Abstract
Food fortification is a cost‐effective and sustainable strategy to prevent or correct micronutrient deficiencies. A double‐blind cluster (bari) randomised controlled trial was conducted in a rural community in Bangladesh to evaluate the impact of consumption of chapatti made of micronutrient‐fortified wheat flour for 6 months by school‐aged children on their vitamin A, haemoglobin and iron status. A total of 43 baris (group of households) were randomly selected. The baris were randomly assigned to either intervention or control group. The intervention group received wheat flour fortified with added micronutrients (including 66 mg hydrogen‐reduced elemental iron and 3030 μg retinol equivalent as retinyl palmitate per kilogram of flour), while the control group received wheat flour without added micronutrients. A total of 352 children were enrolled in the trial, 203 in the intervention group and 149 in the control group. Analyses were carried out on children who completed the study (191 in the intervention group and 143 in the control group). Micronutrient‐fortified wheat flour chapatti significantly increased serum retinol concentration at 6 months by 0.12 μmol L−1 [95% confidence interval (CI): 0.06, 0.19; P < 0.01]. The odds of vitamin A deficiency was significantly lower for children in the intervention group at 3 months [odds ratio (OR) = 0.26; 95% confidence interval (CI): 0.07, 0.89; P < 0.05] and 6 months (OR = 0.21; 95% CI: 0.06, 0.68; P < 0.01). No demonstrable effect of fortified chapatti consumption on iron status, haemoglobin levels or anaemia was observed. Consumption of fortified chapattis demonstrated a significant improvement in the vitamin A status, but not in iron, haemoglobin or anaemia status.
Keywords: controlled trial, Bangladesh, micronutrient fortification, vitamin A, iron, school‐aged children
Introduction
Vitamin A [World Health Organization (WHO) 2009] and anaemia or iron‐deficiency anaemia (IDA; WHO 2008) are two of the most recognised micronutrient‐related persisting global public health problems (Underwood & Smitasiri 1999). In addition to its well‐known effect on prevention of xerophthalmia, adequate vitamin A nutriture may reduce up to a quarter to one‐third of all infection‐related childhood mortality (Fawzi et al. 1993; Glasziou & Mackerras 1993). Whereas evidence indicates that IDA is associated with impaired mental and physical function in children, including reduced physical coordination and capacity, delayed mental development, reduced cognitive abilities and reduced social and emotional development (UNICEF et al. 2006).
World Health Organization (WHO)'s estimates of 2005 suggested that globally, around 5.2 million pre‐school children had been suffering from xerophthalmia and around 190 million from vitamin A deficiency (VAD) in countries with Gross Domestic Product less than USD 15 000 (WHO 2009). In Bangladesh, night blindness, due to lack of vitamin A, among pre‐school children has been reduced from 3.6% in 1983 to 0.04% in 2005 (HKI & IPHN, 2006) as a result of Government initiated massive dose vitamin A supplementation programme. However, the latest national survey conducted in Bangladesh during 2011–2012 still implies VAD as a severe public health problem because as of 2012, prevalence of VAD was still about 21% among pre‐school and school‐aged children (National Micronutrient Survey 2011–2012, unpublished data).
Another form of nutritional deficiency currently ravaging throughout the developing nations is anaemia. It has recently been estimated that globally 1.62 billion people suffered from anaemia, with the highest number of 315.4 million in Southeast Asia region (WHO 2008). In Bangladesh, over one‐third of the school‐aged children (5–11 years) or adolescents (12–19 years) were considered anaemic in the last decade (HKI & IPHN 2002); however, recent survey shows that the prevalence has been reduced to 19.1% (6–11 years) and 17.1% (12–14 years) among the school‐aged children (National Micronutrient Survey 2011–2012, unpublished data).
Nonetheless, globally, it has been assumed that every year, around 27 million DALYs (Disability Adjusted Life Years) are lost due to VAD‐related disorders (Rice et al. 2004) and 35 million DALYs lost due to iron‐deficiency disorders (Stoltzfus et al. 2004). The huge quantity of DALYs lost due to the two nutritional deficiency is subsequently hindering the progress towards attaining MDG 4 (Millennium Development Goal 4) for the developing nations.
Other than large‐scale supplementation programme, fortification of food with micronutrients can be an effective strategy to combat vitamin A and iron deficiency and its related disorders in children. Fortification of foods, such as margarine, milk and bread, has long been practiced in Western countries to combat against deficiency of iodine, iron, vitamin A, D and several B vitamins. In 1920s, Switzerland has introduced the concept of salt iodisation, and sooner many Western countries followed their direction (Burgi et al. 1990). In Guatemala, fortification of sugar with vitamin A substantially improved the vitamin A status of pre‐school children (Arroyave et al. 1981). In Venezuela, consumption of fortified flour (maize, wheat) showed to improve the iron status of its population (Layrisse et al. 1996). Nevertheless, at present, 75 countries globally are using iron/folic acid for fortification of wheat flour (The Flour Fortification Initiative 2012). In Bangladesh, however, the efficacy of food‐based vitamin A or iron fortification with any cereal grain has not been assessed. Rice, the major staple food of Bangladesh, would be a suitable vehicle for fortification. However, rice is often processed from paddy fields in the community at the household level or at small‐scale rice mills, limiting the opportunity for fortification control and safety. On the other hand, wheat, with an increasing trend in consumption among Bangladeshi population, is more often centrally processed, and hence is considered to be a more feasible candidate for fortification.
The primary objective of this study was to evaluate the impact of daily consumption of chapattis made from wheat flour fortified with micronutrients including vitamin A and iron for a duration of 6 months by school‐aged (6–15 years), rural Bangladeshi children on their vitamin A status as reflected in serum retinol (SR) concentration. Furthermore, the secondary objective was to evaluate the impact of fortified chapatti on VAD, haemoglobin (Hb) concentration, anaemia and iron status.
Key messages
Consumption of wheat flour chapatti fortified with multiple micronutrients, including vitamin A (retinyl palmitate) and iron (hydrogen‐reduced elemental iron), improves vitamin A status of school‐aged children but may not improve anaemia or iron status.
To be efficacious, iron fortificant depends largely on careful choice of the iron compound, dose and other environmental factors.
Much needs to be learnt in defining best fortification strategy for reducing anaemia and iron deficiency.
Materials and methods
Study design
This was a double‐blind cluster (bari) randomised controlled trial. The cluster design was chosen to avoid cross‐contamination of the two types of wheat flour among the participants.
The study sites included 7 out of the total 16 unions (approximately 65 villages) of Mirsarai sub‐district in the south‐eastern part of Bangladesh that houses one of the icddr,b.'s demographic surveillance field sites with a population of around 172 300 at that time. All the baris (usually composed of 5–6 adjoining households with a population of about 30–35 relatives) in the study area were listed. There were a total of 4875 baris in the selected unions.
The 80% extracted wheat flour used in the trial was produced by a flourmill with half the amount fortified as a ‘batch process’ at a pharmaceutical company; both infrastructures were located in Dhaka, the capital of Bangladesh. The flour was fortified with multiple micronutrients including 66 mg hydrogen‐reduced elemental iron and 3030 μg retinol equivalent as retinyl palmitate per kilogram of flour. The pharmaceutical company maintained the quality assurance of the fortified flour. Both the fortified and the unfortified flour were packed in identical polyethylene bags, each containing 700 g of flour, and the bags were labelled with blinded code to indicate flour type. Equal numbers of bags containing fortified and unfortified flour were produced. The wheat flour fortifying company sent the flour bags to the study site every 2 weeks.
A pre‐testing of field procedure for collecting information, lasting for 1 month, was conducted in the non‐intervention unions of Mirsarai. Information including handling and storage of wheat flour, making of chapattis by mothers and observing chapatti consumption patterns of the subjects were recorded. The pre‐test revealed high compliance [97.6% (n = 43)] of chapatti consumption by the participating children.
Throughout the intervention period, children belonging to the baris, who received fortified flour, were designated as the intervention group, while baris, who were allocated unfortified flour to be consumed, were considered as the control group.
The primary outcome measure was vitamin A status at 6 months, determined by SR concentration. The secondary outcome measures were SR concentration at 3 months, haemoglobin, serum ferritin (SF) and serum transferrin receptor (STfR) concentrations and proportion of children with VAD, anaemia and iron deficiency at 3 and 6 months. Anthropometric and all other variables mentioned earlier were recorded for baseline comparison.
Sample size and randomisation of baris
In order to calculate sample size, following previously published studies, a difference of 0.175 μmol L−1 in SR concentration between groups, with a standard deviation (SD) of ±0.34 μmol L−1 within groups (Arroyave et al. 1981) and a difference of 7.19 μg L−1 in SF concentration between groups with a SD of ±14.5 μg L−1 within groups (Layrisse et al. 1996) at the end of 6 months of intervention, along with 95% level of significance and 90% statistical power, was considered. Accordingly, a sample of 83 and 87 children per group for SR and SF, respectively, was computed. To adjust for clustering, a design effect of 2 was applied, and finally a sample size of 175 children per group, for a total of 350 children, was calculated.
Children aged below 6 years were excluded as they receive vitamin A supplementation every 6 months on national immunisation or vitamin A days. Severely ill children were also excluded from the study. Furthermore, assuming that 7–9 eligible children (6–15 years) would be available from each bari and using a statistics book generated random number table, a total of 44 baris were randomly selected from the total listed baris for distribution of the flour. Among the 44 selected baris, 22 baris were randomly assigned to the intervention group and 22 baris to the control group (control).
A person not involved with the study assigned the baris to six different codes of flour (A, B, C, D, E and F) for distribution of the flour bags to the baris. During analysis of data, the principal investigator was informed that codes A, C and F were lumped into ‘group A’; and B, D and E into ‘group B’. It was only after completion of the analysis, the groups were unblinded.
Ethical approval
The study was approved by the Institutional Review Board of International Centre for Diarrhoeal Diseases Research, Bangladesh (icddr,b). Written informed consents were obtained from the head of the baris and/or parents and assents were obtained from children >8 years before their enrolment. If a bari head refused to participate at the time of enrolment, an additional bari was randomly selected.
Conduct of the study
Throughout the trial period, the project staff distributed the flour once every week. In order to prevent participants sharing of chapattis with other members of a bari, the same amount of flour was also allocated to other members of that bari during this period. Thus, all residents of the bari were eating the chapattis, although data on consumption were collected only from children enrolled in the study. To improve chapatti consumption compliance, participants were also supplied with condiments [suji (semolina) and sugar to prepare halwa] each week along with the flour.
A total of 65 bari mothers were selected to prepare chapatti and halwa daily for distribution among the participating children. A measuring cup with a capacity of 100 g flour was supplied to the bari mother to ensure that the proper amount of flour was used. Children received chapattis made from 100 g of fortified or unfortified wheat flour daily for 6 months. It was assumed that taking chapattis would not significantly alter their routine dietary intake, even if it did, equal effect was expected to be produced on both groups. A different bari adult (not the bari mother) was assigned to monitor chapatti consumption by the participants during morning feeding sessions and to document the number of chapatti consumed by each participant. This was recorded on a form supplied by the study staff. Study staff visited the baris at least once per week to monitor chapatti consumption during feeding sessions and to collect the forms after verifying them by interviews with bari adult, participants and mothers. In addition, samples of flour and chapatti were collected from the participating baris and were sent to the Institute of Nutrition and Food Science, Dhaka University for analyses. On average, the moisture content of the flour and chapattis were 5.09% and 32%, respectively. Vitamin A content in the fortified flour and chapatti were 100% and 89%, and iron content were 90% and 90% of the added amount on the dry weight basis, respectively. The chapatti‐feeding programme concluded by collection of blood at the end of the 6‐month intervention period.
Measurement
Baseline blood samples were drawn from 352 children to measure SR concentration (vitamin A status) along with SF concentration and transferring receptor concentration (iron status) and haemoglobin concentration. Mid‐point (3 month) and endpoint (6 month) blood samples were collected from 343 (97%) and 334 (95%) subjects, respectively.
About 4.5 mL of blood was collected from the participating children at these time points by venipuncture, and an aliquot (4 mL) was immediately put into a vial covered with aluminum foil to prevent exposure to light and was kept in a rack at room temperature until clotted. Another aliquot (0.5 mL) was placed in a tube coated with ethylenediaminetetraacetic acid for the estimation of haemoglobin. All the samples were transported immediately to a nearby temporary laboratory set‐up at the International Centre for Diarrhoeal Diseases Research, Bangladesh (icddr,b) surveillance centre in Mirsarai for centrifugation, serum preparation and temporary storage. Blood and serum samples were then transported to the Nutritional Biochemistry Laboratory of icddr,b in Dhaka twice a week. Haemoglobin concentration was determined in whole blood immediately thereafter by methemoglobin method (Rice 1967), and serum was stored at −20°C until analysis. The precision of assay, based on coefficient of variation (CV), for haemoglobin was <1%. SR was determined by high‐performance liquid chromatography (Driskell et al. 1982). The CV for SR was <2%. Both SF and transferrin receptor concentration were measured by immunoturbidimetric methods using commercial kits (Tina‐quant Ferritin; Tina‐quant Transferrin Receptor; Roche Diagnostics, Mannheim, Germany). The CV for both tests was <5%.
Study definitions
Anaemia was defined as a haemoglobin concentration <115 g L−1 for children <12 years and <120 g L−1 for those ≥12 years; iron deficiency as SF concentration <20 μg L−1 considering the possibility of sub‐clinical infection in this population and/or transferrin receptor concentration >5 mg L−1. While VAD was defined as SR concentration <0.70 μmol L−1.
Data analysis
All the collected data were checked for inconsistencies. Any discrepancies in recording the data were immediately addressed before finalizing the data set. Distributions of the continuous variables were verified using histograms. SR and Hb were normally distributed, whereas SF and STfR followed approximately normal distribution. Body weight and height measurements were converted to body mass index‐for‐age z‐score (BAZ) using WHO AnthroPlus 2007, v 1.0 software (WHO, Geneva, Switzerland). As the assumption of independence among the subjects was violated due to clustering effect of the individuals nested within baris, multi‐level analyses were performed by incorporating the cluster (bari) as random effects in the mixed‐model analyses. All models were adjusted for child's sex, age and baseline values. Mixed‐model linear regression analyses were performed to assess the intervention effect on the continuous outcomes, while mixed‐model logistic regression analyses were carried out to understand the intervention effect on the status of VAD along with anaemia and iron deficiency using Stata Statistical Software, version 11 (Stata Corp., 2003, College Station, TX, USA). All the analyses were for 3 and 6‐month interval; no other interim interval data were available.
Results
The participants were enrolled for the study during February–March 2002; flour distribution commenced during the last week of March and the consumption of chapatti started during the first week of April 2002. However, the primary starting point of the trial was the day when the respective participants of a bari started consuming chapattis made from the supplied wheat flour, while the primary endpoint was considered to be the day when the participants finished consuming the last bag of the wheat flour provided (total duration 6 months). The last episode of drawing blood from the participants happened within 1–2 days after they finished consuming the last bag of flour provided.
The trial profile is illustrated in Fig. 1. During the baseline data collection, one of the selected baris withdrew their consent, and thus a total of 43 baris participated in the study. Finally, 352 children aged 6–15 years, living in the 43 baris, were included in the study. In total, 203 children were enrolled from 22 baris in the intervention group and 149 children from 21 baris in the control group. The number of children in each bari was not equal, which was accounted for the uneven distribution of children in the two groups. Analysis was carried out on 334 children who completed the study (191 in the intervention and 143 in the control group). Table 1 shows baseline characteristics of the selected children by treatment groups. There was no significant difference between groups with respect to age, sex, weight, height and the outcome variables (SR, SF, STfR, Hb, VAD, anaemia and iron deficiency based on SF), except for iron deficiency based on STfR and nutritional status (BAZ).
Figure 1.
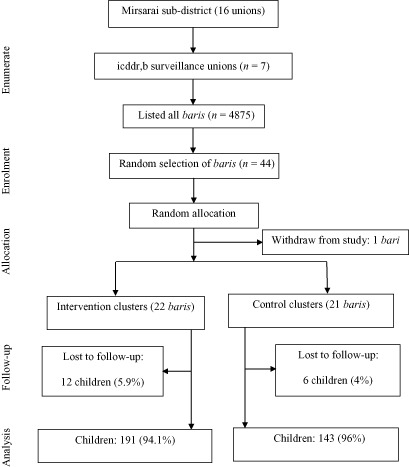
Trial profile.
Table 1.
Baseline characteristics of the study children by treatment group
| Characteristic | Intervention | Control |
|---|---|---|
| Number of clusters (baris) | 22 | 21 |
| Number of children, n (%) | 191 (57.2) | 143 (42.8) |
| Male, n (%) | 95 (49.7) | 74 (51.7) |
| Average number of children in the baris * | 8.7 ± 6.8 | 6.8 ± 4.9 |
| Age (years)* | 10.4 ± 2.68 | 10.3 ± 2.86 |
| Weight (kg)* | 25.3 ± 8.2 | 27.0 ± 9.5 |
| Height (cm)* | 129.6 ± 15.3 | 131.4 ± 16.7 |
| Body mass index‐for‐age z‐score (BAZ) † | −1.63 ± 0.07 ‡ | −1.28 ± 0.07 |
| Serum retinol (μmol L−1) † | 0.96 ± 0.02 | 0.98 ± 0.02 |
| Haemoglobin (g dL−1) † | 12.2 ± 0.07 | 12.1 ± 0.08 |
| Serum ferritin (μg L−1) † | 39.9 ± 1.79 | 36.4 ± 1.75 |
| Serum transferrin receptor (mg L−1) † | 3.69 ± 0.06 | 3.74 ± 0.09 |
| VAD, % (95% CI) | 13.6 (8.7, 18.5) | 15.4 (9.5, 21.3) |
| Anaemia, % (95% CI) | 24.3 (18.2, 30.5) | 30.3 (22.7, 37.8) |
| Iron deficiency: | ||
| Serum ferritin <20 μg L−1, % (95% CI) | 19.4 (13.8, 25) | 24.5 (17.4, 31.5) |
| Serum transferrin receptor >5 mg L−1, % (95% CI) | 4.2 (1.3, 7) ‡ | 10.5 (5.5, 15.5) |
CI, confidence interval; VAD, vitamin A deficiency. *Mean ± SD. †Mean ± SE. ‡Significantly different from control group at P < 0.05.
The amount of micronutrients added to fortified flour and its contribution to the recommended dietary allowances among the intervention group is shown in Table 2.
Table 2.
The amount of micronutrient fortificants in the chapattis (100 g flour) and their contribution (%) to daily requirements
| Nutrient | Contribution of 100 g of flour in meeting dietary requirements of 6–15‐year‐old children | ||
|---|---|---|---|
| Amount | RDA | % RDA | |
| Vitamin A* | 212 μg † | 400–600 μg | 35–53% |
| Iron ‡ | 6.6 mg | 12.6–29.2 mg § | 23–52% |
| Thiamin (vitamin B1) | 0.64 mg | 1.2 mg | 53% |
| Riboflavin (vitamin B2) | 0.40 mg | 1.0 mg | 40% |
| Folic acid | 0.15 mg | 0.25–0.4 mg | 37–60.5% |
| Zinc oxide | 3.3 mg | 10 mg | 33% |
| Niacin as niacinamide | 5.3 mg | 10.4–12.5 mg | 42–51% |
RDA, recommended dietary allowance. *Retinyl palmitate, S/N, United States Pharmacopoeia‐Food Chemical Codex (USP‐FCC). †Assuming 30% loss during storage and chapatti preparation. ‡As hydrogen‐reduced elemental iron, USP‐FCC. §Assuming 5% bioavailability.
The numbers of chapattis (mean ± SD) consumed by the intervention and control groups were 351 ± 21 and 355 ± 16, respectively. Considering the highest possible intake of 366 chapattis, equal or greater than 90% (≥329 chapattis) compliance were achieved by 89% and 93% of the children in fortified and control groups, respectively, and there were no statistical differences in the mean chapatti intake or compliance between the groups.
Vitamin A statuss
There was no significant difference in the mean SR concentration between intervention and the control groups at baseline. However, compared with the control group, SR concentration was significantly higher in the intervention group at 6 months by 0.12 μmol L−1 [95% confidence interval (CI): 0.06, 0.19; P < 0.01] (Table 3). Moreover, the odds of VAD was significantly lower for children in the intervention group at 3 months [odds ratio (OR) = 0.26; 95% CI: 0.07, 0.89; P < 0.05] and at 6 months (OR = 0.21; 95% CI: 0.06, 0.68; P < 0.01) (Table 4). The prevalence of low SR concentration (<1.05 μmol L−1) has decreased from 67% (at baseline) to 48% (at 6 months) in the intervention group and from 63.6% (at baseline) to 60.3% (at 6 months) in the control group, whereas the odds of low SR concentration (<1.05 μmol L−1) was significantly lower for children in the intervention group at 6 months (OR = 0.38; 95% CI: 0.17, 0.87; P < 0.05).
Table 3.
Effect of fortified wheat flour chapatti on serum retinol (SR), serum ferritin (SF), serum transferrin receptor (STfR) and haemoglobin concentrations at 3‐ and 6‐month interval
| Interval | SR concentration* | ||||
|---|---|---|---|---|---|
| Control (n = 143) Mean ± SE (μmol L−1) | Intervention (n = 191) Mean ± SE (μmol L−1) | Intervention effect (95% CI), (μmol L−1) † | Estimated intracluster correlation coefficient † | P‐value | |
| 3 months | 1.04 ± 0.03 | 1.07 ± 0.02 | 0.04 (−0.02, 0.12) | 0.07 | 0.23 |
| 6 months* | 0.94 ± 0.02 | 1.06 ± 0.02 | 0.12 (0.06, 0.19) | 0.13 | 0.00 |
| Interval | SF concentration | ||||
|---|---|---|---|---|---|
| Control (n = 143) Mean ± SE (g L−1) | Intervention (n = 191) Mean ± SE (μg L−1) | Intervention effect (95% CI) (μg L−1) † | Estimated intracluster correlation coefficient † | P‐value | |
| 3 months | 44.0 ± 2.14 | 44.8 ± 2.4 | −1.42 (−7.0, 4.1) | 0 | 0.62 |
| 6 month | 45.6 ± 2.5 | 47.9 ± 2.3 | 0.12 (−5.8, 6.0) | 0.02 | 0.97 |
| Interval | STfR concentration | ||||
|---|---|---|---|---|---|
| Control (n = 143) Mean ± SE (mg L−1) | Intervention (n = 191) Mean ± SE (mg L−1) | Intervention effect (95% CI), (mg L−1) † | Estimated intracluster correlation coefficient † | P‐value | |
| 3 months | 3.86 ± 0.09 | 3.84 ± 0.09 | 0.07 (−0.22, 0.35) | 0.18 | 0.64 |
| 6 months | 3.84 ± 0.1 | 3.79 ± 0.07 | −0.01 (−0.15, 0.13) | 0 | 0.89 |
| Interval | Haemoglobin concentration | ||||
|---|---|---|---|---|---|
| Control (n = 143) Mean ± SE (g dL−1) | Intervention (n = 191) Mean ± SE (g dL−1) | Intervention effect (95% CI) (g dL−1) † | Estimated intracluster correlation coefficient † | P‐value | |
| 3 months | 12.1 ± 0.07 | 12.1 ± 0.07 | −0.04 (−0.18, 0.11) | 0 | 0.62 |
| 6 months | 12.3 ± 0.08 | 12.3 ± 0.07 | −0.16 (−0.4, 0.08) | 0.2 | 0.2 |
CI, confidence interval. *Primary outcome. †Mixed‐model linear regression analyses with cluster (bari) as random effects, adjusted for age, sex and baseline value of the outcome.
Table 4.
Effect of fortified wheat flour chapatti on VAD, iron deficiency and anaemia at 3‐ and 6‐month interval
| Interval | VAD | ||||
|---|---|---|---|---|---|
| Control (n = 143)% | Intervention (n = 191)% | OR (95% CI)* | Estimated intracluster correlation coefficient* | P‐value | |
| 3 months | 16.2 | 7.9 | 0.26 (0.07, 0.89) | 0.25 | 0.03 |
| 6 months | 22.5 | 7.4 | 0.21 (0.06, 0.68) | 0.29 | 0.009 |
| Interval | Iron deficiency (SF ≤ 20 μg L−1) | ||||
|---|---|---|---|---|---|
| Control (n = 143)% | Intervention (n = 191)% | OR (95% CI)* | Estimated intracluster correlation coefficient* | P‐value | |
| 3 months | 15.4 | 15.3 | 1.37 (0.53, 3.54) | 0.19 | 0.52 |
| 6 months | 18.9 | 18.8 | 1.03 (0.41, 2.59) | 0.19 | 0.94 |
| Interval | Iron deficiency (STfR > 5 mg L−1) | ||||
|---|---|---|---|---|---|
| Control (n = 143)% | Intervention (n = 191)% | OR (95% CI)* | Estimated intracluster correlation coefficient* | P‐value | |
| 3 months | 14.0 | 9.52 | 0.9 (0.41, 1.97) | 0.01 | 0.79 |
| 6 months | 14.0 | 8.38 | 0.76 (0.34, 1.7) | 0.003 | 0.51 |
| Interval | Anaemia | ||||
|---|---|---|---|---|---|
| Control (n = 143)% | Intervention (n = 191)% | OR (95% CI)* | Estimated intracluster correlation coefficient* | P‐value | |
| 3 months | 30.8 | 26.1 | 0.89 (0.52, 1.53) | 0.005 | 0.68 |
| 6 months | 24.8 | 26.1 | 1.54 (0.72, 3.35) | 0.1 | 0.27 |
CI, confidence interval; VAD, vitamin A deficiency. *Mixed model logistic regression analysis with cluster (bari) as random effects, adjusted for age, sex and baseline value of the outcome.
Iron, haemoglobin and anaemia status
The mixed‐model linear regression analyses showed no statistically significant effect of fortified chapatti on haemoglobin, SF and transferrin receptor concentrations at 3‐ or 6‐month interval (Table 3). The mixed‐model logistic regression analyses also showed no significant effect of fortified chapatti on the occurrence of anaemia or iron deficiency among the children (Table 4).
Furthermore, although more than a quarter of children were anaemic at baseline (Table 1), no marked change was observed at 6 months (26.1% in intervention and 24.8% in control group were anaemic). At baseline, iron deficiency based on SF level was present in about 19% and 24.5% children in the intervention and control groups, respectively. At 6 months, iron deficiency was still prevalent among 19% of children in both groups. However, iron deficiency was less prevalent in these children if based on transferrin receptor level rather than SF level; nonetheless, 8.4% and 14% children in the intervention and control groups, respectively, had iron deficiency at 6 months based on transferrin receptor (Table 4).
Discussion
The double‐blind randomised controlled efficacy trial of chapattis made from micronutrient‐fortified wheat flour on 6–15‐year‐olds resulted in a significant improvement in vitamin A status, as reflected in increased SR concentration at 6 months and decreased odds of VAD at both 3 and 6 months. The odds of low SR concentration (<1.05 μmol L−1) was also significantly lower in children in the intervention group at 6 months, emphasizing the 19% reduction in the prevalence of low SR concentration in the intervention group compared to a reduction of only 3.3% in the control group from the baseline prevalence. Nonetheless, 48% children in the intervention group still had SR concentration <1.05 μmol L−1 at 6 months. It could be assumed that a longer duration of supplementation of fortified chapatti could have further improved the vitamin A status of the children. However, fortified chapatti had shown no statistically significant effect on haemoglobin, anaemia or iron status.
It should be acknowledged that although the observed effect of fortified chapatti on SR at 6 months was lower than we presumed by citing a previous published study (Arroyave et al. 1981), a significant physiological effect of fortified chapatti on SR concentration was observed at 6 months. In addition, it should also be mentioned that the study was not powered for VAD, a larger sample might have been needed to test the hypothesis associated with this outcome. Due to the lack of any statistically significant effect of fortified chapattis on iron status, haemoglobin concentration and anaemia, it would be noteworthy to mention that several factors may have contributed to the lack of impact, such as the amount of iron consumed by the intervening children from fortified flour was 6.6 mg day–1 which might not be sufficient to reduce iron deficiency and anaemia as their iron consumption from regular diet might also be low. We did not collect dietary intake data as we assumed that because of randomisation of the sample, both groups would have approximately equal amount of micronutrient consumption from their usual diet. In addition, the bioavailability of the iron used to fortify wheat flour might have been lower than originally presumed. The iron compound used in this efficacy trial was hydrogen‐reduced elementary iron. It is evident, being water insoluble and poorly soluble in dilute acid, the bioavailability of this form of iron is less than other forms, e.g. ferrous sulphate (Hurrel 2002). A review of earlier studies observed a wide variability in the bioavailability of hydrogen‐reduced iron ranging from 13% to 148% relative to ferrous sulphate (Hurrel 2002). In addition, another efficacy trial reported that the RBV (relative bioavailability) of hydrogen‐reduced iron is 49% in human subjects (Zimmermann et al. 2005). Moreover, infection caused by Helicobacter pylori, common in Bangladesh (Sarkar et al. 1997), was found to be associated with iron deficiency (Seo et al. 2002) and anaemia (Annibale et al., 1999, Ashorn et al. 2001; Choe et al. 2001) or hypochlorhydria in children (Sarker et al. 2004). Existence of H. pylori infections was not taken under consideration during the design of this trial and hence remains untreated and therefore could be responsible for lack of any improvement in iron status among the subjects. The variations in iron bioavailability may also depend on the iron particle size, presence of hypochlorhydria or other existing causes of iron malabsorption (Hurrel 2002).
Our findings are in concordance to a study in Sri Lanka that failed to demonstrate any improvement in reducing anaemia among different age groups with reduced and electrolytic iron‐fortified flour (Nestel et al. 2004). Moreover, a recent review reported that efficacy trials conducted in four different countries that used hydrogen‐reduced elementary iron for fortification of wheat or maize products did not show any discernible impact on iron and/or haemoglobin status of the participants. Therefore, due to the lack of evidence of significant beneficial effect of the currently available hydrogen‐reduced iron powders on iron status, the reviewers recommended not to use reduced iron powders for the fortification of wheat or maize flours (Hurrell et al. 2010).
It is conceivable that the success of iron fortification programmes depends largely on the careful choice of the iron compound as well as dose and duration of supplementation, along with other environmental factors such as infection or parasitic infestation. It should also be noted that the children of this study did not receive anthelmintic drug since deworming has not been a routine practice in Bangladesh. Therefore, it could be speculated that deworming prior to administration of fortified chapatti might has improved iron status.
Finally, it should be acknowledged that the average per capita consumption of wheat was only 20 g in rural Bangladesh in 1998 (HKI & IPHN 1999), which was far less than the amount of flour (100 g) used in our study. However, wheat production, import and consumption have increased over the years, and in 2011, per capita wheat consumption was estimated to be above 70 g day–1 (Hussain 2012). Moreover, an organoleptic test of chapattis based on hedonic scales of different parameters such as colour, flavour, taste, mouth‐feel and overall satisfaction of consuming chapattis made from fortified wheat flour was found to be highly acceptable (Malek & Bhuyan 2001). Furthermore, vitamin A content retained in the fortified flour, even after baking chapatti was still high (89% of the added amount of vitamin A). Therefore, wheat flour could be a suitable vehicle to be fortified with vitamin A including other micronutrients for targeted as well as general population of this country.
The findings of this study are consistent with those of previous community trials in developing countries where staple food products have been fortified with vitamin A such as substantial improvement in vitamin A status from the use of fortified condiments, like sugar (Darnton‐Hill 1998), monosodium glutamate (Muhilal et al. 1988), margarine (Solon et al. 1996) and wheat flour bun [pandesal] (Solon et al. 2000). Unlike our study, community trials of iron‐fortified staple foods or condiments in developing countries resulted in very small, but statistically significant improvements in the haemoglobin and or iron status (Stuijvenberg et al. 1999; Sari et al. 2001; Zimmermann et al. 2003; WHO 2009).
There is great interest on provision of important micronutrients through food fortification, including initiatives such as the GAIN programme (Global Alliance for Improved Nutrition 2003). However, the results of our study suggest that much needs to be learnt in defining best fortification strategy for reducing anaemia and iron deficiency.
Source of funding
This study was funded by a grant from the MOST project (Contract No. HRN‐AA‐00–98‐00047‐00) and by support to the Mirsarai field area by USAID Cooperation Agreement number 388‐A‐00–97‐00032‐00.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
ASR, TA, MSA, MAW and DAS contributed to the study design. ASR was responsible for field implementation. Data collections were performed by ASR, MSA, FA and TA. The laboratory analysis was carried out by MAW. ASR did the statistical analysis with contribution from TA, FA and DAS. The manuscript was written by ASR and edited by TA, FA, MSA, MAW and DAS.
Acknowledgement
The icddr,b acknowledges with gratitude the cooperation of the people of Mirsarai who participated in this project.
Rahman, A. S. , Ahmed, T. , Ahmed, F. , Alam, M. S. , Wahed, M. A. , and Sack, D. A. (2015) Double‐blind cluster randomised controlled trial of wheat flour chapatti fortified with micronutrients on the status of vitamin A and iron in school‐aged children in rural Bangladesh. Matern Child Nutr, 11: 120–131. doi: 10.1111/mcn.12065.
References
- Annibale B., Marignani M., Monarca B., Antonelli G., Marcheggiano A. & Martino G. (1999) Reversal of iron deficiency anemia after Helicobacter pylori eradication in patients with asymptomatic gastritis. Annals of Internal Medicine 131, 668–672. [DOI] [PubMed] [Google Scholar]
- Arroyave G., Mejia L.A. & Aguilar J.R. (1981) The effect of vitamin A fortification of sugar on the serum vitamin A levels of preschool Guatemalan children: a longitudinal evaluation. American Journal of Clinical Nutrition 34, 41–49. [DOI] [PubMed] [Google Scholar]
- Ashorn M., Ruuska T. & Makipernaa A. (2001) Helicobacter pylori and iron deficiency anemia in children. Scandanavian Journal of Gastroenterology 36, 701–705. [DOI] [PubMed] [Google Scholar]
- Burgi H., Supersaxo Z. & Selz B. (1990) Iodine deficiency diseases in Switzerland one hundred years after Theodor Kocher's survey: a historical review with some new goitre prevalence data. Acta Endocrinologica (Copenhagen) 123, 577–590. [DOI] [PubMed] [Google Scholar]
- Choe Y.H., Kwon Y.S., Jung M.K., Kang S.K., Hwang T.S. & Hong Y.C. (2001) Helicobacter pylori‐associated iron‐deficiency anemia in adolescent female athletes. Journal of Pediatrics 139, 100–104. [DOI] [PubMed] [Google Scholar]
- Darnton‐Hill, I. (1998) Overview: rationale and elements of a successful food‐fortification programme. Food and Nutrition Bulletin 19, 92–100. [Google Scholar]
- Driskell W.J., Neese J.W., Bryant C.C. & Bashor M.M. (1982) Measurement of vitamin A and vitamin E in human serum by high‐performance liquid chromatography. Journal of Chromatography 231, 439–444. [DOI] [PubMed] [Google Scholar]
- Fawzi W.W., Chalmers T.C., Herrera M.G. & Mosteller F. (1993) Vitamin A supplementation and child mortality. A meta‐analysis. Journal of the American Medical Association 269, 898–903. [PubMed] [Google Scholar]
- Glasziou P.P. & Mackerras D.E.M. (1993) Vitamin A supplementation in infectious diseases: a meta‐analysis. BMJ (Clinical Research Ed.) 306, 366–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Alliance for Improved Nutrition (2003) New Global Alliance Brings Food Fortification to World's Poor [Online]. Available at: http://www.who.int/mediacentre/releases/2003/prgain/en/ (Accessed 28 June 2011).
- HKI & IPHN (1999) National and divisional trends among children and households in rural Bangladesh. 1998 NSP Annual Report Dhaka, Bangladesh: Helen Keller International and Institute of Public Health Nutrition.
- HKI & IPHN (2002) Anemia is a severe public health problem in pre‐school children and pregnant women in rural Bangladesh. Nutritional Surveillance Project Bulletin No. 10. Dhaka, Bangladesh: Helen Keller International and Institute of Public Health Nutrition.
- HKI & IPHN (2006) Bangladesh in Facts and Figures: 2005 Annual Report of the Nutritional Surveillance Project. Helen Keller International and Institute of Public Health Nutrition: Dhaka, Bangladesh. [Google Scholar]
- Hurrel R.F. (2002) Fortification: overcoming technical and practical barriers. Journal of Nutrition 132, 806s–812s. [DOI] [PubMed] [Google Scholar]
- Hurrell R., Ranum P., de Pee S., Biebinger R., Hulthen L., Johnson Q. et al (2010) Revised recommendations for iron fortification of wheat flour and an evaluation of the expected impact of current national wheat flour fortification programs. Food and Nutrition Bulletin 31, S7–21. [DOI] [PubMed] [Google Scholar]
- Hussain S.S. (2012) Bangladesh Grain and Feed Annual 2012. Gain Report. USDA Foreign Agricultural Service.
- Layrisse M., Chaves J.F., Mendez‐Castellano V.B., Tropper E. & Bastardo B. (1996) Early response to the effect of iron fortification in the Venezuelan population. American Journal of Clinical Nutrition 64, 903–907. [DOI] [PubMed] [Google Scholar]
- Malek M.A. & Bhuyan M.A.H. (2001) Report of Organoleptic Test of Fortified ATTA. Dhaka: Institute of Nutrition and Food Science, University of Dhaka.
- Muhilal, Murdiana A., Azis I., Saidan S., Jahari A.B. & Karyadi D. (1988) Vitamin A‐fortified monosodium glutamate and vitamin A status: a controlled field trial. American Journal of Clinical Nutrition 48, 1265–1270. [DOI] [PubMed] [Google Scholar]
- Nestel P., Nalubola R., Sivakaneshan R., Wickramasinghe A.R., Atukorala S. & Wickramanayake T. (2004) Use of iron‐fortified wheat flour to reduce anemia among the estate population in Sri Lanka. International Journal for Vitamin and Nutrition Research 74, 35–51. [DOI] [PubMed] [Google Scholar]
- Rice A.L., West K.P. Jr & Black R.E. (2004) Vitamin A deficiency In: Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributes to Selected Major Risk Factors (eds Ezzati M., Lopez A.D., Rodgers A. & Murray C.J.L.), pp 211–256. World Health Organization: Geneva. [Google Scholar]
- Rice E.W. (1967) Rapid determination of total hemoglobin as hemoglobin cyanide in blood containing carboxyhemoglobin. Clinica Chimica Acta 18, 89–91. [DOI] [PubMed] [Google Scholar]
- Sari M., Bloem M.W., de Pee S., Schultink W.J. & Sastroamidjojo S. (2001) Effect of iron‐fortified candies on the iron status of children aged 4–6 y in East Jakarta, Indonesia. American Journal of Clinical Nutrition 73, 1034–1039. [DOI] [PubMed] [Google Scholar]
- Sarkar S.A., Mahalanabis D., Hildebrand P., Raman M.M., Bardhan P.K. & Fuchs G. (1997) Helicobacter pylori: prevalence, transmission, and serum pepsinogen II concentrations in childrenof a poor periurban community in Bangladesh. Clinical Infectious Diseases 25, 990–995. [DOI] [PubMed] [Google Scholar]
- Sarker S.A., Davidson L., Mahmud H., Walczyk T., Hurrel R.F. & Gyr N. (2004) Helicobacter pylori infection, iron absorption, and gastric acid secretion in Bangladeshi children. American Journal of Clinical Nutrition 80, 149–153. [DOI] [PubMed] [Google Scholar]
- Seo J.K., Ko J.S. & Choi K.D. (2002) Serum ferritin and Helicobacter pylori infection in children: a sero‐epidemiologic study in Korea. Journal of Gastroenterology and Hepatology 17, 754–757. [DOI] [PubMed] [Google Scholar]
- Solon F.S., Solon M.S., Mehansho H., West K.P.J., Sarol J. & Perfecto C. (1996) Evaluation of the effect of vitamin A‐fortified margarine on the vitamin A status of preschool Filipino children. European Journal of Clinical Nutrition 50, 720–723. [PubMed] [Google Scholar]
- Solon F.S., Klemm R.D.W., Sanchez L., Dranton‐Hill I., Craft N.E. & Christian P. (2000) Efficacy of a vitamin A‐fortified wheat‐flour bun on the vitamin A status of Filipino schoolchildren. American Journal of Clinical Nutrition 72, 738–744. [DOI] [PubMed] [Google Scholar]
- Stoltzfus R.J., Mullany L. & Black R.E. (2004) Iron deficiency anemia In: Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors (eds Ezzati M., Lopez A.D., Rodgers A. & Murray C.J.L.), pp 163–209. World Health Organization: Geneva. [Google Scholar]
- Stuijvenberg M.E.V., Kvalsvig J.D., Faber M., Kruger M., Kenoyer D.G. & Benade A.J.S. (1999) Effect of iron‐, iodine‐, and β–carotene‐fortified biscuits on the micronutrient status of primary school children: a randomized controlled trial. American Journal of Clinical Nutrition 69, 497–503. [DOI] [PubMed] [Google Scholar]
- The Flour Fortification Initiative (2012) Global Progress [Online]. Available at: http://www.ffinetwork.org/global_progress/index.php (Accessed 25 January 2013).
- Underwood B.A. & Smitasiri S. (1999) Micronutrient malnutrition: policies and programs for control and their implications. Annual Review of Nutrition 19, 303–324. [DOI] [PubMed] [Google Scholar]
- UNICEF, UNU, WHO & MI (1999) Preventing iron deficiency in women and children . Technical consensus on key issues. Technical workshop, October 7–9, 1998. Boston and Ottawa: International Nutrition Foundation and Micronutrient Initiative.
- World Health Organization (WHO) (2008) Worldwide Prevalence of Anaemia 1993–2005: WHO Global Database on Anaemia . WHO: Geneva.
- World Health Organization (WHO) (2009) Global prevalence of vitamin A deficiency in populations at risk 1995–2005. WHO Global Database on Vitamin A Deficiency . WHO: Geneva.
- Zimmermann M.B., Zeder C., Chaouki N., Saad A., Torresani T. & Hurrel R.F. (2003) Dual fortification of salt with iodine and microencapsulated iron: a randomized, double‐blind, controlled trial in Moroccan schoolchildren. American Journal of Clinical Nutrition 77, 425–432. [DOI] [PubMed] [Google Scholar]
- Zimmermann M.B., Winichagoon P., Gowachirapant S., Hess S.Y., Harrington M. & Chavasit V. (2005) Comparison of the efficacy of wheat‐based snacks fortified with ferrous sulfate, electrolytic iron, or hydrogen‐reduced elemental iron: randomized, double‐blind, controlled trial in Thai women. American Journal of Clinical Nutrition 82, 1276–1282. [DOI] [PubMed] [Google Scholar]


