Abstract
A quick‐cooking rice, produced from broken rice, is a convenient ingredient for complementary foods in Thailand. The rice is fortified with micronutrients including iron during the processing procedure, which can cause unacceptable sensory changes. A quick‐cooking rice fortified with ferric ammonium citrate (FAC) or a mixture of ferrous sulphate (FeSO 4) and ferric sodium ethylenediaminetetraacetic acid (NaFeEDTA), with a 2:1 molar ratio of iron from FeSO 4 : iron from NaFeEDTA (FeSO 4 + NaFeEDTA), gave a product that was organoleptically acceptable. The study compared iron absorption by infants and young children fed with micronutrient‐fortified quick‐cooking rice containing the test iron compounds or FeSO 4. Micronutrient‐fortified quick‐cooking rice prepared as a traditional Thai dessert was fed to two groups of 15 8–24‐month healthy Thai children. The iron fortificants were isotopically labelled with 57Fe for the reference FeSO 4 or 58Fe for the tested fortificants, and iron absorption was quantified based on erythrocyte incorporation of the iron isotopes 14 days after feeding. The relative bioavailability of FAC and of the FeSO 4 + NaFeEDTA was obtained by comparing their iron absorption with that of FeSO 4. Mean fractional iron absorption was 5.8% [±standard error (SE) 1.9] from FAC and 10.3% (±SE 1.9) from FeSO 4 + NaFeEDTA. The relative bioavailability of FAC was 83% (P = 0.02). The relative bioavailability of FeSO 4 + NaFeEDTA was 145% (P = 0.001). Iron absorption from the rice containing FAC or FeSO 4 + NaFeEDTA was sufficiently high to be used in its formulation, although iron absorption from FeSO 4 + NaFeEDTA was significantly higher (P < 0.00001).
Keywords: iron bioavailability, complementary food, stable isotope, ferrous sulphate, ferric sodium ethylenediaminetetraacetic acid, ferric ammonium citrate
Introduction
Rice is the staple food for over half of the world's population and is the basic ingredient for complementary foods fed to infants and young children in rice‐eating countries. Rice‐based complementary foods, however, often contain inadequate amounts of iron, calcium, zinc, vitamin A and iodine (Porniammongkol 2001). In Thailand, the low levels of iron and calcium have been reported to be a major concern (Porniammongkol 2001; Souvaphapsopha 2001), and potential deficiencies of zinc, thiamin and folate have also been signalled (World Health Organization 2006; Allen et al. 2006).
Thai mothers often prefer to use commercially manufactured, convenient, quick‐cooking rice as the major ingredient for complementary foods. The rice is made of broken rice pretreated to reduce food preparation time. Chitpan et al. (2005) first developed micronutrient‐fortified quick‐cooking rice containing iron, calcium, thiamin and folate. The micronutrients were added as an aqueous solution to the dried, heated, broken rice grains during manufacture and were drawn into the endosperm as the grains cooled. The first products, however, were not acceptable for commercialization because they rapidly became rancid. Porasuphatana et al. (2008) overcame the sensory problems using sodium citrate as an iron chelator. Sensory quality and shelf stability, however, were only satisfactory with two of the tested iron sources. These acceptable iron sources were ferric ammonium citrate (FAC) and a mixture of ferrous sulphate plus ferric sodium ethylenediaminetetraacetic acid (FeSO4 + NaFeEDTA at molar ratio of 3:1; Fe : EDTA). The level of NaFeEDTA added to the rice provided the children with ≤0.2 mg Fe−1 kg−1 body weight, which meet The Joint FAO/WHO Expert Committee on Food Additives (JECFA) requirement for infant foods fortified with NaFeEDTA (World Health Organization 1999). FeSO4 is a well‐known iron fortificant that is generally used in many kinds of food. Besides, FeSO4 is produced in many countries, which makes it economically feasible. The chelated form of iron in NaFeEDTA is desirable as it has been blocked from being a catalyst for rice lipid oxidation. However, it is not feasible because of high cost and questionable safety in children. Although FAC is another interesting iron fortificant for complementary food, it is generally used in dairy products.
The present study used stable isotope techniques to measure iron absorption by Thai infants and young children fed with micronutrient‐fortified quick‐cooking rice containing FAC, a mixture of FeSO4 + NaFeEDTA or FeSO4 alone. Iron absorption was based on the erythrocyte incorporation of the iron isotope labels.
Key messages
Iron absorption relative to FeSO4 by young Thai children from a traditional Thai meal was 83% from FAC and 145% from the FeSO4 + NaFeEDTA mixture.
Both FAC and FeSO4 + NaFeEDTA mixture were suitable iron fortificants for the quick‐cooking rice.
The fortified quick cooking rice can be potentially used as main ingredient for preparing complementary food.
Materials and methods
Subjects
The study originally planned to have infants and children aged 6–24 months as the subjects; however, there was no infant of age 6–7 months available in the study area during the study period. Therefore, only healthy full‐term infants and children aged 8–24 months old with normal weight (World Health Organization 2006) were recruited from the local population using medical services at the Salawan Primary Care Unit under Phutthamonthon General Hospital, Nakhonpathom Province, Thailand. The subjects were free of gastrointestinal or metabolic disorders, were not receiving regular medication and did not have diarrhoea, fever or apparent infections on the day of test meal feeding. The other criteria for subject recruitment were (1) the child was used to being fed with complementary foods; (2) haemoglobin >10 mg dL−1; and (3) no haemoglobinopathy trait or alpha‐thalassemia trait, and normal complete blood counts (CBC). The subjects' parents or caretakers were asked to review and, if they approved, to sign informed consent forms before participating in the study. The infants and young children did not consume vitamin and mineral supplement for 2 weeks prior to the study and until the last blood sample was drawn.
The 30 recruited subjects were randomly divided into two groups of 15 subjects. Group 1 was fed quick‐cooking rice fortified with FAC (study 1). Group 2 was fed rice fortified with FeSO4 + NaFeEDTA (study 2). The characteristics of groups 1 and 2 are shown in Table 1. There were no statistical differences (P > 0.05) in age, body weight, height, haemoglobin or serum ferritin. Relative bioavailabilities (RBVs) of iron in the fortified iron compounds (either FAC or FeSO4 + NaFeEDTA) compared with FeSO4 were evaluated.
Table 1.
Baseline characteristics of study subjects
| Scores | Baseline characteristics* | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age † (months) | Body weight (kg) | Body height (kg) | Haemoglobin (g dL−1) | Ferritin (μg L−1) | ||||||
| Study 1 | Study 2 | Study 1 | Study 2 | Study 1 | Study 2 | Study 1 | Study 2 | Study 1 | Study 2 | |
| Mean | 11.5 | 11.5 | 9.5 | 9.3 | 74.7 | 74.0 | 12.1 | 11.7 | 88.3 | 75.6 |
| SD | 2.1 | 2.0 | 1.3 | 1.5 | 3.9 | 3.9 | 1.0 | 0.7 | 74.1 | 81.2 |
| Median | 11.0 | 11.0 | 9.1 | 9.2 | 74.0 | 73.0 | 11.9 | 11.7 | 56.0 | 46.5 |
SD, standard deviation. *All baseline characteristics of study 1 and study 2 are not significantly different at P = 0.05. †Age distribution: 6–11 months (17): study 1: 8; study 2: 9; 12–24 months (13): study 1: 7; study 2: 6.
Study design
The Ethical Committees at Mahidol University, Thailand, and the Swiss Federal Institute of Technology (ETH), Zurich, Switzerland, approved the experimental protocol. Two separate iron absorption studies were made. In study 1, iron bioavailability from cooked micronutrient‐fortified quick‐cooking rice containing FAC (test meal 1) was compared with the same cooked rice fortified with FeSO4 (reference meal). In study 2, iron bioavailability from cooked micronutrient‐fortified quick‐cooking rice with FeSO4 + NaFeEDTA (test meal 2) was compared with the same cooked rice fortified with FeSO4 (reference meal). A double‐labelled stable isotope technique was used to determine iron absorption in infants and young children (Walczyk et al. 1997). Within each of the two studies, subjects consumed either of the two test meals (1 or 2) and the reference meal. The reference meal was labelled with 58Fe‐FeSO4. Meal 1 was labelled with 57Fe‐FAC, and meal 2 was labelled with 57Fe‐(FeSO4 + NaFeEDTA).
The labelled 57Fe‐FAC was prepared by Dr. Paul Lohmann™ GmbH, Emmertal, Germany, from enriched elemental iron, following a downscale procedure used to manufacture their equivalent food‐grade commercial product. The labelled 57Fe‐FeSO4 and 58Fe‐FeSO4 were prepared by dissolution of enriched elemental iron (ChemgasTM, Boulogne, France) in diluted sulphuric acid. And the labelled Na57FeEDTA was prepared by dissolution of enriched elemental iron in diluted hydrochloric acid and mixing with equimolar amount of Na2EDTA (Sigma‐AldrichTM, Buchs, Switzerland) solution. Isotopic composition of the labels was measured (five measurements) by negative thermal ionization mass spectrometry (NTIMS) using a magnetic sector field mass spectrometer (MAT262, Finnigan MATTM, Bremen, Germany) equipped with multi‐collector detection. Iron concentration was measured in triplicate by inverse isotopic dilution using an isotopic reference material (IRMM‐014) gravimetric solution as the iron standard of natural isotopic composition. Isotopic ratios were measured by NTIMS (Walczyk 1997).
Iron bioavailability from the iron fortificants was assessed relative to FeSO4 as the reference compound. Subjects were fed two different meals on two consecutive days in the morning after fasting for at least 3 h. Within each study, on day 1, half of the subjects received the reference meal and half either meal 1 or 2; on day 2 the subjects received the alternate meal to that received on day 1. Blood was drawn on day 14 for determination of isotopic composition (Table 2).
Table 2.
Experimental design
| Group | Period | ½ subjects (8) | ½ subjects (7) |
|---|---|---|---|
| 1 (15 subjects) | 2–3 weeks before feeding | Blood drawing for screening and baseline information | |
| 3 h before feeding | Fasting | ||
| First day | FAC | FeSO4 | |
| Second day | FeSO4 | FAC | |
| 3 h after feeding | No feeding | ||
| 14 days after feeding | Blood drawing for analysis of isotopic composition | ||
| 2 (15 subjects) | 2–3 weeks before feeding | Blood drawing for screening and baseline information | |
| 3 h before feeding | Fasting | ||
| First day | FeSO4 + NaFeEDTA | FeSO4 | |
| Second day | FeSO4 | FeSO4 + NaFeEDTA | |
| 3 h after feeding | No feeding | ||
| 14 days after feeding | Blood drawing for analysis of isotopic composition | ||
FAC, ferric ammonium citrate; FeSO4, ferrous sulphate; NaFeEDTA, ferric sodium ethylenediaminetetraacetic acid.
Test meal preparation and feeding
Aliquots (7.5 g) of dried micronutrient‐fortified quick‐cooking rice were prepared 1 day in advance of being cooked and served to each subject. The preparation was performed in individual TeflonTM (DuPont, Wilmington, DE)‐coated pots using individual Teflon‐coated spatulas for each subject in order to avoid iron contamination from cooking utensils and cross contamination. Broken rice was added to the pot, cleaned with deionized water and pre‐dried in a hot air oven at 90°C for 1 h. The pre‐dried broken rice was then soaked in 3 mL of a nutrient fortification solution containing 1.7 mg of iron from different iron fortificants: 0.7 mg from the non‐isotopically labelled compound + 1 mg from the isotopically labelled compound [it was the minimum amount that was estimated based on the assumption that iron can be absorbed at 5–15%. Therefore, 10% of the amount of the isotope form iron after being absorbed should be within the detectable and reliable limit of the operating mass spectrometry (regarding to the specification)], 52.5 mg of calcium, 0.8 mg of zinc, 0.04 mg of thiamine, 5.5 μg of folic acid and 23.8 mg of sodium citrate until the solution was totally absorbed into the rice, which was about 10 min. The soaked rice was dried again in a hot air oven at 70°C for 1.5 h, during which time the spatula was used for turning the rice over when necessary. The heating/drying procedures created more porosity to dried rice and reduced cooking time at home to about 8 min. After drying, the Teflon pot was covered with a glass lid and kept at room temperature until feeding on the next day (Fig. 1). On the feeding day, 105 g of deionized water (1:14) was added to the rice in the pot and the rice was cooked by boiling for 8 min. The rice porridge was then mixed with 7.5 g of UHT coconut milk containing 17% fat (Chaokoh™ brand, Ampol Food Processings Ltd., Nakhonpathom, Thailand), 6 g of canned longan in syrup (Malee™ brand, Malee Sampran (Public), Nakhonpathom, Thailand), 5.2 g of white sugar and pinch of salt. During preparation, individual Teflon spatulas were used for stirring.
Figure 1.

Preparation steps of the fortified quick‐cooking rice used in the study.
The prepared test meal was a traditional Thai dessert called Kao Piak Lamyai, which finally weighed about 100 g after cooking, and was served with a clean plastic spoon in the pot used for the preparation. Ten millilitres of deionized water was served as a drink to each subject. All subjects could finish the provided meal. In addition, complete intake of isotopic label was ensured by rinsing the Teflon pan twice with 10 mL of deionized water, which was subsequently consumed by the subjects. The meals were fed in the morning, after a fasting period of at least 3 h. No food or drink intake was allowed for 3 h following completion of the feeding. The prepared test meal was also analysed for phytate content.
Phytate analysis
Inositol pentaphosphate and inositol hexaphosphate contents were determined using reverse phase high‐performance liquid chromatography with C18 column (Atlantis dC18 5 um, 4.6 × 150 mm2, Waters Corporation, Milford, MA) and mobile phase as methanol : H2O, ratio 3:2, at flow rate of 0.8 mL min−1 at 40°C. Eluted inositol phosphates were detected by a refractive index detector, Walters IR 2414 (Hotz & Gibson 2001).
Blood drawing and analysis
Three millilitres of blood was drawn from each subject via venipuncture into an EDTA‐treated tube and kept frozen until analysis. The first blood drawing was performed 2–3 weeks before the feeding for screening and baseline information. The second blood drawing was performed 14 days after test meal administration to analyse isotopic composition (for the estimation of iron absorption) and to measure haemoglobin, iron status and an inflammation biomarker.
Blood samples were analysed for CBC, haemoglobin concentration and haemoglobin typing at the Thalassemia Research Center, Institute of Science and Technology for Research and Development, Mahidol University, Thailand. CBC and reticulocyte count were analysed using the ADVIATM 120 Hematology System (Bayer Pte Ltd., Raffles Place District, Singapore); haemoglobin typing for β‐globin abnormality was analysed by Bio‐Rad Variant. α‐Globin abnormality (Bio‐Rad Laboratories Inc., Hercules, CA) was analysed by Applied Biosystem™ (Thermo Fisher Scientific corporation, Waltham, MA) GeneAmp™ (Perkin‐Elmer, Norwalk, CT) PCR Systems 9700 and gel electrophoresis system using polymerase chain reaction (GibThai Co., Ltd., Bangkok, Thailand). Aliquots of whole blood were analysed for plasma ferritin (Sigma‐Aldrich) and C‐reactive protein, AssayproTM, catalogue no. EP1311‐1 (Assaypro CLL, Saint Charles, MI) by enzyme‐linked immunosorbent assay. Aliquots of whole blood were shipped frozen to ETH Zurich, Switzerland, for iron isotopic analysis.
Iron isotopic analysis of blood samples
Whole blood samples were mineralized using an HNO3/H2O2 mixture, and microwave digestion followed by separation of the sample iron matrix by anion‐ exchange chromatography and a solvent/solvent extraction step into diethyl ether (Walczyk 1997; Walczyk et al. 1997). All isotopic analyses were performed by NTIMS using a magnetic sector field mass spectrometer (MAT 262, Finnigan MAT) equipped with a multi‐collector system for simultaneous ion beam detection.
Calculation of iron absorption
The amounts of 57Fe and 58Fe isotopic labelled present in the blood 14 days after test meal administrations were calculated based on the principles of isotope dilution, taking into account that the iron isotopic labels are not mono‐isotopic (Walczyk 1997; Walczyk et al. 1997). Circulating iron was calculated based on blood volume and haemoglobin concentration (Kastenmayer et al. 1994). Blood volume calculations were based on body length and weight of the children (Linderkamp et al. 1977). For fractional absorption calculations, 90% incorporation of the absorbed iron into erythrocytes was assumed. RBV was calculated for each pair of data by comparing absorption of the test iron compounds with the absorption of FeSO4.
Statistical analysis
The calculated iron absorption values were logarithmically transformed before statistical analysis to adjust for skewed data distribution. Iron absorption results were presented as geometric mean ± 1 standard deviation (SD). Unpaired t‐test was used to compare the RBV of iron between FAC and FeSO4 + NaFeEDTA. Paired t‐test was used to evaluate individual difference in iron absorption between either FAC or FeSO4 + NaFeEDTA and reference FeSO4. Statistical analysis was performed with SPSS for Windows, version 13.0 (SPSS Inc., Chicago, IL).
Results and discussion
Quick‐cooking rice is a good basis used for preparing complementary food and popular with mothers in Thailand due to its convenience. It is the main source of carbohydrate that is normally used in combination with other locally available foods for preparing foods for infants and children of different age groups. It can be fortified with micronutrients by soaking the dried, heated broken rice grains in an aqueous micronutrient solution and drying again. It can provide young Thai children with the micronutrients most commonly deficient in Thailand including iron, calcium, zinc, thiamin and folate (Porasuphatana et al. 2008). In these early studies, different iron fortification compounds were tested but most led to unacceptable sensory changes. Two exceptions were FAC and a mixture of FeSO4 and NaFeEDTA providing a molar ratio of Fe : EDTA of 3:1. NaFeEDTA alone was also organoleptically acceptable, but the mixture with FeSO4 was necessary to avoid surpassing the acceptable daily intake of EDTA of 2.5 mg kg−1 body weight per day (World Health Organization 1999).
Mean haemoglobin concentration of the 30 healthy term infants and young children was 116 g L−1 (range, 108–139 g L−1). The anaemic subjects were excluded during the screening process using the cut‐off of ≥110 g L−1. Mean plasma ferritin concentration was 79 μg L−1 (range, 2–258 μg L−1). Three subjects were iron deficient based on the cut‐off of <12 μg L−1 (Worwood 1982).
Mean fractional iron absorption in the study 1 (Fig. 2) from FAC by young Thai children, some of whom were iron deficient, was 5.8% [±standard error (SE) 1.9] (range = 2.3–24), which was 83% relative to the absorption of FeSO4, the reference iron fortificant (P = 0.02). In study 2 (Fig. 3), mean fractional iron absorption from the mixture of FeSO4 + NaFeEDTA was 10.3% (±SE 1.9) (range = 4.2–37), 145% relative to the absorption of FeSO4 (P = 0.001). The relative absorption of iron from FeSO4 + NaFeEDTA was 1.75 times higher than the relative absorption of iron from FAC (P = 0.000005). A meal of roughly equivalent amounts of fortified rice, coconut milk, longan fruit and sugar mixed with water provided 1.7 mg of iron or about 98.6 μg of absorbed iron from FAC and 175 μg from the mixture of FeSO4 + NaFeEDTA. Based on the recommended nutrient intake, the contributions from the fortified FAC and FeSO4 + NaFeEDTA were 9% and 18% for the 6–11 months (18.6 and 9.3 mg day−1), and 15% and 19% for 12–24 months (11.6 and 5.8 mg day−1), respectively [World Health Organization (WHO) & Food and Agriculture Organization (FAO) 2004 ].
Figure 2.
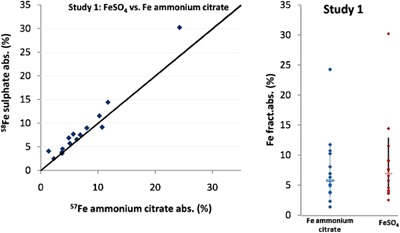
Study 1: absorption of iron in the test meals fortified with ferric ammonium citrate added with sodium citrate.
Figure 3.
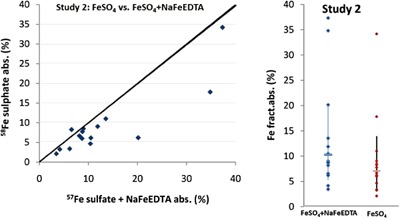
Study 2: absorption of iron in the test meals fortified with FeSO 4 + NaFeEDTA added with sodium citrate as compared with FeSO 4.
In order to optimize the impact of the iron‐fortified rice on ensuring that young Thai children meet their daily iron requirements, the manufacturers of the quick‐cooking micronutrient‐fortified rice can adjust the iron content of either iron fortificant to achieve the desired level of absorbed iron depending on the iron supply from the remainder of the diet, and the number of meals of the fortified rice the children consume per day. Increase in fortified iron, however, might not always proportionally increase iron absorbed. One option to increase the absorption of iron from FAC would be to add ascorbic acid preferably at a 2:1 molar ratio relative to iron (Hurrell 2002) either to the rice or as an ascorbic acid containing ingredient to the meal. The small amount of sodium citrate added to the fortification mix to avoid rancidity caused by the iron compounds would not be expected to influence iron absorption (Walczyk et al. 2005).
The absorption of FAC relative to FeSO4 was 83%, which was slightly but significantly lower (P < 0.05) than for FeSO4. The 83% RBV of iron from FAC by Thai infants consuming the rice meal, however, was higher than the 51% RBV reported for Thai women from the same labelled FAC added to fish sauce and consumed with rice and vegetables (Walczyk et al. 2005). The lower absorption of soluble ferric iron compared with soluble ferrous iron was suggested by these authors to be related to the more ready formation of insoluble, unabsorbable ferric hydroxides in the duodenum (Walczyk et al. 2005). It is also possible that the RBV of soluble ferric salts may vary with the age of the consumer and/or with the composition of the meal. The RBV of insoluble ferric compounds has been reported to vary with the composition of meals (Hurrell & Egli 2010).
NaFeEDTA is the iron compound recommended by the World Health Organization for high phytate foods because of its high RBV. However, the cost is 17 times higher than FeSO4 (Allen et al. 2006). EDTA binds with iron at low pH of the stomach, preventing it from combining with phytic acid, and releases iron for absorption at the higher pH of the duodenum (Bothwell & MacPhail 2004). NaFeEDTA also forms a common pool with other non‐haem iron compounds present in the meal, protecting them from phytate and increasing the fractional absorption of the entire non‐haem iron pool (Mendoza et al. 2001). Other minerals could potentially combine with EDTA although, in young children, NaFeEDTA did not influence the apparent absorption of zinc, copper, calcium or magnesium (Davidsson et al. 2005).
More recently, NaFeEDTA has also been recommended as the iron fortificant for high extraction wheat flours (Hurrell & Egli 2010; Hurrell et al. 2010) and foods containing known inhibitors of Fe absorption such as bran and tea (MacPhail et al. 1981). Indeed, Fe in NaFeEDTA was absorbed two times more efficiently than FeSO4 even in low‐phytate maize meal porridge (MacPhail et al. 1981). This relationship, however, no longer holds in high‐phytate cereal meals where iron absorption is enhanced to a greater extent by the 1:1 molar ratio of Fe : EDTA (Hurrell et al. 2003). Then low‐phytic acid content (undetectable amount) of our rice meal may explain the relatively modest 145% increase in iron absorption in our study from the FeSO4 + NaFeEDTA.
In conclusion, the FeSO4 + NaFeEDTA is the preferred iron fortificant for the quick‐cooking rice. This is because of its higher absorption and its ability to counteract the presence of phytic acid in rice as well as in other foods added to the rice‐based complementary food. However, FAC had also an adequate iron bioavailability and its fortification level could be adjusted to provide infants and young children with sufficient absorbable iron.
Source of funding
International Atomic Energy Agency, Vienna, Austria.
Conflicts of interest
All authors do not have any conflict of interest regarding the topic, methodology, results and discussion of this research.
Acknowledgements
We thank the International Atomic Energy Agency (IAEA) for financial support, and Associate Professor Emorn Wasantwisut, Associate Professor Pattanee Winichagoon and Dr Lena Davidsson for their technical advice.
Chavasit, V. , Porasuphatana, S. , Suthutvoravut, U. , Zeder, C. , and Hurrell, R. (2015) Iron bioavailability in 8–24‐month‐old Thai children from a micronutrient‐fortified quick‐cooking rice containing ferric ammonium citrate or a mixture of ferrous sulphate and ferric sodium ethylenediaminetetraacetic acid. Matern Child Nutr, 11: 179–187. doi: 10.1111/mcn.12167.
References
- Allen L., Benoist B., Dary O. & Hurrell R. (2006) Guidelines on Food Fortification with Micronutrients. World Health Organization: Geneva. [Google Scholar]
- Bothwell T.H. & MacPhail A.P. (2004) The potential role of NaFeEDTA as an iron fortificant. International Journal for Vitamin and Nutrition Research 74 (6), 421–434. [DOI] [PubMed] [Google Scholar]
- Chitpan M., Chavasit V. & Kongkachuichai R. (2005) Development of fortified dried broken rice as a complementary food. Food and Nutrition Bulletin 25 (4), 376–384. [DOI] [PubMed] [Google Scholar]
- Davidsson L., Ziegler E., Zeder C., Walczyk T. & Hurrell R. (2005) Sodium iron EDTA [NaFe(III)EDTA] as a food fortificant: erythrocyte incorporation of iron and apparent absorption of zinc, copper, calcium, and magnesium from a complementary food based on wheat and soy in healthy infants. American Journal of Clinical Nutrition 81, 104–109. [DOI] [PubMed] [Google Scholar]
- Hotz C. & Gibson R.S. (2001) Assessment of home‐base processing methods to reduce the phytate content and phytate/zinc molar ratio of maize (Zima's). Journal of Agricultural and Food Chemistry 49, 692–698. [DOI] [PubMed] [Google Scholar]
- Hurrell R.F. (2002) Fortification: overcoming technical and practical barriers. Journal of Nutrition 132, 806S–812S. [DOI] [PubMed] [Google Scholar]
- Hurrell R.F. & Egli A. (2010) Iron bioavailability and dietary reference values. American Journal of Clinical Nutrition 91, 1461S–1467S. [DOI] [PubMed] [Google Scholar]
- Hurrell R.F., Reddy M.B., Juillerat M.A. & Cook J.D. (2003) Degradation of phytic acid in cereal porridges improves iron absorption by human subjects. American Journal of Clinical Nutrition 77, 1213–1219. [DOI] [PubMed] [Google Scholar]
- Hurrell R.F., Ranum P., Pee S.D., Biebinger R., Hulthen L., Johnson Q. et al (2010) Revised recommendations for iron fortification of wheat flour and an evaluation of the expected impact off current national wheat flour fortification programs. Food and Nutrition Bulletin 31, 7S–21S. [DOI] [PubMed] [Google Scholar]
- Kastenmayer P., Davidsson L., Galan P., Cherouvrier F., Hercberg S. & Hurrell R.F. (1994) A double stable isotope technique for measuring iron absorption in infants. British Journal of Nutrition 71, 411–424. [DOI] [PubMed] [Google Scholar]
- Linderkamp O., Versmold H.T., Riegel K.P. & Betke K. (1977) Estimation and prediction of blood volume in infants and children. European Journal of Pediatrics 125, 227–234. [DOI] [PubMed] [Google Scholar]
- MacPhail A.P., Bothwell T.H., Torrance J.D., Derman D.P., Bezwoda W.R., Charlton R.W. et al (1981) Factors affecting the absorption of iron from Fe(III)‐EDTA. British Journal of Nutrition 45, 215–227. [DOI] [PubMed] [Google Scholar]
- Mendoza C., Viteri F.E., Lonnerdal B., Raboy V., Young K.A. & Brown K.H. (2001) Absorption of iron from unmodified maize and genetically altered, low‐phytate maize fortified with ferrous sulfate or sodium iron EDTA. American Journal of Clinical Nutrition 73, 80–85. [DOI] [PubMed] [Google Scholar]
- Porasuphatana S., Chavasit V., Vasinrapee S., Suthutvoravut U. & Hurrell R.F. (2008) Production and shelf stability of multiple‐fortified quick‐cooking rice as complementary food. Journal of Food Science 73 (7), 359–366. [DOI] [PubMed] [Google Scholar]
- Porniammongkol O. (2001) Development of Appropriate Complementary Food for Infants in Ubon Rachathani Province . Master's Thesis in Food and Nutrition in Development. Faculty of Graduate Studies, Mahidol University: Bangkok.
- Souvaphapsopha S. (2001) Formulation of Canned Complementary Food Using Locally Available Materials . PhD Thesis in Science Nutrition. Faculty of Graduate Studies, Mahidol University: Bangkok.
- Walczyk T. (1997) Iron isotope ratio measurements by negative thermal ionization mass spectrometry. International Journal of Mass Spectrometry and Ion Processes 161, 217–227. [Google Scholar]
- Walczyk T., Davidsson L., Zavaleta N. & Hurrell R.F. (1997) Stable isotope labels as a tool to determine iron absorption by Peruvian school children from a breakfast meal. Fresenius Journal of Analytical Chemistry 359, 445–449. [Google Scholar]
- Walczyk T., Tuntipopipat S., Zeder C., Sirichakwal P., Wasantwisut E. & Hurrell R.F. (2005) Iron absorption by human subjects from different iron fortification compounds added to Thai fish sauce. European Journal of Clinical Nutrition 59, 668–674. [DOI] [PubMed] [Google Scholar]
- World Health Organization (1999) Proceedings of the Fifty‐Third Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA), June 1–10, 1999: International Programme on Chemical Safety. World Health Organization: Rome.
- World Health Organization (2006) WHO Child Growth Standards. World Health Organization: Geneva. [Google Scholar]
- World Health Organization (WHO) & Food and Agriculture Organization (FAO) (2004) Vitamin and Mineral Requirements in Human Nutrition, 2nd edition. Report of a Joint FAO/WHO Expert Consultation, Bangkok, Thailand, 21–30 September 1998. World Health Organization: Geneva.
- Worwood M. (1982) Ferritin in human tissues and serum. Clinics in Haematology 11, 275–307. [PubMed] [Google Scholar]


