Abstract
The effects of prophylactic iron during pregnancy on maternal and child health in developing settings with endemic malaria and high prevalence of HIV remain unclear. This paper describes the rationale, implementation and success of a pragmatic randomised controlled trial comparing routine iron supplementation vs. screening and treatment for anaemia during pregnancy. The setting was two health centres in Maputo, Mozambique. Pregnant women (≥12‐week gestation; ≥18 years old; and not with a high‐risk pregnancy, n = 4326) were recruited. The main outcomes are preterm delivery and low birthweight. The women were randomly assigned to one of two iron administration policies: a routine iron group (n = 2184) received 60 mg of ferrous sulphate plus 400 μg of folic acid daily while a selective iron group (n = 2142) had screening and treatment for anaemia and a daily intake of 1 mg of folic acid. The recruitment, follow‐up, and collection of follow‐up data were successful; both groups were similar to each other in all the trial stages. Collection of delivery data was challenging and data on about 40% of births is missing. These are currently being traced through different hospitals and health centres. The compliance of the study personnel and the women with regard to regular measurement of haemoglobin and intake of the iron and folic acid tablets was high and similar in both trial arms. Taking into account the various constraints encountered, the stages of the present trial prior to delivery were carried out well.
Keywords: iron, clinical trials, micronutrients, pregnancy, pregnancy outcomes, birth outcomes, developing countries, malaria, infections, HIV
Introduction
Iron deficiency remains a major public health concern in most developing countries, particularly among pregnant women and children (Stoltzfus 2001; McLean et al. 2009). Iron‐deficiency anaemia in pregnancy is associated with maternal and child health risks (Brabin et al. 2001; Stoltzfus et al. 2004). On the other hand, high haemoglobin concentrations in late pregnancy also correlate with adverse effects on pregnancy (Lao et al. 2000; Yip 2000). Because of poor intake and low bioavailability of iron from many foods, iron supplementation has been widely recommended during pregnancy (Müngen 2003; Peña‐Rosas & Viteri 2009). Studies show that prophylactic iron supplementation reduces the risk of anaemia (Yip 2000). But beyond the reduction of anaemia risk, the effects of routine iron prophylaxis on maternal and child health in developing settings remain unclear. Cochrane reviews and the World Health Organization (WHO) overviews of randomised controlled trials (RTCs) and a study in Finland comparing routine and selective iron prophylaxis (based on haemoglobin level) have failed to conclude whether routine iron prophylaxis during pregnancy is beneficial or harmful (Hemminki & Rimpelä 1991; Yip 2000; Villar et al. 2003).
The potential of iron to advance infections raises serious concerns for developing settings (Oppenheimer 2001; Gera & Sachdev 2002; Prentice 2008). Infectious agents need iron for replication (Prentice 2008). In sub‐Saharan African countries, a large proportion of maternal and child mortality is attributed to infections during pregnancy (Lawn et al. 2005; Idemyor 2007), malaria and HIV being the main health risks (Idemyor 2007). Malaria may modify the effects of iron therapy, as some evidence suggests differences in the metabolism of iron between malarial and non‐malarial subjects (Gera & Sachdev 2002; Prentice 2008). Some studies show that malaria may be less prevalent and milder among pregnant iron‐deficient women (Kabyemela et al. 2008). A review by Oppenheimer (2001) showed that five out of nine controlled trials among non‐pregnant subjects in malarial areas indicated that iron supplementation increased the rates of clinical episodes of malaria and increased morbidity from other infections. A Tanzanian trial (Sazawal et al. 2006) among young children in an endemic malaria area found that those who received iron plus folic acid were more likely to die or be treated in a hospital than those who received placebo. That study recommended treatment of children after screening rather than routine supplementation. On the contrary, a Cochrane review on iron supplementation for preventing or treating anaemia among children in malaria‐endemic areas concluded that iron does not increase the risk of clinical malaria when regular screening and treatment of malaria are provided (Ojukwu et al. 2009). We found no trial on the effect of iron on HIV infection, but some studies suggest a harmful effect of iron on the progression of HIV infections (Boelaert et al. 1996; Gordeuk et al. 2001).
The effects of iron on health outcomes in populations with concomitant endemic malaria, iron‐deficiency anaemia and on the prevalence of HIV are unexplored (Adetifa & Okomo 2009). There is an urgent need for trials to assess the effects on maternal and child health of prophylactic iron supplementation during pregnancy in populations where iron‐deficiency anaemia, endemic malaria and HIV are prevalent (Adetifa & Okomo 2009).
The aim of this paper was threefold: (1) to give the rationale for a pragmatic randomised trial (PROFEG) comparing the effects of two policies on iron administration during pregnancy (routine prophylactic iron supplementation vs. screening and treatment for anaemia) on maternal and newborn health in Maputo, Mozambique, a setting of endemic malaria and high prevalence of HIV; (2) to describe the study design; and (3) to describe the implementation and compliance.
Key messages
Beyond the reduction of the risk of anaemia, the effects of routine iron prophylaxis on maternal and child health in developing settings remain unclear.
The effects of iron on health outcomes in populations with concomitant endemic malaria, iron‐deficiency anaemia and high prevalence of HIV are unexplored.
We used a pragmatic randomised trial to compare the effects of two policies of iron administration during pregnancy (routine prophylactic iron supplementation vs. screening and treatment for anaemia) on maternal and newborn health in Maputo, Mozambique, a setting of endemic malaria and high prevalence of HIV.
Taking into account the various constraints encountered, the planned trial proved feasible in an ordinary health care setting in Maputo.
Previous trials on iron supplementation during pregnancy
A PubMed search of trials on the effects of iron supplementation during pregnancy and on birth outcomes in developing countries was carried out up to March 2011. Using a combination of key search terms (iron, iron + folic acid, micronutrients, pregnancy, pregnancy outcomes, birth outcomes, developing countries, malaria, infections, HIV), we extracted all relevant RCTs, quasi‐RCTs and other controlled clinical trials. The bibliographies of eligible papers were scrutinised to identify additional potential studies. The appendix shows the flow of the literature search process.
We only included studies that had iron or iron plus folic acid only or in combination with other micronutrients as the intervention and a placebo or alternative that has no iron in it as the control. Some studies examined the effects of micronutrient supplementation during pregnancy on pregnancy and birth outcomes in low‐income countries (Fall et al. 2009). We excluded these studies if iron was both in the intervention and the control groups.
The titles and abstracts of identified studies were checked, and the full text of all potentially eligible studies {except for one unpublished study that had only an extended conference abstract [Juncker et al. American Public Health Association (APHA), Atlanta, 2001] } was assessed. Table 1 presents the features and results from the trials included in this summary. The eligible studies were categorised according to the WHO classification (http://www.malaria.org/ABOUT%20MALARIA/Vaccination%20requirement%20and%20malaria%20situation%20WHO.pdf) into malarial and non‐malarial areas. The Bangladeshi trial [APHA, Atlanta, 2001 (T. Juncker et al., unpublished observations) ] was carried out in rural Dhaka, and we classified it into the malarial areas, considering that most parts of the country have malaria, although the city of Dhaka is classified as a non‐malarial area (WHO International Travel and Health 2011). We contacted (E‐mail correspondence) the authors of that study, but they were unsure of the malaria situation of the trial setting at the time of the study.
Table 1.
Previous trials investigating health outcomes of prophylactic iron supplementation during pregnancy, endemic malaria areas and non‐malarial areas, developing countries
| Author, year of publication (Reference number) | Country | Allocation | Intervention* (n) ‡‡‡ | Control † (n) ‡‡‡ | N ‡ | Start | Population | Results §¶ | Potential problems |
|---|---|---|---|---|---|---|---|---|---|
| Malarial area | |||||||||
| Fleming et al. 1986, ** | Nigeria | RCT | 60 mg iron; 60 mg + 1 mg folate (40) | Placebo; 600 mg chloroquine + 100 mg proguanil (42) | 200 | <24 weeks | 1st pregnancy | No significant effect of iron found. | Compliance was poor; high dropout rates, and exclusions by outcomes |
| Menendez et al. 1994, †† | The Gambia | RCT | 60 mg iron + 1 mg folate (273) | Placebo + 1 mg folate (277) | 757 | <34th week | More than 1 pregnancy | ▴BW | Outcome‐related exclusions 27% (e.g. preterm births, women developing anaemia) |
| Preziosi et al. 1997, ‡‡ | Niger | RCT | 100 mg iron (99) | Placebo (98) | 197 | Mean 28 week (SD 3 weeks) | Healthy women, 66% anemic | ▴BL; ▴AS | Small trial |
| Ndyomugyenyi & Magnussen 2000, §§ | Uganda | RCT | 120 mg iron + 5 mg folate (174) | Placebo (168) | 576 | <28 week | 1st pregnancy, Hb ≥80 g L−1 | ▴BW | Outcome‐related exclusions (women developing anaemia, late baby weighing); many lost to follow‐up |
| APHA, Atlanta, 2001 (T. Juncker et al., unpublished observations) ¶¶ | Bangladesh | Alternative | 66 mg Iron + 250 μg folate (772) | Vitamin B (812) | 2007 | ≥24 week | ≥9.0 g/dL Hb at recruitment | ▴PD; ▾BW | Only extended abstract available |
| Non‐malarial area | |||||||||
| Zeng et al. 2008, *** | China | Cluster RCT | 60 mg iron + 400 μg folate (1565) | 400 μg folate (1705) | 3929 | <28 week | Early pregnancy <28 weeks | ▴GA; ▾PD; ▾MA; ▴BL | Outcome‐related exclusions 20–26% (withdrawal due to nausea and vomiting, foetal loss, and other medical conditions) |
| Christian et al. 2003a,2003b, 2008, 2009a,b, 2010 ††† | Nepal | Cluster RCT | 60 mg iron + 400 μg folate (635) | Vitamin A, 1000 μg (628) | 2008 | Mean 11 week (SD 5.1 weeks) | Recent pregnancy | ▾IH; ▾PS; ▾LBW; ▾IM; ▾CM; ▴BW; ▴ IF; ▴MF | >90% of births happened at home; large numbers of dropouts for most outcomes and reduced number at follow‐ups |
A, abortion; AH, antepartum haemorrhage; ALRI, acute lower respiratory infections; AS, Apgar score; B, bacteriuria; BA, birth asphyxia; BW, birthweight; BL, birth length; CC, chest circumference; CM, child mortality; D, diarrhoea; DL, dysfunctional labour; E, pre‐/eclampsia; FL, foetal loss; GA, gestational age; HC, head circumference; IF, intellectual functioning; IH, intrapartum haemorrhage; IM, 3‐month infant mortality; LBW, low birthweight; M, malaria; MD, mode of delivery; MF, motor functioning; MH, maternal hypertension; MI, maternal infection; ND, neonatal death; O, ophathalmia; P, pyrexia; PD, preterm delivery; PH, post‐partum haemorrhage; PS, puerperal sepsis; PVT, positive venereal disease research laboratory test; PW, placenta weight; RM, preterm premature rupture of membrane; SA, skin abscess; SB, stillbirth; SGA, small‐for‐gestational age; SS, skin sepsis; UCN, umbilical cord around the neck. *If several intervention groups, only the iron or iron‐folate group is presented. †The control group is a placebo or control alternative that excludes iron. ‡Total number of subjects randomised in both iron and control groups. §Comparing the intervention group with the control group. Only results that achieved statistical significance (as reported by the authors) are presented. ¶Symbols: (▴) increase; (▾) decrease. **Outcome assessed: A, ALRI, AS, B, BA, BW, CM, D, E, FL, GA, IH, MD, MH, ND, P, PH, PVT, SA and UCN. ††Outcome assessed: BW, LBW and M. ‡‡Outcome assessed: AS, BW, BL and PW. §§Outcome assessed: BW. ¶¶Outcome assessed: AH, BW, LBW, MH, MI, ND PD and SB. ***Outcome assessed: BW, BL, GA, HC, LBW, PD and SGA. †††Outcome assessed: ALRI, BA, BL, BW, CC, CM, DL, FL, H, HC, IF, IH, IM, LBW, MF, PD, PS, RM and SGA. ‡‡‡Number analysed.
Five trials were carried out in malaria‐endemic areas. A small trial from Nigeria by Fleming et al. (1986) investigated the effects of anti‐malarial, iron and folic acid prophylaxis on maternal and child health, including malaria, among primigravid women in comparison with a control group. No significant effects of iron were seen on any of the outcomes investigated. However, because of poor compliance, large number of dropouts, several exclusions by outcomes, particularly severe anaemia, no viable conclusions can be made from that study. The study by Menendez et al. (1994) among multigravid poor pregnant women from rural Gambia found a beneficial effect of iron on birthweight {56‐g [95% confidence interval (CI) 12–128] increase} and prematurity. However, outcome‐related exclusions (27%), such as preterm delivery and anaemia, were problematic. In a rather small trial from Niger (after recruitment at a mean pregnancy of 28 weeks), iron had a beneficial effect on birth length [0.7‐cm (95% CI 0.05–1.35) increase] and Apgar score [0.4 (95% CI 0.16–0.96) increase], but not on birthweight (Preziosi et al. 1997). However, the small number of women undermines the reliability of the results.
A trial from Uganda found a beneficial effect of iron on birthweight with a 82‐g (95% CI 81–83) increase (2999 vs. 2917 g for the iron and placebo arms of the trial, respectively) Ndyomugyenyi & Magnussen 2000). Because of a large number of dropouts and many outcome‐related exclusions – inclusion of women who developed anaemia or caught malaria, and late weighing of the baby – the study falls short in addressing our research question. The trial from rural Dhaka, Bangladesh enrolled pregnant women at 24 weeks of pregnancy [APHA, Atlanta, 2001 (T. Juncker et al., unpublished observations) ]. The study showed no effect of iron‐folate on low birthweight, maternal hypertension, antepartum haemorrhage, maternal infection, stillbirth or neonatal death. However, in the iron‐folate group, preterm birth [odds ratio (OR), 1.43, 95% CI 1.08–1.89] was more common and birthweight was reduced by 57 g. The results of that study were only available as an extended abstract, so it is difficult to judge the reliability of its findings.
In non‐malarial areas, we found two trials that investigated the effect of prenatal iron prophylaxis on maternal and child health (Christian et al. 2003a,b, 2008, 2009a,b, 2010; Zeng et al. 2008). A trial from rural western China (Zeng et al. 2008) showed a 2‐day longer gestational age, reduced risk of early preterm delivery (OR 0.50, 95% CI 0.27–0.94), and slightly longer birth length [0.3 cm (95% CI 0.09–0.51)] in the iron‐folate group compared with the folate‐only group. No difference was seen in the mean birthweight and head circumference or proportion of low birthweight and small‐for‐gestational age children. A trial from a rural Nepalese district (Christian et al. 2003a,b, 2008, 2009a,b, 2010) followed women from a mean of 11 gestational weeks up to 7 years of follow‐up and found among the iron‐supplemented group a reduced risk for: intrapartum haemorrhage [risk ratio (RR) 0.59, 95% CI 0.35–0.98]; puerperal sepsis (sepsis 1, measured as ≥100.4°F on ≥2 of the first 10 days, with an RR of 0.72, 95% CI 0.54–0.95; or sepsis 2 measured as ≥100.4°F on 2 or more of the first 10 days plus foul‐smelling vaginal discharge for ≥2 days, with an RR of 0.57, 95% CI 0.35–0.91); low birthweight (RR 0.84, 95% CI 72–0.99); 3‐month infant mortality (RR 0.53, 95% CI 0.30–0.92); child mortality from birth up to the age of 7 years (RR 0.69, 95% CI 0.49–0.99). Among children whose mothers were supplemented with iron during pregnancy, the trial found an increase in birthweight (37 g, 95% CI −16–90) and better intellectual and motor functioning. Iron supplementation did not influence a number of other outcomes (dysfunctional labour, eclampsia, preterm premature rupture of membrane, birth length, small‐for‐gestational age, head and chest circumferences, foetal loss, preterm birth, birth asphyxia, acute lower respiratory infections, and hypertension).
In sum, the review of the findings of studies from malarial settings does not allow for a definite conclusion of the benefits of prophylactic iron supplementation on the health of the mother and child. While all of these trials compared iron with either a placebo or a viable control group, no data were available comparing the effects of routine iron supplementation vs. screening and treatment for anaemia. For malaria‐prone settings, the suggestion that iron may invigorate the occurrence of infections brings into question the universally routine use of iron prophylaxis (Sazawal et al. 2006; Ojukwu et al. 2009). None of the previous trials took into account the malaria or HIV status of its participants. Consequently, a clear window of opportunity still exists to further investigate the advantages and disadvantages of routine iron supplementation during pregnancy in settings with concomitant prevalence of malaria and HIV by comparing routine administration of iron to all women vs. administration of iron to only those found to be anaemic.
Methods
Objectives and hypotheses of the PROFEG Trial
The specific objectives of the trial were: (1) to evaluate whether routine iron prophylaxis from the first prenatal visit until delivery (called ‘routine iron’ subsequently) is better than screening and treatment for anaemia (called ‘selective iron’) in regard to maternal and child health, such as preterm delivery, low birthweight and perinatal mortality; (2) to assess whether there is a difference in the effects of iron between high and low seasons of malaria; and (3) to assess whether screening and iron treatment for anaemia is more feasible than routine iron prophylaxis in terms of the use of health care providers and overall compliance in Maputo, Mozambique. Originally, we had had malaria activation as one of the primary outcomes, but the pilot showed it to be unfeasible because the needed equipments were not available in the nurses' office; thus, we collected information on symptoms suggestive of malaria (fever, headache, cold, vomiting/nausea, and body aches during pregnancy).
In line with these objectives, we formulated four working hypotheses: (1) preterm delivery, low birthweight and perinatal mortality are more common among women who receive routine iron prophylaxis; (2) (modified after the pilot) routine iron prophylaxis during pregnancy increases symptoms suggestive of malaria; (3) the health problems in hypotheses 1 and 2 are more prominent in the season of high malaria; and (4) screening and treatment for anaemia is equally feasible than routine iron prophylaxis in terms of use of health care providers and overall compliance.
Study context
The study was carried out in the health centre (clinic settings) of 1° de Maio in Maputo City (the capital) (March 2007–December 2008) and in the health centre of Machava 2 in Maputo Province (June 2007–March 2008), Mozambique; Machava is adjacent to Maputo City. The study centres are urban health centres and were chosen on the basis of the following criteria: they did prenatal care and had maternity ward for child delivery; the two main general hospitals were referral hospitals; the number of births was sufficient to complete the study in the planned time; they had a good accessibility to facilitate the supervision of the study; the centres had an ongoing prevention of vertical transmission of the HIV programme; care providers were interested in the programme; and data collection was feasible. The health profile of Mozambique is typical of sub‐Saharan African countries, with nearly 55% of its 23 million people living below the poverty line (The World Bank 2011). The main causes of morbidity and mortality are infectious and parasitic diseases, with malaria accounting for 30–40% mortality. At the time of the trial, the prevalence of HIV/AIDS was estimated to be around 16% nationally, and around 20% in Maputo City (Measure Demographic and Health Surveys 2009). Health care is administered by the state through district, provincial and national systems (Lindlöw et al. 2004).
Prenatal consultations are recommended from the third month of pregnancy and are usually carried out, along with delivery, by mother‐and‐child health (MCH) nurses. Women who come for their first prenatal consultation with a pregnancy of less than 3 months are not seen and are asked to return when the pregnancy becomes visible. Women with problems prior to the third month are referred to a hospital. Seventy‐five per cent of women in Maputo City have four or more prenatal consultations, with 50% starting their first prenatal visits by the fourth or fifth month (Lindlöw et al. 2004). Like in all public health centres in Mozambique, prenatal care and delivery were free of charge.
Care recommendations at the time of the study included: daily prophylactic iron‐folate supplementation (60 mg + 400 μg) throughout pregnancy, one dose of mebendazol 500 mg (for intestinal parasite), malaria prophylaxis with sulfadoxine pyrimethamine, as well as haemoglobin measurement and syphilis screening at the first prenatal visit. Three doses of tetanus vaccine were recommended: at the fifth and seventh months and at delivery. Malaria was diagnosed during prenatal consultations through a laboratory test and by clinical signs. Voluntary HIV testing was offered in many health centres, including our study centres (Mozambiqan Ministry of Health 2002).
Usually, women arrived at the health centre around 6 am–7 am, with the prenatal consultation ending around 1 pm. At the prenatal sessions, women collectively received information and counselling regarding HIV, vaccinations, and advice on diet. After the collective information session, women were individually attended to by MCH nurses, during which time they had a (voluntary) HIV test and tetanus vaccination. After the individual consultation, the women were sent to the heath centre laboratory to have blood tests for syphilis, haemoglobin, and (primiparous women) blood group determination. Haemoglobin was routinely measured only during the first prenatal consultation, but if a woman presented clinical signs of anaemia, she could have further laboratory tests.
Mothers were given a prenatal card on their first visit and were requested to bring it on subsequent visits. The card was to be completed at each prenatal visit and to be given to the birthplace (called hospitals subsequently). After delivery, in some health centres/hospitals the prenatal card was given back to the woman, while for some it was retained in the hospital archives. The prenatal card also had a section covering births, but not all hospitals completed it. Health centres had no individual records for pregnant women; they had a book of first visits including woman's names, age and date of visit. In addition, only the numbers of subsequent visits were recorded, and these were not linked to individual women.
Data on births were collected using separate forms, which were kept by the hospitals. Furthermore, hospitals had other records (admission books, birth books, books for complications, etc) that varied from one hospital to another. Hospital archiving was variable and unreliable. Often, documents were put into a box and retained in a room containing other things too. Post‐natal visits were not customary, and no form was used in those visits. The main reason for attending the health centre after delivery was for contraception.
Recruitment
In the two study health centres, general information about the study was given to all women attending their first prenatal visit during the routine early morning health‐education sessions. Recruitment occurred during the individual consultations. The physical locations of the two study centres were slightly different: in the 1° de Maio health centre, a room separate from the prenatal visit room was used, while in the Machava centre, it was the same room. In both centres, the women first went for the voluntary HIV testing; the nurses estimated that about 99% of the women had the HIV test.
In the 1° de Maio health centre, the women first had their routine prenatal care consultation with the MCH nurse, followed by the visit to the study nurse. The study nurse checked for eligibility, and those who met the inclusion criteria were asked if they wanted to join the study. In Machava, it was the MCH nurse who asked if the woman wanted to join the trial. If she agreed, the study nurse sat jointly with the MCH nurse when the information on the woman's history was collected and completed the data collection form simultaneously while the routine nurse completed the routine prenatal form. After the consultation, women in the selective iron group had their haemoglobin measured using HemoCue® (Hemocue AB, Ängelholm, Sweden). Women were then supposed to be guided to the laboratory to have the routine tests.
Study nurses were given a study recruitment book into which they entered the following information on the recruited women: name, age, and number of previous pregnancies and births. The study nurses were retired nurses employed by the project. They were given training and a study manual, which they used to carry out the different steps of the study. In the Machava health centre, the MCH nurses collected the data on subsequent visits. The MCH nurses were paid a little incentive ($10.00 to $25.00 per month, depending on the number of women present at each visit) by the project for accommodating the study and for guiding the study nurses. Recruitment and randomisation into the study took place from November 21, 2006 to March 31, 2008.
Exclusion criteria
All pregnant women having their first prenatal visit were the target group. Women excluded from the study were those who missed attending to the study nurses; those too early in pregnancy (<12 weeks), women with high obstetric risk and those less than 18 years of age.
Interventions
Women in the Routine iron group (i.e. routine iron prophylaxis from the first to the last prenatal visit) received 30 tablets (supply of one month) of 60 mg of ferrous sulphate plus 400 μg of folic acid per day. Women in the Selective iron group (i.e. regular screening for haemoglobin level and treatment for anaemia) were given 30 tablets of 1 mg of folic acid per day. At each visit the nurses measured the haemoglobin using a rapid haemoglobin measure (HemoCue Hb 201+). If their haemoglobin was below the cut‐off of <9 g/dL Hb, they received a double dose of iron (60 + 60 mg for treatment of anaemia). The iron plus folic acid tablets were round and red in colour, while the folic acid only tablets were round and yellow in colour. The tablets were given in a plastic bag that had the drug's name and dose on it.
Outcome measures
The main outcomes were preterm delivery (delivery <37 weeks of gestation, estimated from last menstrual period) and low birthweight (<2500 g). Originally, malaria activation was one of the primary outcomes, but as the pilot showed it to be unfeasible, we dropped it. Instead, we collected information on symptoms suggestive of malaria (fever, headache, cold, vomiting/nausea and body aches during pregnancy as secondary outcomes) and self‐reported malaria during pregnancy (the woman was asked by the study nurse whether she has had diagnosed malaria since her last visit). Secondary outcomes were perinatal mortality (as available from the local registers; unlikely to cover early stillbirths or neonatal births occurring at home), complications during pregnancy and labour, and symptoms suggestive of malaria.
Sample size
As there was no prior reliable information on baseline rates or what impact iron might have, we calculated the sample size with various assumptions of the baseline rates, power (85 and 90%), significance level of 5%, and the size of the difference to be detected (20 and 30%) for preterm delivery, low birth rate, (clinical malaria) and perinatal mortality. Based on these calculations and the expected feasibility, the target size chosen was 2000 women for each group. The STATA 7 (StataCorp LP, College Station, TX, USA) was used to estimate the sample size.
Randomisation
Women who agreed to participate and met the inclusion criteria were randomised into either Routine iron group or Selective iron group. The STATA statistical software was used to generate sequential random numbers separately for the two centres, and the women were assigned to either of the groups with a probability of 50%.
The codes for the groups were put into sealed and sequentially numbered opaque envelopes; the woman's study number was repeated on all the documents in the envelope. The envelope contained a study identification card (pink for the Selective group and yellow for the Routine iron group, 10 × 20 cm) and an informed consent form. The envelopes were put into a box and the study nurses were advised to pick them in order. Before the nurse opened the envelope, she wrote the woman's name on it. After opening, the coloured study identification card was stapled to the maternity card.
Informed consent was requested in two stages: first orally, and again after opening the envelope, this time with written confirmation. An envelope was opened for each woman who had orally agreed to join the trial. Women were asked to sign or thumb‐print the informed consent form. Nurses read and explained the text of the form to those who could not read Portuguese. Women were informed about the study on an individual basis. Detailed information was given about the group the woman was assigned to, while it was also explained that the woman had the right not to follow the recommendations. The information included data collection procedures, such as longer first visit and meeting the study nurse at each visit. Those who refused to participate were assured that their decision would not influence their routine care.
Data collection and follow‐up
Data were collected through three methods: (1) abstracting data from mothers' maternity cards and birth records around the time of the visit/hospital stay; (2) asking women questions at prenatal visits; and (3) for birth data only, collecting data from hospital records, death registers, as well as calling women to complete missing data. The first two methods were used mainly for data collection during pregnancy, while the last method (involving mixed methods) was used for collection of delivery data. The first two mentioned methods are described here.
In the 1° de Maio health centre and at the first visit at the Machava health centre, data from prenatal visits were collected by the trained study nurses using data collection forms. In subsequent visits to the Machava health centre, data were collected by the MCH nurses who were giving routine care. Study women were identified by the colour study identification card stapled to the maternity card. Clinical data were abstracted from the maternity cards. Additional questions were asked, for example, on whether the woman had had malaria since the previous visit, whether any malaria prophylaxis was taken, and whether the iron and folic acid tablets were taken by the women. Researchers regularly collected these forms from the health centres; coding and data entry were done by research assistants at the Eduardo Mondlane University using Microsoft Access. The data were later transferred to STATA for data analysis.
The study nurses were given diaries to record any incidents at the health centres, any lack of iron tablets, lack of HIV tests (reagents) or any information they felt was valuable. The information from these diaries was regularly checked by the study coordinators.
At delivery, the study women were identified by the colour identification card stapled to their maternity card. Nurses taking care of deliveries at the study health centres were informed of the study and were requested to tear the study identification card from the maternity card, staple it to the (routine) delivery card, and put the delivery card into a separate study box. The study nurses abstracted data from the delivery cards onto the data collection forms daily. At the two second‐level referral hospitals (Mavalane and Jose Macamo), the MCH nurses were informed of the study and asked likewise to put the delivery cards aside. The study coordinators collected the data from these referral hospitals every 1–2 weeks. We could not organise the birth data collection at the central hospital (third‐level hospital) or other potential birthplaces.
Compliance
The women were instructed and encouraged at each visit to take the tablets they were given. Women allocated to the Routine iron group could refuse to take the iron tables, in which case they were classified as non‐compliant with the intervention. Women who belonged to the Selective iron group and wanted iron (even if their haemoglobin level was not below the cut‐off level) were given iron and were classified as non‐compliant with the intervention. To assess whether nurses had given the tablets and that women had taken the tablets, a few questions were asked on each subsequent visit, including ‘Was haemoglobin measured?’; ‘Was iron/folic acid given to the woman?’; ‘Number of iron/folic acids given?’; and ‘Did the woman take the tablets during the past week?’
Ethical approval
Ethical approval for the study was obtained from the Mozambique Ministry of Health Ethics Committee [CNBS (Ref. 84/CNBS/06) ]. A positive statement was obtained from the National Institute for Health and Welfare, Helsinki, Finland (Dno 2571/501/2007).
Monitoring
The study was monitored for reliable data collection and the safety of the intervention. Decreased haemoglobin levels in the screening group were reported to the local ethics committee. The study nurses kept diaries on ‘any events’, the stock of iron tablets in the health centre, any lack of HIV tests and reagents. They kept a register on the women's attendance to subsequent visits and kept a separate stock of iron tablets purchased for the study; the stock was to be used in the event that the health centre ran out of iron tablets; they reported to the local coordinators. Local coordinators and international coordinators visited the study sites regularly and verified that the study procedures were followed in regard to informing the women, randomisation, recruitment, the technique for measuring haemoglobin using HemoCue, handing out of iron/folic acid tablets and the data collection. Study nurses reported to the local coordinators and local coordinators reported to the international coordinators.
Pilot
A pilot study to study the feasibility of recruitment and follow‐up during pregnancy was carried out between November 2006 and March 2007 in the 1° de Maio health centre to test the feasibility of recruitment (Parkkali et al. 2008). A total of 781 women were enrolled into the pilot study, 134 of whom were followed until delivery; the pilot did not test the completeness of birth data collection.
The setting up of the pilot study was time consuming and administrative issues and authorisations took longer than expected. However, after practical obstacles had been solved, the study design turned out to be feasible. The mean number of women recruited per week was 43. The women came from various nearby areas. Anaemia prevalence (Hb < 9 g/dL) in the selective iron group at recruitment was 36% (n = 140) according to HemoCue. By the standard measurement (Lovibond®; The Tintometer Limited, UK) it was 0.5%. Of the 134 deliveries, 78% (n = 104) took place in the health centre, 17% (n = 23) in the referral hospital (Mavalane) and 5% (n = 7) at home. Home deliveries were recorded in the maternity delivery register at the health centre when the women came with their newborn to have vaccinations and to receive the baby card.
The changes made to the trial protocol included a slight modification to the data collection forms and dropping the aim of collecting data on malaria activation, which had proved unfeasible. The main procedures were not modified.
Statistical analysis
The data were analysed by basically computing descriptive results (means and proportions) of the differences between the iron groups.
Results
Recruitment and exclusions
Figure 1 presents the flow of women in the study. Of the 5778 women present at recruitment (3942 in the 1° de Maio health centre and 1836 in the Machava health centre), the final sample size was 4326 (75%) women, randomised into the two study groups (2184 to the Routine iron and 2142 to the Selective iron group).
Figure 1.
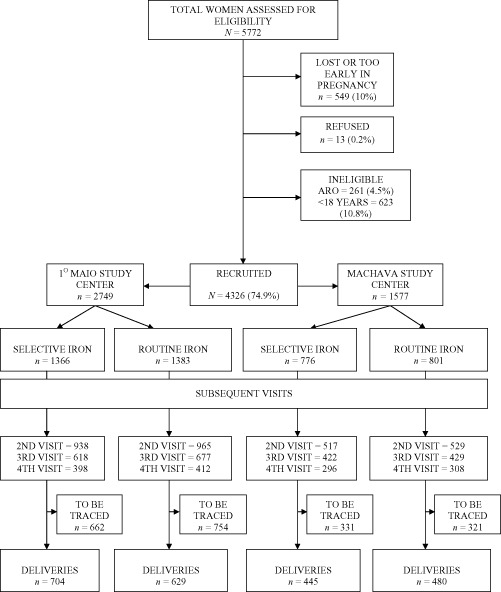
PROFEG Trial flow diagram.
Excluded women were: women not attending the study nurse at all (usually did not find their way) and those who were too early in pregnancy (<12 weeks) (n = 549), refusals (n = 13), those with high‐risk pregnancy (n = 261), and those less than 18 years of age (n = 623). We aimed to collect background information on women who refused, but this did not happen systematically.
Randomisation
Women in both study groups were similar to each other as well as in the two study centres, indicating a successful randomisation (Table 2). Thirty‐three per cent of the women in the Selective iron group had low haemoglobin at enrolment, and were given iron as planned (Table 2).
Table 2.
Characteristics of women at recruitment, by group and centre
| Characteristic | All | 1o de Maio study centre | Machava study centre | |||
|---|---|---|---|---|---|---|
| n = 4326 | n = 2749 | n = 1577 | ||||
| Selective iron* | Routine iron † | Selective iron* | Routine iron † | Selective iron* | Routine iron † | |
| n = 2142 | n = 2184 | n = 1366 | n = 1383 | n = 776 | n = 801 | |
| Maternal age (years), mean (SD) ‡ | 24.8 (5.5) | 24.7 (5.5) | 24.5 (5.5) | 24.2 (5.4) | 25.2 (5.4) | 25.4 (5.5) |
| Maternal age, n (%) | ||||||
| < 20 | 357 (17) | 390 (18) | 247 (18) | 270 (20) | 110 (14) | 120 (15) |
| 20–24 | 859 (40) | 842 (39) | 566 (41) | 559 (40) | 293 (38) | 283 (35) |
| 25–29 | 502 (23) | 510 (23) | 297 (22) | 301 (22) | 205 (26) | 209 (26) |
| 30–34 | 257 (12) | 296 (13) | 145 (11) | 167 (12) | 112 (14) | 129 (16) |
| ≥35 | 146 (7) | 132 (6) | 98 (7) | 73 (5) | 48 (7) | 59 (8) |
| Missing | 21 (1) | 14 (1) | 13 (1) | 13 (1) | 8 (1) | 1 (0) |
| Gestational age (weeks), mean (SD) ‡ | 10.4 (6.0) | 10.2 (5.9) | 9.9 (5.8) | 9.6 (5.6) | 11.2 (6.1) | 11.2 (6.4) |
| Measurement of gestational age, n (%) | ||||||
| Last menstruation | 1850 (86) | 1900 (87) | 1138 (83) | 1165 (84) | 712 (92) | 735 (92) |
| Uterine height | 284 (13) | 272 (12) | 222 (16) | 211 (15) | 62 (8) | 61 (7) |
| Missing | 8 (1) | 12 (1) | 6 (1) | 7 (1) | 2 (0) | 5 (1) |
| Previous abortions, n (%) | ||||||
| Yes | 274 (13) | 298 (14) | 165 (12) | 180 (13) | 109 (14) | 118 (15) |
| No | 1861 (87) | 1881 (86) | 1197 (88) | 1201 (87) | 664 (86) | 680 (85) |
| Missing | 7 (0) | 5 (0) | 4 (0) | 2 (0) | 3 (0) | 3 (0) |
| Previous stillbirths, n (%) | ||||||
| Yes | 172 (8) | 176 (8) | 79 (6) | 94 (7) | 93 (12) | 82 (10) |
| No | 1962 (92) | 2002 (92) | 1284 (94) | 1287 (93) | 678 (87) | 715 (89) |
| Missing | 8 (0) | 6 (0) | 3 (0) | 2 (0) | 5 (1) | 4 (1) |
| Number of previous deliveries, n (%) | ||||||
| None | 631 (30) | 699 (32) | 429 (31) | 476 (34) | 202 (26) | 223 (28) |
| One | 671 (31) | 658 (30) | 434 (32) | 436 (32) | 237 (31) | 222 (28) |
| Two | 399 (19) | 397 (18) | 237 (17) | 236 (17) | 162 (21) | 161 (20) |
| Three or more | 435 (20) | 425 (20) | 262 (19) | 233 (17) | 173 (22) | 192 (24) |
| Missing | 6 (0) | 5 (0) | 4 (1) | 2 (0) | 2 (0) | 3 (0) |
| Number of previous live births, n (%) | ||||||
| None | 652 (30) | 719 (33) | 443 (32) | 493 (36) | 209 (27) | 226 (28) |
| One | 665 (31) | 658 (30) | 430 (32) | 434 (31) | 235 (30) | 224 (28) |
| Two | 402 (19) | 391 (18) | 240 (18) | 234 (17) | 162 (21) | 157 (20) |
| Three or more | 418 (20) | 411 (19) | 249 (18) | 220 (16) | 169 (22) | 191 (24) |
| Missing | 5 (0) | 5 (0) | 4 (0) | 2 (0) | 1 (0) | 3 (0) |
| HIV status, n (%) | ||||||
| Positive | 446 (21) | 428 (20) | 271 (20) | 251 (18) | 175 (23) | 177 (22) |
| Negative | 1696 (79) | 1756 (80) | 1095 (80) | 1132 (82) | 601 (77) | 624 (78) |
| Haemoglobin by HemoCue (g/dL), mean (SD) ‡ | 9.6 (1.7) | 9.6 (1.7) | 9.7 (1.7) | |||
| Haemoglobin by HemoCue (g/dL), n (%) | ||||||
| < 7.0 | 141 (7) | 102 (8) | 39 (5) | |||
| 7.0–8.90 | 535 (25) | 343 (25) | 192 (25) | |||
| 9.0–9.90 | 512 (24) | 336 (25) | 176 (23) | |||
| 10.0–10‐90 | 462 (22) | 294 (22) | 168 (22) | |||
| 11.0–11.90 | 298 (14) | 168 (12) | 130 (17) | |||
| ≥12.0 | 174 (8) | 110 (8) | 64 (8) | |||
| Not measured | 20 (1) | 13 (1) | 7 (1) | |||
| Iron + folic acid given, n (%) | ||||||
| Yes | 708 (33) | 2164 (99) | 464 (34) | 1368 (99) | 244 (32) | 796 (99) |
| No | 1421 (66) | 14 (1) | 892 (65) | 11 (1) | 529 (68) | 3 (0) |
| Missing | 13 (1) | 6 (0) | 10 (1) | 4 (0) | 3 (0) | 2 (0) |
| Only folic acid given, n (%) | ||||||
| Yes | 1426 (67) | 15 (1) | 894 (65) | 10 (1) | 532 (69) | 5 (1) |
| No | 701 (33) | 2159 (99) | 460 (34) | 1367 (99) | 241 (31) | 792 (99) |
| Missing | 15 (0) | 10 (0) | 12 (1) | 6 (0) | 3 (0) | 4 (0) |
| Fever during current pregnancy, n (%) | ||||||
| Yes | 550 (26) | 527 (24) | 405 (30) | 377 (27) | 145 (19) | 150 (19) |
| Headache during current pregnancy, n (%) | ||||||
| Yes | 917 (43) | 944 (43) | 634 (46) | 682 (49) | 283 (37) | 262 (33) |
| Cold/chills during current pregnancy, n (%) | ||||||
| Yes | 412 (19) | 406 (19) | 299 (22) | 298 (22) | 113 (15) | 108 (14) |
| Vomit/nausea during current pregnancy, n (%) | ||||||
| Yes | 612 (29) | 605 (28) | 434 (32) | 444 (32) | 178 (23) | 161 (20) |
| Body aches during current pregnancy, n (%) | ||||||
| Yes | 487 (23) | 488 (22) | 361 (26) | 379 (27) | 126 (16) | 109 (14) |
| Malaria prophylaxis during current pregnancy, n (%) | ||||||
| Yes | 856 (40) | 923 (42) | 481 (35) | 499 (36) | 375 (48) | 424 (53) |
| Malaria during current pregnancy, n (%) | ||||||
| Yes | 125 (6) | 135 (6) | 84 (6) | 86 (6) | 41 (5) | 49 (6) |
| Had malaria test, n (%) | ||||||
| Yes | 159 (7) | 164 (8) | 107 (8) | 107 (8) | 52 (7) | 57 (7) |
*Policy 2: daily intake of 400 μg of folic acid and received iron (120 mg) if their haemoglobin was <9 g/dL. †Policy 1: daily intake of 60 mg ferrous sulphate plus 400 μg of folic acid. ‡Missing data excluded when calculating the mean and standard deviation: maternal age (Selective n = 21, Routine n = 14); gestational age (Selective n = 8, Routine n = 12); haemoglobin (Selective n = 20).
Follow‐up visits and deliveries
The number of visits varied, but was similar in the two groups (Table 3). Most women had only two subsequent visits. The maximum number of visits was seven (about 0.3% of women). For simplicity, the number of subsequent visits were grouped from 1 to 5+ (Table 3).
Table 3.
Number of prenatal visits by study group and centre of study
| Number of visits | All | 1o de Maio study centre § | Machava study centre § | |||
|---|---|---|---|---|---|---|
| n = 4326 | n = 2749 | n = 1577 | ||||
| Selective iron* | Routine iron † | Selective iron* | Routine iron † | Selective iron † | Routine iron † | |
| n = 2142 | n = 2184 | n = 1366 | n = 1383 | n = 776 | n = 801 | |
| One, n (%) ‡ | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Two, n (%) ‡ | 633 (30) | 595 (27) | 424 (31) | 396 (29) | 209 (27) | 199 (25) |
| Three, n (%) ‡ | 462 (22) | 513 (23) | 287 (21) | 319 (23) | 175 (23) | 194 (24) |
| Four, n (%) ‡ | 299 (14) | 321 (15) | 177 (13) | 192 (14) | 122 (16) | 129 (16) |
| Five+, n (%) ‡ | 247 (12) | 247 (11) | 146 (11) | 151 (11) | 101 (13) | 96 (12) |
*Daily intake of 400 μg of folic acid and received iron (120 mg) if their haemoglobin was <9 g/dL. †Daily intake of 60 mg ferrous sulphate plus 400 μg of folic acid. ‡The denominator is the number of subjects in each trial arm. §There were no significant differences between the iron groups and within each study centre.
Table 4 shows the timing of the subsequent visits. At each subsequent visit, most women were between 24 and 36 weeks gestation, and this was similar in both study groups. Consequently, it is possible that most of the women might have delivered before the next visit (Table 4); however, the reasons for absence in subsequent visits and whether the women had delivered or not were not adequately ascertained.
Table 4.
Pregnancy week at subsequent visits after enrolment by study group and centre of study
| Pregnancy week at subsequent visits | All | 1o de Maio study centre | Machava study centre | |||
|---|---|---|---|---|---|---|
| Selective iron* | Routine iron † | Selective iron* | Routine iron † | Selective iron* | Routine iron † | |
| n = 2142 | n = 2184 | n = 1366 | n = 1383 | n = 776 | n = 801 | |
| First subsequent visit, n (%) ‡ | 1455 (68) | 1494 (68) | 938 (69) | 965 (70) | 517 (67) | 529 (66) |
| <30 week § | 991 (68) | 1011 (68) | 635 (68) | 659 (68) | 356 (69) | 352 (66) |
| 30–34 week § | 293 (20) | 309 (21) | 171 (18) | 179 (19) | 122 (24) | 130 (25) |
| ≥35 week § | 83 (6) | 78 (5) | 52 (6) | 40 (4) | 31 (6) | 38 (7) |
| Missing § | 88 (6) | 96 (6) | 80 (8) | 87 (9) | 8 (1) | 9 (2) |
| Second subsequent visit, n (%) ‡ | 1040 (49) | 1106 (51) | 618 (45) | 677 (49) | 422 (54) | 429 (54) |
| <30 week § | 523 (50) | 515 (47) | 329 (53) | 356 (53) | 194 (46) | 159 (37) |
| 30–34 week § | 311 (30) | 377 (34) | 168 (27) | 204 (30) | 143 (34) | 173 (40) |
| ≥35 week § | 163 (16) | 176 (16) | 82 (14) | 81 (12) | 81 (19) | 95 (22) |
| Missing § | 43 (4) | 38 (3) | 39 (6) | 36 (5) | 4 (1) | 2 (1) |
| Third subsequent visit, n (%) ‡ | 694 (33) | 720 (33) | 398 (29) | 412 (30) | 296 (38) | 308 (38) |
| <30 week § | 186 (27) | 173 (24) | 129 (32) | 123 (30) | 57 (20) | 50 (16) |
| 30–34 week § | 265 (38) | 304 (42) | 140 (35) | 180 (44) | 125 (42) | 124 (40) |
| ≥35 week § | 208 (30) | 219 (31) | 98 (25) | 85 (20) | 110 (37) | 134 (44) |
| Missing § | 35 (5) | 24 (3) | 31 (8) | 24 (6) | 4 (1) | 0 (0) |
*Daily intake of 400 μg of folic acid and received iron (120 mg) if their haemoglobin was <9 g/dL. †Daily intake of 60 mg of ferrous sulphate plus 400 μg of folic acid. ‡Frequency and percentage of women at recruitment who attended subsequent visits. §The denominator is the number of those who attended at each subsequent visit.
Thus far, we have not obtained information on all deliveries; currently, we are in the process of locating further delivery data by various methods. Even by excluding estimated late miscarriages (5%), early stillbirths (3%) and estimated home births (10%), we would have expected to obtain data for 3547 women (82%) of the 4326 women who participated in the trial. In the event, we obtained data on only 2258 (64% of 3547) women. We were alerted to these problems too late to be able make adjustments. As delivery cards for our study women were found in all four assumed delivery places, we did not realise the numbers were fewer than expected until the time at which most deliveries would have been expected to have occurred.
Compliance
The compliance of the study nurses is illustrated in Table 5: assessing how many of the women coming for subsequent visits had had their haemoglobin measured and were given iron and folic acid tablets. At each subsequent visit, almost all of the Selective iron women were measured for haemoglobin using the recommended HemoCue method. Almost all women in the Routine iron group were given iron tablets at each subsequent visit. About one‐third of the Selective iron women received iron tablets because of low haemoglobin, while the other two‐thirds received folic acid only (Table 5). HemoCue was also used to measure women in the Routine iron group at the beginning of the trial. Although it was planned not to test the women in this group again in the trial, a misunderstanding meant it was sometimes used later, although for a small number of women.
Table 5.
Measure of compliance by the personnel assessed by frequency of measuring haemoglobin (Selective iron group) and given iron and folic acid (Routine iron group) during subsequent visits, by group and centre
| Subsequent visit after enrolment | All | 1o de Maio study centre | Machava study centre | |||
|---|---|---|---|---|---|---|
| n = 4326 | n = 2749 | n = 1577 | ||||
| Selective iron* | Routine iron † | Selective iron* | Routine iron † | Selective iron* | Routine iron † | |
| n = 2142 | n = 2184 | n = 1366 | n = 1383 | n = 776 | n = 801 | |
| First subsequent visit, n (%) ‡ | 1455 (68) | 1494 (68) | 938 (69) | 965 (70) | 517 (67) | 529 (66) |
| Haemoglobin measured, n (%) § | ||||||
| HemoCue | 1416 (97) | 156 (10) | 919 (98) | 142 (15) | 497 (96) | 14 (3) |
| Iron given, n (%) § | 493 (34) | 1460 (98) | 320 (34) | 948 (98) | 173 (33) | 512 (97) |
| Folic acid only given, n (%) § | 946 (65) | 29 (2) | 603 (64) | 11 (1) | 343 (66) | 18 (3) |
| Second subsequent visit, n (%) ‡ | 1040 (49) | 1106 (51) | 618 (45) | 677 (49) | 422 (54) | 429 (54) |
| Haemoglobin measured, n (%) § | ||||||
| HemoCue | 1000 (96) | 66 (6) | 618 (100) | 52 (8) | 396 (94) | 14 (3) |
| Iron given, n (%) § | 350 (34) | 1090 (99) | 210 (34) | 670 (99) | 140 (33) | 420 (98) |
| Folic acid only given, n (%) § | 671 (65) | 16 (1) | 392 (63) | 7 (1) | 279 (66) | 9 (2) |
| Third subsequent visit, n (%) ‡ | 694 (33) | 720 (33) | 398 (29) | 412 (30) | 296 (38) | 308 (38) |
| Haemoglobin measured, n (%) § | ||||||
| HemoCue | 673 (97) | 16 (2) | 389 (98) | 9 (2) | 284 (96) | 7 (2) |
| Iron given, n (%) § | 185 (27) | 707 (98) | 110 (28) | 405 (98) | 75 (25) | 302 (98) |
| Folic acid only given, n (%) § | 495 (71) | 13 (2) | 277 (70) | 6 (1) | 218 (74) | 7 (2) |
*Daily intake of 400 μg of folic acid and received iron (120 mg) if their haemoglobin was <9 g/dL. †Daily intake of 60 mg of ferrous sulphate plus 400 μg of folic acid. ‡Frequency and percentage of women at recruitment who attended subsequent visits. §The denominator is the number of those who attended at each subsequent visit.
Table 6 shows compliance with taking iron and folic acid tablets during the week previous to each visit based on self‐report. Most women reported taking the tablets regularly as advised and this was similar in the Selective and Routine iron groups in both study centres.
Table 6.
Measure of compliance by women's reports, by group and centre
| Subsequent visit after enrolment | All | 1o de Maio study centre | Machava study centre | |||
|---|---|---|---|---|---|---|
| n = 4326 | n = 2749 | n = 1577 | ||||
| Selective iron* | Routine iron † | Selective iron* | Routine iron † | Selective iron* | Routine iron † | |
| n = 2142 | n = 2184 | n = 1366 | n = 1383 | n = 776 | n = 801 | |
| First subsequent visit, n (%) ‡ | 1455 (68) | 1494 (68) | 938 (69) | 965 (70) | 517 (67) | 529 (66) |
| Tablets taken during the past week, n (%) § | ||||||
| Regularly | 1344 (92) | 1380 (92) | 883 (94) | 912 (95) | 461 (89) | 468 (88) |
| Sometimes | 90 (6) | 88 (6) | 42 (4) | 35 (4) | 48 (9) | 53 (10) |
| No | 15 (1) | 20 (1) | 7 (1) | 14 (1) | 8 (2) | 6 (1) |
| Second subsequent visit, n (%) ‡ | 1040 (49) | 1106 (51) | 618 (45) | 677 (49) | 422 (54) | 429 (54) |
| Tablets taken during the past week, n (%) § | ||||||
| Regularly | 962 (93) | 1031 (93) | 588 (95) | 652 (96) | 374 (87) | 379 (88) |
| Sometimes | 70 (7) | 60 (5) | 24 (4) | 15 (2) | 46 (11) | 45 (10) |
| No | 5 (0.5) | 8 (0.7) | 4 (0.6) | 7 (1) | 1 (0.2) | 1 (0.2) |
| Third subsequent visit, n (%) ‡ | 694 (33) | 720 (33) | 398 (29) | 412 (30) | 296 (38) | 308 (38) |
| Tablets taken during the past week, n (%) § | ||||||
| Regularly | 647 (93) | 670 (93) | 381 (96) | 402 (98) | 266 (90) | 268 (87) |
| Sometimes | 43 (6) | 46 (6) | 15 (4) | 7 (2) | 28 (9) | 39 (13) |
| No | 3 (0.4) | 3 (0.4) | 2 (0.5) | 2 (0.5) | 1 (0.3) | 1 (0.3) |
*Daily intake of 400 μg of folic acid and received iron (120 mg) if their haemoglobin was <9 g/dL. †Daily intake of 60 mg of ferrous sulphate plus 400 μg of folic acid. ‡Frequency and percentage of women at recruitment who attended subsequent visits. §The denominator is the number of those who attended at each subsequent visit.
Discussion
This paper described the rationale, design, and success of a pragmatic RCT on iron prophylaxis during pregnancy in Maputo, Mozambique. Recruitment and randomisation in the study were done well. Follow‐up visits during the study were similar in both trial arms. However, collecting delivery data posed a challenge, and an estimated 36% of institutionalised births were missed. The missed births are now being traced by matching the women to admissions data in the study health centres and referral hospitals. The compliance of the study personnel (with regards to measurement of women's haemoglobin) and the women to the study protocol (uptake of the recommended tablets) was good. Several administrative and practical challenges were encountered during the course of the trial, from planning through to the process of implementation. A pilot trial carried out in the study contexts before the actual trial highlighted areas that needed to be resolved prior to the main trial.
Despite the widespread recommendation of prophylactic iron supplementation during pregnancy, the data available provide insufficient evidence on its benefits to maternal and child health in low‐income settings. In malaria‐prone settings, the small sample sizes, exclusion by outcomes and large dropouts that have characterised previous trials has meant the evidence falls short in clarifying the advantages and disadvantages of prophylactic iron supplementation during pregnancy [APHA, Atlanta, 2001 (T. Juncker et al., unpublished observations) ] (Fleming et al. 1986; Menendez et al. 1994; Preziosi et al. 1997; Ndyomugyenyi & Magnussen 2000). Although the trials from non‐malarial areas generally had large sample sizes and better designs, their results are conflicting (Zeng et al. 2008; Christian et al. 2003a,b, 2008, 2009a,b, 2010), while the results from non‐malarial areas may not be applicable in malarial settings.
Unlike previous studies that employed explanatory designs to test the efficacy of prenatal iron prophylaxis, we utilised the pragmatic trial design so as to compare the effectiveness of two policies for prophylactic iron administration. Pragmatic trials are more suitable to study effects in normal clinical practice; they have the basic aim of informing choice between treatments (Roland & Torgerson 1998; MacPherson 2004). A pragmatic trial design was useful to compare two policies of iron supplementation in a real‐life situation. In these types of trials, placebo and blinding are not customary (Roland & Torgerson 1998; MacPherson 2004). Although several calls have been made to increase the use of pragmatic trials to address clinical questions, they are rarely used and researchers are less experienced with them (Zwarenstein et al. 2008). For this reason, tutoring was necessary for the local research team prior to the trial starting.
As a cut‐off to define anaemia, we used haemoglobin values lower than 9 g/dL. The WHO's haemoglobin cut‐off level for determining anaemia during pregnancy is 11 g/dL (WHO 1972). Thus, our cut‐off value can be questioned. However, the WHO recommended value is based on haemoglobin levels of women in developed countries. For developing countries, clinical signs of symptoms of anaemia usually appear when the haemoglobin level is below 7 g/dL (van den Broek et al. 1999). At the planning stage, we asked health care providers in Maputo about the acceptable cut‐off point for haemoglobin level requiring iron treatment. Their opinions varied between 8 and 11 g/dL. Based on this feedback, we concluded that using a 9 g/dL cut‐off level for treatment with iron would not endanger the woman's or fetus's health, and would also enable us to answer the research questions. An earlier study on anaemia during pregnancy in Mozambique found that 5–15% of pregnant women had haemoglobin values below 9 g/dL and that 58% had levels below 11 g/dL (Liljestrand et al. 1986).
Although the study health centres had previously conducted RCTs in relation to HIV, it nevertheless remained a delicate issue at the time of the study, both at the grass roots level and among higher authorities. Despite this and the voluntary nature of HIV testing, almost all the women (99%) underwent the test. This facilitated our study.
Carrying out this trial was challenging. One key challenge was the sluggishness of administrative and financial procedures. Planning the trial took a long time as did obtaining authorisations from the ethics committees and the local authorities. There was no research infrastructure in the local health care and the existing tradition did not value accurate record keeping. In addition, university facilities were modest and money transfers were cumbersome.
Finding qualified research assistants for the study posed another challenge. We chose retired nurses, as having older and experienced nurses created an environment of trust among the women. However, it took time to train them in the study procedures. Another problem centred on the study nurses receiving a higher salary than MCH nurses, which may have undermined support for the study among MCH nurses. We gave small monthly incentives to the MCH nurses for their collaboration, but that may not have been enough. MCH nurses may not have always informed the women that they needed to see the study nurses. This issue was more likely at the 1° de Maio health centre where the study location was different to that of usual prenatal care consultation.
The number of women participating declined with each subsequent visit. The reasons could not be ascertained, but they may have been due to having no further prenatal visits, women visiting other health centres, or data not being collected. However, considering that a majority of the women were at 30–34 weeks of gestation at their final visit, it is possible that most of them might have delivered already. We are investigating the potential reasons for why women missed subsequent visits. Our data suggest that the compliance of both the study nurses and the women was good. Women were given their tablets at each visit. To assess whether they complied, they were asked in each visit about the frequency of taking their tablets. We cannot be sure how reliably women answered as they might have been intimated by the nurses. The nurses, however, were instructed to encourage the women to tell the truth and they were informed that any answers they provided were acceptable.
The greatest challenge in the trial was gathering birth data by the planned method. This led to changes in the data collection protocol and presently, we are tracing the women's birth data from health centres, hospitals and death registers. This has prolonged the outcome data collection and increased the study costs. Possible reasons for losing birth data include: a miscarriage or home birth with no notification sent to the study health centre; the mother dying or moving from the area before birth; the mother delivering outside the study locations (self‐referrals); delivery nurses not putting the study cards aside; and mothers not having their study cards at delivery.
Conclusions
The planned trial proved feasible in an ordinary health care setting in Maputo. The loss of mothers' delivery data might have been avoided by better surveillance of the process during the trial and better knowledge of the actual patient flow patterns in Maputo.
Source of funding
The study was funded by two grants from the Academy of Finland (2004: 210631; 2010: 139191).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
BIN, SP, EH designed, analysed and prepared the present paper. EH designed and is responsible for the conception of the PROFEG Trial. SP, FA, GS, BC, OA, JC, MD and MN participated in the planning of the PROFEG Trial, made substantial contribution in its execution and participated in the interpretation of results and the critical review of the paper. OA and ER were responsible for data acquisition and preparation, interpretation of results and for the critical review of the paper.
Acknowledgements
We thank Ana Dai (Ministry of Health) for the field work and the study nurses Marcelina Bie, Eulalia Muianga and Rebeca Marulino.
Trial Registration: The trial is registered at ClinicalTrials.gov, number NCT00488579 (June 19, 2007). The first women were randomised to the trial in November 2006.
References
- Adetifa I. & Okomo U. (2009) Iron supplementation for reducing morbidity and mortality in children with HIV. Cochrane Database of Systematic Reviews (1), CD006736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boelaert J.R., Weinberg G.A. & Weinberg E.D. (1996) Altered iron metabolism in HIV infection: mechanisms, possible consequences, and proposals for management. Infectious Agents and Disease 5, 36–46. [PubMed] [Google Scholar]
- Brabin B.J., Hakimi M. & Pelletier D. (2001) An analysis of anemia and pregnancy‐related maternal mortality. Journal of Nutrition 131, 604S–615S. [DOI] [PubMed] [Google Scholar]
- van den Broek N.R., Ntonya C., Mhango E. & White S.A. (1999) Diagnosing anaemia in pregnancy in rural clinics: assessing the potential of the haemoglobin colour scale. Bulletin of World Health Organization 77, 15–21. [PMC free article] [PubMed] [Google Scholar]
- Christian P., Khatry S.K., Katz J., Pradhan E.K., LeClerg S.C., Shrestha S.R. et al (2003a) Effects of alternative maternal micronutrient supplements on low birth weight in rural Nepal: a double blind randomized community trial. British Medical Journal 326, 571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian P., West K.P., Khatry S.K., LeClerg S.C., Pradhan E.K., Katz J. et al (2003b) Effects of maternal micronutrient supplementation on fetal loss and infant mortality: a cluster‐randomized trial in Nepal. American Journal of Clinical Nutrition 78, 1194–1202. [DOI] [PubMed] [Google Scholar]
- Christian P., Darmstadt G.L., Wu L., Khatry S.K., Leclerg S.C., Katz J. et al (2008) The effect of maternal micronutrient supplementation on early neonatal morbidity in rural Nepal: a randomized, control, community trial. Archives Disease in Childhood 93, 660–664. [DOI] [PubMed] [Google Scholar]
- Christian P., Khatry S.K., LeClerq S.C. & Dali S.M. (2009a) Effects of prenatal micronutrient supplementation on complications of labor and delivery and puerperal morbidity in rural Nepal. International Journal of Gynaecology and Obstetrics 106, 3–7. [DOI] [PubMed] [Google Scholar]
- Christian P., Stewart C.P., LeClerq S.C., Wu L., Katz J., West K.P. et al (2009b) Antenatal and postnatal iron supplementation and childhood mortality in rural Neap: a prospective follow‐up in a randomized, controlled community trial. American Journal of Epidemiology 170, 1127–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian P., Murray‐Kolb L.E., Khatry S.K., Katz J., Schaefer B.A., Cole P.M. et al (2010) Prenatal micronutrient supplementation and intellectual and motor function in early school‐aged children in Nepal. Journal of American Medical Association 304, 2716–2723. [DOI] [PubMed] [Google Scholar]
- Fall C.H., Fisher D.J., Osmond C., Margetts B.M. & Maternal Micronutrient Supplementation Study Group (2009) Multiple micronutrient supplementation during pregnancy in low‐income countries: a meta‐analysis of effects on birth size and length of gestation. Food and Nutrition Bulletin 30, S533–S546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A.F., Ghatoura G.B.S., Harrison K.A., Briggs N.D. & Dunn D.T. (1986) The prevention of anaemia in pregnancy in primigravidae in the guinea savanna of Nigeria. Annals of Tropical Medicine and Parasitology 80, 211–233. [DOI] [PubMed] [Google Scholar]
- Gera T. & Sachdev H.P.S. (2002) Effect of iron supplementation on incidence of infectious illness in children: systematic review. British Medical Journal 325, 1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordeuk V.R., Delanghe J.R., Langlois M.R. & Boelaert J.R. (2001) Iron status and the outcome of HIV infection: an overview. Journal of Clinical Virology 20, 111–115. [DOI] [PubMed] [Google Scholar]
- Hemminki E. & Rimpelä U. (1991) A randomized comparison of routine versus selective iron supplementation during pregnancy. Journal of the American College of Nutrition 10, 3–10. [DOI] [PubMed] [Google Scholar]
- Idemyor V. (2007) Human immunodeficiency virus (HIV) and malaria interaction in sub‐Saharan Africa: the collision of two titans. HIV Clinical Trials 8, 246–253. [DOI] [PubMed] [Google Scholar]
- Kabyemela E.R., Fried M., Kurtis J.D., Mutabingwa T.K. & Duffy P.E. (2008) Decreased susceptibility to Plasmodium falciparum infection in pregnant women with iron deficiency. Journal of Infectious Diseases 198, 163–166. [DOI] [PubMed] [Google Scholar]
- Lao T.T., Tam K.F. & Chan L.Y. (2000) Third trimester iron status and pregnancy outcome in non‐anaemic women: pregnancy unfavourably affected by maternal iron excess. Human Reproduction 15, 1843–1848. [DOI] [PubMed] [Google Scholar]
- Lawn J.E., Cousens S., Zupan J. & Lancet Neonatal Survival Steering Team (2005) 4 million neonatal deaths: when? Where? Why? Lancet 365, 891–900. [DOI] [PubMed] [Google Scholar]
- Liljestrand J., Bergström S. & Birgergård G. (1986) Anaemia of pregnancy in Mozambique. Transactions of the Royal Society of Tropical Medicine and Hygiene 80, 249–255. [DOI] [PubMed] [Google Scholar]
- Lindlöw M., Ward P. & Zorzi N. (2004) Primary Health Care in Mozambique: Service Delivery in a Complex Hierarchy . The World Bank: Washington, DC.
- MacPherson H. (2004) Pragmatic clinical trials. Complementary Therapies in Medicine 12, 136–140. [DOI] [PubMed] [Google Scholar]
- McLean E., Cogswell M., Egli I., de Wojdyla D. & Benoist B. (2009) Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutrition. 12, 444–454. [DOI] [PubMed] [Google Scholar]
- Measure Demographic and Health Surveys (2009) Mozambique: Standard AIDS Indicator Survey.
- Menendez C., Todd J., Alonso P.L., Francis N., Lulat S., Ceesay S. et al (1994) The effects of iron supplementation during pregnancy, given by traditional birth attendants, on the prevalence of anaemia and malaria. Transactions of the Royal Society of Tropical Medicine and Hygiene 88, 590–593. [DOI] [PubMed] [Google Scholar]
- Mozambiqan Ministry of Health (May 2002) [Guide to the implementation of the programme; Prevention of Mother to Child Transmission of HIV/AIDS] (Portuguese).
- Müngen E. (2003) Iron supplementation in pregnancy. Journal of Perinatal Medicine 31, 420–426. [DOI] [PubMed] [Google Scholar]
- Ndyomugyenyi R. & Magnussen P. (2000) Chloroquine prophylaxis, iron/folic‐acid supplementation or case management of malaria attacks in primigravidae in western Uganda: effects on congenital malaria and infant haemoglobin concentrations. Annals of Tropical Medicine and Parasitology 94, 759–770. [DOI] [PubMed] [Google Scholar]
- Ojukwu J.U., Okebe J.U., Yahav D. & Paul M. (2009) Oral iron supplementation for preventing or treating anaemia among children in malaria‐endemic areas. Cochrane Database Systematic Reviews (3), CD006589. [DOI] [PubMed] [Google Scholar]
- Oppenheimer S.J. (2001) Iron and its relation to immunity and infectious disease. Journal of Nutrition 131, 616S–633S. [DOI] [PubMed] [Google Scholar]
- Parkkali S., Abacassamo F., Salomé G., Augusto O. & Nikula M. (2008) Routine iron prophylaxis during pregnancy: effects on maternal and child health in Maputo City and the urban part of Maputo Province Mozambique (PROFEG). Stakes Discussion Papers 1/2008. Helsinki: STAKES.
- Peña‐Rosas J.P. & Viteri F.E. (2009) Effects and safety of preventive oral iron or iron + folic acid supplementation for women during pregnancy. Cochrane Database Systematic Reviews (4), CD004736. [DOI] [PubMed] [Google Scholar]
- Prentice A.M. (2008) Iron metabolism, malaria, and other infections: what is all the fuss about? Journal of Nutrition 138, 2537–2541. [DOI] [PubMed] [Google Scholar]
- Preziosi P., Prual A., Galan P., Daouda H., Boureima H. & Hercberg S. (1997) Effect of iron supplementation on the iron status of pregnant women: consequences for newborns. American Journal of Clinical Nutrition 66, 1178–1182. [DOI] [PubMed] [Google Scholar]
- Roland M. & Torgerson D.J. (1998) Understanding controlled trials: what are pragmatic trials? British Medical Journal 316, 285.9472515 [Google Scholar]
- Sazawal S., Black R.E., Ramsan M., Chwaya H.M., Stoltzfus R.J., Dutta A. et al (2006) Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community‐based, randomized, placebo‐controlled trial. Lancet 367, 133–143. [DOI] [PubMed] [Google Scholar]
- Stoltzfus R.J. (2001) Iron‐deficiency anemia: reexamining the nature and magnitude of the public health problem. Summary: implications for research and programs. Journal of Nutrition 131, 697S–700S. [DOI] [PubMed] [Google Scholar]
- Stoltzfus R.J., Mullanny L. & Black R.E. (2004) Iron deficiency anaemia In: Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors (eds Ezzati M., Lopez A.D., Rodgers A. & Murray C.J.L.), Vol. 1, pp 163–209. World Health Organization: Geneva, Switzerland. [Google Scholar]
- The World Bank (2011) Country Indicators, Mozambique . Available at: http://data.worldbank.org/indicator (Accessed 7 March 2011).
- Villar J., Merialdi M., Gülmezoglu M., Abalos E., Carroli G., Kulier R. et al (2003) Nutritional interventions during pregnancy for the prevention or treatment of maternal mortality and preterm delivery: an overview of randomized controlled trials. Journal of Nutrition 133, 1606S–1625S. [DOI] [PubMed] [Google Scholar]
- WHO (1972) Nutritional anaemias . Report of a WHO Group of Experts. (WHO Technical Report Series, No. 503). World Health Organization: Geneva. [PubMed]
- WHO (2011) International Travel and Health 2011 . Available at: http://www.who.int/ith/chapters/ith2011chap7.pdf (Accessed 20 February 2011).
- Yip R. (2000) Significance of an abnormally low or high hemoglobin concentration during pregnancy: special consideration of iron nutrition. American Journal of Clinical Nutrition 72, 272S–279S. [DOI] [PubMed] [Google Scholar]
- Zeng L., Cheng Y., Dang S., Chang S., Kong L. & Yan H. (2008) Impact of micronutrient supplementation during pregnancy on birth weight, duration of gestation, and perinatal mortality in rural western China: double blind cluster randomized controlled trial. British Medical Journal 337, a2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwarenstein M., Treweek S., Gagnier J.J., Altman D.G., Tunis S., Haynes B. et al (2008) Improving the reporting of pragmatic trials: an extension of the CONSORT statement. British Medical Journal 337, a2390. [DOI] [PMC free article] [PubMed] [Google Scholar]


