments highly capable of supporting growth outside of maternal metabolism (Newburg et al. 2010); subsequently, the inverse association between milk adiponectin and infant growth makes sense as alternative environmental resources are available to support such growth.
However, in more marginal or highly seasonal environments, such resources may be unavailable. Here, accelerating growth when maternal metabolism can fully or partially support it may be a strategy for maximising child growth and survival under more limited conditions. This maintenance of infant growth through maternal metabolism may increase maternal costs associated with lactation and may have long‐term effects on maternal reproductive trade‐offs as a faster growing infant will require more milk with subsequent additional maternal metabolic costs compared with a slow growing infant.
However, the infant, given a larger body size as well as potentially improved insulin sensitivity and other aspects of short‐ and long‐term developmental programming, may have improved survival in marginal environments – either because of increased biological sensitivity to the present context, changes to metabolic function or increased fat reserves to ameliorate short‐term, possibly seasonal fluctuations in nutrition. Further, very little is known about the functions of low levels of milk adiponectin on the long‐term development of infant metabolism, fatty acid oxidation and immune function. Even the potential improvement in maintenance of gut barrier function, as the result of localised binding of milk‐borne adiponectin to adiponectin receptor‐1 in the small intestines (Zhou et al. 2005), may be growth promoting under conditions of marginal nutrition and increased pathogenicity. These factors may explain the parabolic relationship between milk adiponectin and infant growth, with low levels targeted primarily at the gut and higher levels allowing for more systemic exposure to milk‐borne adiponectin.
Current understandings of the normal range of variation and physiological function of milk adiponectin may be incomplete and biased towards well‐nourished, well‐growing populations. Similarly, prior studies have also been limited to comparatively young infants, and have not been able to fully investigate the association between milk adiponectin and offspring growth over the first 2 years of life in populations where extended breastfeeding is common. Continued research in more diverse populations with differing nutritional status, infant feeding patterns and child growth patterns will be necessary to more fully understand the function of milk‐borne adiponectin and promote optimal infant health.
Source of funding
This work was supported by the National Science Foundation, Doctoral Dissertation Improvement Grant #0726231; National Science Foundation: BCS‐0746320.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
JA, MC, JO, EQ and KM performed all laboratory analyses and contributed to the writing of the paper. EQ and PD designed the project, assisted with drafting and revising the paper, and conducted the fieldwork.
Acknowledgements
This would not have been possible without the generous participation of the mothers and infants in this study. The authors would like to thank the researchers who worked on this project: Fe Largado was the primary field staff for this study, supported by the staff at the Office of Population Studies at the University of San Carlos, Cebu.
Anderson, J. , McKinley, K. , Onugha, J. , Duazo, P. , Chernoff, M. , and Quinn, E. A. (2016) Lower levels of human milk adiponectin predict offspring weight for age: a study in a lean population of Filipinos. Maternal & Child Nutrition, 12: 790–800. doi: 10.1111/mcn.12216.
References
- Adair L.S. & Guilkey D.K. (1997) Age‐specific determinants of stunting in Filipino children. The Journal of Nutrition 127, 314–320. [DOI] [PubMed] [Google Scholar]
- Adair L.S., Popkin B.M., Akin J.S., Guilkey D.K., Gultiano S., Borja J. et al (2011) Cohort profile: the Cebu longitudinal health and nutrition survey. International Journal of Epidemiology 40, 619–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai‐Sato M., Okamoto M., Endo M., Yoshida H., Murase M., Ikeda M. et al (2006) Hypoadiponectinemia in lean lactating women: prolactin inhibits adiponectin secretion from human adipocytes. Endocrine Journal 53, 555–562. [DOI] [PubMed] [Google Scholar]
- Bartick M. & Reinhold A. (2010) The burden of suboptimal breastfeeding in the United States: a pediatric cost analysis. Pediatrics 125, e1048–e1056. [DOI] [PubMed] [Google Scholar]
- Berg A.H., Combs T.P., Du X., Brownlee M. & Scherer P.E. (2001) The adipocyte‐secreted protein Acrp30 enhances hepatic insulin action. Nature Medicine 7, 947–953. [DOI] [PubMed] [Google Scholar]
- Bernstein R.M. & Dominy N.J. (2013) Mount Pinatubo, inflammatory cytokines, and the immunological ecology of Aeta hunter‐gatherers. Human Biology 85, 231–250. [DOI] [PubMed] [Google Scholar]
- Brunner S., Schmid D., Zang K., Much D., Knoeferl B., Kratzsch J. et al (2015) Breast milk leptin and adiponectin in relation to infant body composition up to 2 years. Pediatric Obesity 10, 67–73. [DOI] [PubMed] [Google Scholar]
- Cesur G., Ozguner F., Yilmaz N. & Dundar B. (2012) The relationship between ghrelin and adiponectin levels in breast milk and infant serum and growth of infants during early postnatal life. The Journal of Physiological Sciences 62, 185–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey K.G. (2003) Is breastfeeding protective against child obesity? Journal of Human Lactation 19, 9–18. [DOI] [PubMed] [Google Scholar]
- Fuglsang J., Sandager P., Møller N., Flyvbjerg A. & Ovesen P. (2010) Alterations in circulating adiponectin levels occur rapidly after parturition. European Journal of Endocrinology 163, 69–73. [DOI] [PubMed] [Google Scholar]
- Gillman M.W. & Mantzoros C.S. (2007) Breast‐feeding, adipokines, and childhood obesity. Epidemiology (Cambridge, Mass.) 18, 730–732. [DOI] [PubMed] [Google Scholar]
- Guardamagna O., Abello F., Cagliero P. & Lughetti L. (2012) Impact of nutrition since early life on cardiovascular prevention. Italian Journal Pediatrics 38, 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloteau P., Zabielski R., Hammon H.M. & Metges C.C. (2009) Adverse effects of nutritional programming during prenatal and early postnatal life, some aspects of regulation and potential prevention and treatments. Journal of Physiology and Pharmacology 60 (Suppl. 3), 17–35. [PubMed] [Google Scholar]
- Harwood H.J.J. (2012) The adipocyte as an endocrine organ in the regulation of metabolic homeostasis. Neuropharmacology 63, 57–75. [DOI] [PubMed] [Google Scholar]
- Hinde K. 2012. Mother's Fat Sends Love Letter to Baby via the Milk Express. Mammals Suck … Milk. Available at: http://mammalssuck.blogspot.com/2012/06/mothers-fat-sends-love-letter-to-baby.html.2014 (Accessed 24 September 2014).
- Knights A.J., Funnell A.P., Pearson R.C., Crossley M. & Bell‐Anderson K.S. (2014) Adipokines and insulin action: a sensitive issue. Adipocyte 3, 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koletzko B., Von Kries R., Monasterolo R.C., Subias J.E., Scaglioni S., Giovannini M. et al (2009) Infant feeding and later obesity risk. Advances in Experimental Medicine and Biology 646, 15–29. [DOI] [PubMed] [Google Scholar]
- Kramer M.S. (2010) ‘Breast is best’: the evidence. Early Human Development 86, 729–732. [DOI] [PubMed] [Google Scholar]
- Kuzawa C.W., Adair L.S., Borja J. & McDade T.W. (2013) C‐reactive protein by pregnancy and lactational status among Filipino young adult women. American Journal of Human Biology: The Official Journal of the Human Biology Council 25, 131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B. & Newburg D.S. (2013) Human milk glycoproteins protect infants against human pathogens. Breastfeeding Medicine: The Official Journal of the Academy of Breastfeeding Medicine 8, 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin L.J., Woo J.G., Geraghty S.R., Altaye M., Davidson B.S., Banach W. et al (2006) Adiponectin is present in human milk and is associated with maternal factors. The American Journal of Clinical Nutrition 83, 1106–1111. [DOI] [PubMed] [Google Scholar]
- Miller E., Aiello M., Fujita M., Hinde K., Milligan L. & Quinn E. (2013) Field and laboratory methods in human milk research. American Journal of Human Biology: The Official Journal of the Human Biology Council 25, 1–11. [DOI] [PubMed] [Google Scholar]
- de Moura E.G. & Passos M.C. (2005) Neonatal programming of body weight regulation and energetic metabolism. Bioscience Reports 25, 251–269. [DOI] [PubMed] [Google Scholar]
- Newburg D.S., Woo J.G. & Morrow A.L. (2010) Characteristics and potential functions of human milk adiponectin. The Journal of Pediatrics 156, S41–S46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L., Binart N., Bohlooly‐Y M., Bramnert M., Egecioglu E., Kindblom J. et al (2005) Prolactin and growth hormone regulate adiponectin secretion and receptor expression in adipose tissue. Biochemical and Biophysical Research Communications 331, 1120–1126. [DOI] [PubMed] [Google Scholar]
- Ozarda Y., Gunes Y. & Tuncer G.O. (2012) The concentration of adiponectin in breast milk is related to maternal hormonal and inflammatory status during 6 months of lactation. Clinical Chemistry and Laboratory Medicine 50, 911–917. [DOI] [PubMed] [Google Scholar]
- Quinn E.A., Largado F., Power M. & Kuzawa C.W. (2012) Predictors of breast milk macronutrient composition in Filipino mothers. American Journal of Human Biology: The Official Journal of the Human Biology Council 24, 533–540. [DOI] [PubMed] [Google Scholar]
- Robinson K., Prins J. & Venkatesh B. (2011) Clinical review: adiponectin biology and its role in inflammation and critical illness. Critical Care 15, 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S. & Fall C. (2012) Infant nutrition and later health: a review of current evidence. Nutrients 4, 859–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruel M.T., Dewey K.G., Martínez C., Flores R. & Brown K.H. (1997) Validation of single daytime samples of human milk to estimate the 24‐h concentration of lipids in urban Guatemalan mothers. The American Journal of Clinical Nutrition 65, 439–444. [DOI] [PubMed] [Google Scholar]
- Savino F., Petrucci E. & Nanni G. (2008) Adiponectin: an intriguing hormone for paediatricians. Acta Paediatrica 97, 701–705. [DOI] [PubMed] [Google Scholar]
- Savino F., Liguori S.A., Fissore M.F. & Oggero R. (2009) Breast milk hormones and their protective effect on obesity. International Journal of Pediatric Endocrinology 2009, 327505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino F., Lupica M.M., Benetti S., Petrucci E., Liguori S.A. & Cordero Di Montezemolo L. (2012) Adiponectin in breast milk: relation to serum adiponectin concentration in lactating mothers and their infants. Acta Paediatrica 101, 1058–1062. [DOI] [PubMed] [Google Scholar]
- Savino F., Benetti S., Liguori S.A., Sorrenti M. & Cordero Di Montezemolo L. (2013) Advances on human milk hormones and protection against obesity. Cellular and Molecular Biology (Noisy‐Le‐Grand, France) 59, 89–98. [PubMed] [Google Scholar]
- Weyermann M., Beermann C., Brenner H. & Rothenbacher D. (2006) Adiponectin and leptin in maternal serum, cord blood, and breast milk. Clinical Chemistry 52, 2095–2102. [DOI] [PubMed] [Google Scholar]
- Weyermann M., Brenner H. & Rothenbacher D. (2007) Adipokines in human milk and risk of overweight in early childhood: a prospective cohort study. Epidemiology (Cambridge, Mass.) 18, 722–729. [DOI] [PubMed] [Google Scholar]
- Woo J.G., Guerrero M.L., Altaye M., Ruiz‐Palacios G.M., Martin L.J., Dubert‐Ferrandon A. et al (2009) Human milk adiponectin is associated with infant growth in two independent cohorts. Breastfeeding Medicine: The Official Journal of the Academy of Breastfeeding Medicine 4, 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo J.G., Guerrero M.L., Guo F., Martin L.J., Davidson B.S., Ortega H. et al (2012) Human milk adiponectin affects infant weight trajectory during the second year of life. Journal of Pediatric Gastroenterology and Nutrition 54, 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon P.W., Black R.E., Moulton L.H. & Becker S. (1996) Effect of not breastfeeding on the risk of diarrheal and respiratory mortality in children under 2 years of age in Metro Cebu, The Philippines. American Journal of Epidemiology 143, 1142–1148. [DOI] [PubMed] [Google Scholar]
- de Zegher F., Sebastiani G., Diaz M., Sanchez‐Infantes D., Lopez‐Bermejo A. & Ibanez L. (2012) Body composition and circulating high‐molecular‐weight adiponectin and IGF‐I in infants born small for gestational age: breast‐ versus formula‐feeding. Diabetes 61, 1969–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Sun X., Jin L., Stringfield T., Lin L. & Chen Y. (2005) Expression profiles of adiponectin receptors in mouse embryos. Gene Expression Patterns 5, 711–715. [DOI] [PubMed] [Google Scholar]


