Abstract
Pre‐pregnancy overweight and obesity is associated with shorter breastfeeding (BF) duration. Whether pre‐pregnancy overweight and obesity is associated with other aspects of infant and young child feeding (IYCF) has not been investigated. We used data from 370 children born January 1999–September 2001 in a semi‐urban community in Morelos, Mexico, where information on how they were fed was available at 1, 3, 6, 9, 12, 18 and 24 months of age. We modified the World Health Organization's dietary diversity indicator to assess the quality of the complementary foods. An index that included BF, quality of complementary foods and other behaviours was constructed to measure IYCF. We used survival analysis to examine the association of pre‐pregnancy body mass index (pBMI) category and BF duration and mixed models for quality of complementary food and IYCF index. Mean maternal pBMI was 24.4 ± 4.1; 31% were overweight, and 9% were obese. pBMI was not associated with BF duration. Quality of complementary food improved over time (6 months, 1.3 ± 1.3; 24 months, 3.8 ± 1.04). Compared with normal‐weight women, overweight and obese women were more likely to feed from more food groups (0.24 ± 0.11 point, P = 0.03), but this did not improve diet diversity from 6 to 24 months. IYCF index decreased throughout follow‐up (1 month, 7.8 ± 2.4; 24 months, 5.5 ± 1.8), and pBMI was not associated with IYCF (−0.11 ± 0.13 point, P = 0.4). We conclude that heavier women were not engaging in IYCF behaviours that were distinct from those of normal‐weight women from 1 to 24 months post‐partum.
Keywords: pre‐pregnancy BMI, infant and young child feeding, breastfeeding duration, complementary foods, Mexico
Introduction
Obesity before conception is associated with greater risk of adverse nutritional outcomes for the offspring, such as increased risk for large‐for‐gestational‐age babies (Baeten et al. 2001; Ray et al. 2001) and childhood obesity (Kitsantas et al. 2010). In addition to these outcomes, maternal obesity has been linked to shorter duration of breastfeeding (BF) (Hilson et al. 1997; Donath & Amir 2000; Baker et al. 2004; Kugyelka et al. 2004; Oddy et al. 2006) and an early introduction of complementary foods (Rasmussen 2007). Also, high maternal body mass index (BMI) before pregnancy has been positively associated with low‐quality complementary diets in infancy (Robinson et al. 2007).
Women who are obese before pregnancy are more likely to abandon exclusive, full and any BF sooner than their normal‐weight counterparts (Oddy et al. 2006). These poor BF outcomes may result from the biological and mechanical difficulties that are unique to obese women, such as inadequate prolactin surge and hyperinsulinaemia (Rasmussen & Kjolhede 2004), large breasts or flat nipples, factors that make it difficult to establish copious milk production (Jevitt et al. 2007).
Pre‐pregnancy obesity may also be associated with the complementary diet. Among Danish women, cessation of BF was associated with earlier introduction of complementary foods by obese women (Baker et al. 2004). In a cohort study of mother–child pairs in the UK, pre‐pregnancy body mass index (pBMI) was associated with inadequate dietary patterns at 6 and 12 months of age (Robinson et al. 2007). The mechanisms underlying an early introduction of foods or infant dietary patterns with pBMI are unknown. It is also unclear if pBMI is associated with infant and young child feeding (IYCF) in middle‐ and low‐income countries.
In Mexico, a middle‐income country with high prevalence of overweight (35%) (25.0–29.9 kg m−2) and obesity (35%) (≥30 kg m−2) (Olaiz‐Fernández et al. 2006), overweight and obese women (BMI >25 kg m−2) were less likely to report exclusive BF at 6 months (15% vs. 25%) and recalled a shorter duration of BF (8 vs. 10 months) than normal‐weight women (González‐Cossío et al. 2003). A high BMI at 1 week post‐partum was associated with perceived milk insufficiency (Perez‐Escamilla et al. 1993) and with poor BF practices at 2 months post‐partum (Segura‐Millan et al. 1994). It is unknown, however, if pBMI in this context is associated with complementary foods or other aspects of IYCF.
In this study, we examined the association of maternal pBMI on IYCF in a cohort of Mexican mothers and their children from 1 to 24 months of age. We first examined BF duration and the quality of the complementary diet as two separate outcomes. Because BF and complementary feeding may be highly related (e.g. women who stop BF may feed a greater variety of solid foods and beverages), we constructed an IYCF index that would allow us to examine BF, complementary foods and the use of bottle and sweetened beverages simultaneously. We hypothesised that pBMI would be negatively associated with BF duration and, consequently, that heavier women would feed a greater variety of complementary foods.
Key messages
In a small low‐income, semi‐urban community in Mexico, high pre‐pregnancy BMI was not associated with risk for termination of breastfeeding
In a small low‐income, semi‐urban community, pre‐pregnancy BMI was associated with diversity of the complementary diet but the magnitude of association was small and is likely insufficient for improving diet diversity.
Further research on the possible associations of pre‐pregnancy obesity on IYCF in Mexico is needed given the increase in the proportion of women of childbearing age who are now overweight or obese and the changes in dietary patterns that have occurred in the 10 years since these data were collected.
Subjects and methods
Subjects
We used data from a randomized, double‐blind micronutrient trial that was conducted among pregnant women and their offspring from a small, semi‐urban community in Morelos, a state in central Mexico (Ramakrishnan et al. 2003). Only details relevant to the present study are included here. Pregnant women received one of two supplements [iron‐only vs. multiple micronutrient (MMN)] daily from as early as possible (<13 weeks) until delivery. From March 1999 until September 2001, women in the pregnancy trial were invited to enroll their 3‐month‐old offspring in a subsequent trial in which children received one of two supplements (iron–vitamin A or MMN) daily from 3 to 24 months of age. Data on IYCF practices were collected when the children were 1, 3, 6, 9, 12, 18 and 24 months old.
For a woman to be included in this secondary analysis, she had to (a) deliver a healthy, full‐term infant; (b) subsequently enroll this infant into the child supplementation trial; (c) be ≥16 years old (d) and have complete information to determine eligibility status (Fig. 1). From those eligible, we excluded (a) women without a weight in the first trimester of pregnancy (b) and the youngest sibling, when a woman had two infants in the study. Of the 370 included infants, 99 (26%) were lost to follow‐up during the 24‐month observation period of the child supplementation trial.
Figure 1.
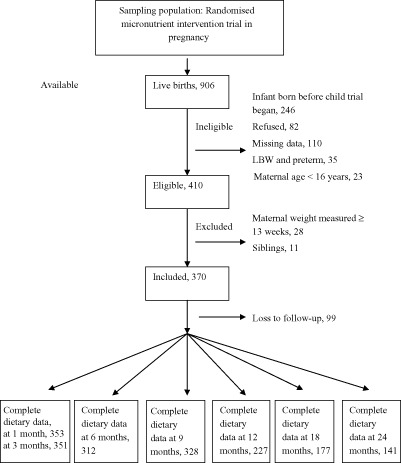
Participant flow chart summarising ineligible, excluded and those with complete dietary data at follow‐up.
Variable creation
Wealth
A measure of household wealth was created by considering household construction (i.e. floor, roof, walls, water, sewage, electricity and bedrooms) and possessions (i.e. car, TV, washer, refrigerator) and smaller household items (i.e. radio, blender, sewing machine) as well as type of cooking fuel (gas or wood) and location of kitchen (indoor or outdoor) (Ramakrishnan et al. 2003). We conducted a principal factors analysis and retained the first factor as it explained 74% of the common variance. The wealth score had a Cronbach's alpha reliability of 0.70.
Maternal pBMI
Weight and height were measured at study enrollment using standardised procedures. Because weight gain in the first trimester is usually less than 2 kg (Hytten & Leitch 1971; IOM 1990), we used weight measured at 4–12 weeks of gestation as a proxy for pre‐pregnancy weight. We adjusted for week of gestation and found that it did not alter the regression estimates between the exposure and the outcomes, so week of gestation was removed from our final models. pBMI was used as a dichotomized variable, with overweight and obese women (n = 148, 40%) compared with all other women (n = 221, 60%).
IYCF index construction
Data were collected using two different instruments – an infant‐feeding questionnaire with yes/no responses for visits between 1 and 9 months of age and 24‐hour dietary recalls collected on two non‐consecutive days for visits between 12 and 24 months of age. The infant‐feeding questionnaire provided general information about categories of foods, beverages and feeding utensils. From 1 to 9 months, responses captured behaviours that occurred between follow‐ups and not just what was consumed in the previous day. After 12 months of age, only information on specific foods and amounts consumed was available from the 24‐hour recall.
We constructed an age‐specific IYCF index based on best practices outlined in the World Health Organization (WHO) Guiding Principles for Complementary Feeding of the Breastfed Child (PAHO 2002) and the WHO Dietary Diversity indicator (WHO 2008). Behaviours in the IYCF index were scored on a bipolar scale, −5 to +5. The numbers are arbitrary, but the intention was to score beneficial practices (e.g. exclusive BF from 0 to 6 months) relatively higher than detrimental practices (e.g. use of bottles). The index was partitioned into three sections: BF, quality of complementary foods and use of bottles and sweetened beverages (Table 1).
Table 1.
Scoring of child feeding behaviours by age in the infant and young child feeding index
| Behaviour | 1–3 months | 6 months | 9 months | 12–24 months |
|---|---|---|---|---|
| Breastfeeding | ||||
| Exclusive breastfeeding | Yes = 5 | Yes = 5 | – | – |
| No = 0 | No = 0 | |||
| Full breastfeeding | Yes = 4 | Yes = 4 | – | – |
| No = 0 | No = 0 | |||
| Any breastfeeding | Yes = 2 | Yes = 2 | Yes = 5 | Yes = 5 |
| No = 0 | No = 0 | No = 0 | No = 0 | |
| Use of bottles | Yes = −5 | Yes = −5 | Yes = −5 | NA−1 |
| No = 0 | No = 0 | No = 0 | ||
| Sweetened beverage | Yes = −2 | Yes = −2 | Yes = −2 | Yes = −2 |
| No = 0 | No = 0 | No = 0 | No = 0 | |
| Carbonated beverage | NA−1 | NA−1 | NA−1 | Yes = −3 |
| No = 0 | ||||
| Complementary food | Yes = −3* | 0–7 † | 0–7 | 0–7 |
| No = 0 | ||||
| Maximum points | −10 to 5 | −7 to 9 | −7 to 12 | −5 to 12 |
*Ever fed solid food. †Complementary food score. NA−1: not applicable because item not available in data.
BF
Exclusive BF (no solids or liquids other than breast milk) was scored the highest, followed by full BF (breast milk along with non‐nutritive liquids, water and tea without sugar) and then any BF.
Bottles
We were only able to score the use of bottles from 1 to 9 months because the 24‐hour recall did not include this information. The use of bottles was considered a negative practice.
Sweetened and carbonated beverages
The use of sweetened beverages was scored negatively on the assumption that beverages would significantly displace breast milk or formula and are not the nutrient‐dense beverages needed for growth. From 0 to 9 months, the sweetened beverage category comprised of tea or water with sugar, any type of fruit juice or milk with sugar. From 12 months, because more complete information was available, this category included sugar and chocolate added to beverages. Unsweetened 100% fruit juice was categorized as fruit, but other types of juice were considered to be sweetened beverages. Carbonated beverages were scored separately because these are inappropriate for infants and young children.
Complementary foods
Feeding solids at 1 and 3 months is a negative practice as young infants are not developmentally ready to receive solid foods. Dietary staples, animal‐source foods, fruits and vegetables should be fed beginning at 6 months (PAHO 2002). The WHO Dietary Diversity indicator is useful for assessing how many food groups the child receives in the previous day (WHO 2008). Dietary diversity captures the quality of the complementary diet (Ruel 2003). The WHO indicator scores seven food groups and categorizes fruits and vegetables on their vitamin A content. We could not categorize fruits and vegetables by their vitamin A content because data were collected as food categories not by food types at 6 and 9 months. Our indicator has seven food groups because we categorized fruits and vegetables separately.
The seven groups scored were grains/tubers (from 1 to 9 months, rice and tortilla only), fruits, vegetables, legumes/nuts (from 1 to 9 months, beans only), egg, flesh foods (from 1 to 9 months, fish consumption was not asked) and dairy (i.e. cheese, cow's milk and infant formula and yogurt, except for yogurt from 1 to 9 months). Categories were scored 1 if the mother reported feeding a food belonging to that category. For cases where mixed dishes were used, individual food components were scored. The categories were summed for each follow‐up visit after 6 months of age. The theoretical range in complementary food scores at each time point was 0 (not given any foods) to 7. For each age ≥12 months, the final score was based on the average of the two non‐consecutive dietary recalls. We also examined energy density of the complementary diets in toddlers. Energy intake from all foods and beverages consumed (except breast milk) in the previous day was estimated from food composition tables from INSP (Safdie M, Barquera S, Porcayo M, Rodriguez S, Ramirez C, Rivera J. et al., Food composition table, 2004, unpublished data). Energy intake was divided by total amount of food and beverages (except breast milk) consumed (g) to obtain an estimate of energy density (kcal g−1). We report the average of the two non‐consecutive dietary recalls at 12, 18 and 24 months by pBMI category.
IYCF index scoring
We took the range in IYCF scores at each follow‐up and anchored them on a scale from 0 to 10 to allow for comparisons over time. For instance, at 1 month, our theoretical range was −10 to 5, so a child with −8 total points received a scaled score of 1.3, and a child with 5 total points received a scaled score of 10. Inasmuch as the index is based on best practices, less optimal feeding behaviours would result in scores closer to zero. For example, at 1 month, women who do not exclusively breastfeed and use bottles would receive low scores for these practices. At 12 months, women who stopped breastfeeding and offered sweetened beverages instead would receive low scores for these behaviours also.
Statistical analysis
To compare participants characteristics at baseline with those who were excluded and those who dropped out, we used independent‐sample t‐tests to assess differences in means and Mann–Whitney U‐test for interval variables that were skewed. Data in text are mean ± SD. Chi‐squared tests with contingency tables were used to assess differences in frequency.
The statistical models described below were adjusted for variables that are associated with pBMI and child feeding in Mexico: education (≤6 years, >6 years), wealth score, multiparity (0, ≥1), maternal age at enrollment, indigenous ethnicity (speak an indigenous language) (González‐Cossío et al. 2003). We adjusted for supplementation group assignment for both mothers and infants. As a robustness check, we ran the models with maternal weight adjusted for height and obtained similar results as with pBMI (data not shown). In our sample, pBMI was uncorrelated with height (r = −0.04).
Analysis of BF duration
To test the hypothesis that pBMI category was associated with shorter duration of full and any BF, we used the complementary log–log (clog–log) model, which is a discrete analogue of the continuous proportional hazard model that is appropriate for time intervals that vary in length (3‐ vs. 6‐month intervals) and for interval censoring (Allison 1995). The interpretation of the regression coefficients in the clog–log models is similar to hazard ratio (Allison 1995). We used the clog–log models to examine time until the termination of full (when the child was first provided other milks or food) and any BF (when a child stopped BF altogether). To detect a 1‐month difference (SD = 1.5) in any BF duration between normal‐weight and overweight and obese women with adequate power, we required 40 women in each group, which were available. The hazard was proportional between the two levels of the independent variable (X2 = −2.47, 1 d.f.).
Analysis of quality of complementary food and IYCF index
We used mixed models to examine the association of pBMI category with complementary food and with the IYCF index across the follow‐up period. Mixed models are useful for both hypothesis testing and for estimating (reporting) the variation in the dependent variable among children given the dependence among repeated responses. We first fitted a random‐intercept model with the dependent variable; child age, pBMI category and other covariates as fixed effects; and random intercepts for children – that is, the intercept was allowed to vary for each child. Then we fitted a two‐level, random‐coefficient model that had random intercepts for children and random slope for child age, but these models failed to converge, suggesting that over time there was little variation in the slope for IYCF index and complementary food score within children. We followed the same procedure for complementary foods and IYCF index. For each model, we verified that the residuals were normally distributed and that the relationship was linear between the predicted and observed values.
As a robustness check on the IYCF index, we examined if the use of bottles or sweetened beverages were each associated with pBMI and found that they were not (data not shown). All 370 children were included in the analysis of breastfeeding duration (full and any), quality of complementary food and the IYCF index for the seven follow‐up visits. There were 2590 person‐time observations available for each analysis. Not all children had complete information for all observation times (Fig. 1), and the models excluded a child at a particular time point if that child had missing data then. In the case of drop‐outs, data were included in the analyses for all observation times before loss to follow‐up. We assume that the missing information for mothers and children could be related to covariates that were measured (e.g. parity, age, wealth, education and ethnicity), that is the data are missing at random. For example, not having a dietary recall could be related to maternal education. We added these covariates to account for potential selection bias. The restricted maximum likelihood estimation used in the mixed models was appropriate for data that are missing at random.
Analyses were done using stata (version 10.1, StataCorp, College Station, TX, USA). We considered differences to be statistically significant at P < 0.05.
Results
The mother–child pairs included in the analysis (n = 370) did not differ from those excluded from the analysis (n = 40) on potential confounding variables (Table 2). A higher proportion of the excluded than the included women delivered vaginally. On average, they were farther along in their pregnancies at the baseline survey, although these differences did not reach statistical significance.
Table 2.
Selected characteristics of mother–child pairs included and excluded from the analysisa
| Characteristic | Included (n = 370) | Excluded (n = 40) | P‐value |
|---|---|---|---|
| Mother | |||
| Parity, n | 1 (0, 11) | 1 (0, 5) | 0.79 |
| Maternal age, years | 23.7 ± 5.1 | 23.8 ± 5.1 | 0.68 |
| Wealth, score | −0.035 ± 0.9 | −0.18 ± 0.8 | 0.28 |
| Education, years | 6 (0, 15) | 7 (0, 16) | 0.70 |
| Weeks pregnant, weeks | 8.6 ± 1.9 | 12.5 ± 3.1 | < 0.001 |
| People in home, n (range) | 5 (1, 23) | 5 (2, 14) | 0.75 |
| Indigenous ethnicity, n (%) | 111 (30) | 11 (27) | 0.69 |
| Married, n (%) | 259 (70) | 28 (72) | 0.58 |
| Vaginal delivery, n (%) | 226 (61) | 30 (74) | 0.098 |
| Weight, kg | 54.0 ± 9.8 | 53.9 ± 10.2 | 0.95 |
| Height, cm | 148.5 ± 4.9 | 149 ± 4.2 | 0.12 |
| Waist circumference, cm | 78.9 ± 8.9 | 77.7 ± 9.2 | 0.51 |
| BMI, kg m−2 | 24.4 ± 0.2 | 24.0 ± 0.7 | 0.56 |
| Underweight (<18.5 kg m−2), n (%) | 14 (4) | 4 (10) | 0.07 |
| Normal‐weight (18.5–24.9 kg m−2), n (%) | 207 (56) | 23 (57) | |
| Overweight (25.0–29.9 kg m−2), n (%) | 116 (31) | 7 (18) | |
| Obese (≥30 kg m−2), n (%) | 32 (9) | 6 (15) | |
| Child | |||
| Birth weight, kg | 3.1 ± 0.3 | 3.1 ± 0.3 | 0.90 |
| Gestational age, weeks | 39.8 ± 0.7 | 39.7 ± 0.8 | 0.40 |
| Girl infant, n (%) | 159 (43) | 16 (40) | 0.49 |
Values are n (%), mean ± SD, median (range).
Women who dropped out during the child supplementation study (n = 99, 26%) were younger (22.6 ± 4.2 vs. 24.2 ± 5.3, P < 0.001) than those who completed the study; but they did not differ in pBMI, education, wealth, parity, indigenous ethnicity or child supplementation group. A greater proportion of the women who dropped out were from the iron‐supplement group (Fe, 30% vs. MMN, 23%, P < 0.001). The most cited reasons for dropping out were child did not like the supplement (n = 14, 14%), and that the mother (n = 27, 27%) or father (n = 23, 23%) did not want to participate.
In this sub‐sample, 30% of the women were primiparous, and another 30% had one child.
pBMI
The pBMI range was 17.3–45.1 kg m−2 (Table 2). pBMI and waist circumference were highly correlated (r = 0.86), and 16% (n = 41) had abdominal obesity (waist circumference >88 cm).
BF, milk feeding and introduction of food between 1 and 6 months
BF was nearly universal (97%) at 1 and 3 months. Full BF was reported by 81% (n = 281) of the women at 1 month and 77% (n = 271) at 3 months. Exclusive BF was reported by only 32% (n = 114) of the women at 1 month and 14% (n = 50) at 3 months. Between 1 and 6 months, 16% of the women reported using infant formula.
Bottles and sweetened beverages
About one‐third of women reported using bottles at 1 and 3 months, and over half did so at 6 months. By 9 months, 30% reported using bottles. Use of sweetened beverages was infrequent from 1 to 6 months (<3%) but increased to 63% (n = 208) by 9 months. The use of carbonated beverages increased through the second year of life, from 29% at 12 months to 56% at 24 months.
Complementary food
At 3 months, 7% of the women (n = 24) reported giving solid food. By 6 months, however, 75% reported the use of solid food (n = 232). At 6 months, 60% of the women had fed fruits, 20% grains and 12% vegetables. At 9 months, 70% had fed fruits, 68% grains and 30% vegetables. By 18 months, feeding grains was nearly universal (≥96%). In the second year, 65% of the women had fed a fruit in the previous day, while less than 6% had fed a vegetable.
The majority of children ≥12 months of age received an animal‐source food in the previous day. At 12 months, 73% of had received egg, flesh food or dairy product, and the proportion increased to 88%, at 18 months and 91% at 24 months. At 12, 18 and 24 months, 16%, 32% and 35% of the children were receiving cow's milk, respectively.
Quality of complementary food improved between 6 and 24 months of age (Table 3). The low score at 6 months likely reflects that most children were just beginning to receive foods. The scores improved at 9 months, but this may be high because it represents intake between 6 and 9 months, not on a single day as is the case for values obtained at 12, 18 and 24 months. Between 12 and 24 months, scores gradually improved as women fed their children more complementary foods, but the scores remained below 4, which is the minimum acceptable score recommended by the WHO.
Table 3.
Unadjusted mean and median for quality of complementary food and infant and young child feeding (IYCF) index for each follow‐upa
| Visit | n | Complementary food | n | IYCF index | ||
|---|---|---|---|---|---|---|
| 1 month | – | – | 353 | 7.8 ± 2.4 | 9.3 (2.0, 10.0) | |
| 3 months | – | – | 351 | 7.5 ± 2.4 | 9.3 (1.3, 10.0) | |
| 6 months | 312 | 1.3 ± 1.3 | 1.0 (0, 6.0) | 307 | 4.3 ± 1.8 | 3.8 (1.3, 8.1) |
| 9 months | 328 | 3.1 ± 2.0 | 3.0 (0, 7.0) | 324 | 6.3 ± 1.7 | 6.8 (0.5, 9.5) |
| 12 months | 227 | 2.5 ± 0.85 | 2.5 (0.5, 5.0) | 227 | 6.6 ± 1.1 | 6.7 (2.3, 8.2) |
| 18 months | 177 | 3.3 ± 0.95 | 3.0 (1.0, 5.5) | 177 | 6.1 ± 1.6 | 6.5 (2.1, 9.1) |
| 24 months | 141 | 3.8 ± 1.04 | 4.0 (1.0, 6.0) | 141 | 5.5 ± 1.8 | 5.2 (1.7, 9.4) |
Values are mean ± SD and median (range).
Total energy density (kcal g−1) did not differ by pBMI category (normal‐weight vs. overweight and obese) at 12 (0.56 ± 0.22 vs. 0.55 ± 0.23, respectively, P = 0.98), 18 (0.67 ± 0.18 vs. 0.62 ± 0.15, respectively, P = 0.20) and 24 months (0.72 ± 0.20 vs. 0.75 ± 0.16, respectively, P = 0.40).
IYCF index
The IYCF index decreased throughout the follow‐up period (Table 3). High IYCF index at 1 and 3 months was largely attributable to good full and exclusive BF behaviours. At 6 months, the use of bottles and transition from full breastfeeding to any breastfeeding negatively impacted this index. The increase in index at 9 months reflects the contribution of the high mean quality of complementary food. From 12 to 24 months, use of carbonated beverages negatively affected IYCF index despite improvements in quality of complementary food.
Associations between pBMI and termination of BF, the quality of complementary foods and IYCF index
pBMI category was not significantly associated with the hazard of stopping full and any BF (Table 4). Baseline covariates were not significantly associated with termination of full and any BF in this sample. The cumulative survival (continuance) of breastfeeding did not differ between the groups (Figs 2 and 3).
Table 4.
Association between pre‐pregnancy BMI category and termination of full and any breastfeeding in the adjusted complementary log‐log multivariate model
| Variables | Full breastfeeding (n = 902*) | Any breastfeeding (n = 1912) | ||
|---|---|---|---|---|
| Hazard ratio (95% CI) | P‐value | Hazard ratio (95% CI) | P‐value | |
| Month | ||||
| 1 | 0.27 (0.18, 0.39) | < 0.001 | 0.017 (0.0035, 0.082) | 0.001 |
| 3 | 0.25 (0.16, 0.37) | < 0.001 | 0.0074 (0.0012, 0.044) | < 0.001 |
| 6 | 1.79 (1.25, 2.54) | < 0.001 | 0.012 (0.0025, 0.067) | < 0.001 |
| 9 | 2.92 (1.87, 4.56) | < 0.001 | 0.022 (0.0046, 0.11) | < 0.001 |
| 12 | – | 0.027 (0.0058, 0.13) | < 0.001 | |
| 18 | – | 0.19 (0.044, 0.78) | 0.021 | |
| 24 | – | 0.23 (0.055, 0.98) | 0.047 | |
| Overweight/obese † | 1.22 (0.95, 1.56) | 0.12 | 1.33 (0.87, 2.04) | 0.19 |
| Maternal age ‡ , years | 1.00 (0.97, 1.03) | 0.86 | 0.95 (0.91, 1.00) | 0.076 |
| Multiparity § | 0.92 (0.67, 1.23) | 0.59 | 1.43 (0.85, 2.40) | 0.17 |
| Education ¶ , <6 years | 1.01 (0.78, 1.28) | 0.20 | 0.88 (0.58, 1.33) | 0.56 |
| Indigenous ethnicity** | 1.07 (0.83, 1.39) | 0.24 | 0.78 (0.50, 1.25) | 0.31 |
| Wealth ‡ , score | 1.10 (0.96, 1.27) | 0.34 | 0.97 (0.85, 2.40) | 0.89 |
| Child supplement †† , MMN | 0.99 (0.79, 1.25) | 0.68 | 1.35 (0.89, 2.02) | 0.15 |
| Mother supplement ‡‡ , MMN | 1.07 (0.85, 1.34) | 0.57 | 1.46 (0.97, 2.16) | 0.069 |
*Person‐time. Reference group: †pBMI < 25 kg m−2. ‡Maternal age centred at mean (23.5 years), wealth centred at mean (−0.035). Reference group: §Primiparity. ¶≥6 years. **Non‐indigenous. ††Fe‐Vit A group assignment. ‡‡Fe group assignment. 95% CI, 95% confidence interval; BMI, body mass index; MMN, multiple micronutrient; pBMI, prepregnancy BMI.
Figure 2.
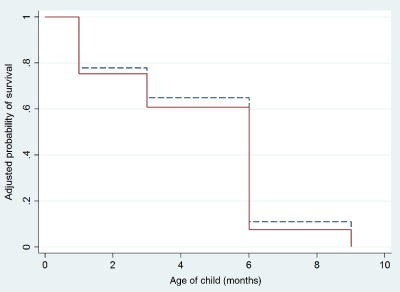
Cumulative survival for duration of full breastfeeding between normal‐weight (dash) and overweight and obese women (solid). Survival probability is adjusted for maternal age, education, wealth, multiparity, indigenous ethnicity and supplementation group assignment for mother and child.
Figure 3.
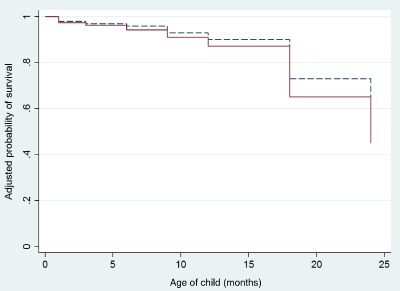
Cumulative survival for duration of any breastfeeding between normal‐weight (dash) and overweight and obese women (solid). Differences in survival probabilities are adjusted for maternal age, education, wealth, multiparity, indigenous ethnicity and supplementation group assignment for mother and child.
In the mixed models, pBMI category was associated with quality of complementary food from 6 to 24 months (Table 5). Compared with normal‐weight women, overweight and obese women fed foods from more of the seven foods groups that were scored. The difference in score, however, was small [0.24 ± 0.12 point, 95% confidence interval (CI): 0.021, 0.46, P = 0.031] on a scale of 0–7. Wealth and multiparity had a small positive association with the complementary food score from 6 to 24 months in this model.
Table 5.
Mixed linear models of the association between pre‐pregnancy BMI category and quality of complementary food and infant and young child feeding (IYCF) index throughout follow‐up
| Complementary food* (n = 1156) | IYCF index † (n = 1836) | |||
|---|---|---|---|---|
| β‐coefficient ± SE | P | β‐coefficient ± SE | P | |
| Constant | 1.83 ± 0.26 | < 0.001 | 7.51 ± 0.31 | < 0.001 |
| Child age ‡ , months | 0.12 ± 0.0067 | < 0.001 | −0.083 ± 0.0075 | < 0.001 |
| Overweight/obese § | 0.24 ± 0.11 | 0.031 | −0.11 ± 0.13 | 0.41 |
| Maternal age ‡ , years | −0.013 ± 0.012 | 0.29 | −0.014 ± 0.015 | 0.34 |
| Education ¶ , <6 years | −0.078 ± 0.11 | 0.47 | −0.017 ± 0.13 | 0.89 |
| Wealth ‡ , score | 0.12 ± 0.062 | 0.044 | −0.010 ± 0.074 | 0.89 |
| Multiparity** | 0.26 ± 0.13 | 0.048 | 0.21 ± 0.16 | 0.17 |
| Indigenous ethnicity †† | 0.050 ± 0.12 | 0.67 | 0.082 ± 0.14 | 0.56 |
| Child supplement ‡‡ , MMN | −0.10 ± 0.10 | 0.33 | −0.21 ± 0.12 | 0.081 |
| Mom supplement §§ , MMN | −0.016 ± 0.10 | 0.87 | −0.25 ± 0.12 | 0.036 |
| Unexplained variance components | ||||
| Among child | 0.38 ± 0.075 | 0.43 ± 0.11 | ||
| Within child | 1.64 ± 0.082 | 4.55 ± 0.17 | ||
*Complementary food: seven food groups: grains/tubers, fruits, vegetables, legumes, egg, flesh foods and dairy (i.e. cheese, cow's milk, infant formula, yogurt). Score range: 0 to 7; random intercept model, N is person‐time. †IYCF Index includes BF (exclusive, full and any), bottles and syringes, sweetened and carbonated beverages, and complementary food; Score range: 0 to 10; random intercept model, N is person‐time. ‡Child age centred at 6 months for complementary food and 1 month for IYCF index. Maternal age centred at mean (23.7 years). Wealth centred at mean (−0.035). Reference group: §pBMI < 25 kg m−2. ¶Education, ≥6 years. **Primiparity. ††Non‐indigenous. ‡‡Fe‐Vit A group assignment. §§Fe group assignment. BMI, body mass index; MMN, multiple micronutrient; pBMI, prepregnancy BMI.
We did not find a statistically significant relationship between pBMI category and IYCF as assessed by our IYCF index (Table 5). The coefficient for pBMI in overweight and obese women was −0.11 ± 0.13 (95% CI: −0.15, 0.37, P = 0.41) point on a scale of 0–10.
Discussion
In this study, maternal pBMI was not associated with BF duration or with IYCF but was positively associated with the complementary food score, even after adjusting for parity, education and wealth. The difference between normal‐weight and overweight and obese women in the complementary foods score was 0.24 point, which is unlikely to have a significant impact on diet diversity from 6 to 24 months. Therefore, we conclude that heavier women were not engaging in IYCF behaviours that were distinct from those of normal‐weight women from 1 to 24 months post‐partum.
Our research was motivated by previous studies in the USA (Li et al. 2003; Hilson et al. 2004; Kugyelka et al. 2004; Rasmussen & Kjolhede 2004), Denmark (Baker et al. 2004, 2007) and Australia (Donath & Amir 2000), all of which showed a positive association between maternal pBMI and early cessation of BF. Furthermore, from cross‐sectional studies, obese women in Mexico were less likely to exclusively BF their infants or breastfed in general for shorter periods (González‐Cossío et al. 2003). Our findings differ from those in the literature for three possible reasons. First, BF in our sample was relatively homogenous; termination of full BF was mostly attributable to the introduction of solid foods, formula use was uncommon and women breastfed well into the second year of life. In contrast, the median BF duration for obese Hispanic women living in an urban area in New York State was 10 days (Kugyelka et al. 2004). We did not have sufficient variation among these Mexican women to detect a difference in any BF duration by pBMI, if one had existed. Covariates associated with BF practices in Mexico, such as maternal education, parity and ethnicity, were not significantly associated with full and any BF duration in this study, which may be due to differences in how breastfeeding was measured in our study compared with other studies in Mexico. The relatively homogenous practices suggest that other unmeasured sociocultural factors, such as a strong cultural consensus for breastfeeding, are important determinants of the intensity and duration of BF in this community (Monterrosa 2010).
Second, we sampled women from a low‐income community in a middle‐income country (Ramakrishnan et al. 2003). Economic constraints may be a powerful motivator to continue BF, despite any physiological or mechanical difficulties women may be experiencing, because the alternatives (formula or solid foods) are expensive. In the USA and Denmark where infant formula is affordable, obese women can choose to feed the alternative beverage and foods and discontinue BF sooner at will. In Mexico, this is also likely to be true among women of higher socioeconomic status who are also obese.
Third, we had a relatively low proportion of obese women (9%), and most of them (82%) were obese class I (BMI 30–35 kg m−2). The proportion of obesity in our sample was lower than the national prevalence of 19% in 1999 (Barquera et al. 2007). Our outcomes, therefore, primarily reflect the performance of overweight women rather than obese women per se. In other studies, overweight women had a lower risk for cessation of BF than obese women (Baker et al. 2007). For example, the risk for cessation of full and any BF among overweight Danish women was low, 7% and 12%, respectively (Baker et al. 2007). Post‐hoc power calculations revealed that our study was underpowered to detect a 20% higher hazard ratio among the overweight/obese than normal‐weight women.
We analysed the quality of the complementary diet because there was evidence in the literature to suggest that maternal obesity was associated with an earlier introduction of food (Baker et al. 2007) or with dietary patterns that were inconsistent with infant feeding recommendations (Robinson et al. 2007). In this present study, we modified the WHO dietary diversity indicator to assess the quality of the complementary foods offered. We observed that the quality of complementary food improved over time. We found a positive association between pBMI and complementary food score from 6 to 24 months, but the small magnitude of this association – 0.24 point of a minimum score of 4 – was not of public health significance. We were unable to assess the timing of introduction of foods because data at 4 or 5 months were not collected, which is when some women would have begun feeding solids (fruits, vegetable or grains).
We also examined the adequacy of the complementary diet through energy density at 12, 18 and 24 months, which were the follow‐up times when 24‐hour recall data were collected. Energy density (kcal g−1) for the total diet did not differ by pBMI category. As additional sensitivity analyses, we examined energy density for the seven foods considered in the complementary food score, and these also were not significantly different by pBMI category (data not shown). That we saw that a significant association in complementary food score but not in energy density is not unexpected given that these outcomes likely measure two different dimensions of complementary feeding. The food score reflects exposure to different food groups, which is driven by household food availability and maternal choice (Monterrosa et al. 2012), while energy density considers the amount of food consumed, which is driven by the infant's appetite, who is likely to self‐regulate its intake (Fox et al. 2006).
Evidence from a study in Denmark had also revealed that, among obese women, BF duration was linked to an earlier introduction of food or greater use of formula (Baker et al. 2007), so we examined the association of pBMI with IYCF comprehensively by constructing an index. This index allowed us to link BF, complementary feeding, formula use and bottle feeding at each time point and make appropriate use of the longitudinal data that had been collected using various instruments. We did not find a significant association between pBMI and IYCF index, or between pBMI and each separate component of the IYCF index. Thus from 1 to 24 months, IYCF did not differ by pBMI category. An ethnographic study from this community showed that IYCF is a cultural practice that is driven by shared knowledge on how to feed infants and young children (Monterrosa et al. 2012). Post‐hoc power calculations showed that we had a sufficiently large sample to detect a 1‐unit change in the IYCF index (α = 0.05, SD = 2, n for sample = 128) had such a change been present.
In this secondary analysis, our inferences about the association of pBMI with IYCF are limited to what had been measured and how it was measured. We could only assess four of the nine best practices outlined in Guiding Principles (PAHO 2002): exclusive BF, introduction of food and continued BF, safe storage of foods (i.e. avoid bottles) and nutrient content of food, which we captured with the quality of complementary food and with sweetened beverages. Also, this index captures different dimensions of IYCF at different times, so high scores at 1 month, for example, are not driven by the same behaviours as high scores ≥12 months. Nonetheless, longitudinal data on feeding practices, along with an objective measure of maternal weight and height in early pregnancy and extensive data on potential confounding factors, provided an opportunity to examine temporal associations between pBMI and IYCF.
We conducted a sensitivity analysis in which we changed the relative scoring of the groups (i.e. assigning a 1 to positive practices and 0 to negative practices), changed the scoring of the groups to reflect hypothesis (i.e. assigning a positive score to bottles and sweetened beverages) or changed the scoring of the groups across time, and none of these approaches changed our main results (data not shown). We also assessed whether the IYCF index was related to weight at 24 months, given that child feeding indices have been associated with growth outcomes (Ruel & Menon 2002; Arimond & Ruel 2004). The association between cumulative IYCF index and weight did not reach statistical significance in either adjusted or unadjusted models (β = −0.035 kg, P = 0.068, adjusted for wealth, n = 93 for children with complete visits).
These analyses have some limitations. We controlled the salient sociodemographic factors that are potential causes of both the outcomes and the independent variable, but we cannot rule out residual confounding, particularly from income because this information was not collected. Money is a key factor for the purchase of flesh foods, fruits and vegetables, and non‐human milk in this community (Monterrosa et al. 2012). Loss to follow‐up in this study was 26%. Loss to follow‐up was not related to pBMI or to child supplement group. In contrast, loss to follow‐up did differ by maternal supplement group. Missing dietary data occurred frequently (40–60%) from 12 to 24 months, and means reported for energy density and complementary food score may be high if those with dietary data had better feeding practices than those without dietary data. We mitigated the possibility of selection bias by including covariates that may have contributed to missing data or loss to follow‐up.
This study was conducted over a decade ago, and the rate of obesity has tripled in this community during this time (Neufeld et al. 2008), coupled with a rapid change in dietary practices in Mexico over the same period (Rivera et al. 2004). As a result, a relationship between pBMI and IYCF could now be present in this community.
In conclusion, pBMI was not associated with BF duration or with IYCF in our sample of mother–child pairs. Children born to heavier women are likely to be exposed to a greater variety of food groups, but from a public health perspective, this did not significantly improve the diet diversity. Nonetheless, given the increases in the prevalence of obesity in Mexico in the last 10 years, and as Mexican women become fatter at a younger age (i.e. heavier nulliparous women), we may observe more women choosing other feeding options, if those are affordable. Thus, research on the association of pre‐pregnancy obesity and IYCF in a larger, contemporary sample of Mexican women, with sufficient variation both in child feeding strategies and socioeconomic status per category of BMI is warranted. Future work should also examine how maternal overweight and obesity influences the complementary diet via exposure to different foods.
Source of funding
Division of Nutritional Sciences Small Grants to ECM. Micronutrient Initiative and Thrasher Research Fund to UR and CONACYT, Mexico to LMN for the original study.
Conflict of interest
The authors declare that they have no conflicts of interest.
Author contributions
ECM and KMR designed the research. ECM, KAE and EAF analysed the data. LMN and UR provided essential database necessary for the analysis. ECM wrote the article and had primary responsibility for the final content. All authors read and approved the final manuscript.
Acknowledgements
The authors acknowledge the statistical advice of F. Vermeylen.
References
- Allison P. (1995) Survival Analysis Using the SAS System: A Practical Guide. SAS Institute Inc.: Cary, NC. [Google Scholar]
- Arimond M. & Ruel M.T. (2004) Dietary diversity is associated with child nutritional status: evidence from 11 Demographic and Health Surveys. Journal of Nutrition 134, 2579–2585. [DOI] [PubMed] [Google Scholar]
- Baeten J.M., Bukusi E.A. & Lambe M. (2001) Pregnancy complications and outcomes among overweight and obese nulliparous women. American Journal of Public Health 91, 436–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker J., Michaelsen K., Rasmussen K. & Sørensen T. (2004) Maternal prepregnant body mass index, duration of breastfeeding, and timing of complementary food introduction are associated with infant weight gain. American Journal of Clinical Nutrition 80, 1579–1588. [DOI] [PubMed] [Google Scholar]
- Baker J.L., Michaelsen K.F., Sorensen T.I. & Rasmussen K.M. (2007) High prepregnant body mass index is associated with early termination of full and any breastfeeding in Danish women. American Journal of Clinical Nutrition 86, 404–411. [DOI] [PubMed] [Google Scholar]
- Barquera S., Peterson K.E., Must A., Rogers B.L., Flores M., Houser R. et al (2007) Coexistence of maternal central adiposity and child stunting in Mexico. International Journal of Obesity (London) 31, 601–607. [DOI] [PubMed] [Google Scholar]
- Donath S.M. & Amir L.H. (2000) Does maternal obesity adversely affect breastfeeding initiation and duration? Breastfeeding Reviews 8, 29–33. [PubMed] [Google Scholar]
- Fox M.K., Devaney B., Reidy K., Razafindrakoto C. & Ziegler P. (2006) Relationship between portion size and energy intake among infants and toddlers: evidence of self‐regulation. Journal of the American Dietetic Association 106, 77–83. [DOI] [PubMed] [Google Scholar]
- González‐Cossío T., Moreno‐Macias H., Rivera J.A., Villalpando S., Shamah‐Levy T., Monterrubio E.A. et al (2003) Breast‐feeding practices in Mexico: results from the Second National Nutrition Survey 1999. Salud Publica Mexico 45 (Suppl. 4), S477–S489. [DOI] [PubMed] [Google Scholar]
- Hilson J., Rasmussen K. & Kjolhede C. (1997) Maternal obesity and breast‐feeding success in a rural population of white women. American Journal of Clinical Nutrition 66, 1371–1378. [DOI] [PubMed] [Google Scholar]
- Hilson J., Rasmussen K. & Kjolhede C. (2004) High prepregnant body mass index is associated with poor lactation outcomes among white, rural women independent of psychosocial and demographic correlates. Journal of Human Lactation 20, 18–29. [DOI] [PubMed] [Google Scholar]
- Hytten F. & Leitch I. (1971) The Physiology of Human Pregnancy. Blackwell Scientific Publications: Oxford. [Google Scholar]
- IOM (1990) Nutrition during Pregnancy. National Academy Press: Washington, DC. [Google Scholar]
- Jevitt C., Hernandez I. & Groër M. (2007) Lactation complicated by overweight and obesity: supporting the mother and newborn. Journal of Midwifery and Women's Health 52, 606–613. [DOI] [PubMed] [Google Scholar]
- Kitsantas P., Pawloski L. & Gaffney K. (2010) Maternal prepregnancy body mass index in relation to Hispanic preschooler overweight/obesity. European Journal of Pediatrics 169, 1361–1368. [DOI] [PubMed] [Google Scholar]
- Kugyelka J.G., Rasmussen K.M. & Frongillo E.A. (2004) Maternal obesity is negatively associated with breastfeeding success among Hispanic but not Black women. Journal of Nutrition 134, 1746–1753. [DOI] [PubMed] [Google Scholar]
- Li R., Jewell S. & Grummer‐Strawn L. (2003) Maternal obesity and breast‐feeding practices. American Journal of Clinical Nutrition 77, 931–936. [DOI] [PubMed] [Google Scholar]
- Monterrosa E.C. (2010) The Influence of Maternal Fatness, Knowledge, and Diet on Infant and Young Child Feeding in Mexico. PhD Thesis. Cornell University: Ithaca, NY. [Google Scholar]
- Monterrosa E.C., Pelto G., Frongillo E. & Rasmussen K.M. (2012) Constructing maternal knowledge frameworks: how mothers conceptualize complementary feeding. Appetite 59, 377–384. [DOI] [PubMed] [Google Scholar]
- Neufeld L.M., Hernandez‐Cordero S., Fernald L.C. & Ramakrishnan U. (2008) Overweight and obesity doubled over a 6‐year period in young women living in poverty in Mexico. Obesity 16, 714–717. [DOI] [PubMed] [Google Scholar]
- Olaiz‐Fernández G., Rivera‐Dommarco J., Shamah‐Levy T., Rojas R., Villalpando‐Hernández S., Hernández‐Avila M. & Sepúlveda‐Amor J. (2006) Encuesta Nacional de Salud y Nutricion 2006. Instituto Nacional de Salud Publica: Cuernavaca, Mexico, 2006. [Google Scholar]
- Oddy W.H., Li J., Landsborough L., Kendall G.E., Henderson S. & Downie J. (2006) The association of maternal overweight and obesity with breastfeeding duration. Journal of Pediatrics 149, 185–191. [DOI] [PubMed] [Google Scholar]
- PAHO (2002) Guiding Principles for Complementary Feeding of the Breastfed Child. Pan American Health Organization: Washington, DC. [Google Scholar]
- Perez‐Escamilla R., Segura‐Millan S., Pollit E. & Dewey K.G. (1993) Determinants of lactation performance across time in an urban population from Mexico. Social Science Medicine 37, 1069–1078. [DOI] [PubMed] [Google Scholar]
- Ramakrishnan U., Gonzalez‐Cossio T., Neufeld L., Rivera J. & Martorell R. (2003) Multiple micronutrient supplementation during pregnancy does not lead to greater infant birth size than does iron‐only supplementation: a randomized controlled trial in a semirural community in Mexico. American Journal of Clinical Nutrition 77, 720–725. [DOI] [PubMed] [Google Scholar]
- Rasmussen K.M. (2007) Association of maternal obesity before conception with poor lactation performance. Annual Review of Nutrition 27, 103–121. [DOI] [PubMed] [Google Scholar]
- Rasmussen K.M. & Kjolhede C.L. (2004) Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics 113, e465–e471. [DOI] [PubMed] [Google Scholar]
- Ray J.G., Vermeulen M.J., Shapiro J.L. & Kenshole A.B. (2001) Maternal and neonatal outcomes in pregestational and gestational diabetes mellitus, and the influence of maternal obesity and weight gain: the DEPOSIT study. QJM: An International Journal of Medicine 94, 347–356. [DOI] [PubMed] [Google Scholar]
- Rivera J.A., Barquera S., Gonzalez‐Cossio T., Olaiz G. & Sepulveda J. (2004) Nutrition transition in Mexico and in other Latin American countries. Nutrition Reviews 62, S149–S157. [DOI] [PubMed] [Google Scholar]
- Robinson S., Marriott L., Poole J., Crozier S., Borland S., Lawrence W. et al (2007) Dietary patterns in infancy: the importance of maternal and family influences on feeding practice. British Journal of Nutrition 98, 1029–1037. [DOI] [PubMed] [Google Scholar]
- Ruel M.T. (2003) Operationalizing dietary diversity: a review of measurement issues and research priorities. Jounal of Nutrition 133, 3911S–33926. [DOI] [PubMed] [Google Scholar]
- Ruel M.T. & Menon P. (2002) Child feeding practices are associated with child nutritional status in Latin America: innovative uses of the Demographic and Health Surveys. Journal of Nutrition 132, 1180–1187. [DOI] [PubMed] [Google Scholar]
- Segura‐Millan S., Dewey K.G. & Perez‐Escamilla R. (1994) Factors associated with perceived insufficient milk in a low‐Income urban population in Mexico. Journal of Nutrition 124, 202–212. [DOI] [PubMed] [Google Scholar]
- WHO (2008) Indicators for Assessing Infant and Young Child Feeding Practices. World Health Organization: Geneva. [Google Scholar]


