Abstract
The wide variety of infant formula available on the market can be confusing for parents and physicians. We aimed to determine associations between predominant type of formula used from birth to 4 months and parental and child characteristics and type of physician consulted, and then to describe relations between type of formula used and growth. Our analyses included 1349 infants from the EDEN mother–child cohort. Infant's feeding mode and type of formula used were assessed at 4 months by maternal self‐report. Infant's weight and height from birth to 4 months, measured in routine follow‐up, were documented by health professionals in the infant's personal health record. Anthropometric z‐scores were calculated by using World Health Organization growth standards. Multinomial logistic regression was used to identify factors associated with the type of formula predominantly used; relations with growth were analysed by linear regressions. Partially hydrolysed formulas were more likely to be used by primiparous women (P < 0.001), those breastfeeding longer (P < 0.001) and for infants with family history of allergies (P = 0.002). Thickened formulas were more often used by mothers returning to employment in the first 4 months (P = 0.05) and breastfeeding shortly (P < 0.001). No significant relation was found between infant's growth and type of formula (P > 0.20). Infants breastfed shorter showed higher weight‐for‐age (P < 0.001) and length‐for‐age (P = 0.001) z‐score changes between birth and 4 months. The use of a specific type of infant formula seems to be mainly related to parental characteristics. Infant's growth in the first 4 months is related to other factors than to the type of formula used.
Keywords: infant, breastfeeding, formula feeding, growth, socio‐demographic factors, longitudinal study
Introduction
The benefits of breastfeeding on child health are well described in the literature (Van Rossum et al. 2001; Ip et al. 2007). The World Health Organization (WHO) and most of the international paediatric societies promote breastfeeding as optimal infant nutrition and recommend exclusive breastfeeding (EBF) until 6 months (Boland 2005; Gartner et al. 2005; Agostoni et al. 2009). Despite these recommendations, many parents use infant formula before 6 months. The wide range of formulas available can be confusing and overwhelming for parents and physicians. Formula companies target physicians with advertising campaigns, advocating functional and beneficial effects of their products for infant health. Thickening agents, prebiotic and probiotics are added in some infant formulas. Prebiotics might have the potential to increase the total number of bifidobacteria present in the gut and to soften stools (Boehm & Moro 2008; Sherman et al. 2009); probiotics might play a role in preventing childhood diseases, especially diarrhoea (Moreau 2001). Their effects on infant's growth are not well known.
Several studies have been conducted to describe determinants of infant‐feeding mode (Butler et al. 2004; Lanting et al. 2005; Bolling et al. 2007; Grjibovski et al. 2008; Kristiansen et al. 2010) and their effects on child growth (Kramer et al. 2007; Griffiths et al. 2009). However, determinants of use of a specific formula compared with others are poorly described in the literature and very few studies (Koletzko et al. 2009) conducted on samples of significant size assess and compare their specific impact on child growth.
The prevalence of overweight children is rising and there is a strong evidence for an association between rapid weight gain in infancy and later obesity (Stettler 2007). Early feeding, especially milk feeding, has been identified as an important factor. Studies relating milk feeding to growth pattern often compare breastfed with formula‐fed infants without distinguishing the different types of formulas. Some formulas may be given specifically to fast‐ or slow‐grower infants and the influence of a specific formula on infant's growth may depend on the characteristics of this formula. Before considering formula‐fed infants as a single group, we aimed to determine the associations between the type of formula used and parental, child's and physician's characteristics, and to describe their relations with infant's growth from birth to 4 months.
Key messages
The use of infant formulas (partially hydrolysed, thickened, enriched in pre‐ or probiotic and others) in the first 4 months of life seems to be essentially related to maternal return to employment, parity and parental history of allergies.
Infant's growth was not related to the type of formula predominantly used in our study.
Infants who were breastfed shorter showed higher growth between birth and 4 months.
Materials and methods
Study design
The EDEN mother–child cohort (study of pre‐ and early post‐natal determinants of child health and development) recruited 2002 pregnant women aged 18–45 years who presented before 24 weeks of gestation for prenatal care at the obstetrics and gynaecology department of Nancy and Poitiers University Hospitals. Enrolment started in February 2003 in Poitiers and September 2003 in Nancy; it lasted 27 months in each centre. Exclusion criteria were multiple pregnancies, history of diabetes, illiteracy and moving outside the region planned in the next 3 years. The study received approval from the ethics committee [Comités Consultatif de Protection des Personnes dans la Recherche Biomédicale (CCPPRB)] of Kremlin‐Bicêtre. Files have been declared to the Commission Nationale de l'Informatique et des Libertés (CNIL). Written consent was obtained from mothers at enrolment, for fathers between mother's enrolment and delivery, and for infants after delivery.
Data collection
At 24–28 weeks of gestation, mothers had a clinical examination performed by research midwives assistants, where their height was measured, using wall Seca 206 stadiometer (Hamburg, Germany) to the nearest 0.2 cm. Maternal education and pre‐pregnancy weight, family income during pregnancy and family history of allergies were obtained by interviewing the mother. Paternal weight and height were measured with the same procedure at some point between mother's inclusion and delivery.
Data were collected from obstetrical and paediatric records on parity, gestational age at delivery, birthweight (measured with electronic Seca scales, Seca 737 in Nancy and Seca 335 in Poitiers), birth length (measured with a wooden somatometer; Testut, Béthune, France) and infant feeding at maternity discharge.
At 4 months, mothers completed questionnaires on which they reported infant's weight and length measured every month since birth in routine follow‐up and documented by health professionals in the infant's personal health record (kept by the mother). Data on mothers' return to employment in the first 4 months, type of physician consulted for the infant, feeding practices and infant health (diarrhoea, regurgitations or colics) were also collected. Mothers reported in the 4‐month questionnaire, if any, the different formula consumed by their infant and the duration of consumption of each: less than a week/between 1 week and 1 month/more than 1 month but less than 4 months/since birth.
Generated variables
In order to classify infants, because of frequent formula changes in the first 4 months in our sample, we estimated from the information collected the total duration of exposure to each type of formula. Infants were included into a specific predominant formula category if the total duration of exposure to that formula was higher than the total exposure to any other formula. Our study focused on regular, partially hydrolysed, thickened (but not enriched in pre‐ or probiotics) and enriched in pre‐ or probiotics (thickened or not) formulas as these four types were the most consumed in our sample (Fig. 1). We created a class ‘others’ with infants who were equally exposed to different formulas, or who had predominantly consumed formulas such as extensively hydrolysed protein formula but were too few to constitute a class of the variable of interest.
Figure 1.
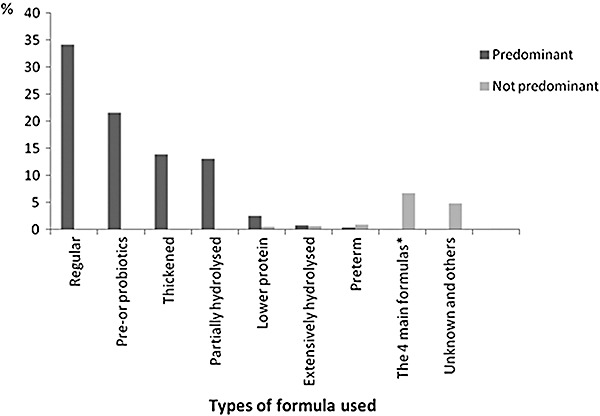
Types of formula used predominantly or not in the EDEN study. Includes 1354 infants exclusively formula‐fed or not in the first 4 months of life. In infants who were in the regular category, regular formulas were used 95% of the time on average. Similarly, in the other groups, pre‐ or probiotics, thickened and partially hydrolysed formulas were used, respectively, 89%, 85% and 95% of the time on average. *Regular, pre‐ or probiotic, thickened and partially hydrolysed formulas: successive or concomitant use.
Duration of EBF (only breast milk as milk feeds) from hospital discharge to 4 months was calculated using the information recorded by midwives at discharge and self‐reported maternal feeding practices in the 4‐month questionnaire for infants who received both breast and formula milk during their first 4 months of life.
As children's measurements were not collected at the same time point, we predicted individual infant weight and length at 4 months using non‐linear mixed effects models. Among the main parametric growth models (Hauspie 1989), Jenss Model [y = a + b × age − exp(c + d × age)] best fitted our weight and length growth data from birth to 1 year of age, according to Akaike information criterion fit parameter and residual distribution over time. The model with a random effect on every parameter allowed having individual equations of the weight (height) growth trajectories by computing each of the four equation's parameters as the fixed‐effect coefficient plus the random‐effect term (Pinheiro & Bates 2000). Using the individual equations, we calculated predicted weight and length at 4 months for all subjects. All available infant weight and height from birth to the 1‐year clinical examination were used in growth modelling. Median and interquartile range of the number of measurements (for weight as well as for height) were 10 (7–12). There was a median of four measurements for the 0–4 month period, three for the 4–8 month period and three after 8 months. Among infants with at least one measurement during the first year, 100% had at least one measurement of weight before 4 months, 81.9% between 4 and 8 months, and 87.2% after 8 months (99.8%, 81.6% and 87.4% for height, respectively). Finally, for the statistical analyses of associations, infants with fewer than four measurements in the first year were excluded as their growth trajectory could be shrunk towards the mean growth trajectory.
In order to facilitate comparisons of our data with other international studies, we used these predicted values to obtain weight‐for‐age (WFA), length‐for‐age (LFA) and weight‐for‐length (WFL) z‐scores at birth and 4 months, according to WHO's growth reference data (WHO Multicentre Growth Reference Study Group 2006). The different z‐scores were then used in the statistical analysis to study the relationships between infant's growth and breast and infant‐formula feeding.
Parental body mass indices (BMIs) were computed as the reported pre‐pregnancy weight (kg)/measured height squared (m2) for the mother and the measured weight (kg)/measured height squared (m2) for the father. When measurements were unavailable from the father, reported weight by the father (14.9%) was used, and reported height by the father (13.9%) or by the mother (7.6%) was used. Underweight was defined as a BMI (kg m−2) of <18.5, normal weight as a BMI between 18.5 and 25, overweight as a BMI between 25 and 30 and obesity as a BMI ≥ 30. Because of the small number of underweight fathers in the EDEN cohort (n = 19; 1.0%), we grouped together fathers in the underweight and normal BMI categories. Parental heights were divided into quintiles.
To handle missing data, we proceeded as follows: when percentage of missing value was lower than 5%, we imputed the modal class value (all except gender, EBF duration, gestational age, birthweight, paternal height and BMI), and when percentage of missing values was higher than 5%, they were grouped into a separate category (paternal height).
Study sample
Of the 2002 recruited women, 96 were excluded because they left the study before or at the time of delivery for personal reasons, 4 because of intrauterine death and 3 because they delivered outside the study hospitals. Data on birthweight were available for 1899 newborns. We excluded 232 infants because the 4‐month questionnaire was unavailable. When compared with the included mothers, the excluded mothers were less educated (29.7% vs. 55.4% had a university degree, P < 0.001) and less often born in France (80.6% vs. 90.4%, P < 0.001). The excluded infants had a gestational age slightly higher than that of the included infants (39.02 vs. 39.29, P = 0.05). There were no statistical difference in infants' gender (P = 0.08) and birthweight (P = 0.15). For the analyses, we selected infants who received formula at least 1 week during the first 4 months and who had information about the type of formula used [missing (n = 27)]. Analyses on formula‐fed infants were therefore based on 1354 infants.
To perform the analyses on post‐natal growth, we selected a subsample of 1239 infants with growth data from birth to 4 months. The 115 pairs excluded at this stage differed from the others by maternal education (university degree: 41.7% vs. 54.1%, P = 0.02), birthweight (2710 g vs. 3319 g, P < 0.001) and gestational age (37 weeks of amenorrhoea vs. 39, P < 0.001).
Statistical analysis
Comparisons of means and proportions by formula group were performed by analysis of variance or chi‐square, respectively (results not shown). Associations between the type of formula (dependent variable) and covariates related to parental, child and health professional characteristics were measured by adjusted odds ratios estimated by multinomial logistic regression.
The relation between growth and type of formula was analysed by multiple linear regressions. The dependent variable was the change in z‐score between birth and 4 months (Δz‐score), which was the difference in z‐scores between birth and 4 months. The models comprised the type of formula; confounding variables that were significantly related to both growth and type of formula (centre, education, family income, mother's return to employment, EBF duration and type of physician consulted); variables highly related to growth (parental heights and BMIs, infant's gender and gestational age); and variables related to the type of formula used that might influence growth (occurrence of diarrhoea and regurgitations). We also adjusted for the average z‐score between 0 and 4 months to consider changes relative to the mean weight or height values.
Analyses were performed with SAS software (version 9.2; SAS Institute, Cary, NC, USA). A P‐value ≤0.05 was considered to indicate statistical significance for all of the analyses.
Results
Description of formula‐fed infants
Mothers were, on average, 29.5 years old and approximately 48% of them were primipara (Table 1). More than half of the mothers had a university degree, and for 91.4% of the families, both parents were born in France. The mean birthweight was 3267 g and 5.8% of the infants were born preterm. The mean EBF duration was 0.9 month. The rate of any breastfeeding was 68.1% at maternity and 21.7% at 4 months. One‐third of the infants were predominantly exposed to regular formula (Fig. 1). For 39% of the infants, the type of formula used never changed in the first 4 months, while about 26% had their formula milk changed twice or more. In infants who received different formulas in the first 4 months, the predominant formula was used 83% of the time, on average, for those whose formula changed once and 77% of the time for those whose formulas changed twice or more.
Table 1.
Characteristics of parents and offspring (n = 1354)
| Variable | n | Mean ± SD or % yes |
|---|---|---|
| Parental characteristics | ||
| Education (% university degree) | 1332 | 53.0% |
| Monthly family income ≤3000€ | 1346 | 72.1% |
| Primiparous | 1351 | 48.0% |
| Mother lives with a partner | 1335 | 95.3% |
| Mother returned to employment in the first 4 months | 1341 | 45.7% |
| Family history of allergy | 1346 | 50.5% |
| Both parents born in France | 1354 | 91.4% |
| Maternal age at delivery (years) | 1354 | 29.5 ± 4.8 |
| Maternal height (cm) | 1334 | 163.4 ± 6.1 |
| Paternal height (cm) | 1344 | 176.8 ± 6.4 |
| Maternal pre‐pregnancy BMI < 25 kg m−2 | 1324 | 71.2% |
| Paternal BMI < 25 kg m−2 | 1252 | 50.1% |
| Child characteristics | ||
| Female gender | 1354 | 47.3% |
| Duration of EBF (months) | 1352 | 0.9 ± 1.1 |
| Gestational age (weeks of amenorrhoea) | 1354 | 39.2 ± 1.7 |
| Birthweight (g) | 1354 | 3267 ± 509 |
| Occurrence of diarrhoea between 0 and 4 months | 1337 | 21.3% |
| Occurrence of regurgitations between 0 and 4 months | 1328 | 62.2% |
| Other variables | ||
| Recruitment centre (% Poitiers) | 1354 | 50.8% |
| Type of physician consulted between 0 and 4 months, General practitioner | 1350 | 28.4% |
BMI, body mass index; EBF, exclusive breastfeeding; SD, standard deviation.
In the subsample with available data on growth (n = 1239), 1.3% of infants had a WFA z‐score >2 standard deviation at birth and 0.3% at 4 months. The mean z‐scores at different ages and by type of formula are presented in Table 2.
Table 2.
z‐Scores at birth and 4 months by type of formula predominantly used in the EDEN cohort study (n = 1239)
| WHO z‐scores | Type of formula milk | ||||
|---|---|---|---|---|---|
| Regular (n = 432) | Partially hydrolysed (n = 165) | Thickened (n = 166) | Pre‐/probiotics (n = 278) | Others (n = 198) | |
| Birth | |||||
| WFA | 0.04 ± 0.88* | 0.12 ± 0.91 | 0.002 ± 0.94 | −0.01 ± 0.89 | −0.07 ± 1.01 |
| LFA | 0.18 ± 0.98 | 0.22 ± 1.08 | 0.07 ± 1.11 | 0.12 ± 1.00 | 0.05 ± 1.20 |
| WFL | −0.07 ± 1.11 | −0.01 ± 1.11 | −0.005 ± 1.15 | −0.07 ± 1.13 | −0.10 ± 1.20 |
| 4 months | |||||
| WFA | −0.25 ± 0.82 | −0.31 ± 0.86 | −0.21 ± 0.78 | −0.26 ± 0.86 | −0.42 ± 0.87 |
| LFA | −0.10 ± 0.90 | −0.03 ± 0.96 | −0.12 ± 0.91 | −0.09 ± 0.92 | −0.18 ± 0.97 |
| WFL | −0.20 ± 0.88 | −0.34 ± 0.95 | −0.11 ± 0.78 | −0.21 ± 0.85 | −0.34 ± 0.85 |
Mean ± standard deviation.
LFA, length‐for‐age; WFA, weight‐for‐age; WFL, weight‐for‐length; WHO, World Health Organization.
Determinants of infant formula use
Partially hydrolysed formulas were twice as likely to be used by the most educated mothers compared with regular formulas, but there was no statistical significant difference for the global comparison of the different formulas with regular formulas according to education level (Table 3). Partially hydrolysed formulas were less likely to be used by multiparous mothers. Thickened formulas were more often used by mothers returning to employment in the first 4 months. Partially hydrolysed and thickened formulas were more likely to be given to infants with family history of allergies than regular formula. Longer period EBF was positively related to the use of partially hydrolysed formulas but negatively related to thickened formulas. Thickened formulas were more likely consumed by infants having regurgitations in the first 4 months. There was no significant association between family income or parent's country of birth and type of formula (all P > 0.19).
Table 3.
Adjusted odds ratios (OR) of the relations between predominant type of formula used and parental and child characteristics, and type of physician consulted (n = 1354)
| Type of formula milk (Regular as reference, n = 463) | Globa l P‐value* | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Partially hydrolysed (n = 176) | Thickened (n = 187) | Pre‐/probiotics (n = 293) | Others (n = 235) | ||||||
| OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Parental characteristics | |||||||||
| Maternal education (Ref: No degree) | 0.10 | ||||||||
| High school degree | 1.26 | 0.68–2.36 | 1.33 | 0.80–2.19 | 1.38 | 0.88–2.17 | 1.07 | 0.65–1.77 | |
| 2‐year university degree | 1.38 | 0.75–2.54 | 0.86 | 0.49–1.49 | 1.20 | 0.75–1.92 | 1.36 | 0.82–2.34 | |
| ≥3‐year university degree | 2.37 | 1.31–4.27 | 0.94 | 0.53–1.64 | 1.31 | 0.81–2.10 | 1.69 | 1.02–2.78 | |
| Monthly family income (Ref: 1501–2300€) | 0.54 | ||||||||
| <1501 | 1.06 | 0.56–2.00 | 0.93 | 0.53–1.61 | 0.81 | 0.49–1.36 | 0.78 | 0.46–1.33 | |
| 2301–3000 | 1.05 | 0.63–1.75 | 0.97 | 0.60–1.58 | 1.50 | 0.83–1.87 | 1.12 | 0.73–1.73 | |
| >3000 | 1.57 | 0.91–2.71 | 1.57 | 0.91–2.70 | 1.51 | 0.95–2.39 | 1.06 | 0.64–1.75 | |
| Parity (Ref: 1) | <0.001 | ||||||||
| 2 | 0.64 | 0.40–0.98 | 1.65 | 1.11–2.46 | 1.28 | 0.91–1.80 | 1.13 | 0.78–1.65 | |
| ≥3 | 0.27 | 0.14–0.50 | 0.71 | 0.40–1.29 | 0.63 | 0.38–1.02 | 0.62 | 0.37–1.03 | |
| Mother returned to employment in the first 4 months (Ref: No) | 0.85 | 0.57–1.28 | 1.49 | 1.01–2.22 | 1.17 | 0.84–1.64 | 0.83 | 0.57–1.19 | 0.05 |
| Family history of allergy (Ref: No) | 2.11 | 1.44–3.08 | 1.49 | 1.04–2.14 | 1.19 | 0.87–1.61 | 1.32 | 0.95–1.83 | 0.002 |
| Country of birth (Ref: Both born in France) | 0.19 | ||||||||
| One of them born outside France | 0.87 | 0.46–1.66 | 1.25 | 0.64–2.45 | 1.70 | 0.91–3.17 | 0.77 | 0.45–1.31 | |
| Child characteristics | |||||||||
| Female gender (Ref: Male) | 0.96 | 0.66–1.40 | 0.82 | 0.57–1.18 | 1.00 | 0.73–1.36 | 1.12 | 0.80–1.55 | 0.68 |
| Duration of EBF (months) | 1.48 | 1.25–1.75 | 0.73 | 0.60–0.89 | 1.02 | 0.88–1.90 | 1.01 | 0.86–1.18 | <0.001 |
| Gestational age (weeks of amenorrhoea) | 0.94 | 0.82–1.09 | 0.93 | 0.82–1.06 | 1.03 | 0.91–1.15 | 0.91 | 0.81–1.02 | 0.32 |
| Birthweight (g) | 1.27 | 0.79–2.03 | 0.87 | 0.55–1.37 | 0.84 | 0.57–1.25 | 0.69 | 0.45–1.05 | 0.20 |
| Occurrence of diarrhoea between birth and 4 months (Ref: No) | 0.74 | 0.46–1.20 | 0.66 | 0.43–1.02 | 0.73 | 0.50–1.08 | 0.94 | 0.63–1.39 | 0.25 |
| Occurrence of regurgitations between birth and 4 months (Ref: No) | 0.99 | 0.68–1.45 | 2.68 | 1.80–3.98 | 1.74 | 1.27–2.39 | 1.48 | 1.06–2.08 | <0.001 |
| Other variables | |||||||||
| Recruitment centre: Nancy (Ref: Poitiers) | 0.58 | 0.39–0.86 | 0.89 | 0.61–1.30 | 0.86 | 0.62–1.18 | 0.92 | 0.65–1.30 | 0.10 |
| Type of physician consulted between birth and 4 months (Ref: GP) | 0.10 | ||||||||
| Paediatrician | 1.46 | 0.84–2.53 | 1.14 | 0.65–1.97 | 1.57 | 1.01–2.46 | 1.82 | 1.11–2.99 | |
| GP and paediatrician | 1.22 | 0.74–1.99 | 1.41 | 0.89–2.23 | 1.16 | 0.77–1.73 | 1.73 | 1.11–2.67 | |
| Specialist with/no GP or paediatrician | 1.95 | 1.13–3.36 | 1.71 | 1.01–2.89 | 1.71 | 1.09–2.69 | 1.98 | 1.20–3.27 | |
Multinomial logistic regression with regular formula as a reference group.
CI, confidence interval; EBF, exclusive breastfeeding; GP, general practitioner.
The use of formulas, even the enriched pre‐ or probiotic formulas, was associated neither with infant characteristics nor with the occurrence of diarrhoea in the first 4 months. Infants consuming other formulas than regular tended to consult more specialists (P = 0.10).
Relation with infant's growth
No significant relation was found between weight and length growth and type of formula consumed predominantly during the first 4 months (Table 4) after adjustment on parental and child characteristics. Nonetheless, infants using partially hydrolysed formula tended to have a lower WFL z‐score change than those consuming regular. Infants that were shorter breastfed showed significant higher WFA and LFA z‐score changes but not WFL z‐score.
Table 4.
Linear regression models with weight‐for‐age, weight‐for‐length and length‐for‐age z‐score change between birth and 4 months as dependent variables and covariates related to parents, child and type of physician consulted (n = 1239)
| ΔWHO z‐scores* | ||||||
|---|---|---|---|---|---|---|
| WFA | WFL | LFA | ||||
| Estimate † | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |
| Child characteristics | ||||||
| Type of formula milk (Ref: Regular) | ||||||
| Partially hydrolysed | −0.07 | −0.22, 0.07 | −0.19 | −0.41, 0.04 | 0.08 | −0.07, 0.24 |
| Thickened | 0.05 | −0.09, 0.19 | 0.03 | −0.19, 0.25 | 0.07 | −0.09, 0.22 |
| Enriched in pre‐ or probiotics | 0.08 | −0.04, 0.20 | 0.06 | −0.12, 0.25 | 0.05 | −0.08, 0.18 |
| Others | −0.04 | −0.18, 0.09 | −0.09 | −0.30, 0.12 | 0.01 | −0.14, 0.15 |
| Duration of EBF (months) | −0.08 | −0.12, 0.04 | −0.04 | −0.11, 0.03 | −0.08 | −0.12, −0.03 |
| Gestational age (weeks of amenorrhoea) | −0.21 | −0.25, −0.17 | −0.12 | −0.17, −0.07 | −0.11 | −0.15, −0.07 |
| Occurrence of diarrhoea between birth and 4 months (Ref: No) | −0.01 | −0.12, 0.10 | 0.04 | −0.13, 0.21 | −0.05 | −0.17, 0.07 |
| Occurrence of regurgitations between birth and 4 months (Ref: No) | 0.01 | −0.08, 0.10 | 0.08 | −0.06, 0.22 | −0.04 | −0.14, 0.06 |
| Parental characteristics | ||||||
| Maternal BMI, kg m−2 (Ref: Normal) | ||||||
| Thin | −0.08 | −0.25, 0.09 | −0.18 | −0.45, 0.08 | – | – |
| Overweight | −0.08 | −0.20, 0.04 | 0.02 | −0.16, 0.21 | – | – |
| Obese | −0.25 | −0.42, −0.09 | −0.10 | −0.35, 0.16 | – | – |
| Paternal BMI, kg m−2 (Ref: Normal) | – | – | ||||
| Missing | 0.21 | 0.03, 0.39 | 0.12 | −0.16, 0.39 | – | – |
| Overweight | 0.11 | 0.01, 0.20 | 0.12 | −0.03, 0.27 | – | – |
| Obese | 0.25 | 0.08, 0.42 | 0.47 | 0.21, 0.74 | – | – |
| Other variables | ||||||
| Type of physician consulted between birth and 4 months (Ref: GP) | ||||||
| Paediatrician | −0.12 | −0.25, 0.01 | −0.31 | −0.51, −0.11 | 0.01 | −0.04, 0.24 |
| GP and paediatrician | −0.03 | −0.15, 0.09 | −0.12 | −0.30, 0.06 | 0.01 | −0.12, 0.13 |
| Specialist with/no GP or paediatrician | −0.01 | −0.15, 0.12 | −0.06 | −0.27, 0.14 | −0.01 | −0.16, 0.13 |
Change in z‐score between birth and 4 months.
Adjusted for recruitment centre, maternal education, monthly family income, mother's return to employment, infant's gender, parental heights (for the analyses on length‐for‐age z‐score) average z‐score between 0 and 4 months.
BMI, body mass index; CI, confidence interval; EBF, exclusive breastfeeding; GP, general practitioner; LFA, length‐for‐age; WFA, weight‐for‐age; WFL, weight‐for‐length; WHO, World Health Organization.
WFA z‐score change of infants of obese mothers was significantly lower than that of infants whose mothers had a normal BMI; there was no association between maternal BMI and WFL z‐score change. While paternal BMI was associated with infant weight gain regardless of BMI category, only paternal obesity seemed to be related to infants' WFL z‐score. There was no significant interaction between EBF period or type of formula used, on the one hand, and gestational age, maternal education, parental heights or BMIs, on the other hand, on infant's growth (all P > 0.10).
In a sensitivity analysis, we ran the same models, excluding premature infants (n = 35, 2.82%) and the results did not change (data not shown). To determine the effects of imputations on our results, we ran the same models, without infants with missing values (n = 88, 5.5%) and the results remained similar to those presented above (data not tabulated).
Discussion
Many studies have been conducted on determinants of feeding practices, especially breastfeeding (Scott et al. 2006; Bonet et al. 2008; Grjibovski et al. 2008; Meedya et al. 2010) and impact of feeding on child growth (Agostoni et al. 1999; Harder et al. 2005), but as far as we are aware, ours is the first to examine the relationships between type of formula used during the first months of life and characteristics related to parents, infants and type of physician consulted, including their associations with infant's growth. We found that types of formula most frequently used in our cohort were related to parity, mother's return to employment, family history of allergy, EBF duration and infant's regurgitations, to infants' characteristics at birth to a lesser extent but not significantly to family income and parents' country of birth. We did not find any significant association between types of formula most frequently used in the first 4 months and infant's growth during the same period.
The prospective nature of the EDEN study allowed us to collect precisely the types of formula used and the variety of information collected led us to examine factors determining their use among mothers who did not want or could not exclusively breastfeed. However, because information about infant formulas prescription by physicians was not collected, we could not determine whether their use was due to physician's advice or to mothers' personal decision. As changes in infant formula are quite frequent between birth and 4 months, we categorised children according to their predominant formula used. We acknowledge that this categorisation does not take account of the reason for choice or for possible formula change and may have weakened the relations, in particular, with growth. Additional analyses were therefore performed to assess whether association between type of formula and growth was different in infants who received only the specific formula over the period or in those who changed formula and received it only as the predominant formula. The occurrence of formula changes in the first 4 months was not significantly related to growth (P‐values: 0.30 for WFA, 0.06 for LFA and 0.73 for WFL z‐score change). There were no significant interactions between change in formula and type of predominant formula in relation to growth (P‐value for the interactions: 0.32 for WFA, 0.10 for LFA and 0.62 for WFL z‐score change). Thus, we believe that our categorisation does not mask any real associations with growth.
Our study population is not representative of the general population. Compared with the national perinatal survey carried out on 14 482 women who delivered in France in 2003 (Blondel et al. 2006), women included in the EDEN study were more educated and more often employed. However, infants' growth in the study fits well with the normal range of the WHO growth curves (WHO Multicentre Growth Reference Study Group 2006) (data not shown) and we believe that the relationships observed are applicable to a general population of infants born in France from middle‐class parents. However, we lacked power to detect associations related to low socio‐economic situations, which may explain that we do not find any association between income levels and type of formula.
Several studies have shown that primiparous mothers breastfed more than multiparous mothers at hospital discharge (Crost & Kaminski 1998; Bonet et al. 2008), and that breastfeeding duration was positively associated with being multiparous (Bolling et al. 2007). Our study goes further, analysing associations between type of formula used and parity, and showing that multiparous (three or more deliveries) use more often regular formula than others. Even if global family income was not related to the type of formula, the previous associations may be related to family income available per child, as regular formulas are often considered as the cheapest on the French market. As already observed with breastfeeding (Butler et al. 2004), mothers' experience with their first child has probably an effect on their practices with the following children.
Most of the paediatric societies recommend, in case of family history of allergy and after breastfeeding cessation, a partially hydrolysed formula (Høst et al. 1999; Committee on Nutrition of American Academy of Pediatrics 2000; Chouraqui et al. 2008a). In our study, consumption of partially hydrolysed formulas was positively associated with family history of allergies and EBF duration, which is consistent with current recommendations.
It has been demonstrated that mothers giving formula milk to their infant returned to employment during the first 4 months after delivery (Stewart‐Knox et al. 2003; Bolling et al. 2007; Hawkins et al. 2007). It has also been shown that adding cereals to babies' bottle to extend sleep bouts, although not recommended, is part of maternal beliefs that can have an influence on feeding practices (Kannan et al. 1999; Kavanagh et al. 2010). We found a positive association between consumption of thickened formula and mother's return to employment, suggesting that mothers may use thickened formula to promote sleep or ‘settle’ their infant.
Research documenting the efficacy of pre‐ or probiotics is still emerging; the benefits of adding them in infant formulas remain unclear (Szajewska et al. 2006; Douglas & Sanders 2008; Thomas & Greer 2010; Braegger et al. 2011). Contrary to other studies (Guarino et al. 1997; Szajewska & Mrukowicz 2001), we found no significant association between using enriched pre‐ or probiotic formulas and digestive disorders, especially the occurrence of diarrhoea in the first 4 months. However, because of our sample size, we had to group together all infants using pre‐ or probiotics (or both) regardless of the type of oligosaccharides or strains included in the formula. That could explain part of this lack of association as the effects of probiotics for instance are strain dependent (Szajewska & Mrukowicz 2005; Canani et al. 2007; Braegger et al. 2011). Our results showed an association between consuming enriched pre‐ or probiotic formulas and occurrence of regurgitations, probably because we grouped together enriched in pre‐ or probiotics formulas and those both thickened and enriched.
Growth parameters were not related to the type of formula after adjustment for parental and child characteristics in our analysis. Formula‐fed infants are known to grow more rapidly than breastfed infants from about the third month in the first year of life (Dewey et al. 1995; Kramer et al. 2004). We showed that infants who were breastfed longer had significantly lower WFA and LFA z‐score changes between 0 and 4 months but not for WFL z‐score, suggesting a slower, but harmonious growth in weight and length. Evidence indicates long‐term effects of feeding practices and of rapid weight gain during early infancy on infant's growth patterns (Ong et al. 2000), but the mechanisms underlying the differences between the patterns are not well known. Regarding the use of infant formula, one might ask the question of the effect of the composition of infant formulas on early growth. A clinical trial showed that lower protein content in infant formula was associated with a lower weight gain during the first 2 years of life (Koletzko et al. 2009). We could not explore the relation between consumption of lower protein formula and growth in our analyses because these formulas have been recently introduced in France and very few infants have used them in our sample (n = 42, Fig. 1). Furthermore, the basic composition of formulas consumed in the EDEN study was almost homogeneous in terms of protein and energy contents, according to the European regulations (European Commission 2006).
The effects of pre‐ or probiotics and of thickened formulas on infant's growth are poorly documented. As most of the studies on the topic (Chouraqui et al. 2008b; Braegger et al. 2011), we found that adding pre‐ or probiotics in infant formulas was not related to infant's weight and length gain. We found a negative relation between type of physician consulted and WFL z‐score change. Infants consulting paediatricians are more likely to be the sickest, which probably explains their significantly lower WFL z‐score change during the study period. However, we acknowledge that our analyses cannot attribute cause and effect.
In our study, maternal obesity was related to a significantly slower weight growth, while paternal BMI was related to a faster infant weight and length growth and parental heights to a faster infant length growth. As we discussed previously (Mok et al. 2008; Regnault et al. 2010), both genetic and pre‐ and post‐natal environmental factors are known to contribute to parental influence on infant's growth. Our analysis showed that these parent–infant relationships, and especially those with parental BMI, are observed whatever the infant‐feeding mode, including the type of formula used.
Conclusion
The range of infant formulas is quite varied and factors related to the infant, such as prematurity, digestive disorders and allergy, may influence the use. Besides these factors, our results pointed out relationships with other factors related to family, such as parity, maternal education level and employment status, which should be taken into account when describing relationships between the use of infant formulas and growth. In our study, after adjusting for these factors, the type of formula used was related neither to infant's growth nor to other health aspects such as diarrhoea. In contrast, EBF duration seems to affect significantly infant's growth between birth and 4 months after taking into account family and child characteristics.
Source of funding
We acknowledge all funding sources for the EDEN study: Fondation pour la Recherche Médicale, French Ministry of Research: Federative Research Institutes and Cohort Program, INSERM Human Nutrition National Research Program, and Diabetes National Research Program (through a collaboration with the French Association of Diabetic Patients), French Ministry of Health, French Agency for Environment Security, French National Institute for Population Health Surveillance, Paris–Sud University, French National Institute for Health Education, Nestlé, Mutuelle Générale de l'Education Nationale, French‐speaking association for the study of diabetes and metabolism, National Agency for Research (non‐thematic program), and National Institute for Research in Public health (IRESP:TGIR cohorte santé 2008 program).
Aisha Betoko was supported by a research grant from the French Ministry for Higher Education and Research.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
The EDEN Study group, coordinated by MAC, was responsible for study design and data collection. AB analysed and interpreted the data and wrote the initial draft of the manuscript. MAC and BLG were involved in all aspects from study conception to manuscript writing. AF, NR, JB and MB participated in data management and data preparation for breastfeeding and infant‐growth analyses. RH, MJSC and all the co‐authors critically reviewed all sections of the text for important intellectual content. MAC is the guarantor of the study. All authors had full access to all of the data in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgements
We thank the heads of the maternity units, the investigators and all the women who participated in the surveys.
References
- Agostoni C. , Grandi F. , Gianni M.L. , Silano M. , Torcoletti M. , Giovannini M. et al . ( 1999. ) Growth patterns of breast fed and formula fed infants in the first 12 months of life: an Italian study . Archives of Disease in Childhood 81 , 395 – 399 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostoni C. , Braegger C. , Decsi T. , Kolacek S. , Koletzko B. , Michaelsen K.F. et al . ( 2009. ) Breast‐feeding: a commentary by the ESPGHAN Committee on Nutrition . Journal of Pediatric Gastroenterology and Nutrition 49 , 112 – 125 . [DOI] [PubMed] [Google Scholar]
- Blondel B. , Supernant K. , Du Mazaubrun C. & Breart G. ( 2006. ) Trends in perinatal health in metropolitan France between 1995 and 2003: results from the National Perinatal Surveys . Journal de Gynecologie, Obstetrique et Biologie de la Reproduction 35 , 373 – 387 . [DOI] [PubMed] [Google Scholar]
- Boehm G. & Moro G. ( 2008. ) Structural and functional aspects of prebiotics used in infant nutrition . The Journal of Nutrition 138 , 1818S – 1828S . [DOI] [PubMed] [Google Scholar]
- Boland M. ( 2005. ) Exclusive breastfeeding should continue to six months . Paediatrics and Child Health 10 , 148 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolling K. , Grant C. , Hamlyn B. & Thornton A. ( 2007. ) Infant Feeding Survey 2005. The Information Centre Part of the Government Statistical Service, London Available at: http://www.ic.nhs.uk/statistics-and-data-collections/health-and-lifestyles-related-surveys/infant-feeding-survey/infant-feeding-survey-2005 (Accessed 16 November 2010 ).
- Bonet M. , Foix L'Hélias L. & Blondel B. ( 2008. ) Exclusive and mixed breastfeeding in maternity unit: situation in France in 2003 . Archives de Pédiatrie 15 , 1407 – 1415 . [DOI] [PubMed] [Google Scholar]
- Braegger C. , Chmielewska A. , Decsi T. , Kolacek S. , Mihatsch W. , Moreno L. et al . ( 2011. ) Supplementation of infant formula with probiotics and/or prebiotics: a systematic review and comment by the ESPGHAN Committee on Nutrition . Journal of Pediatric Gastroenterology and Nutrition 52 , 238 – 250 . [DOI] [PubMed] [Google Scholar]
- Butler S. , Williams M. , Tukuitonga C. & Paterson J. ( 2004. ) Factors associated with not breastfeeding exclusively among mothers of a cohort of Pacific infants in New Zealand . The New Zealand Medical Journal 117 , U908 . [PubMed] [Google Scholar]
- Canani R.B. , Cirillo P. , Terrin G. , Cesarano L. , Spagnuolo M.I. , De Vincenzo A. et al . ( 2007. ) Probiotics for treatment of acute diarrhoea in children: randomised clinical trial of five different preparations . BMJ (Clinical Research Ed.) 335 , 340 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouraqui J.P. , Dupont C. , Bocquet A. , Bresson J.L. , Briend A. , Darmaun D. et al . ( 2008a. ) Feeding during the first months of life and prevention of allergy . Archives de Pédiatrie 15 , 431 – 442 . [DOI] [PubMed] [Google Scholar]
- Chouraqui J.P. , Grathwohl D. , Labaune J.M. , Hascoet J.M. , De Montgolfier I. , Leclaire M. et al . ( 2008b. ) Assessment of the safety, tolerance, and protective effect against diarrhea of infant formulas containing mixtures of probiotics or probiotics and prebiotics in a randomized controlled trial . The American Journal of Clinical Nutrition 87 , 1365 – 1373 . [DOI] [PubMed] [Google Scholar]
- Committee on Nutrition of American Academy of Pediatrics ( 2000. ) Hypoallergenic infant formulas . Pediatrics 106 , 346 – 349 . [PubMed] [Google Scholar]
- Crost M. & Kaminski M. ( 1998. ) Breast feeding at maternity hospitals in France in 1995. National perinatal survey . Archives of Pediatrics 5 , 1316 – 1326 . [DOI] [PubMed] [Google Scholar]
- Dewey K.G. , Peerson J.M. , Brown K.H. , Krebs N.F. , Michaelsen K.F. , Persson L.A. et al . ( 1995. ) Growth of breast‐fed infants deviates from current reference data: a pooled analysis of US, Canadian, and European data sets . Pediatrics 96 , 497 – 503 . [PubMed] [Google Scholar]
- Douglas L.C. & Sanders M.E. ( 2008. ) Probiotics and prebiotics in dietetics practice . Journal of the American Dietetic Association 108 , 510 – 521 . [DOI] [PubMed] [Google Scholar]
- European Commission ( 2006. ) Commission Directive 2006/141/CE of 22 December 2006 on infant formula and follow‐on formula and amending directive 1999/321/EEC of 14 May 1991 .
- Gartner L.M. , Morton J. , Lawrence R.A. , Naylor A.J. , O'Hare D. , Schanler R.J. et al . ( 2005. ) Breastfeeding and the use of human milk . Pediatrics 115 , 496 – 506 . [DOI] [PubMed] [Google Scholar]
- Griffiths L.J. , Smeeth L. , Hawkins S.S. , Cole T.J. & Dezateux C. ( 2009. ) Effects of infant feeding practice on weight gain from birth to 3 years . Archives of Disease in Childhood 94 , 577 – 582 . [DOI] [PubMed] [Google Scholar]
- Grjibovski A.M. , Ehrenblad B. & Yngve A. ( 2008. ) Infant feeding in Sweden: socio‐demographic determinants and associations with adiposity in childhood and adolescence . International Breastfeeding Journal 3 , 23 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarino A. , Canani R.B. , Spagnuolo M.I. , Albano F. & Di Benedetto L. ( 1997. ) Oral bacterial therapy reduces the duration of symptoms and of viral excretion in children with mild diarrhea . Journal of Pediatric Gastroenterology and Nutrition 25 , 516 – 519 . [DOI] [PubMed] [Google Scholar]
- Harder T. , Bergmann R. , Kallischnigg G. & Plagemann A. ( 2005. ) Duration of breastfeeding and risk of overweight: a meta‐analysis . American Journal of Epidemiology 162 , 397 – 403 . [DOI] [PubMed] [Google Scholar]
- Hauspie R.C. ( 1989. ) Mathematical models for the study of individual growth patterns . Revue D'epidemiologie et de Sante Publique 37 , 461 – 476 . [PubMed] [Google Scholar]
- Hawkins S.S. , Griffiths L.J. , Dezateux C. & Law C. ( 2007. ) Maternal employment and breast‐feeding initiation: findings from the Millennium Cohort Study . Paediatric and Perinatal Epidemiology 21 , 242 – 247 . [DOI] [PubMed] [Google Scholar]
- Høst A. , Koletzko B. , Dreborg S. , Muraro A. , Wahn U. , Aggett P. et al . ( 1999. ) Dietary products used in infants for treatment and prevention of food allergy. Joint Statement of the European Society for Paediatric Allergology and Clinical Immunology (ESPACI) Committee on Hypoallergenic Formulas and the European Society for Paediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN) Committee on Nutrition . Archives of Disease in Childhood 81 , 80 – 84 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip S. , Chung M. , Raman G. , Chew P. , Magula N. , DeVine D. et al . ( 2007. ) Breastfeeding and maternal and infant health outcomes in developed countries . In : Evidence Report/Technology Assessment (Full Report No. 153) , pp. 1 – 186 . Agency for Healthcare Research and Quality; : Rockville, MD . [PMC free article] [PubMed] [Google Scholar]
- Kannan S. , Carruth B.R. & Skinner J. ( 1999. ) Cultural influences on infant feeding beliefs of mothers . Journal of the American Dietetic Association 99 , 88 – 90 . [DOI] [PubMed] [Google Scholar]
- Kavanagh K.F. , Habibi M. , Anderson K. & Spence M. ( 2010. ) Caregiver‐ vs infant‐oriented feeding: a model of infant‐feeding strategies among special supplemental nutrition program for women, infants, and children participants in rural east Tennessee . Journal of the American Dietetic Association 110 , 1485 – 1491 . [DOI] [PubMed] [Google Scholar]
- Koletzko B. , Von Kries R. , Closa R. , Escribano J. , Scaglioni S. , Giovannini M. et al . ( 2009. ) Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial . The American Journal of Clinical Nutrition 89 , 1836 – 1845 . [DOI] [PubMed] [Google Scholar]
- Kramer M.S. , Guo T. , Platt R.W. , Vanilovich I. , Sevkovskaya Z. , Dzikovich I. et al . ( 2004. ) Feeding effects on growth during infancy . The Journal of Pediatrics 145 , 600 – 605 . [DOI] [PubMed] [Google Scholar]
- Kramer M.S. , Matush L. , Vanilovich I. , Platt R.W. , Bogdanovich N. , Sevkovskaya Z. et al . ( 2007. ) Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: evidence from a large randomized trial . The American Journal of Clinical Nutrition 86 , 1717 – 1721 . [DOI] [PubMed] [Google Scholar]
- Kristiansen A.L. , Lande B. , Overby N.C. & Andersen L.F. ( 2010. ) Factors associated with exclusive breast‐feeding and breast‐feeding in Norway . Public Health Nutrition 13 , 2087 – 2096 . [DOI] [PubMed] [Google Scholar]
- Lanting C.I. , Van Wouwe J.P. & Reijneveld S.A. ( 2005. ) Infant milk feeding practices in the Netherlands and associated factors . Acta Paediatrica 94 , 935 – 942 . [DOI] [PubMed] [Google Scholar]
- Meedya S. , Fahy K. & Kable A. ( 2010. ) Factors that positively influence breastfeeding duration to 6 months: a literature review . Women and Birth 23 , 135 – 145 . [DOI] [PubMed] [Google Scholar]
- Mok E. , Multon C. , Piguel L. , Barroso E. , Goua V. , Christin P. et al . ( 2008. ) Decreased full breastfeeding, altered practices, perceptions, and infant weight change of prepregnant obese women: a need for extra support . Pediatrics 121 , e1319 – e1324 . [DOI] [PubMed] [Google Scholar]
- Moreau M.C. ( 2001. ) Effets immunomodulateurs des bactéries intestinales: le rôle des bifidobactéries . Journal de Pédiatrie et de Puériculture 14 , 135 – 139 . [Google Scholar]
- Ong K.K. , Ahmed M.L. , Emmett P.M. , Preece M.A. & Dunger D.B. ( 2000. ) Association between postnatal catch‐up growth and obesity in childhood: prospective cohort study . BMJ (Clinical Research Ed.) 320 , 967 – 971 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J.C. & Bates D.M. ( 2000. ) Mixed‐Effects Models in S and S‐PLUS . Springer; : New York . [Google Scholar]
- Regnault N. , Botton J. , Forhan A. , Hankard R. , Thiebaugeorges O. , Hillier T.A. et al . ( 2010. ) Determinants of early ponderal and statural growth in full‐term infants in the EDEN mother‐child cohort study . The American Journal of Clinical Nutrition 92 , 594 – 602 . [DOI] [PubMed] [Google Scholar]
- Scott J.A. , Binns C.W. , Oddy W.H. & Graham K.I. ( 2006. ) Predictors of breastfeeding duration: evidence from a cohort study . Pediatrics 117 , e646 – e655 . [DOI] [PubMed] [Google Scholar]
- Sherman P.M. , Cabana M. , Gibson G.R. , Koletzko B. , Neu J. , Veereman‐Wauters G. et al . ( 2009. ) Potential roles and clinical utility of prebiotics in newborns, infants, and children: proceedings from a global prebiotic summit meeting, New York City, June 27–28, 2008 . The Journal of Pediatrics 155 , S61 – S70 . [DOI] [PubMed] [Google Scholar]
- Stettler N. ( 2007. ) Nature and strength of epidemiological evidence for origins of childhood and adulthood obesity in the first year of life . International Journal of Obesity (2005) 31 , 1035 – 1043 . [DOI] [PubMed] [Google Scholar]
- Stewart‐Knox B. , Gardiner K. & Wright M. ( 2003. ) What is the problem with breast‐feeding? A qualitative analysis of infant feeding perceptions . Journal of Human Nutrition and Dietetics 16 , 265 – 273 . [DOI] [PubMed] [Google Scholar]
- Szajewska H. & Mrukowicz J. ( 2001. ) Diarrhea in Infants and children: a systematic review of published randomized, double‐blind, placebo‐controlled trials . Journal of Pediatric Gastroenterology and Nutrition 33 , S17 – S25 . [DOI] [PubMed] [Google Scholar]
- Szajewska H. & Mrukowicz J.Z. ( 2005. ) Use of probiotics in children with acute diarrhea . Paediatric Drugs 7 , 111 – 122 . [DOI] [PubMed] [Google Scholar]
- Szajewska H. , Setty M. , Mrukowicz J. & Guandalini S. ( 2006. ) Probiotics in gastrointestinal diseases in children: hard and not‐so‐hard evidence of efficacy . Journal of Pediatric Gastroenterology and Nutrition 42 , 454 – 475 . [DOI] [PubMed] [Google Scholar]
- Thomas D.W. & Greer F.R. ( 2010. ) Probiotics and prebiotics in pediatrics . Pediatrics 126 , 1217 – 1231 . [DOI] [PubMed] [Google Scholar]
- Van Rossum C.M.T , Büchner F.L. & Hoekstra J. ( 2001. ) Quantification of health effects of breastfeeding: Review of the literature and model simulation RIVM report 350040001/2005. Available at: http://www.rivm.nl/bibliotheek/rapporten/350040001.pdf (Accessed 26 January 2011 ).
- WHO Multicentre Growth Reference Study Group ( 2006. ) WHO Child Growth Standards: Length/height‐for‐age, weight‐for‐age, weight‐for‐length, weight‐for‐height and body mass index‐for‐age: Methods and development In: Geneva: World Health Organization (312 pages). Available at: http://www.who.int/childgrowth/standards/en/ (Accessed 15 May 2010 ).


