Abstract
The objective of this study was to identify in low‐income Chilean children with normal birthweight which factors occurring during the prenatal period and the first year are associated with overweight (OW)/obesity at 7 years. The sample included 652 7‐year‐olds from a larger cohort study. We collected anthropometric data at 0, 12 and 84 months, maternal pre‐pregnancy and pregnancy characteristics, early feeding practices, number of siblings, birth order, breastfeeding, and timing of solid introduction information. We determined the residuals for z‐scores for body mass index (BMI) (BAZ), weight/age and height/age0–12 months, run univariate analysis (X 2 or t‐test) and multivariate logistic analyses (stepwise approach); P < 0.05 was considered significant. We evaluated the goodness of fit of the model using the Hosmer–Lemeshow test and checked for overdispersion using the Pearson's X 2. The odds of children being OW at 7 years increased if their mothers were OW before pregnancy, if born with a higher BAZ (increase of 18–74% per each additional unit of BAZ) and if their BAZ growth during the first year was higher (62–239% per each unit over the predicted BAZ increase). Higher birth order was protective (6–68% less risk for 2nd birth compared with 1st and 10–73% less for ≥3rd child). All other variables, including gender, were non‐significant (P > 0.1). In low‐income Chilean children with normal birth, four factors during the prenatal period and the first year were associated with OW at 7 years: pre‐pregnancy BMI, BMI at birth, BMI gain between 0 and 12 months, and birth order.
Keywords: childhood obesity, prenatal, birthweight, birth order, weight gain
Introduction
Childhood obesity is considered a serious public health problem in developed as well as in developing nations (Lobstein et al. 2004). Despite efforts being made to tackle this condition, few initiatives have been effective in the long run. In addition, it is has not been well established at what ages preventive strategies are more effective (Dubois & Girard 2006); however, there is ample evidence that the increased prevalence of obesity occurs very early in life (Deckelbaum & Williams 2001).
In Chile, according to the National Association of School Assistance and Scholarships (JUNAEB), in charge of collecting anthropometric data on approximately 70% of children in first grade, obesity has nearly tripled over the last 25 years from 7% in 1987 to 23.1% in 2010 (http://www.junaeb.cl). In the cohort study, in which this paper is based on, we have shown that the prevalence of obesity increases with age. In boys, this prevalence is 10.3%, 16.3% and 18.6% at 2, 3 and 5 years, while in girls, these figures are 10.3%, 12.5% and 12.6%, respectively (Kain et al. 2009a). Although some small‐scale interventions have been effective in reducing obesity (Kain et al. 2009b), the experience accumulated from these studies has not translated into effective initiatives that have impacted obesity rates. On the contrary, childhood obesity has been rising continuously irrespective of preventive interventions (Burrows 2000). Because in this country, 87% of pregnant women as well as their offspring have regular health check‐ups at public health centres until 5 years of age (Chilean Ministry of Health, available at http://www.deis.cl), it is important to determine at the earliest possible, which potential risk factors are associated with later overweight (OW) because of its association to chronic diseases at a young age (Lobstein et al. 2004).
We were interested in the identification of these factors in children who were born with normal birthweight since 85% of babies in Chile are born with a birthweight between 2500 and 4000 g, while 5% are born with a birthweight <2500 g and 10% >4000 g (Chilean Institute of Statistics, available at http://www.ine.cl). Because it has been shown that growth, especially the first year of life, is associated with either a low or a high birthweight (Stettler et al. 2002; Jones‐Smith et al. 2007), we aimed at eliminating this potential risk factor from the study.
Therefore, the main objective of this study was to identify in low‐income Chilean children born with normal birthweight, which factors occurring during the prenatal period and the first year of life are associated with being OW or obese at age 7.
Key messages
Low‐income children born with normal weight from a post‐transitional country like Chile, four factors during the prenatal period and the first year of life were associated with OW at 7 years of age.
The four factors are pre‐pregnancy BMI category, BMI at birth, BMI gain between 0 and 12 months, and birth order.
Identifying these risk factors at the health check‐ups and applying preventive measures early on, especially regarding excessive weight gain after birth, could contribute to halt the continuing rise of obesity among Chilean children.
Methods and procedures
Subjects
The sample included in this study originates from a retrospective and prospective cohort study that began in 2006, with 1196 low‐income Chilean preschool children selected at 3–4 years of age from 54 public kindergartens located in Santiago and that belong to the National Association of Kindergartens. Children who attend these kindergartens belong to low‐income families; those whose mothers work full time and/or whose mother is head of household are given priority (http://www.junji.gob.cl).
The children are presently 9 years of age and will be followed until adolescence with funding provided by Chilean National Research Council (CONICYT). For this study, we restricted the sample to those born between 2500 and 4000 g (as explained earlier, infants with either low or high birthweight may exhibit altered growth patterns during the first year of life), had anthropometric data at 0, 12 and 84 months, and complete information regarding demographic characteristics, pregnancy and feeding practices during the first year of life.
Figure 1 shows the flow of participants according to the inclusion criteria described earlier. The final sample size included 652 7‐year‐old children.
Figure 1.
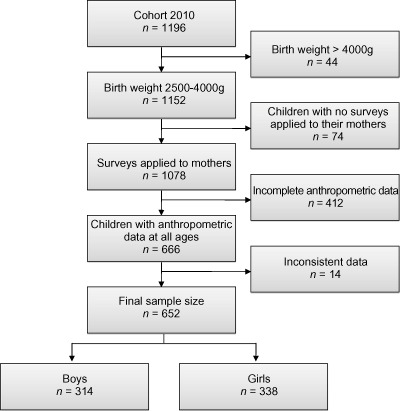
Flow of participants in the study.
This study was approved by the Ethics Committee for Human Studies of the Institute of Nutrition and Food Technology, University of Chile. In addition, a signed informed consent was obtained from one of the parents of every child.
Variables
The information on demographic characteristics, prenatal aspects and feeding practices during the first year of life was obtained from a survey applied directly to the mothers in 2007, when the children were 4 years of age. Variables considered in this study were: pre‐pregnancy weight and height, gestational weight gain (GWG), early feeding practices, smoking during pregnancy, gestational diabetes, other health conditions during pregnancy (hypertension, infections of the urinary tract, cholestasis, respiratory illnesses, risk of preterm delivery), number of siblings, birth order, duration of exclusive and non‐exclusive breastfeeding, smoking and timing of introduction of solids, beverages, candy, and snacks (see variables).
Pre‐pregnancy nutritional status was classified based on body mass index (BMI) (kg m−2) according to World Health Organisation (WHO) standards (WHO 2000), that is, normal weight (BMI 20–25) and OW (BMI > 25) (none of the women had low weight). GWG was divided into three categories (low, adequate and high) according to the increase recommended according to pre‐pregnancy nutritional status. (Institute of Medicine 2009).
Weight and height measurements of the children at birth and 12 months were obtained retrospectively from health records. Birthweight and birth length are determined at maternity hospitals immediately after delivery by trained personnel under standard procedure. After birth, weight and height are assessed by nurses or nutritionists at the health centre. Infant's weight is obtained using a paediatric balance beam scale with a precision of 10 g; recumbent length is obtained with 0.1 cm accuracy. At 84 months of age, weight and height were collected using standardised procedures by two trained registered dietitians of our team. Weight was measured with a Tanita BC 418 bioimpedance (Tanita Corporation, Tokyo, Japan) and height with SECA stadiometer model 222 (SECA Corporation, Hanover, MD, USA) to the nearest 0.1 cm. Intraobserver and interobserver reliability on weight and height measurements were confirmed (intraclass correlation ≥0.75). We calculated z‐scores for BMI (BAZ), height/age (HAZ) and weight/age (WAZ) at birth, at 12 and 84 months, using WHO (2007) references and the residuals for BAZ (ResBAZ), HAZ (ResHAZ) and WAZ (ResWAZ) between 0 and 12 months (this is explained in data analysis). Nutritional status at age 7 was categorised in two groups: normal weight or BAZ ≤ +1, and OW as BAZ > +1 (Onis & Lobstein 2010).
Data analysis
In order to verify whether children included (n = 652) had similar characteristics to those excluded (n = 257), we compared both groups in terms of gender, pre‐pregnancy BMI category, weight gain during pregnancy, nutritional status at birth and at 84 months using chi‐square (X2) for categorical variables and t‐tests for continuous variables. We then compared separately each of the variables considered as a potential risk factor for OW during the prenatal period and the first year of life with bivariate analysis using chi‐square test (X2) for categorical variables and t‐test for continuous variables (P < 0.05 was considered significant). To assess the association between risk factors with OW at age 7, we used logistic regression analysis. For fitting the logistic model, we used the command glm from stats package of r software version 2.15.1 (available at: http://www.R-project.org). In this analysis, we included cases with complete data on all potential risk factors (n = 652). The effect on OW because of the increase in both weight and height during the first year of life was determined by the effect of the observed deviation of BAZ from its predicted value at 12 months (ResBAZ0–12 months). We regressed BAZ at 12 months as outcome and BAZ at birth and sex as covariables. We determined for each child the ResBAZ0–12 months with respect to the prediction of this last model. The same procedure was done for WAZ and HAZ so as to obtain ResWAZ0–12 months and ResHAZ0–12 months. We then performed a stepwise selection procedure with forward selection and backward elimination, with a level of significance of 0.05 and 0.1 for entry and exit of variables from the model, respectively. Gender was included in every step of model building because of its practical importance. We also evaluated all continuous variables for linearity in the logit using fractional polynomials with two terms, each one with two degrees of freedom and with a level of significance of 0.05. Next, all the paired interactions from terms in the previous model were assessed with a level of significance of 0.05 and 0.1 for entry and exit from the model. This step was performed in a similar way as in the previous stepwise step we described. Finally, we evaluated the goodness of fit of the model using the Hosmer–Lemeshow test, and we checked for overdispersion through the Pearson's X2. Although forced to be present in the model, gender was not significant.
Results
Table 1 shows the characteristics of included and excluded children. Gender, pre‐pregnancy BMI category, GWG and birthweight were similar (P > 0.05). No significant differences in birthweight were observed between these two groups with respect to the nutritional status at 84 months (P = 0.651) because birthweight >4000 g was an exclusion criterion.
Table 1.
Characteristics of included vs. excluded participants [mean ± SD or n (%)]
| Included | Excluded | P‐value | ||
|---|---|---|---|---|
| N = 652 | N = 257 | |||
| n (%) or mean ± SD | n for each variable | % or mean ± SD | ||
| Variables | ||||
| Gender | 257 | 0.377 ¶ | ||
| Male | 314 (48.2) | 132 | 51.4 | |
| Female | 338 (51.8) | 125 | 48.6 | |
| PP BMI categories | 220 | 0.52 ¶ | ||
| Normal* | 403 (61.8) | 141 | 64.1 | |
| Overweight † | 249 (38.2) | 79 | 35.9 | |
| GWG ‡ | 204 | 0.995 ¶ | ||
| Deficient | 147 (22.5) | 43 | 21.1 | |
| Adequate | 167 (25.6) | 55 | 27.0 | |
| High | 338 (51.8) | 106 | 52.0 | |
| Birthweight | 3.328 ± 0.33 | 257 | 3.339 ± 0.345 | 0.651** |
| Normal weight at 84 months | 3.289 ± 0.334 | 151 | 3.296 ± 0.354 | 0.82** |
| Overweight at 84 months | 3.382 ± 0.328 | 106 | 3.401 ± 0.323 | 0.605** |
| Nutritional status at 84 months | 0.765 ¶ | |||
| % Normal weight | 376 (57.7) | 151 | 58.8 | |
| % Overweight | 276 (42.3) | 106 | 41.2 | |
PP, pre‐pregnancy; BMI, body mass index; GWG, gestational weight gain; SD, standard deviation. *BMI 19–25. †BMI > 25. ‡Recommendation according to PP BMI category. §Normal or overweight according to WHO 2007. ¶Chi‐square test (X2). **Student's t‐test.
Table 2 shows the characteristics of potential prenatal risk factors of later obesity between normal‐weight and OW children. Most prenatal risk factors were not associated with OW at age 7, except for pre‐pregnancy BMI category and GWG. In normal‐weight children, 69.1% had mothers with normal weight before pregnancy vs. 51.8% of OW children. In contrast, 30.9% of normal‐weight children had mothers who were OW before pregnancy vs. 48.2% of OW children (P < 0.001). Also, we observed that GWG is associated with the nutritional status of the children at 7 years (P = 0.049).
Table 2.
Prenatal characteristics of normal‐weight and overweight 7‐year‐old children
| Sample size | Nutritional status at age 7 | X2 | P | |||
|---|---|---|---|---|---|---|
| Normal | Overweight | |||||
| 376 | 276 | |||||
| N | % | N | % | |||
| Variables | ||||||
| Gender | ||||||
| Male | 179 | 47.6 | 135 | 48.9 | 0.11 | 0.74 |
| Female | 197 | 52.4 | 141 | 51.1 | ||
| PP BMI category | ||||||
| Normal | 260 | 69.1 | 143 | 51.8 | 20.22 | <0.001 |
| Overweight | 116 | 30.9 | 133 | 48.2 | ||
| GWG* | ||||||
| Low | 89 | 23.7 | 58 | 21 | 6.03 | 0.049 |
| Normal | 107 | 28.5 | 60 | 21.7 | ||
| High | 180 | 47.9 | 158 | 57.2 | ||
| Gestational Diabetes | ||||||
| No | 353 | 93.9 | 261 | 94.6 | 0.136 | 0.712 |
| Yes | 23 | 6.1 | 15 | 5.4 | ||
| Other conditions during pregnancy | ||||||
| No | 276 | 73.4 | 190 | 68.8 | 1.619 | 0.203 |
| Yes | 100 | 26.6 | 86 | 31.2 | ||
| Number of siblings | ||||||
| 0 | 115 | 30.6 | 99 | 35.9 | 2.66 | 0.446 |
| 1 | 141 | 37.5 | 93 | 33.7 | ||
| 2 | 68 | 18.1 | 52 | 18.8 | ||
| ≥3 | 52 | 13.8 | 32 | 11.6 | ||
| Birth order | ||||||
| 1 | 152 | 40.4 | 126 | 45.7 | 1.823 | 0.402 |
| 2 | 125 | 33.2 | 82 | 29.7 | ||
| ≥3 | 99 | 26.3 | 68 | 24.6 | ||
| Smoking during pregnancy | ||||||
| No | 317 | 84.3 | 235 | 85.1 | 0.0484 | 0.826 |
| Yes | 59 | 15.7 | 41 | 14.9 | ||
PP, pre‐pregnancy; BMI, body mass index; GWG, gestational weight gain. Values are presented as absolute and relative frequencies. Chi‐square test (X2). *Recommendation according to PP BMI category.
Table 3 shows the characteristics of potential risk factors for later obesity during the first year of life in normal‐weight and OW children at age 7. Differences were observed at birth determined as weight (in grams), BAZ and WAZ (P < 0.001). Also, ResBAZ0–12 months and ResWAZ0–12 months (P < 0.001) were significantly different, while the ResHAZ0–12 months was not (P = 0.16). Although timing of salty snack introduction was significantly different between normal‐weight and OW children (P = 0.031), this occurred at 24.2 months in the OW and 22.3 months in the normal‐weight children.
Table 3.
Anthropometric and feeding practices of normal‐weight and overweight 7‐year‐old children during the first year of life [mean ± SD or n (%)]
| Sample size | Nutritional status at age 7 | Student's t‐test or X2 | P | |
|---|---|---|---|---|
| Normal | Overweight | |||
| 376 (57.7) | 276 (42.3) | |||
| Anthropometric variables | ||||
| Birthweight (kg) | 3.28 ± 0.33 | 3.38 ± 0.33 | 3.55 | <0.001 |
| BAZ0 months | −0.118 ± 0.91 | 0.146 ± 0.90 | 3.66 | <0.001 |
| BAZ12 months | 0.49 ± 0.84 | 1.01 ± 0.98 | 8.42 | <0.001 |
| ResBAZ0–12 months | −0.23 ± 0.83 | 0.319 ± 0.97 | 7.76 | <0.001 |
| HAZ0 months | 0.134 ± 0.84 | 0.18 ± 0.87 | 0.687 | 0.49 |
| HAZ12 months | −0.37 ± 0.93 | −0.25 ± 1.07 | 1.496 | 0.13 |
| ResHAZ0–12 months | −0.057 ± 0.88 | −0.046 ± 1.00 | 1.405 | 0.16 |
| WAZ0 months | −0.017 ± 0.71 | 0.18 ± 0.70 | 3.502 | <0.001 |
| WAZ12 months | 0.145 ± 0.8 | 066 ± 0.93 | 7.752 | <0.001 |
| ResWAZ0–12 months | −0.2 ± 0.77 | 0.24 ± 0.89 | 6.725 | <0.001 |
| Breastfeeding | 0.543 | |||
| Yes (ever breastfed) | 357 (94.9) | 259 (93.8) | ||
| No | 19 (5.1) | 17 (6.2) | ||
| Duration of exclusive breastfeeding (months) | 2.97 ± 2.45 | 2.95 ± 2.54 | −0.129 | 0.898 |
| Duration of total breastfeeding (months) | 12.8 ± 10.4 | 14.3 ± 11.1 | 1.777 | 0.076 |
| Timing of solid food introduction (months) | 6.22 ± 1.99 | 6.31 ± 2.06 | 0.557 | 0.577 |
| Timing of beverage introduction of (months) | 13.26 ± 6.87 | 12.9 ± 6.99 | −0.642 | 0.52 |
| Timing of candy introduction (months) | 17.04 ± 9.4 | 15.87 ± 8.7 | −1.619 | 0.10 |
| Timing of sweet snack introduction (months) | 15.13 ± 9.4 | 14.3 ± 8.54 | −1.147 | 0.25 |
| Timing of salty snack introduction (months) | 24.2 ± 11.45 | 22.3 ± 10.5 | −2.157 | 0.031 |
BAZ, z‐scores for BMI; HAZ, z‐scores for height/age; WAZ, z‐scores for weight/age; Res, residual; SD, standard deviation.
Table 4 summarises the results of the logistic regression model that explains the association of risk factors during the prenatal period and the first year of life with OW at age 7. We observe that gender was not associated with increased odds of OW at age 7 (odds ratio 1.026, 95% confidence interval 0.73–1.44). Significant but negative effects were observed for pre‐pregnancy BMI, BAZ at birth and ResBAZ0–12 months. If mothers were OW before becoming pregnant, there is between 38% and 280% increased odds of their children being OW at age 7. Similarly, babies born with more than 1 unit of BAZ have between 18% and 74% increased odds of being OW at age 7. Babies whose growth rate deviates positively 1 unit of BAZ with respect to the predicted growth rate have between 62% and 239% increased odds of being OW at age 7. Higher birth order is protective; the second‐born child has between 6% and 236% less odds of being OW at age 7, while the third‐born child and higher has between 11% and 267% less (both comparisons are relative to the first‐born).
Table 4.
Association between risk factors during the prenatal period and the first year of life with overweight at age 7
| Variable | Categories | OR (CI 95%) |
|---|---|---|
| Gender | Male vs. female | 1.026 (0.733–1.438) |
| PP BMI | Overweight vs. normal | 1.965 (1.38–2.802) |
| BAZ 0 months | Δ 1 sd in z‐score | 1.433 (1.187–1.738) |
| ResBAZ0–12 months | Δ 1 in z‐score Res | 1.958 (1.618–2.39) |
| Birth order | 2nd vs. 1st | 0.633 (0.423–0.943) |
| Birth order | ≥3rd vs. 1st | 0.584 (0.375–0.901) |
PP BMI, pre‐pregnancy body mass index categories; OR, odds ratio; CI, confidence interval; Res, residual; BAZ, z‐scores for BMI. Log‐likelihood = −399.146, n = 652; Hosmer–Lemeshow = 22.636, d.f. = 18, P = 0.21.
Discussion
The results of this study show that in low‐income Chilean children born with normal weight, four variables are associated with OW at age 7: pre‐pregnancy BMI category, BMI at birth, BMI gain between 0 and 12 months and birth order. These results are consistent with the growing evidence that anthropometric characteristics of mothers and their offspring during the first year of life are related with OW in mid‐childhood (Dubois & Girard 2006; Péneau et al. 2010).
The finding that maternal OW significantly increases the risk of childhood obesity has been reported in many studies (Stettler et al. 2002; Whitaker 2004; Reilly et al. 2005; Kuhle et al. 2010). Tabacchi et al. (2007) reported that maternal obesity increases the transfer of nutrients through the placenta, which may produce permanent changes in programming appetite and energy metabolism. Also, a family history of OW and obesity is one of the most important factors for OW in childhood because not only genes that confer susceptibility to obesity are inherited but also OW parents probably have a distinct pattern of food intake and physical activity related directly to that of their child immediately after birth. In most cases, OW parents may create and maintain an ‘obesogenic’ environment (Ong et al. 2002; Whitaker 2004; Reilly et al. 2005; Tabacchi et al. 2007; Kuhle et al. 2010).
Several studies have shown an association between gestational diabetes and childhood obesity. It has been well established that offspring of diabetic mothers have a higher birthweight and tend to have higher obesity rates. Intrauterine exposure to diabetes per se conveys a high risk for the development of obesity in excess of risk that originates from genetic factors (Pettitt et al. 1993; Dabelea et al. 2000). Maternal smoking during pregnancy has also been found to be a risk factor for childhood obesity. Von Kries et al. (2002) and Toschke et al. (2002) showed in 6 years old German children that there is a dose/dependent association between OW and smoking during pregnancy. These authors also found that smoking after pregnancy was not associated with childhood obesity. In our study, we did not find an association between any of these conditions with OW at 7 years. It is important to remember that the sample does not include macrosomic babies that could partly explain the low prevalence of diabetic mothers (4.4%). Even though the proportion of mothers who smoked during pregnancy was significantly higher than that reported by Toschke et al. (2002) in German mothers (15% vs. 6.2%), smoking was not associated with OW in our sample.
The existing evidence regarding the association between breastfeeding and reduced risk of childhood OW has been inconsistent. In this study, there was no relationship between duration of breastfeeding and OW at age 7, similar to results reported by several authors (Whitaker 2004; Péneau et al. 2010). In studies that have found that breastfeeding is a potential protective factor for childhood obesity (Von Kries et al. 1999; Reilly et al. 2005; Kuhle et al. 2010), results indicate that the effect is observed when breastfeeding is exclusive for at least 4 months. In the present study, duration of breastfeeding (not exclusive) lasted more than a year in both groups, which shows that although exclusive breastfeeding was short, mothers continue with this practice much longer. The average duration of exclusive breastfeeding in both normal‐weight and OW children was slightly less than 3 months, which coincided with the completion of post‐natal leave of Chilean mothers.
It has been shown that early introduction of formula feeding increases the prevalence of childhood obesity (Armstrong & Reilly 2002; Bogen et al. 2004). A recent study (Huh et al. 2011) found that in formula‐fed infants, the introduction of solid foods before the age of 4 months was associated with increased odds of obesity at age 3. In this study, solids were introduced at around 6 months in both groups of children, as recommended by WHO (2003).
Because the relationship between either low or high birthweight with later obesity (Dabelea et al. 2000; Ong et al. 2002; Reilly et al. 2005; Karaolis‐Danckert et al. 2008) has been documented extensively, we decided to include in this study only children with normal weight as is the case of 85% of births in the country. Even with this restriction, birthweight was found to be one of the four variables associated with later obesity.
It has been widely documented that there is an association between early weight gain and childhood OW, being accelerated growth between 0 and 2 years, the period in which most studies have shown to be a critical one for later obesity (Stettler et al. 2002; Whitaker 2004; Jones‐Smith et al. 2007; Tabacchi et al. 2007). Other authors (Dabelea et al. 2000; Stettler et al. 2002; Dubois & Girard 2006; Jones‐Smith et al. 2007) have shown that accelerated growth during the first year is associated with an increased risk of OW in childhood. Recently, Taveras et al. (2011) found that if accelerated growth occurs during the first 6 months of life, there is a higher prevalence of obesity in children 5–10 years of age, and Gillman (2008) reports that the first few weeks or months of life are particularly sensitive for the development of obesity. Baird et al. (2005) published a systematic review of 10 studies that evaluated the relationship between infant weight gain and subsequent obesity. The relative risks of later obesity ranged from 1.17 to 5.70 in children with rapid weight gain during the first year of life. Our results support such evidence.
There are several prenatal and post‐natal risk factors that not only are associated with OW in childhood but can influence the rate of weight gain during the first years of life and in turn body composition. Karaolis‐Danckert et al. (2008) report that among rapid growers between 0 and 2 years who have been bottle‐fed early on, were exposed to tobacco in utero, had an OW mother and were a first‐born child gained significantly more %body fat between 2 and 6 years than rapid growers who did not have these factors.
GWG has been found to be associated with childhood obesity even in women with normal pre‐pregnancy weight (Di Tullio 2003). A recent review article by Adamo et al. (2012) addressing the interplay between maternal obesity, GWG and lifestyle behaviours that can perpetuate the intergenerational cycle of obesity show that although in most studies GWG has been found to be associated with infant adiposity, separating the environmental from the genetic contributions in producing excessive GWG has been very difficult. Because the recommendation of GWG is related to pre‐pregnancy BMI, guidelines recommend a much smaller weight increase for OW and obese women compared with that of normal‐weight women. In a study including US women (Weisman et al. 2010), 40% of normal‐weight women exceeded the recommended GWG compared with 65% of the OW ones. That study showed that OW women were three times more likely to exceed the recommendation as their normal‐weight counterparts. In our study, we found similar results, as 59% of the OW women before pregnancy exceeded the recommended GWG compared with 47.5% of the women with normal weight (not shown). Even though we found an association between GWG and OW in the univariate analysis, in the final regression, this variable lost relevance.
In our study, we observed that the odds of becoming OW in mid‐childhood is significantly less for the second‐born child, or the third‐ and later‐born children compared with the first‐born. This has been found in many studies (Al‐Isa & Moussa 1999; Stettler et al. 2002; Padez et al. 2005; Juresa et al. 2012). In some studies, this variable losses importance in the adjusted final models (Stettler et al. 2002), while in others, it remains significant (38–40). In Italian OW and obese women, Siervo et al. (2011) found that first‐borns have an increased BMI, adiposity and metabolic risk. Because of the high prevalence of OW and obesity in the mothers of our study, this might also occur among their first‐borns.
The main strength of this study is that the sample originates from a contemporary cohort with homogeneous characteristics in terms of socio‐economic status and birthweight. Even though the sample size for the present analysis was small, because of the restrictions we defined, there were no differences between included vs. excluded participants. There are several limitations that have to be noted. These include the fact weight and height at birth and 12 months were collected at the health clinics under a standardised procedure but less stringent than the one used by our research team. The assessment of within group variability and outliers revealed no significant discontinuity. Also, the maternal data used in the analysis (i.e. pre‐pregnancy weight and prenatal variables) are self reported, which might introduce systematic reporting bias; however, mean and prevalence of these variables are consistent with Chilean data (Lopez et al. 2003; Lagos et al. 2004; Parra et al. 2007; Mardones et al. 2008).
We were not able to obtain anthropometric data from the fathers; therefore, the presence of OW as a potential risk factor for childhood obesity could not be addressed. Because of the difficulty in obtaining this information, most studies do not include this data (Dabelea et al. 2000). With respect to maternal educational level, a potential confounder in the development of childhood obesity, some studies have found no association between this variable and obesity in pre‐school and school age probably because participants belonged to the same socio‐economic level (Pettitt et al. 1993; Lamerz et al. 2005); however, other authors have reported that parents' knowledge about feeding practices is critical in preventing rapid weight gain (Lagos et al. 2004; Reilly et al. 2005). In our study, maternal educational level was homogeneously distributed (98% of the mothers did not have a college education).
In conclusion, in our study, four factors during the prenatal period and the first year of life were associated with OW at 7 years of age in low‐income Chilean children with normal birthweight: pre‐pregnancy BMI category, BMI at birth, BMI gain between 0 and 12 months, and birth order. This underscores the fact that birthweight, even within normal range, is associated with childhood obesity. Identifying these risk factors at the health check‐ups and applying preventive measures early on, especially regarding excessive weight gain after birth, could contribute to halt the continuing rise of obesity among Chilean children.
Source of funding
Funding for this study was provided by CONICYT (Proyecto Fondecyt 1090252) and the JUNAEB.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
IR and SC analysed and interpreted the data and wrote the initial draft of the manuscript. CC provided guidance throughout the process, MM provided most of the statistical analyses, and JK participated in the interpretation of the data, critically reviewed all sections and wrote the final manuscript. All the authors have read and approved the manuscript.
Acknowledgements
We thank the children and their mothers for participating in this study since 2006.
Rios‐Castillo, I. , Cerezo, S. , Corvalán, C. , Martínez, M. , and Kain, J. (2015) Risk factors during the prenatal period and the first year of life associated with overweight in 7‐year‐old low‐income Chilean children. Matern Child Nutr, 11: 595–605. doi: 10.1111/mcn.12024.
References
- Adamo K., Ferraro Z. & Brett K. (2012) Can we modify the intrauterine environment to halt the intergenerational cycle of poverty? International Journal of Environmental Research and Public Health 9, 1263–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al‐Isa A. & Moussa M. (1999) Factors associated with overweight and obesity among Kuwaiti kindergarten children 3–5 years. Nutrition and Health 13, 125–139. [DOI] [PubMed] [Google Scholar]
- Armstrong J. & Reilly J.J. (2002) Breastfeeding and lowering the risk of childhood obesity. Lancet 359, 2003–2004. [DOI] [PubMed] [Google Scholar]
- Baird J., Fisher D., Lucas P., Kleijnen J., Roberts H. & Law C. (2005) Being big or growing fast: systematic review of size and growth in infancy and later obesity. BMJ (Clinical Research ed.) 331, 929–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogen D.L., Hanusa B.H. & Whitaker R.C. (2004) The effect of breast‐feeding with and without formula use on the risk of obesity at 4 years of age. Obesity Research 12, 1527–1535. [DOI] [PubMed] [Google Scholar]
- Burrows R. (2000) Prevention and treatment of obesity since childhood: strategy to decrease the non transmissible chronic diseases in adult. Revista Medica de Chile 128, 105–110. [PubMed] [Google Scholar]
- Dabelea D., Hanson R.L., Lindsay R.S., Pettitt D.J., Imperatore G., Gabir M.M. et al (2000) Intrauterine Exposure to diabetes conveys risks for type 2 diabetes and obesity: a study of discordant sibships. Diabetes 49, 2208–2211. [DOI] [PubMed] [Google Scholar]
- Deckelbaum R.J. & Williams C.L. (2001) Childhood obesity: the health issue. Obesity Research 9 (Suppl. 4), 239S–243S. [DOI] [PubMed] [Google Scholar]
- Di Tullio G. (2003) Eating errors in the pathogenesis of childhood obesity. La Medicina Biológica 4, 33–40. [Google Scholar]
- Dubois L. & Girard M. (2006) Early determinants of overweight at 4.5 years in a population‐based longitudinal study. International Journal of Obesity 4, 610–617. [DOI] [PubMed] [Google Scholar]
- Gillman M.W. (2008) The first months of life: a critical period for development of obesity. The American Journal of Clinical Nutrition 87, 1587–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh S., Rifas‐Shiman S., Taveras E., Oken E. & Gillman M. (2011) Timing of solid food introduction and risk of obesity in preschool‐aged children. Pediatrics 127, e544–e551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (2009) Weight Gain during Pregnancy: Reexamination of IOM Pregnancy Weight Guidelines. Available at: http://www.iom.edu/pregnancyweightgain
- Jones‐Smith J.C., Fernald L.C. & Neufeld L.M. (2007) Birth size and accelerated growth during infancy are associated with increased odds of childhood overweight in Mexican children. Journal of the American Dietetic Association 107, 2061–2069. [DOI] [PubMed] [Google Scholar]
- Juresa V., Musil V., Majer M., Ivankovic D. & Petrovic D. (2012) Behavioral patterns of overweight and obese school children. Collegium Antropologicum 36 (Suppl. 1), 139–145. [PubMed] [Google Scholar]
- Kain J., Corvalan C., Lera L., Galvan M. & Uauy R. (2009a) Accelerated growth in early life and obesity in preschool Chilean children. Obesity 17, 1603–1608. [DOI] [PubMed] [Google Scholar]
- Kain J., Leyton B., Cerda R., Vio F. & Uauy R. (2009b) Two‐year controlled effectiveness trial of a school‐based intervention to prevent obesity in Chilean children. Public Health Nutrition 12, 1451–1461. [DOI] [PubMed] [Google Scholar]
- Karaolis‐Danckert N., Buyken A.E., Kulig M., Kroke A., Forster J., Kamin W. et al (2008) How pre‐ and postnatal risk factors modify the effect of rapid weight gain in infancy and early childhood on subsequent fat mass development: results from the Multicenter Allergy Study 90. The American Journal of Clinical Nutrition 87, 1356–1364. [DOI] [PubMed] [Google Scholar]
- Kuhle S., Allen A.C. & Veugelers P.J. (2010) Perinatal and childhood risk factors for overweight in a provincial sample of Canadian Grade 5 students. International Journal of Pediatric Obesity 5, 88–96. [DOI] [PubMed] [Google Scholar]
- Lagos S., Espinoza R. & Orellana J. (2004) Estado nutritivo materno inicial y peso promedio de sus recién nacidos a término. Revista Chilena de Nutricion 31, 52–57. [Google Scholar]
- Lamerz A., Kuepper‐Nybelen J., Wehle C., Bruning N., Trost‐Brinkhues G., Brenner H. et al (2005) Social class, parental education, and obesity prevalence in a study of six‐ year‐old children in Germany. International Journal of Obesity (2005) 29, 373–380. [DOI] [PubMed] [Google Scholar]
- Lobstein T., Baur L. & Uauy R. (2004) IASO International Obesity Task Force Obesity in children and young people: a crisis in public health. Obesity Reviews 5 (Suppl. 1), 4–104. [DOI] [PubMed] [Google Scholar]
- Lopez I., Sepulveda B.H., Jeria C. & Letelier C. (2003) Niños macrosómicos y de peso normal de un consultorio de atención primaria. Comparación de características propias y maternas 1997–2000. Revista Chilena de Pediatria 74, 287–293. [Google Scholar]
- Mardones F., Duran E. & Villarroel L. (2008) Maternal anemia in Concepcion province, Chile: association with maternal nutritional status and fetal growth. Archivos Latinoamericanos de Nutricion 58, 132–138. [PubMed] [Google Scholar]
- Ong K.K., Preece M.A., Emmett P.M., Ahmed M.L. & Dunger D.B. (2002) Size at birth and early childhood growth in relation to maternal smoking, parity and infant breast‐feeding: longitudinal birth cohort study and analysis. Pediatric Research 52, 863–867. [DOI] [PubMed] [Google Scholar]
- Onis M. & Lobstein T. (2010) Defining obesity risk status in the general childhood population: which cut‐offs should we use? International Journal of Pediatric Obesity 5, 458–460. [DOI] [PubMed] [Google Scholar]
- Padez C., Mourao I., Moreira P. & Rosado V. (2005) Prevalence and risk factors for overweight and obesity in Portuguese children. Acta Pediatrica 94, 1550–1557. [DOI] [PubMed] [Google Scholar]
- Parra C.M., San Martín O.A. & Valdés R.E. (2007) Espectro clínico de la preeclampsia: estudio comparativo de sus diversos grados de severidad. Revista Chilena de Obstetricia y Ginecologia 72, 169–175. [Google Scholar]
- Péneau S., Rouchaud A., Rolland‐Cachera M.F., Arnault N., Hercberg S. & Castetbon K. (2010) Body size and growth from birth to 2 years and risk of overweight at 7–9 years. International Journal of Pediatric Obesity 6, e162–e169. [DOI] [PubMed] [Google Scholar]
- Pettitt D.J., Nelson R.G., Saad M.F., Bennett P.H. & Knowler W.C. (1993) Diabetes and obesity in the offspring of Pima Indian women with diabetes during pregnancy. Diabetes Care 16, 310–314. [DOI] [PubMed] [Google Scholar]
- Reilly J.J., Armstrong J., Dorosty A.R., Emmett P.M., Ness A., Rogers I. et al (2005) Early life risk factors for obesity in childhood: cohort study. BMJ (Clinical Research ed.) 330, 1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siervo M., Stephan B., Colantroni A. & Wells J. (2011) First‐borns have a higher metabolic rate and carry a higher metabolic risk in young women attending a weight loss clinic. Eating and Weight Disorders 16, e171–e176. [DOI] [PubMed] [Google Scholar]
- Stettler N., Zemel B.S., Kumanyika S. & Stallings V.A. (2002) Infant weight gain and childhood Overweight status in a multicenter cohort study. Pediatrics 109, 194–199. [DOI] [PubMed] [Google Scholar]
- Tabacchi G., Giammanco S., La Guardia M. & Giammanco M. (2007) A review of the literature and a new classification of the early determinants of childhood obesity: from pregnancy to the first years of life. Nutrition 27, 587–604. [Google Scholar]
- Taveras E.M., Rifas‐Shiman S.L., Sherry B., Oken E., Haines J., Kleinman K. et al (2011) Crossing growth percentiles in infancy and risk of obesity in childhood. Archives of Pediatrics & Adolescent Medicine 165, 993–998. [DOI] [PubMed] [Google Scholar]
- Toschke A.M., Koletzko B., Slikker W. Jr, Hermann M. & von Kries R. (2002) Childhood obesity is associated with maternal smoking in pregnancy. European Journal of Pediatrics 161, 445–448. [DOI] [PubMed] [Google Scholar]
- Von Kries R., Koletzko B., Sauerwald T., von Mutius E., Barnert D., Grunert V. et al (1999) Breast feeding and obesity: cross sectional study. BMJ (Clinical Research ed.) 319, 147–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Kries R., Toschke A.M., Koletzko B. & Slikker W. (2002) Maternal smoking during pregnancy and childhood obesity. American Journal of Epidemiology 156, 954–961. [DOI] [PubMed] [Google Scholar]
- Weisman C., Hillemeier M., Downs D., Chuang C. & Dyer A. (2010) Preconception predictors of weight gain during pregnancy: prospective findings from the Central Pennsylvania. Women's Health Study. Women's Health Issues 20, 126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker R.C. (2004) Predicting preschooler obesity at birth: the role of maternal obesity in early pregnancy. Pediatrics 114, e29–e36. [DOI] [PubMed] [Google Scholar]
- WHO (2000) Obesity: Preventing and Managing the Global Epidemic: Report of a WHO. Consultation. World Health Organization: Geneva, Switzerland. [PubMed]
- WHO (2003) Global Strategy for Infant and Young Child Feeding. Geneva, 2003. Available at: http://www.who.int/nutrition/publications/infantfeeding/en/index.html; http://www.who.int/childadolescent-health/NUTRITION/global_strategy.htm
- WHO (2007) WHO Child Growth Standards. Geneva; 2007 [updated 2007]. Available at: http://www.who.int/childgrowth/en/ (Accessed 5 March 2011).


