Abstract
Leg cramps are common in pregnant women. Currently, there is no standard treatment for pregnancy‐induced leg cramps. The objective of this study was to evaluate the therapeutic efficacy of oral magnesium in pregnant women with leg cramps. This double‐blinded, randomised, placebo‐controlled trial included 86 healthy pregnant women, 14–34 weeks of gestation who had leg cramps at least twice per week. The study period was 4 weeks. Eighty women completed the study. Forty‐one women were assigned to magnesium bisglycinate chelate (300 mg per day) and 39 women to placebo. Details of leg cramps were recorded before beginning the treatment and the fourth week of study. Outcome measure was the reduction of cramp frequency after treatment and cramp intensity measured by 100‐mm visual analogue scale.
Fifty per cent reduction of cramp frequency was significantly higher in the magnesium group than the placebo group (86.0% vs. 60.5%, P = 0.007). The 50% reduction of cramp intensity was also significantly higher in the treatment group than in the placebo group (69.8% vs. 48.8%, P = 0.048). There were no significant differences between the two groups in terms of side effects such as nausea and diarrhoea. These results demonstrated that oral magnesium supplement can improve the frequency and intensity of pregnancy‐induced leg cramps. Therefore, oral magnesium may be a treatment option for women suffering from pregnancy‐induced leg cramps.
Keywords: leg cramps, pregnancy, magnesium bisglycinate chelate, oral, trial
Introduction
Leg cramps are involuntary, localised and usually painful skeletal muscle contractions, which commonly affect calf muscles (Young 2009). Muscle cramps arise from spontaneous discharges of the motor nerves rather than from within the muscle itself (Miller & Layzer 2005).
Leg cramps are common for pregnant women; 30–45% of pregnant women suffer from leg cramps (Hertz et al. 1992; Valbo & Bohmer 1999). Pregnancy‐induced leg cramps tend to be more frequent during the second half of pregnancy, and mostly at night, resulting in sleep disruption, which potentially introduce other complications. For instance, according to Lee & Gay (2004), less sleep during pregnancy (less than 6 h per night) is associated with longer labour and increased rate of operative delivery.
Currently, there is no standard treatment for pregnancy‐induced leg cramps; however, several studies have been conducted so far. Magnesium is the second most abundant intracellular cation and a cofactor for more than 300 metabolic reactions in the body (Elin 1987). As pregnancy is a physiologic state of low serum magnesium compared with non‐pregnant (Cunningham et al. 2005), shortage of magnesium may be one of the causes for cramping, which explains why there is a higher rate of leg cramps in pregnancy. There have been few studies of magnesium for the treatment of leg cramps in pregnancy. Dahle et al. demonstrated the therapeutic effect of magnesium lactate/magnesium citrate against leg cramps in pregnancy when compared with placebo (Dahle et al. 1995). On the contrary, Nygaard et al. found no significant effect of magnesium lactate/magnesium citrate on both frequency and intensity of leg cramps in pregnant women (Nygaard et al. 2008). Because of the conflicting results of two previous studies, this study was performed to evaluate the effect of magnesium.
Thus, the objective of this study was to evaluate the therapeutic effectiveness of oral magnesium bisglycinate chelate as a treatment for pregnancy‐induced leg cramps.
Key messages
Leg cramps are common for pregnant women often resulting in sleep disruption and related complications.
Currently, there is no standard treatment for pregnancy‐induced leg cramps.
Oral magnesium bisglycinate chelate can improve the frequency and intensity of pregnancy‐induced leg cramps and may be a treatment option for women suffering from this condition.
Methods
Pregnant women who attended the antenatal care clinic at the Department of Obstetrics and Gynecology, Faculty of Medicine, Chulalongkorn University, Bangkok, Thailand between June 2010 and August 2011, were invited to join this study if they suffered from leg cramps. Definition of leg cramps was sudden tonic or clonic involuntary contraction of the gastrocnemius muscle associated with severe pain. The eligible pregnant women were those with 14–34 weeks of gestation, having pregnancy‐induced leg cramps at least twice a week, no other medical disease, no concurrent obstetrics complication, no other prescriptions for leg cramps and no history of magnesium allergy. We excluded pregnant women with multifetal gestation, subsequently developed pregnancy‐induced hypertension and preterm labour treated with tocolytic agent.
After the study was approved by the Ethics Committee of the Faculty of Medicine, Chulalongkorn University, eligible women who signed the informed consent were randomised into two groups: treatment or placebo group. A randomisation scheme was generated by random number table using a block‐of‐four technique. The co‐investigator generated the allocation sequence (this was done before the study and the co‐investigator did not have patient contact), and nurses enrolled participants and assigned participants to their groups. When a woman met the study inclusion criteria, the nurses picked a sequentially numbered opaque plastic container.
The opaque plastic container contained 84 tablets of magnesium or placebo (identical in size, shape and colour). The opaque plastic containers were labelled sequentially. Then, each container was distributed in sequential numerical order to ensure randomisation. Both health care providers and women were masked to treatment assignment. Magnesium bisglycinate chelate (Chelated Magnesium®, elemental Mg 100 mg per tablet; Qualimed Co. Ltd., Bangkok, Thailand) was assigned to the treatment group and corresponding placebo to the placebo group. The dosage was one tablet, three times a day with meal. Duration of treatment was for 4 weeks. The treatment assignment was not revealed until data collection was completed.
Participants needed to complete two case record forms (CRF): pretreatment CRF and follow‐up CRF. The pretreatment CRF was completed before beginning the treatment. The pretreatment CRF acquired details about the leg cramps’ characteristics, number of occurrences by week, intensity measured by a 100‐mm visual analogue scale. Background characteristics were also recorded; for example: age, gravida, parity, gestational age, income, education, standing or walking hours per day, pre‐pregnancy body mass index (BMI), antenatal supplement drugs, calcium supplement, blood pressure, leg oedema and varicose vein. For the follow‐up CRF, leg cramps’ characteristics and side effects such as nausea, vomiting and diarrhoea were recorded. Women started recording follow‐up CRF at the beginning of the fourth week and completed it at the end of the fourth week. They recorded in a diary chart. Participants were asked to return follow‐up CRF and the plastic container at the end of the fourth week. Thus, the compliance could be evaluated.
The sample size calculation was based upon the 50% reduction in frequency of leg cramps in both groups obtained from Dahle et al.'s study (Dahle et al. 1995). Fifty per cent reduction in frequency of leg cramps was 50% in the treatment group and 20% in the placebo group. Thus, we needed 39 women in each group to detect statistical difference (α = 0.05, β = 0.1). With adjustments for a withdrawal rate of 10%, a minimum of 43 women in each group were required. The secondary outcomes were the 50% reduction of cramp intensity and side effects.
The statistical package for the social sciences (SPSS) version 17 (SPSS Inc, Chicago, IL, USA) was used for statistical analysis. Analytical statistics (i.e. Chi‐squared test and Fisher's exact test for categorical variables, independent t‐test for continuous variables and Mann–Whitney U‐test for nonparametric variables) were used when appropriate. Chi‐squared test was used to compare 50% reduction of number of leg cramps, 50% reduction of pain score of leg cramps, total cases without leg cramps and nausea between groups. Fisher's exact test was used to compare diarrhoea between groups. Independent t‐test was used to compare mean percentage change of number of leg cramps and mean tablets of returned drug between groups. Mann–Whitney U‐test was used to compare median absolute change in the number of leg cramps and median absolute change of pain score between groups. A P < 0.05 was considered statistically significant. Analysis of the trial was conducted in the intent‐to‐treat (ITT) analysis.
Results
Eighty‐six women enrolled in the study (Fig. 1). Three women quit the study because of personal reasons and other three women were lost to follow‐up. Eighty women completed the study. Forty‐one women were assigned to the magnesium bisglycinate chelate group (300 mg per day) and 39 women were assigned to the placebo group. However, 86 women were included in the ITT analysis by a ‘worst‐case’ scenario (magnesium bisglycinate chelate, n = 43; placebo, n = 43). For background characteristics, there were no significant differences between the groups with respect to age, gravida, parity, gestational age, income, education, hours of standing or walking per day, pre‐pregnancy BMI, antenatal supplement drug, calcium supplement, blood pressure, leg oedema and varicose vein (Table 1). Mean numbers of leg cramps before treatment were 5.4 and 4.2 times per week in the treatment and placebo groups, respectively (P = 0.15). Mean pain score of leg cramps before treatment, which was evaluated by 100‐mm visual analogue scale were 63.7 and 68.5 in the treatment and placebo groups, respectively (P = 0.34) (Table 1).
Figure 1.
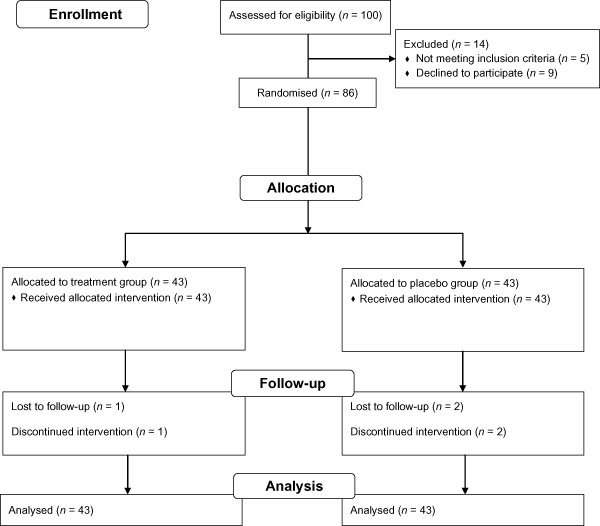
Profile of patient follow‐up following randomisation to either treatment or placebo group.
Table 1.
Background characteristics of the study population
| Treatment group (n = 43) | Placebo group (n = 43) | |
|---|---|---|
| Age (years) | 28.7 ± 5.8 | 28.6 ± 5.1 |
| Gravida | ||
| Primigravida | 14 (32.6%) | 17 (39.5%) |
| Multigravida | 29 (67.4%) | 26 (60.5%) |
| Parity | ||
| Nulliparous | 16 (37.2%) | 22 (51.2%) |
| Multiparous | 27 (62.8%) | 21 (48.8%) |
| Gestational age (weeks) | 28.4 ± 4.9 | 27.7 ± 6.0 |
| Income (USD) | ||
| Less than 400 | 13 (30.2%) | 7 (16.3%) |
| 400–1000 | 17 (39.6%) | 19 (44.2%) |
| more than 1000 | 13 (30.2%) | 17 (39.5%) |
| Education | ||
| Primary school | 3 (7.0%) | 6 (14%) |
| Secondary school | 20 (46.5%) | 22 (51.2%) |
| Bachelor degree or higher | 20 (46.5%) | 15 (34.9%) |
| Standing or walking | ||
| less than 2 h per day | 15 (34.9%) | 20 (46.5%) |
| 2–4 h per day | 17 (39.5%) | 9 (20.9%) |
| more than 4 h per day | 11 (25.6%) | 14 (32.6%) |
| Pre‐pregnancy BMI (kg m−2) | 21.8 ± 3.7 | 21.3 ± 3.9 |
| Drug supplements | ||
| Obimin AZ | 36 (83.7%) | 37 (86.0%) |
| FBC® (Ranbaxy Unichem, Bangkok, Thailand) | 2 (4.7%) | 2 (4.7%) |
| Ferrous sulphate | 5 (11.6%) | 4 (9.3%) |
| Calcium supplement | ||
| No | 39 (90.7%) | 37 (86.0%) |
| Yes | 4 (9.3%) | 6 (14.0%) |
| Systolic BP (mmHg) | 104.8 ± 10.6 | 108.1 ± 10.9 |
| Diastolic BP (mmHg) | 66.3 ± 6.8 | 67.6 ± 7.5 |
| Leg oedema | 3 (7.0%) | 3 (7.0%) |
| Varicose vein | 6 (14.0%) | 5 (11.6%) |
| Mean number of leg cramps per week | 5.4 ± 4.8 | 4.2 ± 2.6 |
| Mean pain score | 63.7 ± 24.5 | 68.5 ± 21.8 |
BMI, body mass index; BP, blood pressure. Data present as mean ± standard deviation or n (%).
After the 4‐week period of treatment, 50% reduction of cramp frequency was significantly higher in the treatment group than the placebo group [37 (95% confidence interval 32, 41) vs. 26 (19, 32) cases, P = 0.007]. The 50% reduction of cramp intensity was also significantly higher in the treatment group than the placebo group [30 (24, 36) vs. 21 (14, 27) cases, P = 0.04] (Table 2). The number needed to treat for 50% reduction of cramp frequency and 50% reduction of cramp intensity were 3.9 and 4.7, respectively. Histogram of percentage change of number of leg cramps is shown in Fig. 2. The number of subjects without leg cramp was significantly higher in the treatment group than the placebo group (48.8% vs. 27.9%, P = 0.04) (Table 2). There were no significant differences between the groups in terms of side effects such as nausea and diarrhoea. Compliance evaluated from returned tablets showed no differences between the groups (P = 0.26) (Table 2).
Table 2.
Leg cramps after treatment, side effects and returned drug
| Treatment group (n = 43) | Placebo group (n = 43) | P value | |
|---|---|---|---|
| 50% reduction of number | 37 (86.0%) | 26 (60.5%) | 0.007* |
| of leg cramps | (75.6, 96.3) | (45.8, 75.1) | |
| 50% reduction of pain score | 30 (69.8%) | 21 (48.8%) | 0.04* |
| of leg cramps | (56, 83.5) | (33.8, 63.7) | |
| Mean percentage change of number of leg cramps (95%CI) | 79 (70.2, 87.8) | 32.4 (3.4, 61.5) | 0.003 ‡ |
| Median absolute change of number of leg cramps (interquartile range) | 1 (0, 4) | 3 (2, 7) | 0.003 § |
| Median absolute change of pain score of leg cramps (interquartile range) | 34 (16, 54) | 53 (10, 71) | 0.16 § |
| Total cases without leg cramp | 21 (48.8%) | 12 (27.9%) | 0.04* |
| (33.8, 63.7) | (14.4, 41.3) | ||
| Side effects | |||
| Nausea | 11 (25.6%) | 6 (14.0%) | 0.27* |
| (12.5, 38.6) | (3.6, 24.3) | ||
| Diarrhoea | 6 (14.0%) | 1 (2.3%) | 0.10 † ; |
| (3.6, 24.3) | (0, 6.7) | ||
| Mean tablets of returned drug | 11.3 ± 10.9 | 14.3 ± 11.4 | 0.26 ‡ |
CI, confidence interval. Data present as mean ± standard deviation or n (%) (95% CI). *Chi‐squared test, †Fisher's exact test, ‡Independent t‐test and §Mann–Whitney U‐test were used.
Figure 2.
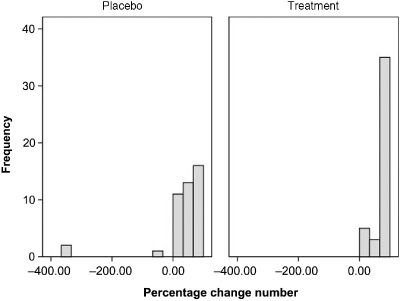
Histogram of percentage change of number of leg cramps.
Discussion
This randomised placebo‐controlled trial demonstrates that supplement of oral magnesium bisglycinate chelate significantly reduced both frequency and intensity of pregnancy‐induced leg cramps than the placebo. The results of this study provided new information regarding the use of magnesium bisglycinate chelate in women with pregnancy‐induced leg cramps.
There have been two previous randomised controlled trials that evaluated the therapeutic effect of magnesium supplement to treat pregnancy‐induced leg cramps (Dahle et al. 1995; Nygaard et al. 2008). Dahle et al. enrolled 73 pregnant women with leg cramps in their study (Dahle et al. 1995). Fifteen millimol per day of magnesium lactate/magnesium citrate or placebo was prescribed for 3 weeks. They found that oral magnesium could decrease leg cramps distress. An average of frequency of leg cramps reduced from every 1.5 days to every 3 days after the 3‐week period of magnesium supplement. Nygaard et al. enrolled 38 pregnant women with leg cramps in their study (Nygaard et al. 2008). Fifteen millimol per day of magnesium lactate/magnesium citrate or placebo was prescribed for 2 weeks. They found no significant effect of oral magnesium on frequency or intensity of leg cramps in pregnant women.
In the present study, the therapeutic effect of magnesium bisglycinate chelate on leg cramps was better because of the form of magnesium and the longer duration of treatment (4 weeks). Because magnesium bisglycinate chelate's absorption rate is 2.2 times better than magnesium lactate or magnesium citrate, it was chosen for this trial. As a result, this study was able to show the higher effectiveness of magnesium in treating leg cramps. The duration of the present study was 4 weeks, which was longer than previous studies (Dahle et al. 1995; Nygaard et al. 2008).
Furthermore, there has been a report that Thai people consumed less dietary magnesium per day compared with the western population. The average daily magnesium consumption in a Thai female (non‐pregnant) is only 42.29% of the recommended dietary allowance (RDA). According to the American RDA, the RDA of magnesium during pregnancy is 350–360 mg per day (Suphiphat et al. 1994; Institute of Medicine 1999). This may be another reason why the magnesium supplement had a higher therapeutic effect in this study.
The strength of this study was a randomised controlled trial of magnesium bisglycinate chelate to treat pregnancy‐induced leg cramps. This study is the first of its kind to use this form of magnesium.
The limitation of this study was that magnesium levels were not assessed. Another limitation was the post‐registration of the trial because of our mistaken idea that we did not want to disclose this study idea. However, the 50% reduction in the frequency of leg cramps was set as a primary outcome. This primary outcome was determined a priori, and the research proposal was sent to the Institute Reviewer Board. This study began after ethical approval.
Conclusion
This present study shows the therapeutic effect of oral magnesium bisglycinate chelate in treating pregnancy‐induced leg cramps. Both frequency and intensity of leg cramps can be reduced without any significant side effects. From this result, we recommend oral magnesium bisglycinate chelate for the treatment of pregnancy‐induced leg cramps.
Source of funding
Internal research grant: Grant for Development of New Faculty Staff, Chulalongkorn University.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
CS and VP contributed to the concept and design. CS assisted by VP, conducted the study and collected the data. CS and VP performed the data analysis and interpretation. CS wrote the draft article and VP critically revised the draft.
Acknowledgements
We acknowledge the assistance of medical and nursing staff of the antenatal care unit, Department of Obstetrics and Gynecology, Faculty of Medicine, Chulalongkorn University for their help in recruiting patients and ensuring the smooth conduct of the trial.
Clinical trial registration: isrctn.org, http://isrctn.org, ISRCTN03989660 (This was registered after trial completion).
References
- Cunningham F.G., Leveno K.J., Bloom S.L., Hauth J.C., Gilstrap L.C.I. & Wenstrom K.D. (2005) Williams Obstetrics. 22nd edn, p 129, 217. McGraw‐Hill: New York. [Google Scholar]
- Dahle L.O., Berg G., Hammar M., Hurtig M. & Larsson L. (1995) The effect of oral magnesium substitution on pregnancy‐induced leg cramps. American Journal of Obstetrics and Gynecology 173, 175–180. [DOI] [PubMed] [Google Scholar]
- Elin R.J. (1987) Assessment of magnesium status. Clinical Chemistry 33, 1965–1970. [PubMed] [Google Scholar]
- Hertz G., Fast A., Feinsilver S.H., Albertario C.L., Schulman H. & Fein A.M. (1992) Sleep in normal late pregnancy. Sleep 15, 246–251. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine (1999) Dietary Reference Intakes: Calcium, Phosphorus, Magnesium, Vitamin D and Fluoride. National Academy Press: Washington DC. [Google Scholar]
- Lee K.A. & Gay C.L. (2004) Sleep in late pregnancy predicts length of labor and type of delivery. American Journal of Obstetrics and Gynecology 191, 2041–2046. [DOI] [PubMed] [Google Scholar]
- Miller T.M. & Layzer R.B. (2005) Muscle cramps. Muscle and Nerve 32, 431–442. [DOI] [PubMed] [Google Scholar]
- Nygaard I.H., Valbo A., Pethick S.V. & Bohmer T. (2008) Does oral magnesium substitution relieve pregnancy‐induced leg cramps? European Journal of Obstetrics, Gynecology, and Reproductive Biology 141, 23–26. [DOI] [PubMed] [Google Scholar]
- Suphiphat V., Rirermvanich L., Sangthumsiri W., Lowhnoo T., Morjaroen N. & Pukboonme I. (1994) A glimpse at the magnesium intake and content in serum and urine of some Thai population. Thai Journal of Parenteral and Enteral Nutrition 5, 77–84. [Google Scholar]
- Valbo A. & Bohmer T. (1999) Leg cramps in pregnancy–how common are they? Tidsskrift for den Norske Laegeforening 119, 1589–1590. [PubMed] [Google Scholar]
- Young G. (2009) Leg cramps. Clinical Evidence (Online) 26, 1113. [Google Scholar]


