Abstract
Our earlier studies both in animals and in humans have indicated that micronutrients (folic acid, vitamin B12) and long‐chain polyunsaturated fatty acids, especially docosahexaenoic acid (DHA), are interlinked in the one‐carbon cycle, which plays an important role in fetal ‘programming’ of adult diseases. The present study examines the levels of maternal and cord plasma fatty acids, maternal folate, vitamin B12 and homocysteine in healthy mothers at various time points during pregnancy and also examine an association between them. A longitudinal study of 106 normal pregnant women was carried out, and maternal blood was collected at three time points, viz., T1 = 16–20th week, T2 = 26–30th week and T3 = at delivery. Cord blood was collected at delivery. Fatty acids were estimated using a gas chromatograph. Levels of folate, vitamin B12 and homocysteine were estimated by the chemiluminescent microparticle immunoassay (CMIA) technology. Maternal plasma folate (P < 0.05), vitamin B12 (P < 0.01) and DHA (P < 0.05) levels were lowest, while maternal homocysteine levels were highest (P < 0.01) at T3. There was a negative association between maternal DHA and homocysteine at T2 (P < 0.05) and T3 (P < 0.01). There was a positive association between plasma DHA in maternal blood at T3 and cord blood. Furthermore, there was a positive association between maternal folate and vitamin B12 at T3 and baby weight, whereas maternal homocysteine at T1 were inversely associated with baby weight at delivery. Our study provides evidence for the associations of folic acid, vitamin B12, homocysteine with DHA and baby weight, suggesting that a balanced dietary supplementation of folate–vitamin B12–DHA during pregnancy may be beneficial.
Keywords: DHA, folate, homocysteine, LCPUFA, vitamin B12
Introduction
Long‐chain polyunsaturated fatty acids (LCPUFAs), especially n‐3 fatty acids, play a vital role in enhancing fetal growth (Olsen et al. 1986, 1990, 1991, 1993; Allen & Harris 2001; Olsen & Secher 2002; Rogers et al. 2004; Guldner et al. 2007). A series of studies in humans carried out at our department also confirms the well‐established association of LCPUFA, especially docosahexaenoic acid (DHA), with poor pregnancy outcome, leading to low‐birthweight (LBW) babies (Kulkarni et al. 2010; Dhobale et al. 2011; Kilari et al. 2011). During pregnancy, fatty acids of both the n‐3 and the n‐6 families play an important role in the fetal growth and development (Innis 2007). During the last trimester of pregnancy, it becomes essential that the fetus receives adequate amount of LCPUFA, especially arachidonic acid (AA) and DHA, due to the rapid synthesis of brain tissue (Clandinin et al. 1980; Martinez 1992). Adequate intake of micronutrients has also been reported to reduce the risk of LBW infants (Cetin et al. 2010).
We have extensively demonstrated in animals that alterations in maternal levels of folate and vitamin B12 affects the levels of its plasma, liver, brain, milk and placental DHA (Rao et al. 2006; Dangat et al. 2011; Kulkarni et al. 2011a; Roy et al. 2012; Sable et al. 2012; Wadhwani et al. 2012). We have discussed through a series of studies that micronutrients such as folic acid, vitamin B12 and DHA are interlinked in the one‐carbon cycle (Kale et al. 2010; Chavan‐Gautam et al. 2011; Sundrani et al. 2011; Kulkarni et al. 2011b; Dhobale & Joshi 2012). Deficiencies of folate, riboflavin, vitamin B6 or vitamin B12 have been consistently shown to be associated with elevated plasma homocysteine concentrations (Allen 2005). Elevated levels of total plasma homocysteine are observed in preeclampsia, placental abruption, premature delivery, LBW and intrauterine growth retardation (Vollset et al. 2000; Murphy et al. 2004; Lindblad et al. 2005).
Our earlier cross‐sectional studies in women with pregnancy complications, such as preeclampsia and preterm pregnancy, indicate altered levels of LCPUFA and micronutrients (folate and vitamin B12) at delivery, which are associated with poor birth outcome (Kilari et al. 2010; Dhobale et al. 2011, 2012; Kulkarni et al. 2011b). Furthermore, we have also reported a negative association between erythrocyte DHA and plasma homocysteine concentrations in preeclampsia (Kulkarni et al. 2011b).
Although there are a number of reports that have examined the fatty acid composition of maternal and cord plasma/erythrocyte throughout pregnancy (van Houwelingen et al. 1992; Hoving et al. 1994; Al et al. 1995; Otto et al. 1997, 2001; Zeijdner et al. 1997; Wijendran et al. 1999; De Vriese et al. 2001, 2003; Matorras et al. 2001; Herrera et al. 2004; Stewart et al. 2007; Dwarkanath et al. 2009), the evidence of human studies examining systematically the association between folate, homocysteine and plasma or blood cell (n‐3) LCPUFA is limited (Crowe et al. 2008). There is therefore a need to undertake longitudinal studies to examine these associations during pregnancy as they are important determinants of the one‐carbon cycle, which play an important role in fetal programming and increase the risk of developing non‐communicable diseases such as type 2 diabetes (Yajnik & Deshmukh 2008) and cardiovascular disease (CVD) (Erkkilä et al. 2008; Martinelli et al. 2009) in later life.
It would be very useful to analyse these levels in early pregnancy to examine changes over time, to understand their role in various pregnancy complications. But before such studies are initiated, it is crucial to examine the changes in these micronutrients and their association with each other in a prospective longitudinal study in a normotensive pregnancy/during an uncomplicated pregnancy. This may open new avenues for supplementation of these nutrients to prevent pregnancy complications and optimise fetal development.
We hypothesise that maternal folate, vitamin B12 and DHA are interlinked in the one‐carbon cycle and influence pregnancy outcome. The role of fatty acids in pregnancy and fetal growth has been well established. But our data for the first time, in addition, link these through one‐carbon cycle to influence pregnancy outcome.
In this study, we report the maternal fatty acids and micronutrient levels at three time points during pregnancy (T1 = 16–20th week, T2 = 26–30th week and T3 = at delivery) in normotensive women delivering at term with baby weight ≥2.5 kg. Association of maternal fatty acids with cord fatty acids is also examined.
Key messages
Our data suggest an association of maternal n‐3 fatty acids, especially DHA and homocysteine concentrations. These further provide the translational mechanistic basis for improving the health of the mother and subsequently improving birth outcome by balanced dietary supplementation of folate–vitamin B12–DHA.
Materials and methods
Subjects
This longitudinal study was conducted at the Department of Obstetrics and Gynaecology, Bharati Hospital, Pune, India. This study was approved by the Bharati Vidyapeeth Medical College Institutional Ethical Committee and a written consent was taken from each subject. This study is part of a large ongoing departmental study, which recruits all healthy women at 16–20 weeks of gestation and follows them throughout pregnancy. The current study includes only those women with singleton pregnancy, delivering at term (total gestation ≥37 weeks and baby weight ≥2.5 kg) and having no medical or obstetrical complications. Women were excluded from the study if there was an evidence of other pregnancy complications, such as multiple gestation, chronic hypertension, type I or type II diabetes mellitus, gestational diabetes, seizure disorder, and renal or liver disease. Pregnant women with alcohol or drug abuse were also excluded from the study.
Fasting plasma samples were obtained at the time of each prenatal visit, scheduled at 4‐week intervals until delivery. The first sample was obtained between 16 and 20 weeks of gestation (T1), the second between 26 and 30 weeks of gestation (T2) and the third sample was taken just before going to the labour room (T3). Umbilical cord was also collected just after delivery. One hundred nine pregnant women were enrolled at 16–20 weeks of gestation. First time point blood sample was obtained from 106 women, second time point blood sample was obtained from 90 women and at delivery sample was obtained from 98 women. Umbilical cord blood sample was obtained from 98 women (Fig. 1).
Figure 1.
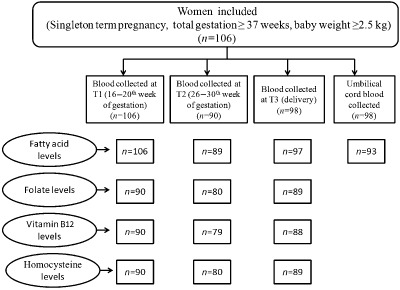
Flow chart showing number of maternal and cord plasma samples analysed for different parameters at various time points (T1 = 16–20th weeks, T2 = 26–30th week, T3 = at delivery).
All women were routinely given iron (100 mg per tablet) and folic acid (1 mg per tablet) tablets during the first trimester of pregnancy as per the National Prophylaxis programme. Gestational age was calculated by last menstrual period and then confirmed by ultrasound. All women were administered a food frequency questionnaire during these three time points to estimate the frequency of intake of foods rich in folic acid, vitamin B12 and DHA, and the details of the questionnaire have been reported by us earlier (Kulkarni et al. 2011b).
Sample collection and processing
The study visits were scheduled in the morning after an overnight fast. At each visit, maternal fasting blood samples (10 mL) were collected into ethylenediaminetetraacetic acid (EDTA) tubes at each time point and at delivery. Cord blood was obtained immediately post‐partum from the umbilical cord. All blood samples were immediately layered on histopaque (Sigma‐Aldrich, St. Louis, MO, USA) and centrifuged at 2000 rpm for 30 min to separate the plasma and erythrocytes and were stored at −80°C until further analysis. As the folate in plasma is known to be unstable with long storage times, analysis for folate, vitamin B12 and homocysteine was carried out immediately. The storage cut‐off was 3 months. Care was taken not to perform analysis on samples that were stored for a longer duration. Furthermore, haemolysed samples were not used for analysis.
Biochemical estimations
All biochemical analyses were performed at laboratories separate from subject recruitment sites. Investigators were blinded to subject identity, which was indicated by a code number maintained by the clinical staff until analysis was completed.
Fatty acid analysis
The procedure for fatty acid analysis used in our study was as reported in our several previous studies (Mehendale et al. 2008; Kilari et al. 2009, 2010; Dangat et al. 2010; Kale et al. 2010; Dhobale et al. 2011; Kulkarni et al. 2011b). Briefly, transesterification of the total plasma fatty acids was performed using hydrochloric acid–methanol. Methyl esters were separated using a PerkinElmer gas chromatograph (SP 2330, 30‐m capillary Supelco column; PerkinElmer, Shelton, CT, USA). Peaks were identified by comparison with standard fatty acid methyl esters (Sigma‐Aldrich). Fatty acids were expressed as g per 100 g fatty acid. The saturated fatty acids (SFAs) include myristic acid (Myr), palmitic acid (Pal) and stearic acid (Ste), while the monounsaturated fatty acids (MUFAs) include myristoleic acid (Myro), palmitoleic acid (Palo), oleic acid (Ole) and nervonic acid (NA). The n‐3 fatty acids included alpha‐linolenic acid (ALA), EPA and DHA, while n‐6 fatty acids included linoleic acid (LA), gamma linolenic acid (GLA), di‐homo‐gammalinolenic acid (DGLA), docosapentaenoic acid (DPA) and AA.
Folate, vitamin B12 and homocysteine estimations
Folate, vitamin B12 and homocysteine levels were estimated by the chemiluminescent microparticle immunoassay (CMIA) technology (Abbott Diagnostics, Abbott Park, IL, USA) (Lee & Griffiths 1985) and described by us earlier (Dhobale et al. 2012). Briefly, 100 μL of plasma was used for analysis of vitamin B12, folate and homocysteine. The vitamin B12, folate and homocysteine assay was a two‐step assay with an automated sample pre‐treatment for determining the presence of vitamin B12, folate and homocysteine in human plasma. The reference range for plasma vitamin B12 assays was 187–883 pg mL−1, for plasma folate assays was 2.34–17.56 ng mL−1, while for homocysteine assay was 5.08–15.39 μmol L−1.
Low plasma folate and vitamin B12 concentrations were defined as <10 ng mL−1 and <150 pg mL−1, respectively, and elevated plasma total homocysteine concentrations as a concentration >10 μmol L−1 (Kulkarni et al. 2011b).
Statistical analysis
Values are reported as mean ± SD. The data were analysed using the SPSS/PC+ package (Version 20, SPSS Inc., Chicago, IL, USA). Skewed variables were transformed to normality using the following transformations: log to the base 10. Mean values of the various parameters were compared using least significance difference estimated from one‐way analysis of variance (ANOVA) (P < 0.05). Correlation between variables was studied using Pearson's correlation analysis after adjusting for gestation, age and body mass index (BMI). Bonferroni adjustment was applied as correction for multiple testing, and after adjusting for multiple comparisons, the acceptance level for statistical significance was 0.016. Statistical analysis was carried out on two sets of data. First set of data consists of all the women who have participated in the study. The second set of data consists of women from whom all the parameters (folic acid, vitamin B12, DHA and homocysteine levels) were analysed for all time points as well as in cord (n = 74). Statistical analysis performed on the first set of data has been reported in this manuscript. Similar results and trends were observed for the second set of data (data not shown). The variable sample number (n) in different measures was due to insufficient sample volume available. Although the data were incomplete, they are unlikely to have biased the results.
Results
Table 1 shows the demographic characteristics of normotensive mothers and their neonates.
Table 1.
Demographic characteristics of normotensive mothers and their neonates
| Mean ± SD | |
|---|---|
| Normotensive women (n = 109) | |
| Maternal characteristics | |
| Age (years) | 24.95 ± 3.94 |
| Height (cm) | 154.86 ± 5.76 |
| Weight (kg) | 50.62 ± 8.15 |
| Body mass index (kg m–2) | 21.17 ± 3.46 |
| Gestation (weeks) | 19.22 ± 2.15 |
| Systolic blood pressure (mm Hg) | 114.62 ± 6.56 |
| Diastolic blood pressure (mm Hg) | 75.46 ± 5.4 |
| Income (Rs.) | 8667.92 ± 5462.21 |
| Neonatal characteristics | |
| Baby weight (kg) | 2.89 ± 0.27 |
| Baby length (cm) | 48.23 ± 2.64 |
| Baby head circumference (cm) | 33.52 ± 1.21 |
| Baby chest circumference (cm) | 32.09 ± 1.63 |
Association between frequency of maternal intake of micronutrient and fatty acid‐rich foods and their plasma values
There was a positive association between the frequency of intake of vitamin B12‐rich foods and plasma vitamin B12 levels at T1 (P < 0.05) and delivery (P < 0.01). There was a positive association between frequency of intake of DHA‐rich foods and plasma DHA levels at T1 (P < 0.01) and T2 (P < 0.01), but not at delivery. There was, however, no association between frequency of consumption of folate‐rich foods and folate levels.
Levels of maternal plasma folate, vitamin B12, homocysteine and fatty acids over time during pregnancy
Plasma folate levels decreased as gestation progressed and was significantly reduced at T3 (P < 0.05) as compared with T1 (Fig. 2). Similar trend was seen for plasma vitamin B12 level and the levels were significantly lower at T2 (P < 0.05) and T3 (P < 0.01) as compared with T1 (Fig. 2). Homocysteine levels, on the other hand, were higher at T3 (P < 0.01) as compared with T1 and T2 (Fig. 2). The plasma folate levels were <10 ng mL−1 in 46.66%, 56.25% and 61.79% of pregnant women at T1, T2 and T3, respectively, and the plasma vitamin B12 levels were <150 pg mL−1 in 22.22%, 31.64% and 42.04% of pregnant women at T1, T2 and T3, respectively, whereas the plasma homocysteine levels were >10 nmol L−1 in 7.77%, 7.5% and 22.47% of pregnant women at T1, T2 and T3, respectively.
Figure 2.
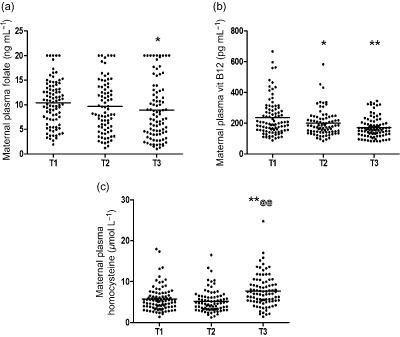
Levels of plasma (a) folate, (b) vitamin B12 and (c) homocysteine at various time points during pregnancy. T1 = 16–20th weeks, T2 = 26–30th week, T3 = At Delivery. **P < 0.01, *P < 0.05 as compared with T1, @@P < 0.01 as compared with T2.
Table 2 shows the fatty acid levels at three time points during pregnancy. The relative amounts of SFA in maternal plasma were higher at T3 (P < 0.01) as compared with T1 and T2. The relative amounts of MUFA in maternal plasma were higher at T2 (P < 0.05) and T3 (P < 0.01) as compared with T1. The relative amounts of LA in maternal plasma were lower at T3 (P < 0.01) as compared with T1 and T2, whereas there was no significant difference observed in the relative amounts of AA. However, the n‐6 fatty acids were lower at T3 (P < 0.01 for both) as compared with T1 and T2. There was no change in the relative amounts of maternal plasma ALA or n‐3 fatty acid throughout pregnancy, but the relative amounts of DHA in maternal plasma were lower at T2 and T3 (P < 0.05) as compared with T1.
Table 2.
Mean (SD) maternal plasma at three time points during normal pregnancy (T1 = 16–20th week, T2 = 26–30th week, T3 = at delivery) and cord plasma fatty acid composition (% total fatty acids)
| Fatty acids (g/100 g fatty acids) | Maternal plasma fatty acids (mean ± SD) | Cord fatty acids (mean ± SD) | Pearson's correlation coefficients between T3 and cord fatty acids (n = 89) | |||
|---|---|---|---|---|---|---|
| T1 (n = 106) | T2 (n = 89) | T3 (n = 97) | Cord (n = 93) | r | P | |
| Myr | 0.62 ± 0.30 | 0.80 ± 0.31** | 0.72 ± 0.30 | 0.76 ± 0.40* | 0.172 | 0.112 |
| Myro | 0.03 ± 0.03 | 0.03 ± 0.02 | 0.04 ± 0.03 | 0.08 ± 0.10**, @@ , ## | 0.364 | 0.001 |
| Pal | 24.77 ± 2.35 | 25.86 ± 2.60* | 27.04 ± 2.69**, @@ | 28.34 ± 2.61**, @@ , ## | 0.191 | 0.077 |
| Palo | 0.59 ± 0.37 | 0.77 ± 0.52 | 1.20 ± 0.72**, @@ | 2.25 ± 0.84**, @@ , ## | −0.117 | 0.281 |
| Ste | 5.81 ± 1.60 | 5.25 ± 0.71 | 6.34 ± 4.16 @ | 10.18 ± 2.45**, @@ , ## | −0.398 | 0.001 |
| Ole | 16.38 ± 2.16 | 17.58 ± 2.40* | 17.80 ± 2.79** | 18.72 ± 3.49**, # | 0.244 | 0.023 |
| LA | 38.72 ± 3.85 | 37.92 ± 4.09 | 33.50 ± 9.26**, @@ | 14.66 ± 7.87**, @@ , ## | −0.777 | 0.001 |
| GLA | 0.07 ± 0.98 | 0.06 ± 0.08 | 0.11 ± 0.10 @ | 0.25 ± 0.15**, @@ , ## | −0.135 | 0.214 |
| ALA | 0.46 ± 0.20 | 0.49 ± 0.24 | 0.44 ± 0.24 | 0.37 ± 0.30 # | 0.130 | 0.226 |
| DGLA | 1.12 ± 032 | 1.11 ± 0.33 | 1.18 ± 0.49 | 1.98 ± 0.60**, @@ , ## | −0.229 | 0.033 |
| AA | 7.08 ± 2.12 | 6.38 ± 1.35 | 7.04 ± 3.46 | 13.80 ± 3.78**, @@ , ## | −0.642 | 0.001 |
| EPA | 0.32 ± 0.35 | 0.27 ± 0.26 | 0.24 ± 0.27 | 0.41 ± 0.50 ## | −0.054 | 0.621 |
| NA | 0.34 ± 0.34 | 0.29 ± 0.22 | 0.35 ± 0.23 | 0.68 ± 0.42**, @@ , ## | −0.128 | 0.232 |
| DPA (n‐6) | 0.20 ± 0.09 | 0.20 ± 0.08 | 0.19 ± 0.11 | 0.21 ± 0.12 | −0.071 | 0.511 |
| DHA | 1.07 ± 0.36 | 0.92 ± 0.36* | 0.95 ± 0.44* | 1.56 ± 0.69**, @@ , ## | 0.351 | 0.001 |
| SFA | 31.22 ± 3.12 | 31.92 ± 2.69 | 34.10 ± 5.90**, @@ | 39.29 ± 3.75**, @@ , ## | −0.3 | 0.004 |
| MUFA | 17.35 ± 2.36 | 18.68 ± 2.54* | 19.40 ± 3.03** | 21.75 ± 3.40**, @@ , ## | 0.213 | 0.046 |
| Total n‐3 | 1.85 ± 0.59 | 1.69 ± 0.58 | 1.64 ± 0.67 | 2.35 ± 0.85**, @@ , ## | 0.417 | 0.001 |
| Total n‐6 | 47.21 ± 4.33 | 45.70 ± 4.14 | 42.04 ± 6.65**, @@ | 30.93 ± 5.30**, @@ , ## | −0.491 | 0.001 |
**P < 0.01, *P < 0.05 as compared with T1, @@P < 0.01, @P < 0.05 as compared with T2, ## P < 0.01, # P < 0.15 as compared with T3. Myr, myristic acid; Myro, myristoleic acid; Pal, palmitic acid; Palo, palmitoleic acid; Ste, stearic acid; Ole, oleic acid; LA, linoleic acid; GLA, gamma linolenic acid; ALA, alpha linolenic acid; DGLA, di‐homo‐gamma linolenic acid; AA, arachidonic acid; EPA, eicosapentaenoic acid; NA, nervonic acid; DPA, n‐6 docosapentaenoic acid; DHA, docosahexaenoic acid. Saturated fatty acids (SFAs): (myristic acid + palmitic acid + stearic acid). Monounsaturated fatty acids (MUFAs): (myristoleic acid + palmitoleic acid + oleic acid + nervonic acid). Total n‐3: (alpha linolenic acid + eicosapentaenoic acid + docosahexaenoic acid). Total n‐6: (linoleic acid + gamma linolenic acid+ di‐homo‐gamma‐linoleic acid + arachidonic acid + docosapentaenoic acid). For Pearson's correlation coefficients, P < 0.05.
Associations between folate, vitamin B12, homocysteine and fatty acids in maternal plasma
There was a negative association between folate and homocysteine in maternal plasma at T1 (r = −0.245, P < 0.05, n = 81). Furthermore, there was a negative association between vitamin B12 and homocysteine in maternal plasma at both T1 and T2 (r = −0.412, P < 0.001, n = 81; r = −0.391, P < 0.01, n = 72, respectively). There was a positive association between folate and DHA (r = 0.225, P < 0.05, n = 77) and between folate and AA (r = 0.300, P < 0.01, n = 77) in maternal plasma at T3. There was, however a negative association between DHA and homocysteine at T2 and T3 (r = −233 0.242, P < 0.05, n = 71; r = −0.381, P < 0.01, n = 77, respectively) and a negative association between n‐3 fatty acids and homocysteine in maternal plasma at T3 (r = −0.240, P < 0.05, n = 77). Similarly, there was a negative association between AA and homocysteine in maternal plasma at T1 and T2 (r = −0.234, P < 0.05, 236, n = 71; r = −0.238, P < 0.05, n = 71, respectively).
Cord plasma fatty acid composition and its association with maternal plasma fatty acids
The relative amounts of both SFA and MUFA in cord plasma were higher (P < 0.01) as compared with maternal values at all time points (Table 2), whereas the total n‐6 fatty acids in cord plasma were lower (P < 0.01) at all time points. The relative amounts of LA in cord plasma were lower (P < 0.01), whereas the relative amounts of AA in cord plasma were higher (P < 0.01) as compared with maternal values at all time points. Total n‐3 fatty acids in cord plasma were higher (P < 0.01) and so were the relative amounts of DHA in cord plasma (P < 0.01) as compared with maternal values at all time points, but the relative amounts of ALA in cord plasma were lower as compared with maternal values at T2 (P < 0.05).
There was a negative association between SFA in cord plasma and maternal plasma at T3 (r = −0.300, P < 0.01, n = 89), whereas there was a positive association between MUFA in cord plasma and maternal plasma at T3 (r = 0.213, P < 0.05, n = 89). There was a negative association between cord plasma and maternal plasma at T3 for LA, AA and n‐6 fatty acids (r = −0.777, P < 0.001, n = 89; r = −0.642, P < 0.001, n = 89; r = −0.491, P < 0.001, n = 89, respectively), whereas there was a strong positive association between cord plasma and maternal plasma at T3 for DHA and n‐3 fatty acids (r = 0.351, P < 0.001, n = 87 and r = 0.417, P < 0.001, n = 87, respectively) (Table 2).
Associations between maternal folate, vitamin B12, homocysteine and LCPUFA and birthweight
Correlation between maternal micronutrient and LCPUFA status was studied after adjusting for gestational age, maternal age and BMI. There was a positive association between baby weight and maternal plasma folate at T1 and T3 (r = 0.240, P < 0.05, n = 75; r = 0.272, P < 0.05, n = 76, respectively) and also for plasma vitamin B12 at T3 (r = 0.223, P < 0.05, n = 76). There was, however, a negative association between maternal homocysteine at T1 and baby weight (r = −0.252, P < 0.05, n = 75). There was a positive association between maternal plasma n‐3 fatty acids at T1 and baby chest circumference (r = 0.236, P < 0.05, n = 92). Furthermore, there was a positive association between baby weight and maternal plasma AA at T1 (r = 0.214, P < 0.05, n = 92) and total n‐6 fatty acids at T1 (r = 0.199, P = 0.055, n = 92).
Discussion
The present study reveals several important and interesting findings in a very large number of unique racial homogenous and with similar lifestyle healthy mothers delivering term: (1) significant reductions in the maternal plasma levels of folate and vitamin B12 as gestation advances; (2) positive association between maternal folate and maternal DHA at T3; (3) negative association between maternal homocysteine and maternal DHA and total n‐3 fatty acids at T2 and T3; (4) positive association between maternal DHA and cord DHA at T3; (5) negative association between maternal homocysteine and baby weight at T1; and (6) positive association between folate, vitamin B12 and baby weight.
In our study, maternal plasma folate levels were reduced as gestation advances and were lowest at T3. Reports on levels of folate during pregnancy are inconsistent with many studies reporting a decrease in serum folate levels as gestation advances (Cikot et al. 2001; López‐Quesada et al. 2003; Milman et al. 2006; Wallace et al. 2008; Ubeda et al. 2011), while others report that folate levels of pregnant women are low initially and then rise later (Parazzini et al. 2011; Ozkan et al. 2012). Takimoto et al. (2007) reported that serum folate concentrations decrease between the first and second trimesters but increase between the second and third trimesters. It has been suggested that vitamin B12 deficiency leads to a folate trap and can interfere with folate metabolism, leading to low serum folate (Klee 2000).
Maternal plasma vitamin B12 levels in our study were reduced as gestation advances and were lowest at T3 and were similar to the findings seen for folate. Many others have also reported that vitamin B12 concentrations progressively decline during pregnancy (Cikot et al. 2001; Velzing‐Aarts et al. 2005; Takimoto et al. 2007; Wallace et al. 2008; Ubeda et al. 2011) and has been attributed to the increased fetal requirement (Gadowsky et al. 1995; Guerra‐Shinohara et al. 2002). Others suggest that an increase in plasma volume and a change in hormonal status can result in low levels of vitamin B12 (Bruinse & van der Berg 1995).
Folate and vitamins B12 and B6 are required for DNA synthesis and cell growth and are involved in homocysteine metabolism (Furness et al. 2013). It is well documented that deficiency of either folate or vitamin B12 results in hyperhomocysteinaemia (Takimoto et al. 2011). In our study, maternal homocysteine levels significantly increased at T3. One study indicates that homocysteine levels are higher in the first trimester as compared with the third trimester in pregnant women (Ozkan et al. 2012). Others indicate that homocysteine concentrations decline slightly in the first trimester and remained approximately constant during the second and third trimesters (Cikot et al. 2001); still others report that it increases in a stepwise manner during pregnancy (Holmes et al. 2005; Wallace et al. 2008). Recent studies indicate that serum homocysteine concentrations are significantly lower in the second trimester and increased significantly in the third trimester of pregnancy (Ubeda et al. 2011).
There are some studies that have examined the levels of LCPUFA at various time points during gestation (Al et al. 1995; Matorras et al. 2001; Otto et al. 2001; De Vriese et al. 2003; Herrera et al. 2004; Stewart et al. 2007; Alvino et al. 2008; Dwarkanath et al. 2009). Most of the reported studies have been carried out on a small sample size (Otto et al. 2001; Alvino et al. 2008) or only during the period of early pregnancy (Otto et al. 2001; van Eijsden et al. 2008) or towards the end of the last trimester of pregnancy (Wijendran et al. 1999; Parra et al. 2002). In our study, relative amounts of maternal plasma LA significantly were reduced at T3 as compared with T1 and T2, whereas the relative amounts of cord LA were reduced significantly as compared with maternal values. Although there was no significant difference in the relative amounts of maternal AA levels at T1, T2 and T3, relative amounts of cord AA significantly increased compared with maternal values. Similar increase in cord blood plasma AA with a decline in the third trimester has been reported earlier (Herrera et al. 2004). Others report a decline in erythrocyte membrane AA (Dwarkanath et al. 2009).
In our study, relative amounts of maternal plasma DHA were reduced at T2 and T3 as compared with T1, whereas the relative amounts of cord DHA increased significantly as compared with maternal values. In contrast, others observed an increase in DHA levels across pregnancy (Stewart et al. 2007); still others report a decrease in DHA during pregnancy (Al et al. 1995; Matorras et al. 2001). A preferential accumulation of AA and DHA in umbilical plasma at birth has been reported earlier (De Vriese et al. 2003).
The current study thus demonstrates lower levels of LA and ALA in maternal plasma and cord plasma and higher levels of AA and DHA in the cord. These results confirm the greater selectivity of placenta for LCPUFA than for essential fatty acids (EFAs) (Santos et al. 2012). It may be possible that there may be an increased conversion of maternal LA to AA and ALA to DHA in the placenta (reduction in cord) as the placenta is, for the greater part, a fetal organ that has a fatty acid composition more similar to that of fetal plasma than of maternal plasma (Chambaz et al. 1985; Al et al. 1990).
Moreover, it is also known that the developing fetal brain requires DHA and AA for incorporation into membranes (Herrera 2002). Therefore, higher levels of AA and DHA in cord vs. maternal plasma can be explained by a selective transfer of these LCPUFAs by the placenta resulting in the ‘biomagnification’ of these fatty acids (Haggarty 2002) and has been extensively reviewed (Duttaroy 2009). Furthermore, others indicated that the fetal synthesis of these fatty acids from their precursors may also be possible (Herrera et al. 2004). Our findings of higher levels of both DHA and AA in the cord and lower LA and ALA in cord support the above studies.
In our study, there was also an increase in the relative amounts of SFA in cord plasma as compared with maternal values. It is known that there is a limited placental transfer for SFAs as compared with PUFA (Campbell et al. 1996; Haggarty et al. 1997) and our findings support the concept of an active lipogenesis in the fetus (Dunlop & Court 1978).
Our study reports, for the first time, a positive association between maternal folate and DHA during pregnancy. Furthermore, there was a negative association between maternal DHA and homocysteine, suggesting the associations of these micronutrients in the one‐carbon cycle as has been reported by us earlier in animal studies (Rao et al. 2006; Dangat et al. 2011; Kulkarni et al. 2011a; Roy et al. 2012; Sable et al. 2012; Wadhwani et al. 2012). Our findings indicate that homocysteine levels in pregnancy are not only determined by folate and vitamin B12 but also by DHA. Durand et al. (1996) suggested that by reducing homocysteine concentrations, folate may reduce the generation of reactive oxygen species and thus spare DHA, which is a target for lipid peroxidation. Furthermore, intervention studies and recent meta‐analysis document that the high consumption of n‐3 PUFA decreases plasma homocysteine (Huang et al. 2011). The role of fatty acids in pregnancy and fetal growth has been well established; however, our data for the first time, in addition, link these through one‐carbon cycle to influence pregnancy outcome.
It is known that gestation (Amini et al. 1994), maternal age (Lee et al. 1988) and maternal BMI (Löf et al. 2008) are associated with birthweight, and hence all associations examined in the current study with birth outcome were adjusted for the above. We observed a strong positive association between maternal and cord n‐3 fatty acids during gestation. Positive associations of fish intake and birthweight have been reported earlier (Olsen et al. 1993; Rogers et al. 2004; Guldner et al. 2007), suggesting that n‐3 fatty acids contribute to enhanced fetal growth (Takimoto et al. 2011). Muthayya et al. (2009) also found increases of between 100 and 200 g in birthweight between the lowest and highest fish/DHA intake groups. We found a negative association between maternal plasma AA levels at T3 and cord AA levels. One possible explanation may be that the placenta preferentially retains AA with respect to other fatty acids (Haggarty et al. 1997). It is also known that the fetus is less dependent on the maternal supply of AA compared with DHA (Haggarty 2010). Furthermore, a negative association between maternal plasma LA levels at T3 and cord LA levels was observed. A recent study suggests that there is a lower placental transfer of LA as a specific mechanism to prevent inhibition of Δ6 desaturase activity and enable higher rates of Δ6 desaturation and acylation of AA and DHA in fetal tissues (Novak et al. 2012).
There was a negative association between maternal homocysteine only at T1 and baby weight and a positive association of maternal folate and vitamin B12 at T3 and baby weight. This may be attributed to the low levels of both folate and vitamin B12 in the Indian population habitually consuming a vegetarian diet. A low maternal RBC folate and high homocysteine values in mid pregnancy are reported to be associated with subsequent reduced fetal growth (Furness et al. 2013). Takimoto et al. (2007) suggested that higher plasma homocysteine in the third trimester is a predictor of LBW. Our findings suggest that supplementation of both folic acid and vitamin B12 may be useful to improve baby weight, especially when diet is insufficient in these micronutrients.
In the current study, while recruiting patients from the hospital, details about education and parity were recorded and were found to be similar. Furthermore, all study participants neither consumed alcohol nor smoked and came from a low socioeconomic background, which represents a large percentage of the Indian population and has been described by us recently (Sundrani et al. 2013). The well‐matching cohort (ethnic, age, dietary habits, lifestyles and socioeconomic) described here eliminates or attenuates the contribution of a large number of variables. This is important as it allows us to propose and test our hypothesis, which are indicative of changes over time in a normotensive pregnancy and are not confounded by other factors. Our separate studies are ongoing to address issues, e.g., socio‐economic and ethnic diversity, that will help understand the contribution of such variables to make our hypothesis relevant to global population.
To discuss the results in order to easily understand the complex inter‐relationships among the studied micronutrients and their possible role in pregnancy and birth outcome, we have first provided a diagrammatic view (Fig. 3) of our hypothesis stated earlier. This hypothesis, we believe with already some isolated published studies including our own studies in human and animals, will be able to explain mechanistically the possible causal relationship of these vital micronutrients. This also will help us understand the significance of some of the key associations. Briefly, the figure shows the key biochemical mechanisms and their consequences. It is known that reduced folate levels affect fetal growth (Furness et al. 2013). In addition, it will affect the restoration of methionine and, together with reduced vitamin B12, will increase levels of homocysteine (Takimoto et al. 2011), in turn, leading to increased oxidative stress (Forges et al. 2007). During oxidative stress, free radicals initiate lipid peroxidation by attacking PUFAs in cell membranes (Madazli et al. 1999). We have previously discussed that reduced DHA may impair the phospholipid methylation and lead to an increase in DNA methylation (Kale et al. 2010), leading to altered programming of critical genes like desaturases (Wadhwani et al. 2012, 2013), resulting in poor pregnancy outcome.
Figure 3.
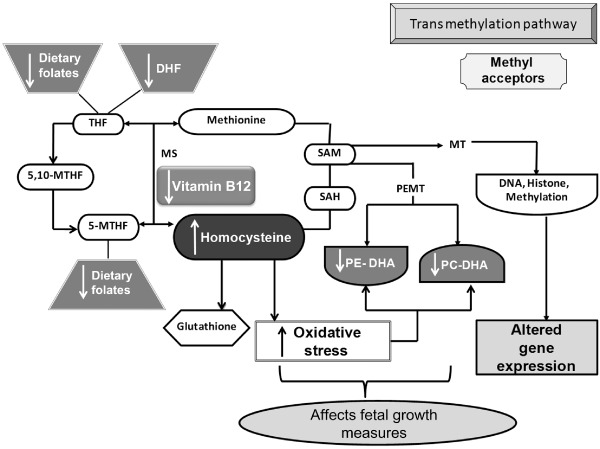
Diagrammatic presentation of mechanisms explaining the interrelationship between folate, vitamin B12 and docosahexaenoic acid (DHA) in one‐carbon cycle. The key metabolic components: THF, tetrahydrofolate, 5,10‐MTHF; 5,10‐methylene tetrahydrofolate; 5‐MTHF, 5‐methylene tetrahydrofolate; B12, vitamin B12; methionine; SAM, S‐adenosyl methionine; SAH, S‐adenosyl homocysteine; homocysteine; glutathione; PE‐DHA, phosphatidylethanolamine with docosahexaenoic acid attached to position 2; PC‐DHA, phosphatidylcholine with docosahexaenoic acid attached to position 2; DNA, deoxyribonucleic acid, histone. Key enzymes: MS, methionine synthase; PEMT, phosphatidyl ethanolamine methyl transferase; MT, methyl transferase. ↑ – increased levels, ↓ – reduced levels.
It is known that a homogenous population provides better control for confounding factors that vary in the general population (Nilsen et al. 2010). The major strengths of our study include the size of the population and the longitudinal design with measurements of several indices of folate, vitamin B12, LCPUFA and homocysteine status at three different time points during pregnancy and cord blood samples. Furthermore, all women were extremely well matched for race and lifestyle patterns with no smoking, drug or alcohol use to reduce confounds to intake and metabolism of these key components. However, as with all observational studies, our study can only suggest associations between various key analytes (e.g. folate, vitamin B12, DHA and homocysteine) and supports their role in the one‐carbon metabolism. Also, one cannot overlook the fact that although many of the correlations are statistically significant, the magnitudes of the correlations are small. Nevertheless, the associations of all the metabolites of the one‐carbon cycle with each other as well as with baby weight, if confirmed on a large sample size, may have implications for increased risk for non‐communicable diseases in adult life.
Source of funding
This study was partially funded by the Department of Biotechnology.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
SRJ, SSM and NSW contributed substantially to conception and design of, or acquisition of data or analysis and interpretation of data. NSW, HRP, SRJ drafted the article or revised it critically for important intellectual content. All authors approved the final version for publication.
Acknowledgements
The authors thank all the subjects who volunteered in this study and nurses of Bharati Hospital who helped in collecting the samples.
Wadhwani, N. S. , Pisal, H. R. , Mehendale, S. S. , and Joshi, S. R. (2015) A prospective study of maternal fatty acids, micronutrients and homocysteine and their association with birth outcome. Matern Child Nutr, 11: 559–573. doi: 10.1111/mcn.12062.
References
- Al M.D., Hornstra G., van der Schouw Y.T., Bulstra‐Ramakers M.T. & Huisjes H.J. (1990) Biochemical essential fatty acid status of mothers and their neonates after normal pregnancy. Early Human Development 24, 239–248. [DOI] [PubMed] [Google Scholar]
- Al M.D., van Houwelingen A.C., Kester A.D., Hasaart T.H., de Jong A.E. & Hornstra G. (1995) Maternal essential fatty acid patterns during normal pregnancy and their relationship to the neonatal essential fatty acid status. The British Journal of Nutrition 74, 55–68. [DOI] [PubMed] [Google Scholar]
- Allen K.G. & Harris M.A. (2001) The role of n‐3 fatty acids in gestation and parturition. Experimental Biology and Medicine (Maywood, N.J.) 226, 498–506. [DOI] [PubMed] [Google Scholar]
- Allen L.H. (2005) Multiple micronutrients in pregnancy and lactation: an overview. The American Journal of Clinical Nutrition 81, 1206S–1212S. [DOI] [PubMed] [Google Scholar]
- Alvino G., Cozzi V., Radaelli T., Ortega H., Herrera E. & Cetin I. (2008) Maternal and fetal fatty acid profile in normal and intrauterine growth restriction pregnancies with and without preeclampsia. Pediatric Research 64, 615–620. [DOI] [PubMed] [Google Scholar]
- Amini S.B., Catalano P.M., Hirsch V. & Mann L.I. (1994) An analysis of birth weight by gestational age using a computerized perinatal data base, 1975–1992. Obstetrics and Gynecology 83, 342–352. [PubMed] [Google Scholar]
- Bruinse H.W. & van der Berg H. (1995) Changes of some vitamin levels during and after normal pregnancy. European Journal of Obstetrics, Gynecology, and Reproductive Biology 61, 31–37. [DOI] [PubMed] [Google Scholar]
- Campbell F.M., Gordon M.J. & Dutta‐Roy A.K. (1996) Preferential uptake of long chain polyunsaturated fatty acids by isolated human placental membranes. Molecular and Cellular Biochemistry 155, 77–83. [DOI] [PubMed] [Google Scholar]
- Cetin I., Berti C. & Calabrese S. (2010) Role of micronutrients in the periconceptional period. Human Reproduction Update 16, 80–95. [DOI] [PubMed] [Google Scholar]
- Chambaz J., Ravel D., Manier M.‐C., Pepin D., Mulliez N. & Bereziat G. (1985) Essential fatty acids interconversion in the human fetal liver. Biology of the Neonate 47, 136–140. [DOI] [PubMed] [Google Scholar]
- Chavan‐Gautam P., Sundrani D., Pisal H., Nimbargi V., Mehendale S. & Joshi S. (2011) Gestation‐dependent changes in human placental global DNA methylation levels. Molecular Reproduction and Development 78, 150. [DOI] [PubMed] [Google Scholar]
- Cikot R.J., Steegers‐Theunissen R.P., Thomas C.M., de Boo T.M., Merkus H.M. & Steegers E.A. (2001) Longitudinal vitamin and homocysteine levels in normal pregnancy. The British Journal of Nutrition 85, 49–58. [DOI] [PubMed] [Google Scholar]
- Clandinin M.T., Chappell J.E., Heim T., Swyer P.R. & Chance G.W. (1980) Fatty acid accretion in fetal and neonatal liver: implications for fatty acid requirements. Early Human Development 5, 1–6. [DOI] [PubMed] [Google Scholar]
- Crowe F.L., Skeaff C.M., McMahon J.A., Williams S.M. & Green T.J. (2008) Lowering plasma homocysteine concentrations of older men and women with folate, vitamin B‐12, and vitamin B‐6 does not affect the proportion of (n‐3) long chain polyunsaturated fatty acids in plasma phosphatidylcholine. The Journal of Nutrition 138, 551–555. [DOI] [PubMed] [Google Scholar]
- Dangat K.D., Mehendale S.S., Yadav H.R., Kilari A.S., Kulkarni A.V., Taralekar V.S. et al (2010) Long‐chain polyunsaturated fatty acid composition of breast milk in pre‐eclamptic mothers. Neonatology 97, 190–194. [DOI] [PubMed] [Google Scholar]
- Dangat K.D., Kale A.A. & Joshi S.R. (2011) Maternal supplementation of omega 3 fatty acids to micronutrient‐imbalanced diet improves lactation in rat. Metabolism 60, 1318–1324. [DOI] [PubMed] [Google Scholar]
- De Vriese S.R., Houwelingen A.C., Hornstra G., Dhont M. & Christophe A.B. (2001) The composition of saturated fatty acids in plasma phospholipids changes in a way to counteract changes in the mean melting point during pregnancy. Lipids 36, 15–20. [DOI] [PubMed] [Google Scholar]
- De Vriese S.R., Dhont M. & Christophe A.B. (2003) FA composition of cholesteryl esters and phospholipids in maternal plasma during pregnancy and at delivery and in cord plasma at birth. Lipids 38, 1–7. [DOI] [PubMed] [Google Scholar]
- Dhobale M. & Joshi S. (2012) Altered maternal micronutrients (folic acid, vitamin B(12)) and omega 3 fatty acids through oxidative stress may reduce neurotrophic factors in preterm pregnancy. The Journal of Maternal‐fetal and Neonatal Medicine 25, 317–323. [DOI] [PubMed] [Google Scholar]
- Dhobale M., Chavan P., Kulkarni A., Mehendale S., Pisal H. & Joshi S. (2012) Reduced folate, increased vitamin B12 and homocysteine concentrations in women delivering preterm. Annals of Nutrition and Metabolism 61, 7–14. [DOI] [PubMed] [Google Scholar]
- Dhobale M.V., Wadhwani N., Mehendale S.S., Pisal H.R. & Joshi S.R. (2011) Reduced levels of placental long chain polyunsaturated fatty acids in preterm deliveries. Prostaglandins, Leukotrienes, and Essential Fatty Acids 85, 149–153. [DOI] [PubMed] [Google Scholar]
- Dunlop M. & Court J.M. (1978) Lipogenesis in developing human adipose tissue. Early Human Development 2, 123–130. [DOI] [PubMed] [Google Scholar]
- Durand P., Prost M. & Blache D. (1996) Pro‐thrombotic effects of a folic acid deficient diet in rat platelets and macrophages related to elevated homocysteine and decreased n‐3 polyunsaturated fatty acids. Atherosclerosis 121, 231–243. [DOI] [PubMed] [Google Scholar]
- Duttaroy A.K. (2009) Transport of fatty acids across the human placenta: a review. Progress in Lipid Research 48, 52–61. [DOI] [PubMed] [Google Scholar]
- Dwarkanath P., Muthayya S., Thomas T., Vaz M., Parikh P., Mehra R. et al (2009) Polyunsaturated fatty acid consumption and concentration among South Indian women during pregnancy. Asia Pacific Journal of Clinical Nutrition 18, 389–394. [PubMed] [Google Scholar]
- van Eijsden M., Hornstra G., van der Wal M.F., Vrijkotte T.G. & Bonsel G.J. (2008) Maternal n‐3, n‐6, and trans fatty acid profile early in pregnancy and term birth weight: a prospective cohort study. The American Journal of Clinical Nutrition 87, 887–895. [DOI] [PubMed] [Google Scholar]
- Erkkilä A., de Mello V.D., Risérus U. & Laaksonen D.E. (2008) Dietary fatty acids and cardiovascular disease: an epidemiological approach. Progress in Lipid Research 47, 172–187. [DOI] [PubMed] [Google Scholar]
- Forges T., Monnier‐Barbarino P., Alberto J.M., Guéant‐Rodriguez R.M., Daval J.L. & Guéant J.L. (2007) Impact of folate and homocysteine metabolism on human reproductive health. Human Reproduction Update 13, 225–238. [DOI] [PubMed] [Google Scholar]
- Furness D., Fenech M., Dekker G., Khong T.Y., Roberts C. & Hague W. (2013) Folate, Vitamin B12, Vitamin B6 and homocysteine: impact on pregnancy outcome. Maternal and Child Nutrition 9, 155–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadowsky S.L., Gale K., Wolfe S.A., Jory J., Gibson R. & O'Connor D.L. (1995) Biochemical folate, B12, and iron status of a group of pregnant adolescents accessed through the Public Health System in Southern Ontario. The Journal of Adolescent Health 16, 465–474. [DOI] [PubMed] [Google Scholar]
- Guerra‐Shinohara E.M., Paiva A.A., Rondo P., Yamasaki K., Terzi C.A. & D'Almeida V. (2002) Relationship between total homocysteine and folate levels in pregnant women and their newborn babies according to maternal serum levels of vitamin B12 . BJOG: An International Journal of Obstetrics and Gynaecology 109, 784–791. [DOI] [PubMed] [Google Scholar]
- Guldner L., Monfort C., Rouget F., Garlantezec R. & Cordier S. (2007) Maternal fish and shellfish intake and pregnancy outcomes: a prospective cohort study in Brittany, France. Environmental Health: A Global Access Science Source 6, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haggarty P. (2002) Placental regulation of fatty acid delivery and its effect on fetal growth – a review. Placenta 23, S28–S38. [DOI] [PubMed] [Google Scholar]
- Haggarty P. (2010) Fatty acid supply to the human fetus. Annual Review of Nutrition 30, 237–255. [DOI] [PubMed] [Google Scholar]
- Haggarty P., Page K., Abramovich D.R., Ashton J. & Brown D. (1997) Long‐chain polyunsaturated fatty acid transport across the perfused human placenta. Placenta 18, 635–642. [DOI] [PubMed] [Google Scholar]
- Herrera E. (2002) Implications of dietary fatty acids during pregnancy on placental, fetal and postnatal development – a review. Placenta 23 (Suppl. A), S9–S19. [DOI] [PubMed] [Google Scholar]
- Herrera E., Ortega H., Alvino G., Giovannini N., Amusquivar E. & Cetin I. (2004) Relationship between plasma fatty acid profile and antioxidant vitamins during normal pregnancy. European Journal of Clinical Nutrition 58, 1231–1238. [DOI] [PubMed] [Google Scholar]
- Holmes V.A., Wallace J.M., Alexander H.D., Gilmore W.S., Bradbury I., Ward M. et al (2005) Homocysteine is lower in the third trimester of pregnancy in women with enhanced folate status from continued folic acid supplementation. Clinical Chemistry 51, 629–634. [DOI] [PubMed] [Google Scholar]
- van Houwelingen A.C., Puls J. & Hornstra G. (1992) Essential fatty acid status during early human development. Early Human Development 31, 97–111. [DOI] [PubMed] [Google Scholar]
- Hoving E.B., van Beusekom C.M., Nijeboer H.J. & Muskiet F.A. (1994) Gestational age dependency of essential fatty acids in cord plasma cholesterol esters and triglycerides. Pediatric Research 35, 461–469. [PubMed] [Google Scholar]
- Huang T., Zheng J., Chen Y., Yang B., Wahlqvist M.L. & Li D. (2011) High consumption of omega‐3 polyunsaturated fatty acids decrease plasma homocysteine: a meta‐analysis of randomized, placebo‐controlled trials. Nutrition 27, 863–867. [DOI] [PubMed] [Google Scholar]
- Innis S.M. (2007) Fatty acids and early human development. Early Human Development 83, 761–766. [DOI] [PubMed] [Google Scholar]
- Kale A., Naphade N., Sapkale S., Raju M., Pillai A., Joshi S. et al (2010) Reduced folic acid, vitamin B12 and docosahexaenoic acid and increased homocysteine and cortisol in never‐medicated schizophrenia patients: implications for altered one‐carbon metabolism. Psychiatry Research 175, 47–53. [DOI] [PubMed] [Google Scholar]
- Kilari A., Mehendale S., Dangat K., Pisal H. & Joshi S. (2011) Associations of long‐chain polyunsaturated fatty acid concentrations with birth outcome in term Indian mothers and their neonates. American Journal of Human Biology 23, 319–324. [DOI] [PubMed] [Google Scholar]
- Kilari A.S., Mehendale S.S., Dangat K.D., Yadav H.R., Kulkarni A.V., Dhobale M.V. et al (2009) Long chain polyunsaturated fatty acids in mothers and term babies. Journal of Perinatal Medicine 37, 513–518. [DOI] [PubMed] [Google Scholar]
- Kilari A.S., Mehendale S.S., Dangat K.D., Yadav H.R., Gupta A., Taralekar V.S. et al (2010) Long chain polyunsaturated fatty acids in mothers of preterm babies. Journal of Perinatal Medicine 38, 659–664. [DOI] [PubMed] [Google Scholar]
- Klee G.G. (2000) Cobalamin and folate evaluation: measurement of methylmalonic acid and homocysteine vs vitamin B12 and folate. Clinical Chemistry 46, 1277–1283. [PubMed] [Google Scholar]
- Kulkarni A., Dangat K., Kale A., Sable P., Chavan‐Gautam P. & Joshi S. (2011a) Effects of altered maternal folic acid, vitamin B12 and docosahexaenoic acid on placental global DNA methylation patterns in Wistar rats. PLoS ONE 6, e17706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni A., Mehendale S., Pisal H., Kilari A., Dangat K., Salunkhe S. et al (2011b) Association of omega‐3 fatty acids and homocysteine concentrations in pre‐eclampsia. Clinical Nutrition 30, 60–64. [DOI] [PubMed] [Google Scholar]
- Kulkarni A.V., Mehendale S.S., Yadav H.R., Kilari A.S., Taralekar V.S. & Joshi S.R. (2010) Circulating angiogenic factors and their association with birth outcomes in preeclampsia. Hypertension Research 33, 561–567. [DOI] [PubMed] [Google Scholar]
- Lee D.S. & Griffiths B.W. (1985) Serum vitamin B12 assay methods. A review. Clinical Biochemistry 18, 261–266. [DOI] [PubMed] [Google Scholar]
- Lee K.S., Ferguson R.M., Corpuz M. & Gartner L.M. (1988) Maternal age and incidence of low birth weight at term: a population study. American Journal of Obstetrics and Gynecology 158, 84–89. [DOI] [PubMed] [Google Scholar]
- Lindblad B., Zaman S., Malik A., Martin H., Ekström A.M., Amu S., Holmgren A. & Norman M. (2005) Folate, vitamin B12, and homocysteine levels in South Asian women with growth‐retarded fetuses. Acta Obstetricia et Gynecologica Scandinavica 84, 1055–1061. [DOI] [PubMed] [Google Scholar]
- Löf M., Hilakivi‐Clarke L., Sandin S. & Weiderpass E. (2008) Effects of pre‐pregnancy physical activity and maternal BMI on gestational weight gain and birth weight. Acta Obstetricia et Gynecologica Scandinavica 87, 524–530. [DOI] [PubMed] [Google Scholar]
- López‐Quesada E., Vilaseca M.A. & Lailla J.M. (2003) Plasma total homocysteine in uncomplicated pregnancy and in preeclampsia. European Journal of Obstetrics, Gynecology, and Reproductive Biology 108, 45–49. [DOI] [PubMed] [Google Scholar]
- Madazli R., Benian A., Gümüştaş K., Uzun H., Ocak V. & Aksu F. (1999) Lipid peroxidation and antioxidants in preeclampsia. European Journal of Obstetrics, Gynecology, and Reproductive Biology 85, 205–208. [DOI] [PubMed] [Google Scholar]
- Martinelli N., Consoli L. & Olivieri O. (2009) A ‘desaturase hypothesis’ for atherosclerosis: janus‐faced enzymes in omega‐6 and omega‐3 polyunsaturated fatty acid metabolism. Journal of Nutrigenetics and Nutrigenomics 2, 129–139. [DOI] [PubMed] [Google Scholar]
- Martinez M. (1992) Tissue levels of polyunsaturated fatty acids during early human development. The Journal of Pediatrics 120, S129–S138. [DOI] [PubMed] [Google Scholar]
- Matorras R., Ruiz J.I., Perteagudo L., Barbazan M.J., Diaz A., Valladolid A. et al (2001) Longitudinal study of fatty acids in plasma and erythrocyte phospholipids during pregnancy. Journal of Perinatal Medicine 29, 293–297. [DOI] [PubMed] [Google Scholar]
- Mehendale S., Kilari A., Dangat K., Taralekar V., Mahadik S. & Joshi S. (2008) Fatty acids, antioxidants, and oxidative stress in pre‐eclampsia. International Journal of Gynaecology and Obstetrics 100, 234–238. [DOI] [PubMed] [Google Scholar]
- Milman N., Byg K.E., Hvas A.M., Bergholt T. & Eriksen L. (2006) Erythrocyte folate, plasma folate and plasma homocysteine during normal pregnancy and postpartum: a longitudinal study comprising 404 Danish women. European Journal of Haematology 76, 200–205. [DOI] [PubMed] [Google Scholar]
- Murphy M.M., Scott J.M., Arija V., Molloy A.M. & Fernandez‐Ballart J.D. (2004) Maternal homocysteine before conception and throughout pregnancy predicts fetal homocysteine and birth weight. Clinical Chemistry 50, 1406–1412. [DOI] [PubMed] [Google Scholar]
- Muthayya S., Dwarkanath P., Thomas T., Ramprakash S., Mehra R., Mhaskar A. et al (2009) The effect of fish and ω‐3 LCPUFA intake on low birth weight in Indian pregnant women. European Journal of Clinical Nutrition 63, 340–346. [DOI] [PubMed] [Google Scholar]
- Nilsen R.M., Vollset S.E., Monsen A.L., Ulvik A., Haugen M., Meltzer H.M. et al (2010) Infant birth size is not associated with maternal intake and status of folate during the second trimester in Norwegian pregnant women. The Journal of Nutrition 140, 572–579. [DOI] [PubMed] [Google Scholar]
- Novak E.M., King D.J. & Innis S.M. (2012) Low linoleic acid may facilitate Δ6 desaturase activity and docosahexaenoic acid accretion in human fetal development. Prostaglandins, Leukotrienes, and Essential Fatty Acids 86, 93–98. [DOI] [PubMed] [Google Scholar]
- Olsen S.F. & Secher N.J. (2002) Low consumption of seafood in early pregnancy as a risk factor for preterm delivery: prospective cohort study. BMJ (Clinical Research Ed.) 324, 447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen S.F., Hansen H.S., Sorensen T.I.A., Jensen B., Secher N.J., Sommer S. & Knudsen L.B. (1986) Intake of marine fat, rich in (n‐3) polyunsaturated fatty acids, may increase birth weight by prolonging gestation. Lancet 2, 367–369. [DOI] [PubMed] [Google Scholar]
- Olsen S.F., Olsen J. & Frische G. (1990) Does fish consumption during pregnancy increase fetal growth? A study of the size of the newborn, placental weight and gestational age in relation to fish consumption during pregnancy. International Journal of Epidemiology 19, 971–977. [DOI] [PubMed] [Google Scholar]
- Olsen S.F., Hansen H.S., Sommer S., Jensen B., Sorensen T.I.A., Secher N.J. et al (1991) Gestational age in relation to marine n‐3 fatty acids in maternal erythrocytes: a study in the Faroe Islands and Denmark. American Journal of Obstetrics and Gynecology 164, 1203–1209. [DOI] [PubMed] [Google Scholar]
- Olsen S.F., Grandjean P., Weihe P. & Viderø T. (1993) Frequency of seafood intake in pregnancy as a determinant of birth weight: evidence for a dose dependent relationship. Journal of Epidemiology and Community Health 47, 436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto S.J., Houwelingen A.C., Antal M., Manninen A., Godfrey K., López‐Jaramillo P. et al (1997) Maternal and neonatal essential fatty acid status in phospholipids: an international comparative study. European Journal of Clinical Nutrition 51, 232–242. [DOI] [PubMed] [Google Scholar]
- Otto S.J., van Houwelingen A.C., Badart‐Smook A. & Hornstra G. (2001) Changes in the maternal essential fatty acid profile during early pregnancy and the relation of the profile to diet. The American Journal of Clinical Nutrition 73, 302–307. [DOI] [PubMed] [Google Scholar]
- Ozkan Y., Yardim‐Akaydin S., Erdem A. & Simşek B. (2012) Variability of total thiol compounds, oxidative and nitrosative stress in uncomplicated pregnant women and nonpregnant women. Archives of Gynecology and Obstetrics 285, 1319–1324. [DOI] [PubMed] [Google Scholar]
- Parazzini F., Chiaffarino F., Ricci E., Improta L. & Monni G. (2011) Homocysteine in Pregnancy Study Group. Homocysteine, red cell, and plasma folate concentrations and birth weight in Italian women: results from a prospective study. The Journal of Maternal‐fetal and Neonatal Medicine 24, 427–431. [DOI] [PubMed] [Google Scholar]
- Parra M.S., Schnaas L., Meydani M., Perroni E., Martínez S. & Romieu I. (2002) Erythrocyte cell membrane phospholipid levels compared against reported dietary intakes of polyunsaturated fatty acids in pregnant Mexican women. Public Health Nutrition 5, 931–937. [DOI] [PubMed] [Google Scholar]
- Rao S., Joshi S., Kale A., Hegde M. & Mahadik S. (2006) Maternal folic acid supplementation to dams on marginal protein level alters brain fatty acid levels of their adult offspring. Metabolism 55, 628–634. [DOI] [PubMed] [Google Scholar]
- Rogers I., Emmett P., Ness A. & Golding J. (2004) Maternal fish intake in late pregnancy and the frequency of low birth weight and intrauterine growth retardation in a cohort of British infants. Journal of Epidemiology and Community Health 58, 486–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Kale A., Dangat K., Sable P., Kulkarni A. & Joshi S. (2012) Maternal micronutrients (folic acid and vitamin B12) and omega 3 fatty acids: implications for neurodevelopmental risk in the rat offspring. Brain and Development 34, 64–71. [DOI] [PubMed] [Google Scholar]
- Sable P.S., Dangat K.D., Joshi A.A. & Joshi S.R. (2012) Maternal omega 3 fatty acid supplementation during pregnancy to a micronutrient‐imbalanced diet protects postnatal reduction of brain neurotrophins in the rat offspring. Neuroscience 217, 46–55. [DOI] [PubMed] [Google Scholar]
- Santos F.S., Chaves C.R., Costa R.S., Oliveira O.R., Santana M.G., Conceição F.D. et al (2012) Status of cis and trans fatty acids in Brazilian adolescent mothers and their newborns. Journal of Pediatric and Adolescent Gynecology 25, 270–276. [DOI] [PubMed] [Google Scholar]
- Stewart F., Rodie V.A., Ramsay J.E., Greer I.A., Freeman D.J. & Meyer B.J. (2007) Longitudinal assessment of erythrocyte fatty acid composition throughout pregnancy and post partum. Lipids 42, 335–344. [DOI] [PubMed] [Google Scholar]
- Sundrani D., Khot V., Pisal H., Mehendale S., Wagh G., Joshi A. & Joshi S. (2013) Gestation dependant changes in angiogenic factors and their associations with fetal growth measures in normotensive pregnancy. PLoS ONE 8, e54153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundrani D.P., Chavan Gautam P.M., Mehendale S.S. & Joshi S.R. (2011) Altered metabolism of maternal micronutrients and omega 3 fatty acids epigenetically regulate matrix metalloproteinases in preterm pregnancy: a novel hypothesis. Medical Hypotheses 77, 878–883. [DOI] [PubMed] [Google Scholar]
- Takimoto H., Mito N., Umegaki K., Ishiwaki A., Kusama K., Abe S. et al (2007) Relationship between dietary folate intakes, maternal plasma total homocysteine and B‐vitamins during pregnancy and fetal growth in Japan. European Journal of Nutrition 46, 300–306. [DOI] [PubMed] [Google Scholar]
- Takimoto H., Hayashi F., Kusama K., Kato N., Yoshiike N., Toba M. et al (2011) Elevated maternal serum folate in the third trimester and reduced fetal growth: a longitudinal study. Journal of Nutritional Science and Vitaminology 57, 130–137. [DOI] [PubMed] [Google Scholar]
- Ubeda N., Reyes L., González‐Medina A., Alonso‐Aperte E. & Varela‐Moreiras G. (2011) Physiologic changes in homocysteine metabolism in pregnancy: a longitudinal study in Spain. Nutrition 27, 925–930. [DOI] [PubMed] [Google Scholar]
- Velzing‐Aarts F.V., Holm P.I., Fokkema M.R., van der Dijs F.P., Ueland P.M. & Muskiet F.A. (2005) Plasma choline and betaine and their relation to plasma homocysteine in normal pregnancy. The American Journal of Clinical Nutrition 81, 1383–1389. [DOI] [PubMed] [Google Scholar]
- Vollset S.E., Refsum H., Irgens L.M., Emblem B.M., Tverdal A., Gjessing H.K. et al (2000) Plasma total homocysteine, pregnancy complications, and adverse pregnancy outcomes: the Hordaland homocysteine study. The American Journal of Clinical Nutrition 71, 962–968. [DOI] [PubMed] [Google Scholar]
- Wadhwani N.S., Manglekar R.R., Dangat K.D., Kulkarni A.V. & Joshi S.R. (2012) Effect of maternal micronutrients (folic acid, vitamin B12) and omega 3 fatty acids on liver fatty acid desaturases and transport proteins in Wistar rats. Prostaglandins, Leukotrienes, and Essential Fatty Acids 86, 21–27. [DOI] [PubMed] [Google Scholar]
- Wadhwani N.S., Dangat K.D., Joshi A.A. & Joshi S.R. (2013) Maternal micronutrients and omega 3 fatty acids affect placental fatty acid desaturases and transport proteins in Wistar rats. Prostaglandins, Leukotrienes, and Essential Fatty Acids 88, 235–242. [DOI] [PubMed] [Google Scholar]
- Wallace J.M., Bonham M.P., Strain J., Duffy E.M., Robson P.J., Ward M. et al (2008) Homocysteine concentration, related B vitamins, and betaine in pregnant women recruited to the Seychelles Child Development Study. The American Journal of Clinical Nutrition 87, 391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijendran V., Bendel R.B., Couch S.C., Philipson E.H., Thomsen K., Zhang X. et al (1999) Maternal plasma phospholipid polyunsaturated fatty acids in pregnancy with and without gestational diabetes mellitus: relations with maternal factors. The American Journal of Clinical Nutrition 70, 53–61. [DOI] [PubMed] [Google Scholar]
- Yajnik C.S. & Deshmukh U.S. (2008) Maternal nutrition, intrauterine programming and consequential risks in the offspring. Reviews in Endocrine and Metabolic Disorders 9, 203–211. [DOI] [PubMed] [Google Scholar]
- Zeijdner E.E., van Houwelingen A.C., Kester A.D. & Hornstra G. (1997) Essential fatty acid status in plasma phospholipids of mother and neonate after multiple pregnancy. Prostaglandins, Leukotrienes, and Essential Fatty Acids 56, 395–401. [DOI] [PubMed] [Google Scholar]


