Abstract
Peanut milk‐based ready‐to‐use therapeutic food (P‐RUTF) primarily used to treat severe acute malnutrition at community setting is expensive. We developed an alternative milk‐free soybean–maize–sorghum‐based RUTF (SMS‐RUTF) using locally grown ingredients that have the potential to support local economy and reduce the cost of RUTF. We describe the production process and results of acceptability of the new product. Acceptability and tolerance of SMS‐RUTF was compared with P‐RUTF among 45 children aged 4–11 years old based on a cross‐over design. Each child consumed 250 g RUTF for 10 days followed by a five‐day washout period and a subsequent 10‐day period on the second RUTF. The SMS‐RUTF was as acceptable as the P‐RUTF among normal children aged 4–11 years of age with no associated adverse effects. SMS‐RUTF was stable for at least 12 months without detectable microbiological or chemical deterioration. The major challenge encountered in SMS‐RUTF development was the difficulty to accurately determine key nutrient composition due to its high oil content. Use of diversified locally available ingredients to produce RUTF is feasible. The SMS‐RUTF meets expected standards and is acceptable to children aged 4–11 months old. Effectiveness and cost‐effectiveness of SMS‐RUTF is required.
Keywords: acceptability, severe acute malnutrition, milk‐free soya–maize–sorghum‐RUTF, shelf stability
Introduction
Severe acute malnutrition (SAM) is widespread in resource‐poor settings (Collins et al. 2006). Over the last decade, a ready‐to‐use therapeutic food (RUTF) composed of a mixture of milk powder, sugar, vegetable oil, peanut butter, vitamins and minerals (Manary 2006) has been effectively used in the treatment of SAM among children in developing countries (Diop et al. 2004; Sandige et al. 2004; 2005, 2006; Linneman et al. 2007), especially sub‐Saharan Africa.
Despite the positive developments in RUTF production and evidence that it works in treatment of SAM, there are issues that need addressing.
First, local production of RUTF in any particular setting is mainly limited by ingredient availability. Milk constitutes 25–35% w/w of peanut‐based RUTF (P‐RUTF) formula. Milk powder is expensive and often must be imported, and in some countries, the cost of milk powder represents over half the cost of the final RUTF (Collins & Henry 2004). Currently, there is a dearth of data on production and acceptability of RUTF manufactured without addition of milk while using legumes other than peanuts. Initial research into the use of RUTF formulation based on chickpea, sesame and maize with low milk content has proved effective in addressing acute malnutrition in human immunodeficiency virus (HIV)‐positive adults (Bahwere et al. 2009), but there is no adequate evidence among children. Such alternative RUTF formulations may offer affordable options for management of SAM in resource poor settings. Second, non‐peanut formulations may be more attractive due to potentially lesser risk of aflatoxin contamination in P‐RUTFs. Because locally grown peanuts are highly prone to aflatoxin contamination (Owino 2009), peanut paste for RUTF production in Africa is mostly imported.
P‐RUTF is mainly processed through the blending of roasted peanuts with the rest of the ingredients. However, other thermal processing techniques such as extrusion cooking have not been applied for pre‐cooking of main ingredients for P‐RUTF. Extrusion cooking is a short‐time high‐temperature cooking process during which food is subjected to high shear pressure at a temperature of up to 150°C for 20–30 s. Extrusion cooking is associated with improved protein digestibility and bioavailability of sulphur‐containing amino acids (Singh et al. 2007). This process is easier to automate and may thus be easier to scale up.
RUTF formulation development and acceptability among target populations have not been adequately described in peer reviewed literature.
This study aimed to describe the formulation and processing of non‐milk RUTF based on soybean (Glycine max), maize (Zea mais) and sorghum (Sorghum bicolor) and to assess whether the milk‐free soya–maize–sorghum (SMS)‐RUTF may be as acceptable as P‐RUTF with milk among children aged 4–11 years.
Key messages
-
•
Affordable RUTF formulations are needed, but such new formulations must be acceptable and not associated with any adverse health effects among target children with SAM.
-
•
There is need to standardize nutrient analysis techniques in RUTF, especially in laboratories based in low income settings.
-
•
It is feasible to formulate acceptable milk free RUTF using non peanut ingredients, but the adoption of such new formulations should be based on proven efficacy among target populations.
Materials and methods
Soybean, maize and sorghum were chosen as they are widely grown and consumed in Africa. Soybean is an important source of high quality, inexpensive protein (38%) and oil (18%). In sub‐Saharan Africa, soybean is mostly grown by small‐scale farmers either as a sole crop or mixed with sorghum, maize or cassava. The per capita consumption of maize in sub‐Saharan Africa is 60 kg, providing about one‐third of the mean calorie intake. Sorghum is a main staple food in Africa and is consumed in the form of stiff or thin porridges as a steam‐cooked product, such as couscous, or as a beverage (Consultative Group on International Agricultural Research: http://www.cgiar.org/impact/research/soybean.html).
All soybean, maize and sorghum were obtained from stocks approved and pre‐purchased by Insta Products EPZ, Athi River, Kenya. Icing sugar was purchased from Ken Afric Industries Ltd and Patco Industries Ltd, both based in Nairobi, Kenya. Palm olein was purchased from Kapa Oil Refineries, Nairobi, Kenya. Palm stearin was purchased from Ciranda Inc, Hudson, WI, USA. All plastic jars with plastic lids and aluminium liners for paste packaging were purchased from SafePak Ltd, Nairobi, Kenya. Cardboard carton boxes for external packaging were purchased form Elgon Ltd, Nairobi, Kenya. Vitamin and mineral premix was purchased from DSM Nutritional Products PTY, Isando, South Africa.
Recipe formulation
The theoretical formulation of SMS‐RUTF food components was based on the use of the following: linear programming (LP) using Microsoft Excel software (Microsoft Corporation, Redmond, WA, USA); the nutritional composition databases of soybean, maize, sorghum, oil and sugar; and ingredient costs. The LP mathematical modelling target was set to achieve the cheapest formulation while matching both United Nations (UN) Organization nutritional requirements for RUTF [World Health Organization (WHO) et al. 2007] and palatability. The source of the food composition database was World Food Dietary Assessment – data base 2.0 (FAO/INFOODS 2007). Whenever data were not available, other international food composition databases were used (USDA 2007; Nutrisurvey 2007). The vitamins and mineral premix were designed to match the requirements of RUTF suggested by UN guidelines (WHO et al. 2007). The micronutrients premix was designed allowing for the endogenous minerals and vitamins of the food ingredients, potential partial loss during extrusion cooking (Killeit 1994) and storage (WHO & FAO 2007).
Industrial extrusion cooking
SMS‐RUTF was entirely processed at Insta Products EPZ. Insta Products EPZ is internationally certified by UNICEF to supply both corn soy blend powder and RUTF. The SMS‐RUTF was processed in two stages namely, extrusion cooking of soya, maize and sorghum blend and mixing of the extruded blend with oil, sugar and mineral and vitamin premix to form a paste. The extrusion cooking phase involved five distinct steps: thus, manual mixing of dry soya, maize and sorghum; pre‐extrusion milling; extrusion cooking and pellets cooling; post‐extrusion coarse milling; and fine milling. Manual mixing of dry ingredients was preceded by accurate weighing of maize, sorghum and soybean before blending using clean spades on a clean tarpaulin surface. The blended dry ingredients were then milled using a Hammer Mill type 650/630B (Cimbria, Thisted, Denmark). The pre‐milled blend was then collected in clean gunny bags and transferred manually to extruder hoppers. The blend was extruded using single‐screw Insta Pro Dry Extruder Model 2000RC (Insta‐Pro International, Urbandale, IA, USA) with a capacity of 12 kg min–1 and set at 140–160°C and 115–135°C barrel and product temperatures, respectively. Extruded pellets were cooled to 20–26°C in a Futtermittel Model K200.2S cooler (Insta‐Pro International, Urbandale, IA, USA). The cooled pellets were milled using a Hammer Mill 650/630B (Cimbria) prior to fine milling using an Alpine pin mill type A‐160‐2 (Hosokawa Micron Ltd, Runcorn, Cheshire, UK) set at 160 mm rotor diameter and 14 000 rpm. Finely milled extruded SMS blend was packed in clean polyethylene bags and transferred for use in the paste processing stage. To form the SMS‐RUTF paste, the SMS blend, sugar, vegetable oil (85:15 w/w palm olein and palm stearin mix) and mineral and vitamin premix were accurately weighed and mixed in a Hobert Mixer Model NCM40 (The Hobart MFG Co. Ltd, London, UK) for a total 15 min at three varying speeds (31 rpm, 78 rpm and 137 rpm, respectively), each lasting 5 min. The resulting SMS‐RUTF was packed in food‐grade 250 g polyethylene jars (SafePak Ltd, Nairobi, Kenya) by scooping with table spoon.
Product stability and quality control
Storage stability of SMS‐RUTF was assessed over 18 months between 2008 and 2010. Microbiological (total viable count, yeasts, moulds, coliforms, Escherichia coli), chemical [peroxide value (PV), total aflatoxin], nutritional composition (protein, fat, energy, carbohydrates) and micronutrient composition (iron, vitamin A and vitamin C) were determined at SGS Kenya Ltd, Mombasa based on standard procedures (AOAC 1995).
To ensure that the level of micronutrients added were as per the declared premix formulation, iron and zinc levels were analysed at the Institute of Food, Nutrition and Health, Zurich, Switzerland and compared with theoretical amounts. Additionally, phytic acid (mg/100 g) was analysed and the results were used to calculate phytic acid to iron molar ratio and phytic acid to zinc molar ratio.
-
1
Phy : Fe molar ratio was calculated as follows:
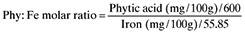
-
2
Phy : Zn molar ratio was calculated as follows:

Acceptability and tolerance test
The aim of this phase was to measure the acceptability and tolerance of SMS‐RUTF compared with P‐RUTF with milk. Acceptability and tolerance was assessed among healthy school‐going children aged 4–11 years old in Lusaka, Zambia. Although RUTF is mainly used among younger children (6–59 months) with SAM, older and healthier children were chosen for ethical reason and because they could give verbal feedback. In this study, RUTF was deemed acceptable based on two criteria, namely, the child consumed at least 75% of the serving of the test RUTF and the frequency of ill effects associated with the food requiring withdrawal from the trial did not exceed 10% of total number of children.
Using a crossover design, a total of 45 children from two pre‐schools, namely, John Howards Community School (JHCS) and Society for Women and AIDS in Zambia (SWAAZ), received SMS‐RUTF and P‐RUTF in two phases each lasting 10 days. The two schools were randomly assigned to first receive either SMS‐RUTF or P‐RUTF for the first phase, which lasted for 10 days. Then the schools automatically switched to the second RUTF after a 5‐day washout break. Anthropometry data [weight, height, mid‐upper arm circumference (MUAC)] were collected on days one, six and 10 in each phase, while 24‐h morbidity and data relating to acceptability (taste perception and consumption) were collected daily. Both SMS‐RUTF and P‐RUTF were packed in 250‐g plastic jars in order to minimize any bias due to the nature of the packaging. A five‐scaled hedonic scale was used to assess characteristics of the two products.
Statistical analysis
Data for nutritional composition of the foods and shelf‐life stability were entered and analysed using Microsoft Excel version 2007. Data were expressed as means and standard deviations where applicable. RUTF acceptability data were analysed using the STATA Data Analysis and Statistical Software (version 10.0, StataCorp LP, Texas, CA, USA). Differences in consumption and acceptability of SMS‐RUTF vs. P‐RUTF were determined using one‐way analysis of variance and least square differences.
Results
Table 1 presents theoretical and analysed values for energy, protein, fat and carbohydrate content and the energy composition per 100 g SMS‐RUTF. All values obtained by analysis were slightly lower than those estimated based on theoretical calculation using data from food composition tables. Both theoretical and analytical values for the proportion of energy from protein and fat matched the reference specifications (WHO et al. 2007) for RUTF namely 10–12% and 45–60% for protein and fat, respectively. The absolute fat content was also in agreement with reference (WHO et al. 2007) values (30–35 g/100 g). The theoretical protein digestibility corrected amino acid score for SMS‐RUTF was estimated to be 83% compared with the reference (Golden et al. 1995) value of at least 70% showing that protein digestibility was optimum.
Table 1.
Proximate, mineral and vitamin premix composition per 100 g SMS‐RUTF
| Nutrient | Amount 100 g−1 SMS‐RUTF | |
|---|---|---|
| Theoretical | Analysed* | |
| Energy (kcal) | 519 | 503.5 ± 25.1 |
| Protein (g) | 15.3 | 13.6 ± 2.0 |
| Fat (g) | 32.7 | 30.5 ± 2.7 |
| Carbohydrates (g) | 50.0 | 55 ± 5.0 |
| Percent energy from protein | 11.8 | 10.5 |
| Percent energy from fat | 56.7 | 53.9 |
SBS, soybean–maize–sorghum ready‐to‐use therapeutic food. *Values are means of 10 determinations. Figures in parentheses are standard deviations.
Table 2 shows, where applicable, the analysed and theoretical values for phytic acid, iron, zinc composition and the resultant phytic acid : iron and phytic acid : zinc molar ratios based on 100 g SMS‐RUTF. The theoretical estimate for iron content was lesser than the analysed value. The phytate : iron molar ratio for SMS‐RUTF (0.8) was lower than the maximum limit (1) (Hallberg et al. 1989). The observed phytate : zinc molar ratio (2.5) was much lower than the maximum limit of 15 (Ma et al. 2005). Phytate : zinc molar ratio of at most 10 is acceptable in providing adequate dietary zinc, and daily ratios consistently above 20 may jeopardize zinc status (Oberleas & Harland 1981). The analysed vitamin C content was much higher than the theoretical value likely due to the fact that the latter did not include intrinsic vitamin C in the ingredients.
Table 2.
Phytic acid, iron, zinc, phy : iron, phy : zinc molar ratios (mg/100 g) and vitamin C content 100 g–1
| Parameter | Amount 100 g−1 SMS‐RUTF | |
|---|---|---|
| Analysed* | Theoretical | |
| Iron (mg) | 52.4 ± 1.7 | 41.4 |
| Zinc (mg) | 18.7 ± 0.1 | 18.4 |
| Phytic acid (mg) | 480 ± 19 | ND |
| Phytic acid : iron molar ratio | 0.8 | ND |
| Phytic acid : zinc molar ratio | 2.5 | ND |
| Vitamin C | 168.9 ± 47 | 126.9 |
SBS, soybean–maize–sorghum ready‐to‐use therapeutic food; ND, not determined. *Values are means of five determinations. Figures in parentheses are standard deviations.
Eighteen‐month storage stability of SMS‐RUTF
Storage stability assessment was initially designed to determine nutrient stability based on vitamin C as the indicator micronutrient as it is highly unstable to heat and oxidation. Moisture content and microbiological growth were measured throughout the storage period. Additionally stability to lipid oxidation was determined based on PV. Figure 1 shows values for protein, moisture and PV at 0, 3, 5, 8, 13 and 19 months of storage at ambient temperature (25°C). PV remained below the maximum value (10 meq kg–1) with an initial decrease followed by a sharp rise before decreasing again. The moisture content remained fairly stable with an all time average of 2.5 ± 0.6 g 100 g–1 and was compatible with reference (WHO et al. 2007) specification.
Figure 1.
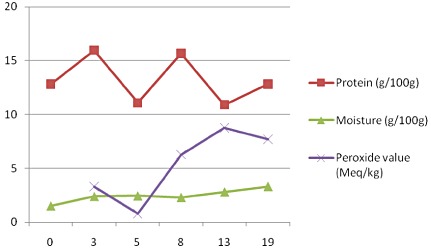
Variation in mean protein, moisture and peroxide value content of duplicate SMS‐RUTF samples at 0, 3, 5, 8, 13 and 19 months of storage at ambient temperature (25°C).
There was wide variation in protein content with all time average being 13.2 ± 2.2. It was not expected that SMS‐RUTF protein content would vary much over 18 months of storage. However, in the course of time, it was observed that there was wide variation in protein content when both intra‐ and inter‐laboratory figures were compared. There was a tendency towards reporting of lower protein values compared with the theoretical values, especially towards the end of the shelf stability trial, which coincided with oil separation. Two anonymous samples of each of SMS‐RUTF that had been stored for 13 and 19 months, respectively, were sent to three different laboratories for protein analysis. Figure 2 shows that there was wide intra‐ and inter‐laboratory variation in reported protein content. Laboratory 1 reported protein content that most closely approached the expected theoretical value. Laboratory 2 (Lab 2) reported the lowest protein content on first analysis attempt. On probing, the research team learnt that the technicians at lab 2(a) had determined protein content in the top phase of the SMS‐RUTF paste, which was composed mainly of separated oil. It is likely that lab 3 followed the same procedure as lab 2(a). After discussions with the research team, lab 2 (b) reported much increased protein values that approached the expected value. It is likely that lab 1 and lab 2(b) analysed for protein after homogeneously blending the SMS‐RUTF paste. To determine intra‐laboratory variation, values for duplicate samples were compared for lab 1 and lab 2. The absolute differences in protein content (g) reported at 13 months were 0.6, 1.6 and –1.1 and 0.35, 0.7 and 1.2 at 19 months, respectively, for LAB 1, LAB 2(A) AND LAB 2(b). Figure 3 depicts variation in vitamin C content over 18 months of storage. The observed figures were much higher than the theoretical value (126.9). This is likely due to analytical error and potential fractionation of the RUTF mass as oil separated overtime.
Figure 2.
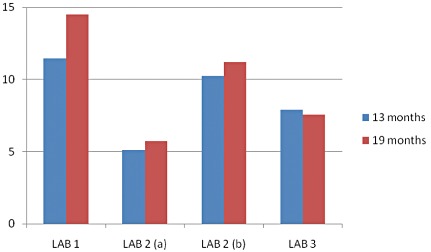
Variations by laboratory in the mean protein content of duplicate SMS‐RUTF samples at 13 and 19 months.
Figure 3.
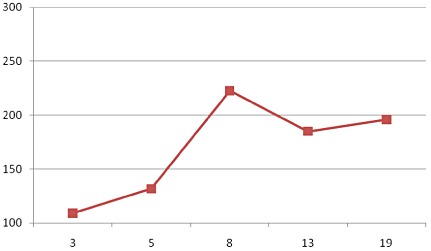
The mean vitamin C content of duplicate SMS‐RUTF samples over 18 months of storage.
Total viable count was 2 × 102, 2.5 × 103 and 2.5 × 102 cfu g–1 at 3, 12 and 19 months of storage, respectively. Yeasts and moulds count remained at <10 cfu g–1 throughout the 18‐month storage period. Aflatoxin content varied from 1 to 1.7 µg kg–1 over the storage period with most of the analyses showing no aflatoxin detection.
SMS‐RUTF acceptability assessment
Twenty‐one children from SWAAZ and 24 from JHCS took part in the acceptability and tolerance study. Fifty five per cent of the children were females. The mean age, MUAC and weight of the children on admission were 7.0 ± 1.9 years, 16.1 ± 2.2 cm and 19.7 ± 5.0 kg, and 6.9 ± 2.0 years, 16.3 ± 1.6 cm and 19.8 ± 5.4 kg for SWAAZ and JHCS, respectively. No carry‐over effect was detected.
Nine children (20.0%) reported at least one episode of diarrhoea while on SMS‐RUTF and seven (15.6%) children while on P‐RUTF; the difference was not significant (P = 0.6). Five children (11.1%) reported vomiting: two (4.4%) while on SMS‐RUTF and three (6.7%) while on PN‐RUTF, respectively. The difference was not statistically significant (P = 1.0). Occurrence of skin rash was reported among 6 (13.3%) children on P‐RUTF; all of them were from JHCS. No case of skin rash was reported for children on SMS‐RUTF during the entire study. The observed skin rash was not specific to certain parts of the body and was itchy and appeared as mild rash with no pustules or vesicles.
The mean observation time was comparable between the two RUTFs: 25.15 min for SMS‐RUTF and 26.31 min for P‐RUTF. The mean consumption of RUTF during this period for SMS‐RUTF and P‐RUTF were 7.3 g kg–1 body weight (bw) and 7.4 g kg–1 bw, respectively; the difference was not statistically significant (P = 0.6). Children were more eager to eat SMS‐RUTF at the beginning evidenced by the higher early consumption pattern compared with P‐RUTF. However, the consumption pattern of SMS‐RUTF decreased over time compared with P‐RUTF. This gradual decline in the consumption of SMS‐RUTF happened following accidental contamination of children in SWAAZ school with P‐RUTF during SMS‐RUTF phase. RUTF colour, taste, sweetness and texture preference was also assessed. One child was excluded from this analysis due to poor communication in most days. Two outstanding reasons for liking SMS‐RUTF were the sweetness and soya content of the product. There was no significant difference in colour preference (P = 0.16) between SMS‐RUTF and P‐RUTF with over 90% in both groups stating that they were okay, happy or very happy with either product. Similarly, no differences were observed in preferences for taste (P = 0.4) and sweetness (P = 0.33) between the two groups. There was a significant difference (P = 0.002) in preference for texture with more children (50%) expressing satisfaction with P‐RUTF compared with only 35.5% for SMS‐RUTF.
At the end of the study, 39 children who were present on the last day were asked which RUTF they preferred to have, and 66.7% (26/39) of the children chose P‐RUTF, 25.6% (10/39) chose SMS‐RUTF as their preference and 7.8% (3/38) children liked both equally.
Discussion
This paper demonstrates that it is possible to formulate a RUTF that meets international standards with no milk addition and based on non‐peanut ingredients. Moreover, SMS‐RUTF has a 12‐month shelf‐life. The SMS‐RUTF was as acceptable as the P‐RUTF with milk among normal children aged 4–11 years of age. No adverse effects were associated with consuming SMS‐RUTF among the study children. No carry‐over effects were observed.
Results from acceptability assessment show that SMS‐RUTF is palatable and that there are no serious adverse effects associated with its consumption among children. The 24‐h morbidity assessment and analysis is supportive of the fact that neither P‐RUTF nor SMS‐RUTF used in this study was associated with major medical problems. Cooking oil, maize meal and sweetness of SMS‐RUTF and texture and presence of large particles in SMS‐RUTF were areas children reported during the course of the acceptability as reason for not liking the product. The cooking oil might have resulted from the high oil separation leading to children being exposed to concentrated amounts of oil during initial consumption. In addition, the texture of the SMS‐RUTF was not acceptable to the study children. In order to overcome this, a fine sieve was used in subsequent productions.
The main limitations in this study included the fact that although SMS‐RUTF was designed to treat SAM, we tested acceptability among normal children. Acceptability testing among children with SAM nested in a large randomized effectiveness trial in Lusaka (data not included) – that followed this initial acceptability among older children has shown that the SMS‐RUTF is also acceptable among vulnerable children. Additionally, the absence of any side effects on consumption of SMS‐RUTF by normal children may be used as a basis to try it on vulnerable children. We, however, acknowledge that the sample size in this study was small, and safety‐related results ought to be interpreted cautiously. Additionally, we did not include vitamin A analysis in this study due to high analytical cost. Vitamin A is highly sensitive to environmental factors, mainly light. Currently, there is no study on nutrient stability in RUTF and we recommend that future studies include vitamin A assay. Because this study was done as part of a large randomized trial to determine the effectiveness of SMS‐RUTF vs. P‐RUTF in the treatment of SAM, data comparing the nutrient composition of the two products are reported separately (A. Irene et al., unpublished). However, nutrient composition of SMS‐RUTF was designed to match that of P‐RUTF. Detailed composition of P‐RUTF is publicly available (WHO et al. 2007). The variations observed between theoretical and analysed nutrient levels may be attributable to analytical error.
The processing of SMS‐RUTF in the current study differed from the existing P‐RUTF manufacturing process in three distinct ways: namely, no peanuts were used, and for the first time, cereals (maize and sorghum) were included in the recipe; no milk protein was added; and processing was based on industrial extrusion cooking as opposed to roasting that is used for P‐RUTF manufacture. A study done in Malawi determined the acceptability of non‐peanut RUTF with no milk based of chickpea and sesame, but the processing was based on roasting and the assessment was done among HIV‐infected adults. A recent study from India compared acceptability and energy intake from P‐RUTF vs. gruel made from rice and green grams (khichri) (Dube et al. 2009) and found that children preferred the latter food compared with RUTF. A study from Senega found no difference in the acceptability of locally processed vs. imported peanut butter among severely acutely malnourished children, but both RUTFs were peanut‐based. The observation that SMS‐RUTF closely matched international specifications for nutrients, was stable over 12 months of storage and was acceptable among children shows that there is a potential to diversify RUTF ingredients and processing techniques, thus allowing for contextualization of the RUTF‐based interventions. Numerous studies on the efficacy and safety of RUTF, both locally processed and imported, for the management of SAM have recently been reviewed (Gera 2010; Owino 2010). However, there are few published studies reporting RUTF acceptability and shelf‐life in cases where the formulations are based on non‐peanut ingredients. Our study provides a reference point for RUTF recipe formulation, shelf‐life and acceptability assessment.
Although SMS‐RUTF was apparently stable during storage over 18 months, we have conservatively used 12 months as the most optimum usage time based on current practice with locally processed P‐RUTF. However, recent development such as nitrogen flushing of RUTF packages has increased imported RUTF shelf life to at least 24 months (Beesabathuni & Natchu 2010). Such technologies are unlikely to be practical in resource poor settings.
In this study, we have reported the formulation of a nutritious, highly acceptable RUTF from maize, soybeans and sorghum with a shelf stability of at least 12 months. This is likely to provide an affordable alternative RUTF for treatment of children with SAM in developing countries if proved efficacious in ongoing randomized trials. The use of diversified, locally available ingredients is likely to stimulate small scale agriculture and hence, encourage self‐reliance among food crop producers in resource‐poor settings. Development of standardized RUTF shelf stability assessment and nutrient analysis is highly recommended.
Source of funding
Supported by funds from Irish Aid, Department of Foreign Affairs, Republic of Ireland.
Conflicts of interest
Valid Nutrition designed and produced the SMS‐RUTF. VOO is an employee of Valid Nutrition. SC is the unpaid director of Valid Nutrition. Valid International is the sister company of Valid Nutrition. Irish Aid had no say on the design, implementation and interpretation of the results. Valid Nutrition administered the study grant, while staff from Valid International implemented the study.
Contributions
SC conceived study idea and provided technical oversight throughout the trial including data collection and preparation of this manuscript. AHI, VOO and FD designed the study protocol and implemented data collection, entry and analysis; FD participated in manuscript preparation. All authors contributed throughout the stages of the study and contributed to the write‐up of the manuscript. All authors have read and approved the manuscript.
Acknowledgements
We are grateful to Insta Products EPZ (Athi River) Ltd for availing their factory for SMS‐RUTF production trials. We thank Paul Karanja Warigi and Peter Ochieng Akomo, the two food technologists who supervises SMS‐RUTF production. In addition, we thank all the production assistants who worked hard to produce the SMS‐RUTF. We express gratitude to Immaculate Wafula and Benard Otieno, both Valid Nutrition staff in Nairobi for the administrative support during this work. We thank all Valid Nutrition field staff in Zambia for making this work possible. We are grateful to Irish Aid for the funding that made this entire work.
References
- AOAC (Association of Official Analytical Chemists) (1995) Official Methods of Analysis, 16th edn. AOAC International: Arlington, TX. [Google Scholar]
- Bahwere P., Sadler K. & Collins S. (2009) Acceptability and effectiveness of chickpea sesame ready‐to‐use‐therapeutic food in malnourished HIV‐positive adults. Patient Preferences and Adherence 3, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesabathuni K.N. & Natchu U.C.M. (2010) Production and distribution of a therapeutic nutritional product for severe acute malnutrition in India: opportunities and challenges. Indian Pediatrics 47, 702–706. [DOI] [PubMed] [Google Scholar]
- Ciliberto M.A., Sandige H., Ndekha M.J., Ashorn P., Briend A., Ciliberto H.M. et al (2005) Comparison of home‐based therapy with ready‐to‐use therapeutic food with standard therapy in the treatment of malnourished Malawian children: a controlled, clinical effectiveness trial. American Journal of Clinical Nutrition 81, 864–870. [DOI] [PubMed] [Google Scholar]
- Ciliberto M.A., Manary M.J., Ndekha M.J., Briend A. & Ashorn P. (2006) Home‐based therapy for oedematous malnutrition with ready‐to‐use therapeutic food. Acta Paediatrica 95, 1012–1015. [DOI] [PubMed] [Google Scholar]
- Collins S. & Henry J. (2004) Alternative RUTF Formulations (Special Supplement 2) Field Exchange (Suppl. 2), 35. Available at: http://fex.ennonline.net/102/4-3-2.aspx (Accessed 6 July 2010).
- Collins S., Dent N., Binns P., Bahwere P., Sadler K. & Hallam A. (2006) Management of severe acute malnutrition in children. Lancet 368, 1992–2000. [DOI] [PubMed] [Google Scholar]
- Diop E.I., Dossou N.I., Briend A., Yaya M.A., Ndour M.M. & Wade S. (2004) Home‐based rehabilitation for severely malnourished children using locally made ready‐to‐use therapeutic food (RTUF). In Proceedings of the 2nd World Congress on Pediatric Gastroenterology Hepatology, and Nutrition. Paris, France, July 3‐7.
- Dube B., Rongsen T., Mazumder S., Taneja S., Rafique F., Bhandari N. et al (2009) Comparison of ready‐to‐use therapeutic food with cereal legume‐based khichri among malnourished children. Indian Pediatrics 46, 383–388. [PubMed] [Google Scholar]
- FAO/INFOODS (Food and Agricultural Organization International Network of Food Data Systems) (2007) World Food Dietary Assessment System: an Overview Available at: http://www.fao.org/infoods/software_overview_en.stm (Accessed 3 May 2006).
- Gera T. (2010) Efficacy and safety of therapeutic nutrition products for home based therapeutic nutrition for severe acute malnutrition: a systematic review. Indian Pediatrics 47, 709–718. [DOI] [PubMed] [Google Scholar]
- Golden M.H.N., Breind A. & Grellety Y. (1995) Report of meeting on supplementary feeding programmes with particular reference to refugee populations. European Journal of Clinical Nutrition 49, 137–145. [PubMed] [Google Scholar]
- Hallberg L., Brune M. & Rossander L. (1989) Iron absorption in man: ascorbic acid and dose‐dependent inhibition by phytate. American Journal of Clinical Nutrition 49, 140–144. [DOI] [PubMed] [Google Scholar]
- Killeit U. (1994) Vitamin retention in extrusion cooking. Food Chemistry 49, 149–155. [Google Scholar]
- Linneman Z., Matilsky D., Ndekha M., Maleta K. & Manary M.J. (2007) A large‐scale operational study of home‐based therapy with ready‐to‐use therapeutic food in childhood malnutrition in Malawi. Maternal and Child Nutrition 3, 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G., Jin Y., Piao J., Kok F., Guusje B. & Jacobsen E. (2005) Phytate, calcium, iron, and zinc contents and their molar ratios in foods commonly consumed in China. Journal of Agricultural and Food Chemistry 53, 10285–10290. [DOI] [PubMed] [Google Scholar]
- Manary M.J. (2006) Local production and provision of ready‐to‐use therapeutic food (RUTF) spread for the treatment of severe childhood malnutrition. Food and Nutrition Bulletin 27 (3 Suppl.), S83–S89. [DOI] [PubMed] [Google Scholar]
- Nutrisurvey (2007). Nutrition Surveys and Calculations. Guidelines, Software and Additional Information Available at: http://www.nutrisurvey.de/ (Accessed 7 April 2009).
- Oberleas D. & Harland B.F. (1981) Phytate content of foods: effect on dietary zinc availability. Journal of the American Dietetic Association 79, 433–436. [PubMed] [Google Scholar]
- Owino V. (2010) Why lipid‐based ready to use foods (RUF) must be key components of strategies to manage acute malnutrition in resource poor settings. African Journal of Food, Agriculture, Nutrition and Development. Available at: http://www.ajfand.net/Issue33/PDFs/CommentaryOwino.pdf (Accessed 10 December 2010). [Google Scholar]
- Owino V.O. (2009) Adaptation of glass columns for clean‐up in RP‐HPLC determination of aflatoxins with post‐column derivatisation with bromine and fluorescence detection. African Journal of Food, Agriculture, Nutrition and Development. Available at: http://www.ajfand.net/Volume9/No3/Owino9215.pdf (Accessed 19 December 2011). [Google Scholar]
- Sandige H., Ndekha M.J., Briend A., Ashorn P. & Manary M.J. (2004) Home‐based treatment of malnourished Malawian children with locally produced or imported ready‐to‐use food. Journal of Pediatric Gastroenterology and Nutrition 39, 141–146. [DOI] [PubMed] [Google Scholar]
- Singh S., Gamlath S. & Wakeling L. (2007) Nutritional aspects of food extrusion: a review. International Journal of Food Science and Technology 42, 916–929. [Google Scholar]
- USDA (United States Department of Agriculture) (2007) National Nutrient Database for Standard Reference, Release 20 Available at: http://www.ars.usda.gov/main/docs.htm?docid=15869 (Accessed 7 April 2009).
- WHO & FAO (2007) Guidelines on Food Fortification with Micronutrients. WHO: Geneva. [Google Scholar]
- WHO, WFP, UN/SCN & UNICEF (World Health Organization, World Food Programme, United Nations Standing Committee on Nutrition, The United Nations Children's Fund) (2007) Community‐Based Management of Severe Acute Malnutrition. WHO, WFP, UN/SCN, UNICEF: Geneva, Rome, New York. [Google Scholar]


