Abstract
The relationship between breastfeeding and the loss of weight gained during pregnancy remains unclear. This study aimed to investigate the association between breastfeeding and maternal weight changes during 24 months post‐partum. We studied a dynamic cohort comprising 315 women living in two cities in the state of Bahia, Brazil. The outcome variable was change in the post‐partum weight; the exposure variable was the duration and intensity of breastfeeding. Demographic, socio‐economic, environmental, reproductive and lifestyle factors were integrated in the analysis as covariates. The data were analysed using multiple linear regression and linear mixed‐effects models. The average cumulative weight loss at 6 months post‐partum was 2.561 kg (SD 4.585), increasing at 12 months (3.066 kg; SD 5.098) and decreasing at 18 months (1.993 kg; SD 5.340), being 1.353 kg (SD, 5.574) at 24 months post‐partum. After adjustment, the data indicated that for every 1‐point increase in breastfeeding score, the estimated average post‐partum weight loss observed was 0.191 kg at 6 months (P = 0.03), 0.090 kg at 12 months (P = 0.043), 0.123 kg at 18 months (P < 0.001) and 0.077 kg at 24 months (P = 0.001). Based on these results, we concluded that despite the low expressiveness, the intensity and duration of breastfeeding was associated with post‐partum weight loss at all stages of the study during the 24‐month follow‐up.
Keywords: weight loss, breastfeeding, post‐partum, obesity, prospective study
Introduction
Obesity is one of the most important health problems in the world. The complex process of determining overweight/obesity has been expressed differently between sexes in different countries (WHO 2011), including Brazil (Instituto Brasileiro de Geografia e Estatística 2010), with prevalence being higher in women.
Among the risk factors associated with post‐partum obesity, parity (Coitinho et al. 2001; Lacerda & Leal 2004), being overweight pre‐pregnancy, gestational weight gain above the recommended by Institute of Medicine (Nohr et al. 2008; Siega Riz et al. 2009; Mamun et al. 2010; Fraser et al. 2011) and the absence or short duration of lactation (Baker et al. 2008) have been highlighted.
The results of studies evaluating the influence of breastfeeding on the loss of weight gained during pregnancy have been contradictory (Gunderson & Abrams 1999). Dewey et al. (1993), Janney et al. (1997), Kac et al. (2004), Baker et al. (2008) and Stuebe et al. (2010) have identified associations between breastfeeding and post‐partum weight loss. However, Haiek et al. (2001), Gigante et al. (2001), Sichieri et al. (2003) and Onyango et al. (2011) have found that associations are negligible or non‐existent. These contradictions have contributed to the inconclusive results presented so far (Ip et al. 2007).
Among the clarifications put forward for the contradictory results, of note are the different methodologies used to analyse breastfeeding variables (Lederman 2004); variations in the follow‐up of cohorts, with the concentration being greatest between 6 and 12 months post‐partum; and insufficient control of confounding variables and effect modifiers (Gunderson & Abrams 1999; Fraser & Grimes 2003; Lacerda & Leal 2004; Ip et al. 2007).
During the post‐partum period, differing behaviour has been observed among women regarding the loss of weight gained during pregnancy. Some lost all of the weight gained during pregnancy, notably after 6 months; others needed up to 12 months to do so, while still others did not return to their pre‐pregnancy weights (Riley 2011), resulting in the gradual increase of weight, particularly in the event of other pregnancies.
Estimates indicate that 2–5 kg of fat are accumulated during pregnancy (Anderson 2007), serving as energy reserves that can be mobilised and used during lactation. Thus, prolonged breastfeeding by women with adequate pre‐pregnancy weight and recommended weight gain during pregnancy is expected to promote a return to their previous weights, possibly by the first 6 months post‐partum. However, studies have indicated that some women, especially those who became pregnant while overweight and/or exceeded the recommended weight gain during pregnancy, do not reach this goal at the expected time (Nohr et al. 2008).
Therefore, this study aimed to investigate the association between breastfeeding and maternal weight changes at 6, 12, 18 and 24 months post‐partum.
Key messages
Breastfeeding appears to exert influence on the mother's weight loss during the 24 months post‐partum.
Encouragement of exclusive breastfeeding until the child is 6 months old and its continuity as the main milk source until the child is at least 24 months old may favour post‐partum weight loss.
Studies should be directed towards developing methods for collection and analysis of breastfeeding information in a way that encourages the creation of variables that estimate energy expenditure resulting from lactation more accurately for use in population studies.
Material and methods
We studied a dynamic cohort originating from the Breastfeeding and Complementary Feeding in Weaning – State of Nutrition and Health in the First Two Years of Life project involving women living in the cities of Laje and Mutuípe in the state of Bahia, Brazil. These cities have predominantly agricultural economies and similar geographic, demographic, social, economic and health features. When the survey was carried out, these cities had a human development index of 0.657 (Mutuípe) and 0.654 (Laje) (PNUD, 2000).
The selection of the participating cities was based on logistics to guarantee sample capture and the implementation of procedures throughout the logistics field defined in the study while participants were still in the delivery room and nursery, as well as follow‐up of the cohort with the least possible loss. Cohort capture occurred in only two hospitals, with all of the women who agreed to participate in the study living in the researched municipalities and giving birth from March 2005 to October 2006. The women were followed up for 24 months.
Among the 528 women originally in the cohort, we excluded from this study those aged <18 years (83), with gestational age <37 weeks (14), who became pregnant during follow‐up (47), who delivered children with low birthweight (<2500 g) (20), who had twin pregnancy (1), who had a history of chronic non‐communicable diseases (1), who did not raise their children (3), with only one weight measurement during follow‐up (33) and those who did not have their weight measured at baseline (11), which numbered 213 women in total. Therefore, 315 women were eligible for this study.
Outcome variable and time‐dependent covariates
Change in post‐partum weight, breastfeeding and the interval between measurements were treated as time‐dependent variables.
The outcome variable weight change post‐partum (kg) was obtained by the absolute difference between the women's weight (kg) measured at each stage of follow‐up (6, 12, 18 and 24 months) and their weight at day 1 post‐partum (baseline).
The main exposure variable, the duration and intensity with which breast milk was provided to the child, was represented by scores derived from the sum of points assigned to each type of breastfeeding as adapted from Ohlin & Rössner (1990) and Baker et al. (2008) as follows: 0 point was allocated for each kind of breastfeeding when the child was breastfed for 15 days only. After that, each month of exclusive and predominant breastfeeding was awarded 2 points, complementary breastfeeding was awarded 1.5 points and mixed feeding was awarded 1 point. When the child was 13 months old onwards, 0.5 point was awarded per month for any type of breastfeeding until the child was 24 months old.
Therefore, defining the maternal breastfeeding variable by the attribution of points, valuing time and diets that use greater amounts of breast milk per day with a gradual decrease in the points as other foods are incorporated for feeding the child, favours the construction of a variable that can reflect the variation in energy expenditure for the production of breast milk (Baker et al. 2008).
The energy cost for breast milk production in well‐nourished women with appropriate gestational weight gain is approximately 500 kcal day–1 (WHO Report of a joint FAO/WHO/UNU expert consultation 2001). Thus, given the difficulty of obtaining a direct estimate of the energy cost of lactation in population studies, considering the time associated with the type of breastfeeding adopted is of great importance because it indirectly represents the demand for energy mobilised during lactation (Baker et al. 2008).
Type of breastfeeding was defined according to the World Healt Organization (WHO 2010) as follows: exclusive, when breast milk was the only source of nourishment provided to the child, without water, tea, juice or any other food; predominant, when breast milk was the only source of milk, and water, tea, juice or other liquid were also provided; partial, breast milk plus other kinds of milk, and may include other foods; artificial, milk other than breast milk, associated with other foods, including infant formula feeding. The breastfeeding type was considered ‘complemented’ when breast milk was the only dairy source accompanied by other foods (WHO 1991).
The score of the intensity and duration of breastfeeding was used as a categorical variable (those below and above the median) in the descriptive analysis of the association with post‐partum weight change and as a continuous variable in the statistical modelling.
The covariate time since delivery was derived from the difference between the date of each interview and the date of the weight 1 day post‐partum.
Time‐independent covariates
The following socio‐economic and demographic variables were selected as time‐independent covariables: social programmes for income transfer (yes, no) was defined by families receiving financial support from governmental programmes, e.g. ‘Bolsa Família’; residence zone (rural, urban); mother's age (<24 years, ≥24 years); mother's self‐reported skin colour (clear, brown, dark); marital status (living with a partner, single); mother's education (incomplete elementary, complete elementary, secondary/post‐secondary); gender of the head of the family (male, female); number of residents in the household (≤4, >4); work post‐partum (yes, no); and sanitary conditions in the home environment (SCHE) (appropriate, semi‐appropriate, inappropriate).
The SCHE was built with the following variables: sanitary drainage; garbage disposal; source of water supply; existence of faucets, kitchen and bathroom; type of wall; and number of inhabitants per room in the household, as adapted from Oliveira et al. (2006). The best SCHE was awarded 4 points, and the worst, 0 point. The point totals were grouped into tertiles, and the families were classified as having SCHE that were inappropriate (≤15 points), semi‐appropriate (16–24 points) and appropriate (≥25 points).
We considered post‐partum physical activity per week (no activity, <3 days, ≥3 days) and smoking or had smoked post‐partum (yes, no) as variables related to lifestyle.
Reproductive and anthropometric variables were type of delivery (vaginal, Caesarean); parity (primiparous, 2–3 children, >3 children); prenatal consultations (<6, ≥6); gender (male, female) and weight of the child at birth (2500–2999, 3000–3500, >3500 g); intravenous rehydration during delivery (none, ≤500 mL, >500 mL), pre‐pregnancy body mass index (BMI) (pre‐pregnancy BMI, i.e. pre‐pregnancy weight/height2), which was categorised as underweight (<18.5 kg m−2), normal weight (18.5 kg m−2 > pre‐pregnancy BMI < 25.0 kg m−2), overweight (≥25 kg m−2) (Institute of Medicine 2009), and mother's height (<1.59 m, ≥1.59 m).
For categorical covariates, code 0 was cited for the reference categories and code 1 for the indication of risk. Those with more than two categories were treated as dummy variables.
Data collection
The data were collected by nutritionists and trained health care professionals integrated with the work proposal.
Maternal weight was initially measured at the maternity hospital, and in subsequent steps, it was measured at the local health centre or at home with an E‐150/3P Filizola microelectronic scale with a capacity of up to 150 kg (Filizola Industrial Scales, São Paulo, Brazil). The women were weighed standing, barefoot and wearing light clothing. The maximum acceptable inter‐observer variation for weight and height were 0.100 g and 0.1 cm, respectively. Health care professionals measured birthweight in the delivery room using a Filizola digital scale with a capacity of up to 15 kg and intervals of 10 g. Measurements were performed in duplicate using standard techniques (Lohman et al. 1988).
The collection of breastfeeding information during the first month post‐partum took place at the mother's homes. Other information collection sessions took place monthly until the child was 6 months of age; subsequently, information collection took place once every 6 months until the end of the follow‐up. In each contact between the mother and the project team, the mothers provided information regarding the status of breastfeeding and a description of the diet pattern adopted. We used semi‐quantitative food frequency and 24‐h recall methods for this purpose. Mothers filled up a specific questionnaire about breastfeeding at each interview, answering complementary questions about the kind of milk provided to the child: whether the child received breast milk only, breast milk plus another kind of milk, or another kind of milk only. In the latter case, the mother was asked how long the other kind of milk had been introduced to the child.
Pre‐pregnancy weight was derived from the pregnancy‐monitoring card. The weight that appeared on the card was obtained by measuring the mother's weight when she first started prenatal care, before week 13 of pregnancy. In its absence, the self‐reported weight was used. Information about the type of delivery and intravenous rehydration during delivery was acquired from hospital records.
The socio‐economic and demographic characteristics were collected during the first home visit (first month post‐partum), and information regarding lifestyle at the end of follow‐up. However, subsequent evaluations were performed at the Health Unit or at home if the mother missed a follow‐up visit.
Ethical considerations
The women who agreed to participate in the research signed a consent form. Those who were illiterate signed using their thumbprints. The Ethics Committee of the Maternity Climério of Oliveira from the Federal University of Bahia approved the study (Technical Advice No. 74/2005).
Statistical analysis
A chi‐square test for proportions was used to compare the main characteristics between the accompanied women and those lost during the follow‐up. Fisher's exact test was applied when any category presented n < 5.
The evaluation of data consistency, linearity and normality of variables was based on histograms, scatterplots, p‐plots and Kolmogorov–Smirnov test.
A paired t‐test was used to compare the average post‐partum weight change between the different intervals of the study, as well as among the weight change at 6 and 12, 12 and 18, and 18 and 24 months post‐partum. The t‐test was adopted for means of equality when comparing the average weight change according to the breastfeeding score, whether it was lower, equal to or greater than the median.
To investigate the association between duration and intensity of breastfeeding and post‐partum weight change in the first 6 months of monitoring, the multiple linear regression model was used when considering only one measurement of weight change during the period. Up to 12, 18 and 24 months post‐partum, the linear mixed‐effects model was used, a technique suitable for repeated measures in the time and for unbalanced data; it uses both fixed and time‐dependent variables, allowing the estimation of weight variation over time (Fausto et al. 2008). The correlation structure adopted for the linear regression analysis with mixed effects was the exchangeable structure estimated by the method of restricted maximum likelihood. In this structure, the correlations between subsequent measurements are assumed to be the same, independent of the time interval (Twisk 2003).
Considering the purpose of the study, we built four regression models. Initially, bivariate analyses were processed with the aim of selecting the variables that individually contributed to explaining the post‐partum weight change (P < 0.20). These variables integrated the multivariate models. Considering the available knowledge and the statistical significance of the bivariate analyses, the covariates that altered the estimated parameter by more than 10% were defined as confounders (Hosmer & Lemeshow 2000), and candidates to integrate the final model with the interaction terms. The final model was built using the stepwise procedure and backward elimination when the variables with less statistical significance and less epidemiological relevance were eliminated gradually. In the end, variables with P < 0.05 and the confounders were selected by statistical parameter, although they did not present statistical significance in the multivariate analysis, they remained in the model as adjustment variables.
The multicollinearity between independent variables was evaluated by factor inflation variance, adopting values in the 1–2 range (Stine 1995). Residue analysis was used in the diagnostic model, and adjustment by R 2 determined the regression module for the follow‐up at 6 months. The Akaike information criterion was used to identify the best model for the mixed‐effects models (Pinheiro & Bates 2000). Statistical analysis was performed with SPSS version 17.0 (SPSS Inc., Chicago Illinois, USA) and SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
The loss percentages of the 315 women eligible for the study were 10.2% (32), 17.1% (54), 14% (44) and 10.2% (32) at 6, 12, 18 and 24 months post‐partum, respectively. In some instances, women who did not participate in some stages of the follow‐up were rescued in the subsequent phases. Failure to participate was due to relocation to another city, the team's inability to access some residences during the monsoon season, temporary absence from the cities because of children's diseases or extended visits to relatives in other cities.
The homogeneity between the characteristics of the followed women and those lost to follow‐up with regard to age, mother's education, marital status, parity, nutritional status at baseline and pre‐pregnancy BMI were evaluated at each step of the follow‐up. The groups were similar for most of the selected variables, except for age at 18 (P = 0.024) and 24 months (P = 0.026) (data not shown).
The characterisation of the sample regarding socio‐economic, demographic, anthropometric and lifestyle variables are presented in Table 1.
Table 1.
Demographic, socio‐economic, reproductive, characteristics and lifestyle (Mutuípe/Laje, 2005–2008)
| Variables | n = 315 | % |
|---|---|---|
| Zone of residence | ||
| Rural | 223 | 70.8 |
| Urban | 92 | 29.2 |
| Mother's age (years) | ||
| <24 | 158 | 50.2 |
| ≥24 | 157 | 49.8 |
| Mother's education | ||
| Elementary (1st cycle) | 122 | 38.7 |
| Complete elementary | 138 | 43.8 |
| High school/college | 55 | 17.5 |
| Mother's marital status | ||
| Single | 76 | 24.1 |
| Living with a partner | 239 | 75.9 |
| Skin colour | ||
| Clear | 145 | 46.2 |
| Brown | 72 | 22.9 |
| Dark | 97 | 30.9 |
| Social programmes for income transfer* | ||
| Yes | 148 | 47.4 |
| No | 164 | 52.6 |
| Post‐partum work †‡ | ||
| Yes | 244 | 80.3 |
| No | 60 | 19.7 |
| Gender of the head of family | ||
| Male | 262 | 83.2 |
| Female | 53 | 16.8 |
| Schooling of head of family | ||
| Elementary (1st cycle) | 101 | 32.1 |
| Complete elementary | 132 | 41.9 |
| High school/college | 82 | 26.0 |
| Inhabitants per household | ||
| 1–4 | 133 | 42.2 |
| >4 | 182 | 57.8 |
| SCHE | ||
| Inappropriate | 96 | 30.5 |
| Semi‐appropriate | 116 | 36.8 |
| Appropriate | 103 | 32.7 |
| Smoking post‐partum †‡ | ||
| Yes | 21 | 7.0 |
| No | 281 | 93.0 |
| Post‐partum physical activity †‡ | ||
| Yes | 45 | 14.9 |
| No | 258 | 85.1 |
| Prenatal (No. of consultations) | ||
| <6 | 176 | 44.1 |
| ≥6 | 139 | 55.9 |
| Type of delivery | ||
| Vaginal | 222 | 70.5 |
| Caesarean | 93 | 29.5 |
| Parity (No. of children) | ||
| 1 | 134 | 42.5 |
| 2–3 | 139 | 44.1 |
| ≥4 | 42 | 13.3 |
| Pre‐pregnancy BMI ‡ | ||
| Underweight | 33 | 11.2 |
| Appropriate | 211 | 71.5 |
| Overweight | 51 | 17.3 |
| Birthweight (g) | ||
| 2500–2999 | 98 | 31.1 |
| 3000–3500 | 133 | 42.2 |
| >3500 | 84 | 26.7 |
| Child's gender | ||
| Male | 167 | 53.0 |
| Female | 148 | 47.0 |
| Mother's height (cm) | ||
| >159 | 137 | 43.5 |
| ≥159 | 178 | 56.5 |
| Intravenous rehydration during delivery (mL) | ||
| ≤500 | 119 | 37.8 |
| >500 | 85 | 27.0 |
| No | 111 | 35.2 |
BMI, body mass index. SCHE, sanitary conditions in home environment. *Families receiving financial support from governmental programmes. †Information collected at the end of follow‐up. ‡Losses due to inconsistencies of the information or non‐response.
Although the data from Table 2 have no statistical significance, they indicated a decrease in the frequency of overweight and obesity between 6 and 12 months post‐partum, increasing at 18 months, affecting 33.2% of women by the end of the study. In parallel, there was an increase in the frequency of underweight women up to 12 months post‐partum (13.8%), decreasing after that, and reaching 7.4% at 24 months post‐partum. We also observed a median of 120 days for exclusive/predominant breastfeeding. At 24 months follow‐up, 32% of women were still breastfeeding.
Table 2.
Behaviour of the time‐varying variables over 6, 12, 18 and 24 months post‐partum (Mutuípe/Laje, 2005–2008)
| Variables | Time of post‐partum follow‐up (months) | ||||
|---|---|---|---|---|---|
| Baseline (n = 315) | 6 (n = 283) | 12 (n = 261) | 18 (n = 271) | 24 (n = 283) | |
| BMI % (n)* | |||||
| Underweight | 3.2 (10) | 9.5 (27) | 13.8 (36) | 8.5 (23) | 7.1 (20) |
| Appropriate | 60.0 (189) | 64.0 (181) | 60.2 (157) | 60.5 (164) | 59.7 (169) |
| Overweight | 32.7 (103) | 20.8 (59) | 19.5 (51) | 24.0 (65) | 25.8 (73) |
| Obesity | 4.1 (13) | 5.7 (16) | 6.5 (17) | 7.0 (19) | 7.4 (21) |
| Type of feeding % (n) | |||||
| BF exclusive/Predominant | – | 27.6 (87) | – | – | – |
| Outros tipos BF | – | 56.8 (179) | 67.9 (207) | 51.5 (156) | 32.2 (97) |
| Artificial | – | 15.6 (49) | 32.1 (100) | 48.5 (147) | 67.8 (205) |
| BF †‡ | – | 10 (0–14) | 15 (0–22) | 19 (0–30) | 20 (0–33) |
| Time interval between since delivery (days) ‡ | – | 184 (169–231) | 366 (346–421) | 550 (516–616) | 731 (676–824) |
BF, breastfeeding; BMI, body mass index. *Comparing of proportion test (P > 0.05; frequency comparing on each BMI category in different stages of the study). †Breastfeeding (and intensity score duration since parturition). ‡Breastfeeding: median (minimum–maximum).
By the end of the study, the average weight loss was 1.353 kg (SD 5.557). Therefore, weight loss was more accentuated in the period between baseline and the first 12 months post‐partum (3.066 kg; SD 5.098) when compared to the other evaluated periods (P = 0.001). The average weight loss in the group with a breastfeeding score equal to or greater than the median proved to be higher at all moments of evaluation when compared to that of the group that scored lower than the median in the same period, despite demonstrating a significant statistical difference only up to 18 months post‐partum (P < 0.01) (Fig. 1).
Figure 1.
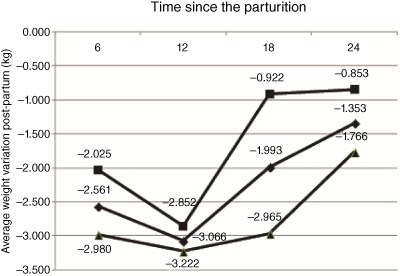
Average weight change post‐partum by duration and intensity of breastfeeding at 6, 12, 18 and 24 months post‐partum (Mutuípe/Laje, 2005–2008). (■) Breastfeeding duration and intensity below the median (n = 124, SD = 4.84; n = 110, SD = 5.71; n = 129 SD = 6.34 and n = 128, SD = 6.63 at 6, up to 12, 18 and 24 months, respectively). (▴) Breastfeeding duration and intensity above the median (n = 159 SD = 4.34; n = 151 SD = 4.62; n = 142, SD = 4.00 and n = 155, SD = 4.50 at 6, up to 12, 18 and 24 months, respectively). t‐Test for equality of means for comparisons between weight change average according breastfeeding scores below and above the median for 6 (P = 0.08), 12 (P = 0.56), 18 (P < 0.01) and 24 (P = 0.17) months post‐partum. (♦) Paired t‐test (P = 0.01) for comparisons between weight average for different periods of the study the exception of the comparison between 6 and 18 months (P = 0.07).
Analysing the weight changes between different time intervals in the study (Fig. 2), we observed higher weight loss between 1 day and 6 months post‐partum (−2.56 kg; SD 4.58; P < 0.01) compared to the interval between 6 and 12 months (−0.509; SD 2.35). We also observed weight gain from 12 to 18 months (P < 0.001) and from 18 to 24 months (0.64 kg; SD 2.64); for the latter period, the decline observed in the average weight gain was not statistically significant (P = 0.084).
Figure 2.
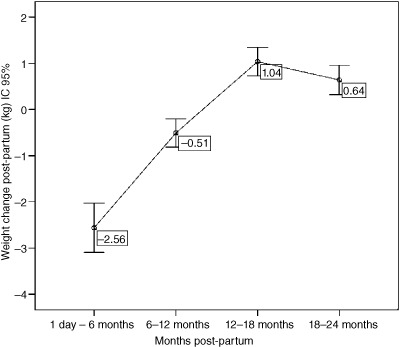
Mothers' average weight change during the 24 months post‐partum (Mutuípe/Laje, 2005–2008). Paired t‐test for comparison among averages weight change between 6 (n = 283) and 12 (n = 233); 12 and 18 (n = 240) months has resulted to P < 0.01; and 18 −24 (n = 262), P = 0.08.
The covariates area of residence, mother's level of education, ethnicity, work post‐partum, SCHE, participation in social programmes of income transfer, birthweight, interval between measurements, mother's height, delivery type, parity, pre‐pregnancy BMI category, intravenous rehydration during delivery and number of prenatal visits were included in the multivariate analysis by presenting them as P < 0.20 in the bivariate analysis (data not shown).
Interactions between the pre‐pregnancy BMI category, parity, skin colour, interval between measurements from the baseline and breastfeeding were tested in the multivariate models and did not present statistically significant differences.
Three linear mixed‐effects models (from baseline until 12, 18 and 24 months post‐partum) and one multiple linear regression model (at 6 months) were built to identify the influence of breastfeeding on weight changes at each stage of follow‐up as adjusted by covariates that presented P < 0.05, including also the confounders with P > 0.05 (Table 3).
Table 3.
Final linear regression model at 6 months and mixed‐effects models up to at 12, 18 and 24 months of the association between breastfeeding and weight change post‐partum (Mutuípe/Laje, 2005–2008)
| Variables | Estimate | SE | P‐value | Evaluation of the models |
|---|---|---|---|---|
| Model 1: At 6 months of follow‐up* | ||||
| Constant | −3.184 | 1.040 | 0.002 | 0.193 † |
| Breastfeeding ‡ | −0.191 | 0.088 | 0.032 | |
| Model 2: At 12 months of follow‐up § | ||||
| Intercept | −3.417 | 1.0453 | <0.001 | 2671.8 ¶ |
| Breastfeeding ‡ | −0.090 | 0.0443 | 0.043 | |
| Model 3: At 18 months of follow‐up** | ||||
| Intercept | −3.732 | 0.9695 | <0.001 | 3888.3 ¶ |
| Breastfeeding ‡ | −0.123 | 0.0299 | <0.001 | |
| Model 4: At 24 months of follow‐up †† | ||||
| Intercept | −4.635 | 0.7475 | <0.001 | 5384.3 ¶ |
| Breastfeeding ‡ | −0.077 | 0.0235 | 0.011 |
*Model 1 = model adjusted for sanitary conditions in home environment (SCHE), parity, type of delivery, pre‐pregnancy body mass index (BMI) categories, birthweight and intravenous rehydration during delivery. † R 2 adjusted. ‡Duration and intensity of breastfeeding (continuum score). §Model 2 = mixed‐effects regression adjusted for SCHE, parity, social programmes for income transfer, type of delivery, intravenous rehydration during delivery, mother's education, work post‐partum, skin colour and birthweight. ¶Akaike information criterion. **Model 3 = mixed‐effects regression adjusted for SCHE, parity, social programmes for income transfer, skin colour, mother's education, type of delivery, intravenous rehydration during delivery and pre‐pregnancy BMI categories. ††Model 4 = mixed‐effects regression adjusted for SCHE, parity, skin colour, type of delivery, intravenous rehydration during delivery and pre‐pregnancy BMI categories.
In all four models, breastfeeding was associated with post‐partum weight loss, indicating that for each 1‐point increase in the breastfeeding score, we observed a loss of 0.191 kg (P = 0.03) at 6 months, 0.090 kg (P = 0.043) at 12 months, 0.123 kg (P < 0.001) at 18 months and 0.077 kg (P = 0.001) at 24 months in the average of weight change post‐partum (Table 3).
Discussion
In this study, breastfeeding was associated with weight loss at 6, 12, 18 and 24 months post‐partum after adjustment. In studies that used the breastfeeding score and weight retention, associations were observed as well at 6 and 18 months (Baker et al. 2008), between 2.5 and 6 months (Ohlin & Rossner 1990), and at 12 months post‐partum (Rossner 1997). Using the same approach in relation to breastfeeding, but considering the time post‐partum and for a longer period, Linné et al. (2003) observed that the breastfeeding score was lowest among women who became obese at 15 years post‐partum compared to those of adequate weight. Amorim et al. (2007) also observed an association at 6 and 12 months and at 15 years post‐partum, emphasising that lactation appears to be more significant in the long term.
Statistically significant associations were also found in studies that did not use the breastfeeding score, but which addressed breastfeeding considering the time and intensity associated with weight retention between 6 and 12 months (Dewey et al. 1993), 9 months (Kac et al. 2004), 5 years (Gigante et al. 2001), and between 3 and 6 months post‐partum (Krause et al. 2010).
Despite the methodological similarities with this study in terms of the follow‐up period and the use of scores to evaluate breastfeeding and variable weight change outcomes, data from the Multicentre Growth Reference Study (Onyango et al. 2011), involving 1542 women living in six countries (Brazil, Ghana, India, Norway, Oman and the United States), indicated no association when the breastfeeding score was used to describe post‐partum weight change patterns associated with the duration and intensity of breastfeeding among women in the participating countries. Further, the data revealed that the median duration of breastfeeding for Brazilian women was 8.3 months, which is well below the median of 16.8 months that was observed in Brazilian women also in the present study.
It is noteworthy that the majority of studies reported no association between breastfeeding and post‐partum retention or weight loss observed low rates of breastfeeding until 1 year post‐partum such as the study by Boardley et al. (1995). In this study, 67.9% of women still breastfed their children at 12 months, and 32.2% continued it up until the child was 24 months old, which may have contributed to the results observed in all periods.
Despite the low expressiveness of the post‐partum weight loss assigned to breastfeeding and reported in this study, the women experienced a wide variation in weight change at all stages of follow‐up: −14.8 to 11.2 kg, −16.2 to 21.0 kg, −14.9 to 24.9 kg and −16.2 to 23.2 kg were recorded at 6, 12, 18 and 24 months post‐partum, respectively. These variations revealed different situations in the patterns of weight change, indicating that some women lost all of the weight gained during pregnancy and remained at that weight. Others did not lose enough weight, while the rest gained weight, and it remains possible that some lost all of the weight gained during pregnancy during the first months post‐partum, but regained it by the end of the study.
Thus, the present data illustrate different weight change patterns throughout follow‐up between the different time intervals adopted for the interviews, with weight gain being particularly at 12 and 18 months post‐partum. This change in the average weight post‐partum throughout follow‐up may be clarified not only by the modification of the child's diet regarding breastfeeding but also by the complex web of factors associated with post‐partum weight change.
It is important to mention the possible limitations of this study. The first is related to the use of the measured weight on day 1 post‐partum, despite the understanding that approximately 14 days are required for the stabilisation of body fluid changes that take place during pregnancy (Amorim et al. 2008). The adoption of this procedure took into account the ease of measuring the weight when a woman was still at the maternity health care facility. It is possible that including the amount of intravenous rehydration provided during delivery in the analysis helped minimise the bias attributed to this condition in a certain way.
We consider the self‐reported pre‐pregnancy weight (83.1%) and the weight stated in the pregnancy follow‐up cards (16.9%) the second limitation of this study. The former is a limitation due to the possibility of underestimation or overestimation by women who were overweight or underweight before pregnancy, respectively (Stevens‐Simon et al. 1992), and the latter is a limitation based on the uncertain reliability of the measuring equipment and techniques used. Pre‐pregnancy weight was not used in the construction of the outcome variable in this study; instead, we used the BMI and added pre‐pregnancy BMI categories to the model, minimising the bias effect generated in this study (Gunderson & Abrams 1999).
The percentage of losses during follow‐up at each monitoring stage is considered acceptable in a cohort study. Furthermore, there was similarity between the groups of women monitored and the women lost during follow‐up regarding the social, demographic and reproductive variables selected. Regarding age, it is possible that the statistical differences observed at 18 and 24 months follow‐up were not sufficient to establish selection bias in the study, considering that the sample consisted of adult women aged 18–43 years, and that here was no statistically significant association among age and weight change in any of the four models evaluated in the analysis.
We also wish to highlight the fact that the mixed‐effects regression model used in this study is considered appropriate because it permits better estimation of time‐dependent variables (Pinheiro & Bates 2000).
Finally, this study also points out the obvious contribution of breastfeeding to post‐partum weight loss during all of the studied periods, particularly at 18 and 24 months post‐partum, a theme underexplored by most studies. It also highlighted the quality and accuracy of prospectively collected information regarding breastfeeding, and that the use of a consistent methodology according to Baker et al. (2008) can represent the duration and intensity of breastfeeding.
We conclude that despite the low expressiveness, breastfeeding was associated with weight loss 1 throughout the 24‐month follow‐up. However, we consider it important to perform further new studies that contribute to the construction of breastfeeding‐related variables that can estimate the energy expenditure required for the mobilisation of fat accumulated during pregnancy more accurately, enabling identification of the true weight loss attributed to lactation, and increasing the support provided to this biological plausibility. It is also important that these studies contemplate other determinant factors of overweight/obesity, such as the eating habits and cultural factors.
Source of funding
This project was developed by the School of Nutrition of the Federal University of Bahia and was supported by funds from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Process No. 505971/04‐6, Centro Colaborador em Alimentação e Nutrição Nordeste II/Ministry of Health of Brazil and the FAPESB/Brazil.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
MCMS and AMOA were responsible for the conception and study design. AMOA was responsible for funding and the field implementation. SMCP did the statistical analysis with contribution from MCMS, AMOA and TRPC. Data collections were performed by MCMS and AMOA. The manuscript was written and revised by MCMS, AMOA, SMCP, LPMO and TRPC.
Acknowledgements
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), the Ministry of Health of Brazil and the Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB)/Brazil for financial support for project development. They also thank the Governments of Mutuípe and Laje, and the women who participated in this study.
da Silva, M. Cã. M. , Oliveira Assis, A. M. , Pinheiro, S. M. C. , de Oliveira, L. P. M. , and da Cruz, T. R. P. (2015) Breastfeeding and maternal weight changes during 24 months post‐partum: a cohort study. Matern Child Nutr, 11: 780–791. doi: 10.1111/mcn.12071.
References
- Amorim A.R., Rossner S., Neovius M., Lourenco P.M. & Linné Y. (2007) Does excess pregnancy weight gain constitute a major risk for increasing long‐term BMI? Obesity (Silver Spring, Md.) 15, 1278–1286. [DOI] [PubMed] [Google Scholar]
- Amorim A.R., Linné Y., Kac G. & Lourenço P.M. (2008) Assessment of weight changes during and after pregnancy: practical approaches. Maternal and Child Nutrition 4, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson A. (2007) Pre‐pregnancy, pregnancy and lactation In: Essentials of Human Nutrition (eds Mann J. & Truswell A.S.), 3rd edn, p. 599 Oxford University Press: Oxford. [Google Scholar]
- Baker J.L., Gamborg M., Heitmann B.L., Lissner L., Sørensen T.I.A. & Rasmussen K.M. (2008) Breastfeeding reduces postpartum weight retention. The American Journal of Clinical Nutrition 88, 1543–1551. [DOI] [PubMed] [Google Scholar]
- Boardley D.J., Sargent R.G., Coker A.L., Hussey J.R. & Sharpe P.A. (1995) The relationship between diet, activity, and other factors, and postpartum weight change by race. Obstetrics and Gynecology 86, 834–838. [DOI] [PubMed] [Google Scholar]
- Coitinho D.C., Sichieri R. & D'Aquino Benício M.H. (2001) Obesity and weight change related to parity and breast‐feeding among parous women in Brazil. Public Health Nutrition 4, 865–870. [DOI] [PubMed] [Google Scholar]
- Dewey K.G., Heining M.J. & Nommsen L.A. (1993) Maternal weight‐loss patterns during prolonged lactation. The American Journal of Clinical Nutrition 58, 162–166. [DOI] [PubMed] [Google Scholar]
- Fausto M.A., Carneiro M., Antunes C.M.F., Pinto J.A. & Colosimo E.A. (2008) O modelo de regressão linear misto para dados longitudinais: uma aplicação na análise de dados antropométricos desbalanceados. Cadernos de Saúde Pública 24, 513–524. [DOI] [PubMed] [Google Scholar]
- Fraser A.B. & Grimes D.A. (2003) Effect of lactation on maternal body weight: a systematic review. Obstetrical and Gynecological Survey 584, 265–269. [DOI] [PubMed] [Google Scholar]
- Fraser A., Tilling K., Macdonald‐Wallis C., Hughes R., Sattar N., Nelson S.M. et al (2011) Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: the avon longitudinal study of parents and children (ALSPAC). American Journal Clinical Nutrition 93, 1285–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigante D.P., Victora C.G. & Barros F.C. (2001) Breast‐feeding has a limited long‐term effect on anthropometry and body composition of Brazilian mothers. The Journal of Nutrition 131, 78–84. Available at: http://jn.nutrition.org (Accessed 12 May 2011). [DOI] [PubMed] [Google Scholar]
- Gunderson E.P. & Abrams B. (1999) Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiologic Reviews 21, 261–275. [DOI] [PubMed] [Google Scholar]
- Haiek L.N., Kramer M.S., Ciampi A. & Tirado R. (2001) Postpartum weight loss and infant feeding. Journal of the American Board of Family Practice 14, 85–94. [PubMed] [Google Scholar]
- Hosmer D.W. & Lemeshow S. (2000) Applied logistic regression, 2nd edn, Wiley: New York. [Google Scholar]
- Institute of Medicine . (2009) Weight Gain During Pregnancy: Reexamining The Guidelines. National Academies Press: Washington, DC. [PubMed] [Google Scholar]
- Instituto Brasileiro de Geografia e Estatística (2010) Antropometria e estado nutricional de crianças, adolescentes e adultos no Brasil Pesquisa de Orçamentos Familiares 2008–2009. Rio de Janeiro. Available at: http://www.ibge.gov.br/home/estatistica/populacao/condicaodevida/pof/2008_2009_encaa/pof_20082009_encaa.pdf (Accessed 10 January 2010).
- Ip S., Chung M., Raman G., Chew P., Magula N., DeVine D. et al (2007) Breastfeeding and maternal and infant health outcomes in developed countries Evidence Report/Technology Assessment 153. Available at: http://www.ncbi.nlm.nih.gov/books/NBK38337/ (Accessed 17 October 2011). [PMC free article] [PubMed]
- Janney C.A., Zhang D. & Sowers M.F. (1997) Lactation and weight retention. The American Journal of Clinical Nutrition 66, 116–124. Available at: http://www.ajcn.org (Accessed 27 January 2011). [DOI] [PubMed] [Google Scholar]
- Kac G., Benicio M.H.A., Velasquez‐Melendez G., Valente J.G. & Struchiner C.J. (2004) Breastfeeding and postpartum weight retention in a cohort of Brazilian women. The American Journal of Clinical Nutrition 79, 487–493. [DOI] [PubMed] [Google Scholar]
- Krause K.M., Lovelady C.A., Peterson B.L., Chowdhury N. & Østbye T. (2010) Effect of breast‐feeding on weightretention at 3 and 6 months postpartum: data from the North Carolina WIC Programme. Public Health Nutrition 13, 2019–2026. [DOI] [PubMed] [Google Scholar]
- Lacerda E.M.A. & Leal M.C. (2004) Fatores associados com a retenção e o ganho de peso pós‐parto: uma revisão sistemática. Revista Brasileira de Epidemiologia 7, 187–200. [Google Scholar]
- Lederman S.A. (2004) Influence of lactation on body weight regulation. Nutrition Reviews 62, S112–S119. [DOI] [PubMed] [Google Scholar]
- Linné Y., Dye L., Barkeling B. & Rossner S. (2003) Weight development over time in parous women – The SPAWN study – 15 years follow‐up. International Journal of Obesity 27, 1516–1522. [DOI] [PubMed] [Google Scholar]
- Lohman T.G., Roche A.F. & Martorell R. (1988) Anthropometric Standardization Reference Manual. Human Kinetics Books: Champaign, IL. [Google Scholar]
- Mamun A.A., Kinarivala M., O'Callaghan M.J., Williams G.M., Najman J.M. & Callaway L.K. (2010) Associations of excess weight gain during pregnancy with long‐term maternal overweight and obesity: evidence from 21 y postpartum follow‐up. The American Journal of Clinical Nutrition 91, 1336–1341. [DOI] [PubMed] [Google Scholar]
- Nohr E.A., Vaeth M., Baker J.L., Sorensen T.I.A., Olsen J. & Rasmussen K.M. (2008) Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. The American Journal of Clinical Nutrition 87, 1750–1759. Available at: http://www.ajcn.org (Accessed 13 March 2011). [DOI] [PubMed] [Google Scholar]
- Ohlin A. & Rössner S. (1990) Maternal body weight development after pregnancy. International Journal of Obesity 14, 159–173. [PubMed] [Google Scholar]
- Oliveira V.A., Assis A.M.O., Pinheiro S.M.C. & Barreto M.L. (2006) Determinantes dos déficits ponderal e de crescimento linear de crianças menores de dois anos. Revista de Saúde Pública 40, 874–882. [DOI] [PubMed] [Google Scholar]
- Onyango A.W., Nommsen‐Rivers L., Siyam A., Borghi E., Onis M., Garza C. et al (2011) Post‐partum weight change patterns in the WHO Multicentre Growth Reference Study. Maternal and Child Nutrition 7, 228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J.C. & Bates D.M. (2000) Mixed‐Effects Models in S and S‐Plus. Springer‐Verlag: New York. [Google Scholar]
- Programa das Nações Unidas para o Desenvolvimento (PNUD) (2000) Ranking decrescente do IDH‐M dos municípios do Brasil; 2000. Atlas do Desenvolvimento Humano. Acessado em 11 de outubro de 2008.
- Riley H. (2011) Weight management before, during and after pregnancy – what are the ‘rules’. Nutrition Bulletin 36, 212–215. [Google Scholar]
- Rossner S. (1997) Weight gain in pregnancy. Human Reproduction 12, S110–S115. [DOI] [PubMed] [Google Scholar]
- Sichieri R., Field A.E., Rich‐Edwards J. & Willett W.C. (2003) Prospective assessment of exclusive breastfeeding in relation to weight change in women. International Journal of Obesity and Related Metabolic Disorders 27, 15–20. [DOI] [PubMed] [Google Scholar]
- Siega Riz A.M., Herring A.H., Carrier K., Evenson K.R., Dole N. & Deierlein A. (2009) Sociodemographic, perinatal, behavioral, and psychosocial predictors of weight retention at 3 and 12 months postpartum. Obesity (Silver Spring, Md.) 18, 1996–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens‐Simon C., Roghmann K.J. & McAnarney E.R. (1992) Relationship of self‐reported prepregancy weight and weight gain during pregnancy to maternal body habitus and age. Journal of the American Dietetic Association 92, 85–87. [PubMed] [Google Scholar]
- Stine R.A. (1995) Graphical interpretation of variance inflation factors. The American Statistician 49, 53–56. [Google Scholar]
- Stuebe A.M., Kleinman K., Gillman M.W., Rifas‐Shiman S.L., Gunderson E.P. & Rich‐Edwards J. (2010) Duration of lactation and maternal metabolism at 3 years postpartum. Journal of Women's Health 19, 941–950. Available at: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2924789/pdf/jwh.2009.1660.pdf (Accessed 3 November 2011). DOI: 10.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twisk J.W.R. (2003) Applied Longitudinal Data Analysis for Epidemiology: A Practical Guide. Cambridge University Press: Cambridge, UK. [Google Scholar]
- WHO (1991) Division of child health and development. Indicators for assessing breastfeeding practices. Reprinted report of an informal meeting. World Health Organization. Geneva.
- WHO (2010) Indicators for assessing infant and young child feeding practices. Part 3. Definitions. World Health Organization.
- WHO (2011) Global status report on noncommunicable diseases 2010 Available at: http://www.who.int/nmh/publications/ncd_report_full_en.pdf). (Accessed 26 October 2011).
- WHO Report of a joint FAO/WHO/UNU expert consultation (2001) Human energy requirements. Food and Nutrition Technical Report Series 17–24.


