Abstract
Post‐partum weight retention (WR) occurs in 60–80% of women with some retaining ≥10 kg with contributing factors reported as pre‐pregnancy body mass index (BMI), gestational weight gain (GWG) and breastfeeding. A longitudinal study of pregnancy, with 12‐month post‐partum follow‐up was conducted to determine factors associated with WR. Pregnant women (n = 152) were recruited from the John Hunter Hospital antenatal clinic in New South Wales, Australia. Pre‐pregnancy weight was self‐reported; weight was measured four times during pregnancy (for GWG) and in the first 12 months post‐partum. Infant feeding data were obtained via questionnaires. Breastfeeding was categorised as exclusive, predominant, complementary or not breastfeeding. Linear mixed models tested the predictors of WR, with and without adjustment for potential confounders. Compared with pre‐pregnancy weight, 68% of women retained weight at 12 months, median (interquartile range) [4.5 kg (2.1–8.9)]. After adjustment, GWG was positively associated with WR (P < 0.01), but pre‐pregnancy weight did not predict WR. For each additional week of any breastfeeding, 0.04 kg less weight was retained. Compared with women who retained weight, those women who did retain had higher rates of exclusive breastfeeding at three months (P < 0.05), but the number of weeks of exclusive breastfeeding failed to predict WR for all women. WR following childbirth is common and associated with GWG, while the number of weeks of ‘any’ breastfeeding contributed to post‐partum weight loss. Whether these factors are modifiable strategies to optimise the weight status of women at this life stage requires further research.
Keywords: body mass index, breastfeeding, obesity, pregnancy, weight gain, weight retention
Introduction
Thirty‐five per cent of women in Australia, aged 25–35 years, are overweight or obese [body mass index (BMI) >25 kg m−2] (Callaway et al. 2006). Many women attribute weight gain to childbearing, particularly with successive pregnancies (Harris et al. 1997; Kac et al. 2004; Walker et al. 2005; Gunderson 2009). Therefore, pregnancy and the post‐natal period could be an important time to motivate and educate women to make healthy lifestyle changes (Phelan 2010). However, women require knowledge, skills and/or support to undertake these changes (Furness et al. 2011). Clinicians, such as obstetricians and midwives, have regular contact with women during pregnancy and are well placed to assist women to achieve a healthy weight and healthy lifestyle. However, further knowledge of the factors predicting greater post‐partum weight retention (WR) will help identify those at greatest risk.
Previous studies have reported that 50–80% of women retain 1.4–5 kg up to 12 months after birth, which may become permanent (Ohlin & Rossner 1990; Schauberger et al. 1992; Olson et al. 2003; Rothberg et al. 2011). Furthermore, 20–50% will retain 5–10 kg 12 months after birth (Olson et al. 2003; Althuizen et al. 2011; Rothberg et al. 2011). Excessive weight gain in pregnancy is associated with later overweight and obesity (Amorim et al. 2007; Mamun et al. 2010), which is a risk factor for obstetric complications in subsequent pregnancies as well as cardiovascular disease, metabolic syndrome and diabetes (Gunderson 2009; Sirimi & Goulis 2010).
Gestational weight gain (GWG) has been associated with both short‐ and long‐term WR, which has been confirmed in more than 10 observational studies, one systematic review and a meta‐analysis (Greene et al. 1988; Walker 1996; Kac 2004; Baker et al. 2008; Viswanathan et al. 2008; Mamun et al. 2010; Fraser et al. 2011; Huang et al. 2011; Nehring et al. 2011; Rothberg et al. 2011). The meta‐analysis of nine observational studies demonstrated that women who gained above the Institute of Medicine (IOM) GWG recommendations are on average 4.7 kg heavier up to 15 years post‐partum compared with those whose weight gain matched with the recommendations (Nehring et al. 2011). Results from a longitudinal study of 2356 British women found those who had excessive GWG (based on the IOM guidelines) had adverse health outcomes 16 years after birth compared with women who gained within recommendations (Fraser et al. 2011). Those with excessive GWG were 3.6 times more likely to have a of BMI ≥ 25 kg m−2 [95% confidence interval (CI) 2.6–4.9] and 2.8 times more likely to have a waist circumference ≥80 cm (95% CI 1.8–4.0) 16 years post‐partum (Fraser et al. 2011).
The evidence is equivocal as to the association between pre‐pregnancy BMI and WR. A lower pre‐pregnancy BMI has been associated with higher WR (coefficient = −0.5; P‐value ≤ 0.001) (Kac et al. 2004). However, the inverse has also been reported (Linné et al. 2003; Walker 2009). These results are based on observational studies with differing sample sizes (n = 247–1423), socioeconomic status, country of origin (Brazil, USA and Stockholm) and length of follow‐up (9 months to 15 years), indicating that research is needed (Linné et al. 2003; Kac et al. 2004; Walker 2009). While the relationship between pre‐pregnancy BMI and WR is unclear, there is considerable evidence that a high BMI increases the risk of adverse maternal and infant outcomes (Solomon et al. 1997; Sebire et al. 2001; O'Brien et al. 2003; Rowlands et al. 2010; Sathyapalan et al. 2010). For example, a high pre‐pregnancy BMI increases the risk for adverse outcomes including gestational diabetes [relative risk (RR) = 2.1; 95% CI 1.7–2.7 for BMI>25–29.9 kg m−2 and RR = 2.1; 95% CI 2.2–3.9 for BMI ≥ 30 kg m−2) (Solomon et al. 1997; Sathyapalan et al. 2010), pre‐eclampsia [odds ratio (OR) = 2.1–5.2 for overweight and obese] (O'Brien et al. 2003), delivery intervention (Rowlands et al. 2010), macrosomia (OR 1.6; 95% CI 1.5–1.6 for BMI >25–30 kg m−2 and 2.4; 95% CI 2.2–2.5 for BMI ≥ 30 kg m−2) (Sebire et al. 2001) and reduced rates breastfeeding initiation and duration (Donath & Amir 2000; Mamun et al. 2011). Excessive post‐partum WR has also been linked with increased health risks including overweight and obesity (Nehring et al. 2011). Furthermore, the post‐partum period has been associated with disordered eating, diminished self‐esteem and depression (Devine et al. 2000; Clark et al. 2009; Pedersen et al. 2011), all of which can compound WR (Yanovski 2003; Luppino et al. 2010).
Breastfeeding should theoretically reduce WR due to the energy cost of lactation of approximately 2100–2800 kJ day–1 (Allen & Hector 2005; Butte & King 2005; Stuebe & Rich‐Edwards 2009). However, research on the relationship between breastfeeding and WR has shown minimal or no effect (Manning‐Dalton & Allen 1983; Haiek et al. 2001). Ohlin & Rossner (1990) found a significant but very weak correlation (r = −0.09, P < 0.01) between breastfeeding and weight loss at 12 months post‐partum (Ohlin & Rossner 1990), while Baker et al. (2008) found that for every one unit increase in breastfeeding score (1 point given for each week of full breastfeeding), WR was decreased by 0.01–0.04 kg (Baker et al. 2008). Two additional studies found that women exclusively breastfeeding were 1 kg (Brewer et al. 1989) and 2 kg (Dewey et al. 1993) lighter at 6 months and 12 months, respectively, compared with those formula feeding (P < 0.05). A methodological problem is that these studies define breastfeeding as ‘any’ breastfeeding, without using the standard World Health Organization (WHO) definitions of exclusive, predominant, complementary or no breastfeeding (Brewer et al. 1989; Dewey et al. 1993, Walker, 2009) (Table 1).
Table 1.
Criteria for the World Health Organization infant feeding categories
| Breastfeeding category | Food sources the infant can receive | Food sources the infant cannot receive |
|---|---|---|
| Exclusive | Breast milk as the main food source, medicine, vitamins, minerals | Anything else |
| Predominant | Breast milk as the main food source, liquids (water, fruit juice, oral rehydration salts, ritual fluids, medicine, vitamins, minerals) | Anything else |
| Complementary | Breast milk AND solid/semi solid food or any other liquid (non‐human milk, food based fluid, water, fruit juice, oral rehydration salts, ritual fluids, drops, syrups) | – |
| No breastfeeding | Solid/semi solid food, liquid (non‐human milk, food based fluid, water, fruit juice, oral rehydration salts, ritual fluids, drops, syrups) | Breast milk |
Binns C.W. et al. (2009) Defining exclusive breastfeeding in Australia. Journal of Paediatrics and Child Health 45, 174–180.
Therefore the aim of this study was to assess the contribution of factors associated with WR up to 12 months post‐partum, including pre‐pregnancy BMI, GWG and breastfeeding variables, including breastfeeding category and duration.
Key messages
An improved understanding of why WR occurs and its impact on the health of the mother and infant is crucial to the development of guidelines on how to manage post‐partum WR.
Ante‐ and post‐natal factors, including excessive GWG and decreased time of any breastfeeding, are associated with WR and should be considered in ante‐ and post‐natal weight management advice.
Many women are heavier at 12 months post‐partum, which can increase their BMI category. Women who are overweight or obese have an increased risk of unfavourable short‐ and long‐term health outcomes, including unfavourable pregnancy outcomes, diabetes and cardiovascular disease and research is urgently required to address this within current maternal services.
Materials and methods
A detailed description of the methods has been previously published (Hure et al. 2012). Briefly, the Women and their Children's Health (WATCH) study is an ongoing prospective cohort spanning pregnancy and early childhood conducted at the John Hunter Hospital, Newcastle, Australia (Hure et al. 2008). Women who were less than 18 weeks pregnant were considered eligible to participate, including those receiving maternal care from private obstetricians or shared‐care from general practitioners. The WATCH Study received ethics approval from the Hunter New England and the University of Newcastle Human Research Ethics Committees. Recruitment occurred from July 2006 to December 2007 with a total of eight study visits by 1 year after birth; four scheduled during pregnancy at approximately 19, 25, 30 and 36 weeks gestation and four during the first year post‐partum at 3, 6, 9 and 12 months.
Anthropometric assessments were performed by a dietician with Level One Anthropometry accreditation from the International Society for the Advancement of Kinanthropometry (2004). Pre‐pregnancy weight was self‐reported at the first study visit and subsequent weights were measured on annually calibrated electronic scales at each visit (A&D FV‐150 K, A&D Mercury Pty Ltd, Thebarton, South Australia). Height (cm) was measured at two assessments using the same wall‐mounted stadiometer (Seca Deutschland, Hamburg, Germany) and the mean was used in analysis. BMI was calculated [weight (kg)/height2 (m2)]. GWG was calculated by subtracting the weight at 36 weeks gestation from the pre‐pregnancy weight. WR was calculated by subtracting the pre‐pregnancy weight from the 12 months post‐partum weight. Participants were divided into two groups based on WR: (1) those who retained >0 kg (known as retainers) and (2) those who retained ≤ 0 kg (known as non‐retainers).
Infant feeding data were collected during the first year after birth using two questionnaires: an Infant Feeding Recall, and a Current Feeding Practices questionnaire, modified from 2004). These questionnaires were interviewer‐administered by accredited practising dieticians who have been trained to administer the questionnaires in a standardised manner. Responses were used to determine breastfeeding initiation, duration and exclusivity. Time taken to complete the interview was 5–10 min. Breastfeeding initiation was a binary outcome (Yes/No) and phrased as ‘Has your child ever been breastfed?’ Breastfeeding duration was measured as ‘Is your child currently being breastfed?’ (Yes/No) at 3, 6, 9 and 12 months post‐partum, and ‘What is the total time for which your child was breastfed (weeks)?’ The questionnaires were used to determine the number of weeks of any breastfeeding and of exclusive breastfeeding. Breastfeeding exclusivity was determined using responses from 24 questions from the Infant Feeding Recall and Current Feeding Practices (Hector et al. 2004), that asked about intake of breast milk, infant formula, tinned/powered/fresh milk, plain/sweet water, juice, medicine, vitamins, minerals, oral rehydration salts and solids. Breastfeeding was further defined by the WHO infant feeding categories (Binns et al. 2009) displayed in Table 1.
A range of socioeconomic and maternal outcomes were captured via questionnaire at study visit one or were recorded in the Obstetrix database. Obstetrix is the major repository in New South Wales for recording antenatal data, patient and family history, and birth outcomes (LeMay 2005). Data on marital status, education level, country of birth, Index of Relative Socioeconomic Disadvantage (IRSAD) based on postcode, smoking status, age at conception, parity, gestational age at birth and infant sex were recorded. The IRSAD uses national census data including household income and employment, to derive a measure of social advantage and disadvantage based on area of residence (postcode) (Adhikari 2006).
Statistical analyses
To be included in the analyses, women in the WATCH study had to have attended one or more post‐partum follow‐up visits. Women were excluded if they became pregnant in the post‐partum follow‐up period (n = 11) or had twins (n = 1). One repeat participant only had data from her first pregnancy (of two) included in the analyses.
Data were analysed using the statistical software package Intercooled Stata, version 11 (StataCorp LP, College Station, TX, USA) and statistical significance was set at α = 0.05. All data was tested for normality and the median [interquartile range (IQR) ] was recorded for non‐normally distributed data. Chi‐squared, Fisher's exact and rank sum tests were used to determine the characteristics of women who differed by WR groups. Linear mixed models with random effects were used to determine the variables that predicted WR at over the 12 months post‐partum. Pre‐pregnancy BMI, GWG, breastfeeding categories and weeks spent breastfeeding were used as the main predictors of WR (as a continuous variable), with and without adjustment for potential confounders. The potential confounders that were tested comprised of infant sex, maternal age at conception, smoking during pregnancy, IRSAD, education level, marital status and parity. Model 1 was analysed without adjustment and Model 2 was adjusted for age, smoking, IRSAD and parity as these were the confounders which remained in the model at P < 0.2.
We conducted an additional comparative analysis between those who withdrew and those who remained in the study. This was done to determine any differences in socio‐demographic, weight and breastfeeding variables. The variables tested were maternal age, marital status, country of birth, education level, IRSAD, parity, pre‐pregnancy weight and BMI, gestational weight, breastfeeding initiation and duration.
Women who did not attend a post‐partum visit or those who withdrew from the study during pregnancy were excluded from the comparative analysis. They were excluded on the basis that they did not have any available data on WR. We conducted a comparative analysis to determine any differences in socio‐demographic, weight and breastfeeding variables between those excluded and included from the analysis. The variables tested were maternal age, marital status, country of birth, education level, IRSAD, parity, pre‐pregnancy weight and BMI, gestational weight, breastfeeding initiation and duration.
Results
The proportion of women who were still enrolled in the WATCH study at the 12‐month follow‐up was 81% (n = 146). Reasons for withdrawal included ‘too busy’ (n = 9), had moved (n = 4), fetal or child death (n = 4), lost contact (n = 4), ‘too much effort to participate’ (n = 3) or other reasons (n = 9). Results of the comparative analysis between withdrawals and those who remained in the study revealed there was only one difference between the groups, which was age (P = 0.03). Participants who had withdrawn were younger [mean + standard deviation (SD), 26.7 + 5.5 years] compared with those who remained in the study (mean + SD, 29.2 + 5.5). In addition, results of the comparative analysis between those excluded and included from the comparative analysis in Table 2 revealed there was only one difference between the groups, which was marital status (P = 0.03). Excluded participants were less likely to be in a de facto or married relationship (30%) compared with included participants (13%).
Table 2.
Characteristics of all participants (n = 152) † , non‐retainers and retainers at 6 (n = 129) and 12 (n = 114) months
| Characteristic | All (n = 152) † | WR ‡ at 6 months (n = 129) | P‐value | (WR) ‡ at 12 months (n = 114) | P‐value | Test | ||
|---|---|---|---|---|---|---|---|---|
| Baseline | ≤0 kg (n = 29) | >0 kg (n = 100) | ≤0 kg (n = 37) | >0 kg (n = 77) | ||||
| Age § | 28.6 (25.2–32.8) | 29.8 (26.7–36.6) | 28.9 (25.6–31.9) | 0.17 | 29.4 (26.2–34.0) | 29.6 (26.3–33.2) | 0.70 | Rank sum |
| Height § | 164.3 (160.1–169.2) | 165.2 (161.5–170.0) | 164.4 (160.0–168.2) | 0.34 | 162.9 (158.8–166.9) | 165.0 (160.0–16.9) | 0.37 | Rank sum |
| Born in Australia, n (%) | 142 (93.4) | 25 (86.2) | 94 (94.0) | 0.16 | 35 (94.6) | 70 (93.5) | 0.59 | Fishers exact |
| Married or de facto, n (%) | 132 (86.8) | 25 (86.2) | 87 (87.0) | 0.56 | 32 (86.5) | 68 (88.3) | 0.78 | Chi2/Fishers exact |
| Education ≥ year 12, n (%) | 104 (71.7) | 21 (72.4) | 71 (71.0) | 0.70 | 27 (73.0) | 59 (76.6) | 0.69 | Chi2 |
| Infant sex, n (%) male | 77 (50.7) | 17 (58.6) | 49 (49.0) | 0.36 | 15 (40.5) | 41 (53.3) | 0.20 | Chi2 |
| Smoking, n (%) | 18 (11.8%) | 3 (10.3) | 13 (13.0) | 0.49 | 3 (8.1) | 9 (11.7) | 0.41 | Exact |
| Weight variables | ||||||||
| Pre‐pregnancy weight § | 65.0 (58.0–78.8) | 75.0 (63.0–83.5) | 65.0 (56.5–75.5) | <0.01* | 66.0 (59.0–80.0) | 65.0 (58.0–76.0) | 0.62 | Rank sum |
| Pre‐pregnancy BMI § | 24.3 (21.3–29.0) | 25.8 (21.1–26.6) | 23.6 (21.1–26.5) | 0.02* | 23.6 (21.3–30.1) | 24.1 (21.1–26.9) | 0.60 | Rank sum |
| GWG § , ¶ | 12.7 (8.9–17.1) | 8.8 (6.4–12.2)* | 14.6 (10.5–18.1) | <0.01* | 10.3 (6.8–14.3)* | 14.7 (1142–18.1) | <0.001* | Rank sum |
| Weight at each time § | 69.2 (60.3–79.6) | 71.0 (61.3–83.4) | 69.6 (60.5–80.8) | 0.90 | 62.9 (57.1–78.5)* | 70.4 (63.1–83.5) | 0.01* | Rank sum |
| BMI at each time § | 25.5 (22.4–30.4) | 24.8 (21.8–31.1) | 25.5 (22.6–30.4) | 0.72 | 22.7 (20.8–29.1)* | 26.1 (23.3–30.6) | 0.01* | Rank sum |
| WR at each time § | 2.3 (−0.7–6.8) | −1.8 (−5.7–1.35) | 4.3 (2–8) | <0.01* | −1.8 (−4.4−0.9) | 4.5 (2.1–8.9) | <0.001* | Rank sum |
| Breastfeeding variables | ||||||||
| Initiated breastfeeding, n (%) | 136 (93.2) | 27 (93.1) | 94 (94.0) | 0.57 | 36 (97.3) | 71 (92.2) | 0.27 | Fishers exact |
| Exclusive breastfeeding at 3 months n (%) | 76 (52.1) | 17 (58.6) | 51 (52.0) | 0.82 | 25 (67.6)* | 37 (48.1) | 0.046* | Fishers exact |
| Any breastfeeding at each time n (%) | 34 (28.8) | 16 (55.2) | 57 (57.0) | 0.86 | 14 (37.8) | 20 (26.0) | 0.20 | Chi2 |
| Total weeks breastfeeding § | 29.0 (7.0–52.0) | 7.0 (0.0–14.0) | 26.0 (0.6–26.0) | 0.17 | 40.0 (7.0–52.0) | 26.0 (8.0–52.0) | 0.36 | Rank sum |
| Number of weeks exclusively breastfeeding § | 24.0 (20.0–26.0) | 24.0 (21.0–25.0) | 24.0 (20.0–26.0) | 0.50 | 24.0 (20.0–25.0) | 24.0 (20.0–26.0) | 0.59 | Rank sum |
*Indicates statistical significance α < 0.05. †This excludes those who withdrew during pregnancy and who were enrolled but did not attend any post‐partum visits. ‡Weight retained is pre‐pregnancy weight (kg) subtracted from weight at three, six, nine and 12 months post‐partum. §Median (25th–75th percentile) reported for non‐normally distributed continuous data. ¶Gestational weight gain (GWG) is pre‐pregnancy body weight subtracted from weight at 36 weeks gestation.
The baseline characteristics of the cohort in these analyses are presented in Table 2. The majority of participants had a high school education of year 12 or more, were in a married or de facto relationship and were born in Australia. At 12 months post‐partum, the median weight of participants had increased by 4.2 kg from the pre‐pregnancy weight of 65.0 kg (IQR: 58.0–78.8). The median BMI at 12 months post‐partum had increased by 1.2 kg m−2 compared with the pre‐pregnancy (rank sum:W = −5.17, P < 0.001). This increase meant that the median BMI category moved from the normal weight category into the overweight category in 12 months.
Of those who attended the 6‐month follow‐up, 22.4% (n = 29) had returned to or weighed less than their pre‐pregnancy weight, defined as non‐retainers, while 77.5% (n = 100) remained above their pre‐pregnancy weight, defined as retainers (Table 2). Non‐retainers at 6 months had a significantly higher median pre‐pregnancy BMI [25.8 kg (IQR: 21.1–26.6)] and pre‐pregnancy weight [75 kg (IQR: 63.0–83.5)], but lower GWG [8.8 (IQR: 6.4 −12.2)] compared with retainers (rank sum: W = 2.31, P = 0.02 and rank sum: W = −4.67, P < 0.001, respectively). There were no differences in baseline height, demographics; or breastfeeding (category or number of weeks) characteristics between the two groups.
At 12 months post‐partum, only 32.5% (n = 37) of participants had returned to their pre‐pregnancy weight or lower (Table 2). There were no significant differences in baseline demographic characteristics between retainers and non‐retainers at 12 months after birth. Retainers were 7.5 kg heavier [70.4 kg (IQR: 63.1–83.5); rank sum: W = −2.52, P = 0.01] and had a higher BMI [26.1 (IQR: 23.3–30.6); rank sum: W = −2.47, P = 0.01] compared with non‐retainers at 12 months post‐partum. Weight retainers at 12 months post‐partum also gained 4.4 kg more during pregnancy [median 14.7 kg (IQR: 11.4–18.1)] and a lower proportion were exclusively breastfeeding (49.3%) at three months post‐partum, compared with non‐retainers (rank sum: −4.09, P < 0.001 and Fisher's exact: no test statistic, P = 0.046, respectively). However, there was no difference in the total number of weeks of exclusive or any breastfeeding between groups.
Figure 1 displays the 25th, median and 75th percentiles of WR over 12 months, divided into weight retainers and non‐retainers. The trajectories of median weight loss were very different across the groups. On average, non‐retainers had predominantly achieved this within the first 3 months post‐partum, while retainers had a median of 5.3 kg (IQR: 2.9–9.9) above their pre‐pregnancy weight at 3 months post‐partum. A further 2.3 kg was lost between three and 12 months after birth in non‐retainers, compared with less than a 1‐kg loss in retainers.
Figure 1.
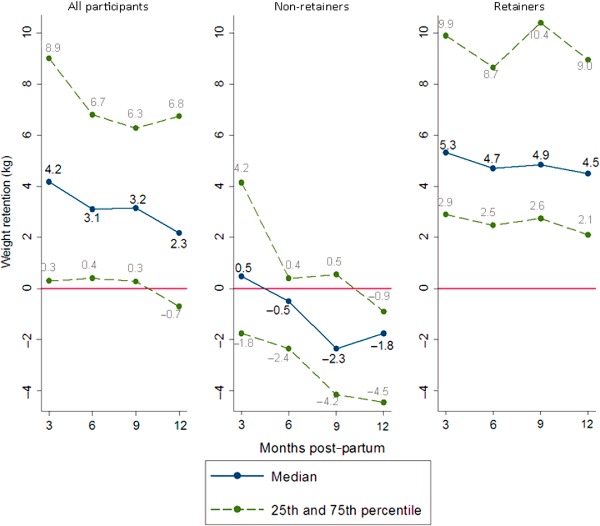
Percentiles of post‐partum weight retention (WR) for all participants (n = 152) and by WR status.
In the adjusted linear mixed model, GWG significantly predicted WR over the 12 months (β = 1.5, P < 0.001). The number of weeks spent breastfeeding was inversely associated with WR and for every 1‐week increase in time spent breastfeeding, WR was reduced by 0.04 kg (P < 0.001). The type of breastfeeding (e.g. exclusive, partial, complementary or not breastfeeding) was not associated with WR. For every child a woman had, WR was increased by 1.5 kg (P < 0.001).
Discussion
The aim of this study was to examine the determinants of WR in a cohort of pregnant women who were followed up to 12 months post‐partum. We found 68% of women from this cohort had retained some of their pregnancy weight gain at 12 months post‐partum, with GWG being the main predictor of WR. The results for the breastfeeding variables were less clear. In the comparison of retainers vs. non‐retainers, there were no differences in the number of weeks of ‘any’ or exclusive breastfeeding. However, results from the longitudinal analyses (linear mixed models) showed that the number of weeks of ‘any’ breastfeeding did inversely predict WR. The median WR for the entire cohort after 1 year was just over 2 kg, with more than a third of women still 5 kg above their self‐reported pre‐pregnancy weight. This is of concern as weight gained by adult women increases the risk of developing type 2 diabetes and cardiovascular disease. Results from the Nurses' Health Study (n > 230 000) found that every kilogram gained from the ages of 18–28 years starting from a normal weight range BMI of 18 kg m−2, increased the risk for developing type 2 diabetes by 49% and 3.1% for cardiovascular disease (Colditz et al. 1990; Willett et al. 1995; Resnick et al. 2000).
The median 12‐month WR of 2.3 kg in this study concurs with the literature that indicates women retain from 1.5 to 4 kg up to 12 months post‐partum (Ohlin & Rossner 1990; Olson et al. 2003; Amorim Adegboye et al. 2008). Previous studies report 14–25% of women (Europe and USA) retain 5 kg or more, and even up to 20 kg (Ohlin & Rossner 1990; Schauberger et al. 1992; Olson et al. 2003; Amorim Adegboye et al. 2008; Althuizen et al. 2011), which can be carried into subsequent pregnancies and also move a woman into the higher BMI categories of overweight and obesity. A high BMI category is associated with adverse antenatal, birth and post‐natal outcomes including miscarriage, preeclampsia, gestational diabetes, macrosomia, preterm delivery, caesarean section and long‐term maternal as well as infant overweight and obesity (Galtier‐Dereure et al. 2000; Gunderson 2009; Rowlands et al. 2010; Sirimi & Goulis 2010; Mamun et al. 2011). Additionally, WR has been shown to accumulate at central rather than peripheral sites (Smith et al. 1994), which is an independent risk factor of cardiovascular disease in middle‐aged women (RR = 3.1; 95% CI 1.5–6.1 for a waist circumference ≥96.5 cm and RR = 3.3; 95% CI 1.8–6.0 for a waist to hip ratio of ≥0.88) (Rexrode et al. 1998).
In this study, when participants were divided into retainers and non‐retainers, pre‐pregnancy BMI was not a predictor of WR. Previous studies are inconsistent when examining this association. A USA cohort of 985 women found self‐reported pre‐pregnancy BMI to be associated with long‐term (median 2 years), but not short‐term (6 weeks post‐partum) WR (Gunderson et al. 2001). Women with a higher pre‐pregnancy BMI had greater two years post‐partum WR (post‐partum weight loss −4.2 ± 0.2, −3.4 ± 0.6, – 0.3 ± 0.7 kg for normal weight, overweight and obese women, respectively; P < 0.001) after adjustment for confounders including ethnicity, smoking, age, parity and mode of delivery (Gunderson et al. 2001). Also in the USA (n = 2006), women who had a high self‐reported pre‐pregnancy BMI were 40% less likely to retain more than 4.5 kg compared with normal and underweight women at 3 months post‐partum (RR = 0.6; 95% CI 0.4–0.8 and RR = 0.4; 95% CI 0.3–0.6 for overweight and obese women, respectively) (Siega‐Riz et al. 2010). At 12 months, underweight women were twice as likely to retain 0.5–4.5 kg compared with normal weight women (RR = 2.0; 95% CI 1.6–2.7) after adjustment for marital status, parity, income, delivery mode and depression score (Siega‐Riz et al. 2010).
In contrast, there is convincing evidence to support the association between greater GWG with short‐ (Greene et al. 1988; Harris et al. 1999; Butte et al. 2003; Kac et al. 2004; Walker et al. 2004; Althuizen et al. 2011) and long‐term WR (Linne et al. 2004; Mamun et al. 2010; Fraser et al. 2011). A recent meta‐analysis of nine observational studies involving >65 000 women (Nehring et al. 2011) demonstrated that women who had a GWG above the IOM guidelines were 3.1 kg (95% CI 1.5–4.6 kg) and 4.7 kg (95% CI 2.9–6.5 kg) heavier at 3 and 15 years post‐partum, respectively, compared with women who gained within the guidelines (Nehring et al. 2011). Five studies had short‐term follow‐up (1.5–12 months post‐partum), while four studies had longer‐term follow‐up (3–15 years) (Nehring et al. 2011). Pooled results remained consistent after adjustment for social class; however, adjustment for confounding within the individual studies was poor (Nehring et al. 2011). As a result, the true effect of GWG on WR requires further investigation with consideration given to the confounders of weight gain in women.
The revised 2009 IOM guidelines recommend appropriate GWG based on pre‐pregnancy BMI with the aim of reducing the adverse maternal and infant outcomes of excessive weight gain, such as gestational diabetes, pre‐eclampsia, delivery complications and post‐partum WR (Institute of Medicine 2009). Excessive GWG (≥16 kg) increases the risk of pre‐eclampsia (OR = 2.8; 95% CI 2.4–3.2) (Nohr et al., 2008), gestational diabetes (OR = 1.7; 95% CI 1.2–2.6) (Hedderson et al. 2010), caesarean section delivery (OR = 1.4; 95% CI 1.3–1.5) (Nohr et al., 2008) and babies that are large for gestational age (OR = 2.6; 95% CI 2.4–2.8) (Nohr et al., 2008). The guidelines were revised in 2009 as a result of the recognition that a number of characteristics of pregnant women in America have changed since the previous recommendations in 1990 (Institute of Medicine 2009). These include an increase in the average age of childbearing, pre‐pregnancy weight, GWG and infant birthweight (Institute of Medicine 2009). The guidelines also include a range of weight gain for each BMI category with the recognition of diversity between women, including stature and ethnicity (Institute of Medicine 2009).
The IOM guidelines are an international standard that is frequently cited. However, a number of experts consider there are too few quality studies informing these guidelines to justify recommending changes in clinical practice (Poston et al. 2011). This is mainly due to the evidence consisting of predominantly observational studies and only cohorts of women with similar demographics, suggesting the guidelines are not applicable to a range of demographics (National Heath and Medical Research Council 2000). Despite this, there is evidence to suggest weight gain above the IOM guidelines is a risk factor for long‐term WR and adverse pregnancy outcomes (Gunderson 2009; Sirimi & Goulis 2010).
The association between breastfeeding and WR in this cohort was variable. The greatest decrease in WR for all women was observed during the first 3 months and non‐retainers had higher rates of exclusive breastfeeding at 3 months. However, when infant feeding was further categorised (exclusively breastfeeding, predominantly breastfeeding, complementary or not breastfeeding) it was not predictive of WR after adjustment for demographic, social and pre‐ and antenatal weight variables. Although women who are exclusively breastfeeding have a significantly greater energy output than those not breastfeeding, evidence suggests weight loss may be minimised because of compensatory increases in appetite and energy intake (Chou et al. 1999). In the current study, when examining total duration of ‘any’ breastfeeding over 12 months measured in weeks, the longer a woman breastfed the less weight retained. Women who maintained any breastfeeding over the 12 months had a 2‐kg lower WR. Previous studies have found that during the first 3–6 months post‐partum, compared with not breastfeeding, exclusive breastfeeding can lead to weight loss of up to 2 kg (Dewey et al. 1993; Janney et al. 1997; Baker et al. 2008), although a greater weight loss (−1.4 kg) in women who are formula feeding compared with those breastfeeding up to 3 months post‐partum has also been reported (Manning‐Dalton & Allen 1983). Two studies found no relationship between breastfeeding and WR (Dugdale & Evans 1989; Haiek et al. 2001).
Comparison of results between studies is problematic due to the inconsistent definitions of breastfeeding status. Women may be referred to as ‘exclusively’, ‘fully’, ‘predominantly’ or ‘prolonged’ breastfeeding, and as an example ‘exclusively’ could be defined as any of the following: (1) breast milk as a measurement of infant's energy intake (Janney et al. 1997); (2) measuring breast milk plus up to 120 mL day−1 of other milk (Dewey et al. 1993); or (3) solely breast milk with the inclusion of vitamins, minerals and/or water (Baker et al. 2008). Three studies defined exclusivity as breastfeeding with no introduction of formula, other milk or fluids (Dewey et al. 1993; Sichieri et al. 2003; Hatsu et al. 2008), and two studies did not provide definitions for the infant feeding categories (Brewer et al. 1989; Walker 2009). As a result, no comparison of the different analyses of energy expenditure in relation to output of milk can be made.
In the current study, the WHO infant feeding categories (Table 1) to define breastfeeding patterns were used (Binns et al. 2009) as these are considered the gold standard definitions. We propose future research uses the WHO infant feeding guidelines to standardise research in this area.
The limitations of the current study need to be acknowledged. Post‐partum weight change is multifactorial and a limitation of the current study is that it has not adjusted for all known factors that may influence weight gain, such as dietary intake and physical activity. Without including these factors (such as dietary intake and exercise levels), it is difficult to determine what truly affects post‐partum WR. There are no known studies that include data on energy intake, energy expenditure (including physical activity), post‐partum smoking, hormonal changes influencing weight and the energy cost of lactation production and output all in the one study. Other factors that could also be considered include psychological health, hours of sleep, social support, lactation support, contraceptive use and birthing experience (mode of delivery, length of hospital stay, infant hospitalisation). Furthermore, reliable and validated data collection techniques need to be considered rather than the use of subjective self‐report methods. The small sample size of the present study also needs to be recognised when determining associations and may explain some of the differences between the findings of this study and others mentioned in the discussion.
An additional limitation is the difference between those who withdrew and those who remained in the study. We found that the withdrawals were younger, on average, is comparison with those who remained in the study. This introduces a potential selection bias indicating that our study sample may not be representative of the true population. As a result, interpretation of the data should be done with caution and also emphasises the need for further research on WR in post‐partum women. Similarly, there was a difference in marital status between those who were excluded and included from the comparative analysis in Table 2. Similarly, there was a difference in marital status between those who were excluded and included from the comparative analysis in Table 2. This introduces a potential bias that should be considered when extrapolating the data to other populations.
In conclusion, we found that the majority of women in the WATCH cohort retained excess weight 1 year after childbirth. Excessive GWG, parity and less time spent breastfeeding were associated with WR. These factors could be targeted as part of routine ante‐ and post‐natal care within current maternal health services, as a strategy to optimise health and well‐being of mothers and their offspring. Women can be more motivated during pregnancy to make positive lifestyle changes and this, combined with regular clinician contact, presents an ideal opportunity to target improvements in their health behaviours (Morin & Reilly 2007). This is important because research suggests behavioural changes adopted during pregnancy can be maintained throughout the post‐partum period and long term (Clark & Ogden 1999; Tanentsapf et al. 2011). However, clinicians have difficulty in educating women about weight before and during pregnancy because of time constraints, inadequate training, apprehension because of the potentially sensitive nature of the topic, self‐perception of body weight, inability to believe the patient can lose weight or a belief that treatment will be ineffective (Cogswell et al. 2010; Stotland et al. 2010; Furness et al. 2011). During the post‐partum period, paediatricians and child and family health nurses are in a position to provide brief advice and/or to make referrals to dieticians, exercise physiologists and physiotherapists who can then provide education on nutrition, behavioural management and physical activity.
Source of funding
CC is funded by a Career Development Award from the National Health and Medical Research Council. The WATCH study was funded by the University of Newcastle, the John Hunter Hospital Charitable Trust and the Newcastle Permanent Charitable Foundation.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
AH, RS and CC were involved in the study design and AH collected the data. AH and JM entered the data and JM conducted the statistical analysis with significant input from AH. All authors discussed the results and implications. JM wrote the manuscript and all authors made significant intellectual contributions to the manuscript at all stages. All authors gave final approval of the version to be published.
Acknowledgements
We thank the research midwives, Trish Engel, Therese Finnegan and Annie Wright for their efforts in recruiting the study participants. We thank all families for their participation in this study. We also thank statisticians Patrick Mcelduff and Julia Smith for their assistance with data analysis.
References
- Adhikari P. (2006) Socio‐economic indexes for areas: introduction, use and future directions. In: Statistics, A.B.O. (ed.). Canberra.
- Allen J. & Hector D. (2005) Benefits of breastfeeding. New South Wales Public Health Bulletin 16, 42–46. [DOI] [PubMed] [Google Scholar]
- Althuizen E., van Poppel M.N.M., De Vries J.H., Seidell J.C. & van Mechelen W. (2011) Postpartum behavior as predictor of weight change from before pregnancy to one year postpartum. BMC Public Health 11, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amorim A.R., Rossner S., Neovius M., Lourenco P.M. & Linne Y. (2007) Does excess pregnancy weight gain constitute a major risk for increasing long‐term BMI. Obesity (Silver Spring, Md.) 15, 1278–1286. [DOI] [PubMed] [Google Scholar]
- Amorim Adegboye A., Linne Y. & Lourenco P. (2008) Diet or exercise, or both, for weight reduction in women after childbirth (Review). Cochrane Collaboration 4, 1–40. [DOI] [PubMed] [Google Scholar]
- Baker J.L., Gamborg M., Heitmann B.L., Lissner L., SøRensen T.I.A. & Rasmussen K.M. (2008) Breastfeeding reduces postpartum weight retention. The American Journal of Clinical Nutrition 88, 1543–1551. [DOI] [PubMed] [Google Scholar]
- Binns C.W., Fraser M.L., Lee A.H. & Scott J. (2009) Defining exclusive breastfeeding in Australia. Journal of Paediatrics and Child Health 45, 174–180. [DOI] [PubMed] [Google Scholar]
- Brewer M.M., Bates M.R. & Vannoy L.P. (1989) Potpartum changes in maternal weight and body fat depots in lactating vs nonlactating women. The American Journal of Clinical Nutrition 49, 259–265. [DOI] [PubMed] [Google Scholar]
- Butte N.F. & King J.C. (2005) Energy requirements during pregnancy and lactation. Public Health Nutrition 8, 1010–1027. [DOI] [PubMed] [Google Scholar]
- Butte N.F., Ellis L.J., Wong W.W., Hopkinson J.M. & Smith E.O. (2003) Composition of gestational weight gain impacts maternal fat retention and infant birth weight. American Journal of Obstetrics and Gynecology 189, 1423–1432. [DOI] [PubMed] [Google Scholar]
- Callaway L.K., Prins J.B., Chang A.M. & Mcintyre H.D. (2006) The prevalence and impact of overweight and obesity in an Australian population. The Medical Journal of Australia 184, 56–59. [DOI] [PubMed] [Google Scholar]
- Chou T.‐W., Chan G.M. & Moyer‐Mileur L. (1999) Postpartum body composition changes in lactating and non‐lactating primiparas. Nutrition 15, 481–484. [DOI] [PubMed] [Google Scholar]
- Clark A., Skouteris H., Wertheim E.H., Paxton S.J. & Milgrom J. (2009) The relationship between depression and body dissatisfaction across pregnancy and the postpartum. Journal of Health Psychology 14, 27–35. [DOI] [PubMed] [Google Scholar]
- Clark M. & Ogden J. (1999) The impact of pregnancy on eating behaviour and aspects of weight concern. International Journal of Obesity 23, 18–24. [DOI] [PubMed] [Google Scholar]
- Cogswell M.E., Power M.L., Sharma A.J. & Schulkin J. (2010) Prevention and management of obesity in nonpregnant women and adolescents: beliefs and practices of U.S. obstetricians and gynecologists. Journal of Women's Health 19, 1625–1634. [DOI] [PubMed] [Google Scholar]
- Colditz G.A., Willett W.C., Stampfer M.J., Manson J.E., Hennekens C.H., Arky R.A. et al (1990) Weight as a risk factor for clinical diabetes in women. American Journal of Epidemiology 132, 501–513. [DOI] [PubMed] [Google Scholar]
- Devine C.M., Bove C.F. & Olson C.M. (2000) Continuity and change in women's weight orientations and lifestyle practices through pregnancy and the postpartum period: the influence of life course trajectories and transitional events. Social Science & Medicine 50, 567–582. [DOI] [PubMed] [Google Scholar]
- Dewey K.G., Heinig M.J. & Nommsen L.A. (1993) Maternal weight‐loss patterns during prolonged lactation. The American Journal of Clinical Nutrition 58, 162–166. [DOI] [PubMed] [Google Scholar]
- Donath S.M. & Amir L.H. (2000) Does maternal obesity adversely affect breastfeeding initiation and duration? Journal of Paediatrics and Child Health 36, 482–486. [DOI] [PubMed] [Google Scholar]
- Dugdale A.E. & Evans J.E. (1989) The effect of lactation and otehr factors on post‐partum changes in body‐weight and triceps skinfold thickness. The British Journal of Nutrition 61, 149–153. [DOI] [PubMed] [Google Scholar]
- Fraser A., Tilling K., Macdonald‐Wallis C.M., Hughes R., Sattat N., Nelson S.M. et al (2011) Associations of gestational weight gain with maternal body mass index, waist circumference, and blood pressure measured 16 y after pregnancy: the AVON Longitundinal Study of Parents and Children. The American Journal of Clinical Nutrition 93, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness P.J., Mcseveny K., Arden M.A., Garland C., Dearden A.M. & Soltani H. (2011) Maternal obesity support services: a qualitative study of the perspectives of women and midwives. BMC Pregnancy and Childbirth 11, 69–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtier‐Dereure F., Boegner C. & Bringer J. (2000) Obesity and pregnancy: complications and cost. The American Journal of Clinical Nutrition 71, 1242S–1248S. [DOI] [PubMed] [Google Scholar]
- Greene G.W., Smiciklas‐Wright H., Scholl T.O. & Karp R.J. (1988) Postpartum weight change: how much of the weight gained in pregnancy will be lost after delivery? Obstetrics and Gynecology 71, 701–707. [PubMed] [Google Scholar]
- Gunderson E.P. (2009) Childbearing and obesity in women: weight before, during, and after pregnancy. Obstetrics and Gynecology Clinics of North America 36, 317–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunderson E.P., Abrams B. & Selvin S. (2001) Does the pattern of postpartum weight change differ according to pregravid body size? International Journal of Obesity 25, 853–862. [DOI] [PubMed] [Google Scholar]
- Haiek L.N., Kramer M.S., Ciampi A. & Tirado R. (2001) Postpartum weight loss and infant feeding. Journal of the American Board of Family Medicine 14, 85–94. [PubMed] [Google Scholar]
- Harris H.E., Ellison G.T.H. & Holliday M. (1997) Is there an independent association between parity and maternal weight gain? Annals of Human Biology 24, 507–519. [DOI] [PubMed] [Google Scholar]
- Harris H.E., Ellison G.T.H. & Clement S. (1999) Do the psychosocial and behavioral changes that accompany motherhood influence the impact of pregnancy on long‐term weight gain. Journal of Psychosomatic Obstetrics and Gynaecology 20, 65–79. [DOI] [PubMed] [Google Scholar]
- Hatsu I.E., Mcdougald D.M. & Anderson A.K. (2008) Effect of infant feeding on maternal body composition. International Breastfeeding Journal 3, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hector D., Webb K. & Lymer S. (2004) State of Food and Nutrition in NSW Series: report on breastfeeding in NSW 2004, Sydney, CPHN/NSW Health Department.
- Hedderson M.M., Gunderson E.P. & Ferrara A. (2010) Gestational weight gain and risk of gestational diabetes mellitus. Obstetrics and Gynecology 115, 597–604. 10.1097/AOG.0b013e3181cfce4f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T., Yeh C. & Tsai Y. (2011) A diet and physical activity intervention for preventing weight retention among Taiwanese childbearing women: a randomised controlled trial. Midwifery 27, 257–264. [DOI] [PubMed] [Google Scholar]
- Hure A., Smith R. & Collins C. (2008) A recruiting failure turned success. BMC Health Services Research 8, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hure A.J., Collins C.E., Giles W.B., Wright I.M.R. & Smith R. (2012) Protocol for the Women and their Children's Health (WATCH) study: a cohort of pregnancy and beyond. Journal of Epidemiology 22, 267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Institute of Medicine (2009) Weight Gain during Pregnancy: Reexamining the Guidelines. National Academy of Sciences: Washington, DC. [Google Scholar]
- International Society for the Advancement of Kinanthropometry (ed.) (2001) International Standards for Anthropometric Assessment. ISAK: Underdale, South Australia. [Google Scholar]
- Janney C.A., Zhang D. & Sowers M. (1997) Lactation and weight retention. The American Journal of Clinical Nutrition 66, 1116–1124. [DOI] [PubMed] [Google Scholar]
- Kac G., Benico M.H.D.A., Velasquez‐Melendez G., Valente J.G. & Struchiner C.J. (2004) Gestational weight gain and prepregnancy weight influence postpartum weight retention in a cohort of Brazilian women. The Journal of Nutrition 134, 6. [DOI] [PubMed] [Google Scholar]
- Kac G., Benício M.H.D.A., Velásquez‐Meléndez G., Valente J.G. &Struchiner C.J. (2004) Gestational weight gain and prepregnancy weight influence postpartum weight retention in a cohort of Brazilian women. The Journal of Nutrition 134, 661–666. [DOI] [PubMed] [Google Scholar]
- Lemay R. (2005) NSW mothers to get state‐wide database [Online]. Australia: ZDNet Available at: http://www.zdnet.com.au/nsw-mothers-to-get-state-wide-database-139181965.htm (Accessed 12 March 2011).
- Linné Y., Dye L., Barkeling B. & Rossner S. (2003) Weight development over time in parous women – the SPAWN study – 15 years follow up. International Journal of Obesity 27, 1516–1522. [DOI] [PubMed] [Google Scholar]
- Linne Y., Dye L., Barkeling B. & Rossner S. (2004) Long‐term weight development in women: a 15‐year follow‐up of the effects of pregnancy. Obesity (Silver Spring, Md.) 12, 1166–1178. [DOI] [PubMed] [Google Scholar]
- Luppino F.S., De Wit L.M., Bouvy P.F., Stijnen T., Cuijpers P., Penninx B.W.J.H. et al (2010) Overweight, obesity, and depression: a systematic review and meta‐analysis of longitudinal studies. Archives of General Psychiatry 67, 220–229. [DOI] [PubMed] [Google Scholar]
- Mamun A.A., Kinarivala M., O'callaghan M.J., Williams G.M., Najman J.M. & Callaway L.K. (2010) Associations of excess weight gain during pregnancy with long‐term maternal overweight and obesity: evidence from 21 y postpartum follow‐up. The American Journal of Clinical Nutrition 91, 1336–1341. [DOI] [PubMed] [Google Scholar]
- Mamun A., Callaway L., O'Callaghan M., Williams G., Najman J., Alati R., et al (2011) Associations of maternal pre‐pregnancy obesity and excess pregnancy weight gains with adverse pregnancy outcomes and length of hospital stay. BMC Pregnancy and Childbirth 11, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning‐Dalton C. & Allen L.H. (1983) The effects of lactation on energy and protein consumption, postpartum weight change and body composition of well nourished North American women. Nutrition Research (New York, N.Y.) 3, 293–308. [Google Scholar]
- Morin K.H. & Reilly L. (2007) Caring for obese pregnant Women. Journal of Obstetric, Gynecologic, and Neonatal Nursing 36, 482–489. [DOI] [PubMed] [Google Scholar]
- National Heath and Medical Research Council (2000) How to Use the Evidence: Assessment and Application of Scientific Evidence [Online]. Commonwealth of Australia. Available at: http://www.nhmrc.gov.au/guidelines/publications/cp65 (Accessed 18 Janurary 2012).
- Nehring I., Schmoll S., Beyerlein A., Hauner H. & Von Kries R. (2011) Gestational weight gain and long‐term postpartum weight retention: a meta‐analysis. The American Journal of Clinical Nutrition 94, 1225–1231. [DOI] [PubMed] [Google Scholar]
- Nohr E.A., Vaeth M., Baker J.L., SøRensen T. I. A., Olsen J. & Rasmussen K.M. (2008) Combined associations of prepregnancy body mass index and gestational weight gain with the outcome of pregnancy. The American Journal of Clinical Nutrition 87, 1750–1759. [DOI] [PubMed] [Google Scholar]
- O'brien T.E., Ray J.G. & Chan W.‐S. (2003) Maternal body mass index and the risk of preeclampsia: a systematic overview. Epidemiology (Cambridge, Mass.) 14, 368–374. [DOI] [PubMed] [Google Scholar]
- Ohlin A. & Rossner S. (1990) Maternal body weight development after pregnancy. International Journal of Obesity 14, 159–173. [PubMed] [Google Scholar]
- Olson C.M., Strawderman M.S., Hinton P.S. & Pearson T.A. (2003) Gestational weight gain and postpartum behaviors associated with weight change from early pregnancy to 1 y postpartum. International Journal of Obesity and Related Metabolic Disorders 27, 117–127. [DOI] [PubMed] [Google Scholar]
- Pedersen P., Baker J.L., Henriksen T.B., Lissener L., Heitmann B., Sorensen T.I.A. et al (2011) Influence of psychosocial factors on postpartum weight retention. Obesity (Silver Spring) 19, 639–646. [DOI] [PubMed] [Google Scholar]
- Phelan S. (2010) Pregnancy: a ‘teachable moment’ for weight control and obesity prevention. American Journal of Obstetrics and Gynecology 202, 135.e1–135.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poston L., Harthoorn L.F. & Van Der Beek E.M. (2011) Obesity in pregnancy: implications for the mother and lifelong health of the child. A consensus statement. Pediatric Research 69, 175–180. [DOI] [PubMed] [Google Scholar]
- Resnick H.E., Valsania P., Halter J.B. & Lin X. (2000) Relation of weight gain and weight loss on subsequent diabetes risk in overweight adults. Journal of Epidemiology and Community Health 54, 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexrode K.M., Carey V.J., Hennekens C.H., Walters E.E., Colditz G.A., Stampfer M.J. et al (1998) Abdominal adiposity and coronary heart disease in women. JAMA: The Journal of the American Medical Association 280, 1843–1848. [DOI] [PubMed] [Google Scholar]
- Rothberg B.E.G., Magriples U., Kershaw T.S., Rising S.S. & Ickovics J.R. (2011) Gestational weight gain and subsequent postpartum weight loss among young, low‐income, ethnic minority women. American Journal of Obstetrics and Gynecology 204, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowlands I., Graves N., de Jersey S., McIntyre H.D. & Callaway L.K. (2010) Obesity in pregnancy: outcomes and economics. Seminars in Fetal & Neonatal Medicine 15, 94–99. [DOI] [PubMed] [Google Scholar]
- Sathyapalan T., Mellor D. & Atkin S.L. (2010) Obesity and gestational diabetes. Seminars in Fetal & Neonatal Medicine 15, 89–93. [DOI] [PubMed] [Google Scholar]
- Schauberger C.W., Rooney B.L. & Brimer L.M. (1992) Factors that influence weight loss in the puerperium. Obstetrics and Gynecology 79, 424–429. [DOI] [PubMed] [Google Scholar]
- Sebire N.J., Jolly M., Harris J.P., Wadsworth J., Joffe M., Beard R.W. et al (2001) Maternal obesity and pregnancy outcome a study of 287 213 pregnancies in London. International Journal of Obesity 25, 1175–1182. [DOI] [PubMed] [Google Scholar]
- Sichieri R., Field A.E., Rich‐Edwards J.W. & Willett W.C. (2003) Prospective assessment of exclusive breastfeeding in relation to weight change in women. International Journal of Obesity 27, 815–820. [DOI] [PubMed] [Google Scholar]
- Siega‐Riz A.M., Herring A.H., Carrier K., Evenson K.R., Dole N. & Deierlein A. (2010) Sociodemographic, perinatal, behavioral, and psychosocial predictors of weight retention at 3 and 12 months postpartum. Obesity (Silver Spring, Md.) 18, 1996–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirimi N. & Goulis D.G. (2010) Obesity in pregnancy. Hormones 9, 299–306. [DOI] [PubMed] [Google Scholar]
- Smith D.E., Lewis C.E., Caveny J.L., Perkins L.L., Burke G.L. & Bild D.E. (1994) Longitudinal changes in adiposity associated with pregnancy. Journal of the American Medical Association 271, 1747–1751. [PubMed] [Google Scholar]
- Solomon C.G., Willett W.C., Carey V.J., Rich‐Edwards J., Hunter D.J., Colditz G.A. et al (1997) A prospective study of pregravid determinants of gestational diabetes mellitus. Journal of the American Medical Association 278, 1078–1083. [PubMed] [Google Scholar]
- Stotland N.E., Gilbert P., Bogetz A., Harper C.C., Abrams B. & Gerbert B. (2010) Preventing excessive weight gain pregnancy: how do prenantal care providers approach counselling? Journal of Women's Health 19, 807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe A.M. & Rich‐Edwards J.W. (2009) The reset hypothesis: lactation and maternal metabolism. American Journal of Perinatology 26, 081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanentsapf I., Heitmann B. & Adegboye A. (2011) Systematic review of clinical trials on dietary interventions to prevent excessive weight gain during pregnancy among normal weight, overweight and obese women. BMC Pregnancy and Childbirth 11, 1–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan M., Siega‐Riz A.M., Moos M.‐K., Deirlein A., Mumford S., Knaack J. et al (2008) Outcomes of maternal weight gain, evidence report/technology assessment. Agency for Healthcare Research and Quality. [PMC free article] [PubMed]
- Walker L.O. (1996) Predictors of weight gain at 6 and 18 months after childbirth: a pilot study. Journal of Obstetric, Gynecologic, and Neonatal Nursing 25, 39–48. [DOI] [PubMed] [Google Scholar]
- Walker L.O. (2009) Low‐income women's reproductive weight patterns. Empirically based clusters of prepregnant, gestational and postpartum weight. Women's Health Issues 19, 398–405. [DOI] [PubMed] [Google Scholar]
- Walker L.O., Timmerman G.M., Sterling B.S., Kim M. & Dickson P. (2004) Do low‐income women attain their pre‐pregnant weight by the 6th week postpartum? Ethnicity and Disease 14, 119–126. [PubMed] [Google Scholar]
- Walker L.O., Sterling B.S. & Timmerman G.M. (2005) Retention of pregnancy‐related weight in the early postpartum period: implications for women's health services. Journal of Obstetric, Gynecologic, and Neonatal Nursing 34, 418–427. [DOI] [PubMed] [Google Scholar]
- Willett W.C., Manson J.E., Stampfer M.J., Colditz G.A., Rosner B., Speizer F.E. et al (1995) Weight, weight change, and coronary heart disease in women. Journal of the American Medical Association 273, 461–465. [DOI] [PubMed] [Google Scholar]
- Yanovski S.Z. (2003) Binge eating disorder and obesity in 2003: could treating an eating disorder have a positive effect on the obesity epidemic? The International Journal of Eating Disorders 34, S117–S120. [DOI] [PubMed] [Google Scholar]


