Abstract
Convergent evidence from neuronal biology and hominin brain hypertrophy suggests that omega‐3 fatty acids are a limiting resource for neural and cognitive development in Homo sapiens, and therefore that children from populations with higher omega‐3 availability should display superior cognitive performance. Using multiple regression, we tested this prediction in a sample of 28 countries, with Programme for International Student Assessment (PISA) math scores in 2009 as an index of cognitive performance, and country‐specific breast milk levels of omega‐3 docosahexaenoic acid (DHA) as an index of omega‐3 availability. Breast milk DHA makes a highly significant contribution to math scores (β = 0.462, P = 0.006), greater in magnitude than the control variables of per capita Gross Domestic Product (PCGDP) and educational expenditures per pupil. Together, dietary fish (positively) and total fat (negatively) explain 61% of the variance in maternal milk DHA in a larger sample of 39 countries.
Keywords: docosahexaenoic acid, brain function, child nutrition, education, childhood diet, human milk
Introduction
One the most derived features of Homo sapiens is our allometrically large brain (Jerison 1973). While many nutrients are important to brain growth and function, essential dietary omega‐3 fatty acids – especially docosahexaenoic acid (DHA) – are particularly important and are relatively scarce in terrestrial environments. DHA comprises roughly 10% of the dry weight of the human brain (Makrides et al. 1994) and is critical to all aspects of neurodevelopment and brain function, including neurogenesis, neurite proliferation and growth, nerve impulse transmission via the sodium–potassium pump, neuronal integrity and vitality, blood glucose transport and gene expression in the brain (Ryan et al. 2010; Bradbury 2011; Simopoulos 2011; de Souza et al. 2011; Harbeby et al. 2012; Lassek & Gaulin 2012). Consistent with these roles, animal studies have shown that DHA availability is strongly related to cognitive development and learning ability; and dietary DHA insufficiency may also compromise neurodevelopment in infants and children (Cohen et al. 2005; Oken et al. 2008; Ryan et al. 2010; de Souza et al. 2011; Lassek & Gaulin 2011).
Because there is strong metabolic competition between omega‐3 and omega‐6 fats in the diet (Mohrhauer & Holman 1963; Boudreau et al. 1991; Friesen & Innis 2010; Bradbury 2011), maternal milk DHA is an especially valuable indicator, given that it represents the DHA that survives this competition and is available to the mother and her offspring. Oils from seeds and grains including maize, soybean, sunflower, and safflower oils are especially high in the omega‐6 fats that compete with omega‐3 (Godley et al. 1996; Astorg et al. 2008). The DHA in fish is unavailable when cooked with such vegetable oils (Elmadfa et al. 2006; Chung et al. 2008). The meat, milk and eggs of animals consuming these grains are also higher in omega‐6 and lower in omega‐3 than for animals fed grass (Wood et al. 2004; McAfee et al. 2011; Lassek & Gaulin 2012; Martin et al. 2012).
While many measures of cognitive performance have moderate to high heritabilities within populations (Plomin et al. 2008), between‐population differences may be less heritable and are known to have environmental correlates. For example, across nations, cognitive performance is positively related to measures of economic well‐being such as the per capita gross domestic product (GDP/PC) (Lynn & Vanhanen 2002, 2012; Chiu 2007). Controlling for such environmental factors, previous work has shown that individual American children who consumed more dietary omega‐3 and less omega‐6 performed significantly better on four cognitive tests, including a mathematics achievement test (Lassek & Gaulin 2011).
Such findings raise the question of whether there is an ecological cross‐national relationship between dietary omega‐3 and cognitive performance. Country‐specific dietary omega‐3 levels can be estimated by using maternal breast milk DHA values as in Hibbeln (2002), and cognitive performance can be assessed by standardised international tests administered to school children. We predict that children in countries with higher levels of maternal milk DHA will have better test performance. We also predict that milk DHA levels in different countries will be positively related to dietary fish and seafood and negatively related to vegetable fats and oils, which are generally higher in omega‐6 and lower in omega‐3 fats.
Key messages
Maternal breast milk DHA levels explain more that 20% of the variance in performance on an international cognitive test.
The variance explained by breast milk DHA is unique in that it is not explained by measured socio‐economic variables.
The best positive dietary predictors of breast milk DHA levels were various measures of fish consumption; dietary levels of total fat, vegetable oils, beef and milk are all significant negative predictors of breast milk DHA.
Materials and methods
Variable selection
To measure cognitive performance, we selected the recently published 2009 mathematics test scores from the Programme for International Student Assessment (PISA) of the Organization for Economic Cooperation and Development (OECD) because it is a single test that is administered in a consistent way to 15‐year‐old students in 59 countries (OECD 2011).
National averages of maternal milk DHA content from published reports provided our measure of DHA availability to children. We searched MEDLINE combining MESH search terms ‘milk’ and ‘fatty acids’ and thereby identified 128 studies providing maternal milk DHA values as percentages of total fatty acids in 50 countries (see Supporting Information http://onlinelibrary.wiley.com/doi/10.1111/mcn.12060/suppinfo). Percentages of arachidonic acid, the omega‐6 fatty acid active in the brain, were also obtained. Although somewhat different methods may be used for extracting and analysing milk fatty acids in different studies, these percentages are less sensitive to such differences.
To account for socio‐economic influences on cognitive performance, we used two control variables: GDP/PC and educational expenditures per pupil. Recent data for GDP/PC were obtained from United Nations sources (United Nations Statistics Division 2011) and for public educational expenditures per pupil from the OECD (2012).
To control for the possible effect of dietary macronutrients on maternal milk DHA levels, we included 2005 estimates of daily per capita calories, fat, protein and carbohydrates for each country in the analysis. These are available from Food Balance Sheets compiled by the United Nations Food and Agricultural Organization (UN FAO 2012 and are frequently used for cross‐national comparisons (Sasaki & Kesteloot 1992).
If breast milk DHA levels explain significant variance in national cognitive performance, it would be of interest to discover the dietary correlates of breast milk composition. To explore these relationships, data for food disappearance were used in a larger sample of 39 countries as math scores were not needed for this analysis. Food items included fish and seafood, known to be high in DHA, as well as eggs, milk, poultry and red meats, which may also contain DHA, depending on animal feeds. Because of the possible competition between omega‐6 fats and omega‐3 fats, we also included total, animal and vegetable fat, and vegetable oils.
Analytical methods
Linear bivariate regression was used to measure the independent influence of DHA, GDP/PC, per‐pupil school expenditures and macronutrients on math scores; multiple regression was used to measure the joint effects of DHA together with each of these economic control variables and the macronutrients. In a larger sample of 39 countries, including some which lacked PISA math scores, bivariate correlation and multiple regression were used to determine the relationship between dietary items and maternal milk DHA. SPSS‐20 was used throughout (SPSS Inc., Chicago, IL, USA).
Results
There were 28 countries with all data elements. For these countries, there were 114 separate population samples with breast milk data, with a mean of 4.2 ± 4.3 samples per country. Five countries had a single sample, four countries had two samples and the rest had three or more samples. Most of these samples date from 1995 to 2012. There were 39 countries that had both dietary and breast milk data; 11 of these lacked PISA scores (for details, see Supporting Information http://onlinelibrary.wiley.com/doi/10.1111/mcn.12060/suppinfo).
Maternal milk DHA and PISA math test scores
Maternal milk DHA, GDP/PC and expenditures per pupil were all significant independent predictors of math scores, whereas none of the macronutrients were. Table 1 shows the results of three bivariate and two multivariate regressions. The predictive value of milk DHA is highly significant and only slightly attenuated when either of the two socio‐economic variables is added in multiple regression format. Milk DHA consistently accounts for more variance than either economic factor. When added to the regression, milk arachidonic is negatively related to math scores, but not significantly and hence is not shown in Table 1. Likewise, none of the macronutrient variables were significant as bivariate predictors of test scores, nor when added to the regressions. To illustrate the observed relationship, Fig. 1 shows a scatterplot of milk DHA percentages and PISA math scores for the 28 countries in the complete data set.
Table 1.
Standardised regression coefficients and r 2 for significant predictors of 2009 PISA math scores in 28 countries
| β coefficients | Adjusted r 2 | ||||||
|---|---|---|---|---|---|---|---|
| Maternal milk DHA | Per capita GDP | Per pupil expenditure | |||||
| β | P | β | P | β | P | r 2 | P |
| 0.487 | 0.009 | 0.208 | 0.009 | ||||
| 0.444 | 0.015 | 0.167 | 0.015 | ||||
| 0.413 | 0.028 | 0.171 | 0.028 | ||||
| 0.472 | 0.006 | 0.396 | 0.018 | 0.393 | 0.002 | ||
| 0.462 | 0.006 | 0.431 | 0.009 | 0.376 | 0.001 | ||
DHA, docosahexaenoic acid; GDP, gross domestic product; PISA, Programme for International Student Assessment.
Figure 1.
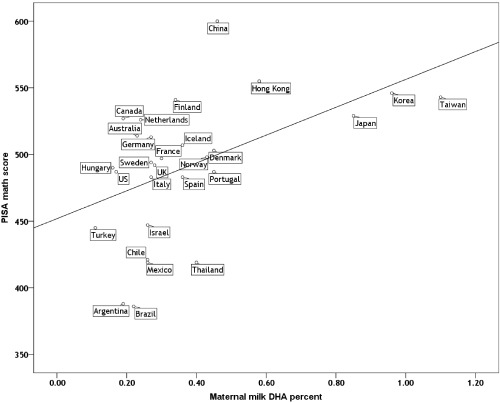
Scatterplot of maternal milk docosahexaenoic acid (DHA) content and Programme for International Student Assessment (PISA) math test scores for 28 nations.
Dietary correlates of maternal milk DHA
Table 2 shows the significant bivariate correlations of maternal milk DHA with grams per capita per day of food items listed on national Food Balance Sheets. Not surprisingly, maternal DHA is very strongly positively related to dietary fish and seafood, the richest sources of DHA. By contrast, total dietary fat, vegetable oils, beef and milk – items that are rich in omega‐6 fatty acids (especially when derived from grain‐fed animals) – are all negatively related to maternal milk DHA.
Table 2.
Significant bivariate correlations between maternal milk DHA and dietary items in 39 countries
| Food category | r | P |
|---|---|---|
| Pelagic fish | 0.61 | <0.001 |
| Total fish | 0.59 | <0.001 |
| Total seafood | 0.60 | <0.001 |
| Total fat | −0.39 | 0.016 |
| Vegetable oils | −0.34 | 0.034 |
| Beef | −0.33 | 0.043 |
| Milk | −0.44 | 0.006 |
For list of countries and sources, see Supporting Information http://onlinelibrary.wiley.com/doi/10.1111/mcn.12060/suppinfo.
When added to a regression with either seafood or total fish, total, vegetable and animal fats were still significantly negatively related to milk DHA, with β coefficients ranging from −0.43 to −0.55. The combination that explains the most variance in maternal milk DHA is total fish (β = 0.71) and total fat (β = −0.55), which together explain 61% of the variance in cross‐national maternal milk DHA levels (all P < 0.0001). Any regression including total fish or seafood, and vegetable oils, animal fat or milk consistently explains at least half of the variance in milk DHA, with fish or seafood having positive βs and the remainder having negative βs.
Discussion
This study suggests that a country's balance of dietary omega‐3 and omega‐6 fatty acids – as reflected in maternal milk DHA – is strongly related to its students' performance on a standardised mathematics test. The observed relationship between milk DHA and cognitive performance remains highly significant even when controlling for national wealth, investment in education and macronutrient intake. This study is the first to show that these effects are both significant and substantial in cross‐national comparisons. Although, as expected, socio‐economic factors explain considerable variance in performance on a standardised test of mathematical performance, the availability of DHA from human breast milk explains a larger and essentially non‐overlapping fraction of the national differences on this cognitive measure. Maternal milk DHA, in turn, is strongly related to per capita fish and seafood intake, but negatively related to the intake of total fat, vegetable fat, vegetable oil and beef, suggesting competition from fats high in omega‐6.
In addition to the inherent limitations of ecological studies, this study is limited by the sources of the data used. Maternal milk DHA values for each country are based on one or more modest‐sized samples that may not closely reflect the values for the population as a whole. The methods used for extracting and measuring milk fatty acids may vary in different studies, although the use of percentages of total fatty acids may compensate for this to some degree. Math test results are also subject to sampling errors, although these derive from samples designed to be representative of the national population. Per capita consumption of individual foods is also subject to error because FAO estimates are based on food disappearance rather than foods actually consumed. There may also be other confounders that affect both milk DHA and math scores other than those accounted for in the analysis. The strong correlation of maternal milk DHA percentages with independently measured dietary sources of DHA provides some support for the validity of both sources. Moreover, there is no a priori reason to assume that any of these potential sources of error would be biased in a way that would produce a spurious correlation between breast milk DHA and PISA math scores.
This study supports the view that omega‐3 fatty acids – and especially DHA – play a critical role in supporting human neuro‐cognitive development, a view supported by theoretical (Crawford et al. 1999; Gomez‐Pinilla 2008), paleontological (Broadhurst et al. 2002; Richards et al. 2005; Marean et al. 2007; Bradbury 2011), correlational (Lassek & Gaulin 2011; Oken et al. 2008) and experimental studies (Cohen et al. 2005). Using a similar methodology, breast milk DHA was shown to be inversely related to rates of post‐partum depression in a previous cross‐national study (Hibbeln 2002). It would be desirable to test this relationship in other populations and, if possible, with direct measurement of DHA in the blood.
In their implications for food policy, these findings pose challenges. The inverse relationship between maternal milk DHA and dietary fats vividly illustrates the metabolic competition between omega‐3 and omega‐6 fats derived – directly or indirectly through their use as animal feeds – from seeds and grains. In countries like the United States where such crops and oils predominate in processed foods and animal feeds, dietary ratios of omega‐6 to omega‐3 are extremely elevated, and levels of DHA in breast milk are correspondingly depressed (Hibbeln 2002; Yuhas et al. 2006; Martin et al. 2012). As similar methods of food production are adopted by other nations, dietary support for optimal cognitive development will require attention both to augmenting omega‐3 supplies and to reducing the oversupply of omega‐6 fats.
Source of funding
There was no external funding for this paper.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
Both authors contributed to the conception and design of the study. WDL was responsible for data acquisition, analysis and the initial draft. Both authors contributed to subsequent revisions and gave final approval of the version submitted for publication.
Supporting information
Appendix S1 Supplementary references for ‘Maternal milk DHA content predicts cognitive performance in a sample of 28 nations’.
Acknowledgements
We gratefully acknowledge the valuable comments and suggestions of two anonymous reviewers.
Lassek, W. D. , and Gaulin, S. J. C. (2015) Maternal milk DHA content predicts cognitive performance in a sample of 28 nations. Matern Child Nutr, 11: 773–779. doi: 10.1111/mcn.12060.
References
- Astorg P., Bertrais S., Laporte F., Arnault N., Estaquio C., Galan P. et al (2008) Plasma n‐6 and n‐3 polyunsaturated fatty acids as biomarkers of their dietary intakes: a cross‐sectional study within a cohort of middle‐aged French men and women. European Journal of Clinical Nutrition 62, 1155–1161. [DOI] [PubMed] [Google Scholar]
- Boudreau M.D., Chanmugam P.S., Hart S.B., Soo H.L. & Hwang D.H. (1991) Lack of dose response by dietary n‐3 fatty acids at a constant ratio of n‐3 to n‐6 fatty acids in suppressing eicosanoid biosynthesis from arachidonic acid. American Journal of Clinical Nutrition 54, 111–117. [DOI] [PubMed] [Google Scholar]
- Bradbury J. (2011) Docosahexaenoic acid (DHA): an ancient nutrient for the modern human brain. Nutrients 3, 529–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadhurst C.L., Wang Y.Q., Crawford M.A., Cunnane S.C., Parkington J.E. & Schmidt W.F. (2002) Brain‐specific lipids from marine, lacustrine, or terrestrial food resources: potential impact on early African Homo sapiens . Comparative Biochemistry and Physiology. B, Comparative Biochemistry 131, 653–673. [DOI] [PubMed] [Google Scholar]
- Chiu M.M. (2007) Families, economies, cultures, and science achievement in 41 countries: country‐, school‐, and student‐level analyses. Journal of Family Psychology 21, 510–519. [DOI] [PubMed] [Google Scholar]
- Chung H., Nettleton J.A., Lemaitre R.N., Barr R.G., Tsai M.Y. & Tracy R.P. (2008) Frequency and type of seafood consumed influence plasma (n‐3) fatty acid concentrations. Journal of Nutrition 138, 2422–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J.T., Bellinger D.C., Connor W.E. & Shaywitz B.A. (2005) A quantitative analysis of prenatal intake of n‐3 polyunsaturated fatty acids and cognitive development. American Journal of Preventive Medicine 29, 366–374. [DOI] [PubMed] [Google Scholar]
- Crawford M.A., Bloom M., Broadhurst C.L., Schemidt W.F., Cunnane S.C., Galli C. et al (1999) Evidence for the unique function of docosahexaenoic acid during the evolution of the modern hominid brain. Lipids 34, s39–s47. [DOI] [PubMed] [Google Scholar]
- Elmadfa I., Al‐Saghir S., Kanzler S., Frisch G., Majchrzak D. & Wagner K.H. (2006) Selected quality parameters of salmon and meat when fried with or without added fat. International Journal for Vitamin & Nutrition Research 76, 238–246. [DOI] [PubMed] [Google Scholar]
- Friesen R.W. & Innis S.M. (2010) Linoleic acid is associated with lower long‐chain n‐6 and n‐3 fatty acids in red blood cell lipids of Canadian pregnant women. American Journal of Clinical Nutrition 91, 23–31. [DOI] [PubMed] [Google Scholar]
- Godley P.A., Campbell M.K., Gallagher P., Martinson F.E., Mohler J.L. & Sandler R.S. (1996) Biomarkers of essential fatty acid consumption and risk of prostatic carcinoma. Cancer Epidemiology, Biomarkers & Prevention 5, 889–895. [PubMed] [Google Scholar]
- Gomez‐Pinilla F. (2008) Brain foods: the effects of nutrients on brain function. Nature Reviews Neuroscience 9, 568–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harbeby E., Jouin M., Alessandri J.M., Lallemand M.S., Linard A., Lavialle M. et al (2012) n‐3 PUFA status affects expression of genes involved in neuroenergetics differently in the fronto‐parietal cortex compared to the CA1 area of the hippocampus: effect of rest and neuronal activation in the rat. Prostaglandins, Leukotrines, and Essential Fatty Acids 86, 211–220. [DOI] [PubMed] [Google Scholar]
- Hibbeln J.R. (2002) Seafood consumption, the DHA content of mother's milk and prevalence rates of postpartum depression: a cross‐national, ecological analysis. Journal of Affecive Disorders 69, 15–29. [DOI] [PubMed] [Google Scholar]
- Jerison H.J. (1973) The Evolution of the Brain and Intelligence. Academic: New York. [Google Scholar]
- Lassek W.D. & Gaulin S.J.C. (2011) Sex differences in the relationship of dietary fatty acids to cognitive measures in children. Frontiers in Evolutionary Neuroscience 3, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassek W.D. & Gaulin S.J.C. (2012) Why Women Need Fat. Hudson Street Press: New York. [Google Scholar]
- Lynn R. & Vanhanen T. (2002) IQ and the Wealth of Nations. Praeger: Westport, CT. [Google Scholar]
- Lynn R. & Vanhanen T. (2012) National IQ's: a review of their educational, cognitive, economic, political, demographic, sociological, epidemiological, geographic and climatic correlates. Intelligence 40, 226–234. [Google Scholar]
- Makrides M., Neumann M.A., Byard R.W., Simmer K. & Gibson R.A. (1994) Fatty acid composition of brain, retina, and erythrocytes in breast‐ and formula‐fed infants. American Journal of Clinical Nutrition 60, 189–194. [DOI] [PubMed] [Google Scholar]
- Marean C.W., Bar‐Matthews M., Bernatchez J., Fisher E., Goldberg P., Herries A.I.R. et al (2007) Early human use of marine resources and pigment in South Africa during the Middle Pleistocene. Nature 449, 905–909. [DOI] [PubMed] [Google Scholar]
- Martin M.A., Lassek W.D., Gaulin S.J.C., Evans R.W., Woo J.G., Geraghty S.R. et al (2012) Fatty acid composition in the mature milk of Bolivian forager‐horticulturalists: controlled comparisons with a US sample. Maternal & Child Nutition 8, 404–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfee A.J., McSorley E.M., Cuskelly G.J., Fearon A.M., Moss B.W., Beattie J.A. et al (2011) Red meat fromanimals offered a grass diet increases plasma and platelet n‐3 PUFA in healthy consumers. British Journal of Nutrition 105, 80–89. [DOI] [PubMed] [Google Scholar]
- Mohrhauer H. & Holman R.T. (1963) Effect of linolenic acid upon the metabolism of linoleic acid. Journal of Nutrition 81, 67–74. [DOI] [PubMed] [Google Scholar]
- Organization for Economic Cooperation and Development (OECD) (2011) Programme for International Student Assessment 2009 Database Available at: http://www.oecd.org/dataoecd/54/12/46643496.pdf
- Organization for Economic Cooperation and Development (OECD) (2012) Education at a Glance: OECD Indicators Available at: http://www.oecd-ilibrary.org/education/education-at-a-glance-2012_eag-2012-en Table B1 1a.
- Oken E., Radesky J.S., Wright R.O., Bellinger D.C., Amarasiriwardena C.J., Kleinman K.P. et al (2008) Maternal fish intake during pregnancy, blood mercury levels and child cognition at age 3 years in a US cohort. American Journal of Epidemiology 167, 1171–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plomin R., DeFries J.C., McClearn G.C. & McGuffin P. (2008) Behavioral Genetics. Worth: New York. [Google Scholar]
- Richards M.P., Jacobi R., Cook J., Pettitt P.B. & Stringer C.B. (2005) Isotope evidence for the intensive use of marine foods by Late Upper Palaeolithic humans. Journal of Human Evoution 49, 390–394. [DOI] [PubMed] [Google Scholar]
- Ryan A.S., Astwood J.D., Gautier S., Kuratko C.N., Nelson E.B. & Salem N., Jr (2010) Effects of long‐chain polyunsaturated fatty acid supplementation on neurodevelopment in childhood: a review of human studies. Prostaglandins, Leukotrines, and Essential Fatty Acids 82, 305–314. [DOI] [PubMed] [Google Scholar]
- Sasaki S. & Kesteloot H. (1992) Value of Food and Agriculture Organization data on food‐balance sheets as a data source for dietary fat intake in epidemiologic studies. American Journal of Clinical Nutrition 56, 716–723. [DOI] [PubMed] [Google Scholar]
- Simopoulos A.P. (2011) Evolutionary aspects of diet: the omega‐6/omega‐3 ratio and the brain. Molecular Neurobiology 44, 203–215. [DOI] [PubMed] [Google Scholar]
- de Souza A.S., Fernandes F.S. & das Graças Tavares Do Carmo M. (2011) Effects of maternal malnutrition and postnatal nutritional rehabilitation on brain fatty acids, learning and memory. Nutition Reviews 69, 132–144. [DOI] [PubMed] [Google Scholar]
- United Nations Food and Agricultural Organization (UN FAO) (2012) Food Balance Sheets Available at: http://faostat3.fao.org/home/index.html
- United Nations Statistics Division (2011) Social Indicators, Income and Economic Activity, Table 5a Available at: http://unstats.un.org/unsd/demographic/products/socind/
- Wood J.D., Richardson R.I., Nute G.R., Fisher A.V., Campo M.M., Kasapidou E. et al (2004) Effects of fatty acids on meat quality: a review. Meat Science 66, 21–32. [DOI] [PubMed] [Google Scholar]
- Yuhas R., Pramuk K. & Lien E.L. (2006) Human milk fatty acid composition from nine countries varies most in DHA. Lipids 41, 851–858. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Supplementary references for ‘Maternal milk DHA content predicts cognitive performance in a sample of 28 nations’.


