Abstract
Birth size is an important gauge of fetal and neonatal health. Birth size measurements were collected within 72 h of life for 16 290 live born, singleton infants in rural Bangladesh from 2004 to 2007. Gestational age was calculated based on the date of last menstrual period. Newborns were classified as small‐for‐gestational age (SGA) based on a birthweight below the 10th percentile for gestational age, using three sets of US reference data. Birth size distributions were explored based on raw values as well as after z‐score standardisation in reference to World Health Organization (WHO) 2006 growth standards. Mean (SD) birthweight (g), length (cm) and head circumference (cm) measurements, completed within [median (25th, 75th percentile)] 15 (8, 23) h of life, were 2433 (425), 46.4 (2.4) and 32.4 (1.6), respectively. Twenty‐two per cent were born preterm. Over one‐half (55.3%) of infants were born low birthweight; 46.6%, 37.0% and 33.6% had a weight, length and head circumference below −2 z‐scores of the WHO growth standard at birth; and 70.9%, 72.2% and 59.8% were SGA for weight based on Alexander et al., Oken et al. and Olsen et al. references, respectively. Infants in this typical rural Bangladesh setting were commonly born small, reflecting a high burden of fetal growth restriction and preterm birth. Our findings, produced by active birth surveillance, suggest that low birthweight is far more common than suggested by cross‐sectional survey estimates. Interventions that improve fetal growth during pregnancy may have the largest impact on reducing SGA rates.
Keywords: birth, weight, growth, preterm birth, small‐for‐gestational age, intrauterine growth restriction
Introduction
Birth size is an important gauge of fetal and neonatal health at both an individual and a population level. Birthweight in particular is strongly associated with fetal, neonatal, post‐neonatal and young child mortality (McCormick 1985). Bangladesh is one of the few countries to carry out a national birthweight survey to estimate low‐birthweight (LBW, <2500 g) rates, one of the world's highest at 35.6% based on a 2003–2004 nationally representative survey (Bangladesh Bureau of Statistics 2005).
Most developing countries rely on estimates of LBW to characterise intrauterine growth failure; however, these estimates have limited value owing to two major limitations related to how LBW estimates are derived and the absence of data on gestational duration. Firstly, LBW rates derived from national household surveys (e.g. Demographic and Health Surveys and Multiple Indicator Cluster Survey) are often based on recall of birthweight. Moreover, in settings where infants are born at home, they are rarely weighed at or soon after birth. Attempts to adjust estimates by using maternal perception of infant size at birth have often systematically underestimated LBW incidence (Blanc & Wardlaw 2005). Additionally, LBW estimates derived from facility‐based records may be biased if only a small and selective proportion of births occur in facilities.
In addition to fetal growth, size at birth also reflects the duration of gestation. The absence of valid and reliable gestational‐age estimates preclude discerning between the two underlying biologic causes of LBW. Thus, there is a need for population‐based data of newborn size coupled with estimates of gestational duration to better inform decision making at both clinical and public health levels.
In addition to birthweight, investigators have used birth length and birth head, chest and/or mid‐upper arm circumferences (MUAC) to characterise birth size. With multiple birth size measures, indices of body proportionality, such as Rohrer's ponderal index (PI), can be calculated to provide information about whether a newborn appears relatively ‘thin’ or ‘fat’ at birth (Walther & Ramaekers 1982). Coupled with gestational‐age data, these measures can be used to characterise intrauterine growth and can provide important diagnostic and prognostic information beyond that provided by birthweight alone.
From 2004 to 2007, we conducted a newborn vitamin A supplementation trial (Klemm et al. 2008) nested within a larger placebo‐controlled maternal vitamin A and β‐carotene supplementation trial (West et al. 2011) in northwestern Bangladesh among ∼16 000 neonates, during which gestational‐age estimates were obtained and several dimensions of size at birth were measured. While newborn dosing was performed after birth, neither maternal vitamin A nor β‐carotene supplementation had an impact on birth size or risk of LBW, preterm birth or small‐for‐gestational age (SGA) (Christian 2013). This paper combines data from all three treatment groups to describe the birth size distributions, including weight, length, and head, chest and arm circumference of these infants, and assess these distributions relative to birth‐size‐for‐gestational‐age distributions derived from accepted reference populations.
Key messages
In Bangladesh, birthweights obtained by active birth surveillance show higher low birthweight prevalence than by cross‐sectional survey estimates.
Using low birthweight incidence as a proxy underestimated the magnitude of fetal growth restriction by an absolute 15.7%.
The low birth size and high rates of small‐for‐gestational age and preterm infants suggest a high burden of fetal growth restriction and preterm which is likely related to inadequate maternal nutrition, including poor pre‐pregnancy status, as well as other environmental risk factors.
Methods
Study population
The data used in this analysis represent all live born, singleton infants enrolled in a community‐based newborn vitamin A supplementation study (Klemm et al. 2008) conducted from January 2004 to July 2007 in rural northwest Bangladesh. This study was nested into and balanced across treatment arms of an ongoing placebo‐controlled, weekly maternal VA or β‐carotene supplementation trial (West et al. 2011). Pregnant women were identified and recruited into the parent trial via a five weekly, home‐visit surveillance system that was based on a history of the last menstrual period (LMP) combined with urine‐based pregnancy testing. Live born infants of consented mothers were visited by field staff as soon as possible after birth [median (interquartile range, IQR): 7 (2–18) h of age] and administered a coded supplement based on randomisation unit containing either 50 000 IU vitamin A or placebo.
At enrolment into the parent trial, an interview was conducted by trained staff at ∼9 weeks gestation to ascertain date of LMP, obtain family socio‐economic status information and assess mid‐upper arm circumference (MUAC) as an indicator of wasting malnutrition. Other information was also obtained, but is not pertinent to the analyses presented here.
Birth size measurements
Immediately following supplementation, one of the trained team of 56 female anthropometrists conducted a home visit to measure the infant for weight, length and mid‐upper arm, head and chest circumferences. Birthweight was measured to the nearest 10 g using a Tanita BD‐585 digital paediatric scale (Tanita Corporation, Tokyo, Japan). Length was measured to the nearest 0.1 cm using an affixed head board and movable foot plate that had been fashioned for use with the Tanita scale. Circumferential measurements were made with a Ross insertion tape (Abbott Laboratories, Columbus, OH, USA). Three independent measurements of length, head, chest and MUAC were taken. During the study's preparatory phase, anthropometrists were trained on standard measurement techniques and were certified against one, gold standard, anthropometry expert. Field activities were regularly overseen by a quality control team of anthropometrists, with each field‐based anthropometrist being randomly observed and her anthropometric measurement rechecked at least once every 3 months.
Gestational age was assessed using the reported first date of the LMP. The recall of this date was aided by the 5‐weekly assessment of menstrual histories that were used to administer pregnancy tests and the provision of calendars to facilitate recall and documentation of the onset of menses. The LMP date was ascertained at the time of the pregnancy enrolment interview, which was soon after detection of pregnancy, thereby shortening the recall period. Almost 85% of the pregnancies were enrolled within 12 weeks of gestation. Local events calendars were used to facilitate recall. In addition, we also cross‐checked the reported LMP with the date of the positive urine test.
Statistical analysis
All (n = 16 290) singleton, live born babies for whom birth size measurements were obtained within 72 h of birth are included in this analysis. LBW was defined as birthweight <2500 g (World Health Organization 2011a). Underweight, stunted, wasted and small head circumference were defined as weight‐ (WAZ), length‐(LAZ) and head circumference (HCZ)‐at‐birth being ≤2 z‐scores below the 2006 WHO child growth birth standards, respectively (World Health Organization 2011b). Wasted status was not calculated for infants whose birth lengths were <45 cm because of a lack of a standard definition for newborns of this size. Because the WHO growth standards do not include reference values for MUAC <3 months of age, a MUAC <9.0 cm defined low MUAC at birth (Goto 2011). PI was calculated using the formula 100 times the birthweight (g)/birth length (cm)3.
Gestational age, calculated as the number of completed weeks, was computed from the reported LMP obtained at the pregnancy enrolment interview and the date of birth of the live born infant. Birth size and gestational‐age data were available for 94.6% (15 435/16 290) of newborns. Term, mild, moderate and very preterm births were defined as gestation age in weeks ≥37, 34–36, 32–33 and <32, respectively (Kramer et al. 2000).
Estimates of appropriate‐for‐gestational age (AGA) and SGA with respect to infant weight were obtained using three separate reference populations because of the lack of a single universally accepted prescriptive standard describing optimal fetal growth and newborn nutritional status. AGA and SGA were defined as infants whose birth weight was ≥10th or <10th percentile, respectively, using Alexander et al. (1996) as the primary reference population, and Oken et al. (2003) and Olsen et al. (2010) as secondary reference populations. Severe SGA with respect to weight was defined as infant birthweight <3rd percentile using Oken et al. (2003) and Olsen et al. (2010), because the Alexander et al. reference does not provide third percentile values. SGA newborns were classified as having an adequate PI, representing symmetric growth retardation in both weight and length, or a low PI defined as <10th percentile on a reference chart of PI for each gestational‐age category (Lubchenco et al. 1966), representing asymmetric growth retardation. Because Olsen et al. (2010) provided gender‐ and gestational‐age distributions for newborn length and head circumference, we also estimated the proportion of infants who were SGA‐for‐length and for‐head circumference using a cut‐off of <10th percentile.
Distributions were compared by sex and severity of preterm birth using Student's t‐tests and one‐way analysis of variance, respectively.
There was no treatment effect on birth size for the parent maternal supplementation or an imbalance in the nested newborn vitamin A supplementation trials, and therefore, all birth size information was pooled across the intervention groups. The number of consenting pregnant women in this analysis (n = 22 719) differs from that reported in the main effects paper of the parent trial (n = 59 666) (West et al. 2011) because we only measured birthweight on infants in the nested trial, which began 3.5 years after the parent trial. The number of infants in this analysis (n = 16 290) also differs from that reported in the nested trial (n = 15 937). This is because we included infants born to consenting women after our Data Safety and Monitoring Board recommended halting the main newborn vitamin A study for reasons of efficacy of the intervention in reducing infant mortality before the attainment of the planned sample size (Klemm et al. 2008).
All analyses were completed using Stata version 11.2 (StataCorp 2009).
Ethical approval for the parent and nested studies referred to above was provided by the Bangladesh Medical Research Council, an autonomous body under the Ministry of Health and Family Welfare, Government of Bangladesh, and the Institutional Review Board at the Johns Hopkins Bloomberg School of Public Health, Baltimore, MD.
Results
Among the 22 719 pregnant women from whom a consent for birth assessment was obtained (96% of 23 627 enrolled in the maternal supplementation trial), there were 16 290 singleton, live born infants with birth size measurements obtained within 72 h of birth (Fig. 1). The median (IQR) time of birth size measurements was 15 (8, 23) h since birth. These infants, 51% male, were born to mothers predominantly less than 20 years of age, nulliparous [median (IQR) parity: 1 (0, 2)], and with some education (58.5% with any years of schooling) (Table 1). Mean (SD) gestational age was 37.8 (2.9) weeks. Ninety‐one per cent of births occurred at home. Twenty‐nine per cent of mothers had a MUAC < 22.0 cm, indicative of undernutrition (Gibson 2005).
Figure 1.
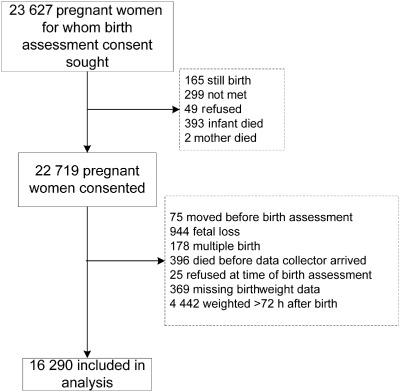
Flow chart of participant selection.
Table 1.
Participant's household and demographic characteristics (n = 16 290 infants)
| Characteristic | n | % |
|---|---|---|
| Infant sex | ||
| Male | 8 296 | 50.9 |
| Female | 7 994 | 49.1 |
| Maternal age (years)* | ||
| <20 | 9 559 | 58.7 |
| 20–29 | 5 769 | 35.5 |
| ≥30 | 947 | 5.8 |
| Parity | ||
| 0 | 7 058 | 43.3 |
| 1–2 | 6 694 | 41.1 |
| 3–4 | 2 013 | 12.4 |
| ≥5 | 525 | 3.2 |
| Mid‐upper arm circumference | ||
| <22.0 cm | 4 812 | 29.5 |
| ≥22.0 cm | 11 478 | 70.5 |
| Place of delivery | ||
| Home | 14 796 | 90.8 |
| Hospital or private clinic | 363 | 2.4 |
| Other | 1 131 | 6.9 |
| Household size, people* | ||
| 1–3 | 6 854 | 42.2 |
| 4–5 | 6 108 | 37.6 |
| ≥6 | 3 298 | 20.3 |
| Maternal educational (years)* | ||
| 0 | 6 749 | 41.5 |
| 1–9 | 8 610 | 53.0 |
| ≥10 | 892 | 5.5 |
| Occupation of head of family* | ||
| Business | 6 066 | 37.3 |
| Labourers | 4 438 | 27.3 |
| Farmer | 3 974 | 24.5 |
| Private | 1 147 | 7.1 |
| House construction materials | ||
| Tin/wood plank | 8 428 | 52.1 |
| Thatch, grass, sticks, branches | 4 648 | 28.8 |
| Mud | 2 450 | 15.2 |
| Stone | 631 | 3.9 |
| Household asset ownership* | ||
| Cattle | 6 755 | 41.6 |
| Goats | 3 730 | 22.9 |
| Radio | 3 122 | 19.2 |
| Electricity | 2 488 | 15.3 |
| Irrigation pump | 1 538 | 9.5 |
Missing data: maternal age, n = 15; maternal education, n = 39; occupation of head of household, n = 665; household size, n = 30; house construction material, n = 124. *Each household can own more than one item.
Mean birthweight (SD) was 2433 (425) g with over half of babies born low birthweight (LBW, n = 9000, 55.3%) (Table 2). The mean (SD) weight‐at‐birth z‐score for term babies was −1.82 (0.97). Females were born at mean (SD) 80 (10) g lighter than males (P < 0.0001) and had higher rates of LBW (59.7% vs. 50.9%, P < 0.0001). Mean (SD) birth length was 46.4 (2.4) cm, and mean (SD) circumferential measurements for head, chest and mid‐upper arm were 32.4 (1.6), 30.4 (2.1) and 9.3 (0.9) cm, respectively. Boys were born, on average, 0.5 cm longer, and with a 0.6 and 0.2 cm larger head and chest, respectively. MUAC and PI did not differ by infant sex.
Table 2.
Birth size measurements, z‐scores and percent below established cut‐offs, relative to 2006 WHO child growth standards and acceptable size‐for‐gestational age distributions
| All | Gestational age (weeks) † | Sex | |||||
|---|---|---|---|---|---|---|---|
| n = 16 290 | ≥37 | 34 to <37 | 32 to <34 | <32 | Boys | Girls | |
| n = 12 042 | n = 2419 | n = 600 | n = 374 | n = 8296 | n = 7994 | ||
| Birth size, mean (SD) | |||||||
| Weight (g) | 2 433 (425) | 2 498 (392) | 2240 (411) | 2094 (493) | 2055 (553) | 2473 (437) | 2392* (408) |
| Length (cm) | 46.4 (2.4) | 46.8 (2.2) | 45.3 (2.5) | 44.4 (3.0) | 44.2 (3.3) | 46.7 (2.5) | 46.1* (2.3) |
| Head circumference (cm) | 32.4 (1.6) | 32.6 (1.5) | 31.6 (1.6) | 30.9 (2.1) | 30.9 (2.5) | 32.6 (1.7) | 32.1* (1.6) |
| Chest circumference (cm) | 30.4 (2.1) | 30.7 (1.9) | 29.4 (2.1) | 28.7 (2.6) | 28.4 (3.1) | 30.5 (2.1) | 30.3* (2.1) |
| Mid‐upper arm circumference (cm) | 9.3 (0.9) | 9.4 (0.8) | 9.0 (0.8) | 8.7 (1.0) | 8.7 (1.1) | 9.3 (0.9) | 9.3 (0.9) |
| Ponderal index, 100 × g cm–3 | 2.42 (0.30) | 2.43 (0.30) | 2.40 (0.30) | 2.35 (0.27) | 2.35 (0.34) | 2.42 (0.31) | 2.43 (0.29) |
| Birth size relative to WHO standard, mean (SD) | |||||||
| WAZ | −1.99 (1.07) | −1.82 (0.97) | −2.48 (1.07) | −2.87 (1.28) | −2.99 (1.46) | −1.99 (1.09) | −1.99 (1.06) |
| LAZ | −1.72 (1.29) | −1.52 (1.15) | −2.32 (1.32) | −2.78 (1.60) | −2.90 (1.78) | −1.75 (1.31) | −1.68* (1.26) |
| HCZ | −1.54 (1.32) | −1.33 (1.17) | −2.14 (1.33) | −2.70 (1.72) | −2.71 (2.04) | −1.50 (1.32) | −1.58* (1.32) |
| Low birthweight ¶ | 9 000 (55.2) | 6 042 (50.2) | 1774 (73.3) | 456 (76.0) | 290 (77.5) | 4224 (50.9) | 4776* (59.7) |
| ≤2 WAZ ¶ | 7 593 (46.6) | 4 924 (40.9) | 1604 (66.3) | 432 (72.0) | 266 (71.1) | 3829 (46.0) | 3773 (46.6) |
| ≤2 LAZ ¶ | 5 892 (37.0) | 3648 (30.9) | 1380 (58.4) | 372 (64.1) | 220 (62.5) | 3115 (38.5) | 2777* (35.5) |
| ≤2 HCZ ¶ | 5 431 (33.6) | 3 296 (27.6) | 1298 (54.1) | 363 (61.0) | 221 (60.4) | 2581 (31.4) | 2850* (35.9) |
| Low MUAC §§ ¶ | 5 065 (31.3) | 3 176 (26.5) | 1115 (46.5) | 331 (55.7) | 209 (58.1) | 2545 (30.9) | 2520 (31.7) |
| Low PI ¶¶ | 2 712 (18.4) | 2 318 (20.5) | 335 (13.8) | 43 (7.2) | 16 (4.3) | 1388 (18.4) | 1324 (18.5) |
| Gestational age at birth n (%) | |||||||
| SGA for weight n (%) | n = 15 435 | n = 12 042 | n = 2419 | n = 600 | n = 374 | n = 7881 | n = 7554 |
| Alexander** | 10 944 (70.9) | 9 647 (80.1) | 1169 (48.3) | 112 (18.7) | 16 (4.3) | 5524 (70.1) | 5420* (71.7) |
| Oken †† | 11 444 (72.2) | 9 892 (82.1) | 1146 (47.4) | 97 (16.2) | 9 (2.4) | 5623 (71.3) | 5521* (73.1) |
| Olsen ‡‡ | 8 533 (59.8) | 7 825 (72.0) | 625 (25.8) | 71 (11.8) | 12 (3.2) | 4290 (58.5) | 4243* (61.2) |
| Oken †† | 7 455 (48.3) | 6 986 (58.0) | 443 (18.3) | 26 (4.3) | 0 (0.0) | 3681 (46.7) | 3774* (50.0) |
| Olsen ‡‡ | 5 110 (35.8) | 4 847 (44.6) | 229 (9.5) | 32 (5.3) | 2 (0.5) | 2550 (34.8) | 2560* (36.9) |
| SGA with low PI n (%) (n = 15 435) ¶¶ | 2 543 (24.5) | 2 244 (24.8) | 268 (22.9) | 25 (22.3) | 6 (37.5) | 1302 (24.8) | 1241 (24.3) |
| SGA for length n (%) | 6423 (46.1) | 5916 (55.6) | 456 (19.3) | 47 (8.1) | 4 (1.1) | 3246 (45.5) | 3177 (46.8) |
| SGA for head circumference n (%) | 4361 (30.8) | 3862 (35.8) | 426 (17.8) | 59 (9.9) | 14 (3.8) | 2112 (29.1) | 2249* (32.3) |
SGA, small‐for‐gestational age; WAZ, weight‐for‐age z‐score; LAZ, length‐for‐age z‐score; HCZ, head circumference‐for‐age z‐score; PI, ponderal index; MUAC, mid‐upper arm circumference. *Indicates girls differ from boys at P < 0.05. †Missing data: gestational age and size for gestational age, n = 855; length and LAZ, n = 374; head circumference and HCZ, n = 135; arm circumference, n = 107; chest circumference, n = 273; Ponderal index, n = 374. ¶Percentages reflect proportion of those within the same gestational age category at birth and are based on number with available data. **Based on Alexander et al. (1996). ††Based on Oken et al. (2003). ‡‡Based on Olsen et al. (2010). §§Low MUAC defined as MUAC < 9 cm based on Goto (2011). ¶¶Low PI was defined as <10th percentile of reference chart of PI for each gestational‐age category based on Lubchenco et al. (1966); PI missing for n = 563 infants with gestational age >42 weeks and n = 521 for infant without gestational‐age data.
At birth, infant mean (SD) WAZ, LAZ and HCZ were −1.99 (1.07), −1.72 (1.29) and −1.54 (1.30) relative to the WHO growth standard, respectively, and the proportion of newborns with z‐scores less than −2 for these indices was 46.6%, 37.0% and 33.6%, respectively. Girls had significantly lower LAZ and HCZ scores at birth relative to boys. Almost one‐third of newborns had a MUAC < 9 cm, and 18.4% had a low PI.
The overall prevalence of preterm births was 22%, with births occurring between 34 and <37 weeks, 32 to <34 and <32 weeks being 15.7%, 3.9% and 2.4%, respectively. Depending on the reference population, 70.9% (Alexander et al. 1996), 72.2% (Oken et al. 2003) and 59.8% (Olsen et al. 2010) of newborns were SGA for weight, respectively, which were lower among males (Table 2). Overall SGA for length and head circumference was 46.1% and 30.8%, respectively. Among full‐term infants, 80.1%, 82,1% and 72% were SGA based on the three reference populations, whereas among preterm infants 36.7%, 36.9% and 20.9% were SGA, respectively. Severe SGA was 48.3% (Oken et al. 2003) and 35.8% (Olsen et al. 2010), and significantly higher in girls (50.0% and 36.9%) than boys (46.7% and 34.8%), respectively. Among the SGA births, 75.0% had an adequate PI and 25.0% had a low PI with no significant difference across gestational age categories or sex.
Fetal growth restriction is strikingly progressive after ∼34 weeks gestation as noted by rates of SGA and severe SGA‐for‐weight rates that are, respectively, ∼4–5 and ∼8–14 times higher for full‐term newborns, depending on the reference population used. There also appears to be substantial sparing of linear and head circumference growth after ∼34 weeks as noted by the high prevalence of SGA‐for‐length (55.6%) and SGA‐for‐head‐circumference (35.8%) among full‐term newborns compared with <10% among infants born <34 weeks gestation.
Eighty‐six per cent of SGA and 53% of AGA infants were born full‐term. The proportion of LBW due to SGA alone, SGA and preterm, and preterm alone is 70.5%, 15.2% and 14.3%, respectively (Figs 2 and 3). Over 50% of non‐LBW infants were SGA, all of whom were term infants.
Figure 2.
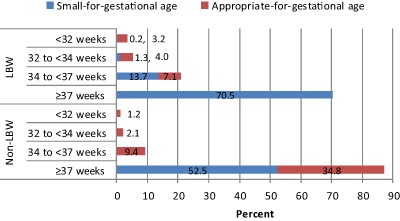
Appropriate‐for‐gestational age (AGA) and small‐for‐gestational age (SGA) by birthweight and gestational‐age categories.
LBW, low birthweight (i.e. birthweight <2500 g); non‐LBW, non‐low‐birthweight (i.e. birthweight ≥2500 g).
Figure 3.
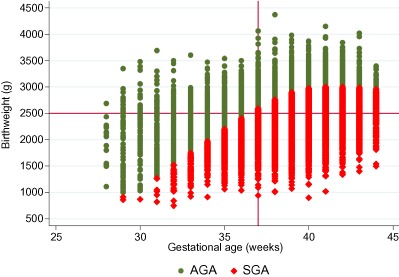
Distribution of birthweight (gram) by gestational age at birth (week) categorised by small‐ and appropriate‐for‐gestational age based on weight as defined by Alexander et al. (1996).
AGA, appropriate‐for‐gestational age; SGA, small‐for‐gestational age.
Relative to the mean weight of 2500 g among infants weighed <6 h after birth, mean weights declined sharply with age of birthweight measurement through 48–53 h to 2360 g; thereafter, mean birthweights rose to 2470 g through 71 h (Fig. 4). Correspondingly, estimates of LBW prevalence varied by timing of birthweight measurements ranging from 49% for infants weighed <6 h after birth to 60% for infants weighed 24–53 h after birth (Fig. 4). LBW prevalence estimates based on measurements taken on a newborn's first (i.e. 0–23 h), second (24–47 h) or third day (48–71 h) of life are 54%, 60% and 56% (data not shown).
Figure 4.
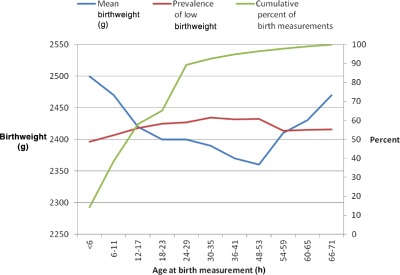
Mean birthweight (g), prevalence of low birthweight (%) by age (h) at measurement and cumulative percent of birth measurements.
Discussion
In rural northwest Bangladesh, measuring over 16 000 newborns at home within 72 h of birth, we found LBW and preterm prevalence rates of 55.2% and 22.0%. Relative to WHO birth size standards (World Health Organization, Multicentre Growth Reference Study Group 2006), 46.6%, 37.3% and 33.6% of infants were born ≤2 WAZ, LAZ and HCZ, respectively. SGA and severe SGA rates based on birthweight were between 59.8–72.2% and 35.8–48.3%, respectively, depending on the reference population. SGA for length and head circumference were 46.1% and 30.8%, respectively. Individually and collectively, these indicators suggest a much higher burden of fetal growth restriction than previously reported in Bangladesh (Goodburn et al. 1994; Arifeen et al. 2000; Bangladesh Bureau of Statistics 2005).
Birthweight
The high prevalence of LBW is consistent with findings from other studies in Bangladesh (Goodburn et al. 1994; Ferro‐Luzzi et al. 1998; Arifeen et al. 2000; Bangladesh Bureau of Statistics 2005), but at 55.2% prevalence, it is the highest rate published to date and exceeds the 2003–2004 district prevalence estimate of 37.3% reported for Rajshahi where our study was conducted. Our study was not designed to be representative of Bangladesh or Rajshai district, but the study area was typical of rural Bangladesh and selected based on its exhibiting average‐to‐below average socio‐economic status, health service utilisation, and maternal malnutrition and morbidity (Labrique et al. 2011). Potential explanations for the large differences in LBW prevalence estimates include methodological variation in birth capture, timing and/or precision of birth size measurement and/or regional and seasonal variations in birth size. However, all birth size data used in the present analysis were measured with a digital scale precise to 10 g, with a median time of 15 h and a maximum time of <72 h since birth. Moreover, birth size was measured at the place of birth by a team of certified anthropometrists who used routinely calibrated, sensitive and precise equipment. Thus, we feel our estimate provides a true characterisation of LBW incidence in this population.
Effect of age at measurement
Neonatal weight loss in the first hours and days of birth is universally recognised, and also is evident in our findings where mean birthweights decrease within the first 2 days of life and then increase thereafter. Timing of measurement can affect LBW prevalence particularly when the populations' mean birthweight is near the 2500 g LBW cut‐off. The LBW prevalence in our sample was 49% among infants weighed within 6 h of birth, but 60% among infants weighed on the second day of life. This suggests that when reporting LBW prevalence, the median age of the birth measurement should be reported (in our study, 50% of infants were measured by 15 h post‐birth). It would also be useful to report LBW prevalence by day of birth measurement. Based on our data, there was a 11% relative difference in LBW prevalence between infants weighed on their first day of life (LBW prevalence of 54%) relative to those weighed on their second day of life (LBW prevalence of 60%).
Comparison with national LBW estimates
Our findings suggest that LBW via fetal growth restriction is far more common than suggested by cross‐sectional survey estimates regularly invoked and used by many international groups to track, model and draw widely accepted inferences about apparent improvements in this vital aspect of newborn health. For example, commonly used UNICEF SOWC data suggest that the LBW rate in Bangladesh is <30%, which, as our data suggest, is severely underestimating its extent, and may help explain the seeming paradox of sustained high mortality in Bangladesh in the presence of seemingly much improved birthweight. The answer is likely that birthweight due to chronic fetal malnutrition (and likely infection and inflammation) remains a very large problem.
Sex differences in birthweight
We report significant differences in birth size between boys and girls. At birth, boys were, on average, 81 g heavier, 0.6 cm longer, and had a head and chest circumference of 0.5 and 0.2 cm larger than girls. Sex differences in fetal growth rates have long been recognised (e.g. Lubchenco et al. 1963), but biological mechanisms underlying these differences are not well understood.
Wasting prevalence
Wasting prevalence was high and constant from 32 to 34 weeks onward, suggesting inadequate nutriture to support cell differentiation and tissue growth that also plays out with respect to linear growth, which steadfastly slowed relative to the reference to term.
Small‐for‐gestational age
Our study design and methodology permitted reliable and timely assessment of menstrual date histories used to estimate gestational age. The process of eliciting menstrual date histories every 5 weeks as part of the study's pregnancy surveillance system made remembering the menstrual date a habit among women. We also identified and enrolled women early in gestation [median (IQR) gestational age at enrolment was 8 (6–11) weeks], thereby reducing the recall period between the menstrual date and enrolment interview. While estimating gestational age from menstrual date histories is not a gold standard method, we are confident that regular menstrual date elicitation, combined with a short recall period, provided reliable and valid gestational‐age estimates.
Using both birth size and gestational‐age estimates, we were able to assess birth size in relation to gestational age and quantify the magnitude of fetal growth restriction. Our data show that the incidence of LBW underestimates the overall magnitude of fetal growth restriction because it does not account for infants whose weight falls below the 10th percentile but who weighs ≥2500 g at birth. Overall, 70.9% of study infants were SGA based on birthweight when accounting for gestational age, exceeding the LBW rate by 15.7% and the underweight rate (i.e. ≤2 WAZ) based on WHO growth standards by 24.3%. These large differences illustrate the starkly different impressions of adequacy of intrauterine growth obtained by using references that adjust or fail to adjust for gestational age.
Using the Olsen reference population, the rates of SGA were 59.8%, 46.1% and 30.8% for weight, length and head circumference‐for‐gestational age, respectively. The much lower rates of small head circumference, relative to weight and length, renders some support to the observations of brain sparing on pups born to undernourished dams reported from animal studies (Joshi et al. 2003). Similarly, the low length‐for‐gestational‐age rate relative to weight suggests that skeletal growth is also being preserved in spite of maternal undernutrition. About 82% of SGA infants had an adequate PI. This high proportion points to the influence on intrauterine growth of factors operative in early pregnancy or before.
Implications of high SGA rates
The high (80.1%) proportion of term infants born SGA suggests that the largest reductions in SGA would come from interventions that increase fetal growth during the third trimester, particularly weight gain. Because nutrient supply to the fetus is a key factor in the regulation of fetal growth, these high rates of SGA suggest that maternal nutrition, both before and during stages of pregnancy, is an important determinant of fetal growth and size at birth (Harding & Johnston 1995). Evidence of maternal undernutrition in our study population shows a 30% of the women having MUAC < 22 cm, an indicator of maternal undernutrition. Other studies from Bangladesh point to inadequate dietary intake during the second and third trimesters of pregnancy, with estimates of maternal energy intake of only 1464 kcal day−1 (Alam et al. 2003). Studies in India have shown that maternal nutrition is an important determinant of fetal growth and size at birth (Yajnik 2006), and highlight the need for improving maternal diet through micronutrient rich foods (Fall et al. 2003). The data also suggest that micronutrient deficiencies are common and concurrent among rural pregnant women in some South Asian contexts (Jiang et al. 2005), and that their diets are frequently deficient in energy, protein and different micronutrients (Kontic‐Vucinic et al. 2006a, 2006b). While maternal nutrition clearly plays a role in the regulation of fetal growth, more research is needed to understand the mechanisms of nutrient supply to the fetus. Evidence to date suggests that the effects of maternal nutrition on birth size vary with the severity and timing of nutritional insult.
Summary
In summary, in this large community‐based study, we described the birth size distributions of rural Bangladeshi newborns and documented high rates of LBW, SGA and preterm among rural infants in Bangladesh. The low birth size and high rates of SGA and preterm suggest a high burden of fetal growth restriction and preterm that is likely related to inadequate maternal nutrition, including poor pre‐pregnancy status, as well as other environmental risk factors. The risks associated with poor fetal growth are high levels of mortality and morbidity in infancy and childhood, and a growing body of evidence points to small size of birth being associated with increased risk of hypertension, cardiovascular disease, type 2 diabetes and other health risks in later life. We demonstrated that birthweight and LBW prevalence is a poor proxy for assessing fetal growth restriction. We also noted the high rates of SGA among term babies, suggesting that interventions that improve fetal growth during the second and third trimesters of pregnancy are likely to have the largest impact on reducing SGA rates. Finally, the results emphasise the importance of improving our understanding of the mechanisms underlying feto‐placental metabolic and endocrine adaptation, and testing their response to nutritional interventions. In this rural Bangladeshi setting, infant size at birth may be one of the most important determinants of subsequent growth status during infancy.
Source of funding
Funding for this study was from a Global Research Activity cooperative agreement between JHU and the Office of Health and Nutrition, US Agency for International Development (USAID), Washington DC (GHS‐A‐00‐03‐00019‐00 and Micronutrients for Health Cooperative Agreement HRN‐A‐00‐97‐00015) and a grant from the Bill and Melinda Gates Foundation, Seattle, Washington (Global Control of Micronutrient Deficiency, Grant No. 614). Additional direct or in‐kind support was provided by Sight and Life (Basel, Switzerland), the Sight and Life Research Institute (Baltimore, MD), Nutrilite Health Institute (Nutrilite Division, Access Business Group, LLC, 5600 Beach Blvd, Buena Park, CA 90621), the Canadian International Development Agency (CIDA), and the National Integrated Population and Health Program (NIPHP) of the Ministry of Health and Family Welfare of the Government of the People's Republic of Bangladesh.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
RK had full access to the data, was involved in all aspects of the study design, analyzed the data and wrote the manuscript. RM assisted with data analysis, contributed to and reviewed the manuscript. LW assisted with data management, produced the flow chart of participant selection and edited the manuscript. AAS and HA were involved in training, implementation, supervision, and quality control of all aspects of the fieldwork, and reviewed the manuscript. AL served as project scientist, was involved in all aspects of the study management and implementation, and reviewed the manuscript. PC was involved in all aspects of the study design, helped with data interpretation and edited the manuscript. KPW served as the PI of the study, is the guarantor for the study, and helped write and edit the manuscript.
Acknowledgements
We acknowledge the entire field research team in Bangladesh who participated in data collection, supervision, data entry and management, and provided logistical, mapping, laboratory and administrative support. We acknowledge Darrell Mast and Allan Massie for providing programming support.
Klemm, R. D. W. , Merrill, R. D. , Wu, L. , Shamim, A. A. , Ali, H. , Labrique, A. , Christian, P. , and West, K. P. Jr (2015) Low‐birthweight rates higher among Bangladeshi neonates measured during active birth surveillance compared to national survey data. Matern Child Nutr, 11: 583–594. doi: 10.1111/mcn.12041.
References
- Alam D.S., Van Raaij J.M., Hautvast J.G., Yunus M. & Fuchs G.J. (2003) Energy stress during pregnancy and lactation: consequences for maternal nutrition in rural Bangladesh. European Journal of Clinical Nutrition 57, 151–156. [DOI] [PubMed] [Google Scholar]
- Alexander G.R., Himes J.H., Kaufman R.B., Mor J. & Kogan M. (1996) A United States national reference for fetal growth. Obstetrics and Gynecology 87, 163–168. [DOI] [PubMed] [Google Scholar]
- Arifeen S.E., Black R.E., Caulfield L.E., Antelman G., Baqui A.H., Nahar Q. et al (2000) Infant growth patterns in the slums of Dhaka in relation to birth weight, intrauterine growth retardation, and prematurity. The American Journal of Clinical Nutrition 72, 1010–1017. [DOI] [PubMed] [Google Scholar]
- Bangladesh Bureau of Statistics (2005) National Low Birth Weight Survey of Bangladesh, 2003–2004.
- Blanc A.K. & Wardlaw T. (2005) Monitoring low birth weight: an evaluation of international estimates and an updated estimation procedure. Bulletin of the World Health Organization 83, 178–185. [PMC free article] [PubMed] [Google Scholar]
- Christian P., Klemm R., Shamim A.A., Ali H., Rashid M., Shaikh S., Wu L., Mehra S., Labrique A., Katz J. & West K.P. Jr. (2013) Effects of vitamin A and β‐carotene supplementation on birth size and length of gestation in rural Bangladesh: a cluster‐randomized trial. The American Journal of Clinical Nutrition 97, 188–194. [DOI] [PubMed] [Google Scholar]
- Fall C.H., Yajnik C.S., Rao S., Davies A.A., Brown N. & Farrant H.J. (2003) Micronutrients and fetal growth. The Journal of Nutrition 133 (5 Suppl. 2), 1747S–1756S. [DOI] [PubMed] [Google Scholar]
- Ferro‐Luzzi A., Ashworth A., Martorell R. & Scrimshaw N. (1998) Report of the IDECG Working Group on effects of IUGR on infants, children and adolescents: immunocompetence, mortality, morbidity, body size, body composition, and physical performance. European Journal of Clinical Nutrition 52 (Suppl. 1), S97–S99. [PubMed] [Google Scholar]
- Gibson R.S. (2005) Principles of Nutritional Assessment. 2nd edn, Oxford University Press: New York. [Google Scholar]
- Goodburn E., Chowdhury M. & Gazi R. (1994) Low birth weight in rural Bangladesh. Journal of Tropical Pediatrics 40, 123. [DOI] [PubMed] [Google Scholar]
- Goto E. (2011) Meta‐analysis: identification of low birthweight by other anthropometric measurements at birth in developing countries. Journal of Epidemiology/Japan Epidemiological Association 21, 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding J.E. & Johnston B.M. (1995) Nutrition and fetal growth. Reproduction, Fertility, and Development 7, 539–547. [DOI] [PubMed] [Google Scholar]
- Jiang T., Christian P., Khatry S.K., Wu L. & West K.P. (2005) Micronutrient deficiencies in early pregnancy are common, concurrent, and vary by season among rural Nepali pregnant women. The Journal of Nutrition 135, 1106–1112. [DOI] [PubMed] [Google Scholar]
- Joshi S., Garole V., Daware M., Girigosavi S. & Rao S. (2003) Maternal protein restriction before pregnancy affects vital organs of offspring in Wistar rats. Metabolism: Clinical and Experimental 52, 13–18. [DOI] [PubMed] [Google Scholar]
- Klemm R.D., Labrique A.B., Christian P., Rashid M., Shamim A.A., Katz J. et al (2008) Newborn vitamin A supplementation reduced infant mortality in rural Bangladesh. Pediatrics 122, e242–e250. [DOI] [PubMed] [Google Scholar]
- Kontic‐Vucinic O., Sulovic N. & Radunovic N. (2006a) Micronutrients in women's reproductive health: I. Vitamins. International Journal of Fertility and Women's Medicine 51, 106–115. [PubMed] [Google Scholar]
- Kontic‐Vucinic O., Sulovic N. & Radunovic N. (2006b) Micronutrients in women's reproductive health: II. Minerals and trace elements. International Journal of Fertility and Women's Medicine 51, 116–124. [PubMed] [Google Scholar]
- Kramer M.S., Demissie K., Yang H., Platt R.W., Sauve R. & Liston R. (2000) The contribution of mild and moderate preterm birth to infant mortality. Fetal and Infant Health Study Group of the Canadian Perinatal Surveillance System. JAMA: the Journal of the American Medical Association 284, 843–849. [DOI] [PubMed] [Google Scholar]
- Labrique A.B., Christian P., Klemm R.D., Rashid M., Shamim A.A., Massie A. et al (2011) A cluster‐randomized, placebo‐controlled, maternal vitamin A or beta‐carotene supplementation trial in Bangladesh: design and methods. Trials 12, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubchenco L.O., Hansman C., Drssler M. & Boyd E. (1963) Intrauterine growth as estimated from liveborn birth weight data at 24 to 42 weeks of gestation. Pediatrics 32, 793–800. [PubMed] [Google Scholar]
- Lubchenco L.O., Hansman C. & Boyd E. (1966) Intrauterine growth in length and head circumference as estimated from live births at gestational ages from 26 to 42 weeks. Pediatrics 37, 403–408. [PubMed] [Google Scholar]
- McCormick M.C. (1985) The contribution of low birth weight to infant mortality and childhood morbidity. The New England Journal of Medicine 312, 82–90. [DOI] [PubMed] [Google Scholar]
- Oken E., Kleinman K.P., Rich‐Edwards J. & Gillman M.W. (2003) A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatrics 3, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen I.E., Groveman S.A., Lawson M.L., Clark R.H. & Zemel B.S. (2010) New intrauterine growth curves based on United States data. Pediatrics 125, e214–e224. [DOI] [PubMed] [Google Scholar]
- StataCorp (2009) Stata Statistical Software: Release 11. [11.2], StataCorp LP, College Station, TX.
- Walther F.J. & Ramaekers L.H. (1982) The ponderal index as a measure of the nutritional status at birth and its relation to some aspects of neonatal morbidity. Journal of Perinatal Medicine 10, 42–47. [DOI] [PubMed] [Google Scholar]
- West K.P. Jr, Christian P., Labrique A.B., Rashid M., Shamim A.A., Klemm R.D. et al (2011) Effects of vitamin A or beta carotene supplementation on pregnancy‐related mortality and infant mortality in rural Bangladesh: a cluster randomized trial. JAMA: the Journal of the American Medical Association 305, 1986–1995. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2011a) International Statistical Classification of Diseases and Related Health Problems.
- World Health Organization (2011b) The WHO Child Growth Standards. [Online].
- World Health Organization, Multicentre Growth Reference Study Group (2006) WHO Child Growth Standards: Length/Height‐for‐age, Weight‐for‐age, Weight‐for‐length, Weight‐for‐height and Body Mass Index‐for‐age: Methods and Development. World Health Organization: Geneva. [Google Scholar]
- Yajnik C. (2006) Nutritional control of fetal growth. Nutrition Reviews 64 (5 Pt 2), S50–S51; discussion S72–S91. [DOI] [PubMed] [Google Scholar]


