Abstract
The cost of ready‐to‐use therapeutic food (RUTF) used in community‐based management of acute malnutrition has been a major obstacle to the scale up of this important child survival strategy. The current standard recipe for RUTF [peanut‐based RUTF (P‐RUTF )] is made from peanut paste, milk powder, oil, sugar, and minerals and vitamins. Milk powder forms about 30% of the ingredients and may represent over half the cost of the final product. The quality of whey protein concentrates 34% (WPC34) is similar to that of dried skimmed milk (DSM) used in the standard recipe and can be 25–33% cheaper. This blinded, parallel group, randomised, controlled non‐inferiority clinical trial tested the effectiveness in treating severe acute malnutrition (SAM) of a new RUTF formulation WPC‐RUTF in which WPC34 was used to replace DSM. Average weight gain (non‐inferiority margin Δ = −1.2 g kg−1 day−1) and recovery rate (Δ = −10%) were the primary outcomes, and length of stay (LOS) was the secondary outcome (Δ = +14 days). Both per‐protocol (PP) and intention‐to‐treat (ITT) analyses showed that WPC‐RUTF was not inferior to P‐RUTF for recovery rate [difference and its 95% confidence interval (CI) of 0.5% (95% CI –2.7, 3.7) in PP analysis and 0.6% (95% CI –5.2, 6.3) in ITT analysis] for average weight gain [0.2 (−0.5; 0.9) for both analyses] and LOS [−1.6 days (95% CI, −4.6, 1.4 days) in PP analysis and −1.9 days (95% CI, −4.6, 0.8 days) for ITT analysis]. In conclusion, whey protein‐based RUTF is an effective cheaper alternative to the standard milk‐based RUTF for the treatment of SAM.
Keywords: malnutrition, undernutrition, community‐based, randomised controlled trial, whey protein, therapeutic feeding
Introduction
Over the last decade following the introduction of the community‐based management of acute malnutrition (CMAM) using ready‐to‐use therapeutic foods (RUTF), there have been major improvements in the survival of children with severe acute malnutrition (SAM) treated in outpatient therapeutic programmes (Collins et al. 2006a,b; WHO et al. 2007). The use of RUTF, coupled with improved access to CMAM services, has been associated with high recovery rates, lower case‐fatalities and greater weight gain of children with SAM (Ciliberto et al. 2005; Chaiken et al. 2006; Collins et al. 2006a,b; Linneman et al. 2007; Lapidus et al. 2009; Yebyo et al. 2013). In addition, providing RUTF in a dosage tailored to body weight has been shown to increase the rate of catch‐up growth in children with SAM compared with those treated with F100, the World Health Organisation (WHO) recommended a milk‐based diet (Diop et al. 2003). However, the worldwide scale up of the CMAM approach requires appropriately formulated, effective, cheap and readily available RUTFs (Collins and Henry 2004; Dibari et al. 2012). The current standard recipe for RUTF is made from peanut paste, milk powder, oil, sugar, and minerals and vitamins. Milk powder forms about 30% of the ingredients and represents over half the cost of the final product in small‐scale local production units, usually between $3500–4000 per metric ton (Manary 2006). At this price, the purchase of this RUTF referred to as peanut‐based RUTF (P‐RUTF) accounts for a large proportion of total programme costs, and this has been a major obstacle to the scale up of its use in the treatment of SAM given the limited health budgets of most developing countries (Manary 2006; Black et al. 2008, 2010). Unfortunately, it is unlikely that it will be possible to decrease the cost of the current milk powder‐based formulations given the high cost of the milk powder required for its manufacture. Thus, there is an urgent need to develop and test the effectiveness of alternative formulations that minimise the level of milk powder and to make the outcomes of these studies available to the international nutrition community. Among the options to be explored include the development and testing of RUTF that uses an alternative source of protein to milk protein. This could include a combination of cereals and pulses, soya flour, soya concentrates proteins or a cheaper protein of animal source such as whey protein concentrates (WPCs) (Collins and Henry 2004; Dibari et al. 2012; Hoppe et al. 2008; Irena et al. 2013; Oakley et al. 2010).
Whey is a by‐product of cheese making, and it refers to the serum or liquid part of milk that remains dissolved in the aqueous portion after the removal of fat and casein during the cheese‐making process (Hoppe et al. 2008). Whey has a protein density of 0.7–0.8% and contains five major proteins: α‐lactoglobulin, β‐lactalbumin, glycomacropeptide proteose peptone 3, immunoglobulins and serum albumin (Hoppe et al. 2008). Whey also contains a high level of milk lactose, is rich in minerals and is commercially transformed into a dry powder product which has a typical protein content of 11–14.5% and lactose content of 63–75% (Hoppe et al. 2008). The powder is usually processed into WPC with different concentrations of protein ranging from 34% to 80% (Hoppe et al. 2008). The quality of WPC 34% (WPC34) is similar to that of dried skimmed milk (DSM) and can be 25–33% cheaper (Hoppe et al. 2008). This study tests the effectiveness of a new RUTF formulation (WPC‐RUTF) in which WPC34 is used to replace DSM with the other ingredients being similar to that of the standard P‐RUTF/milk‐based RUTF (Briend et al. 1999; Diop et al. 2003).
Although whey proteins have been used in ready‐to‐use foods for childhood malnutrition previously (de Pee & Bloem 2009; LaGrone et al. 2012), they have not been used as the main source of protein and have always been combined with either soy or skim milk (de Pee & Bloem 2009). Whey protein also contains bioactive factors that have beneficial effects on the immune system and muscle synthesis, and it has been shown to promote loss of adipose tissue while maintaining lean body mass (Ha & Zemel 2003; Zemel 2005; Paul 2009). Thus, there is a potential for whey to improve weight gain and linear growth during recovery from SAM. However, the higher satiety effect of WPC ingestion may in theory lead to a reduction of total energy intake leading to lower weight gain (Luhovyy et al. 2007), although it is possible that this could be compensated by better efficiency of WPC in promoting lean mass deposition compared with other proteins from milk products (Ha & Zemel 2003).
Key messages
Replacing dried skimmed milk by whey protein concentrate (WPC) does not affect acceptability and tolerance of ready‐to‐use therapeutic food (RUTF).
WPC can be used to replace dried skimmed milk in RUTF to reduce the price as WPCs are cheaper than milk powder, and WPC‐RUTF is not inferior to peanut‐based RUTF.
Further research on the effect of such replacement on the composition of weight gain is still needed.
Methods
Setting
The study was conducted in Central Malawi between March 2010 and March 2011 in Lilongwe Health District within the Chileka and Kabudula health management areas. These areas have three hospitals and a CMAM programme for the treatment of SAM that supports 17 outpatient treatment programme (OTP) sites. The three hospitals provided inpatient care services for SAM with complications. On average, the total number of children with SAM admitted for treatment from these health management areas is 956 per year, with most admissions occurring between December and April.
Study design
This was a blinded, parallel group, randomised, controlled non‐inferiority clinical trial in which individuals were evenly randomised to either WPC‐RUTF (intervention) or P‐RUTF (active control) arms. The non‐inferiority hypothesis was assumed because the protein quality of the WPC34 is said to be similar to that of the DSM used in P‐RUTF. Children with SAM in both arms were treated according to the Malawi national guidelines for the management of SAM (Government of Malawi and Ministry of Health 2007).
Study population
All children were aged between 6 and 59 months and had been diagnosed at one of the 17 participating OTP centres as suffering from SAM without complications. The diagnostic criteria for SAM were a mid‐upper arm circumference (MUAC) <11.0 cm or pitting oedema of grade 1 (+) or 2 (++) (Collins 2007). Complications were defined as either medical, dehydration or the absence of appetite. Medical complications were diagnosed using the WHO's Integrated Management of Childhood Illness (IMCI) standard definitions (Nicoll 2000). Dehydration was diagnosed if the caregiver reported the occurrence of more than three watery stools per day and sunken eyes that started after the commencement of the watery stools. Appetite was assessed by asking the mother to sit quietly with the child for 15 min during which time she offered RUTF. If the mother reported that the child ate the RUTF, then appetite was assessed as good; if not, then the appetite was assessed as poor.
Children with SAM who presented with complications were referred to one of the three hospitals serving as inpatient stabilisation units and were eligible for inclusion in the study only when referred back to the OTP. Children who developed complications during the course of the treatment remained in the programme. Children previously discharged from the study with a recovered outcome that later relapsed and who then presented again at one of the participating OTPs with a new episode of SAM were not eligible for enrolment in the study for a second time. Children with any neurological or gastro‐intestinal chronic disability were also not eligible.
Randomisation and masking
After confirming the diagnosis of SAM, children were randomised by a closed‐envelope method either to receive WPC‐RUTF or P‐RUTF. A computer‐generated sequentially numbered randomisation list (with variable block sizes) that contained both allocations and codes for 700 children was pre‐prepared by the trial statistician based outside Malawi and sent to the national study coordinator who then prepared 700 opaque, sealed and consecutively numbered randomisation envelopes. The envelopes were distributed to the enumerator team leaders at study sites in a block of 20 envelopes. In the field, the caretakers were asked to randomly pick a sealed opaque envelope containing the arm code.
The two RUTFs were manufactured by Valid Nutrition in Lilongwe, Malawi. They were packaged in identical 250‐g pots and were labelled with a letter code. The RUTFs were similar in colour, texture and smell. The investigators directly involved in supervision, child recruitment, and management and outcome assessment were blinded to the identity of the letter codes. All non‐participating staff of the research sites and the caregivers of enrolled children were also blinded to the identity of the RUTFs. Upon the end of data collection, Valid Nutrition provided code identification to the statistician for the purpose of data analysis.
Data collection and follow‐up
The study used a combination of specially trained study nurses, nurses and community health workers from participating health facilities as enumerators. In the week preceding the start of data collection, all enumerators received a refresher training on the diagnosis of SAM, its management and the follow‐up of cases in the community. A standardised modified version of the standard OTP individual monitoring card was used for data collection and follow‐up. It contained background information, anthropometric information, medical history and physical examination, and follow‐up sections.
All children diagnosed with SAM were re‐examined by a trained study nurse enumerator to ascertain the diagnosis of SAM using MUAC, weight and bilateral pedal pitting oedema and confirm eligibility. MUAC was measured using graduated MUAC tapes on the left upper arm of each child. This was done twice for each child by two different enumerators, and the average of the two measures was recorded to the nearest 0.1 cm. Weight in minimal clothing was measured to the nearest 100 g using Salter scales (Salter Weight‐Tronix Ltd, West Bromwich, West Midlands, UK). Bilateral pitting oedema was assessed twice, once at the screening stage and then again at diagnostic confirmation by pressing for 3 s on the dorsum of the foot. Once a diagnosis of SAM was established, a medical history and physical examination were undertaken, and children found to have medical complications and/or no appetite were referred to the nearest inpatient stabilisation centre. All other cases of SAM were admitted to the OTP and included in the trial. Human immunodeficiency virus (HIV) positivity was not considered as a complication, and an HIV test was offered to all caregivers but not performed systematically. At the time of the study, the country had not yet adopted the provider‐initiated HIV testing approach. Previous studies have revealed an HIV prevalence below 3% among children with SAM admitted to therapeutic feeding units of Central Malawi (Bahwere et al. 2008; Thurstans et al. 2008).
All enrolled children were asked to return to the OTP site for a follow‐up visit once each week until they were discharged from the programme. At each follow‐up visit, MUAC, oedema and weight were recorded, and the children were screened for medical problems, including possible side effects of the food and the presence of appetite. Caregivers were interviewed at each visit about the acceptability of the RUTF. Caregivers were also asked about whether the child had eaten an RUTF formulation other than the one they had been allocated. No other means was used to assess for non‐adherence to the allocated RUTF.
Treatment protocols
After admission into the study, all children received a 5‐day course of amoxicillin, a single 100 mg dose of mebendazole, a 1‐week ration of RUTF, and health and nutrition advice. The RUTF ration was calculated to provide around 175 kcal kg−1. At each weekly visit, a repeat 1‐week ration of RUTF was provided at the same dosage and identified medical conditions treated according to IMCI guidelines or other relevant national protocols (Nicoll 2000).
Outcomes
Average weight gain and recovery rate were the primary outcomes of interest. The secondary outcome of interest was length of stay (LOS). Children exited the study in one of five ways: recovery (cure), death, default, transfer out of the catchment area and non‐recovery. For children admitted with a MUAC <11.0 cm, recovery was defined as follows: a weight gain of at least 15%, MUAC >11.0 cm, no medical complication, the absence of bilateral pitting oedema and a minimum stay in the programme of 1 month (Government of Malawi and Ministry of Health 2007). In the case of children admitted with bilateral pitting oedema, recovery was defined as the absence of bilateral pitting oedema, being clinically well and a MUAC >11.0 cm (Government of Malawi and Ministry of Health 2007).
A child was considered to have defaulted if they were absent for three consecutive visits. Defaulters were followed up by trained volunteers and invited back into the programme. Those who returned were given a new outcome based on their status when they exited the programme.
Children whose condition deteriorated in the course of the outpatient treatment were referred to the nearest of the three hospitals serving as inpatient stabilisation centres. Once stabilised, the children returned to the OTP site to complete their treatment, and an outcome was allocated based on their exit status from the OTP site. Those children who died or defaulted while undergoing inpatient treatment were given an outcome status of ‘death’ or ‘Defaulter’, respectively. Children who did not reach the recovery discharge criteria after 4 months of treatment were given the outcome status of non‐recovery.
Food products used in the study
The WPC‐RUTF and P‐RUTF content are presented in Table 1. The development of the WPC‐RUTF was carried out using linear programming, in accordance to a method described by Dibari et al. (Dibari et al. 2012). The method determined the quantity of WPC34 needed to replace skimmed milk powder and the mineral and vitamin specifications to include into the premix. The final product matched the WHO 2007 requirements for RUTF recommended mineral and vitamin composition. The WPC34 was imported from United States, and the information on its composition used in linear programming was obtained from ‘Reference Manual for U.S. Whey and Lactose Products’ available at US Dairy Export Council (USDEC) website. Table 1 shows that the WPC‐RUTF meets WHO specifications (WHO et al. 2007). Subsequently, a production test demonstrated similar consistency and colour to that of P‐RUTF, with a mixing procedure and sequences similar to that used for the P‐RUTF (Fellows 2004).
Table 1.
Ingredients and nutrients of the study foods
| Ingredients/Nutrients | WPC‐RUTF a | P‐RUTF b | UN specifications | |
|---|---|---|---|---|
| Ingredients | ||||
| WPC34 | (g/100g) | 24.0 | 0.0 | |
| Dried Skim Milk | (g/100g) | 0.0 | 25.0 | |
| Sugar | (g/100g) | 27.9 | 27.4 | |
| Peanut paste | (g/100g) | 27.0 | 26.0 | |
| Soybean oil | (g/100g) | 19.5 | 20.0 | |
| Vitamin and minerals Premix | (g/100g) | 1.6 | 1.6 | |
| Nutrients | ||||
| Energy | (Kcal/100g) | 530 | 530 | 520–550 |
| Protein/Energy ratio | (%) | 12.0 | 12.0 | 10–12 |
| Fat/Energy ratio | (%) | 56.0 | 56.0 | 45–60 |
| Omega‐6/Energy ratio | (%) | 1.4 | 3–10 | |
| Omega‐3/Energy ratio | (%) | 1.5 | 0.3–2.5 | |
| Omega‐6/Omega‐3 ratio | 22.1 | 5–9 | ||
| Cysteine | (g/100g) | 0.3 | 0.1 | |
| Methionine | (g/100g) | 0.2 | 0.3 | |
| Vitamin A | (μg/100g) | 1423.4 | 910 | 810–1100 |
| Vitamin C | (mg/100g) | 74.9 | 53 | ≥50 |
| Vitamin D | (μg/100g) | 26.3 | 16 | 15–20 |
| Vitamin E | (mg/100g) | 29.8 | 20 | ≥20 |
| Thiamin (Vitamin B1) | (mg/100g) | 0.7 | 0.6 | ≥0.5 |
| Riboflavin (Vitamin B2) | (mg/100g) | 2.3 | 1.8 | ≥1.6 |
| Niacin (Vitamin B3) | (mg/100g) | 6.7 | 5.3 | ≥5 |
| Pantothenic acid (Vitamin B5) | (mg/100g) | 4.4 | 3.1 | ≥3 |
| Pyridoxine (Vitamin B6) | (mg/100g) | 0.9 | 0.6 | ≥0.6 |
| Biotin (Vitamin B7) | (μg/100g) | 86.7 | 65 | ≥60 |
| Folates (Vitamin B9) | (μg/100g) | 298.4 | 210 | ≥200 |
| Cobalamin (Vitamin B12) | (μg/100g) | 2.4 | 1.8 | ≥1.6 |
| Vitamin K | (μg/100g) | 32.9 | 21 | 15–30 |
| Calcium | (mg/100g) | 450 | 315 | 300–600 |
| Phosphorus | (mg/100g) | 450 | 370 | 300–600 |
| Magnesium | (mg/100g) | 110 | 86 | 60–140 |
| Potassium | (mg/100g) | 1250 | 1140 | 1100–1400 |
| Sodium | (mg/100g) | 214.8 | 110 | <290 |
| Copper | (mg/100g) | 1.6 | 1.7 | 1.4–1.8 |
| Iodine | (μg/100g) | 136.5 | 100 | 70–140 |
| Iron | (mg/100g) | 12.0 | 12.0 | 10–14 |
| Zinc | (mg/100g) | 12.5 | 11.1 | 11–14 |
| Anti‐nutrients | ||||
| Phytic acid | (mg/100g) | 370 | 255 | <100 |
| Phytic acid/Zinc ratio | 2.9 | 2.2 | <15 | |
| Phytic acid/Iron ratio | 2.6 | 1.9 | <1 | |
aWPC‐RUTF = Whey Protein Concentrates 34% Based Ready‐To‐Use Therapeutic Food; bP‐RUTF = Peanut paste based Ready‐To‐Use Therapeutic Food. [Correction made on the 7th of March after online publication: Table 1 was replaced.]
The production test was followed by an acceptability trial conducted using a crossover design as previously published by this group (Dibari et al. 2012). The acceptability trial used 24 normally nourished Malawian children aged between 6 and 15 years (average 10.6 years) recruited from a Lilongwe primary school. Criteria for inclusion were that the children could express their opinions regarding the taste, colour, texture and smell of both RUTFs and rate their feeling about the products using a 5‐point hedonic scale. The trial assessed the acceptability and tolerance of the product at a dosage of 250 g day−1.
In this acceptability trial, the majority (19/20) met the acceptability criteria of eating at least 75% of the daily serving of WPC‐RUTF and P‐RUTF (19/22) for more than 75% of 14 successive days of evaluation. The rating of both RUTFs was excellent or very good for colour (19/20 for WPC‐RUTF and 19/22 for P‐RUTF), smell (19/20 for WPC‐RUTF and 16/22 for P‐RUTF) and texture (16/20 for WPC‐RUTF and 18/22 for P‐RUTF). For the taste, 18/20 rated the WPC‐RUTF as having an excellent or very good taste, whereas 2/20 rated the taste as good or fair. The rating for P‐RUTF was excellent or very good for 10/22 children, good or fair for 11/22 children and bad to very bad for one child. Transient abdominal discomfort (4/18 for WPC‐RUTF and 3/20 for P‐RUTF), vomiting (1/18 for WPC‐RUTF and 2/20 for P‐RUTF), diarrhoea (4/18 for WPC‐RUTF and 3/20 for P‐RUTF) and oral thrush (0/18 for WPC‐RUTF and 2/20 for P‐RUTF) were reported. Five children dropped out during the course of the acceptability trial because they did not like the RUTF: four during the P‐RUTF phase and one during the WPC‐RUTF phase.
Sample size calculation
We calculated the sample size to demonstrate that WPC‐RUTF was not inferior to P‐RUTF for weight gain and recovery rate among children with SAM discharged as cured from a CMAM programme. Based on the literature, we assumed a margin of non‐inferiority of 1.2 g kg−1 day−1 and a standard deviation (SD) of 4.6 (Ashworth 2006). Considering a beta = 0.1 and an alpha = 0.05, a total of 600 children including 504 who were discharged cured were required to be 90% sure that the lower limit of a one‐sided 95% confidence interval (CI) was above the non‐inferiority limit of −1.2 (Julious 2004). This sample size was also sufficient to demonstrate non‐inferiority in recovery rate using the same alpha and beta and a margin of non‐inferiority of minus 10%. (Blackwelder 1982). The non‐inferiority margins were selected according to literature and medical judgment of a clinically appropriate and acceptable margin (Ashworth 2006).
Data management and statistical analysis
Child data were entered into an Epidata database prepared for this study (Lauritsen and Bruus 2003). Data were entered by two enumerators and cleaned, coded and then exported to stata‐11 (StataCorp. 2009) for analysis. In accordance with recommendations for analysing and reporting equivalence and non‐inferiority studies, both intention‐to‐treat (ITT) and per‐protocol (PP) analyses were performed (Le Henanff et al. 2006; Piaggio et al. 2006). The ITT analyses included all children enrolled in the study. The PP analyses for weight gain included only the children who were discharged cured as this is how this indicator of effect of therapeutic feeding on growth velocity is usually calculated in programmes and reported in literature. For recovery rate, PP analyses excluded children who defaulted, transferred out of the programme and were lost to follow‐up after inpatient transfer, but included children discharged as cured, died or non‐cured.
Summary enrolment characteristics for each arm were calculated as mean ± SD or median (interquartile range) for continuous or discrete measures and as n (%) for categorical measures. Means were compared using t‐test, the median using the Mantel–Haenszel test and proportions using the Student's chi‐squared test. Rates of weight gain during the entire period of follow‐up were estimated in g kg−1 day−1 and compared between the study arms. These were calculated by dividing the weight gain (weight at exit – weight at admission) expressed in grams by the weight at admission (in kilograms) and the LOS (in days). Recovery rate was calculated as a percentage of total recovered children divided by total exits. Difference in the estimated marginal means between the treatment arms along with bootstrapped 95% CI was estimated to draw inference on non‐inferiority. For the comparison of recovery rates, generalised regression models with zero‐inflated negative binomial distribution were used. We used a logistic regression to test for interactions between the recovery rate and other variables. These variables were sex, age group (<24 vs. ≥24 months), immunisation status (fully immunised vs. not fully immunised), travelling time from home to the Health Centre (HC) (>30 vs. ≤30 min), number of children below 5 years in the household (≤2 vs. >2), admission criterion and presence of oedema. We used a linear regression to test the interaction between weight gain and the variables listed above. The differences between two incidence rates were done with the ‘MidPoint Rule method’. A data safety monitoring board was assigned to perform an ongoing review of study outcomes, but no formal interim analysis was planned.
Ethical considerations
Permission for the trial to be conducted was obtained from Malawi National Health Sciences Research Committee (NHSRC) of the Ministry of Health prior to randomisation. At the time of admission, each child's parent or carer was informed about the nature and purpose of the study, the different RUTFs, and randomisation process and asked for their informed written consent for their child to be included in the study and for their medical information to be used for research purposes. In addition to the authorisation for carrying out of the study, an authorisation to publish the findings was sought and obtained from Malawi NHRSC.
Results
The study enrolment and movement of children from preliminary screening to data analysis are shown in Fig. 1. Between March 2010 and March 2011, a total of 619 eligible children were screened, of whom 600 were randomised to either P‐RUTF (n = 292) or WPC‐RUTF (n = 308). Nineteen eligible children were excluded prior to randomisation either because of being allocated to an arm by the HC staffs before receiving the code from the central level (n = 18) or because the child was considered has a return from defaulting as he had recently been under SAM treatment but he discontinued treatment before cure (n = 1). Five children randomised to the WPC‐RUTF group were excluded after randomisation because either critical baseline data including weight, MUAC or oedema were not collected prior to the initiation of the nutrition rehabilitation (n = 3) or because the verification of age indicated that the child was older than 59 months.
Figure 1.
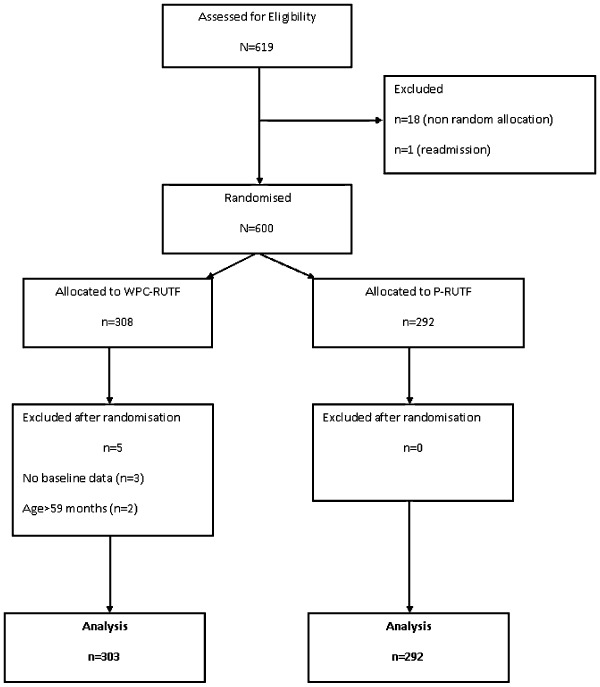
Chart showing enrolment, randomisation and follow‐up of study participants.
Baseline characteristics of children included in the ITT analyses for each study group are shown in Table 2. There was no difference between the two groups at baseline in terms of maternal vital status, child age, proportion of boys, main carer, time to the clinic, proportion receiving humanitarian food support, nutrition parameters such as weight and presence of infection. However, the prevalence of HIV was higher in the WPC‐RUTF group than in the P‐RUTF group. Oedematous malnutrition was the dominant form of SAM with one to five children having at least two plus oedema on admission. Diarrhoea and fever were common at baseline, but less than 5% of the children required initial inpatient treatment.
Table 2.
Baseline demographic and clinical characteristics of the 595 children randomly assigned to WPC‐RUTF or P‐RUTF group
| Criteria | WPC‐RUTF | P‐RUTF | ||
|---|---|---|---|---|
| n | 303 | 292 | ||
| Socio‐demographic parameters | ||||
| Male, n (%) | 145 | (47.8) | 154 | (52.7) |
| Age (months), means (SD) | 25.0(11.0) | 24.5(10.3) | ||
| Mother alive, n (%) (n = 269/267) † | 264 | (98.1) | 250 | (97.3) |
| Mother main carer, n (%) | 226 | (74.6) | 215 | (73.6) |
| Distance home to HC (minutes), means ± SD | 92.6(54.4) | 98.4(50.4) | ||
| Receiving humanitarian food package, n (%) (n = 233/214) † | 51 | (21.9) | 48 | (22.4) |
| Nutrition parameters | ||||
| Previous episode of SAM, n (%) | 16 | (5.3) | 8 | (2.8) |
| Oedema being admission criteria, n (%) | 249 | (82.2) | 241 | (82.5) |
| Oedema ≥+2, n (%) | 64 | (21.1) | 60 | (20.6) |
| Baseline weight (kg), means (SD) | 8.7(2.0) | 8.7(2.1) | ||
| Baseline height (cm), means (SD) | 76.1(8.6) | 76.0(8.8) | ||
| Baseline MUAC (mm), means (SD) | 125.7(16.0) | 124.9(14.7) | ||
| Baseline weight‐for‐height (z‐score), means (SD) | −1.32(1.42) | −1.30(1.64) | ||
| Baseline height‐for‐age (z‐score), means (SD) | −3.26(2.02) | −3.30(2.36) | ||
| Baseline weight‐for‐age (z‐score), means (SD) | −2.75(1.32) | −2.68(1.44) | ||
| Need of initial inpatient care, n (%) | 9 | (3.0) | 8 | (2.7) |
| Infectious parameters | ||||
| Complaint of diarrhoea, n (%) (n = 288/282) † | 71 | (24.6) | 68 | (24.1) |
| Complaint of fever, n (%) (n = 283/279) † | 94 | (33.2) | 95 | (34.0) |
| HIV sero‐positivity, n (%) (n = 129/112) † | 17 | (13.2) | 6 | (5.4) |
HC, Health Centre; HIV, human immunodeficiency virus; MUAC, mid‐upper arm circumference; P‐RUTF, peanut‐based ready‐to‐use therapeutic food; SAM, severe acute malnutrition; SD, standard deviation; WPC‐RUTF, whey protein concentrate ready‐to‐use therapeutic food. †Different n because of missing data.
Within‐group analyses showed that children of both study groups met the international minimum standard for recovery rate (>70%), mortality rate (<10%) and defaulter rate (<15%). For children in the WPC‐RUTF group, the recovery rate, mortality rate and defaulter rate for ITT analyses were 84.8% (257/303), 1.6% (5/303) and 12.2% (37/303), respectively. For those of the P‐RUTF group, the figures were 84.2% (246/292), 0.7% (2/292) and 12.2% (37/292), respectively. Average weight gain was inferior to 4 g kg−1 day−1 for both groups and was 3.1 g kg−1 day−1 for children of the WPC‐RUTF group and 2.9 g kg−1 day−1 for those of the P‐RUTF group. For children discharged as cured, the average weight gain was 3.5 g kg−1 day−1 for children in the WPC‐RUTF group and 3.4 g kg−1 day−1 for those in the P‐RUTF group. For the PP analyses the international minimum standards were also met in both groups for recovery rate (257/266 = 96.5 for the WPC‐RUTF group and 246/256 = 96.1) and mortality rate (5/266 = 1.9% for the WPC‐RUTF group and 2/256 = 0.8% for the P‐RUTF group).
Both PP and ITT analyses showed that the recovery rate of the WPC‐RUTF group was not inferior to the recovery rate of the P‐RUTF group as the 95% CI of the difference between the two groups was above the pre‐defined non‐inferiority margin of Δ = −10% (Fig. 2). The observed differences were 0.5% (95% CI –2.7, 3.7) in PP analysis and 0.6% (95% CI –5.2, 6.3) in ITT analysis.
Figure 2.
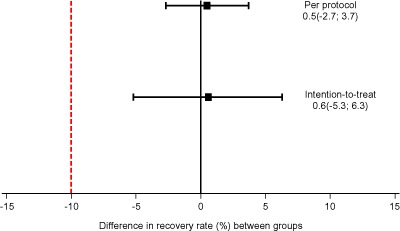
Difference in recovery rate between whey protein concentrate ready‐to‐use therapeutic food (WPC‐RUTF) and peanut‐based RUTF (P‐RUTF).
Similarly, both PP and ITT between‐group analyses showed that the average weight gain of the WPC‐RUTF group was not inferior to average weight gain of the P‐RUTF group as their 95% CIs were above the pre‐defined non‐inferiority margin of Δ = −1.2 g kg−1 day−1 (Fig. 3). The difference was 0.2 (–0.5; 0.9) for both analyses.
Figure 3.
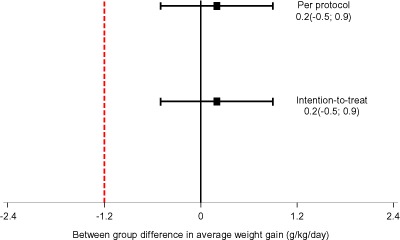
Difference in average weight gain between whey protein concentrate ready‐to‐use therapeutic food (WPC‐RUTF) and peanut‐based RUTF (P‐RUTF).
The subgroup analysis demonstrated no interaction between age groups (<24 vs. ≥24 month) or type of malnutrition (oedematous or non‐oedematous) for either weight gain or the recovery rate.
Overall, the LOS (pre‐defined non‐inferiority margin of Δ = +14 days) in the ITT analysis for children recruited into WPC‐RUTF group was 32.6 days (95% CI, 30.8–34.5 days), and for P‐RUTF, it was 34.5 days (95% CI, 32.5–36.5), giving a difference of −1.9 days (95% CI, −4.6, 0.8 days). For the PP analysis, it was 34.2 days (95% CI, 32.8–36.8 days) for WPC‐RUTF group, 35.8 days (95% CI, 33.8–36.3) for P‐RUTF, with a difference of −1.6 days (95% CI, −4.6, 1.4 days). For children who were discharged as recovered, the figure was 34.8 days (95% CI, 32.8–36.8 days) for WPC‐RUTF, 36.1 days (95% CI, 33.8–36.3) for P‐RUTF and −1.3 days (95% CI, −4.7, 1.7 days) for the difference. All these differences in LOS were not statistically significant.
The proportion of children who had a history of diarrhoea at the first weekly follow‐up did not significantly differ according to RUTF group (44/291 = 15.1% for the WPC34 group and 49/258 = 19.0% for the P‐RUTF group; P = 0.227), and the average duration of symptoms was 3.3(2.5) days for children of WPC34 group and 2.9(1.7) days (P = 0.478). The overall incidence rate of history of diarrhoea did not differ between the two groups (P = 0.795) and was 11.5 episodes per 100 child visits for children of WPC34 group (146 episodes for 1270 child visits), 11.8 episodes per 100 child visits for children of P‐RUTF group (142 episodes for 1198 child visits). Children of the WPC34 group had significantly lower frequency of fever 27/278 = 9.1% for the WPC34 group and 43/253 = 17.0% for the P‐RUTF group; P = 0.013) and a trend of lower frequency of cough 33/278 = 11.9% for the WPC34 group and 43/250 = 17.2% for the P‐RUTF group; P = 0.082) at the first weekly follow‐up.
Discussion
This study has demonstrated that an RUTF in which all the DSM is replaced by WPC, a by‐product of cheese making, is not inferior to the standard P‐RUTF and milk‐based RUTF in terms of organoleptic acceptability, tolerance, weight gain and recovery rate.
For both groups, the recovery rate observed exceed the SPHERE minimum standards of >70% and is better or comparable with the recovery rates of between 60% to 88% reported in other studies that evaluated outcomes in community‐based programmes implanted in Malawi and other African countries (Ciliberto et al. 2005, 2006; Collins et al. 2006b; Bahwere et al. 2008; Sadler et al. 2008; Kerac et al. 2009; Irena et al. 2012; Trehan et al. 2013; Yebyo et al. 2013). This high recovery rate and the non‐inferiority of WPC‐RUTF when compared with P‐RUTF and the fact that the PP and ITT analyses gave a similar result are robust evidence showing that WPC‐RUTF is indeed an excellent alternative to P‐RUTF in the management of SAM (Kaul & Diamond 2006).
In both groups, the weight gain observed in this study is lower than the recommended >5 g kg−1 day−1 but compares favourably with weight gains observed in other studies carried out in Malawi. Ciliberto et al. found a rate of weight gain of 2.8 g kg−1 day−1 in oedematous children from Southern Malawi despite calculating the weight gain for the first 4 weeks of treatment only, a period during which the highest weight gain is typically observed (Ciliberto et al. 2006). In an earlier study, the same author documented a weight gain for the first 4 weeks of 3.5 kg kg−1 day−1 among children, 44% of whom presented with oedematous malnutrition (Ciliberto et al. 2005). This suggests that the weight gain observed in this study can be considered as satisfactory, particularly where a large proportion of children with SAM present with oedema. It is important to note that oedematous children tend to lose weight during the early phase of treatment. In outpatient programmes, the true lowest weight is difficult to establish because with this initial weight loss, the lowest weight often occurs in between the two weekly visits. The result is that the rates of weight gain reported based upon the admission weight are likely to underestimate the real rates of weight gain in oedematous children had these been calculated using the lowest weight attained in the centre. In addition, we believe the conclusion of non‐inferiority of the WPC‐RUTF to be robust because both the PP and ITT gave a similar result, and the weight gain observed with P‐RUTF is in the range of weight gain reported in the literature in well‐run programmes (Kaul & Diamond 2006).
WPC34 is deemed to have similar nutritional qualities to that of DSM; thus, the non‐inferiority results are not surprising. What is surprising is, that although WPC34 is 25–33% cheaper than DSM (Hoppe et al. 2008), it is not currently used as main source of protein in RUTF production despite the universal concern about the cost of RUTF and the contribution of milk powder to this cost (Manary 2006). This is probably because of the absence of a study that provides evidence of the effectiveness of WPC‐based RUTF and of its performance in comparison with the standard skimmed milk powder‐based RUTF. The results of this study fill this knowledge gap and provide evidence that WPC‐RUTF is a credible alternative to P‐RUTF for cost reduction. The potential savings through use of a WPC‐RUTF have been documented in the range of 10–20% and are particularly attractive because WPC price in the international market seems more predictable and fluctuates less than DSM (Michaelsen et al. 2009; Rosenberg et al. 2011). At the 2013 average‐weighted UNICEF procurement price of $US4064 per metric ton and the planned 32 000 metric tons procurement, a 10% reduction on the final price would have resulted in a saving of around $US13m for UNICEF. The 13 million can be an underestimation of saving according to the summary of 2013 price trends in the United States. Indeed, figures from the US Department of Agriculture's Agricultural Marketing Service summarised by Consultancy and Market Research Food and Dairy and accessed on their website in early November show that in the US market, the 2013 average price of WPC34 (€2231 metric ton−1) has been 21% lower to that of DSM (€2817 metric ton−1). In addition to the potential reduction in cost, several other properties of WPC are favourable for the production of RUTF. Whey protein is comprised of a number of individual bioactive components, or fractions, which include α‐lactalbumin, β‐lactoglobulin, serum proteins, lactoferrin and a series of immunoglobulins (Marshall 2004). Individually, these fractions are known to be immune‐system enhancer. In addition, they support a wide range of bioactive functions, such as iron binding, tissue repair, maintenance of intestinal integrity, resistance to pathogens and elimination of toxins (Marshall 2004). For example, the higher concentration of the branched‐chain amino acids leucine, isoleucine and valine give WPC34 a comparative advantage over DSM given the important role of these amino acids in promoting protein synthesis and counteracting muscle protein breakdown and treatment goal in SAM management and of promoting new lean tissue synthesis for the replenishment of the body mass cells (Marshall 2004). Both RUTFs provided daily amount of cysteine and methionine higher than the 391.2 and 496.5 μmol kg−1 day−1 associated with rapid restoration of glutathione homeostasis (Jahoor et al. 1988; Solak & Akin 2012). However, children taking P‐RUTF had a cysteine intake of 385 μmol kg−1 day−1 lower than that of those taking WPC34 which was 825 μmol kg−1 day−1. Our study was not designed to assess the effect of incidence of morbidity, time to resolution of oedema or lean mass deposition, but theoretically, the extra intake of cysteine taken by children of the WPC34 arm did probably lead to an improvement of these outcomes especially in children admitted with oedematous malnutrition as they have a very high cysteine requirement (Jahoor et al. 1988; Marshall 2004; Badaloo et al. 2012). In the context of possible association between environmental enteropathy and gut microbiome with the occurrence of oedematous malnutrition and the response to treatment, the higher concentration in transforming growth factor‐β (TGFβ1) in WPC34, a whey protein bioactive component that strengthens the intestinal barrier and of glutamine which serves as food for fast dividing cells such as those of intestinal wall, is in favour of the use WPC34 instead of DSM (Noyer et al. 1998; Bushen et al. 2004; Hering et al. 2011; Prendergast & Kelly 2012; Smith et al. 2013). Indeed, previous studies demonstrated that TGFβ1 up‐regulates the epithelial barrier function towards barrier protection even when barrier properties are not impaired, and supplementation with glutamine accelerates intestinal wall repair during nutrition rehabilitation (Noyer et al. 1998; Bushen et al. 2004; Hering et al. 2011). However, in this study. there was no difference in frequency of history of diarrhoea between the two groups.
Whey proteins are increasingly used as ingredients in infant formulas and specialised protein supplements because they contain all the essential amino acids and have the highest protein quality rating among other protein sources (Yalcin 2006; Solak & Akin 2012). Indeed, recent literature suggests that the use of whey proteins is associated with improving lean body mass deposition (Morr & Ha 1993; Ha & Zemel 2003). Although the initial protocol included the assessment of body composition, this objective was later dropped because of financial constraints. Thus, one of the limitations of this study is the absence of data on body composition that limits inference about deposition of lean mass in children fed WPC‐RUTF compared with those fed P‐RUTF. However, the catch up growth resulting in an average time to recovery of less than the 42 days usually observed in CMAM programmes (Collins et al. 2006b) suggests efficient deposition of lean mass in both groups.
Although lactose intolerance is not a significant clinical problem with P‐ RUTF, the use of whey protein, by supporting a reduction of the lactose content of the RUTF, could help to minimise the risk of lactose intolerance and reduce the incidence of diarrhoea at the initiation of nutrition rehabilitation (Barth & Behnke 1997; Hoppe et al. 2008; Ali et al. 2013). Indeed, some caretakers and patients list diarrhoea as a relatively common side effect of RUTF consumption (Ali et al. 2013).
Unpublished data from Valid International's work in Bangladesh and Malawi have shown that poor tolerance of RUTF usually results in children halting consumption. In this study, there was no evidence of more children stopping consumption in the WPC arm than in the P‐RUTF in either the acceptability of effectiveness trials, and the defaulter rate in effectiveness trial was similar in both arms.
The recipe development phase showed that the same manufacturing processes can be used to make both standard recipe and WPC RUTFs. As for the production of standard RUTF, the main challenge for producing WPC‐RUTF in Africa is the difficulty in procuring WPC powder, and based on the findings of this trial, efforts should be made to increase availability of WPC in developing countries, especially in those ready to get involved in production of high‐quality complementary and therapeutic foods.
The main limitation of the present study is the absence of the monitoring of the RUTF intake and the weekly follow‐up design. The absence of intake monitoring prevents us from distinguishing between effect of the products and operational constraints. For example, it prevents from comparing the intra‐household sharing pattern of the two products and from determining the relationship between actual intake and the weight gain. Our inability to document the weight of the child at the time of oedema disappearance certainly affected the estimation of weight gain and limited our ability to assess the potential impact of WPC34 on duration of oedema. Future studies should address these design limitations by opting for an efficacy trial rather than effectiveness when assessing food therapeutic value. Future studies should also assess the effect of WPC34 on morbidity. Indeed, although some data on morbidity have been presented with the indication that WPC34 may synergise with antibiotics to lower morbidity as already suggested in the literature, these results are from post hoc analyses and should be confirmed by a study specifically including this objective before a firm conclusion is made.
In conclusion, the present study demonstrates that a whey protein‐based RUTF is an effective alternative to the standard milk‐based RUTF for the treatment of SAM. This result is important because it provides governments and the humanitarian community with an alternative to the standard product which is both effective and less costly. The cost of RUTF has been a major obstacle to the scale up of treatment of SAM given the limited health budgets of most developing countries that suffer a high burden of malnutrition (Manary 2006; Collins et al. 2006a,b). We suggest that WPC‐RUTF has an important role to play in both improving access to treatment for the 25 million children currently suffering from SAM that have no access to treatment and in making progress towards achieving millennium development goal 4 that aims to reduce child mortality in 2015. More research is required to assess the potential beneficial impact of other WPC properties on nutrition recovery and absorption of key micronutrients including zinc and iron.
Source of funding
USDEC through Clinton Foundation provided funding for the study.
Conflicts of interest
VO was an employee of Valid Nutrition. SC is the unpaid director of Valid Nutrition. Valid International is the sister company of Valid Nutrition that promotes the development and promotion of RUTF. Clinton Foundation and USDEC had no say on the design, implementation and interpretation of the results.
Contributions
SC and PB conceived study idea and provided technical oversight throughout the trial including data collection, data analysis and preparation of this manuscript. FD and VOO participated in study design especially in the development of WPC‐RUTF and in the preparation of the manuscript. KS, TB, GN and SB contributed in data collection, data analysis and manuscript preparation. All authors have read and approved the manuscript
Acknowledgements
We would like to thank the Lilongwe district health management team and the Malawi Ministry of Health for the support rendered to us throughout the study. Our gratitude goes to the health personnel involved in CMAM service, our research team and all the beneficiaries. Last but not least, we would like to thank Clinton foundation and the US Dairy Export Council (USDEC) for the financial support.
References
- Ali E., Zachariah R., Shams Z., Manzi M., Akter T., Alders P., Allaouna M., Delchevalerie P. & Harries A.D. (2013) Peanut‐based ready‐to‐use therapeutic food: how acceptable and tolerated is it among malnourished pregnant and lactating women in Bangladesh? Maternal and Child Nutrition. doi: 10.1111/mcn.12050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth A. (2006) Efficacy and effectiveness of community‐based treatment of severe malnutrition. Food and Nutrition Bulletin 27 (Suppl. 3), S24–S48. [DOI] [PubMed] [Google Scholar]
- Badaloo A., Hsu J.W., Taylor‐Bryan C., Green C., Reid M., Forrester T. & Jahoor F. (2012) Dietary cysteine is used more efficiently by children with severe acute malnutrition with edema compared with those without edema. American Journal of Clinical Nutrition 95, 84–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahwere P., Piwoz E., Joshua M.C., Sadler K., Grobler‐Tanner C.H., Guerrero S. & Collins S. (2008) Uptake of HIV testing and outcomes within a Community‐based Therapeutic Care (CTC) programme to treat severe acute malnutrition in Malawi: a descriptive study. BMC Infectious Diseases 8, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barth C.A. & Behnke U. (1997) Nutritional physiology of whey and whey components. Die Nahrung 41, 2–12. [DOI] [PubMed] [Google Scholar]
- Black R.E., Allen L.H., Bhutta Z.A., Caulfield L.E., de Onis M., Ezzati M., Mathers C. & Rivera J. (2008) Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371, 243–260. [DOI] [PubMed] [Google Scholar]
- Black R.E., Cousens S., Johnson H.L., Lawn J.E., Rudan I., Bassani D.G., Jha P., Campbell H., Walker C.F., Cibulskis R., Eisele T., Liu L. & Mathers C. (2010) Global, regional, and national causes of child mortality in 2008: a systematic analysis. Lancet 375, 1969–1987. [DOI] [PubMed] [Google Scholar]
- Blackwelder W.C. (1982) ‘Proving the null hypothesis’ in clinical trials. Controlled Clinical Trials 3, 345–353. [DOI] [PubMed] [Google Scholar]
- Briend A., Lacsala R., Prudhon C., Mounier B., Grellety Y. & Golden M.H.N. (1999) Ready‐to‐use therapeutic food for treatment of marasmus [letter]. Lancet 353, 1767–1768. [DOI] [PubMed] [Google Scholar]
- Bushen O.Y., Davenport J.A., Lima A.B., Piscitelli S.C., Uzgiris A.J., Silva T.M., Leite R., Kosek M., Dillingham R.A., Girao A., Lima A.A. & Guerrant R.L. (2004) Diarrhea and reduced levels of antiretroviral drugs: improvement with glutamine or alanyl‐glutamine in a randomized controlled trial in northeast Brazil. Clinical Infectious Diseases 38, 1764–1770. [DOI] [PubMed] [Google Scholar]
- Chaiken M.S., Deconinck H. & Degefie T. (2006) The promise of a community‐based approach to managing severe malnutrition: a case study from Ethiopia 15. Food and Nutrition Bulletin 27, 95–104. [DOI] [PubMed] [Google Scholar]
- Ciliberto M.A., Manary M.J., Ndekha M.J., Briend A. & Ashorn P. (2006) Home‐based therapy for oedematous malnutrition with ready‐to‐use therapeutic food. Acta Paediatrica 95, 1012–1015. [DOI] [PubMed] [Google Scholar]
- Ciliberto M.A., Sandige H., Ndekha M.J., Ashorn P., Briend A., Ciliberto H.M. & Manary M.J. (2005) Comparison of home‐based therapy with ready‐to‐use therapeutic food with standard therapy in the treatment of malnourished Malawian children: a controlled, clinical effectiveness trial. American Journal of Clinical Nutrition 81, 864–870. [DOI] [PubMed] [Google Scholar]
- Collins S. (2007) Treating severe acute malnutrition seriously. Archives of Disease in Childhood 92, 453–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S., Dent N., Binns P., Bahwere P., Sadler K. & Hallam A. (2006a) Management of severe acute malnutrition in children. Lancet 368, 1992–2000. [DOI] [PubMed] [Google Scholar]
- Collins S. & Henry C.J.K. Alternative RUTF formulations. Emergency Nutrition Network special supplement[2], 35–37. 2004. Ref Type: Serial (Book, Monograph).
- Collins S., Sadler K., Dent N., Khara T., Guerrero S., Myatt M., Saboya M. & Walsh A. (2006b) Key issues in the success of community‐based management of severe malnutrition. Food and Nutrition Bulletin 27, S49–S82. [DOI] [PubMed] [Google Scholar]
- de Pee S. & Bloem M.W. (2009) Current and potential role of specially formulated foods and food supplements for preventing malnutrition among 6‐ to 23‐month‐old children and for treating moderate malnutrition among 6‐ to 59‐month‐old children. Food and Nutrition Bulletin 30 (Suppl. 3), S434–S463. [DOI] [PubMed] [Google Scholar]
- Dibari F., Diop El H.I., Collins S. & Seal A. (2012) Low‐cost, ready‐to‐use therapeutic foods can be designed using locally available commodities with the aid of linear programming. The Journal of Nutrition 142, 955–961. [DOI] [PubMed] [Google Scholar]
- Diop E.H.I., Dossou N.I., Ndour M.M., Briend A. & Wade S. (2003) Comparison of the efficacy of a solid ready to use food and a liquid milk‐based diet for the rehabilitation of severely malnourished children: a randomized trial. American Journal of Clinical Nutrition 78, 302–307. [DOI] [PubMed] [Google Scholar]
- Fellows , P. Local Production of RUTF. Emergency Nutrition Network Special supplement[2], 33–35. 2004. Ref Type: Generic.
- Government of Malawi & Ministry of Health . Interim guidelines for the management of acute malnutrition through community‐based therapeutic care. 2007. Ref Type: Generic.
- Ha E. & Zemel M.B. (2003) Functional properties of whey, whey components, and essential amino acids: mechanisms underlying health benefits for active people (review). The Journal of Nutritional Biochemistry 14, 251–258. [DOI] [PubMed] [Google Scholar]
- Hering N.A., Andres S., Fromm A., van Tol E.A., Amasheh M., Mankertz J., Fromm M. & Schulzke J.D. (2011) Transforming growth factor‐beta, a whey protein component, strengthens the intestinal barrier by upregulating claudin‐4 in HT‐29/B6 cells. The Journal of Nutrition 141, 783–789. [DOI] [PubMed] [Google Scholar]
- Hoppe C., Andersen G.S., Jacobsen S., Molgaard C., Friis H., Sangild P.T. & Michaelsen K.F. (2008) The use of whey or skimmed milk powder in fortified blended foods for vulnerable groups. Journal of Nutrition 138, 145S–161S. [DOI] [PubMed] [Google Scholar]
- Irena A., Bahwere P., Owino V.O., Diop E.H.I., Bachmann M.O., Mbwili M., Dibari F. & Collins S. Milk‐free soy‐maize‐sorghum based ready‐to‐use therapeutic food is non‐inferior to standard RUTF with 25% milk among severely acutely malnourished Zambian children; cluster randomized trial. 2012. Ref Type: Unpublished Work. [DOI] [PMC free article] [PubMed]
- Irena A.H., Bahwere P., Owino V.O., Diop El H.I., Bachmann M.O., Mbwili‐Muleya C., Dibari F., Sadler K. & Collins S. (2013) Comparison of the effectiveness of a milk‐free soy‐maize‐sorghum based ready‐to‐use therapeutic food to standard ready‐to‐use therapeutic food with 25% milk in nutrition management of severely acutely malnourished Zambian children: an equivalence non‐blinded cluster randomized controlled trial. Maternal and Child Nutrition. doi: 10.1111/mcn.12054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahoor F., Klein S., Miyoshi H. & Wolfe R.R. (1988) Effect of isotope infusion and sampling sites on glucose kinetics during a euglycemic clamp. The American Journal of Physiology 255 (6 Pt 1), E871–E874. [DOI] [PubMed] [Google Scholar]
- Julious S.A. (2004) Sample sizes for clinical trials with normal data. Statistics in Medicine 23, 1921–1986. [DOI] [PubMed] [Google Scholar]
- Kaul S. & Diamond G.A. (2006) Good enough: a primer on the analysis and interpretation of noninferiority trials. Annals of Internal Medicine 145, 62–69. [DOI] [PubMed] [Google Scholar]
- Kerac M., Bunn J., Seal A., Thindwa M., Tomkins A., Sadler K., Bahwere P. & Collins S. (2009) Probiotics and prebiotics for severe acute malnutrition (PRONUT study): a double‐blind efficacy randomised controlled trial in Malawi. Lancet 374, 136–144. [DOI] [PubMed] [Google Scholar]
- LaGrone L.N., Trehan I., Meuli G.J., Wang R.J., Thakwalakwa C., Maleta K. & Manary M.J. (2012) A novel fortified blended flour, corn‐soy blend ‘plus‐plus’, is not inferior to lipid‐based ready‐to‐use supplementary foods for the treatment of moderate acute malnutrition in Malawian children. American Journal of Clinical Nutrition 95, 212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidus N., Minetti A., Djibo A., Guerin P.J., Hustache S., Gaboulaud V. & Grais R.F. (2009) Mortality risk among children admitted in a large‐scale nutritional program in Niger, 2006. PLoS ONE 4, e4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauritsen J.M. & Bruus M. Epidata entry version 3.02:A comprehensive tool for validated entry and documentation of data. [3.02]. 2003. The Epidata Association. Ref Type: Computer Program.
- Le Henanff A., Giraudeau B., Baron G. & Ravaud P. (2006) Quality of reporting of noninferiority and equivalence randomized trials. JAMA: the journal of the American Medical Association 295, 1147–1151. [DOI] [PubMed] [Google Scholar]
- Linneman Z., Matilsky D., Ndekha M., Manary M.J., Maleta K. & Manary M.J. (2007) A large‐scale operational study of home‐based therapy with ready‐to‐use therapeutic food in childhood malnutrition in Malawi. Maternal and Child Nutrition 3, 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhovyy B.L., Akhavan T. & Anderson G.H. (2007) Whey proteins in the regulation of food intake and satiety. Journal of the American College of Nutrition 26, 704S–712S. [DOI] [PubMed] [Google Scholar]
- Manary M.J. (2006) Local production and provision of ready‐to‐use therapeutic food (RUTF) spread for the treatment of severe childhood malnutrition. Food and Nutrition Bulletin 27 (Suppl. 3), S83–S89. [DOI] [PubMed] [Google Scholar]
- Marshall K. (2004) Therapeutic applications of whey protein. Alternative Medicine Review 9, 136–156. [PubMed] [Google Scholar]
- Michaelsen K.F., Hoppe C., Roos N., Kaestel P., Stougaard M., Lauritzen L., Molgaard C., Girma T. & Friis H. (2009) Choice of foods and ingredients for moderately malnourished children 6 months to 5 years of age. Food and Nutrition Bulletin 30 (Suppl. 3), S343–S404. [DOI] [PubMed] [Google Scholar]
- Morr C.V. & Ha E.Y. (1993) Whey protein concentrates and isolates: processing and functional properties. Critical Reviews in Food Science and Nutrition 33, 431–476. [DOI] [PubMed] [Google Scholar]
- Nicoll A. (2000) Integrated management of childhood illness in resource‐poor countries: an initiative from the World Health Organization. Transactions of the Royal Society of Tropical Medicine and Hygiene 94, 9–11. [DOI] [PubMed] [Google Scholar]
- Noyer C.M., Simon D., Borczuk A., Brandt L.J., Lee M.J. & Nehra V. (1998) A double‐blind placebo‐controlled pilot study of glutamine therapy for abnormal intestinal permeability in patients with AIDS. The American Journal of Gastroenterology 93, 972–975. [DOI] [PubMed] [Google Scholar]
- Oakley E., Reinking J., Sandige H., Trehan I., Kennedy G., Maleta K. & Manary M. (2010) A ready‐to‐use therapeutic food containing 10% milk is less effective than one with 25% milk in the treatment of severely malnourished children. Journal of Nutrition 140, 2248–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul G.L. (2009) The rationale for consuming protein blends in sports nutrition. Journal of the American College of Nutrition 28 (Suppl.), 464S–472S. [DOI] [PubMed] [Google Scholar]
- Piaggio G., Elbourne D.R., Altman D.G., Pocock S.J. & Evans S.J. (2006) Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA: the journal of the American Medical Association 295, 1152–1160. [DOI] [PubMed] [Google Scholar]
- Prendergast A. & Kelly P. (2012) Enteropathies in the developing world: neglected effects on global health. The American Journal of Tropical Medicine and Hygiene 86, 756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg I., Tilahun J., Schlossman N., Bagriansky J., Johnson Q., Webb P., Rogers B. & Masterson A.R. (2011) Nutritional enhancement of US Title II food aid products. Food and Nutrition Bulletin 32 (Suppl. 3), S134–S151. [DOI] [PubMed] [Google Scholar]
- Sadler K., Kerac M., Collins S., Khengere H. & Nesbitt A. (2008) Improving the Management of Severe Acute Malnutrition in an Area of High HIV Prevalence. Journal of Tropical Pediatrics 54, 364–369. [DOI] [PubMed] [Google Scholar]
- Smith M.I., Yatsunenko T., Manary M.J., Trehan I., Mkakosya R., Cheng J., Kau A.L., Rich S.S., Concannon P., Mychaleckyj J.C., Liu J., Houpt E., Li J.V., Holmes E., Nicholson J., Knights D., Ursell L.K., Knight R. & Gordon J.I. (2013) Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 339, 548–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solak B.B. & Akin N. (2012) Health benefits of whey protein: a review. Journal of Food Science and Engineering 2, 129–137. Available at: http://www.davidpublishing.com/davidpublishing/Upfile/4/24/2012/2012042401763045.pdf (Accessed 26 June 2013) [Google Scholar]
- StataCorp (2009) Stata Release 11. Statistical Software. StataCorp LP: College Station, TX. [Google Scholar]
- Thurstans S., Kerac M., Maleta K., Banda T. & Nesbitt A. (2008) HIV prevalence in severely malnourished children admitted to nutrition rehabilitation units in Malawi: geographical & seasonal variations a cross‐sectional study. BMC Pediatrics 8, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trehan I., Goldbach H.S., LaGrone L.N., Meuli G.J., Wang R.J., Maleta K.M. & Manary M.J. (2013) Antibiotics as part of the management of severe acute malnutrition. The New England Journal of Medicine 368, 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO, UNICEF, & SCN Joint statement on the community‐based management of severe malnutrition in children. 2007. Ref Type: Hearing.
- Yalcin A.S. (2006) Emerging therapeutic potential of whey proteins and peptides. Current Pharmaceutical Design 12, 1637–1643. [DOI] [PubMed] [Google Scholar]
- Yebyo H.G., Kendall C., Nigusse D. & Lemma W. (2013) Outpatient therapeutic feeding program outcomes and determinants in treatment of severe acute malnutrition in tigray, northern ethiopia: a retrospective cohort study. PLoS ONE 8, e65840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemel M.B. (2005) The role of dairy foods in weight management. Journal of the American College of Nutrition 24 (Suppl. 6), 537S–546S. [DOI] [PubMed] [Google Scholar]


