Abstract
Adherence to supplementation provided during an intervention trial can affect interpretation of study outcomes. We compared different approaches for estimating adherence to small‐quantity lipid‐based nutrient supplements (SQ‐LNS) and dispersible tablets in a randomised clinical trial in Burkina Faso. A total of 2435 children (9–18 months) were randomly assigned to receive daily 20 g SQ‐LNS with varying contents of zinc and a dispersible tablet containing 0 or 5 mg zinc. Adherence to SQ‐LNS and tablets was assessed for all children through weekly caregiver interviews, and disappearance rate was calculated based on empty and unused packages returned during home visits. Additional adherence data were collected in different randomly selected subgroups of children: 12‐h home observations were completed for children 11 and 16 months of age (n = 192) to assess consumption of SQ‐LNS and dispersible tablets, and plasma zinc concentration was measured at baseline and 18 months (n = 310). Apparent adherence to SQ‐LNS and dispersible tablets differed according to the assessment method used. Average daily caregiver‐reported adherence to both SQ‐LNS and dispersible tablets was 97 ± 6%. Disappearance rates showed similarly high average weekly adherence (98 ± 4%). In contrast, only 63% and 54% of children at 11 and 16 months, respectively, received SQ‐LNS during the 12‐h home observation periods, and fewer (32% and 27%) received a tablet. The lack of change in plasma zinc concentration after 9 months of supplementation suggests low adherence to the zinc tablet. Better methods are needed to assess adherence in community‐based supplementation trials.
Keywords: small‐quantity lipid‐based nutrient supplements, zinc, adherence, report, disappearance rate, home observation, plasma zinc concentration, caregivers, infants, Burkina Faso
Introduction
Growth faltering with respect to international growth standards occurs frequently among young children in lower income countries, in part because of inadequate intake of nutrient‐rich complementary foods (Caulfield et al. 1999). Small‐quantity lipid‐based nutrient supplements (SQ‐LNS) consisting of peanut paste enriched with milk protein, essential fatty acids, and a range of vitamins and minerals were developed to prevent undernutrition and promote children's growth and development during the complementary feeding period (Arimond et al. 2013). We recently completed a community‐based, partially double‐masked, placebo‐controlled, randomised efficacy trial among young children in Burkina Faso to determine the optimal dose of zinc to be included in SQ‐LNS to promote linear growth and development (Hess et al. 2013). As part of this trial, we assessed adherence to study supplements using a variety of methods.
As with any clinical trial, a lack of adherence to study intervention protocols can compromise the outcomes and complicate interpretation of the study results (De Roos et al. 2001; Geltman et al. 2004). Poor adherence can be defined as failure of study participants to take the prescribed intervention as indicated by the trial protocol (Kehoe et al. 2009); this can include incomplete consumption of the recommended dose as well as issues related to the timing of the dose in relation to meals (Pullar et al. 1989).
Various methods are used in clinical trials to assess adherence. The most widely used methods, such as participant reporting and product disappearance rate, are usually the most feasible and least costly, but they have important limitations (Pullar et al. 1989; Kehoe et al. 2009). Participants (or caregivers in the case of children) may over‐report adherence to gain the approval of study staff (Paulhus 1991; Kehoe et al. 2009), or underreport because of lapses in memory. Disappearance rates can also be erroneous, for example, if remaining supplements are discarded or given to non‐trial participants (Pullar et al. 1989; Kehoe et al. 2009). Direct observation of subjects consuming the supplements is a more objective method to assess adherence, although this may not reflect true adherence during non‐observation days and may lead to a decreased willingness to participate because of the additional burden on the participants (Huybregts et al. 2009; Kehoe et al. 2009). Moreover, consumption may occur outside the observation period or beyond the observers' perception. Extended periods of home observations are also quite costly and logistically difficult. Other options include the measurement of a metabolically inactive tracer (Pullar et al. 1989; De Roos et al. 2001) or a dietary biomarker (Hedrick et al. 2012). However, these methods can be logistically challenging because of the need to collect additional biological specimens, and expensive to implement in large community‐based trials. Additionally, interactions with food and non‐linear dose–response relationships may influence the adherence estimates (Aaron et al. 2011; Lo et al. 2011).
In the present study, we assessed adherence using a variety of methods and at different time points throughout the intervention period. The objective of the current analyses is to compare the results of the different approaches for estimating adherence.
Key messages
Lack of adherence to study intervention protocols can compromise outcomes and complicate interpretation of the study results.
Important differences in adherence to SQ‐LNS and dispersible tablets were found based on the assessment method used.
Caregiver report and disappearance rate likely overestimated adherence, while 12‐h home observation and plasma zinc concentration suggested lower adherence to SQ‐LNS and dispersible tablets than reported.
Lower reported adherence during child illness confirms reliability of reported non‐adherence.
Objective tools to assess adherence should be adopted in randomised controlled trials to allow for better interpretation of the study outcomes.
Materials and methods
Study design
The iLiNS‐ZINC study was a community‐based, partially double‐masked, placebo‐controlled, randomised clinical trial conducted in the Dandé health district in southwestern Burkina Faso. Ethical approval was provided by the Institutional Review Boards of the Centre Muraz in Bobo‐Dioulasso (Burkina Faso) and the University of California, Davis (USA). The study was registered with the U.S. National Institutes of Health (http://www.ClinicalTrials.gov; NCT00944281).
Nine‐month‐old children were identified during periodic censuses conducted in 34 participating communities; 25 communities were stratified to intervention cohort (IC) and 9 to non‐IC (NIC). Further details of the study design are reported elsewhere (Hess et al. 2013). For the present analyses, only children in the IC are considered. Eligible children in these communities were randomly assigned to receive one of the following interventions from 9 to 18 months of age: (1) SQ‐LNS without zinc and a placebo tablet (LNS‐Zn0); (2) SQ‐LNS with 5 mg zinc and a placebo tablet (LNS‐Zn5); (3) SQ‐LNS with 10 mg zinc and a placebo tablet (LNS‐Zn10); or (4) SQ‐LNS without zinc and a 5 mg zinc tablet (LNS‐TabZn5). A second randomisation was completed at the concession level to select subgroups for the different adherence assessment methods [12‐h home observations at 11 and 16 months; knowledge, attitudes and practices (KAP) interview at 15 months; and baseline and final plasma zinc concentration (PZC)]. The first child from each concession was eligible to participate in just one of the adherence sub‐studies (Fig. 1).
Figure 1.
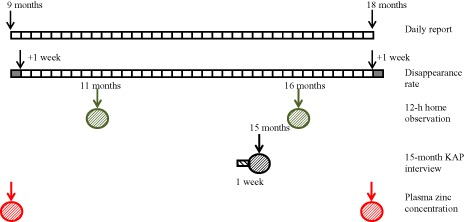
Scheme of timing of different adherence assessment methods.
Administration of SQ‐LNS and dispersible tablets
Caregivers were instructed to give a total of 20 g of SQ‐LNS/day in two separate doses. At the beginning of the study, SQ‐LNS was distributed in 140 g cups for weekly use; this was later changed to 20 g sachets for daily administration. For the cups, caregivers were given a teaspoon and instructed to give one spoonful of SQ‐LNS at each of two separate times during the day, mixed in a small portion of the child's meal (such as porridge) to ensure that the child consumed the full dose of SQ‐LNS. Similarly, for the sachets, caregivers were instructed to squeeze half of the sachet into a small portion of the child's meal. For the placebo/zinc dispersible tablets, caregivers were instructed to disperse the tablet in one teaspoon of drinking water or breast milk, and to give the child the entire dose once a day, at least 30 min after a meal. The purpose of the latter instruction was to reduce potential inhibition of zinc absorption if the tablet was given with food (Brown et al. 2007). Both SQ‐LNS and tablets were colour coded by study group and provided by Nutriset SAS (Malaunay, France). In addition to the dietary supplementation instructions, brief feeding messages were provided to promote continued breastfeeding and diverse, nutritious child diets. Instructions on how to give the SQ‐LNS and dispersible tablets were repeated monthly, while general feeding instructions were repeated irregularly.
Sample size
Daily reported adherence and disappearance rates were assessed in the whole IC. The sample size estimates were based on the number of children needed in each group to detect (with a significance of P < 0.05 and power >0.80) an effect size of >0.22 for diarrhoea incidence, malaria incidence and physical growth, assuming an attrition rate of 15%. The sample size estimate for change in PZC was based on an effect size of 0.6 and an attrition rate of 20%. For both 12‐h home observations and the 15‐month KAP interview, sample sizes of 10% and 20%, respectively, were chosen for convenience. Timing and frequency of data collection in both of the latter subsamples were chosen based on considerations of participant burden, cost constraints, and time needed for the data collection and quality control supervision.
Reported daily adherence
Field workers visited the children weekly for morbidity surveillance using standardised data collection tools and delivery of SQ‐LNS and tablets. In case of reported illnesses, treatment was provided free of charge for confirmed cases of malaria, non‐malaria fever and diarrhoea. At the same time, information on consumption of SQ‐LNS and dispersible tablets was collected by interviewing the mother or another adult caregiver (N = 2418) for the period since the previous home visit. Caregivers were asked to recall the child's daily SQ‐LNS consumption (morning and afternoon, yes or no) and tablet consumption (yes or no). SQ‐LNS daily adherence was calculated by summing all consumption episodes each day. Any special case (e.g. SQ‐LNS/tablet served but not consumed or vomited) was recorded separately as a comment. To facilitate recall, a pictorial chart was distributed weekly, on which the caregivers were encouraged to record the consumption of SQ‐LNS/tablet and any morbidity symptoms each day, using simple tallying marks, which was used as memory aid during the interview.
Disappearance rate
During the same weekly visit, field workers collected both empty and unused SQ‐LNS and tablet packages. The field workers recorded the number of unused packages as a proportion of the total distributed during the previous visit. This was done by estimating the percentage remaining in the cup or by counting the number of unused sachets for SQ‐LNS, and by counting the number of unused tablets. Daily disappearance rate was calculated as the difference between the distributed SQ‐LNS or tablets and the unused packages divided by the number of observation days.
Twelve‐hour home observation
In a randomly selected subsample (N = 192), field workers spent 12 h in the family home on two occasions when the child was approximately 11 and 16 months of age to observe the child. Three data collectors were trained to collect the 12‐h home observation data unobtrusively. Caregivers were told that the main purpose of the observation was to record the child's activity level. Data collectors emphasised upon arrival that the caregivers should not change their behaviour towards the child and should follow their usual routine. While present in the home, the data collectors also recorded the consumption of SQ‐LNS and dispersible tablets, breastfeeding, and feeding of other solid or liquid food. Field workers were trained not to show any particular interest in child feeding and/or SQ‐LNS and tablet consumption (e.g. not to move closer to examine the SQ‐LNS pot/sachet, the tablet or the child's plate). During each home visit, the child was observed by two data collectors alternating the observations from 6 am to 6 pm and recording the relevant activities every 5 min using a standardised tool on a personal digital assistant (PDA, Hewlett‐Packard Development Company, L.P., Palo Alto, CA, USA). Child observations were carried out when the children's general health status was reported as normal, and were rescheduled in case of illness. Information recorded about SQ‐LNS and dispersible tablet adherence included whether the supplement was served, the time of administration, the estimated amount consumed by the child, the way the products were offered (with food or alone) and any sharing with other family members. The consumed amounts of SQ‐LNS and tablets were calculated as the sum of all portions administered and consumed.
Fifteen‐month KAP interview
At 15 months, a separate data collection team interviewed the child's caregiver to assess the caregiver's knowledge, attitude and practices related to complementary feeding (N = 349). KAP interviews were conducted by study personnel who were not involved in the distribution of SQ‐LNS or tablets, so less reporting bias might be expected during these interviews. In particular, caregivers were interviewed on: (1) SQ‐LNS consumption during the previous week; (2) SQ‐LNS consumption on the previous day; (3) acceptance of SQ‐LNS by the child and any reasons for non‐acceptance; (4) sharing of SQ‐LNS with other household members; and (5) method of serving the SQ‐LNS during the previous week (with any liquid or solid food). No information was collected on tablet consumption during these interviews on child feeding practices.
Plasma zinc concentration
Venous blood samples were collected from children in a randomly selected subgroup (N = 310) at enrollment (age 9 months) and after 9 months of intervention (age 18 months) using specimen collection and processing methods recommended by the International Zinc Nutrition Consultative Group (Brown et al. 2004). Children had to be reported free from fever and diarrhoea symptoms during the 2 days preceding the blood draw. At both time points, blood was drawn 1–2 h after the last breastfeeding episode. Blood was collected in trace element‐free, lithium heparin vacutainer tubes (Sarstedt AG & Co, Nümbrecht, Germany). Blood samples were stored on ice and transported to the field laboratory, where plasma was separated by centrifuging at 2800 rpm for 10 min and stored at −20°C until analyses. PZC was measured with inductively coupled plasma optical emission spectrophotometry (Vista; Varian Inc, Walnut Creek, CA, USA) at the Children's Hospital of Oakland Research Institute (Killilea & Ames 2008; Wessells et al. 2012). Acute phase proteins (C‐reactive protein and α‐1‐acid glycoprotein) were analysed by enzyme‐linked immunosorbent assay (DBS‐Tech, Willstaett, Germany) (Erhardt et al. 2004) to adjust PZC for the effect of subclinical inflammation (Thurnham et al. 2010).
Baseline socio‐economic status (SES) characteristics
Baseline data on maternal age, education level and marital status, number of children in the household, and data on household food insecurity access scale (Coates et al. 2007) were collected via interview for all study households within 2 weeks of enrollment.
Statistical analysis
Children with less than 1 week of data collected (n = 17) were not included in the analysis. All the data were checked for consistency during cleaning and analysis. Inconsistent data were excluded based on pre‐defined criteria. Outcomes for the different adherence assessment methods (reported daily adherence and disappearance rate, 12‐h home observation, 15‐month KAP interview and adjusted PZC) were compared by study group using analysis of covariance for continuous outcome variables and logistic regression for categorical outcome variables. Additionally, outcomes for the 12‐h home observation were analysed using mixed model analysis to account for repeated measurements from the same subject. Group was used as the main effect, and age, initial PZC and sex as covariates for PZC analysis. All the analyses accounted for the random effect of the community, and for the family compound (i.e. concession) in case of daily reported adherence and disappearance rate. Group means were compared post hoc using least‐square means with the Tukey–Kramer test.
Associations between reported daily adherence and disappearance rate, reported daily adherence and 12‐h home observation, and between reported daily adherence and 15‐month KAP interview of the same child and during the same observation day were calculated by non‐parametric Spearman correlation. Prevalence of reported non‐adherence and its association with illness days (fever, diarrhoea, malaria, vomiting, anorexia or hospitalisation) were analysed using mixed model adjusted for the random effect of the village and the concession. All statistical analyses were carried out using SAS software for Windows (9.3, SAS Institute, Cary, NC, USA).
Results
Participant characteristics
The characteristics of participants have been described previously (Hess et al. 2013). Briefly, the children resided in rural agricultural communities in southwestern Burkina Faso. Most of the children's primary caregivers were non‐literate (58%) or had low or informal educational levels (32%). At baseline, when the children were 9 months of age, they had moderate degrees of stunting (prevalence of children with length‐for‐age z‐score <−2 SD = 22.9%) and wasting (prevalence of children with length‐for‐weight z‐score <−2 SD = 16.2%). The four intervention groups did not differ with regard to child age and length, and most household SES characteristics.
Reported daily adherence
Reported adherence to the recommended daily doses of SQ‐LNS and dispersible tablets was very high, with an average per child adherence rate of 97 ± 6% (n = 2418 children; 562 020 and 565 955 days of SQ‐LNS and dispersible tablet consumption, respectively) for both types of supplements across all the groups. There were no differences in reported adherence by study group (P = 0.22 and P = 0.35 for average daily SQ‐LNS and dispersible tablet intake, respectively, Table 1), and there were no changes in reported adherence rates during the course of the 9‐month study period. SQ‐LNS consumption reportedly occurred only once a day (vs. the recommended twice per day) on 0.9% of days (n = 5076 days) because: (1) the full dose of SQ‐LNS was given in a single feeding (n = 876 days); (2) the child refused to consume it during the second feeding (n = 358 days); (3) the caregiver refused or forgot to give the second dose (n = 205 days); or (4) due to traveling, vomiting or sharing (n = 67 days). The child reportedly received no SQ‐LNS on 2.3% of days (n = 13 021 days) mainly because the child refused to consume it (n = 2490 days), the caregiver refused or forgot to administer the dose (n = 1824 days), or due to traveling (n = 1286 days).
Table 1.
Adherence to SQ‐LNS and dispersible tablets by study group and method of assessment
| Assessment methods | LNS‐Zn0 | LNS‐Zn5 | LNS‐Zn10 | LNS‐TabZn5 | P‐value for the difference between groups § | All |
|---|---|---|---|---|---|---|
| Reported daily adherence* | ||||||
| N of children | 596 | 609 | 600 | 613 | 2418 | |
| Mean N of days of observation/child | 239 ± 63 | 237 ± 63 | 240 ± 61 | 238 ± 62 | 0.932 | 239 ± 63 |
| Mean % of days reported consuming SQ‐LNS/child | 96.7 ± 6.6 | 96.5 ± 7.5 | 97.1 ± 5.2 | 96.8 ± 6.6 | 0.225 | 96.8 ± 6.5 |
| Mean % of days reported consuming tablets/child | 97.5 ± 6.1 | 97.1 ± 7.5 | 97.7 ± 5.1 | 97.3 ± 6.6 | 0.353 | 97.4 ± 6.4 |
| Disappearance rate* | ||||||
| Mean N of days with returned SQ‐LNS package/child | 227 ± 64 | 224 ± 65 | 228 ± 63 | 224 ± 63 | 0.882 | 226 ± 64 |
| Mean % of days SQ‐LNS emptied/child | 97.9 ± 5.2 | 98.2 ± 3.9 | 98.1 ± 4.4 | 98.0 ± 4.7 | 0.684 | 98.1 ± 4.6 |
| Mean N of days with returned capsule packages/child | 235 ± 64 | 232 ± 65 | 235 ± 62 | 234 ± 63 | 0.896 | 234 ± 63 |
| Mean % of days capsule packages emptied/child | 99.0 ± 4.1 | 99.2 ± 2.4 | 99.1 ± 2.7 | 98.9 ± 3.1 | 0.567 | 99.1 ± 3.2 |
| Twelve‐hour home observation at 11 and 16 months † | ||||||
| N of children observed at 11 and/or 16 months | 44 | 45 | 45 | 45 | – | 179 |
| Total number of days of observation at 11 and 16 months combined | 72 | 69 | 71 | 68 | – | 280 |
| Number of days SQ‐LNS consumed, n (%) | 32 (44.4)b | 50 (72.5)a | 41 (57.7)ab | 41 (60.3)ab | 0.019 | 164 (58.6) |
| Daily % SQ‐LNS consumption when offered | 78.9 ± 59.2 | 108.6 ± 80.6 | 84.4 ± 71.0 | 90.7 ± 66.3 | 0.354 | 92.5 ± 71.3 |
| Number of days tablets consumed, n (%) | 16 (22.2) | 29 (42.0) | 21 (29.6) | 17 (25.0) | 0.049 | 83 (29.6) |
| Daily % tablet intake when offered | 90.6 ± 20.2 | 95.7 ± 15.0 | 89.3 ± 24.5 | 92.6 ± 21.2 | 0.702 | 92.5 ± 71.3 |
| Fifteen‐month KAP interview ‡ | ||||||
| N of children | 87 | 83 | 92 | 87 | – | 349 |
| % of children who reportedly consumed SQ‐LNS on previous day, n (%) | 81 (95.3) | 76 (92.7) | 87 (97.7) | 85 (100.0) | 0.521 | 329 (96.5) |
| Mean N of days study child consumed SQ‐LNS during previous week | 6.5 ± 1.4 | 6.6 ± 1.3 | 6.7 ± 1.4 | 6.6 ± 1.4 | 0.945 | 6.6 ± 1.3 |
KAP, knowledge, attitudes and practices interview; SQ‐LNS, small‐quantity lipid‐based nutrient supplements. *Assessed during weekly home visit. Children were considered in the analysis when they had at least 1 week of follow‐up. †Assessed at 11 and at 16 months of age during the 12‐h home visit. ‡At 15 months of age, knowledge, attitude and practices interview regarding consumption of SQ‐LNS during the previous day/week. §Values presented are unadjusted means ± SD, and proportions n (%). P‐values are for chi‐square tests for comparing proportions across observation time, and mixed model adjusted for the random effect of village and concession for means. Values in the same row with different superscripts are significantly different (P < 0.05).
Dispersible tablets were reportedly not consumed on 2% of days (n = 11 804 days). The reasons for non‐adherence were mainly caregiver's refusal or forgetfulness (n = 1932 days), child refusal (n = 1712 days), travel (n = 1272 days) or vomiting (n = 423 days).
Adherence based on supplement disappearance rate
Adherence based on returned unused portions of SQ‐LNS and tablets was also very high and did not differ by study group (Table 1). Disappearance rate and reported daily adherence during the supplementation period were significantly correlated for both SQ‐LNS (r = 0.82, P < 0.0001 reported daily adherence vs. disappearance rate) and dispersible tablet (r = 0.94, P < 0.0001).
Overall, 95.1% of the SQ‐LNS packages that were distributed were returned, and 95.6% of these were empty packages. About 98.6% of the tablet blister packages were returned, of which 97.2% were empty.
Adherence during 12‐h home observations
A total of 147 children were included in the 12‐h home observations at 11 months of age, and 133 of these children participated again at 16 months of age. Children randomly selected for these observations did not differ significantly from those who were not selected for any of the baseline characteristics (data not shown). A total of 67 observation days were dropped from the analyses because the children were not observed consuming any food or breast milk during the 12‐h observation period, which was deemed to be implausible.
Because there were no significant differences among study groups for most of the data recorded during the 12‐h home observations, the groups were combined for the analyses at each time point (Table 2). It has to be noted that our sample size was not powered to detect differences among groups. Among the children included in these analyses, 63% of those observed were offered SQ‐LNS during the 12‐h observation period at 11 months, and 54% were offered SQ‐LNS at 16 months. At both time points, there were no significant relationships between the percent of children who were offered SQ‐LNS and the number of days since the previous supplement distribution day (P = 0.104). The average proportion of the daily recommended SQ‐LNS dose (20 g) that was consumed was high (93 ± 71%) at both time points among those who were offered SQ‐LNS. Despite the recommendation that SQ‐LNS should be mixed with food, it was given alone (as a snack) in 60% and 86% of the observed servings at 11 and 16 months, respectively. More than 20 g was served on only a few of the observation days (2% and 1% at 11 and 16 months, respectively), mainly during the period when SQ‐LNS was packaged in a single 140 g cup for use during the whole week. Sharing of SQ‐LNS was observed in 8% of SQ‐LNS feeding observations. In 4 of the 19 instances of sharing, the SQ‐LNS was shared with another child at the same time that the study child was eating it. In all other cases (15/19), only the leftover portion was shared, mainly with other children. For most SQ‐LNS feeding observations, the child's mother administered the SQ‐LNS (87% and 51% at 11 and 16 months, respectively). Eight per cent of the children were feeding themselves with SQ‐LNS at 11 months and 36% were self‐fed at 16 months.
Table 2.
Consumption of SQ‐LNS, dispersible tablets, breastfeeding and complementary feeding practices as recorded during 12‐h home observation at 11 and 16 months of age
| Indicators | 11 months | 16 months | P‐value for the difference between the two times for all groups* |
|---|---|---|---|
| Number of children | 147 | 133 | |
| Child age (months) | 12.4 ± 0.7 | 17.1 ± 0.7 | |
| Number of breastfeeding episodes † | 9.9 (9.0, 10.8)a | 9.2 (8.4, 9.9)b | 0.017 |
| Number of feeding episodes of solid foods other than LNS | 3.8 (3.3, 4.2)a | 2.5 (2.1, 2.9)b | 0.0002 |
| SQ‐LNS | |||
| Number of LNS servings per day | 0.9 (0.7, 1.1)a | 0.7 (0.5, 0.8)b | 0.013 |
| Child offered any LNS (%) | 92 (62.6) | 72 (54.1) | 0.109 |
| Mean % LNS consumed when offered | 90.0 ± 72.0 | 95.8 ± 70.7 | 0.740 |
| Frequency of SQ‐LNS offered alone (vs. with porridge or other food), n (%) | 79 (59.8) | 79 (85.9) | – |
| Dispersible tablet | |||
| Child offered dispersible tablet, n (%) | 47 (32.0) | 36 (27.1) | 0.370 |
| Mean % tablet consumed when offered | 88.8 ± 23.8b | 97.2 ± 11.6a | 0.039 |
| Dispersible tablet served >30 min from meal/LNS, n (%) | 23 (48.9) | 22 (61.1) | 0.273 |
| Tablet offered with n (%): | – | ||
| Water | 30 (63.8) | 17 (47.2) | |
| Maternal milk/milk | 10 (21.2) | 17 (47.2) | |
| Porridge | 4 (8.5) | – | |
| Solid tablet | 2 (4.2) | 1 (2.8) |
SQ‐LNS, small‐quantity lipid‐based nutrient supplements. *Values presented are unadjusted means (95% CI), means ± SD and proportions n (%). P‐values are for chi‐square tests for comparing proportions across observation time, and mixed model adjusted for the random effect of village for means. Values in the same row with different superscript are significantly different (P < 0.05). †A breastfeeding episode started when the child latched on, and finished when the child stopped breastfeeding for at least 5 min. If he/she latched on again before 5 min, it was considered as one episode.
The dispersible tablet was given fewer times than the SQ‐LNS during the 12‐h home observations at both time points (32% and 27% at 11 and 16 months, respectively), but the whole tablet was consumed when served. The number of days since the last weekly round of delivery of the supplements to the home was not related to the likelihood of consumption (P = 0.235). On 51% and 39% of the times that the tablet was offered at 11 and 16 months, respectively, the tablet was given less than 30 min after a meal or SQ‐LNS had been served, potentially compromising zinc absorption. As with SQ‐LNS, the mother was the person who usually administered the dispersible tablet to the child (96% and 89% at 11 and 16 months, respectively).
Adherence according to KAP interview at 15 months
In general, the characteristics of the 349 children who were randomly selected for the KAP interview did not differ by treatment group (except for baseline maternal marital status, P = 0.043) nor did they differ from the cohort as a whole, except for some maternal characteristics (data not shown). In particular, mothers of children in this subgroup were slightly more likely to have BMI > 25 kg m−2 (9% vs. 6%, P = 0.015).
Adherence to SQ‐LNS during the previous day and week, as reported during the KAP interview, was as high as the adherence reported to the weekly surveillance team (Table 1). For the small number of cases where SQ‐LNS was reportedly not served, the caregivers stated that this was due to child refusal 25% of the time. Caregivers reported that SQ‐LNS was served alone half of the time, and the rest of the time it was served according to the protocol (i.e. with porridge or other family food). Sharing of SQ‐LNS during the previous week was reported during half (49.7%) of the interviews, with the SQ‐LNS being shared with a sibling (94/173 cases), other children (45/173) and the mother (38/173).
Plasma zinc concentration
Among the children selected for participation in the biochemistry subgroup, 58% were included in the present analysis because they provided blood samples at both 9 and 18 months. Children who provided the blood samples did not differ from those not selected (data not shown). Final PZC did not differ among study groups after adjusting for acute phase proteins and other covariates (Table 3).
Table 3.
Plasma zinc concentration in children at 9 and 18 months by study group
| Adjusted plasma zinc concentration* | LNS‐Zn0 | LNS‐Zn5 | LNS‐Zn10 | LNS‐TabZn5 | P‐value between groups † |
|---|---|---|---|---|---|
| N of children with samples analysed at both time points | 84 | 74 | 79 | 73 | |
| Adjusted plasma zinc concentration at 9 months (μg dL−1) | 68.1 ± 1.2 | 68.1 ± 1.2 | 71.0 ± 1.2 | 71.3 ± 1.2 | 0.208 |
| Adjusted plasma zinc concentration at 18 months (μg dL−1) | 64.1 ± 1.2 | 65.1 ± 1.2 | 64.5 ± 1.2 | 65.0 ± 1.2 | 0.842 |
*Values presented are means ± SD, adjusted for acute phase proteins as described in text. † P‐values are for mixed model adjusted for the random effect of village, and for time of blood draw, time since last breastfed, log CRP, log AGP, sex and age at baseline, and for adjusted plasma zinc concentration at 9 mo and other covariates at 18 mo.
Comparison of adherence results assessed by different methods
SQ‐LNS adherence as reported to the surveillance field worker was compared with 12‐h home observation on the same day for the same child. Daily reported adherence to SQ‐LNS was not significantly correlated with the 12‐h home observation data (r = 0.035, P = 0.593). On the observed days, caregiver reported that SQ‐LNS was consumed on 237 days (97%), and the child was indeed observed consuming a portion of or the total dose of SQ‐LNS during the 12‐h observation period during 123 of these days (51%). In four cases (2%), the caregiver reported that no SQ‐LNS was consumed, and the child also did not consume any SQ‐LNS during the 12‐h home observation; in two cases (1%), the caregiver reported there was no SQ‐LNS given to the child, but the child was observed consuming SQ‐LNS; and in 112 cases (46%), the caregiver reported that the child consumed either half (two cases) or all (110 cases) of the SQ‐LNS dose, but the child was not given any SQ‐LNS while observed. Similarly, daily reported adherence to the dispersible tablet was also only weakly associated with the 12‐h home observation data on the same day (r = 0.11, P = 0.067). In 66 cases (27% of total cases), the caregiver reported that the tablet was administered to the child, and the child was observed taking the tablet. However, in 167 cases (69%), the observations did not confirm the reported tablet consumption.
Data from the 15‐month KAP interview, and reported daily adherence from the same day showed a high agreement. SQ‐LNS was reportedly consumed in 316 cases (95% of total cases) by daily report and 15‐month KAP interview. In three cases (1%), the caregiver reported on the daily report that SQ‐LNS was not consumed by the child, but reported during the 15‐month KAP interview that the child was given SQ‐LNS. In 12 cases (4%), the caregiver reported on the daily report that SQ‐LNS was consumed by the child, while reporting during the 15‐month KAP interview that the child was not given SQ‐LNS.
Child illness and reported consumption of SQ‐LNS and dispersible tablet
Reported adherence to both supplements was significantly less on days with malaria, fever, diarrhoea, vomiting, anorexia or hospitalisation (Table 4). During illness days, reported adherence to SQ‐LNS was reduced slightly more than adherence to the tablet (prevalence of non‐adherence was 9.9% for SQ‐LNS vs. 6.9% for tablet during fever; 7.4% vs. 5.0% during diarrhoea; 15.5% vs. 10.5% during anorexia; 16.5% vs. 12.8% during vomiting; 7.4% vs. 6.9% during malaria; P < 0.0001 for all).
Table 4.
Number of days reportedly not consuming SQ‐LNS and tablets by illness status
| Number of days of observation, n | Number of days of illness or hospitalisation, n (%) | % of healthy days with reported non‐adherence ‡‡ | % of days of illness or hospitalisation with reported non‐adherence ‡‡ | Difference in means of prevalence ‡‡ | |
|---|---|---|---|---|---|
| Reported non‐adherence for SQ‐LNS | |||||
| Fever † | 569 856 | 20 177 (3.5) | 1.9 | 10.3 | −8.3 (−9.0, −7.7)* |
| Diarrhoea ‡ | 569 817 | 16 973 (3.0) | 2.1 | 7.7 | −5.6 (−6.3, −5.0)* |
| Anorexia § | 569 875 | 20 311 (3.6) | 1.7 | 16.0 | −14.2 (−15.2, −13.3)* |
| Vomiting ¶ | 569 810 | 6 326 (1.1) | 2.1 | 17.5 | −15.5 (−16.8, −14.1)* |
| Malaria** | 568 663 | 8 595 (1.5) | 2.0 | 8.9 | −6.9 (−7.8, −6.0)* |
| Hospitalisation †† | 593 733 | 141 (0.02) | 2.8 | 74.5 | −71.8 (−83.1, −60.4)* |
| Reported non‐adherence for dispersible tablet | |||||
| Fever | 569 856 | 20 177 (3.5) | 1.8 | 7.1 | −5.3 (−5.9, −4.7)* |
| Diarrhoea | 569 817 | 16 973 (3.0) | 1.9 | 5.2 | −3.3 (−3.9, −2.8)* |
| Anorexia | 569 875 | 20 311 (3.6) | 1.7 | 10.8 | −9.1 (−9.9, −8.3)* |
| Vomiting | 569 810 | 6 326 (1.1) | 1.9 | 13.4 | −11.5 (−12.8, −10.3)* |
| Malaria | 568 663 | 8 595 (1.5) | 1.8 | 6.3 | −4.5 (−5.3, −3.7)* |
| Hospitalisation | 593 733 | 141 (0.02) | 2.5 | 70.1 | −67.5 (−79.4, −55.6)* |
SQ‐LNS, small‐quantity lipid‐based nutrient supplements. *P < 0.0001. †Fever can be reported or confirmed: (1) reported fever was defined as any reported fever by the caregiver, whether or not confirmed by measured elevated temperature; (2) confirmed fever was defined as measured temperature >37.5°C by auricular thermometer. ‡Diarrhoea was defined as the reported presence of three or more liquid or semi‐liquid stools during 24‐h period. §Reported by caregiver, and defined as reduced or no intake of breast milk and other complementary foods during the reported day. ¶Child reportedly vomited one or more times per day. **An episode of malaria was defined as the presence of a new episode of reported or confirmed fever and a positive malaria rapid diagnostic test (RDT) obtained 21 days after any previous malaria episode. ††Hospitalisation was defined as at least 24‐h stay in health centre because of illness. ‡‡Values presented are adjusted means of prevalence of reported non‐adherence during healthy or unhealthy days and the difference in means of prevalence (95% confidence intervals for the difference in means). P‐values are for mixed model, adjusted for the random effect of village and concession, and weighted for number of adherence days,
Discussion
We found that different methods of obtaining information on adherence to recommended supplements of SQ‐LNS and dispersible tablets for young children provided substantially different estimates of consumption. Reported adherence to study supplements was very high, as has been observed in other efficacy trials of SQ‐LNS (Adu‐Afarwuah et al. 2007) and dispersible tablets (Becquey et al. 2013), and supplement disappearance rates were similarly high. By contrast, observers based in the home for 12‐h periods noted supplement consumption during a much lower proportion of observation days, which was consistent with the lack of change in PZC found in all the groups, including those children who received zinc as a dispersible tablet. In a previous study in the same region of Burkina Faso and using the same zinc tablets as in the present study, we found a sizeable increase in PZCs following directly observed consumption of tablets provided to fasting children (Wessells et al. 2012).
Strengths of the current study include the use of multiple strategies commonly used in clinical trials to assess adherence to the study products, the large sample size, the thorough training and close supervision of the different data collection teams, the measures taken in the field to control for the quality of the data, and the different statistical analyses used to compare the results obtained by each method. However, some specific limitations have to be considered when evaluating the adherence methods in the context of our study. Except for the daily reporting and disappearance assessments, we collected information from three independent subgroups to avoid respondent burden. Thus, we were not able to compare intra‐individual differences for all children. Moreover, the number of returned packages was considered to be a quality control tool for the daily report and not an independent assessment method (i.e. the field worker would revise the caregiver report if the number of unused packages did not correspond with the daily report). This likely explains the high correlation between the two methods. Another limitation of the home observations was that children were observed only during 12 h on just 2 days. It is possible that the supplements could have been consumed before or after the observer was present in the home or outside of the observer's sight. Furthermore, the use of PDAs to collect these observations was challenging because of frequent malfunctions in the field, which resulted in some loss of data (corrupted files). Although the low level of observed supplement consumption is consistent with the level of change in PZC, this conclusion may only be valid for the group assigned to zinc tablets, because previous studies have found that PZC may not respond to additional zinc provided in food, despite the consistent positive response to supplements (Brown et al. 2002, 2007; Aaron et al. 2011; Lo et al. 2011).
Strengths and limitations of methods used to assess adherence
Daily reporting is an easy method to implement in a large‐scale trial with weekly data collection. However, we believe for several reasons that over‐reporting occurred. In particular, we think that over‐reporting occurred because of the limited variability during the 9‐month period (Kehoe et al. 2009), the large discrepancy with the 12‐h home observations and the reports of sharing during the KAP interviews. A possible reason for over‐reporting adherence is to gain approval of the study workers who were responsible for distributing the study supplements. This may not translate across all settings, though, and there could be less bias in populations with higher income and education levels or in different cultural contexts. For example, a low adherence (30–63%) to both ferrous fumarate sprinkles and ferrous sulphate drops administered to 6‐month‐old infants was reported by low‐income caregivers in the United States (Geltman et al. 2009). Furthermore, only 7–9% of 6–18‐month‐old children reportedly consumed all of the recommended amounts of plumpydoz (a LNS product that provides daily 247 kcal to young children) during an efficacy trial in rural Honduras (Siega‐Riz et al. 2014), although 70% of the children reportedly consumed at least some of the supplement.
Disappearance rate calculated according to the number of returned product packages resulted in similar adherence estimates compared with daily reported adherence for both SQ‐LNS and dispersible tablets. However, disappearance rate is also susceptible to manipulation (Appelgren et al. 2010). Because the disappearance rate and the daily reported adherence rate were high, and we suspect that the daily reported adherence rate was inflated, we believe that sharing may have occurred frequently in case of SQ‐LNS.
By using an independent KAP team to collect data on reported adherence, our objective was to obtain information on reported adherence in a potentially more ‘objective’ way, as this team was not involved in distributing the supplements. Adherence data obtained during the 15‐month KAP interview were generally consistent with the high reported daily adherence, which could indicate that the daily reported adherence gave realistic numbers. However, it is also possible that the caregivers still viewed the KAP team as a part of the broader study team, so they continued to adjust their report to please the study staff. Half of caregivers reported during this interview that the SQ‐LNS was shared among household members, which confirmed our belief that it was a fairly common practice in study households. This frequency of sharing is substantial and should be taken into consideration in planning further interventions. Except for the information about sharing, the KAP interview did not add value to the adherence information collected by the daily report. Because caregivers seem willing to provide information on sharing, a question about sharing and reasons for non‐adherence could be added systematically to the weekly questionnaire instead of using an independent team for assessing reported adherence.
In contrast to the daily reported adherence, the 12‐h home observation indicated a considerably lower proportion of supplement consumption. There are several explanations for this discrepancy with the three aforementioned assessment methods: (1) possible non‐administration of SQ‐LNS and the dispersible tablet and over‐reporting by interviews; (2) difficulty observing the child at all times during the 12‐h period (especially when the child went into private rooms); (3) supplementation occurring outside the 12‐h observation period; and (4) recording errors by the field staff conducting the observations. It is important to note that 67 observation days were dropped because no food consumption or breastfeeding episodes were recorded, presumably because the children were eating elsewhere or observers failed to record the activity when it occurred. Nevertheless, the 12‐h home observation was the method that gave the most complete details about adherence to the recommended study supplementation practices, including feeding behaviours and timing of supplementation, both of which are essential for understanding the delivery of the intervention. Additionally, based on the relatively low observed adherence at both time points, we think caregivers were not intentionally changing their behaviours. Furthermore, the hypothesis that supplement administration might have occurred during the first days following their weekly distribution was rejected, as our analysis revealed no association between the day of supplement delivery to the home and observed consumption of SQ‐LNS and tablets. Although home observation is more objective in assessing adherence, it has some limitations primarily related to logistics: the difficulty in observing the child continuously during 12 h; the lack of information on the activities that occurred outside this observation window, i.e. before 6 am and after 6 pm; and the high cost required for this method, especially when children live in multiple, widely dispersed villages.
The lack of change in PZC for children supplemented with zinc tablets in the present study is unexpected and could indicate low adherence to the dispersible tablet, either in its administration or its timing in relation to meals. The lack of change in PZC from baseline among children who received additional zinc through SQ‐LNS could be due to low adherence or to the effect of food on zinc absorption or post‐absorptive metabolism, as was found in other trials providing 5 mg of zinc mixed with food (Zlotkin et al. 2003; Brown et al. 2007; Adu‐Afarwuah et al. 2008). However, two studies found a significant increase in PZC after supplementing young children with 5 and 10 mg zinc in micronutrient powder provided in food (Samadpour et al. 2011; Jack et al. 2012). Because of the contradictory findings when supplementing with zinc in food, we are not able to draw a firm conclusion about adherence to SQ‐LNS containing 5 or 10 mg zinc based on PZC.
Feeding practices during illness
Caregivers in many settings stop feeding children during illness, and/or the children refuse to eat (Caulfield et al. 1999). Caregivers in our study reported higher non‐adherence when the child was ill, and this was more pronounced for SQ‐LNS than for the dispersible tablets. This suggests that reports of non‐adherence may be reliable, even though we suspect over‐reporting on non‐illness days. Another possible explanation for lower reported supplement consumption on illness days is that the caregivers feared the supplements were contributing to the illness symptoms despite information to the contrary provided during study enrollment.
Alternative methods to assess adherence
Our comparison of the most commonly used methods to assess adherence during field trials demonstrated several limitations of these methods and the need to develop other approaches. Alternative methods that could provide more relevant information are: (1) providing the study products under the study workers' supervision or controlled supplementation (Brown et al. 2007; Huybregts et al. 2009; Wessells et al. 2012); (2) use of biomarkers of response to supplementation; (3) use of external tracers; and (4) use of a recently developed digital health feedback system. Controlled supplementation has some limitations, including the cost of daily follow‐up in a large‐scale trial and during a long time period, and the high burden to both study field workers and caregivers that is associated with daily visits. It is also not suitable for effectiveness trials because this level of attention is unrealistic for most programs. The use of biomarkers may be more objective compared with other methods of adherence assessment. Depending on the type of supplements and the study population, certain biomarkers can be measured in the plasma or urine. To be useful, the metabolism of the biomarker must be understood, and it should not be affected by non‐dietary factors, and should reflect long‐term vs. recent intake. These criteria can be difficult to meet for some nutrients, so appropriate biomarkers may not be available for use in every trial. Additionally, the high cost of obtaining and analyzing biological samples could limit extensive use of this method. Examples of external tracers that have been used previously in clinical trials with adult subjects are lithium (De Roos et al. 2001) and para‐amino benzoic acid (Roberts et al. 1990). While both do not enter into the metabolism and their recovery in urine is complete, the need for 24‐h urine collection makes this method impractical for large‐scale trials, especially in young children. To the best of our knowledge, no research exists addressing the question on whether external tracer/creatinine ratio of a spot urine sample is indicative of 24‐h urinary excretion. However, it may be possible to measure blood levels instead to make this method more feasible. As an example, De Roos et al. (2001) and Donahoo et al. (2004) validated a method to measure serum lithium after intake of lithium‐marked food in adherence assessments in adults. Finally, the use of digital health feedback system (Proteus Digital Health, CA, USA) is a promising technology which uses an ingestible sensor in the supplement, which is activated upon ingestion. The activated sensor sends information to a patch, worn on the body, which sends information on the time of intake to a mobile device. This system has been used for tracking adherence to medications but could be adapted for large‐scale nutritional trials as well. One limitation of this method is that the full intake is not a condition for recording an event, so it is possible to monitor frequency of intake, but not actual amounts consumed. Furthermore, the cost of this approach may be prohibitive in large‐scale trials, and acceptability to caregivers would have to be assessed.
In conclusion, important differences in adherence to SQ‐LNS and dispersible tablets were found depending on the assessment method used. Caregiver report and disappearance rate seemed to overestimate adherence. Twelve‐hour home observation and PZC suggested that intake of SQ‐LNS and tablets was lower than reported, and/or that administration was not consistent with the instructions. This was especially true for the dispersible tablet. In the context of our study, there was no best method that could serve as a ‘gold standard’ for measuring adherence to SQ‐LNS or dispersible tablets. Objective tools to assess adherence should be adopted in randomised controlled trials to allow for better interpretation of the study outcomes.
Source of funding
This article is based on research funded by a grant to the University of California, Davis from the Bill & Melinda Gates Foundation. The findings and conclusions contained within are those of the authors and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation.
Conflicts of interest
KHB has worked as a consultant and later as employee for the Bill & Melinda Gates Foundation. SA reports grants from the Bill & Melinda Gates Foundation during the conduct of the study, and non‐financial support from Nutriset, outside the submitted work. None of the other authors have a conflict of interest to declare.
Contributions
KHB and SYH were responsible for the design of the study. SA, EYJ, JWS and RMG implemented the research and SYH, KHB, SAV and JBO supervised data collection. SA completed the statistical analyses and drafted the manuscript. EYJ, SYH and KHB edited the manuscript. KHB had primary responsibility for final content. All authors read and approved the final manuscript.
Acknowledgements
Our thanks go to Janet M. Peerson (UC Davis) for statistical advice, Zinéwendé P. Ouédraogo for contributing in the coordination of the iLiNS‐Zinc study, Karim Hayoro for supervising the 12‐h home observation data collection, and to the iLiNS project steering committee (http://www.ilins.org). Finally, we sincerely appreciate the support of the participating children and their parents, the local communities, and the staff of the Health District of Dandé.
Abbeddou, S. , Hess, S. Y. , Yakes Jimenez, E. , Somé, Jô. W. , Vosti, S. A. , Guissou, R. M. , Ouédraogo, J.‐B. , and Brown, K. H. (2015) Comparison of methods to assess adherence to small‐quantity lipid‐based nutrient supplements (SQ‐LNS) and dispersible tablets among young Burkinabé children participating in a community‐based intervention trial. Matern Child Nutr, 11: 90–104. doi: 10.1111/mcn.12162.
References
- Aaron G.J., Ba Lo N., Hess S.Y., Guiro A.T., Wade S. & Brown K.H. (2011) Plasma zinc concentration increases within 2 weeks in healthy Senegalese men given liquid supplemental zinc, but not zinc‐fortified wheat bread. Journal of Nutrition 141, 1369–1374. [DOI] [PubMed] [Google Scholar]
- Adu‐Afarwuah S., Lartey A., Brown K.H., Zlotkin S., Briend A. & Dewey K.G. (2007) Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: effects on growth and motor development. The American Journal of Clinical Nutrition 86, 412–420. [DOI] [PubMed] [Google Scholar]
- Adu‐Afarwuah S., Lartey A., Brown K.H., Zlotkin S., Briend A. & Dewey K.G. (2008) Home fortification of complementary foods with micronutrient supplements is well accepted and has positive effects on infant iron status in Ghana. The American Journal of Clinical Nutrition 87, 929–938. [DOI] [PubMed] [Google Scholar]
- Appelgren K.E., Nietert P.J., Hulsey T.C., Hollis B.W. & Wagner C.L. (2010) Analyzing adherence to prenatal supplement: does pill count measure up? International Journal of Endocrinology 2010, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arimond M., Zeilani M., Jungjohann S., Brown K.H., Ashorn P., Allen L.H. et al (2013) Considerations in developing lipid‐based nutrient supplements for prevention of undernutrition: experience from the International Lipid‐Based Nutrient Supplements (iLiNS) Project. Maternal & Child Nutrition 6, 12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becquey E., Ouedraogo C.T., Hess S.Y., Rouamba N., Prince L., Vosti S.A. et al (2013) Comparison of preventive and therapeutic zinc supplementation program effects on diarrhea and febrile illnesses among young children: a randomized trial. The FASEB Journal 27, 845.19.23450005 [Google Scholar]
- Brown K.H., Peerson J.M., Rivera J. & Allen L.H. (2002) Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta‐analysis of randomized controlled trials. The American Journal of Clinical Nutrition 75, 1062–1071. [DOI] [PubMed] [Google Scholar]
- Brown K.H., Rivera J.A., Bhutta Z., Gibson R.S., King J.C., Lonnerdal B. et al (2004) International Zinc Nutrition Consultative Group (IZiNCG) technical document #1. Assessment of the risk of zinc deficiency in populations and options for its control. Food and Nutrition Bulletin 25, S99–S203. [PubMed] [Google Scholar]
- Brown K.H., Lopez De Romana D., Arsenault J.E., Peerson J.M. & Penny M.E. (2007) Comparison of the effects of zinc delivered in a fortified food or a liquid supplement on the growth, morbidity, and plasma zinc concentrations of young Peruvian children. The American Journal of Clinical Nutrition 85, 538–547. [DOI] [PubMed] [Google Scholar]
- Caulfield L.E., Huffman S.L. & Piwoz E.G. (1999) Interventions to improve intake of complementary foods by infants 6 to 12 months of age in developing countries: impact on growth and on the prevalence of malnutrition and potential contribution to child survival. Food and Nutrition Bulletin 20, 183–200. [Google Scholar]
- Coates J., Swindale A. & Bilinsky P. (2007) Household food insecurity access scale (HFIAS) for measurement of food access: indicator guide. Washington, DC. [Google Scholar]
- De Roos N.M., De Vries J.H. & Katan M.B. (2001) Serum lithium as a compliance marker for food and supplement intake. The American Journal of Clinical Nutrition 73, 75–79. [DOI] [PubMed] [Google Scholar]
- Donahoo W.T., Bessesen D.H., Higbee D.R., Lei S., Grunwald G.K. & Higgins J.A. (2004) Serum lithium concentration can be used to assess dietary compliance in adults. Journal of Nutrition 134, 3133–3136. [DOI] [PubMed] [Google Scholar]
- Erhardt J.G., Estes J.E., Pfeiffer C.M., Biesalski H.K. & Craft N.E. (2004) Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C‐reactive protein by an inexpensive, sensitive, and simple sandwich enzyme‐linked immunosorbent assay technique. Journal of Nutrition 134, 3127–3132. [DOI] [PubMed] [Google Scholar]
- Geltman P.L., Meyers A.F., Mehta S.D., Brugnara C., Villon I., Wu Y.A. et al (2004) Daily multivitamins with iron to prevent anemia in high‐risk infants: a randomized clinical trial. Pediatrics 114, 86–93. [DOI] [PubMed] [Google Scholar]
- Geltman P.L., Hironaka L.K., Mehta S.D., Padilla P., Rodrigues P., Meyers A.F. et al (2009) Iron supplementation of low‐income infants: a randomized clinical trial of adherence with ferrous fumarate sprinkles versus ferrous sulfate drops. Journal of Pediatrics 154, 738–743. [DOI] [PubMed] [Google Scholar]
- Hedrick V.E., Dietrich A.M., Estabrooks P.A., Savla J., Serrano E. & Davy B.M. (2012) Dietary biomarkers: advances, limitations and future directions. Nutrition Journal 11, 1475–2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess S.Y., Abbeddou S., Yakes Jimenez E., Some J.W., Prado E., Ouedraogo Z.P. et al (2013) Small‐quantity lipid‐based nutrient supplements together with malaria and diarrhea treatment improve growth and neurobehavioral development in young Burkinabe children. Annals of Nutrition and Metabolism 63, 24. [Google Scholar]
- Huybregts L., Roberfroid D., Lanou H., Menten J., Meda N., Van Camp J. et al (2009) Prenatal food supplementation fortified with multiple micronutrients increases birth length: a randomized controlled trial in rural Burkina Faso. The American Journal of Clinical Nutrition 90, 1593–1600. [DOI] [PubMed] [Google Scholar]
- Jack S.J., Ou K., Chea M., Chhin L., Devenish R., Dunbar M. et al (2012) Effect of micronutrient sprinkles on reducing anemia: a cluster‐randomized effectiveness trial. Archives of Pediatrics & Adolescent Medicine 166, 842–850. [DOI] [PubMed] [Google Scholar]
- Kehoe S.H., Chheda P.S., Sahariah S.A., Baird J. & Fall C.H. (2009) Reporting of participant compliance in randomized controlled trials of nutrition supplements during pregnancy. Maternal & Child Nutrition 5, 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killilea D.W. & Ames B.N. (2008) Magnesium deficiency accelerates cellular senescence in cultured human fibroblasts. Proceedings of the National Academy of Sciences of the United States of America 105, 5768–5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo N.B., Aaron G.J., Hess S.Y., Dossou N.I., Guiro A.T., Wade S. et al (2011) Plasma zinc concentration responds to short‐term zinc supplementation, but not zinc fortification, in young children in Senegal. The American Journal of Clinical Nutrition 93, 1348–1355. [DOI] [PubMed] [Google Scholar]
- Paulhus D.L. (1991) Measurement and control of response bias In: Measures of Personality and Social Psychological Attitudes (eds Robinson J.P. & Shaver P.R.), pp. 17–59. Academic Press: San Diego, CA. [Google Scholar]
- Pullar T., Kumar S. & Feely M. (1989) Compliance in clinical trials. Annals of the Rheumatic Diseases 48, 871–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S.B., Morrow F.D., Evans W.J., Shepard D.C., Dallal G.E., Meredith C.N. et al (1990) Use of p‐aminobenzoic acid to monitor compliance with prescribed dietary regimens during metabolic balance studies in man. The American Journal of Clinical Nutrition 51, 485–488. [DOI] [PubMed] [Google Scholar]
- Samadpour K., Long K.Z., Hayatbakhsh R. & Marks G.C. (2011) Randomised comparison of the effects of Sprinkles and Foodlets with the currently recommended supplement (Drops) on micronutrient status and growth in Iranian children. European Journal of Clinical Nutrition 65, 1287–1294. [DOI] [PubMed] [Google Scholar]
- Siega‐Riz A.M., Estrada Del Campo Y., Kinlaw A., Reinhart G.A., Allen L.H., Shahab‐Ferdows S. et al (2014) Effect of supplementation with a lipid‐based nutrient supplement on the micronutrient status of children aged 6–18 months living in the rural region of Intibuca, Honduras. Paediatric and Perinatal Epidemiology 13, 12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurnham D.I., McCabe L.D., Haldar S., Wieringa F.T., Northrop‐Clewes C.A. & McCabe G.P. (2010) Adjusting plasma ferritin concentrations to remove the effects of subclinical inflammation in the assessment of iron deficiency: a meta‐analysis. The American Journal of Clinical Nutrition 92, 546–555. [DOI] [PubMed] [Google Scholar]
- Wessells K.R., Ouedraogo Z.P., Rouamba N., Hess S.Y., Ouedraogo J.B. & Brown K.H. (2012) Short‐term zinc supplementation with dispersible tablets or zinc sulfate solution yields similar positive effects on plasma zinc concentration of young children in Burkina Faso: a randomized controlled trial. Journal of Pediatrics 160, 129–135. [DOI] [PubMed] [Google Scholar]
- Zlotkin S., Arthur P., Schauer C., Antwi K.Y., Yeung G. & Piekarz A. (2003) Home‐fortification with iron and zinc sprinkles or iron sprinkles alone successfully treats anemia in infants and young children. Journal of Nutrition 133, 1075–1080. [DOI] [PubMed] [Google Scholar]


