Abstract
In‐home fortification of infants with micronutrient powders (MNPs) containing 12.5 mg iron may increase morbidity from infections; therefore, an efficacious low‐dose iron‐containing MNP might be advantageous. Effects of iron‐containing MNPs on infant growth are unclear. We assessed the efficacy of a low‐iron MNP on iron status and growth and monitored safety in a randomised, controlled, double‐blind 1‐year trial in 6‐month‐old infants (n = 287) consuming daily a maize porridge fortified with either a MNP including 2.5 mg iron as NaFeEDTA (MNP + Fe) or the same MNP without iron (MNP − Fe). At baseline, after 6 and 12 months, we determined haemoglobin (Hb), iron status [serum ferritin (SF), soluble transferrin receptor (sTfR) and zinc protoporphyrin (ZPP)], inflammation [C‐reactive protein (CRP)] and anthropometrics. We investigated safety using weekly morbidity questionnaires asking for diarrhoea, cough, flu, bloody or mucus‐containing stool and dyspnoea, and recorded any other illness. Furthermore, feeding history and compliance were assessed weekly. At baseline, 71% of the infants were anaemic and 22% iron deficient; prevalence of inflammation was high (31% had an elevated CRP). Over the 1 year, Hb increased and SF decreased in both groups, without significant treatment effects of the iron fortification. At end point, the weight of infants consuming MNP + Fe was greater than in the MNP − Fe group (9.9 vs. 9.5 kg, P = 0.038). Mothers of infants in the MNP + Fe group reported more infant days spent with cough (P = 0.003) and dyspnoea (P = 0.0002); there were no significant differences on any other of the weekly morbidity measures. In this study, low‐dose iron‐containing MNP did not improve infant's iron status or reduce anaemia prevalence, likely because absorption was inadequate due to the high prevalence of infections and the low‐iron dose.
Keywords: iron fortification, infant, anaemia, growth, micronutrient powder, sub‐Saharan Africa
Introduction
Iron deficiency (ID) is the most common micronutrient deficiency worldwide, particularly in infants (Stoltzfus 1998). Iron deficiency anaemia (IDA) can lead to poor cognitive and motor development (Stoltzfus 1998; Sachdev et al. 2005). Infants' iron stores at birth are generally sufficient to cover their needs over the first 4–6 months, but during the subsequent weaning period complementary foods rich in iron are needed to meet the increased requirements for growth (Zimmermann & Hurrell 2007). Thus, iron fortification of complementary foods is recommended to prevent ID during infancy and early childhood (Fontaine 2007; Dube et al. 2010).
Untargeted iron supplementation at high doses (0.8–1.2 mg Fe/kg body weight) increased morbidity in pre‐school children in Tanzania (Sazawal et al. 2006). An earlier systematic review of randomised controlled trials suggested iron supplementation may increase risk of diarrhoea (Gera & Sachdev 2002) and some experts suggest food fortification with low doses of iron as the safest iron intervention (Oppenheimer 2001; Gera & Sachdev 2002). Micronutrient powders (MNPs) for in‐home fortification containing high doses of iron are efficacious in improving iron status in infants and school children (Zlotkin et al. 2003; Adu‐Afarwuah et al. 2008). However, their safety in malarial areas has been recently questioned. A large intervention study in Pakistani infants reported that a MNP containing a high dose of iron (12.5 mg day−1) reduced anaemia and iron deficiency, but increased diarrhoea and respiratory morbidities (Soofi et al. 2013). The same dose of iron has been reported to increase hospitalisation, possibly due to diarrhoea, in Ghanaian infants (Zlotkin et al. 2013).
Iron from NaFeEDTA is highly bioavailable, even in phytate‐rich whole flours often used in complementary porridges, and may allow the use of lower, safer iron dosages for infants and children (Andang'o et al. 2007; Troesch et al. 2009). However, its efficacy in complementary foods has not yet been assessed in infants.
Iron is essential for growth, but meta‐analyses have shown limited or no effect of iron interventions and/or MNPs on child growth (Ramakrishnan et al. 2004; Sachdev et al. 2006). Furthermore, studies in breastfed and iron‐replete infants have reported negative effects of iron on growth (Dewey et al. 2002) and on weight‐for‐age z‐scores (WAZ) (Lind et al. 2008). The intervention in Pakistani infants reported a small positive effect of high‐dose iron fortification (12.5 mg day−1) on linear growth (Soofi et al. 2013).
We aimed to assess the efficacy of a low‐iron MNP (containing 2.5 mg iron as NaFeEDTA) in 6‐month‐old Kenyan infants. Primary outcome was haemoglobin (Hb) and secondary outcomes were iron status, growth and the safety of the low‐iron MNP based on recording weekly morbidity.
Key messages
In‐home fortification of complementary feeding in Kenyan infants was well accepted and compliance was high.
Low‐dose iron MNP did not improve iron status or haemoglobin in this rural African infant population with high rates of infection and inflammation.
After the 1‐year intervention, infants consuming maize porridge fortified with iron‐containing MNP had greater weight gain compared with controls.
Future large‐scale studies are needed to evaluate safe and effective iron fortification strategies in infants.
Subjects and methods
Study site
The study was conducted between February 2010 and August 2012 in the capture area of the Kikoneni Health Center (Msambweni County) in southern coastal Kenya. Since this rural area is sparsely populated, recruitment was done continuously and completed by August 2011. The intervention period included three long rainy seasons from April to July (2010–2012) and two short rainy seasons from October to November (2010 and 2011). The main economic activity in the area is subsistence farming with maize as the staple food crop. The typical local weaning food is a liquid maize porridge ‘uji’; a regular portion consists of about 8–10 g maize flour boiled in 100–150 mL water and sweetened with sugar.
Study design and participants
This efficacy study was embedded in a randomised, double‐blind, controlled trial of a low‐dose iron‐containing MNP, with cognitive and motor development as primary outcome (J. Kvalsvig unpublished observations). Inclusion criteria were: (1) age of 6 months (± 3 weeks); (2) Hb ≥ 70 g L−1; and (3) no acute or chronic illness. The study was explained by the study nurse to one of the caretakers and written informed consent was obtained. Random assignment was performed using a computer‐generated list and eligible subjects were allocated to one of the four colour‐labelled groups [two containing iron (MNP + Fe) and two containing no iron (MNP − Fe)]. After a baseline assessment, 379 eligible infants were enrolled into the cognition study. For this efficacy trial, the primary outcome was Hb, and the sample size was powered on the full sample size included at start of the trial. We included all subjects that completed the intervention in the evaluation without a formal power calculation prior analysis. Post analysis, we estimate that the observed variation in Hb would allow detecting a difference at end point between MNP + Fe and MNP − Fe of 6 g L−1 with 80% power. The detectable difference in total body iron stores at end point can be estimated to be 14 mg with 80% power.
The intervention was a daily consumed maize porridge fortified with the allocated MNP. The mothers were instructed on how to prepare the maize porridge daily, and to take a portion of porridge that could easily be eaten by the infant and fortify it with the full content of MNP sachet. The MNP (MixMe, DSM Nutritional Products Europe Ltd, Basel, Switzerland) was packed in four different coloured sachets, two for each treatment group [MNP with 2.5 mg NaFeEDTA (MNP + Fe) and MNP without iron (MNP − Fe)]. The composition of the MNPs is shown in Table 1. Before the intervention started, we tested the acceptability of the MNPs and potential sensory changes using a triangle test (Meilgaard et al. 2007). The MNPs (MNP + Fe and MNP − Fe) were indistinguishable and acceptability was high among the local community (data not shown). Samples of the maize flour were analysed in triplicate at the Laboratory of Human Nutrition at the ETH Zurich for their iron and phytate content; these were 1.15 ± 0.06 mg and 310 ± 20 mg per 100 g, respectively.
Table 1.
Composition of micronutrient powder used in the intervention study
| Nutrient | Amount per 1 g sachet |
|---|---|
| Vitamin A | 100 μg |
| Iron | 0 or 2.5 mg |
| Vitamin D | 5 μg |
| Copper | 0.34 mg |
| Tocopherol equivalent | 5 mg |
| Iodine | 30 μg |
| Vitamin K1 | 30 μg |
| Selenium | 17 μg |
| Thiamine | 0.5 mg |
| Zinc | 2.5 mg |
| Riboflavin | 0.5 mg |
| Pyridoxine | 0.5 mg |
| Folic acid anhydrous | 90 μg |
| Niacinamide | 6 mg |
| Vitamin B12 | 0.9 μg |
| Vitamin C | 60 mg |
Weekly, the mothers collected 2–3 kg maize flour (Dola, Kitui Flour Mills Ltd, Mombasa, Kenya) and seven micronutrient sachets at the nearest of six distribution points. Compliance was assessed by collecting and recording empty and full MNP sachets from the previous week. Feeding history and the child's overall health status were assessed using a weekly recall covering the previous 7 days. If the mother reported any current illness, the child was examined by the study nurse at the health center of Kikoneni or referred to the district hospital in Msambweni. In addition, the study nurse probed for prior and current episodes of malaria, diarrhoea and respiratory tract infections at baseline, after 6 and 12 months. Infant weight was measured to the nearest 100 g using a hanging scale (Salter 235‐6S, 25 kg × 100 g; Salter Brecknell, Smethwick, UK) and recumbent length to the nearest 0.1 cm using a measurement board (Shorr Production, LLC, Olney, MD, USA).
There was a labelling error from the first to the second production batch of the MNP, where two of the four colours (one MNP + Fe and one MNP − Fe) were interchanged. This was only discovered during unblinding after the study was completed. Data from all subjects affected by this labelling error were excluded (MNP + Fe: n = 45; MNP − Fe: n = 47) and 287 infants were included in the final analysis (Fig. 1, trial profile). There were no differences between the baseline characteristics [age, weight, length, Hb, serum ferritin (SF), soluble transferrin receptor (sTfR), zinc protoporphyrin (ZPP) and C‐reactive protein (CRP)] of the infants excluded due to the labelling error and those remaining in the analysis using independent samples t‐tests.
Figure 1.
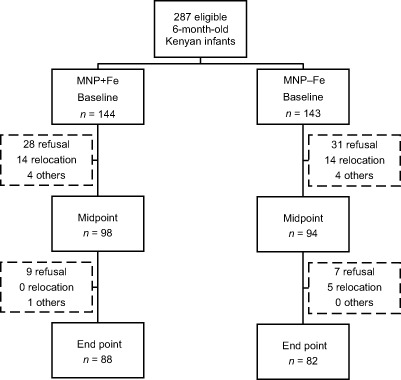
Trial profile with group allocation and drop outs in the two trial arms over the 12‐months intervention. Others: missed drugs, official withdrawal, one death in MNP + Fe before midpoint that was not associated with the treatment (included in ‘others’). MNP + Fe, micronutrient powder with iron; MNP − Fe, micronutrient powder without iron.
Biochemical indicators
At baseline, after 6 and 12 months, venous blood samples (3 mL) were drawn by the study nurse using heparin vacutainers and butterfly needles. Hb was measured using a HemoCue (HemoCue AB, Ängelholm, Sweden) or a HemoControl device (EKF Diagnostics Sales GmbH, Barleben/Magdeburg, Germany). Serum was separated by centrifugation on collection day. The remaining erythrocytes were washed three times with normal saline, and the ZPP to haem ratio was measured using a calibrated AVIV hematofluorometer (AVIV Biomedical, Lakewood, CA, USA). SF, sTfR and CRP were measured at Lancet Laboratories in Nairobi by using the Cobas Integra (Roche, Basel, Switzerland). We converted the Roche sTfR concentration to the Flowers assay (Flowers et al. 1989) using the regression equation by Pfeiffer (Flowers = 1.5 * Roche + 0.35) (Pfeiffer et al. 2007).
Body iron stores (mg kg−1 body weight) were calculated from the ratio of sTfR to SF according to the equation by Cook et al. (2003) (body iron (mg/kg) = −[log10 (sTfR * 1000/SF) −2.8229)]/0.1207) and total body iron stores (mg) by multiplying with the body weight. The following cut‐offs were used: (1) anaemia: Hb < 110 g L−1 (WHO 2001); (2) ID: body iron stores < 0 mg kg−1; and (3) inflammation: CRP ≥ 4.1 mg L−1 (manufacturer's reference range).
Ethics
This study was approved by the ethics and research committees of the Kenyatta National Hospital/University of Nairobi (P167/6/2009), the University of KwaZulu‐Natal (BF121/08) and the Swiss Federal Institute of Technology Zurich (EK 2009‐N‐53). Caregivers signed an informed consent. This study is registered at http://clinicaltrials.gov as NCT01111864.
Statistical analysis
Data were analysed using IBM SPSS Statistics 20.0.0 (SPSS Inc., Chicago, IL, USA) and Microsoft Office EXCEL 2010 (Microsoft, Redmond, WA, USA). Data were double entered and its distribution checked for normality (Kolmogorov–Smirnov test); not normally distributed data were log transformed. We obtained geometric means and corresponding standard deviations or standard errors of the mean for absolute concentrations by taking the antilog of these values. We calculated an adjusted SF variable, if CRP ≥ 4.1, by dividing the SF by a factor of 1.59. This factor derived from the median of all SF if CRP ≥ 4.1 (31.7 μg L−1) divided by the median of all SF if CRP < 4.1 (19.9 μg L−1). Weight‐for‐age (WAZ), height‐for‐age (HAZ) and weight‐for‐height (WHZ) were calculated by using the WHO Anthro software (version 3.2.2) and standards. The definition for stunting was a HAZ < −2, for underweight WAZ < −2, and for wasting a WHZ < −2 (WHO 2007).
Baseline, mid‐ and end point differences between the treatment groups were assessed using independent samples t‐test for continuous variables and Pearson's chi‐square test for ID and IDA prevalence. Estimated intervention effects on iron status (SF, sTfR, ZPP and SF adjusted for CRP ≥ 4.1), Hb and CRP were assessed using general linear models (GLMs), baseline values were used as covariates, and a P‐value of < 0.05 was considered significant. A subgroup analysis according to baseline iron stores was done to assess the intervention effects in iron‐deficient (body iron stores < 0) and iron‐replete (body iron stores ≥ 0) infants separately.
The effect of the treatment on the total days with diarrhoea, cough, flu, bloody or mucus‐containing stool and dyspnoea was compared using Poisson regression. The effect of the treatment on diarrhoea and malaria incidence was assessed using Pearson's chi‐square test.
Results
Study participants
At baseline, the overall prevalence of anaemia was 70.7%; 21.8% of infants were iron deficient; and 31.0% had an elevated CRP (Table 2). The prevalence of underweight was 7.8%, while 13.5% of infants were stunted. Mothers indicated that 62.1% of the infants were born at home and 26.2% in hospital; nine infants were born preterm (3.2%) and four were delivered by Caesarian section (1.4%). Nearly all infants were still being breastfed (98.2%, n = 277) but 90.1% (n = 254) had already been introduced to complementary foods, predominantly sweetened maize gruel (‘uji’), starting on average at 4 months of age.
Table 2.
Baseline characteristics by intervention group of per protocol analysis
| MNP + Fe | MNP − Fe | Both | |
|---|---|---|---|
| n | 144 | 143 | 287 |
| Age (months) | 6.0 ± 1.1 | 6.0 ± 1.1 | 6.0 ± 1.1 |
| Sex (male) | 74 (51.4%) | 72 (50.3%) | 146 (50.9%) |
| Haemoglobin (g L–1) | 104.1 ± 10.5 | 102.1 ± 10.6 | 103.0 ± 10.5 |
| Zinc protoporphyrin (μmol mol–1 haem) | 85.1 ± 1.6* | 103.8 ± 1.8 | 94.1 ± 1.7 |
| Serum ferritin (ng mL–1) | |||
| All children | 34.1 ± 1.8 | 27.8 ± 2.0 | 30.9 ± 1.9 |
| Children without inflammation † | 30.5 ± 1.7 | 25.2 ± 2.1 | 27.8 ± 1.9 |
| Soluble transferrin receptor (mg L–1) | 8.6 ± 1.2* | 9.6 ± 1.2 | 9.1 ± 1.2 |
| C‐reactive protein (mg L–1) | 4.9 ± 1.7 | 3.7 ± 1.4 | 4.3 ± 1.6 |
| Inflammation † | 45 (31.2%) | 44 (30.8%) | 89 (31.0%) |
| Anaemia ‡ | 99 (68.8%) | 104 (72.7%) | 203 (70.7%) |
| ID § | 18 (12.8%)** | 43 (30.9%) | 61 (21.8%) |
| IDA ¶ | 15 (10.6%)** | 38 (27.3%) | 53 (18.9%) |
| Weight baseline (kg) | 7.3 ± 1.1 | 7.3 ± 1.1 | 7.3 ± 1.1 |
| Length baseline (cm) | 64.8 ± 1.0 | 64.1 ± 1.1 | 64.4 ± 1.1 |
| Birthweight (kg) | 3.2 ± 1.0 | 3.1 ± 1.0 | 3.2 ± 1.0 |
| Body iron stores (mg kg–1) | 3.26 ± 1.0* | 1.86 ± 1.0 | 2.57 ± 1.0 |
Data are geometric means ± SD or number (%) unless indicated otherwise. ID, iron deficiency; IDA, iron deficiency anaemia; MNP + Fe, micronutrient powder with iron; MNP − Fe, micronutrient powder without iron. *Different from control (MNP − Fe), P < 0.05 using t‐test. **Different from control (MNP − Fe), P < 0.05 using Pearson's chi‐square test. †CRP ≥ 4.1 mg L–1. ‡Hb <110 g L–1. §Body iron stores (mg kg–1) < 0. ¶Concurrent ID and anaemia.
Although randomisation was checked by comparing baseline Hb, weight and length, which did not differ between groups, there were significant differences in sTfR (P = 0.004), ZPP (P = 0.005, Table 2), body iron stores (P = 0.004), and the prevalence of ID (P = 0.0003) and IDA (P = 0.002) between the two treatment groups at baseline.
In total, 170 (MNP + Fe = 88, MNP − Fe = 82) children completed the intervention (Fig. 1). The main reasons for discontinuing the study were refusal and relocation. The overall compliance to the MNP, assessed through collection of full and empty sachets, of all participants in the study was 99.4% (99.3% in MNP + Fe and 99.6% in MNP − Fe). The most frequent reason for a child not to consume the sachet was absence/travel or sickness.
Iron status and inflammation
There was no effect of iron on any of the iron status or inflammation markers adjusted for baseline differences, except for a decrease in SF (P = 0.0001) and CRP (P = 0.012) and an increase in sTfR (P = 0.001) in the MNP + Fe group at midpoint (Table 3). Adjusting the SF values for the elevated CRP values did not change this effect, which remained significant (P = 0.003). Anaemia prevalence decreased during the intervention in both treatment groups (MNP + Fe and MNP − Fe) from 68.8% and 72.7% at baseline, to 67.4% and 61.9% after 6 months, and 55.8% and 41.4% after 12 months, respectively. The prevalence of IDA (negative body iron stores and Hb < 110 g L−1) differed significantly at baseline 10.4% in the MNP + Fe and 26.6% in the MNP − Fe (P = 0.001), and was not different at midpoint 32.1% and 18.5% (P = 0.130), and differed again at end point 32.9% and 12.9% (P = 0.035), in MNP + Fe and MNP − Fe, respectively, using Pearson's chi‐square tests.
Table 3.
Changes in iron status and inflammation and weight during the 12‐month intervention
| Gender | Time point (month) | Hb (g L–1) | ZPP (μmol mol–1 haem) | sTfR (mg L–1) | SF (μg L–1) | SF adjusted (μg L–1) † | CRP (mg L–1) | Weight (kg) | |
|---|---|---|---|---|---|---|---|---|---|
| MNP + Fe | Male (n = 74) | Baseline (0) | 104.0 ± 10.5 | 92.7 ± 1.6** | 9.0 ± 1.2** | 29.8 ± 1.8** | 26.3 ± 1.8** | 4.5 ± 1.8 | 7.6 ± 1.1 |
| Midpoint (6) | 106.0 ± 10.5 | 106.3 ± 1.7 | 10.0 ± 1.1 | 18.8 ± 1.6 | 17.7 ± 1.6 | 2.4 ± 1.3 | 9.0 ± 1.1 | ||
| End point (12) | 108.9 ± 10.5 | 81.2 ± 1.8 | 8.9 ± 1.2 | 23.0 ± 1.9 | 20.9 ± 1.8 | 2.9 ± 1.6 | 10.0 ± 1.1 | ||
| Female (n = 70) | Baseline (0) | 104.2 ± 10.5 | 77.8 ± 1.5 | 8.3 ± 1.1 | 39.2 ± 1.8 | 33.6 ± 1.7 | 5.3 ± 1.7 | 7.1 ± 1.1 | |
| Midpoint (6) | 103.0 ± 10.5* , ** | 97.7 ± 1.7* | 10.1 ± 1.3* , ** | 16.1 ± 1.5* , ** | 14.3 ± 1.4* , ** | 3.4 ± 1.7 | 8.8 ± 1.1 | ||
| End point (12) | 104.8 ± 10.8* , ** | 90.0 ± 1.6* , ** | 9.1 ± 1.2* | 16.1 ± 1.5* , ** | 13.6 ± 1.3* , ** | 2.9 ± 1.4 | 9.9 ± 1.1 | ||
| Both (n = 144) | Baseline (0) | 104.1 ± 10.5 | 85.1 ± 1.6** | 8.6 ± 1.2** | 34.1 ± 1.8 | 29.7 ± 1.8 | 4.9 ± 1.7 | 7.3 ± 1.1 | |
| Midpoint (6) | 104.6 ± 10.5 | 102.2 ± 1.7 | 10.0 ± 1.2* | 17.5 ± 1.6* , ** | 16.0 ± 1.5* , ** | 2.9 ± 1.5* | 8.9 ± 1.1 | ||
| End point (12) | 107.1 ± 10.6 | 85.0 ± 1.7 | 9.0 ± 1.2 | 19.7 ± 1.7 | 17.4 ± 1.6 | 2.9 ± 1.5 | 9.9 ± 1.1** | ||
| MNP − Fe | Male (n = 72) | Baseline (0) | 101.2 ± 10.6 | 118.7 ± 1.8 | 10.6 ± 1.2 | 22.1 ± 1.8 | 19.0 ± 1.8 | 3.9 ± 1.5 | 7.7 ± 1.1 |
| Midpoint (6) | 103.3 ± 10.6 | 120.1 ± 1.7 | 10.4 ± 1.2 | 21.9 ± 1.7 | 18.9 ± 1.6 | 3.5 ± 1.4 | 9.3 ± 1.1 | ||
| End point (12) | 105.7 ± 10.8 | 88.2 ± 1.8 | 10.5 ± 1.3 | 21.2 ± 1.7 | 18.9 ± 1.6 | 2.3 ± 1.3 | 9.6 ± 1.1 | ||
| Female (n = 71) | Baseline (0) | 102.9 ± 10.6 | 90.5 ± 1.8 | 8.7 ± 1.2 | 34.8 ± 2.2 | 30.5 ± 2.2 | 3.6 ± 1.4 | 7.0 ± 1.1 | |
| Midpoint (6) | 109.6 ± 10.5 | 86.3 ± 1.7 | 8.2 ± 1.1 | 26.7 ± 1.6 | 22.5 ± 1.6 | 5.4 ± 1.7 | 8.5 ± 1.1 | ||
| End point (12) | 113.8 ± 10.5 | 96.8 ± 1.6 | 8.2 ± 1.1 | 26.1 ± 1.5 | 23.6 ± 1.5 | 2.8 ± 1.4 | 9.3 ± 1.1 | ||
| Both (n = 143) | Baseline (0) | 102.1 ± 10.6 | 103.8 ± 1.8 | 9.6 ± 1.2 | 27.8 ± 2.0 | 24.2 ± 2.0 | 3.7 ± 1.4 | 7.3 ± 1.1 | |
| Midpoint (6) | 106.8 ± 10.6 | 99.9 ± 1.7 | 9.1 ± 1.2 | 24.5 ± 1.6 | 20.8 ± 1.6 | 4.5 ± 1.5 | 8.8 ± 1.1 | ||
| End point (12) | 110.3 ± 10.7 | 77.2 ± 1.7 | 9.2 ± 1.2 | 24.0 ± 1.6 | 21.5 ± 1.5 | 2.6 ± 1.3 | 9.5 ± 1.1 |
Data are geometric means ± SD. CRP, C‐reactive protein; Hb, haemoglobin; MNP + Fe, micronutrient powder with iron; MNP − Fe, micronutrient powder without iron; SF, serum ferritin; sTfR, soluble transferrin receptor; ZPP, zinc protoporphyrin. *Different from control (MNP – Fe), P < 0.05 using univariate GLM with baseline variable as covariate. **Different from control (MNP − Fe), P < 0.05 using t‐test. †SF was adjusted with a factor of 1.59 if CRP ≥ 4.1.
In the gender‐specific analysis, boys' iron status and inflammation did not differ between groups (SF, P = 0.068 and CRP, P = 0.089), while girls of the MNP + Fe had lower SF along with a tendency for lower CRP at midpoint compared with those in the MNP − Fe group (SF midpoint: P = 0.0001 and end point: P = 0.0005; CRP midpoint: P = 0.068 and end point: P = 0.603). This effect was slightly less distinct but still significant in the SF adjusted for and elevated CRP (midpoint: P = 0.003, end point: P = 0.001). Furthermore, in girls consuming MNP + Fe we identified higher levels of ZPP (midpoint: P = 0.033, end point: P = 0.022) and sTfR (midpoint: P = 0.002, end point: P = 0.048) and lower levels of Hb (midpoint: P = 0.001, end point: P = 0.003, Table 3) compared with girls consuming MNP − Fe.
Baseline body iron stores as a subgroup analysis criterion
Infants with negative iron stores at baseline increased them (Fig. 2A) irrespective of the treatment, whereas infants with positive iron stores at baseline appeared to stabilise them at about 1–1.6 mg Fe/kg body weight (Fig. 2B). Figure 2C shows the changes in body iron stores in all infants.
Figure 2.

Body iron stores ± SD (mg kg–1, geometric means) over the 12‐month intervention in (A) in infants with negative iron stores at baseline [MNP + Fe (n = 18) and MNP − Fe (n = 43)]; (B) in infants with positive iron stores at baseline [MNP + Fe (n = 123) and MNP − Fe (n = 96)]; and (C) all infants [MNP + Fe (n = 141) and MNP − Fe (n = 139)]. The differences between the groups from baseline to midpoint were significant in the graphs B (P = 0.000034) and C (P = 0.0001) using GLM, but not from baseline to end point. MNP + Fe, micronutrient powder with iron; MNP − Fe, micronutrient powder without iron.
Growth
There was no significant effect of iron on weight using GLM statistics (P = 0.185). However, after the 1‐year intervention, infants in the MNP + Fe group were significantly heavier (9.9 kg vs. 9.5 kg, P = 0.038, using independent samples t‐test, Table 3). The gender‐specific analysis showed: girls in the MNP + Fe were borderline significant heavier than in the MNP − Fe (9.9 kg vs. 9.3 kg, P = 0.067), but not boys (10.0 kg vs. 9.6 kg, P = 0.324). The prevalence of underweight did not differ between groups and was 9.2% and 6.4% at baseline, 6.2% and 7.8% at midpoint, and 14.9% and 21.2% at end point in the MNP + Fe and MNP − Fe, respectively. Furthermore, changes in WHZ and HAZ were not different between the two groups. The height did not differ between the treatment groups.
Morbidity
During the 1‐year intervention, mothers from infants in the MNP + Fe group reported more days spent with cough (7.4 vs. 6.4; P = 0.003) and dyspnoea (2.2 vs. 1.5; P = 0.0002). Days spent with diarrhoea, flu, bloody or mucus‐containing stool, and any other illness did not differ between the two treatment groups. On average, mothers reported 2 days with diarrhoea, 7 days cough, 8 days flu, 1 day bloody or mucus‐containing stool, and 2 days dyspnoea over the 12‐month study period. Further, the incidence of treated malaria and diarrhoea did also not differ between the treatment groups. The incidence of treated malaria from baseline to midpoint was 5.4% and 7.0% in the MNP + Fe and the MNP − Fe, respectively, and increased from midpoint to end point to 13.8% (MNP + Fe) and 8.9% (MNP − Fe). The incidence of diarrhoea from baseline to midpoint was 13.5% and 14.1% in the MNP + Fe and the MNP − Fe, respectively, and 12.5% (MNP + Fe) and 15.2% (MNP − Fe) from midpoint to end point.
Discussion
In this study, in‐home fortification of maize porridge with micronutrients and 2.5 mg iron as NaFeEDTA daily for 1 year did not improve infant's iron status or reduce their anaemia prevalence compared to fortification with micronutrients only. Previous fortification studies using higher iron doses (11–12.5 mg day−1) as ferrous fumarate have reduced ID in infants in sub‐Saharan Africa (Faber et al. 2005; Adu‐Afarwuah et al. 2008). In older children (1–5 years of age), 2.5 mg NaFeEDTA daily for 4 months reduced prevalence of iron deficiency and anaemia (Macharia‐Mutie et al. 2012). There are several possible explanations why the lower dose of iron used in this study did not improve infants' iron status.
First, although iron bioavailability from NaFeEDTA in inhibitory meals is high, the iron dose may have been too low. The phytic acid to iron molar ratio of the plain porridge was 22.4:1; fortification reduced this ratio to 0.9:1. Former absorption studies using similar meals in adults estimated an absorption of NaFeEDTA of 4.4% (Troesch et al. 2009) and 5.2% (Hurrell et al. 2000) for a SF of 40 and 39 ng mL−1; extrapolating this to our mean SF of 31 ng mL−1 at baseline (Cook et al. 1991), we anticipated an absorption of ≈5–6%. This means 0.14–0.16 mg of the provided iron was expected to be absorbed, covering ≈20% and ≈30% of the daily requirement of 6‐ to 12‐ (0.72 mg) and 12‐ to 18‐ (0.46 mg) month‐old children, respectively (WHO 2001). We recently reported that a MNP containing 12.5 mg iron as ferrous fumarate improved iron status in infants from this same catchment area in southern Kenya (Jaeggi et al. 2014). In a study by Macharia‐Mutie et al. (2012), the same MNP (2.5 mg iron as NaFeEDTA) daily for 4 months reduced IDA in Kenyan 1–5 year old children. These contradicting results may be due to the overall higher food intake in older children, which, combined with low‐dose fortification, may allow to reach sufficient bioavailable iron intake at similar estimated daily requirements (WHO 2001).
The regulations of JECFA (Joint FAO/WHO Expert Committee on Food Additives) limit the use of EDTA to 1.9 mg kg–1 body weight. The rationale for this restriction is the estimated absorption of 5% of the EDTA which can negatively affect the metabolism of minerals by increased urinary excretion (Candela et al. 1984; Hurrell et al. 1994). Therefore, in our study population with an average baseline body weight of 7.3 kg, we could not add more than 2.5 mg iron as NaFeEDTA (containing 13 mg EDTA). In young Beninese children, iron absorption from a lipid‐based complementary food mixed with millet porridge and fortified with a mixture of NaFeEDTA and FeSO4 was significantly lower than from meals fortified with FeSO4 only (Cercamondi et al. 2013). The authors explained this rather surprising outcome by the possibility that the ascorbic acid provided enhanced absorption of FeSO4 but not NaFeEDTA. Furthermore, EDTA has been shown to reduce the absorption of native iron in the diet (Cook & Monsen 1976). However, since the complementary food in this study did not provide a considerable amount of iron this effect may be negligible.
Second, inflammation is a major determinant for hepcidin expression and therefore iron absorption (Nemeth & Ganz 2009). In our study population, malaria and other infectious diseases were common and one‐third of the infants had an elevated CRP at baseline. In contrast to this, in Macharia‐Muties's study, the prevalence of inflammation was ≈10 times lower in the iron group (3.4%) and significantly elevated in the control group (16.5%) at baseline (Macharia‐Mutie et al. 2012). A cross‐sectional analysis of our infant population indicated that inflammation increases hepcidin concentration even in iron‐deficient subjects (Jaeggi et al. 2013). However, a subgroup analysis in infants without elevated CRP did not show an effect of iron treatment on iron status (data not shown).
Third, despite randomisation and equivalent Hb and anthropometrics at baseline, prevalence of ID and IDA was lower in the iron‐fortified group than in the control group. Although we attempted to correct for these effects using baseline values as covariates, these may have resulted in smaller treatment effects, as iron status is a major determinant of dietary iron absorption. Further, it may have caused regression towards the mean (Bland & Altman 1994a, 1994b), where values being significantly different at baseline (such as iron status) may become more similar over time.
A recent study in Pakistani infants reported a small but significant positive effect of iron‐containing MNP on length gain (Soofi et al. 2013). Former meta‐analyses have shown an effect of micronutrients (Ramakrishnan et al. 2004), but not of iron alone on child growth (Ramakrishnan et al. 2004; Sachdev et al. 2006). However, in one of these meta‐analyses, a trend was suggested for an increase in weight through iron supplementation in children from malarial endemic areas (Sachdev et al. 2006). In a study in South African school children, maize porridge fortified with micronutrients including phytase and 2.5 mg NaFeEDTA daily for 5 months showed a small treatment effect on WAZ along with an increase in body iron stores compared with unfortified porridge (Troesch et al. 2011). In the current study, infants consuming iron‐fortified maize porridge weighed significantly more at the end of the 1‐year intervention (0.4 kg). There are several potential explanations for this higher weight in the MNP + Fe group at end point. Differences in infant feeding practices between the two groups could have had an effect, though the trial was randomised, and we have no evidence that there were feeding differences between groups. Another possibility might be that EDTA from the NaFeEDTA increased the bioavailability of the zinc from the MNP (as zinc oxide) and/or from the maize (Hettiarachchi et al. 2004), improving zinc status and contributing to increased weight gain (Brown et al. 2009). However, we did not measure serum zinc concentration in this study. Furthermore, iron has shown to increase the population of short‐chain fatty acids (SCFAs) producing bacteria in rats (Dostal et al. 2012). These SCFAs can be a significant energy source for the host, contributing up to 10% of the daily caloric requirements of humans (McNeil 1984).
In this study, we found a small but significant increase in the reported days spent with cough and dyspnoea (+1 and 0.7 days, respectively) in infants consuming the MNP with iron compared to control. Days spent with diarrhoea, blood or mucus in the stool, and flu, as well as malaria and diarrhoea incidence over the first and second half of the study did not differ between groups. This effect on respiratory morbidity is similar to a recent fortification trial in Pakistani infants (Soofi et al. 2013) where use of a higher iron dose (12.5 mg iron daily) in a MNP increased chest indrawing. However, the current study was not powered to detect effects of treatment on morbidity outcomes; these were rather assessed for ethical and safety reasons.
We conclude that in‐home fortification with a MNP containing 2.5 mg iron as NaFeEDTA did not improve iron status in infants in an area with high burden of infectious disease, likely because of the combination of low dose and high prevalence of inflammation. However, infants consuming maize porridge fortified with MNP + Fe had a higher weight after the 1‐year trial compared with infants consuming maize porridge fortified with MNP − Fe. Future large‐scale studies are needed to confirm these results in infants from rural African settings.
Source of funding
This project was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (Award Number U01HD064921), and realised in collaboration with the INSTAPA Project, funded by the European Union's Seventh Framework Programme (Fp7/2007–2013) under Grant Agreement No. 211484.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
TBJ, DM, JK, PAH, JN, AM, MKC, CL and MBZ designed the study. TBJ, JK, PAH, JN and MKC collected the data. TBJ, DM and MBZ performed the statistical analysis. TBJ, DM and MBZ wrote the paper. All authors read and approved the final manuscript.
Acknowledgements
We thank the families who participated in this study, the project staff for their hard work and dedication, and Gary M. Brittenham and Luciano Molinari for their valuable input during data analysis and interpretation.
Barth‐Jaeggi, T. , Moretti, D. , Kvalsvig, J. , Holding, P. A. , Njenga, J. , Mwangi, A. , Chhagan, M. K. , Lacroix, C. , and Zimmermann, M. B. (2015) In‐home fortification with 2.5 mg iron as NaFeEDTA does not reduce anaemia but increases weight gain: a randomised controlled trial in Kenyan infants. Matern Child Nutr, 11: 151–162. doi: 10.1111/mcn.12163.
References
- Adu‐Afarwuah S., Lartey A., Brown K.H., Zlotkin S., Briend A. & Dewey K.G. (2008) Home fortification of complementary foods with micronutrient supplements is well accepted and has positive effects on infant iron status in Ghana. The American Journal of Clinical Nutrition 87, 929–938. [DOI] [PubMed] [Google Scholar]
- Andang'o P.E., Osendarp S.J., Ayah R., West C.E., Mwaniki D.L., De Wolf C.A. et al (2007) Efficacy of iron‐fortified whole maize flour on iron status of schoolchildren in Kenya: a randomised controlled trial. Lancet 369, 1799–1806. [DOI] [PubMed] [Google Scholar]
- Bland J.M. & Altman D.G. (1994a) Regression towards the mean. BMJ (Clinical Research Ed.) 308, 1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland J.M. & Altman D.G. (1994b) Some examples of regression towards the mean. BMJ (Clinical Research Ed.) 309, 780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K.H., Peerson J.M., Baker S.K. & Hess S.Y. (2009) Preventive zinc supplementation among infants, preschoolers, and older prepubertal children. Food and Nutrition Bulletin 30, S12–S40. [DOI] [PubMed] [Google Scholar]
- Candela E., Camacho M.V., Martinez‐Torres C., Perdomo J., Mazzarri G., Acurero G. et al (1984) Iron absorption by humans and swine from Fe(III)‐EDTA. Further studies. The Journal of Nutrition 114, 2204–2211. [DOI] [PubMed] [Google Scholar]
- Cercamondi C.I., Egli I.M., Mitchikpe E., Tossou F., Hessou J., Zeder C. et al (2013) Iron bioavailability from a lipid‐based complementary food fortificant mixed with millet porridge can be optimized by adding phytase and ascorbic acid but not by using a mixture of ferrous sulfate and sodium iron EDTA. The Journal of Nutrition 143, 1233–1239. [DOI] [PubMed] [Google Scholar]
- Cook J.D. & Monsen E.R. (1976) Food iron absorption in man II. The effect of EDTA on absorption of dietary non‐heme iron. The American Journal of Clinical Nutrition 29, 614–620. [DOI] [PubMed] [Google Scholar]
- Cook J.D., Dassenko S.A. & Lynch S.R. (1991) Assessment of the role of nonheme‐iron availability in iron balance. The American Journal of Clinical Nutrition 54, 717–722. [DOI] [PubMed] [Google Scholar]
- Cook J.D., Flowers C.H. & Skikne B.S. (2003) The quantitative assessment of body iron. Blood 101, 3359–3364. [DOI] [PubMed] [Google Scholar]
- Dewey K.G., Domellof M., Cohen R.J., Landa Rivera L., Hernell O. & Lonnerdal B. (2002) Iron supplementation affects growth and morbidity of breast‐fed infants: results of a randomized trial in Sweden and Honduras. The Journal of Nutrition 132, 3249–3255. [DOI] [PubMed] [Google Scholar]
- Dostal A., Chassard C., Hilty F.M., Zimmermann M.B., Jaeggi T., Rossi S. et al (2012) Iron depletion and repletion with ferrous sulfate or electrolytic iron modifies the composition and metabolic activity of the gut microbiota in rats. The Journal of Nutrition 142, 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube K., Schwartz J., Mueller M.J., Kalhoff H. & Kersting M. (2010) Iron intake and iron status in breastfed infants during the first year of life. Clinical Nutrition (Edinburgh, Scotland) 29, 773–778. [DOI] [PubMed] [Google Scholar]
- Faber M., Kvalsvig J.D., Lombard C.J. & Benade A.J. (2005) Effect of a fortified maize‐meal porridge on anemia, micronutrient status, and motor development of infants. The American Journal of Clinical Nutrition 82, 1032–1039. [DOI] [PubMed] [Google Scholar]
- Flowers C.H., Skikne B.S., Covell A.M. & Cook J.D. (1989) The clinical measurement of serum transferrin receptor. The Journal of Laboratory and Clinical Medicine 114, 368–377. [PubMed] [Google Scholar]
- Fontaine O. (2007) Conclusions and recommendations of the WHO Consultation on prevention and control of iron deficiency in infants and young children in malaria‐endemic areas. Food and Nutrition Bulletin 28, S621–S627. [DOI] [PubMed] [Google Scholar]
- Gera T. & Sachdev H.P. (2002) Effect of iron supplementation on incidence of infectious illness in children: systematic review. BMJ (Clinical Research Ed.) 325, 1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hettiarachchi M., Hilmers D.C., Liyanage C. & Abrams S.A. (2004) Na2EDTA enhances the absorption of iron and zinc from fortified rice flour in Sri Lankan children. The Journal of Nutrition 134, 3031–3036. [DOI] [PubMed] [Google Scholar]
- Hurrell R.F., Ribas S. & Davidsson L. (1994) NaFe3 + EDTA as a food fortificant: influence on zinc, calcium and copper metabolism in the rat. The British Journal of Nutrition 71, 85–93. [DOI] [PubMed] [Google Scholar]
- Hurrell R.F., Reddy M.B., Burri J. & Cook J.D. (2000) An evaluation of EDTA compounds for iron fortification of cereal‐based foods. The British Journal of Nutrition 84, 903–910. [PubMed] [Google Scholar]
- Jaeggi T., Moretti D., Kvalsvig J., Holding P.A., Tjalsma H., Kortman G.A. et al (2013) Iron status and systemic inflammation, but not gut inflammation, strongly predict gender‐specific concentrations of serum hepcidin in infants in rural Kenya. PLoS ONE 8, e57513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi T., Kortman G.A., Moretti D., Chassard C., Holding P., Dostal A. et al (2014) Iron fortification adversely affects the gut microbiome, increases pathogen abundance and induces intestinal inflammation in Kenyan infants. Gut [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Lind T., Seswandhana R., Persson L.A. & Lonnerdal B. (2008) Iron supplementation of iron‐replete Indonesian infants is associated with reduced weight‐for‐age. Acta Paediatrica 97, 770–775. [DOI] [PubMed] [Google Scholar]
- Macharia‐Mutie C.W., Moretti D., Van den Briel N., Omusundi A.M., Mwangi A.M., Kok F.J. et al (2012) Maize porridge enriched with a micronutrient powder containing low‐dose iron as NaFeEDTA but not amaranth grain flour reduces anemia and iron deficiency in Kenyan preschool children. The Journal of Nutrition 142, 1756–1763. [DOI] [PubMed] [Google Scholar]
- McNeil N.I. (1984) The contribution of the large intestine to energy supplies in man. The American Journal of Clinical Nutrition 39, 338–342. [DOI] [PubMed] [Google Scholar]
- Meilgaard M.C., Carr B.T. & Civille G.V. (2007) Sensory Evaluation Techniques, 4th edn, Taylor Francis Group: Boca Raton, FL. [Google Scholar]
- Nemeth E. & Ganz T. (2009) The role of hepcidin in iron metabolism. Acta Haematologica 122, 78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oppenheimer S.J. (2001) Iron and its relation to immunity and infectious disease. The Journal of Nutrition 131, 616S–633S. [DOI] [PubMed] [Google Scholar]
- Pfeiffer C.M., Cook J.D., Mei Z., Cogswell M.E., Looker A.C. & Lacher D.A. (2007) Evaluation of an automated soluble transferrin receptor (sTfR) assay on the Roche Hitachi analyzer and its comparison to two ELISA assays. Clinica Chimica Acta 382, 112–116. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan U., Aburto N., McCabe G. & Martorell R. (2004) Multimicronutrient interventions but not vitamin a or iron interventions alone improve child growth: results of 3 meta‐analyses. The Journal of Nutrition 134, 2592–2602. [DOI] [PubMed] [Google Scholar]
- Sachdev H., Gera T. & Nestel P. (2005) Effect of iron supplementation on mental and motor development in children: systematic review of randomised controlled trials. Public Health Nutrition 8, 117–132. [DOI] [PubMed] [Google Scholar]
- Sachdev H., Gera T. & Nestel P. (2006) Effect of iron supplementation on physical growth in children: systematic review of randomised controlled trials. Public Health Nutrition 9, 904–920. [DOI] [PubMed] [Google Scholar]
- Sazawal S., Black R.E., Ramsan M., Chwaya H.M., Stoltzfus R.J., Dutta A. et al (2006) Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community‐based, randomised, placebo‐controlled trial. Lancet 367, 133–143. [DOI] [PubMed] [Google Scholar]
- Soofi S., Cousens S., Iqbal S.P., Akhund T., Khan J., Ahmed I. et al (2013) Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster‐randomised trial. Lancet 382, 29–40. [DOI] [PubMed] [Google Scholar]
- Stoltzfus R. (1998) Guidelines for the use of iron supplements to prevent and treat iron deficiency anemia.
- Troesch B., Egli I., Zeder C., Hurrell R.F., de Pee S. & Zimmermann M.B. (2009) Optimization of a phytase‐containing micronutrient powder with low amounts of highly bioavailable iron for in‐home fortification of complementary foods. The American Journal of Clinical Nutrition 89, 539–544. [DOI] [PubMed] [Google Scholar]
- Troesch B., van Stuijvenberg M.E., Smuts C.M., Kruger H.S., Biebinger R., Hurrell R.F. et al (2011) A micronutrient powder with low doses of highly absorbable iron and zinc reduces iron and zinc deficiency and improves weight‐for‐age Z‐scores in South African children. The Journal of Nutrition 141, 237–242. [DOI] [PubMed] [Google Scholar]
- WHO (2001) Iron Deficiency and Anemia: Assessment Prevention and Control. A Guide for Programme Managers. World Health Organization: Geneva, Switzerland. [Google Scholar]
- WHO (2007) Anthro for Personal Computers. Version 2. Software for Assessing Growth and Development of the World's Children, World Health Organization: Geneva: Available at: http://www.who.int/childgrowth/software/en [Google Scholar]
- Zimmermann M.B. & Hurrell R.F. (2007) Nutritional iron deficiency. Lancet 370, 511–520. [DOI] [PubMed] [Google Scholar]
- Zlotkin S., Arthur P., Schauer C., Antwi K.Y., Yeung G. & Piekarz A. (2003) Home‐fortification with iron and zinc sprinkles or iron sprinkles alone successfully treats anemia in infants and young children. The Journal of Nutrition 133, 1075–1080. [DOI] [PubMed] [Google Scholar]
- Zlotkin S., Newton S., Aimone A.M., Azindow I., Amenga‐Etego S., Tchum K. et al (2013) Effect of iron fortification on malaria incidence in infants and young children in Ghana: a randomized trial. JAMA: The Journal of the American Medical Association 310, 938–947. [DOI] [PubMed] [Google Scholar]


