Abstract
Low‐ to middle‐income countries may experience the occurrence of a dual burden of under and overnutrition. To better understand the overall progression of body mass index (BMI) during childhood, we estimated average BMI‐for‐age z‐score (BAZ) growth curves in a population‐based longitudinal study of 255 children living in the Brazilian Amazon. Children were aged 0.1–5.5 years at recruitment (2003). We collected data on socio‐economic and maternal characteristics, children's birthweight and infant feeding practices. Child anthropometric measurements were taken in 2003, 2007 and 2009. BAZ differences among categories of exposure variables were calculated at 6 and 12 months, and 2, 7 and 10 years. At baseline, the mean (standard deviation) age was 2.6 (1.4) years; 12.9% were overweight and 3.9% thin. After adjustment, mean BAZ estimates were mostly negative. Boys were close to the median value for BAZ until 12 months, whereas girls were below the median (P = 0.05). Children from households above the wealth median were 0.36 z‐ and 0.49 z‐less underweight than poorer children at 7 and 10 years, respectively (P < 0.01). Maternal BMI was positively associated with children's BAZ since 12 months old; BAZ in children from overweight mothers was higher by 0.69 compared with their counterparts at 10 years (P < 0.01). Birthweight was positively related to BAZ up until 2 years (P = 0.01). Socio‐economic background and maternal nutritional status are important predictors of BAZ throughout childhood. Although excessive weight gain is a public health concern, it is critical to restrict inequities, while promoting healthier growth in developing countries.
Keywords: child growth, BMI‐for‐age z‐score, trajectory, school‐age years, developing countries
Introduction
Low‐ to middle‐income countries have been experiencing modifications in the nutritional profile of their populations because of alterations in environmental, socio‐economic and demographic factors during the last decades (Popkin 2001). Consequently, excessive weight gain has affected adults (Finucane et al. 2011), as well as children and adolescents (Wang & Lobstein 2006), in both developed and developing settings worldwide. It is debatable regarding how the current stage of nutrition transition affects changes in body mass index (BMI) in low‐ to middle‐income countries. Some studies concluded that excessive weight gain is still concentrated among the wealthier groups of these countries (Neuman et al. 2011). In contrast, other investigations have suggested that a faster increase in overweight rates occurs in the lower socio‐economic strata, which could be an indication of a shift in the overnutrition burden to the poor (Jones‐Smith et al. 2012).
Studies on BMI determinants from birth to school‐age years have prioritised the investigation of factors associated with overweight and obesity (Li et al. 2007; Kleiser et al. 2009; Manios et al. 2010). However, there is consistent evidence that developing countries commonly face critical social and health inequities (Barros et al. 2010). This can result in the occurrence of a dual burden of concomitant under and overnutrition from early childhood, increasing short‐ to long‐term morbidity and mortality risks because of higher susceptibility to infectious diseases (Black et al. 2008) or to the development of chronic metabolic disorders (Han et al. 2010). As a result, it is important to better understand the mechanisms determining the overall progression of body weight during childhood in low‐ to middle‐income countries, rather than to solely assess specific categories of child nutritional status. Furthermore, it would be informative to make use of a normative measure for growth and development when examining BMI values among children, because this might assist the efforts for adequate implementation of public health policies.
This study aimed to investigate socio‐economic, maternal and child early life determinants of BMI up to the age of 10 years in a population‐based cohort study of children living in the Brazilian Amazon. For this purpose, we examined growth trajectories of BMI‐for‐age and sex z‐scores (BAZ) throughout childhood according to the World Health Organization (WHO) international references. We particularly focused on BAZ estimates during infancy (up until 2 years old), the period associated with adiposity rebound (occurs up to 7 years old) and late childhood (10 years old).
Key messages
Socio‐economic background and maternal nutritional status are the most important predictors of BMI throughout childhood in a population‐based cohort study in the Brazilian Amazon.
Because BMI‐for‐age estimates were mostly negative, the positive associations with socio‐economic and maternal characteristics found in our study indicate that children in the upper categories of wealth and maternal nutritional status were not more overweight, but less underweight than their counterparts.
Although being overweight and obese have become a major public health concern worldwide, public health policies should consider the reduction of inequities to promote healthier child growth in low‐ to middle‐income settings.
Materials and methods
Study design and population
This longitudinal study was conducted in the urban area of Acrelândia, a town located 100 km from Rio Branco, the capital of the state of Acre, in the Western Brazilian Amazon region. Covering a territory of 1607.5 km2, the main economic activities in this town are commercial agriculture and raising cattle. By 2003, Acrelândia had 8697 inhabitants, of whom 43% resided in the urban area.
In January 2003, we conducted a population‐based cross‐sectional study in Acrelândia with the assistance of local teams of the Family Health Program of the Brazilian Ministry of Health (Muniz et al. 2007). All households from this urban area with children aged <5 years were identified and invited to participate (n = 334), and only two declined participation. Data were collected from 332 households (99.4%) involving a total of 489 children. Complete anthropometric information was available for 467 children (95.5% of those eligible).
As reported elsewhere (Garcia et al. 2011), in December 2007, a second population‐based cross‐sectional survey was carried out in the same area among all children aged <10 years, and included 250 of the children who had previously been examined in 2003. In December 2009, another follow‐up assessment included 205 of the children who had been evaluated in 2003 and/or 2007. The current longitudinal analyses comprised 255 children with valid weight and length/height measurements in 2003 and at least at one other time point. These children contributed a total of 703 anthropometric measurements. The age distribution of the number of measurements in children was as follows: 0 to <6 months: 20; 6 to <12 months: 27; 12 months to <2 years: 47; 2 to <7 years: 261; 7 to <10 years: 274; and ≥10 years: 74.
Written informed consent for participation was obtained from parents or guardians before enrolment. This study was approved by the ethical review board of the School of Public Health, University of São Paulo, Brazil.
Data collection and anthropometry
At baseline (2003), trained fieldworkers performed structured face‐to‐face interviews with each child's mother or guardian during household visits. Information was collected on child's sex, age and race, presence of household assets, maternal age, education level and occurrence of hypertension during pregnancy, and child's age at introduction of weaning foods. Birthweight was retrieved from child health cards (Muniz et al. 2007).
Trained research assistants obtained anthropometric measurements from the children at a local family health clinic (in the 2003 and 2007 surveys) or at the households (in 2009), using standardised procedures and calibrated equipment (Lohman et al. 1988). The date of birth was recorded directly from birth certificates or child health cards. In 2003, among children aged <24 months, weight was measured in light clothing and without shoes to the nearest 10 g on an electronic paediatric scale (Tanita model 1583, Tanita Corporation, Tokyo, Japan), and recumbent length was measured to the nearest millimetre with a locally made infant measuring board. For children aged ≥2 years, weight was measured in light clothing and without shoes to the nearest 100 g on an electronic scale (Tanita model HS‐302), and height was measured to the nearest millimetre with a stadiometre (Seca, Hamburg, Germany in 2003 and 2007; WCS, Curitiba, Brazil in 2009) affixed to a flat surface on a wall, without a baseboard and perpendicular to the floor. Children were positioned barefoot in the vertical standing position in the middle of the stadiometre, with their head, shoulders, buttocks and heels against the wall. Mother's weight and height were subsequently measured by the research assistants, following the same standardised procedures (Lohman et al. 1988). Each measurement was repeated, and the mean value was calculated.
Data management
BMI was computed as weight in kg divided by length/height in m2. We then calculated BAZ, our main outcome of interest, according to the WHO Child Growth Standards (WHO 2006) for children aged 0–5 years and the WHO Growth Reference Data (de Onis et al. 2007) for children >5 years. The measured exposures comprised baseline household socio‐economic status, maternal characteristics and child's birthweight. We also examined associations with infant feeding practices and height at baseline.
To assess the household socio‐economic status, we performed a principal component analysis to generate a wealth index based on the presence of 14 home appliances (Muniz et al. 2007). After standardising the weight of household assets, scores were added to produce an estimated index of household wealth (Filmer & Pritchett 2001). Predictors were categorised according to previously used cut‐off points in this population. The wealth index was examined in quartiles, tertiles, and as less than or as greater than or equal to the median. Because similar results were observed, we opted to present the associations for this variable according to the latter classification. Maternal nutritional status was classified according to BMI categories as non‐overweight (<25 kg m−2) and overweight (≥25 kg m−2), because only 16 mothers had BMI values ≥30 kg m−2. Child's birthweight was categorised as ≤2500, 2501–3500 or >3500 g. The age at introduction of cow's milk, an indicator of infant feeding practices, was classified as <3 vs. ≥ 3 months. According to the WHO growth curves, stunting at baseline was defined as a height‐for‐age z‐score <−2, thinness as BAZ <−2 and risk of overweight or obesity as BAZ >1.
From a total of 467 children included at baseline (2003), the distribution of sex, age, weight, length/height, and socio‐economic, maternal, and child characteristics of children who were followed‐up in the 2007 and/or 2009 assessments, and therefore were included in the longitudinal analyses (n = 255), was similar to the distribution of those who were not followed (n = 212).
Statistical analysis
First, we compared the distribution of BAZ by categories of socio‐economic, maternal and child characteristics at baseline, using tests of trend for ordinal predictors and the Wilcoxon rank–sum test for dichotomous predictors.
We then examined the associations between the exposures of interest and BAZ by estimating average BAZ‐for‐age growth curves for each category of the predictors with the use of mixed‐effect models for repeated measurements with restricted cubic splines (see Appendix 1). Cubic splines represent non‐linear terms for age at each assessment that allow smoothing of the relation between BAZ and age. Piecewise cubic polynomials are smoothly joined at joint points or ‘knots’ (Durrleman & Simon 1989; Lourenço et al. 2012). Knots were placed at the ages 0.25, 0.75, 1.50, 3.50 and 9 years, because these ages appear to be important reference points in the curvilinear segments of the WHO growth curves (de Onis et al. 2007, WHO 2006). In each model, the outcome was BAZ, and covariates comprised the predictor of interest, linear and spline terms for child age in decimal years, and predictor category × age interaction terms. Random effects for the intercept and the linear term for age (slope) were included to account for the within‐person correlation of measurements in the estimation of the variance (Diggle et al. 2002). These methods do not require an equal number of measurements in all children, nor that measurements must be obtained at exactly the same time points on every participant; therefore, all available measurements were included in the models. Because the age distribution of children at baseline ranged from 0 to 5 years, we tested for possible birth cohort effects on the construction of the curves by including terms for year of birth. These terms were not statistically significant and their introduction did not change the magnitude of the associations. Estimates also remained similar when we considered additional adjustment for potential correlations among siblings within the same household.
Adjusted mean BAZ‐for‐age curves were obtained using multivariable mixed‐effect models. Variables were included in the model if they were considered conceptually relevant or if there was a clear association with the outcome in the unadjusted analyses. Statistical significance was an additional criterion for retaining a variable in the model. Missing observations were included in the multivariable model by creating missing‐value categories. We compared results from the model with missing‐value categories with those from a complete case analysis. Because magnitude and direction of all associations were similar, we decided on the first approach to preserve all 255 children in the multivariable model.
We estimated BAZ from the growth curves at the ages of 6 months, 12 months, 2 years, 7 years and 10 years, as the predicted values of the spline function, with values of predictor covariates at the reference category. Differences in the values of BAZ and their 95% confidence intervals (CIs) were calculated among the categories of each predictor at these ages. All reported P‐values are two‐tailed. We used SAS 9.2 (SAS Institute Inc., Cary, NC, USA) for all analyses.
Results
At baseline, among 467 children with complete anthropometric information, the mean (standard deviation) age of children was 2.6 (1.4) years (range: 0.1–5.5 years), 50.8% were male, and 88.1% were mulatto. BAZ was positively associated with male sex and maternal BMI (Table 1). In 2003, the prevalence of thinness was 3.9% (4.4% among girls and 3.4% among boys), and 12.9% of the children were considered at risk of being overweight or obese (9.6% among girls and 16.0% among boys).
Table 1.
Mean body mass index‐for‐age z‐score according to baseline characteristics of children with complete anthropometric information [Acrelândia, Brazil (2003)]
| n (%)* | Mean BAZ (SD) † | P ‡ | Missing (%) | |
|---|---|---|---|---|
| Child's sex | <0.001 | 0.0 | ||
| Female | 230 (49.2) | −0.33 (1.08) | ||
| Male | 237 (50.8) | −0.06 (1.12) | ||
| Child's age (months) | <0.001 | 0.0 | ||
| 0–5 | 32 (6.9) | −0.17 (1.05) | ||
| 6–11 | 53 (11.3) | 0.29 (1.03) | ||
| 12–23 | 80 (17.1) | 0.44 (1.19) | ||
| 24–35 | 100 (21.4) | −0.32 (1.00) | ||
| ≥36 | 202 (43.3) | −0.51 (1.01) | ||
| Wealth index | 0.32 | 1.5 | ||
| Below median | 245 (53.3) | −0.23 (1.15) | ||
| Above median | 215 (46.7) | −0.13 (1.07) | ||
| Mother's educational level (years) | 0.88 | 39.6 | ||
| 0–4 | 148 (52.5) | −0.23 (1.07) | ||
| ≥5 | 134 (47.5) | −0.19 (0.99) | ||
| Mother's age (years) | 0.07 | 31.5 | ||
| ≤20 | 44 (13.8) | 0.09 (0.99) | ||
| 21–30 | 194 (60.6) | −0.20 (1.03) | ||
| >30 | 82 (25.6) | −0.30 (1.17) | ||
| Mother's BMI (kg m−2) | 0.05 | 10.3 | ||
| Non‐overweight (<25) | 278 (66.4) | −0.26 (1.08) | ||
| Overweight (≥25) | 141 (33.6) | −0.04 (1.15) | ||
| Hypertension during pregnancy | 0.04 | 6.2 | ||
| No | 392 (89.5) | −0.21 (1.07) | ||
| Yes | 46 (10.5) | 0.19 (1.35) | ||
| Child's birthweight (g) | 0.09 | 3.0 | ||
| ≤2500 | 44 (9.7) | −0.07 (1.45) | ||
| 2501–3500 | 277 (61.2) | −0.34 (0.98) | ||
| >3500 | 132 (29.1) | 0.11 (1.15) | ||
| Age at cow's milk introduction (months) | 0.54 | 9.0 | ||
| <3 | 136 (32.0) | −0.11 (1.21) | ||
| ≥3 | 289 (68.0) | −0.21 (1.06) | ||
| Stunting at baseline | 0.75 | 0.0 | ||
| No | 420 (89.9) | −0.20 (1.12) | ||
| Yes | 47 (10.1) | −0.12 (0.99) |
BMI, body mass index; SD, standard deviation. *Totals may be less than 467 because of missing values. †BAZ: BMI‐for‐age z‐scores, calculated according to the World Health Organization (WHO) growth curves (de Onis et al. 2007, WHO 2006). ‡Test for linear trend for ordinal predictors; for dichotomous predictors, Wilcoxon rank–sum test.
The median follow‐up time for the 255 children who were evaluated in 2007 and/or 2009 was 6.9 years (range: 4.9–7.5 years), during which time a median of three anthropometric measurements was collected for each child (range: 2–3, 62 children had two and 193 children had three measurements). In 2007 and 2009, 9.6 and 17.6% of the children were at risk of being overweight or obese, respectively.
In unadjusted analyses, male sex was positively associated with mean BAZ from 6 months to 7 years. Household wealth and maternal BMI were also positively related to children's mean BAZ at ages 7 and 10 years. Children weighing >3500 g at birth had significantly higher BAZ values compared with children who weighed 2501–3500 g up until 2 years of age (Table 2).
Table 2.
Body mass index‐for‐age z‐scores according to age and baseline characteristics. Acrelândia, Brazil, unadjusted analysis (2003–2009)
| n * | Mean BAZ (SE) according to age †‡ | |||||
|---|---|---|---|---|---|---|
| 6 months | 12 months | 2 years | 7 years | 10 years | ||
| Child's sex | 255 | |||||
| Female | −0.27 (0.24) | 0.09 (0.21) | −0.28 (0.11) | −0.57 (0.09) | −0.14 (0.10) | |
| Male | 0.44 (0.22) | 0.93 (0.20) | 0.04 (0.13) | −0.09 (0.10) | 0.06 (0.13) | |
| Difference (95% CI) | 0.71 (0.07, 1.35) | 0.84 (0.27, 1.41) | 0.32 (−0.01, 0.64) | 0.48 (0.22, 0.74) | 0.20 (−0.12, 0.52) | |
| Wealth index | 253 | |||||
| Below median | 0.03 (0.14) | 0.42 (0.17) | −0.20 (0.12) | −0.47 (0.09) | −0.25 (0.11) | |
| Above median | 0.08 (0.34) | 0.58 (0.24) | −0.05 (0.12) | −0.17 (−0.10) | 0.18 (0.12) | |
| Difference | 0.05 (−0.68, 0.78) | 0.16 (−0.43, 0.74) | 0.15 (−0.18, 0.48) | 0.30 (0.03, 0.56) | 0.43 (0.11, 0.75) | |
| Mother's BMI (kg m−2) | 229 | |||||
| Non‐overweight (<25) | −0.04 (0.19) | 0.28 (0.17) | −0.22 (0.10) | −0.50 (0.08) | −0.27 (0.10) | |
| Overweight (≥25) | 0.32 (0.43) | 1.20 (0.27) | 0.13 (0.16) | 0.05 (0.15) | 0.41 (0.15) | |
| Difference | 0.36 (−0.56, 1.29) | 0.92 (0.28, 1.56) | 0.35 (−0.02, 0.72) | 0.55 (0.22, 0.88) | 0.68 (0.33, 1.04) | |
| Hypertension during pregnancy | 228 | |||||
| No | 0.10 (0.17) | 0.35 (0.16) | −0.16 (0.09) | −0.36 (0.07) | −0.08 (0.09) | |
| Yes | 0.13 (0.49) | 1.21 (0.35) | 0.18 (0.22) | 0.06 (0.24) | 0.37 (0.25) | |
| Difference | 0.03 (−0.99, 1.05) | 0.86 (0.10, 1.61) | 0.34 (−0.14, 0.81) | 0.42 (−0.06, 0.91) | 0.45 (−0.06, 0.97) | |
| Child's birthweight (g) | 249 | |||||
| a. ≤2500 | 0.96 (0.47) | 1.00 (0.43) | −0.05 (0.35) | −0.30 (0.37) | 0.14 (0.39) | |
| b. 2501–3500 | −0.31 (0.19) | 0.15 (0.19) | −0.27 (0.10) | −0.39 (0.08) | −0.09 (0.09) | |
| c. >3500 | 0.67 (0.23) | 1.02 (0.22) | 0.18 (0.15) | −0.18 (0.11) | 0.05 (0.17) | |
| Difference (a – b) | 1.27 (0.27, 2.27) | 0.85 (−0.06, 1.77) | 0.22 (−0.50, 0.94) | 0.09 (−0.65, 0.83) | 0.23 (−0.55, 1.02) | |
| Difference (c – b) | 0.98 (0.40, 1.57) | 0.87 (0.31, 1.43) | 0.45 (0.09, 0.81) | 0.21 (−0.07, 0.49) | 0.14 (−0.24, 0.52) | |
| Age at cow's milk introduction (months) | 241 | |||||
| <3 | 0.34 (0.22) | 0.89 (0.27) | 0.03 (0.16) | −0.18 (0.13) | 0.01 (0.15) | |
| ≥3 | −0.03 (0.33) | 0.32 (0.18) | −0.17 (0.10) | −0.40 (0.08) | −0.10 (0.10) | |
| Difference | 0.37 (−0.40, 1.15) | 0.57 (−0.07, 1.21) | 0.20 (−0.17, 0.58) | 0.22 (−0.08, 0.52) | 0.11 (−0.24, 0.47) | |
| Stunting at baseline | 255 | |||||
| No | 0.00 (0.18) | 0.49 (0.15) | −0.14 (0.09) | −0.36 (0.07) | −0.06 (0.08) | |
| Yes | 0.77 (0.78) | 1.25 (1.09) | −0.29 (0.23) | 0.56 (0.50) | 0.21 (0.21) | |
| Difference | 0.77 (−0.79, 2.34) | 0.76 (−1.39, 2.91) | −0.15 (−0.61, 0.31) | 0.92 (−0.07, 1.90) | 0.27 (−0.14, 0.68) | |
BMI, body mass index; CI, confidence interval; SE, standard error. *Totals may be less than 255 due to missing values. †BAZ: BMI‐for‐age z‐scores,calculated according to the World Health Organization (WHO) growth references (de Onis et al. 2007,WHO 2006). ‡Unadjusted mean values and standard errors were estimated from restricted cubic spline regression models.
In the multivariable model (Table 3), boys had a significantly higher BAZ than girls until 12 months and at 7 years. Socio‐economic status remained significantly associated with BAZ during school‐age years. Children from households above the wealth median had BAZ values higher by 0.36 (95% CI: 0.10, 0.61) at 7 years and by 0.49 (95% CI: 0.19, 0.81) at 10 years compared with children from households below the wealth index median. Maternal BMI was associated with children's BAZ since 12 months of age. By the age of 10 years, children whose mothers were overweight had a mean BAZ value higher by 0.69 (95% CI: 0.35, 1.04) in relation to children from non‐overweight mothers. Birthweight also remained positively related to BAZ during the first 2 years of life after multivariable adjustment. From 6 months to 2 years, the difference in BAZ between children weighing >3500 g and those weighing 2501–3500 g at birth ranged from 0.92 (95% CI: 0.26, 1.59) to 0.44 (95% CI: 0.10, 0.80). This difference considerably decreased to 0.20 z at 7 years old and 10 years old, and was not significant at this time. Low‐birthweight babies had a higher mean BAZ value at 6 months compared with that in babies in the reference category (difference: 1.40; 95% CI: 0.21, 2.58).
Table 3.
Multivariable model for body mass index‐for‐age z‐scores by socio‐economic, maternal and child characteristics [Acrelândia, Brazil (2003–2009)]
| Adjusted mean (SE) BAZ according to age*† (n = 255) | |||||
|---|---|---|---|---|---|
| 6 months | 12 months | 2 years | 7 years | 10 years | |
| Child's sex | |||||
| Female | −0.92 (0.35) | −0.42 (0.26) | −0.59 (0.15) | −0.94 (0.11) | −0.66 (0.14) |
| Male | −0.10 (0.27) | 0.23 (0.30) | −0.35 (0.17) | −0.47 (0.13) | −0.45 (0.16) |
| Difference (95% CI) | 0.82 (0.10, 1.54) | 0.65 (0.08, 1.23) | 0.24 (−0.10, 0.59) | 0.47 (0.21, 0.72) | 0.21 (−0.09, 0.52) |
| Wealth index | |||||
| Below median | −0.92 (0.35) | −0.42 (0.26) | −0.59 (0.15) | −0.94 (0.11) | −0.66 (0.14) |
| Above median | −0.62 (0.35) | −0.22 (0.28) | −0.39 (0.15) | −0.58 (0.12) | −0.17 (0.14) |
| Difference | 0.30 (−0.38, 0.98) | 0.20 (−0.33, 0.75) | 0.20 (−0.14, 0.54) | 0.36 (0.10, 0.61) | 0.49 (0.19, 0.81) |
| Mother's BMI (kg m−2) | |||||
| Non‐overweight (<25) | −0.92 (0.35) | −0.42 (0.26) | −0.59 (0.15) | −0.94 (0.11) | −0.66 (0.14) |
| Overweight (≥25) | −0.80 (0.43) | 0.31 (0.36) | −0.23 (0.21) | −0.40 (0.17) | 0.03 (0.19) |
| Difference | 0.12 (−0.57, 0.79) | 0.73 (0.11, 1.35) | 0.36 (0.01, 0.73) | 0.54 (0.22, 0.85) | 0.69 (0.35, 1.04) |
| Child's birthweight (g) | |||||
| a. ≤2500 | 0.48 (0.51) | 0.09 (0.47) | −0.39 (0.36) | −0.70 (0.35) | −0.28 (0.38) |
| b. 2501–3500 | −0.92 (0.35) | −0.42 (0.26) | −0.59 (0.15) | −0.94 (0.11) | −0.66 (0.14) |
| c. >3500 | 0.00 (0.26) | 0.35 (0.31) | −0.15 (0.20) | −0.74 (0.14) | −0.46 (0.20) |
| Difference (a – b) | 1.40 (0.21, 2.58) | 0.51 (−0.46, 1.48) | 0.20 (−0.53, 0.94) | 0.24 (−0.47, 0.94) | 0.38 (−0.39, 1.15) |
| Difference (c – b) | 0.92 (0.26, 1.59) | 0.77 (0.24, 1.30) | 0.44 (0.10, 0.80) | 0.20 (−0.06, 0.45) | 0.20 (−0.16, 0.56) |
Early introduction of cow's milk was not associated with BAZ after adjustment for sex, socio‐economic status, maternal nutritional status and birthweight. A positive association between stunting at baseline and BAZ was significant only at 7 years (difference: 0.98; 95% CI: 0.04, 1.90).
To assess the influence of the most important early life predictors on the overall progression of children's body weight, we present adjusted mean BAZ‐for‐age curves up to the age of 10 years using restricted cubic splines (Fig. 1). Mean BAZ estimates for all categories of exposure variables were mostly negative. While the influence of being born weighing >3500 g appeared to be more substantial during infancy, children from households above the wealth index median and from overweight mothers presented higher BAZ values, especially during school‐age years compared with the growth trajectory for the reference category.
Figure 1.
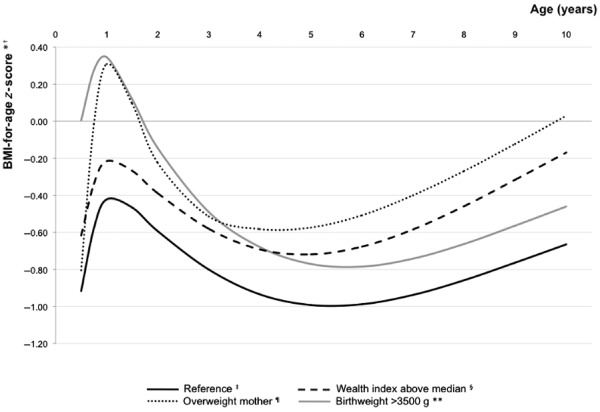
Mean adjusted body mass index (BMI)‐for‐age z‐score (BAZ) growth trajectories according to early life predictors, Acrelândia, Brazil. *Curves estimated from a restricted cubic splines multivariable model (Table 3). Covariates in the model: sex, wealth index, mother's BMI and child's birthweight.† BMI‐for‐age z‐scores calculated according to the World Health Organization (WHO) growth references (de Onis et al. 2007, WHO 2006). ‡Reference category: wealth index below median, mother's BMI < 25 kg m−2 and child's birthweight 2501–3500 g. §Wealth index above the median predicts higher BAZ at 7–10 years. ¶Mother's BMI ≥ 25 kg m−2 predicts higher BAZ at 12 months to 10 years. **Child's birthweight > 3500 g predicts higher BAZ at 6 months to 2 years.
Discussion
Using data from a population‐based prospective study of children residing in the Brazilian Amazon, we found that the BAZ during childhood was positively associated with the male sex, household wealth, maternal BMI and a child's birthweight. At baseline, the prevalence of being overweight in Acrelândia was lower than that observed in more affluent countries (Kipping et al. 2008; Kleiser et al. 2009) and wealthier regions of Brazil (Wang et al. 2002), and it increased by approximately 5% until the last follow‐up assessment performed in 2009.
In our study, boys were close to the WHO median value for BAZ at 6 and 12 months of age, whereas girls were below this value. Previous evidence suggests that boys may be heavier than girls during childhood in some developed (Eriksson et al. 2001) and developing countries (Li et al. 2007; Mushtaq et al. 2011), but this is not a consensus (Kleiser et al. 2009; Maddah & Nikooyeh 2009). Social and cultural factors could affect childbearing practices and favour a specific sex group, especially at earlier ages. However, trend analyses comparing national cross‐sectional surveys in the last decades have shown consistently higher increases in BMI for boys during childhood and adolescence in Brazil (Veiga et al. 2004) and in the United States (Ogden et al. 2012).
We found that higher household wealth was related to a greater BAZ at and after the age of 7 years. The association of socio‐economic indicators with BMI may substantially differ according to the study's setting. While in high‐income countries, there is a well‐established inverse relationship (Howe et al. 2011), in low‐ to middle‐income countries, greater BMI is usually related to higher socio‐economic status (Griffiths et al. 2008; Maddah & Nikooyeh 2009). Notably, despite the positive association with household wealth, we observed in the present analysis, the mean BAZ value in the better‐off group was still below the WHO reference median at any age. At the time of our study, Acrelândia had an estimated human development index of 0.68, which was considered intermediate and below the Brazilian national mean estimate of 0.75 (UNDP, Brazil 2000). Therefore, it is expected that affording proper and continuous access to food may be challenging in this region. In 2006, the prevalence of household food insecurity in the Brazilian macroregion that comprises the town of Acrelândia was 53.0% (Brazilian Ministry of Health 2009). By 2009, 53.9% of the children in this cohort were in households with food insecurity (M. Cardoso, unpublished observations). Because mean BAZ values were mostly negative, our results indicate that children from households above the wealth index median are not more overweight, but are less underweight than their counterparts, particularly during school‐age years. Therefore, although the ‘obesity epidemic’ has become a major public health problem worldwide, it seems important to acknowledge within‐country inequities affecting child populations from low‐ to middle‐income countries (Barros et al. 2010), in spite of their overall emergent economic development.
In our study, maternal nutritional status was positively associated with children's BAZ since at a young age. This is an indication that shared genetic and environmental factors, as well as behavioural influences, may act concomitantly in determining BMI in the offspring (Bouchard 2009; Fontaine et al. 2011). Moreover, in light of the socio‐economic context of our study's population, it is noteworthy that children from overweight mothers had a BAZ that was nearly 0.70 higher than that of children from non‐overweight mothers, yet it was not significantly above zero. These findings are consistent with other longitudinal studies showing that maternal BMI is a strong predictor of child's BMI and a major factor in the intergenerational transfer of body weight status (Cnattingius et al. 2011; Jääskeläinen et al. 2011).
In the current study, birthweight was positively related to a child's BAZ up until 2 years old, and this association was no longer statistically significant at 7 years or 10 years of age. While high birthweight has been positively associated with BMI during childhood in cross‐sectional studies (Kleiser et al. 2009), our results are similar to a follow‐up study in a Finnish rural community, where birthweight was not a good predictor of BMI at 7 years and 15 years of age, even though it was associated with BMI during infancy (Fuentes et al. 2003). Another population‐based longitudinal investigation showed that birthweight could not satisfactorily explain the BMI distribution during school‐age years (Rughlom et al. 2005).
In agreement with other reports (Lourenço & Cardoso 2009), early introduction of cow's milk was not related to BAZ during childhood in our study. Some surveys have shown that babies who have been breastfed for longer periods may exhibit slower weight gain (Karaolis‐Danckert et al. 2007), but a long‐term effect of infant feeding practices on the mean BMI from childhood to early adulthood is not supported by evidence from prospective studies (Victora et al. 2003; Bonuck et al. 2010). We also did not find consistent associations between stunting at baseline and BAZ. A cross‐sectional metabolic study that provided a self‐selection menu to shantytown pre‐pubertal children suggested that growth‐stunted individuals might eat opportunistically and display signs of impaired regulation of energy intake (Hoffman et al. 2000). Conversely, a longitudinal study in South Africa showed that urban children who were stunted at 2 years of age had no differences in BMI and body composition 7 years later compared with non‐stunted children (Cameron et al. 2005).
Our study has some limitations. First, this was a population‐based study at baseline, but our follow‐up rate was mainly affected by the high mobility of Acrelândia's residents in search for job offers in the region. The inability to contact participants because of migration out of the study's area was probably related to the sociodemographic context of their families. Therefore, caution should be taken when extrapolating our findings to the general population. Nevertheless, children included in the analyses (255 of 467 children who participated at baseline) were not different from those who were not included with respect to all the exposure variables observed at baseline, including socio‐economic, maternal and child characteristics. Although it is not possible to ascertain that dropout was not related to unobserved covariates, losses to follow‐up were also not differential with regard to child's sex, age and observed BAZ, our main outcome of interest. Second, lack of information on some exposure variables could potentially lead to the occurrence of missing data bias; however, we believe our results might not be influenced on average because the proportion of missing information for these variables in our longitudinal analysis was low (<10%). Third, even though information on children's fathers was not available, a recent large study with objectively measured data from both parents concluded that the maternal influence is stronger than the paternal effect on the intergenerational transmission of weight status (Whitaker et al. 2010). Fourth, birthweight was not directly measured by the research team, but there is evidence that child health records are valid in Brazil (Mascarenhas & Gomes 2011). Lastly, while the use of cubic splines provides flexibility to data, there is some indication that overfitting may be possible (especially with a large number of knots or a very small number of observations). In our analysis, the cubic spline function was constrained to be linear at the tails (i.e. before the first knot and after the last knot) and we considered the fewest possible number of knots, which were placed at essential age points considering the WHO growth curves. Our study also has several strengths, including its longitudinal design, a long follow‐up period and the use of direct and standardised weight and length/height measurements in both children and their mothers.
In conclusion, the present findings suggest that the socio‐economic background and the maternal nutritional status are important predictors of a child's BAZ throughout childhood. In view of the magnitude of estimates in our study's population, it is possible that the nutritional status of children from the poorest families still suffer the consequences of a lack of resources from a young age, and public health policies should consider the reduction of economic and health inequities to promote healthier child growth. Given the increase in the percentage of children classified at risk of overweight from baseline to the last follow‐up assessment, additional follow‐up will be required to ascertain whether these exposures may actually lead to excessive weight gain in later years and adulthood.
Source of funding
This study was funded by the Brazilian National Counsel of Technological and Scientific Development, CNPq (Grant nos. 551359/2001‐3, 502937/2003‐3, 307728/2006‐4 and 470573/2007‐4) and the São Paulo Research Foundation, FAPESP (Grant no. 2007/53042‐1). BHL received PhD scholarships from the FAPESP (Grant no. 2008/57796‐3) and the Organization of American States (BR Self Grad 2010/11, ID 20100656).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Contributions
BHL contributed to the study design and data collection; BHL, EV and RAA participated in statistical data analyses; BHL conducted data analyses, interpreted results and wrote the initial draft of the paper; MAC implemented and supervised all study protocols and was responsible for project management; BHL, EV and MAC participated in data interpretation and were involved in the review of the paper. All authors read and approved the final paper.
Acknowledgements
The authors gratefully acknowledge all families and health professionals in Acrelândia as well as our research team members, for their collaboration in this study.
Appendix 1
Use of restricted cubic splines to estimate growth curves
The use of smoothing splines is a relatively simple method to avoid problems that arise from inadequate linearity assumptions for regression models. The cubic spline function is a piecewise polynomial of degree n = 3, constrained in its two first derivatives to be continuous at the joint points, or knots. The number (K) and position (t 1 < t 2 < … < t K) of the knots are fixed according to how the phenomenon under study varies over its covariate space. ‘Restricted’ cubic splines are cubic splines constrained to be linear at the tails (i.e. before the first knot t 1 and after the last knot t K). The use of restricted cubic splines in a multivariable model implies the introduction of K − 2 new variables, and results in the estimation of K − 1 regression coefficients. Using the ‘+’ notation to indicate that a+ = a if a > 0, or a+ = 0 if a ≤ 0, the restricted cubic spline for a variable x is represented by:
When estimating growth curves, the piecewise cubic polynomials represent non‐linear terms connected across different intervals of the linear term for age, and the placement of knots should consider how growth rates are expected to vary along the years. This can be of special interest because growth trajectories are usually complex and may not be accurately represented by a linear function.
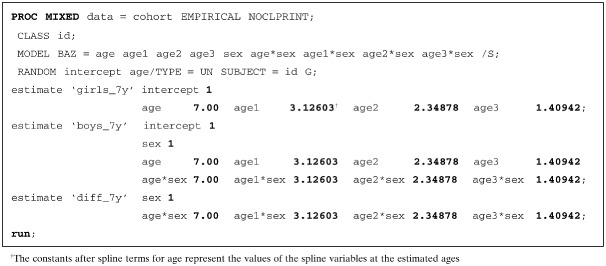
In our study, knots were placed at five age points (0.25, 0.75, 1.50, 3.50 and 9 years); therefore, three new spline variables were generated (namely, age1, age2 and age3). The spline variables were included in the mixed‐effect models along with each predictor of interest, the linear term for child age, and the interaction terms between predictor categories and linear and spline variables for age. An example of the SAS code is provided below, with child's sex as the predictor of interest and the estimation of BAZ values for girls (reference category, coded as ‘0’), boys (coded as ‘1’), and the difference among these categories, at age 7 years.
It is possible to use cubic splines with multiple covariates in the model (as shown in Table 3) and to determine the significance of non‐linearity by comparing the log‐likelihood for a model with spline variables to the log‐likelihood of a model with the linear variable only.
References
- Barros F.C., Victora C.G., Scherpbier R. & Gwatkin D. (2010) Socioeconomic inequities in the health and nutrition of children in low/middle income countries. Revista de Saude Publica 44, 1–16. [DOI] [PubMed] [Google Scholar]
- Black R.E., Allen L.H., Bhutta Z.A., de Caufield L.E., Onis M., Ezzati M. et al (2008) Maternal and child undernutrition: global and regional exposures and health consequences. Lancet 371, 243–260. [DOI] [PubMed] [Google Scholar]
- Bonuck K.A., Huang V. & Fletcher J. (2010) Inappropriate bottle use: an early risk for overweight? Literature review and pilot data for a bottle‐weaning trial. Maternal & Child Nutrition 6, 38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C. (2009) Childhood obesity: are genetic differences involved? The American Journal of Clinical Nutrition 89, 1494S–1501S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazilian Ministry of Health (2009) National Survey on Demography and Health of Women and Children – PNDS 2006: Dimensions of Reproduction and Child Health. Brazilian Ministry of Health: Brasília. [Google Scholar]
- Cameron N., Wright M.M., Griffiths P.L., Norris S.A. & Pettifor J.M. (2005) Stunting at 2 years in relation to body composition at 9 years in African urban children. Obesity Research 13, 131–136. [DOI] [PubMed] [Google Scholar]
- Cnattingius S., Villamor E., Lagerros Y.T., Wikström A.K. & Granath F. (2011) High birth weight and obesity – a vicious circle across generations. International Journal of Obesity. doi: 10.1038/ijo.2011.248. [DOI] [PubMed] [Google Scholar]
- Diggle P.J., Heagerty P., Liang K.Y. & Zeger S.L. (2002) Analysis of Longitudinal Data, 2nd edn, Oxford University Press: Oxford. [Google Scholar]
- Durrleman S. & Simon R. (1989) Flexible regression models with cubic splines. Statistics in Medicine 8, 551–556. [DOI] [PubMed] [Google Scholar]
- Eriksson J., Forsén T., Tuomilehto J., Osmond C. & Baker D. (2001) Size at birth, childhood growth and obesity in adult life. International Journal of Obesity 25, 735–740. [DOI] [PubMed] [Google Scholar]
- Filmer D. & Pritchett L.H. (2001) Estimating wealth effects without expenditure data‐or tear: an application to educational enrolments in states of India. Demography 38, 115–132. [DOI] [PubMed] [Google Scholar]
- Finucane M.M., Stevens G.A., Cowan M.J., Danaei G., Lin J.K., Paciorek C.J. et al (2011) National, regional, and global trends in body mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country‐years and 9.1 million participants. Lancet 377, 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine K.R., Robertson H.T., Holst C., Desmond R., Stunkard A.J., Sorensen T.I.A. et al (2011) Is socioeconomic status of the rearing environment causally related to obesity in the offspring? PLoS ONE 6, e27692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes R.M., Notkola I.L., Shemeikka S., Tuomilehto J. & Nissinen A. (2003) Tracking of body mass index during childhood: a 15‐year prospective population‐based family study in eastern Finland. International Journal of Obesity 27, 716–721. [DOI] [PubMed] [Google Scholar]
- Garcia M.T., Granado F.S. & Cardoso M.A. (2011) Alimentação complementar e estado nutricional de crianças menores de dois anos atendidas no Programa Saúde da Família em Acrelândia, Acre, Amazônia Ocidental Brasileira. Cadernos de Saúde Pública 27, 305–316. [DOI] [PubMed] [Google Scholar]
- Griffiths P.L., Rousham E.K., Norris S.A., Pettifor J.M. & Cameron N. (2008) Socio‐economic status and body composition outcomes in urban South African children. Archives of Disease in Childhood 93, 862–867. [DOI] [PubMed] [Google Scholar]
- Han J.C., Lawlor D.A. & Kimm S.Y.S. (2010) Childhood obesity. Lancet 375, 1737–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman D.J., Roberts S.B., Verreschi I., Martins P.A., Nascimento C., Tucker K.L. et al (2000) Regulation of energy intake may be impaired in nutritionally stunted children from the shantytowns of São Paulo, Brazil. The Journal of Nutrition 130, 2265–2270. [DOI] [PubMed] [Google Scholar]
- Howe L.D., Tilling K., Galobardes B., Davey Smith G., Ness A.R. & Lawlor D.A. (2011) Socioeconomic disparities in trajectories of adiposity across childhood. International Journal of Pediatric Obesity 6, e144–e153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jääskeläinen A., Pussinen J., Nuutinen O., Schwab U., Pirkola J., Kolehmainen M. et al (2011) Intergenerational transmission of overweight among Finnish adolescents and their parents: a 16‐year follow‐up study. International Journal of Obesity 35, 1289–1294. [DOI] [PubMed] [Google Scholar]
- Jones‐Smith J.C., Gordon‐Larsen P., Siddiqi A. & Popkin B.M. (2012) Is the burden of overweight shifting to the poor across the globe? Time trends among women in 39 low‐ and middle‐income countries (1991–2008). International Journal of Obesity 36, 1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaolis‐Danckert N., Gunther A.L.B., Kroke A., Hornberg C. & Buyken A.E. (2007) How early dietary factors modify the effect of rapid weight gain in infancy on subsequent body‐composition development in term children whose birth weight was appropriate for gestational age. The American Journal of Clinical Nutrition 86, 1700–1708. [DOI] [PubMed] [Google Scholar]
- Kipping R.R., Jago R. & Lawlor D.A. (2008) Obesity in children. Part 1: epidemiology, measurement, risk factors, and screening. British Medical Journal 337, a1824. [DOI] [PubMed] [Google Scholar]
- Kleiser C., Rosario A.S., Mensink G.B.M., Prinz‐Langenohl R. & Kurth B.M. (2009) Potential determinants of obesity among children and adolescents in Germany: results from the cross‐sectional KiGGS study. BMC Public Health 9, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhai F., Yang X., Schouten E.G., Hu X., He Y. et al (2007) Determinants of childhood overweight and obesity in China. The British Journal of Nutrition 97, 210–215. [DOI] [PubMed] [Google Scholar]
- Lohman T.G., Roche A.F. & Martorell R. (1988) Anthropometric Standardization Reference Manual. Human Kinetics Books: Champaign, IL. [Google Scholar]
- Lourenço B.H. & Cardoso M.A. (2009) Infant feeding practices, childhood growth and obesity in adult life. Arquivos Brasileiros de Endocrinologia e Metabologia 53, 528–539. [DOI] [PubMed] [Google Scholar]
- Lourenço B.H., Villamor E., Augusto R.A. & Cardoso M.A. (2012) Determinants of linear growth from infancy to school‐aged years: a population‐based follow‐up study in urban Amazonian children. BMC Public Health 12, 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddah M. & Nikooyeh B. (2009) Factors associated with overweight in children in Rasht, Iran: gender, maternal education, skipping breakfast and parental obesity. Public Health Nutrition 13, 196–200. [DOI] [PubMed] [Google Scholar]
- Manios Y., Moschonis G., Grammatikaki E., Anastasiadou A. & Liarigkovinos T. (2010) Determinants of childhood obesity and association with maternal perceptions of their children's weight status: the ‘GENESIS’ Study. Journal of the American Dietetic Association 110, 1527–1531. [DOI] [PubMed] [Google Scholar]
- Mascarenhas M.D. & Gomes K.R. (2011) Reliability of data available in the Information System for Live Birth in the city of Teresina, Piauí State, Brazil 2002. Ciência & Saúde Coletiva 16, 1233–1239. [DOI] [PubMed] [Google Scholar]
- Muniz P.T., Castro T.G., Araújo T.S., Nunes N.B., Da Silva‐Nunes M., Hoffmann E.H. et al (2007) Child health and nutrition in the Western Brazilian Amazon: population‐based surveys in two counties in Acre State. Cadernos de Saúde Pública 23, 1283–1293. [DOI] [PubMed] [Google Scholar]
- Mushtaq M.U., Gull S., Abdullah H.M., Shahid U., Shad M.A. & Akram J. (2011) Prevalence and socioeconomic correlates of overweight and obesity among Pakistani primary school children. BMC Public Health 11, 724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman M., Finlay J.E., Davey Smith G. & Subramanian S.V. (2011) The poor stay thinner: stable socioeconomic gradients in BMI among women in lower‐ and middle‐income countries. The American Journal of Clinical Nutrition 94, 1348–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden C.L., Carrol M.D., Kit B.K. & Flegal K.M. (2012) Prevalence of obesity and trends on body mass index among US children and adolescents, 1999–2010. The Journal of the American Medical Association 307, 483–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Onis M., Onyango A.W., Borghi E., Siyam A., Nishida C. & Siekmann J. (2007) Development of a WHO growth reference for school‐aged children and adolescents. Bulletin of the World Health Organization 85, 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin B.M. (2001) The nutrition transition and obesity in the developing world. The Journal of Nutrition 131, 871S–873S. [DOI] [PubMed] [Google Scholar]
- Rughlom S., Baker J.L., Olsen L.W., Schack‐Nielsen L., Bua J. & Sorensen T.I.A. (2005) Stability of the association between birth weight and childhood overweight during the development of the obesity epidemic. Obesity Research 13, 2187–2194. [DOI] [PubMed] [Google Scholar]
- United Nations Development Programme (UNDP), Brazil (2000) Human Development Index Ranking of Municipalities in Brazil, HTML version . Available at: http://www.pnud.org.br/atlas/ranking/IDH_Municipios_Brasil_2000.aspx?indiceAccordion=1&li=li_Ranking2003 (Accessed 10 September 2012).
- Veiga G.V., Cunha A.S. & Sichieri R. (2004) Trends in overweight among adolescents living in the poorest and richest regions of Brazil. American Journal of Public Health 94, 1544–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora C.G., Barros F., Lima R.C., Horta B.L. & Wells J. (2003) Anthropometry and body composition of 18‐year‐old men according to duration of breast feeding: birth cohort study from Brazil. British Medical Journal 327, 901–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. & Lobstein T. (2006) Worldwide trends in childhood overweight and obesity. International Journal of Pediatric Obesity 1, 11–25. [DOI] [PubMed] [Google Scholar]
- Wang Y., Monteiro C. & Popkin B.M. (2002) Trends of obesity and underweight in older children and adolescents in the United States, Brazil, China and Russia. The American Journal of Clinical Nutrition 75, 971–977. [DOI] [PubMed] [Google Scholar]
- Whitaker K.L., Jarvis M.J., Beeken R.J., Boniface D. & Wardle J. (2010) Comparing maternal and paternal intergenerational transmission of obesity risk in a large population‐based sample. The American Journal of Clinical Nutrition 91, 1560–1567. [DOI] [PubMed] [Google Scholar]
- World Health Organization (WHO) (2006) WHO child growth standards based on length/height, weight and age. Acta Paediatrica 450, S76–S85. [DOI] [PubMed] [Google Scholar]


