Abstract
Lipid‐based nutrient supplements (LNS) can help treat undernutrition; however, the dietary adequacy of children supplemented with LNS, and household utilisation patterns are not well understood. We assessed diet adequacy and the quality of complementary foods by conducting a diet assessment of 128 Ugandan children, ages 6–59 months, who participated in a 10‐week programme for children with moderate acute malnutrition (MAM, defined as weight‐for‐age z‐score < −2). Caregivers were given a weekly ration of 650 kcal day−1 (126 g day−1) of a peanut/soy LNS. Two 24‐h dietary recalls were administered per child. LNS was offered to 86% of targeted children at least once. Among non‐breastfed children, over 90% met their estimated average requirement (EAR) cut‐points for all examined nutrients. Over 90% of breastfed children met EAR cut‐points for nutrient density for most nutrients, except for zinc where 11.7% met cut‐points. A lower proportion of both breastfed and non‐breastfed children met adjusted EARs for the specific nutritional needs of MAM. Fewer than 20% of breastfed children met EAR nutrient‐density guidelines for MAM for zinc, vitamin C, vitamin A and folate. Underweight status, the presence of a father in the child's home, and higher programme attendance were all associated with greater odds of feeding LNS to targeted children. Children in this community‐based supplemental feeding programme who received a locally produced LNS exhibited substantial micronutrient deficiencies given the special dietary needs of this population. These results can help inform programme strategies to improve LNS targeting, and highlight potential nutrient inadequacies for consumers of LNS in community‐based settings.
Keywords: child malnutrition, lipid‐based nutrient supplements, diet adequacy, household allocation, sharing
Introduction
Supplemental feeding programmes can be an effective strategy to address undernutrition (Dewey & Adu‐Afarwuah 2008). Generally, these programmes seek to address the underlying and proximate factors related to undernutrition – food insecurity, chronic dietary inadequacy, poor childcare and infection – through a combination of dietary supplementation and education (Black et al. 2008). Lipid‐based nutrient supplements (LNS) are energy‐dense, lipid‐based pastes that do not require cooking or refrigeration that have increasingly been used to support supplemental feeding programmes in community‐based settings for the prevention and treatment of malnutrition (Maleta et al. 2004; Isanaka et al. 2009). While it has long been understood that supplemental food rations distributed in resource‐restricted settings are likely to be diverted to other household members, the proportion of distributed rations that are received by targeted children is largely undocumented, because of lack of consumption data in most programme reports and studies (Beaton & Ghassemi 1982; Navarro‐Colorado et al. 2008). Characterising the patterns of LNS consumption can provide important insights into tailoring dosage levels for such programmes, or in modifying programmes to maximise the nutrient delivery to intended recipients.
Previously, we have discovered that caregivers enrolled in a supplemental feeding programme in Western Uganda were challenged to feed a targeted LNS supplement to only their enrolled child, and that caregiver preparations of the LNS may impair the intended nutrient density of the supplement (Jilcott et al. 2010; Ickes et al. 2012). Further, caregivers in this remote setting found it difficult to attend weekly programme sessions because of the opportunity costs of foregone household responsibilities, as well as a long commuting distance. Caregivers reported that children enjoyed the taste of the LNS supplement and credited the supplement with helping their malnourished children to recover by gaining weight and improving their health. The goal of this paper was to extend earlier qualitative findings to quantify the proportion of supplemental food that does not reach the intended beneficiary, and to assess the dietary adequacy of children enrolled in a supplemental feeding programme based in a community with a stunting prevalence of nearly 45% (Jilcott et al. 2007; UBOS and Macro International, Inc. 2012).
To that end, this study aimed to (1) examine the nutrient contribution of the LNS to the overall diet; (2) quantify the proportion of distributed LNS that was consumed by the targeted child; (3) assess the diet quality of supplemental feeding participants; and (4) assess the socio‐demographic, behavioural, and anthropometric factors associated with LNS consumption among programme beneficiaries. These aims were addressed among caregivers and children enrolled in the Byokulia Bisemeye mu Bantu (BBB) programme in Bundibugyo, Uganda, which is Lubwisi for ‘Good food to Good People’.
Key messages
A nutrition education programme supported by a locally produced lipid‐based nutrient supplement (LNS) contributed substantially to children's overall nutrient intakes.
A large proportion of the distributed LNS ration was not offered to targeted children; of that which was offered, nearly all was consumed.
Substantial nutrient inadequacies still existed among children supplemented with LNS. These inadequacies were more pronounced for children <12 months.
The amount of LNS offered to children did not vary by child age, indicating that caregivers may require additional information about appropriate serving sizes of nutrient‐dense complementary foods for older children.
Subjects and methods
Participants and setting
Bundibugyo is one of four districts in Uganda's Western Region. At the time of the study, Bundibugyo was the only district in the region with no paved roads or electricity. With the Democratic Republic of Congo to the west and the Rwenzori mountains to the east, the district is geographically isolated from the rest of Uganda.
The Bakonjo and Babwisi are the two predominant people groups in the 290 000‐person district, which includes 52 500 (18%) children less than 5 years (UBOS and Macro International, Inc. 2012). The stunting prevalence (height‐for‐age z‐score < −2) of 43.9% is the second highest in the country, and well above the national prevalence of 33.4% (Jilcott et al. 2007; UBOS and Macro International, Inc. 2012). The total fertility rate of 6.4 is higher than the national mean of 6.2. Uganda as a whole has the highest total fertility rate in East and Southern Africa, and one of the highest birth rates worldwide (UBOS & Macro International, Inc. 2011).
Data collection
Children are regularly recruited for BBB programme enrolment at local schools and churches and at routine outpatient weighing at health centres. All caregivers who enrolled in the 10‐week programme between June 2008 and June 2009 were eligible for participation in the study (n = 138). Study personnel were present during each weekly programme session for the duration of the study. Beginning at the fourth week of each programme cycle, a random sample of the caregivers in attendance at the programme was recruited to provide 24‐h dietary recall information for their enrolled child. Caregivers who were absent from the programme were recruited at their homes using information provided in the programme register. Recalls were conducted through week 9 of the program, with the goal of complete recruitment into the study of all mothers who contributed to and participated in the programme. Verbal informed consent was obtained prior to the dietary assessment. Demographic surveys and dietary recalls were conducted in either Lubwisi or Lukonjo, the two primary local languages, depending on the preference of the caregiver. After agreeing to participate, caregivers were interviewed following the daily program, or at a later day within the same week. About 70% of recalls were administered on weekdays in order to provide a proportional assessment of weekday and weekend dietary patterns. However, child diets do not vary much according to the day of the week and are generally very monotonous. Follow‐up recalls were scheduled at the conclusion of the first recall, and were conducted on non‐consecutive days within a 10‐day period to approximate usual food consumption, and to account for within‐person variation. This method of scheduling recalls allowed us to obtain dietary information for children on most days of the week in order to provide variation in the elapsed time since LNS distribution. Three study personnel trained in diet assessment methods (S.I., B.C., K.M.) performed all dietary recall measurements.
Information collected during dietary recall included the name and time of each meal, and the ingredients and preparation method for each food item. Morbidity was assessed simply through a single question on whether the child's diet was typical diet on the day of recall. We included dietary recalls from children who were ill, but this affected only 2% (five total) recalls. Next, the portion size offered and the amount consumed by the child of interest were estimated using standard local utensils (e.g. tablespoon, 800‐mL plate, 500‐mL cup). Cups and plates were marked with fraction lines that divided the utensil into fifths to assist caregivers in estimating portion sizes. De‐identified dietary recalls and demographic surveys were scanned and entered into an electronic database for analysis.
Demographic data were collected during the first recall with each caregiver using a modified questionnaire from the Uganda Demographic and Health Survey (UBOS & Macro International, Inc. 2007). Demographic questions included caregiver parity; the number of children living at the compound; and the education, presence, occupation, and marital status of the index child's parents. Socio‐economic constructs included building materials of the home, participation in a small business, primary means of food acquisition and whether the household cultivated cash crops. Access to health services and sanitation was measured by caregiver estimates of the distance in meters to the nearest clean water source and health centre. Information on the frequency of visits to health facilities was not collected.
Trained health centre staff assessed children's anthropometric measurements. Weights were measured to the nearest 0.1 kg with a hanging Salter scale. Length was measured to the nearest 0.1 cm using a length board for children 6–24 months or who were shorter than 65 cm. Height for children >24 months or who were taller than 65 cm was measured using fixed measuring tapes with a headboard. Scales and length boards were calibrated twice monthly during the course of the programme. The University of North Carolina at Chapel Hill Institutional Review Board (Study # 08–1100) and the Bundibugyo District Health Office approved all study protocol.
Programme components of the BBB supplemental feeding programme
The BBB programme is managed by World Harvest Mission in the Bundibugyo District of Uganda and operates in two health centres and enrols 50 underweight [weight‐for‐age z‐score (WAZ) < −2] children ages 6–59 months per 10‐week cycle. Deworming medicine is offered at 5‐week intervals during the programme cycle. Anthelminthic treatment has been found to improve appetite when administered quarterly (Stoltzfus et al. 2004). As this population was well covered for de‐worming, the proximity of anthelminthic treatment to the day of dietary recalls was unlikely to influence the level of dietary intake. At each weekly visit, caregivers receive child growth monitoring and promotion, a weekly supply of LNS, children's multivitamin with iron, and nutrition education. Education is delivered by community health workers and health centre staff to emphasise: (1) the impact of early nutrition on school performance later in life; (2) healthy antenatal nutrition; (3) the importance of breastfeeding; (4) healthy complementary feeding practices; (5) using an attentive, responsive child feeding style; (6) feeding children during and after illness; and (7) hygiene practices. Caregivers receive 650 kcal day−1 of a peanut‐ and soy‐based LNS. This high dosage of LNS, particularly for children <2 years, was standard across age strata to simplify distribution logistics. If consumed without wastage or leakage, the daily LNS dose of 650 kcal day−1 would provide 63.5% of the mean total daily energy needs for children under 2 years, and 96.6% for children 1 year of age (FNB & IOM 2005).
The LNS formula is fortified with dried moringa leaf powder, but no additional micronutrients. Therefore, children were also given a weekly supply of a children's daily multivitamin with iron at each programme session. The multivitamin was a standard children's chewable vitamin with iron. Parents were instructed to feed children half a tablet per day. Multivitamins contained vitamins A, C, D, E, B6, B12, thiamin, riboflavin, niacin, folic acid, pantothenic acid, sodium and iron. Adherence to the multivitamin was not formally assessed; however, anecdotes from programme staff indicated that adherence might have been low. Further, as the goal of our study was to examine the impact of LNS‐based supplementation on dietary adequacy, the micronutrient contribution from multivitamins was not considered in our analysis.
Caregivers were instructed to feed the LNS to the enrolled child only, either directly without cooking or as a thick porridge, at each feeding episode (Jilcott et al. 2010). Although caregivers were not instructed with a specific dosage, the amount of LNS used in demonstration preparations and feedings was 2–4 Tb.
Nutrient composition of lipid‐based nutrient supplement
LNS was made locally using hand‐powered grinders to prepare two products: (1) roasted groundnut (peanut) paste fortified with dried Moringa oleifera leaf powder (440 g); and (2) roasted soy flour (440 g). Each product was distributed in a plastic bag and was mixed by caregivers into a single bag at the conclusion of each programme session. The resulting product resembles thick peanut butter. To examine the composition of the LNS, three separate samples were analysed at the Department of Food Science and Technology, Makerere University, Uganda in June 2008, for moisture content (oven method), proximate composition (crude fat, crude protein, dietary fiber and ash; AOAC, 1999 method), energy (bomb calorimeter method), vitamin C and A (AOAC 1999 method) and aflatoxin content (VICAM fluorometer method) (Jilcott et al. 2010). The product was found to be free from aflatoxin. The 128 g day−1 ration provides 100% of the estimated average requirement (EAR) for vitamin A, 45% for vitamin C, 120% for folate, 34% for calcium, and 150% for zinc, for children 12–36 months (FNB & IOM 1997, 1998a, 1998b, 2000a, 2000b, 2005). Per 100 g, the LNS product contained 532 kcal. Table 1 summarises the nutrient composition of the LNS product.
Table 1.
Nutrient composition of the lipid‐based nutrient supplement, per 100 g
| Nutrient | Amount |
|---|---|
| Fat | 39.6 g |
| Protein | 27.9 g |
| Fiber | 11.8 g |
| Vitamin C | 49.8 mg |
| Vitamin A | 176 IU |
| Vitamin B6 | 0.43 g |
| Folate | 124.5 g |
| Calcium | 142.8 g |
| Iron | 4.3 g |
| Zinc | 3.2 g |
Assessment of dietary diversity and infant and young child feeding (IYCF) practices
World Health Organization (WHO) Indicators for the assessment of IYCF were used to measure whether children ages 6–24 months were fed a ‘minimum acceptable diet’. Breastfed children met this criterion if they were fed four or more food groups and the minimum number of times per day (≥2 for children 6–8 months, ≥3 for children ages 9–23 months). Non‐breastfed children met this criterion if they were fed: (1) four or more food groups (apart from milk); (2) milk or milk‐based products two or more times daily; and (3) four or more times per day (WHO 2008). Breastfeeding status was assessed through self‐report. As the focus of the study was to determine nutrient adequacy of children during supplementation with LNS, the rationale for not breastfeeding was not explored in the present study. A food group was counted if a child consumed at least 1 g from any of the following seven groups: (1) grains, roots, and tubers, including porridge or fortified baby food from grains, including matoke/plantains; (2) legumes and nuts; (3) dairy products, including milk other than breast milk, cheese, yogurt, or other milk products; (4) flesh foods such as meat, poultry, fish, shellfish, organ meats; (5) eggs; (6) vitamin A‐rich fruits or vegetables; and (7) other fruits and vegetables. To draw comparisons among age groups, a dietary diversity score (DDS) was calculated as the sum of foods consumed within these seven food groups, resulting in a 0 = low diversity; 7 = high diversity. The DDS was identical to that used in the WHO IYCF Indicator assessment (WHO 2008). Scores were based on seven food groups and thus range from 0 to 7. These scores are similar, but not identical to FAO DDSs, which range from 0 to 12 for households and 0 to 9 for women (FAO & WHO 2002; WHO 2008).
Assessment of dietary adequacy
Nutrient values were represented as the mean from the two 24‐h recalls, obtained on non‐consecutive days. Food items were assigned nutrient values from the Tanzania Food Consumption Table (FCT) (Lukmanji et al. 2008), Malawi FCT (Ferguson et al. 2004) and United States Department of Agriculture (USDA) Nutrient Database (USDA Agricultural Research Service 2009). Portion weights were estimated by multiplying the estimated portion size by the unit weight in grams (obtained from field measurements taken in February 2009, or from the USDA or Tanzania FCT, when available). Total daily nutrient intakes were calculated for selected nutrients that are of particular interest to child growth: energy, protein, vitamin C, vitamin A, vitamin B6, zinc, iron and folate. We conducted two dietary recalls on non‐consecutive days to approximate usual intake of children. We calculated correlation coefficients between the two daily nutrient values. The correlation for macronutrients was moderate for fat (0.50), total energy (0.33) and total protein (0.29). The correlation for micronutrients was high for iron (0.74), moderate for folate (0.41) and low for vitamin C (0.26). Vitamin A (0.24) and calcium (0.24) intakes were least correlated among observations. To estimate the nutrient contribution of LNS to the overall diet, the mean amount of selected nutrients consumed from LNS was divided by the mean total amount consumed of that nutrient over 2 days of recall. The correlation coefficient between total energy intake from LNS on both days of recall was 0.56. While this correlation indicates variation between the amount of LNS consumed over 2 days, the goal of our study was to estimate the average contribution that LNS made to children's overall diets, recognising that intake will vary on a daily basis for a variety of factors. Calculating the mean LNS consumption from non‐consecutive days in a community‐based setting allows us to minimise extreme consumption values that may result from an early depletion of food rations.
Dietary adequacy was assessed separately for non‐breastfed and breastfed children, because the breast milk intake for breastfed children in our study population was unknown. For non‐breastfed children, the probability of adequate intake (AI) for each selected nutrient was calculated using the cut‐point method, which compared each child's nutrient consumption with his or her age‐specific EAR or AI, in the case of calcium (FNB & IOM 2000b; Murphy et al. 2006). Dietary reference intake and AI values were obtained from the Food and Nutrition Board and Institute of Medicine guidelines (FNB & IOM 1997, 1998a, 1998b, 2000a, 2000b, 2005). Absorbed calcium was estimated by multiplying the total grams of calcium consumed by an absorption factor of 0.32 for all food groups. Iron absorption was calculated using absorption factors of 0.06 and 0.11 from plant and animal sources, respectively (Working Group on Infant and Young Child Feeding Indicators 2006). For breastfed children whose breast milk intake amount was unknown, we assessed dietary adequacy by examining the nutrient density of complementary foods. Nutrient densities per 100 kcal were calculated as follows:
The nutrient density of complementary foods was compared against the age‐specific nutrient densities guidelines that are specified by the WHO standards, including the specific requirements of children with moderate acute malnutrition (MAM) (FAO & WHO 2002; Dewey & Brown 2003; Golden 2009).
Statistical analysis
All data analysis was conducted on Stata 12.0 (Stata Corp, College Station, TX, USA). Potential confounders in the regression models were selected based on findings from an earlier qualitative study (Ickes et al. 2012) and theoretical knowledge of factors that influence children's diets in developing countries (Black et al. 2008). Regression coefficients were considered statistically significant if the 95% confidence interval did not overlap with the null. Comparisons of mean nutrient intakes across age strata were made using a one‐way analysis of variance test. Differences were considered statistically significant if P < 0.05.
Modelling dietary LNS consumption
A multivariate regression model was created to estimate the association of selected socio‐demographic factors with LNS consumption. Because of the substantial proportion of children who did not consume any LNS over the days of observation (14.1%), two separate regression models were used. First, we applied logistic regression to examine the factors associated with no LNS consumption by comparing non‐consumers with consumers among children who consumed a minimum of 1 g of LNS on either day. Second, we applied linear regression to examine the factors associated with the level of LNS consumption (coded continuously), conditional on consuming at least 1 g of LNS in either study day. As the factors associated with no daily consumption may differ markedly from the factors associated with varying levels of LNS consumption, we tested factors separately in each scenario. Both regression models controlled for age, gender, nutritional status (by WAZ score), presence of the father in the home, maternal education and primary means of food acquisition.
Results
Study population
Of the 180 children who participated in the programme between June 2008 and June 2009, we recruited 128 child–caregiver dyads into the study. Over 87% of children were stunted and 24% were wasted. Over 95% of maternal caregivers had received less than a primary school education in their lifetimes. The mean ± standard error (SE) number of children at caregiver compounds (the house or connected houses where caregivers and their dependent child reside) was 7.9 ± 0.4 (Table 2). Over half (55.4%) of mothers were in monogamous marriages.
Table 2.
Demographic characteristic of study participants (n = 128)*
| Number (%) | |
|---|---|
| Child age in months at dietary assessment (mean ± SE), months | 17.8 (0.9) |
| 6 to <9 | 14 (10.9%) |
| 9 to <12 | 28 (21.9%) |
| 12 to <24 | 66 (51.6%) |
| 24 to <37 | 11 (8.6%) |
| 37 to <60 | 9 (7.0%) |
| Percentage still breastfed | 84 (62.2%) |
| Child anthropometry† | |
| Percentage stunted (height‐for‐age z‐score < −2) | 112 (87.5%) |
| Percentage underweight (weight‐for‐age z‐score < −2) | 103 (80.5%) |
| Percentage wasted weight‐for‐length z‐score < −2) | 31 (24.2%) |
| Percentage male | 73 (57.0%) |
| Percentage with living father | 122 (95.3%) |
| Percentage with father present in the home | 75 (58.6%) |
| Number (%) | |
|---|---|
| Highest paternal education | 5.70 (0.33) |
| None | 22 (17.2%) |
| Some primary | 71 (55.5%) |
| Secondary or higher | 34 (26.6%) |
| Percentage with living mother | 125 (97.7%) |
| Percentage with mother or grandmother present in the home | 127 (99.2%) |
| Highest maternal education, mean years (SE) | 2.8 (0.24) |
| None | 52 (40.6%) |
| Some primary | 71 (55.5%) |
| Secondary or higher | 5 (3.9%) |
| Building materials of home | |
| Percentage with tin roof | 94 (73.4%) |
| Percentage with mud walls, no cement or bricks | 125 (97.7%) |
| Number of birth children, mean (SE) | 4.2 (0.21) |
| One | 12 (9.4%) |
| Two to four | 66 (51.6%) |
| Five or more | 50 (39.1%) |
| Number of children at compound, mean (SE) | 7.9 (0.42) |
| Percentage > 500 m from water source | 56 (43.75%) |
| Percentage > 3000 m from health facility | 59 (46.1%) |
| Caregiver marital status (%) | |
| Married, monogamous | 71 (55.4%) |
| Separated/divorced/widowed/polygamous | 57 (44.6%) |
| Primary means of food acquisition (%) | |
| Garden only | 6 (4.7%) |
| Garden and some market purchase | 51 (39.8%) |
| Garden and market transfers | 64 (50.0%) |
| Percentage growing cash crops | 122 (95.3%) |
*Unless otherwise noted, reported values are Number (%). †Anthropometric indices were calculated using the new World Health Organization (WHO) growth standards (WHO Multicentre Growth Reference Study Group, 2006).
LNS consumption patterns
A high proportion of children (111 of 128, or 86.7%) consumed LNS on at least one of the observation days, while 75.8% of children (n = 97 of 128) consumed LNS on both observation days. Figure 1 shows distribution of the mean amount of LNS offered to children, based on the average of two non‐consecutive dietary recalls. The mean ± SE amount offered to index children was 41.5 ± 2.4 g day−1 (226.2 ± 13.0 kcal),
Figure 1.

Mean grams of lipid‐based nutrient supplement (LNS) consumed by targeted children. Caregivers of targeted children were given 128 g day−1 of LNS. Values are mean portion sizes offered to children over 2 days of recall. Percentage refers to the percentage of children in the study sample (n = 128).
Caregivers offered LNS 1.3 ± 0.07 (mean ± SE) times per day, and LNS was included in 45.7% ± 2.8 (mean ± SE) of index children's total meals. Of the 650 kcal day−1 ration, 34.7% was offered to targeted children. This amount did not vary substantially according to children's age: infants 6–8 months were offered 239.3 ± 37.6 kcal day−1, while children 36–59 months were offered 233.2 kcal day−1. The amount of LNS offered to children also did not vary by the degree of underweight: pair wise comparisons according to WAZ score category with WAZ < −3 as the referent indicated no significant differences (results not shown). Of children offered any LNS (n = 98), 90.7% ± 1.0 (mean ± SE) of the amount offered to the child was consumed, according to parental report.
Figure 2 shows that the contribution of the LNS to the children's total energy intakes (including children who did not consume LNS) declined with increasing child age, ranging from 29.5% of total calories consumed by infants (6–8 months) to 15.7% of total calories consumed among toddlers (36–59 months). The percentage of total protein calories from LNS varied similarly across age strata. As estimated over 2 days of dietary recall, LNS contributed 55% of total calories from protein to children's diets among children 6–8 months, and 28.7% of total protein energy among children 36–59 months. Similar trends across age strata were observed for each of the selected micronutrients.
Figure 2.
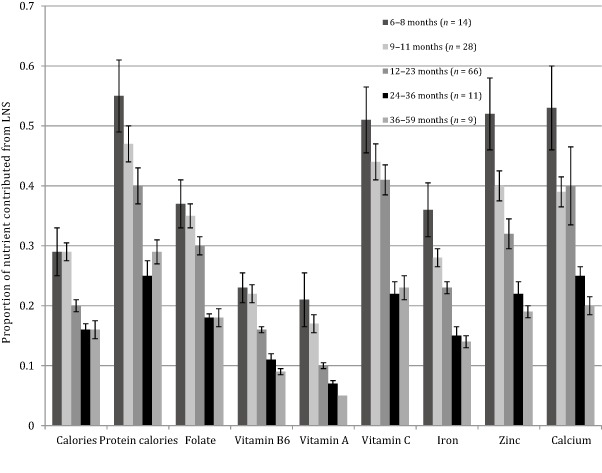
Contribution of lipid‐based nutrient supplement (LNS) to overall child diet for selected nutrients, by age group (n = 128). Nutrient contribution of LNS calculated as the mean nutrient amount from LNS consumed divided by the mean total amount of the nutrient consumed from all complementary foods over 2 days of dietary recall.
IYCF practices during supplementation
Feeding frequency
The minimum number of daily feeding occasions was met by 64% of children in the study sample (82 of 128). The probability of meeting the requirement for the minimal number of daily meals varied significantly by age: 70.3% of children 6–24 months met their age‐specific feeding frequency requirement, compared with only 34.6% of children over 24 months (P = 0.007).
Dietary diversity
Over 70% (93 of 128, 72.6%) of children were fed four or more food groups per day, based on the mean DDS over 2 days of recall. The mean ± SE DDS was 3.7 (0.81). DDS increased with age. DDS means ± SE within each age strata were: 3.3 ± 0.80 for children 6–8 months, 3.4 ± 0.88 for children 9–11 months, 3.8 ± 0.75 for children 12–23 months, 3.9 ± 0.72 for children 24–36 months and 4.3 ± 0.5 for children 36–59 months. Dietary diversity did not vary according to children's underweight status, nor based on whether caregivers offered LNS to the index child. The mean DDS ± SE was 3.6 (0.96) for children with WAZ > −2, and 3.7 (0.76) for children with WAZ < −2 (P = 0.684). The mean DDS ± SE was 3.82 (0.76) for non‐LNS consumers, and 3.7 (0.82) for LNS consumers (P = 0.387).
Minimally acceptable diet
Among children ages 6–24 months, 52 of 108 (48.2%) were fed a ‘minimally acceptable diet’ on at least 1 day of recall; all of the children who met the criteria on 1 day of recall also met the indicator requirements on the second day of recall. Seventy‐six of 108 (70.3%) of children were fed the minimum number of times per day, based on their age and breastfeeding status. Among children under 12 months, 40 of 52 (76.9%) were still breastfeeding. Among all children under 24 months who were not breastfed (31 of 108, 28.7%), none were fed milk or milk products.
Dietary adequacy during supplementation
Table 3 shows the dietary adequacy of non‐breastfed children (n = 51) during supplementation with LNS, according to the percentage of children who achieved age‐specific EARs. Nearly all (98%) of the non‐breastfed children met their EAR for protein, and 72.5% of non‐breastfed children met the elevated EAR protein cut‐point for MAM. More than 90% of non‐breastfed children met their EAR for folate, vitamin B6, vitamin A, vitamin C, and iron, and zinc. When assessing adequacy with MAM cut‐points, the estimated adequacy was lower: 72.5% of non‐breastfed children met protein cut‐points, and more than half met requirements for folate, vitamin B6, vitamin A, vitamin C and iron. Very few non‐breastfed children (2.0%) met their EAR for zinc. Mean calcium intakes increased slightly with age, with mean intake values well below the AI for healthy and moderately malnourished populations. Estimations of adequacy stratified by age indicated that children ages 6 to <12 months were more likely than older children to meet their EAR for key nutrients when assessed using the adjusted values for moderate malnutrition (Table 3).
Table 3.
Percentage of non‐breastfed children, ages 6–59 months, achieving age‐specific EAR while receiving the lipid‐based nutrient supplement (n = 51)* , †
| Nutrient | All non‐breastfed children – general EAR (n = 51) (%) | All non‐breastfed children – MAM EAR (n = 51) (%) | 6–11 months general EAR (n = 9) (%) | 6–11 months MAM EAR (n = 9) (%) | 12–23 months general EAR (n = 22) (%) | 12–23 months MAM EAR (n = 22) (%) | 24–59 months general EAR (n = 20) (%) | 24–59 months MAM EAR (n = 20) (%) |
|---|---|---|---|---|---|---|---|---|
| Protein | 98.0 | 72.5 | 100.0 | 100 | 95.5 | 63.6 | 100.0 | 70.0 |
| Folate | 98.0 | 56.9 | 100.0 | 88.9 | 95.5 | 54.6 | 100.0 | 45.0 |
| Vitamin B6 | 100.0 | 90.2 | 100.0 | 88.9 | 100.0 | 86.4 | 100.0 | 95.0 |
| Vitamin A | 94.1 | 56.9 | 77.8 | 66.7 | 95.5 | 59.1 | 100.0 | 50.0 |
| Vitamin C | 96.1 | 52.9 | 77.8 | 77.8 | 100.0 | 50.0 | 100.0 | 45.0 |
| Iron | 94.1 | 62.8 | 77.8 | 77.8 | 95.5 | 59.1 | 100.0 | 60.0 |
| Zinc | 92.2 | 2.0 | 77.8 | 0.0 | 90.9 | 4.6 | 100.0 | 0.0 |
| Calcium ‡ | 159.51 (17.8) mg | 137.63 (74.2) | 164.3 (25.1) | 164.2 (34.9) | ||||
EAR, estimated average requirement; MAM, moderate acute malnutrition. *EARs obtained from the FNB &IOM (1997, 1998a, 1998b, 2000a, 2000b, 2002, 2005). EAR recommendations for children with moderate acute malnutrition obtained from Golden (2009). Proposed recommended nutrient densities for moderately malnourished children. Food and Nutrition Board (30)S267‐S342. †Nutrient intakes calculated as the mean of two 24‐h recalls taken on non‐consecutive days. ‡For calcium, the mean (standard deviation) intake is presented as the adequate intake value would overestimate the prevalence of inadequacy in the sample. The adequate intake recommendations for healthy populations are 400 mg for 6–11‐month‐olds; 500 for 12–23 months and 600 mg for 24–59 months. The adequate intake recommendations for food‐based consumption of calcium in moderately malnourished populations are 400 for 6–11‐month‐olds; 570 for 12–23 months and 740 mg for 24–59 months (Golden 2009).
Table 4 shows the dietary adequacy of breastfed children ages 6–24 months (n = 77), assessed as the proportion of children who achieved age‐specific nutrient density EAR guidelines while receiving the LNS supplement. Apart from zinc (11.7%), adequacy estimates were high using nutrient density guidelines for healthy children. Estimates of adequacy were considerably lower when specific requirements for MAM were applied. While over half (63.6%) of children met MAM‐specific nutrient density EAR for protein, only 10.4% exceeded their cut‐point for vitamin A, 3.9% for folate, 15.6% for vitamin C and 2.6% for zinc. Mean (SE) calcium intake for breastfed children was 141.8 mg (16.6), which is less than the AI for healthy and moderately malnourished children in this age group, which ranges from 400 to 570 mg (Table 4).
Table 4.
Percentage of breastfed children, ages 6–24 months, achieving age‐specific nutrient density guidelines while receiving the lipid‐based nutrient supplement
| Nutrient | Percent of breastfed children achieving general nutrient density requirements (n = 77) | Percent of breastfed children achieving nutrient density requirements for moderate acute malnutrition (n = 77) |
|---|---|---|
| Protein | 98.7% | 63.6% |
| Folate | 100.0% | 3.9% |
| Vitamin B6 | 100.0% | 100% |
| Vitamin A | 77.9% | 10.4% |
| Vitamin C | 98.7% | 15.6% |
| Iron | 100% | 100% |
| Zinc | 11.7% | 2.6% |
| Calcium | 263.1 (191.2) mg | – |
Nutrient density recommendations obtained from Food and Agricultural Organization/World Health Organization Joint Expert Consultation. Vitamin and Mineral requirements in human nutrition. Geneva (FAO & WHO 2002). Nutrient density recommendations for children with moderate acute malnutrition obtained from Golden (2009). Proposed recommended nutrient densities for moderately malnourished children. Food and Nutrition Board (30)S267‐S342. Because nutrient density recommendations are not available for breastfed children >24 months, the seven breastfed children >24 months in the full study sample were excluded from nutrient density analysis. Dietary reference intakes obtained from the FNB & IOM (1997, 1998a, 1998b, 2000a, 2000b, 2002, 2005). Nutrient intakes calculated as the mean of two 24‐h recalls taken on non‐consecutive days. For calcium, the mean (standard deviation) intake is presented as the adequate intake value would overestimate the prevalence of inadequacy in the sample. The adequate intake recommendations for healthy populations are 400 mg for 6–11‐month‐olds; 500 for 12–23 months. The adequate intake recommendations for food‐based consumption of calcium in moderately malnourished populations are 400 for 6–11‐month‐olds and 570 for 12–23 months (Golden 2009).
Demographic predictors of LNS consumption
Three factors were found to be associated with whether a child was fed any amount of LNS. Children who were underweight were 5.4 times as likely to be fed any LNS over the 2 days of recall compared with children who were not underweight [odds ratio (OR) (95%) = 5.42 (1.55, 18.89), P = 0.008]. The presence of the father in the home marginally increased the odds of feeding any LNS to intended recipients [OR (95%) = 3.86 (1.01, 14.65), P = 0.049]. Programme attendance was also associated with a greater likelihood of feeding LNS to children [OR (95%) = 1.54 (1.11, 2.16), P = 0.009]. The logistic regression model simultaneously included age, gender, WAZ score and presence of the father in the home, maternal education and primary means of food acquisition as predictors of feeding LNS (Table 5). Among caregivers who fed any amount of LNS, no demographic variables were significantly related to the amount of LNS offered to children (results not shown).
Table 5.
Results of logistic regression analysis of factors associated with feeding any LNS to targeted child*
| Demographic, behavioral factor | Variable type | OR | 95% CI | SE | z | P > |z| |
|---|---|---|---|---|---|---|
| Number of biological children | Number, continuous | 0.91 | 0.72, 1.15 | 0.11 | −0.82 | 0.454 |
| Number of weeks attended program | Weeks, continuous | 1.54 | 1.11, 2.16 | 0.26 | 2.60 | 0.013 † |
| Targeted child age | Months, continuous | 1.04 | 0.96, 1.12 | 0.04 | 1.02 | 0.367 |
| Targeted child gender (female is referent) | Binary | 0.62 | 0.19, 2.05 | 0.38 | −0.78 | 0.397 |
| Weight‐for‐age z‐score (normal weight is referent) | Coded, 4 category | 5.42 | 1.56, 18.9 | 3.45 | 2.66 | 0.007 † |
| Presence of father in home | Binary | 3.86 | 1.01, 14.65 | 2.62 | 1.99 | 0.049 † |
| Maternal education | Years, continuous | 0.99 | 0.79, 1.24 | 0.12 | −0.06 | 0.943 |
| Primary means of food acquisition ‡ | Coded, 3 category | 0.60 | 0.22, 1.67 | 0.33 | −0.98 | 0.416 |
CI, confidence interval; LNS, lipid‐based nutrient supplement; OR, odds ratio; SE, standard error. *Feeding any LNS coded as ‘0’ for no LNS fed to child, or ‘1’ if any LNS was fed to child over two days of diet recall. †Variable was significantly associated with whether LNS was fed to child, P < 0.05. ‡Primary means of food acquisition was coded as (1) acquire food only from personal garden (5%); (2) acquire food from purchases and self‐produced from garden (42%), and (3) acquire solely through purchase (53%).
Discussion
The purposes of this study were to (1) examine child feeding patterns with LNS; (2) assess the contribution of LNS to children's overall diets; (3) determine the nutrient adequacy of children's diets while receiving LNS during an ongoing community‐based supplemental feeding programme; and (4) explore whether socio‐demographic factors influenced the likelihood of feeding LNS to index children.
Results from this study indicate that LNS was offered in some quantity to most of the study participants; however, a large amount intended LNS dose did not reach targeted children. Of the portion of LNS that was offered to the enrolled children, nearly all was consumed. While the LNS made considerable contributions to children's overall energy, protein and micronutrient intakes, substantial nutrient inadequacies were present among supplemented children. Given our exclusion of the multivitamin contribution to children's diets, we may have overestimated the level of inadequacy for iron and some other problem nutrients. As programme staff reports indicate that the distribution and adherence of the multivitamin were erratic and low, we believe this overestimate of inadequacy is at most modest. Moreover, given that our study seeks to understand how adequate children's diets can be during supplementation with a locally produced LNS, the contribution of the multivitamin is theoretically unimportant for the study, especially if programmes seek to provide only LNS to beneficiaries.
To our knowledge, this is the first study to examine LNS consumption patterns and the dietary adequacy of programme beneficiaries in an ongoing community‐based programme. A similar proportion of the distributed LNS rations was given to targeted children in a Malawi‐based experimental growth trial: Maleta et al. reported that underweight and stunted children ages 42–60 months who were provided with 543 kcal day−1 of peanut, oil, sugar and milk‐powder, or of 531 kcal day−1 of maize and soy‐flour blend, received only 30% of the supplementary LNS, and 43% of the maize and soy flour blend provided (Maleta et al. 2004). The authors suggest that diversion of supplies within households was likely, despite the provision of similar rations to all young malnourished children in the household. Flax et al. further examined the diversion of supplies within households in their 2010 study, which compared feeding patterns of underweight children in Malawi using LNS and corn–soy blend. Their findings showed that while 98% of LNS offered to participating children was consumed, there were leftover portions of the supplement in 21% of feeding episodes. Most often, leftovers were eaten by the participating child's siblings or cousins. Presence of leftovers was negatively associated with change in WAZ (Flax et al. 2010).
A Malawi‐based study examined the post‐supplemental effects of LNS on stunting, wasting and diet adequacy of the beneficiaries, HIV− children born to HIV+ mothers. The study compared nutrition status of 15–18–month‐old children who had been given LNS as a substitute to breast milk from 28 to 48 weeks with matched community children. While LNS supplementation protected against malnutrition, children still expressed a high prevalence of inadequacy for total energy, fat and several micronutrients important for child growth after supplementation was discontinued (Parker et al. 2013). Another study by the same group indicated that while families place a high value on complementary foods, the quality of these foods is often low in energy and nutrient density (Parker et al. 2011). Their findings suggest that LNS appears to provide some benefit to children's complementary food intake, but does not solve all nutrient gaps nor provide adequate diets post supplementation.
The BBB community nutrition programme provides the same LNS ration to all enrolled children, irrespective of age, in order to simplify programme administration logistics. However, the fact that identical amounts of LNS were given to all children allowed us to explore the contribution of LNS to children's diets, given a constant amount of LNS distributed to children. We found that while total energy consumption increased with age, the amount of LNS consumed by children did not vary across age groups. This finding suggests that a culturally driven ceiling may exist, which may limit the amount of high‐quality, protein‐rich complementary foods given to children. In the study context, caregivers may prioritise feeding special foods to infants and children less than 24 months. It is also possible that caregivers with older underweight children are likely to have additional young children to feed, which may reduce the likelihood of feeding a larger dose to the index child. In either case, this finding underscores the importance of providing nutrition education that emphasises the changing dietary needs of children as they grow and age, including the type of foods and nutrients needed, and the need to increase the frequency of child meals.
To our knowledge, this is the first study in Uganda that has observed a failure of caregivers to provide increased amounts of energy‐dense complementary foods as children age. The potential ceiling effect observed in our study has been observed in at least one other context. A recent randomised trial based in Honduras examined the impact of LNS supplementation over 1 year on micronutrient status in a 6–18‐month‐old population (Siega‐Riz et al. 2014). The intervention provided food vouchers at the household level given the high level of food insecurity in the region in order to offset sharing of LNS with other children in the intervention families. Caretakers were counselled to feed different amounts of LNS depending on child age. The authors found low adherence to LNS treatment and no increase in the amount of LNS given to children ageing over the course of intervention. The mean LNS intake dropped by 23.7 g per day between 9 and 12 months of supplementation. Among children who met the protocol for adherence, the mean daily consumption was between 82 g (12 months) and 105 g (9 months). Consumption amounts among a subsample of children who consumed any Plumpy'doz was between 34 and 50 g over the course of 12‐month study, which was similar to the mean consumption of 42 in our study sample. A second study based in rural Zimbabwe found that tailored education messages were useful in improving the underlying child diet independent of supplementation with LNS (Paul et al. 2012). Together, these studies underscore the importance of tailoring complementary feeding education and recognising the nutrition needs of other household members in food‐insecure populations.
The failure of most children in the study sample to meet the AI levels for calcium highlights this as a key problem nutrient in this region of Uganda, where dairy cattle are sparse and animal milk is not fed to infants and young children. Processed complementary food products that do not contain milk powder do little to address the calcium deficiency in this population. The impact of calcium supplementation, alone or in combination with phosphorous, among children in developing countries with insufficient calcium consumption has been shown to have no impact on improvements in weight and length gain (Prentice & Bates 1994). Therefore, strategies to address low calcium intake should consider food‐based supplementation approaches. It may be particularly important to support the diets of young children who are not breastfed with milk‐based or calcium‐fortified complementary foods as this subgroup was especially vulnerable to inadequate calcium intake. In our sample, non‐breastfed children were unable to meet criteria for a ‘minimally acceptable diet’ as none of these children in the study sample were fed milk or milk‐based products – a specific requirement for this indictor of diet quality (WHO 2008).
By linking demographic, programme attendance and nutrient intake data, our study design enabled us to examine how caregiver characteristics influenced whether caregivers successfully fed a food supplement to a targeted child. Findings from our previous qualitative work were supported in the present study: caregivers with more children were less likely to feed LNS to the targeted child (Ickes et al. 2012). In addition, better programme attendance was associated with a greater likelihood of feeding LNS to the targeted children. Caregivers who attend the programme more regularly were obviously more likely to obtain the supplemental food. However, this finding may also suggest the role of education in providing clear messages for how supplemental food products should be used. In either case, addressing factors that influence programme attendance is a critical hurdle in the last mile of maximising intervention coverage and scale‐up. Appreciation should be given to the opportunity cost of attending nutrition programmes that is born by caregivers who carry demanding childcare and domestic responsibilities. Interesting, the presence of a father in the home, and the current nutrition status of a child was associated with whether LNS was offered to targeted children. These findings support recent efforts to engage fathers in child nutrition programming, and indicate that caregiver perception of children's nutritional status may influence feeding practices with specialised supplementary foods.
Limitations
Our study applies the EAR cut‐point method, which can sometimes overestimate the prevalence of inadequacy in some circumstances compared with the probability of adequacy method. However, the cut‐point method provides a good approximation of the probability of adequacy approach when intakes are (1) accurately measured; (2) the actual prevalence of inadequacy is neither very low nor very high; (3) estimated intakes for individuals are independent of each individual's requirement; (4) the distribution of requirements is approximately symmetrical; and (5) variability in intakes among individuals in the group is greater than the variability in requirements of the individuals (FNB & IOM 2000a). Our study population meets these criteria. Empirical evidence suggests that the expected bias from using the EAR cut‐point method is likely to be low as long as the correlation between intakes and requirements is moderate – no larger than 0.25 or 0.30 (FNB & IOM 2000a). Correlation between individual requirements and estimated usual intakes was low for our study, ranging from 0.003 for vitamin C to 0.211 for total energy.
The logistical challenge of reaching caregiver homes led us to conduct the majority (65%) of recalls at health centres during the programme day, when most of the enrolled caregivers were present at one location. The recalls conducted at the health centres assessed the child's food consumption on the day immediately prior to the programme, and may represent a worst‐case scenario of LNS consumption, given that the rations may have been depleted earlier in the week. Note was made on data forms if LNS rations had been exhausted earlier in the week, which occurred in only one instance. Morbidity of children was only crudely measured in the dietary recall process, and some recalls were conducted among ill children. However, only five recalls of the 270 recalls indicates that children were ill on the day of recall, so this limitation most likely did not substantially influence the estimate of normal feeding practices.
Two dietary recalls collected on non‐consecutive days improves the ability to account for within‐person variation in food consumption (Kennedy et al. 2007). However, we were limited by inherent weaknesses of dietary adequacy assessment reported elsewhere: imperfect estimates of portion quantities, inability to account for the inherent nutrient variation within foods and uncertainty about the actual shape of nutrient requirement distributions used to estimate the probability of adequacy (Daniels et al. 2009). The error in reporting amounts of LNS in recipes may have varied according to the method of preparation in such a way that the ability to recall the amount of specific ingredients may decline for recipes with more ingredients.
Social desirability bias may have misrepresented a child's dietary quality and the amount of LNS consumption. However, the moderate proportion (14%) of caregivers who did not feed any LNS to children on either day of recall suggests that such bias may have been minimal, as not feeding LNS is contradictory to the BBB programme messages.
Our study found no demographic variables that were significantly associated with the amount of LNS offered to children among caregivers who offered any amount of LNS to children. This finding may have been limited by the relatively small degree of variation in the mean amount of LNS offered over 2 days of recall (Fig. 1). Because dietary consumption data was not collected for other members of the household, it is unknown who consumed the remaining portion of LNS.
Conclusions
While LNS is well‐received by caregivers and offered to the majority of underweight children enrolled in an ongoing supplemental feeding programme, a large proportion of the distributed LNS was not offered to targeted children. Several important results regarding LNS supplementation were found in this population. First, LNS contributed substantially to nutrient intakes and may have helped caregivers achieve indicators of healthy complementary feeding, but perhaps not as much as it should have given the relatively large distributed ration. Second, older children were less likely to meet their EARs than children under 1 year of age. Finally, the LNS dosage offered to children did not vary by child age. Thus, while the nutrient requirements for older children are obviously higher, the failure to increase the amount of LNS to older children may have resulted in a greater probability of dietary inadequacy in these children. These findings support recent efforts to engage fathers in child nutrition programming, and indicate that caregiver perception of children's nutritional status may influence feeding practices with specialised supplementary foods.
It is clear that a 650‐kcal day−1 ration of supplemental LNS does not solve the problem of household food insecurity, particularly in large households. Education messages to accompany LNS distribution may help improve LNS consumption by targeted children. Understanding caregiver LNS feeding patterns and addressing issues that may prevent rations from getting to targeted children may improve LNS programmes in regions of moderately high food insecurity.
Sources of funding
This work was supported by a GlaxoSmithKline Duke‐UNC Global Health Research Award (Research Triangle Park, North Carolina), a Carolina Center for Public Service Entrepreneurship in Public Service Award (Chapel Hill, North Carolina), and a Smith Research Award and Off‐Campus Dissertation Fellowship from the University of North Carolina at Chapel Hill (Chapel Hill, North Carolina).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
SI, MB, LA, AA, JM and HT, designed the research; SI and BC conducted the research; SI and CB analysed data; SI wrote the paper and had primary responsibility for final content; BC, JA, MB, LA, AA, and HT, provided critical insight into the content of the paper. All authors read and approved the final paper.
Acknowledgements
The authors thank Linda Chamiec‐Case, Jane Ickes, and Scott Myhre for management of the study data. We thank Katie Morris for assistance in data collection. We thank the two anonymous reviewers who provided helpful feedback to an earlier version of this manuscript. Finally, we thank the mothers who participated in this study.
Ickes, S. B. , Adair, L. S. , Brahe, C. A. , Thirumurthy, H. , Charles, B. , Myhre, J. A. , Bentley, M. E. , and Ammerman, A. S. (2015) Impact of lipid‐based nutrient supplementation (LNS) on children's diet adequacy in Western Uganda. Matern Child Nutr, 11: 163–178. doi: 10.1111/mcn.12164.
References
- AOAC (1999) Official Methods of Analysis. 16 Ed. 5th Rev. Method 967.21. Gaithersburg, Md.
- Beaton G.H. & Ghassemi H. (1982) Supplementary feeding programs for young children in developing countries. The American Journal of Clinical Nutrition 35, 863–916. [DOI] [PubMed] [Google Scholar]
- Black R.E., Allen L.H., Bhutta Z.A., Caulfied L.E., de Onis M., Ezzati M. et al (2008) Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371 (9608), 243–260. [DOI] [PubMed] [Google Scholar]
- Daniels M.C., Adair L.S., Popkin B.M. & Truong Y.K. (2009) Dietary diversity scores can be improved through the use of portion requirements: an analysis in young Filipino children. European Journal of Clinical Nutrition 63, 199–208. [DOI] [PubMed] [Google Scholar]
- Dewey K.G. & Adu‐Afarwuah S. (2008) Systematic review of the efficacy and effectiveness of complementary feeding interventions in developing countries. Maternal and Child Nutrition 4, 24–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewey K.G. & Brown K.H. (2003) Update on technical issues concerning complementary feeding of young children in developing countries and implications for intervention programs. Food and Nutrition Bulletin 24, 5–28. [DOI] [PubMed] [Google Scholar]
- Ferguson E.L., Gadowsky S.L., Huddle J.M., Cullinan T.R., Lehrfeld J., Gibson R.S. (2004) An interactive 24‐h recall technique for assessing the adequacy of trace mineral intakes of rural Malawian women; its advantages and limitations. European Journal of Clinical Nutrition 1995; 49: 565–578. [PubMed] [Google Scholar]
- Flax V.L., Phuka J., Cheung Y.B., Ashorn U., Maleta K. & Ashorn P. (2010) Feeding patterns and behaviors during home supplementation of underweight Malawian children with lipid‐based nutrient supplements or corn‐soy blend. Appetite 54 (3), 504–511. [DOI] [PubMed] [Google Scholar]
- Food and Agriculture Organization (FAO) & World Health Organization (WHO) (2002) Joint Expert Consultation on Vitamin and Mineral Requirements in Human Nutrition, World Health Organization: Geneva. [Google Scholar]
- Food and Nutrition Board (FNB) & Institute of Medicine (IOM) (1997) Dietary Reference Intakes for Calcium, Phosphorus, Magnesium, Vitamin D, and Fluoride, National Academy Press: Washington. [PubMed] [Google Scholar]
- Food and Nutrition Board (FNB) & Institute of Medicine (IOM) (1998a) Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline, National Academy Press: Washington. [PubMed] [Google Scholar]
- Food and Nutrition Board (FNB) & Institute of Medicine (IOM) (1998b) A Risk Assessment Model for Establishing Upper Intake Levels for Nutrients, National Academy Press: Washington. [PubMed] [Google Scholar]
- Food and Nutrition Board (FNB) & Institute of Medicine (IOM) (2000a) Dietary Reference Intakes: Applications in Dietary Assessment, National Academy Press: Washington. [Google Scholar]
- Food and Nutrition Board (FNB) & Institute of Medicine (IOM) (2000b) Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids, National Academy Press: Washington. [PubMed] [Google Scholar]
- Food and Nutrition Board (FNB) & Institute of Medicine (IOM) (2002) Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc : A Report of the Panel on Micronutrients, National Academy Press: Washington. [Google Scholar]
- Food and Nutrition Board (FNB) & Institute of Medicine (IOM) (2005) Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids, National Academy Press: Washington. [DOI] [PubMed] [Google Scholar]
- Golden M. (2009) Proposed recommended nutrient densities for moderately malnourished children. Food and Nutrition Bulletin 30, S267–S342. [DOI] [PubMed] [Google Scholar]
- Ickes S.B., Jilcott S.B., Adair L.S., Bentley M.E., Thirumurthy H., Myhre J.A. et al (2012) Examination of barriers and facilitators to home‐based supplemental feeding with ready‐to‐use‐food for underweight children in western Uganda. Maternal and Child Nutrition 8, 115–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isanaka S., Nombela N., Djibo A., Poupard M., Van Beckhoven D., Gaboulaud V. et al (2009) Effectiveness of preventive supplementation with ready‐to‐use‐therapeutic food on the nutrition status mortality, and morbidity of children aged 6 to 60 months in Niger: a cluster randomized trial. Journal of the American Medical Association 301, 277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilcott S.B., Masso K.L., Ickes S.B., Myhre S.D. & Myhre J.A. (2007) Surviving but not quite thriving: anthropometric survey of children ages 6–59 months in a rural Western Uganda district. Journal of the American Dietetic Association 107, 1983–1988. [DOI] [PubMed] [Google Scholar]
- Jilcott S.B., Ickes S.B., Ammerman A.S. & Myhre J.A. (2010) Iterative design, implementation and evaluation of a supplemental feeding program for underweight children ages 6–59 months in Western Uganda. Maternal and Child Health 14, 299–306. [DOI] [PubMed] [Google Scholar]
- Kennedy G.L., Pedro M.R., Sehieri C., Nantel G. & Brouwer I. (2007) Dietary diversity score is useful indicator of micronutrient intake in non‐breastfeeding Filipino children. Journal of Nutrition 137, 472–477. [DOI] [PubMed] [Google Scholar]
- Lukmanji Z., Hertzmark E., Mingi N., Assey V., Ndossi G. & Fawzi W. (2008) Tanzania Food Composition Tables, 1st edn, Tanzania Food and Nutrition Centre and Harvard School of Public Health: Boston, MA. [Google Scholar]
- Maleta K., Kuittinen J., Duggan M.B., Briend A., Manary M., Wales J. et al (2004) Supplementary feeding of underweight, stunted Malawian children with a ready‐to‐use food. Journal of Pediatric Gastroenterology & Nutrition 38, 152–158. [DOI] [PubMed] [Google Scholar]
- Murphy S.P., Guenther P.M. & Kretsch M.J. (2006) Using the dietary reference intakes to assess intakes of groups: pitfalls to avoid. Journal of the American Dietetic Association 106, 1550–1553. [DOI] [PubMed] [Google Scholar]
- Navarro‐Colorado C., Mason F. & Shoham J. (2008) Measuring the effectiveness of supplementary feeding programmes in emergencies. Humanitatrian Practice Network 63, 1–28. [Google Scholar]
- Parker M.E., Bentley M.E., Chasela C., Adair L., Piwoz E.G., Jamieson D.J. et al (2011) The acceptance and feasibility of replacement feeding at 6 months as an HIV prevention method in Lilongwe, Malawi: results from the BAN Study. AIDS Education and Prevention 23 (3), 281–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M.E., Tembo M., Adair L., Chasela C., Piwoz E.G., Jamieson D.J. et al (2013) The health of HIV‐exposed children after early weaning. Maternal and Child Nutrition 9 (2), 217–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul K.H., Muti M., Chasekwa B., Mbuya M.N.N., Madzima R.C., Humphrey J.H. et al (2012) Complementary feeding messages that target cultural barriers enhance both the use of lipid‐based nutrient supplements and underlying feeding practices to improve infant diets in rural Zimbabwe. Maternal and Child Nutrition 8, 225–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentice A. & Bates C.J. (1994) Adequacy of dietary mineral supply for human bone growth and mineralisation. European Journal of Clinical Nutrition 48, S161–S176. [PubMed] [Google Scholar]
- Siega‐Riz A.M., Estrada Del Campo Y., Kinlaw A., Reinhart G.A., Allen L.H., Shahab‐Ferdows S. et al (2014) Effect of supplementation with a lipid‐based nutrient supplement on the micronutrient status of children aged 6–18 months living in the rural region of Intibucá, Honduras. Paediatric and Perinatal Epidemiology 28, 245–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus R.J., Chway H.M., Montresor A., Tielsch J.M., Jape J.K., Albonico A. et al (2004) Low dose daily iron supplementation improves iron status and appetite but not anemia, whereas quarterly anthelminthic treatment improves growth, appetite and anemia in Zanzibari preschool children. Journal of Nutrition 134, 348–356. [DOI] [PubMed] [Google Scholar]
- Uganda Bureau of Statistics (UBOS) and Macro International Inc. (2007) Uganda Demographic and Health Survey 2006. Calverton, Maryland: UBOS and Macro International Inc.
- Uganda Bureau of Statistics (UBOS) and Macro International Inc. (2011) Uganda Demographic and Health Survey 2010. Calverton, Maryland: UBOS and Macro International Inc.
- Uganda Bureau of Statistics (UBOS) and Macro International, Inc. (2012) Uganda Demographic and Health Survey 2011, UBOS and Macro International, Inc.: Calverton, Maryland. [Google Scholar]
- United States Department of Agriculture (USDA) Agricultural Research Service (2009) National Nutrient Databases for Standard Reference. Available at: http://www.nal.usda.gov/fnic/foodcomp/search
- Working Group on Infant and Young Child Feeding Indicators (2006) Developing and Validating Simple Indicators of Complementary Food Intake and Nutrient Density for Infants and Young Children in Developing Countries: Protocol for Data Analysis, The Food and Nutrition Technical Assistance Project and Academy for Educational Development: Washington, DC. [Google Scholar]
- World Health Organization (WHO) Multicentre Growth Reference Study Group (2006) WHO Child Growth Standards: Length/height‐for‐age, weight‐for‐age, weight‐for‐length, weight‐for‐height and body mass index‐for‐age: Methods and development. Geneva: World Health Organization.
- World Health Organization (WHO) (2008) Indictors for Assessing Infant and Young Child Feeing Practices: Conclusions of a Consensus Meeting held 6–8 November 2007 in Washington, D.C., USA, World Health Organization: Geneva. [Google Scholar]


